SUMMARY
Oxytocin (OT) is a neuropeptide elaborated by the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei. Magnocellular OT neurons of these nuclei innervate numerous forebrain regions and release OT into the blood from the posterior pituitary. The PVN also harbors parvocellular OT cells that project to the brainstem and spinal cord, but their function has not been directly assessed. Here, we identified a subset of approximately 30 parvocellular OT neurons, with collateral projections onto magnocellular OT neurons and neurons of deep layers of the spinal cord. Evoked OT release from these OT neurons suppresses nociception and promotes analgesia in an animal model of inflammatory pain. Our findings identify a new population of OT neurons that modulates nociception in a two tier process: (1) directly by release of OT from axons onto sensory spinal cord neurons and inhibiting their activity and (2) indirectly by stimulating OT release from SON neurons into the periphery.
INTRODUCTION
Oxytocin (OT), a neuropeptide that plays an important role in sociability, is produced in the brain exclusively in the hypothalamic paraventricular (PVN), supraoptic (SON), and intermediate accessory nuclei (Swanson and Sawchenko, 1983). OT neurons can be classified in magnocellular OT (magnOT) and parvocellular OT (parvOT) neurons, which are distinct in size and shape, subnuclear location, the amount of OT production, and involvement in distinct circuitries and functions (Armstrong et al., 1980; Swanson and Kuypers, 1980; Sofroniew, 1983; Swanson and Sawchenko, 1983).
According to a long-held dogma, magnOT neurons provide systemic OT supply by release into the blood via the posterior pituitary (Scharrer, 1928; Scharrer and Scharrer, 1940, Bargmann and Scharrer, 1951). Simultaneously, magnOT neurons innervate the forebrain, including the nucleus accumbens (Ross et al., 2009; Knobloch et al., 2012; Dölen et al., 2013) and the central nucleus of the amygdala (Knobloch et al., 2012). The forebrain fibers, as exemplarily studied in the central amygdala, allow for focal release and discrete, modulatory action of OT (Knobloch et al., 2012). These characteristics might account for the distinct impact of OT on numerous types of brain-region specific behaviors (Lee et al., 2010).
In contrast to magnOT neurons, parvOT neurons project to distinct brainstem nuclei and different regions of the spinal cord (SC) (Swanson and Sawchenko, 1983; Sawchenko and Swanson, 1982). Based on the location of parvOT axons and the effects of externally applied OT, it has been proposed that OT from parvOT axonal terminals contributes to modulation of cardiovascular functions, breathing, feeding behavior, and nociception (Mack et al., 2002; Petersson, 2002; Condés-Lara et al., 2003; Atasoy et al., 2012). However, no selective and specific genetic access to parvOT neurons has been available and, hence, there was no evidence for the capacity of parvOT axons to release endogenous OT and to selectively modulate the above-mentioned functions. Moreover, it has remained unknown how parvOT neurons are incorporated into the entire OT system and functionally interact with magnOT neurons.
Based on recent reports that OT-modulated nociception and pain response comprise a peripheral (Juif and Poisbeau, 2013) and a central component (Juif et al., 2013; González-Hernández et al., 2014), it is tempting to propose that these components are dependent on different OT cell types. The central component results from parvOT innervation of SC targets (Swanson and McKellar, 1979), whereas peripherally acting OT, in contrast, is provided to the blood stream by magnOT neurons and presumably targets C-type fibers in the dorsal root ganglion (DRG; Juif and Poisbeau, 2013). We therefore hypothesized that the complementary, analgesic OT action—at central and peripheral levels—depends on the communication between magnOT and parvOT neurons residing in spatially segregated OT nuclei. Our present results reveal that the modulation of pain signals by OT is triggered by only a handful of parvOT neurons that innervate simultaneously “sensory wide dynamic range” (WDR) neurons in the deep laminae of the SC, expressing neurokinin-1 (NK1R) and OT receptors (OTR), and SON neurons that secrete OT in the periphery. We show that these separate innervations underlie a two-tier modulation of pain by OT reaching the SC through fast, direct neuronal projections and a slower, indirect peripheral pathway.
RESULTS
Intrahypothalamic Axonal Trees of the OT System
To examine the intrahypothalamic OT system, we used recombinant adeno-associated virus (rAAV), allowing cell-type specific fluorescent labeling of OT neurons with 98%–100% cell-type specificity, as reported in Knobloch et al. (2012). To compare the OT system with the vasopressin (VP) system, we used AAV carrying different fluorescent markers driven by an evolutionarily conserved VP promoter (for specificity, see Table S1; Figure S1A).
After injection of rAAV expressing Venus, under the control of an OT promoter (Figure 1A), we observed that OT neurons of the PVN give rise to fibers connecting to the ipsi- and even contralateral SON and form a pronounced plexus (Figures 1A4 and 1A5). Interconnections within the intrahypothalamic VP system, in contrast, were absent (Figure S1C). The OT plexus might stem from PVN OT neurons projecting above the third ventricle to the contralateral PVN (Figure 1A3). OT connectivity from the PVN to SON was present in females and males (Figure S2A). The connection between the OT nuclei was one-way: the SON-arising OT fibers reached only marginally the ipsi- (Figure 1A9) and never the contralateral SON (Figure 1A7) or PVN (Figure 1A8).
Figure 1. Anatomical and Functional Connectivity between OT Neurons of the PVN and SON.
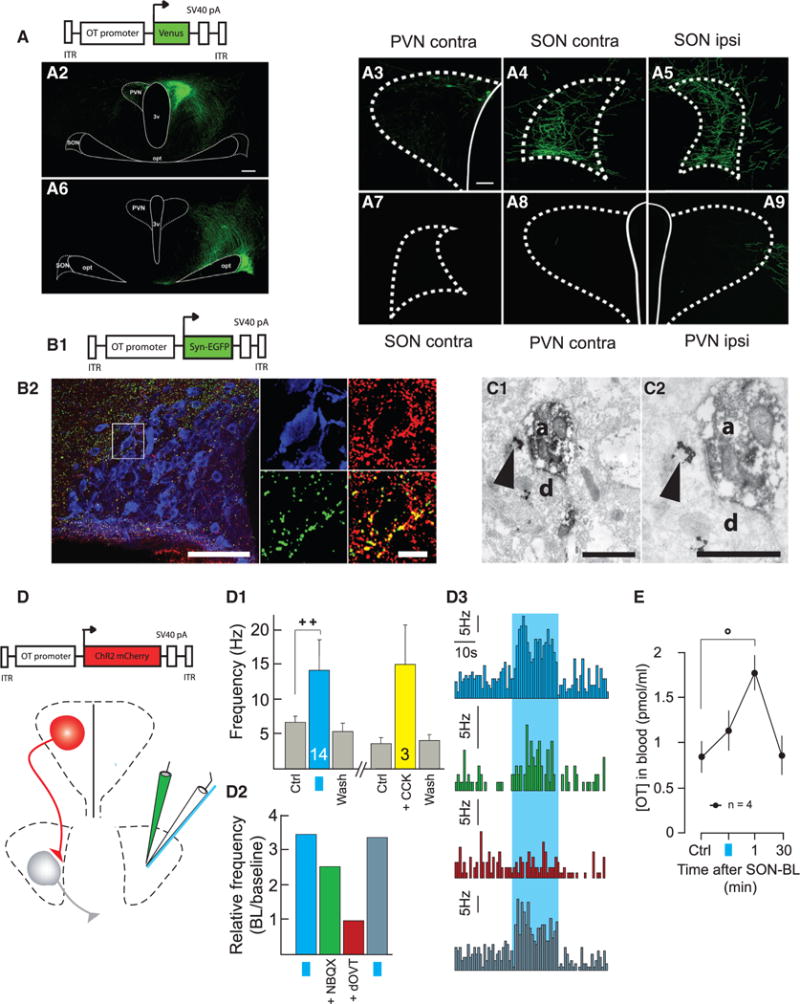
(A) OT projections from PVN to SON.
(A1) Scheme of the viral vector used to infect PVN neurons.
(A2–A9) PVN OT neurons infected with cell-type specific viral vector project Venus-positive axons to contralateral PVN (A3) and to contra- and ipsilateral SON
(A4 and A5). OT neurons of the SON (A6) do not project Venus axons to contralateral SON (A7) or PVN (A8) and only marginally enter the external border of ipsilateral PVN (A9). The scale bars represent 200 μm (left) and 50 μm (right).
(B) OT axon terminals contain vGluT2.
(B1) Scheme of viral vector.
(B2) GFP-positive terminals in the area of the SON (left). In the magnified inset (right), the OT neuron (blue) is surrounded by GFP terminals, which also contain vGlut2 (red). Both of the immunosignals overlap (yellow) in virtually all of the terminals. The scale bars represent 100 μm (left) and 25 μm (right).
(C–C2) Electron microscopy OT axon terminals (Venus visualized as diaminobenzidine [DAB] endproduct, OT, as a silver-gold-intensified DAB) form asymmetric synapses on OT-ir dendrite within the SON. The OT-immunoreactivity (clusters of silver particles, arrows) are shown in the presynaptic axon (a) terminal and postsynaptic dendrite (d) at lower (C1) and higher magnifications (C2). The scale bar represents 0.5 μm.
(D) Scheme of the viral vector and setup of in vivo electrophysiological recordings (white pipette) in SON, together with SON-BL stimulation (blue fiber) and drug infusion (green pipette).
(D1–D3) Functional connection between PVN and SON OT neurons. (D1) Average spike frequencies of SON OT neurons before (Ctrl), after either SON-BL (n = 14, blue bar) or systemic injection of CCK (n = 3, yellow bar), and after washout effect (Wash). (D2) Relative frequency increase induced by SON-BL in control condition (blue bar), after infusion of NBQX (1 μM, 0.5 μl; green bar), after additional infusion of dOVT (1 μM, 0.5 μl; red bar), and after 30 min washout of the drugs (dark blue bar). (D3) Histograms of the frequency rates recorded under conditions described in (D2).
(E) Effect of unilateral SON-BL effect on OT blood concentration at the end of SON-BL, 1 min and 30 min after (n = 4). All results are expressed as average ± SEM. The statistical significances: ++ p < 0.01 and Wilcoxon’s test. (°p < 0.05, Friedman’s test followed by Dunn post hoc test) The blue squares represent 20 s BL stimulation at 30 Hz with 10 ms pulses of BL stimulation.
The OT PVN-SON connection was reconstructed using light sheet microscopy. As presented in Figure S2B, descending fibers from the PVN mainly project rostro-ventrally, turn horizontally at the level of the SON, and enter the SON from the rostral position, to run caudally along the whole extent of the nucleus.
PVN OT Neurons Innervate the SON and Control MagnOT Neuron Activity to Induce OT Release into Blood Circulation
At the light microscopic level, Venus-labeled OT axons that arose from the PVN formed tight appositions to dendrites and somata of magnOT SON neurons resembling synaptic contacts. To assess if synapses were present, we injected the PVN with rAAV that expresses the synaptic marker synaptophysin fused to the green fluorescent marker EGFP in PVN OT neurons (Figure 1B1). GFP-positive puncta were found in the SON. The vast majority of terminals with GFP signal overlapped with VGluT2 signal (red, Figure 1B2). GFP/VGluT2 terminals engulfed OT cell bodies and dendrites (blue, Figure 1B2). We found that EGFP signals overlapped with VGluT2 in 92.6% ± 8.3% of all terminals (Figure 1B2). These light microscopic observations suggested the presence of synaptic contacts, which we further confirmed at the electron microscopic level: EGFP-positive OT axons from the PVN (EGFP: greyish filling) formed asymmetric (presumably glutamatergic) synapses on OT dendrites of the SON (OT: dark aggregate in pre- and postsynaptic elements; Figures 1C1 and 1C2).
Based on the anatomical evidence for OT connections between PVN and SON neurons, we aimed for a functional characterization of these connections. We expressed the blue-light (BL)-sensitive ChR2 protein (Nagel et al., 2003) fused to mCherry in PVN OT neurons (for construct validation, see Knobloch et al., 2012). In vivo extracellular recordings in anaesthetized animals revealed the expression of functional ChR2 in the PVN, as evident from BL-induced (PVN-BL, 20 s at 30 Hz with 10 ms pulses), reversible, and reproducible increases of spike frequencies in these PVN neurons (on average the frequency increased from 4.1 ± 0.7 to 7.8 ± 0.7 Hz; data not shown).
We then further tested in vivo whether exposure to BL of PVN-OT axons in the SON (SON-BL, scheme in Figure 1D) could also activate, ipsilaterally, SON neurons. SON-BL exposure evoked a reversible increase in spike frequencies of SON neurons, from 6.7 ± 1.5 to 14.1 ± 2.7 Hz, confirming that BL stimulation of parvOT PVN axon terminals could excite SON neurons (Figure 1D1). To verify that OT was the main transmitter involved, we recorded the response of a single neuron to the SON-BL in the absence of any drug, or after sequential infusion of AMPA and OTR antagonists (respectively, NBQX and dOVT) into the SON, and after their washout (Figures 1D2 and 1D3). Interestingly, while NBQX decreased the baseline frequency of SON neurons, SON-BL paired to NBQX application still efficiently increased the relative frequency of discharge of the recorded neuron. Subsequent dOVT infusion totally blocked the SON-BL response, with full recovery 30 min after washout (Figures 1D2 and 1D3). These results are in accordance with our previous observations in the central amygdala (Knobloch et al., 2012).
We aimed at providing functional evidence for the OT nature of the SON neurons that were contacted by the PVN. To this purpose, we first of all injected into the blood circulation cholecystokinin (CCK) a hormone inducing the activation of OT neurons (Verbalis et al., 1986). CCK induced a prominent increase in spike frequencies of SON-BL responding neurons from 3.6 ± 0.8 to 15.0 ± 5.9 Hz (Figure 1D1), establishing an indirect argument of the OT identity of the in vivo recorded SON neuron. Second, as magnOT neurons are known to release OT in the blood, we performed a time-dependent measurement of OT concentrations in plasma by mass-spectrometry after SON-BL. This revealed a significant increase of OT plasma concentrations at 60 s after SON-BL (from 0.84 ± 0.17 to 1.76 ± 0.22 pmol/ml; Figure 1E). Taken together, these findings provide evidence for an OT identity of the SON neurons that are activated by axonal terminals originating from OT neurons in the PVN.
OT Neurons Projecting to SON MagnOT Neurons Are ParvOT Neurons Displaying Distinct Anatomical and Electrophysiological Characteristics
To identify PVN neurons projecting to the SON, we injected into the SON retrogradely transported and monosynaptically transmitted canine adenovirus 2 (CAV2). After counting of sections containing the entire PVN, we identified in total a very small population of GFP/OT-positive neurons residing bilaterally (Table S2; 31.5 ± 8.5 neurons). CAV spread occurs within 200 μm of the injection site (Schwarz et al., 2015), making unlikely the diffusion of the virus from the SON to PVN (the distance between these two nuclei is about 1.5 mm; see Paxinos and Watson, 1998).
To characterize the magno- versus parvocellular nature of back-labeled PVN cells, we combined CAV2 with systemic administration of Fluorogold (Figure S3A). Fluorogold, when injected intraperitoneal (i.p.), is taken up by neurons projecting beyond the blood brain barrier, for example, by magnOT neurons, thus allowing to distinguish them from the parvOT (Fluorogold-negative) neurons (Luther et al., 2002; Table S2). Notably, all magnOT neurons of the SON were Fluorogold-positive (data not shown). After neuron counting in sections containing the entire PVN, we established that the vast majority of the 31.5 ± 8.5 GFP/OT-positive neurons (90%) did not contain Fluorogold (Table S2). In addition to the detection of back-labeled GFP-positive neurons in the PVN, we observed GFP neurons in other structures typically known to innervate the SON, further confirming the specificity of our retrograde labeling (Miselis, 1981; Cunningham and Sawchenko, 1988; Figure S3B).
To characterize the parvOT neurons projecting to the SON, we next injected into the SON CAV2 expressing Cre recombinase and into the PVN rAAV carrying a double-floxed inverted open reading frame (ORF) (DIO) of GFP under the control of the OT promoter (Figure 2A1). By this combination, we limited GFP expression exclusively to SON-projecting parvOT neurons. In line with previous results, this revealed a unique position of back-labeled GFP neurons in the dorso-caudal PVN (Figures 2A2, 2A3, S2B, and S2C). Individual GFP neurons have bipolar spindle-like morphology (Figure 2A4) distinct from neighboring magnOT neurons (Figure 2A5). The number of back-labeled PVN GFP (exclusively OT) neurons was comparable (33.4 ± 9.1), with the estimation of non-selectively labeled PVN neurons identified by costaining with OT antibodies.
Figure 2. Anatomical and Electrophysiological Characteristics of PVN OT Neurons Projecting to the SON.
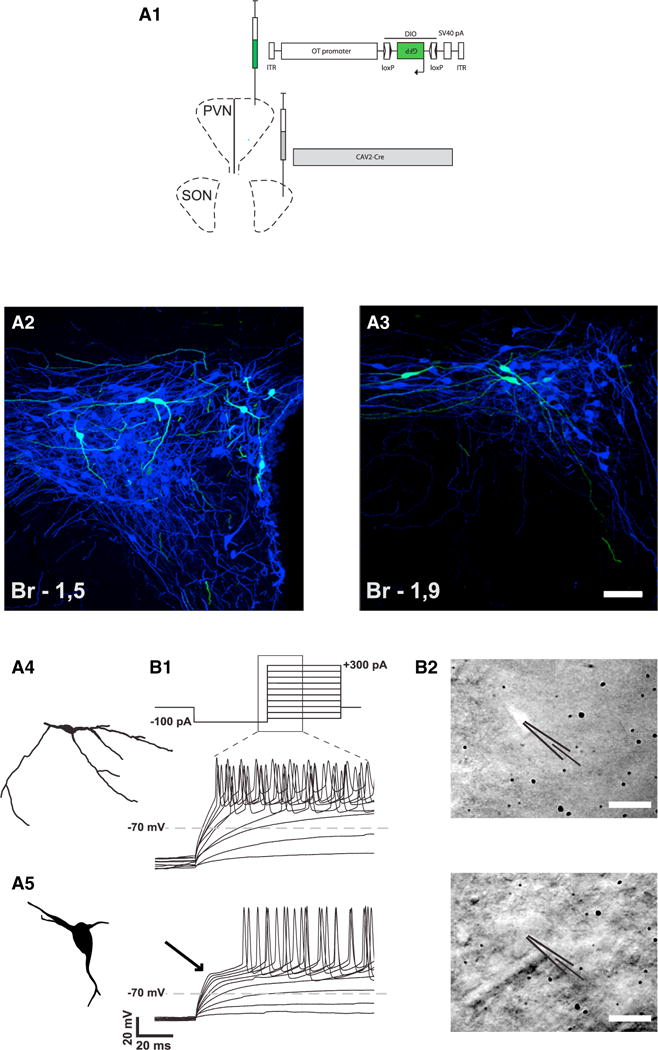
(A) Identification of a subset of OT neurons projecting from PVN to SON.
(A1) Scheme showing the injection of viruses in the SON and PVN.
(A2 and A3) Defined subset of back-labeled OT neurons (green) in dorso-caudal PVN displays consistent morphology: small oval somas (12 to 20 μm in diameter) with predominantly longer horizontal axes. The scale bar represents 50 μm in (A2) and 50 μm in (A3).
(A4 and A5) The morphology of these cells is clearly distinct from the typical magnocellular neurons with large cell bodies and less branching processes (A5).
(B) Functional differentiation of this subset of PVN OT neurons.
(B1) Current steps protocol starting from a hyperpolarizing current chosen to reach −100 mV (here 100 pA) followed by progressively more depolarizing current injections (upper trace). The representative changes in membrane potential for the parvOT and magnOT PVN neurons during the part of the current steps as indicated by the zoomed area are shown (lower traces). The ParvOT neurons (middle trace) do not display the transient outward rectification specific for the magnOT neurons (lower trace, arrow).
(B2) Photographs of a GFP-fluorescent parvOT neuron (upper) in the PVN (labeled by injection of CAV2-Cre into the SON and OT-DIO-GFP AAV in the PVN) and in the same area a typical magnOT neuron (lower) as indicated by the patch pipettes. The scale bars represent 20 μm.
OT neurons similar in morphology and location were obtained in our initial study (data not shown) with the application of latex retrobeads (Katz and Iarovici, 1990) in the SON, which, however, labeled only few cells in the PVN (and other structures innervating the SON; Figure S3), precluding quantitative analysis.
We determined the electrophysiological characteristics of fluorescently labeled neurons in the PVN to assess their parvocellular nature. We conducted whole-cell patch clamp recordings in slices (Figure 2B) in current clamp applying a protocol of depolarizing current injections (Figure 2B1). This was aimed to determine the presence of a transient outward rectification, which is typically found in magnOT, but not in parvOT neurons (Luther et al., 2002). We recorded in a total of seven animals 11 fluorescent putative parvOT, and found that none, as expected, exhibited a hyperpolarizing notch. Conversely, all of the 13 non-fluorescent neurons from the same region (putative mag-nOT) showed the typical transient outward rectifying current, as known as (aka) “notch”. Quantification of these differences was made by analyzing the time to spike (spike delay) and rise slope, which was the slope measured between beginning of the depolarization and the peak time of the first action potential. Both of these parameters showed highly significant differences between the two groups of neurons (Table S4; Figure S4). Differences in the spike frequency also showed a tendency, though with less significance than previously reported (Luther et al., 2000). The electrophysiological responses were in agreement with the morphology of the cells. Neurons classified electrophysiologically as parvOT had a small soma and a more elongated shape, while the ones classified as magnOT had a big soma and were more rounded (Figure 2B2).
ParvOT Neurons Innervating MagnOT Neurons Also Project Specifically to NK1R/OTR Positive WDR Neurons in the Deep Layers of the SC
The above established exclusive labeling of parvOT neurons and all their processes using a combination of CAV2-Cre with OT cell typed-specific Cre-dependent rAAV (Figures 3A1–3A3) allowed us to follow projections of this OT cell population up to the distal (L5) segments of the SC. After labeling presumably all PVN OT neurons, axons can be visualized in both superficial and deep SC layers (Figure S5A). In contrast, we found that synaptophysin-GFP-filled terminals from parvOT only were loosely and sparsely distributed in superficial laminae, but heavily innervate deep laminae, in close proximity to neurokinin 1 receptor (NK1R)-positive large cells (diameter 30–40 μm; Figure 3A2) identified as sensory WDR neurons (Ritz and Greenspan, 1985). Importantly, in an additional labeling (on separate slices because both OTR and NK1R antibodies had been raised in the same species), we found in this same region cells that expressed OTR (Figure 3A3). The specificity of the antibody was confirmed in transfected HEK cells (Figure S5B1) and brainstem sections of OTR knockout mice (Figure S5B2), in agreement with a previous study using these antibodies in mouse cortex (Marlin et al., 2015). To show the co-localization of NK1R and OTR in the same cells, we performed fluorescent in situ hybridization and found the presence of respective mRNAs in the same neurons of deep layers of the SC (Figures 3B1–3B4). As a next step, we wanted to demonstrate that NK1R-positive neurons of deep SC laminae could be activated by sensory/pain stimulation. Bilateral injection of capsaicin in the hindpaws indeed induced c-Fos expression in large NK1R neurons (Figures 3C1–3C3). Furthermore, back-labeled parvOT neurons were also activated by capsaicin (data not shown).
Figure 3. ParvOT Neurons Project to SC and Innervate NK1R/OTR WDR Neurons in Deep Laminae.
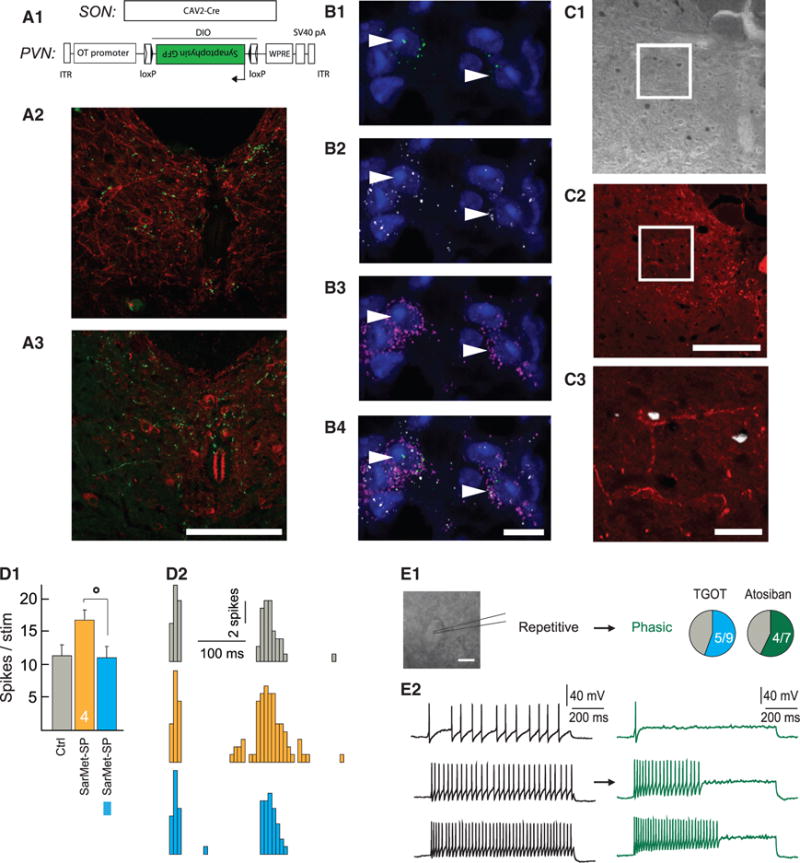
(A) ParvOT projections to the SC.
(A1) Scheme of the viruses injected into the SON and PVN.
(A2) Detection of synaptophysin-GFP containing terminals (green) in close proximity to NK1R-positive neurons (red) in SC deep laminae.
(A3) Synaptophysin-GFP terminals locate close to OTR-positive neurons of deep laminae. The scale bars represents 500 μm in (A2) and 500 μm in (A3).
(B–B4) Colocalization of NK1R and OTR mRNAs in the same neurons of SC deep laminae. Immunofluorescent in situ hybridization revealed the presence of OTR mRNA (green dots; B1 and B4) and NK1R mRNA (white dots; B2 and B4) in the same neurons, which were visualized by detection of vGlut1/2/3 mRNAs in their somas (pink/violet dots; B3 and B4). The nuclei of cells were stained by DAPI. The arrow heads point NK1R/OTR double positive neurons. The scale bars represent 10 μm.
(C–C3) NK1R-positive SC neurons start to express c-Fos after intraplantar injection of capsaicin in the hindpaw. The c-Fos signal (DAB) was detected in deep laminae of SC (C1), where the NK1R (red) were located (C2). The digital overlay of the two signals demonstrates localization of c-Fos in the NK1R-postive neuron
(C3). The scale bars represent 500 μm in (C1) and (C2) and 50 μm in (C3).
(D) WDR C-fiber evoked spikes in response to a series of isolated hindpaw stimulations in control condition (Ctrl), during application of the specific agonist of NK1R SarMet-SP (orange), and during SarMet-SP paired with BL (blue).
(D1) Average of C-fiber evoked spikes (n = 5).
(D2) Representative traces.
(E) Discharge profile of putative WDR recorded in current clamp applying a protocol of depolarizing current injections before (black) and after bath application of 1 μM TGOT (blue, n = 9) or 1 μM Atosiban (green, n = 7).
(E1) Proportion of putative WDR neurons discharge pattern changed from repetitive to phasic after TGOT or Atosiban bath application.
(E2) Example response of putative WDR neuron to 20 pA (top), 40 pA (middle), and 60 pA (bottom) current injection before (black) and after (green) Atosiban bath application. The scale bar represents in (E1) 30 μm. All results are expressed as average ± SEM. The statistical significance: °p < 0.05, Friedman’s test followed by Dunn post hoc test.
To show that NK1R WDR neurons are functionally modulated by both NK1R specific agonist (SarMet-SP) and parvOT-deriving OT, we measured in vivo the WDR C-fiber evoked spikes in response to a series of isolated hindpaw stimulations. We found that the C-fiber evoked spikes were increased in the presence of SarMet-SP, as expected (Budai and Larson, 1996). Interestingly, BL-activation of ChR2 expressing parvOT fibers in the SC (SC-BL; schemes in Figures 5A and 5B) upon SarMet-SP significantly reduced the number of C-fiber evoked spikes from 16.8 ± 1.1 to 11.2 ± 1.5 (Figures 3D1 and 3D2). These findings show that release of OT from parvOT axons can effectively inhibit the activity of WDR neurons potentiated by NK1R activation.
Figure 5. Stimulation of ParvOT PVN Axons in SON and SC Modulates Responses of WDR Neurons.
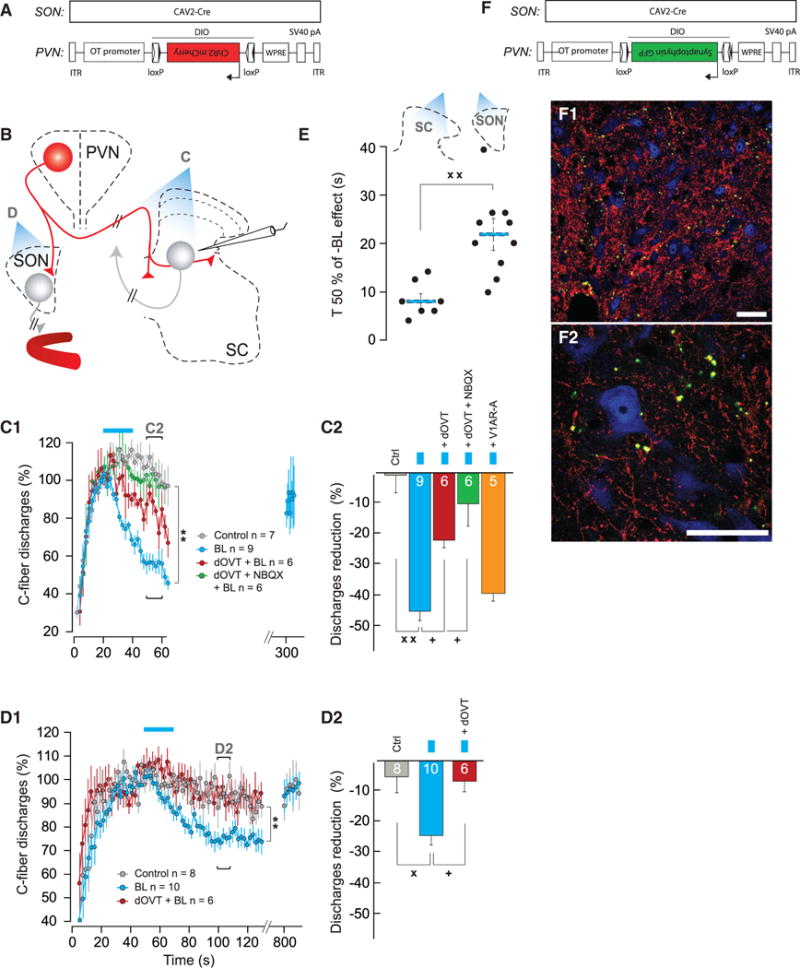
(A) Viruses injected into the SON and PVN.
(B) Scheme of the experimental procedures.
(C) Effect of SC-BL on WDR-C discharges.
(C1) Time course of WDR-C in control condition (n = 7), when shining SC-BL alone (n = 9), after local dOVT application (n = 6), or local dOVT + NBQX application (n = 6).
(C2) Average discharge reduction of WDR-C on Ctrl (n = 7), when shining SC-BL alone (n = 9), after local dOVT application (n = 6), local dOVT + NBQX application (n = 6), or local V1AR-A application (n = 5). The statistical significance of drug modulation of the SON-BL effect was assessed by comparing the effect of SON-BL on the same neuron before and after drug injection.
(D) Effect of SON-BL on WDR-discharges.
(D1) Time course of WDR-C in control condition (n = 8), measured 30 s after shining SON-BL (as indicated in C1) alone (n = 10), or after systemic dOVT systemic injection (n = 6).
(D2) Average discharge reduction of WDR-C on Ctrl (n = 8), when shining SON-BL alone (n = 10), or after systemic dOVT injection (n = 6). The statistical significance of dOVT modulation of the SON-BL effect was assessed by comparing the effect of SON-BL on the same neuron before and after dOVT injection (n = 6).
(E) Comparison between individual (black dots) and average T 50% (blue bar) effect of SON-BL (n = 10) and SC-BL (n = 7) on recorded WDR.
(F) Viruses injected into the SON and PVN.
(F1 and F2) Axonal terminals containing synaptophysin-GFP fusion protein in proximity to SC L5 neurons.
(F1) Overview of fiber distribution within SC: VGluT2 (red), synaptophysin-GFP (green), and NeuN (blue).
(F2) A zoom-in shows the green signal (green) largely overlaps with the VGluT2 signal (red) in terminals surrounding cell bodies. The scale bars represent 50 μm in (F1) and (F2). All results are expressed as average ± SEM. The statistical significance: °p < 0.05, Friedman with Dunn post hoc test; + p < 0.05, ++ p < 0.01, and Wilcoxon’s test; xx p < 0.01 and Kruskal and Wallis test; and ** p < 0.01 BL versus Control, two-way ANOVA.
Then, we analyzed the inhibitory effect of OT on WDR neuron firing properties. To do so, we performed in vitro whole-cell patch clamp recordings in current clamp applying a protocol of depolarizing current injections (Figure 3E; Breton et al., 2009). Putative WDR neurons, identified by their large cell body and repetitive firing related to stimulation intensities (Figure 3E; Ritz and Greenspan, 1985), were located around the central canal (cc) and in deep layers V–VI. As expected, [Thr4,Gly7]-OT (TGOT) was able to change the firing properties of these neurons, from repetitive to phasic in 5/9 of recorded cells (Figure 3E), similar to what was described in superficial layers (Breton et al., 2009). Importantly, the application of a specific biased agonist for OTR linked to Gi subunit, Atosiban (Busnelli et al., 2012), induced the exact same effects in 4/7 recorded cells (Figure 3E). This experiment demonstrates for the first time on living tissue that OTR can functionally bind a Gi protein, thus elucidating the inhibitory mechanism of OT on the firing properties of WDR neurons.
Finally, to demonstrate that the population of identified parvOT neurons is a single anatomical unit and that the same cells project collaterals to both the SON and SC, we injected a CAV2-Cre virus in deep laminae of L5 and Cre-responder AAV expressing GFP under the OT promoter in the PVN (Figure 4A1). We detected back-labeled cell bodies of GFP/OT neurons in the PVN and their axonal projections in close proximity to somas and dendrites of magnOT neurons of the SON (Figures 4A2–4A5).
Figure 4. ParvOT-MagnOT-SC Anatomical Unit.
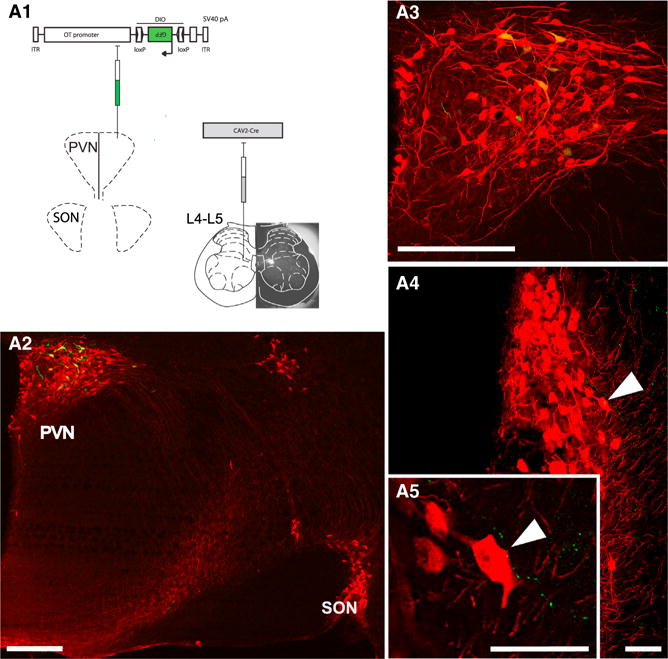
(A) Scheme of viruses injected into the SC and PVN. The actual SC injection site (fluorescent latex bead accumulation) is shown as an insert underlying SC drawing.
(A2–A5) PVN parvocellular cells back-labeled from SC (green). The GFP-positive cell bodies were found in the caudal portion of the PVN and always colocalized OT (red) (A3, magnification from A2). Fibers, projecting from back-labeled PVN OT neurons to SON (arrow in A4, more caudal to A2) GFP-expressing varicouse axons in close proximity to cell bodies and dendrites of SON mag-noOT neurons (high magnification in A5) are shown. The scale bars represent 500 μm in (A2) and (A3) and 75 μm in (A4) and (A5).
ParvOT Neurons Projecting to MagnOT SON Neurons and NK1R/OTR Positive WDR Neurons in Deep Layers of the SC Control the Central Nociceptive Processing
To test whether the specific population of PVN-OT neurons projecting to both the SON and SC indeed acts on nociceptive input, we recorded SC neuronal responses in vivo during electrical stimulation of their hindpaw receptive field. The coding properties and short-term potentiation (wind-up; WU) following repetitive receptive field stimulation were calculated from the response of WDR neurons in deep laminae. Recordings include the deep laminae, which integrate convergent peripheral sensory information from fast-conducting (A-type) and slow-conducting (C-type) primary afferent fibers (Figures 5 and S6).
We first tested the inhibitory action of OT released from parvOT-hypothalamo-spinal terminals by shining BL directly onto the dorsal surface of the SC (SC-BL). In this set of experiments, we used the same combination of viruses (CAV2-Cre and rAAV carrying OT promoter-DIO-ChR2-mCherry) to elicit OT release from parvocellular PVN fibers. SC-BL efficiently reduced the WDR discharges from C- (−44.6% ± 3.7%; Figures 5C1 and 5C2) and Aδ- (−36.3% ± 4.5%), but not from Aβ- fibers (−0.3% ± 2.8%; Figure S6G2). The half-efficacy of SC-BL inhibition was 8.3 ± 1.3 s (Figure 5E). The WU returned to control values ~300 s after SC-BL (Figure 5C1). SC-BL had no effect on superficial layer neuron activity in the same recording condition as for WDR neurons (Figure S6F). The OTR antagonist dOVT, directly applied to the surface of the SC, significantly, but not entirely, reduced Aδ- and C-fiber mediated discharges (Figures 5C1 and 5C2). In contrast, the VP receptor type 1A antagonist applied on SC failed to change the SC-BL inhibition of WU intensity (Figure 5C2), whereas it could efficiently block the effect of exogenously applied AVP (data not shown). Since VGluT2 was detected in synaptophysin-GFP-containing (Figures 5F1 and 5F2; overlap of GFP and vGluT2 signals was found in 89% ± 7.4% GFP terminals) axonal terminals of parvOT neurons near cell bodies of WDR-like neurons (Figure 5F2), we assessed the effect of NBQX in vivo. Coapplication of both dOVT and NBQX entirely blocked the SC-BL effects (Figures 5C1 and 5C2). Thus, stimulation of parvOT axons in SC deep layers leads to a fast, short-lasting decrease in nociceptive processing which is mediated by central OTR, and to a lesser extent by ionotropic Glut receptors.
We then assessed the efficiency of OT release from parvOT neurons onto magnOT SON neurons in modulating nociception (Figures 5B and S6A). Eliciting OT release from parvOT fibers in SON by BL (SON-BL) significantly reduced the WDR discharges evoked by slow-conducting C-type fibers (−24.9% ± 3.1%; Figures 5D1 and 5D2) and fast-conducting fibers Aδ- (−30.0% ± 6.8%), but not by non-nociceptive, fast-conducting Aβ- fibers (−6.4% ± 3.5%; Figure S6G1). The half-efficacy of SON-BL induced inhibition of WU was 22.2 ± 3 s (T 50%; Figure 5E), a value which was significantly higher than the SON-BL effect (Figure 5E). The WU intensity returned to control values only 800 s after SON-BL (Figure 5D1). Moreover, to further confirm that the reduction in WU intensity was related to the elevated level of blood OT (see Figure 1E), we injected the OTR antagonist dOVT intravenously before applying SON-BL. As expected, this abolished the SON-BL inhibition of WDR discharges that were evoked by both Aδ- and C-fibers (Figures 5D1, 5D2, and S6G1). Thus, the central release of OT from parvOT axons targeting magnOT SON neurons leads to a systemic release of OT, which reduces nociceptive processing by WDR neurons. This effect was slow to appear and long-lasting.
In summary, the subpopulation of PVN OT parvOT neurons projecting both to magnOT SON neurons and to NK1R/OTR WDR neurons from deep layers of SC exerts an inhibition of spinal nociceptive processing by fast action on SC neurons and a relatively slower effect on peripheral targets by stimulation of SON neurons and subsequent induction of OT release into blood.
Activation of ParvOT Neurons Results in Analgesia
In the last part of our work, we analyzed the functional importance of these parvOT neurons in the processing of inflammatory compared to nerve injury-induced neuropathic pain. To this purpose, we measured both the effects of stimulation or inhibition of parvOT neurons on the symptoms of either a peripheral painful inflammatory sensitization triggered by a single unilateral intraplantar injection of complete Freund adjuvant (CFA) or a nerve injury-induced neuropathy induced by the cuffing of the sciatic nerve (Cuff; Pitcher et al., 1999; Figure S7C1). To this purpose, we used rats that expressed either ChR2 or hM4Di (Zhu and Roth, 2014) restricted to parvOT PVN neurons synapsing on magnOT SON neurons (Figure 6A). The efficiency of ChR2-mediated activation and hM4Di-mediated inhibition of OT neurons was assessed respectively by targeting unilaterally the PVN by BL or by i.p. administration of CNO and was confirmed both in vitro (Figures S7A1 and S7A2) and in vivo (Figures S7B1–S7B4).
Figure 6. Activation/Inhibition of ParvOT PVN Neurons Modulates Mechanical Threshold and Thermal Hot Latency in Animals Subjected to Complete Adjuvant Injection.
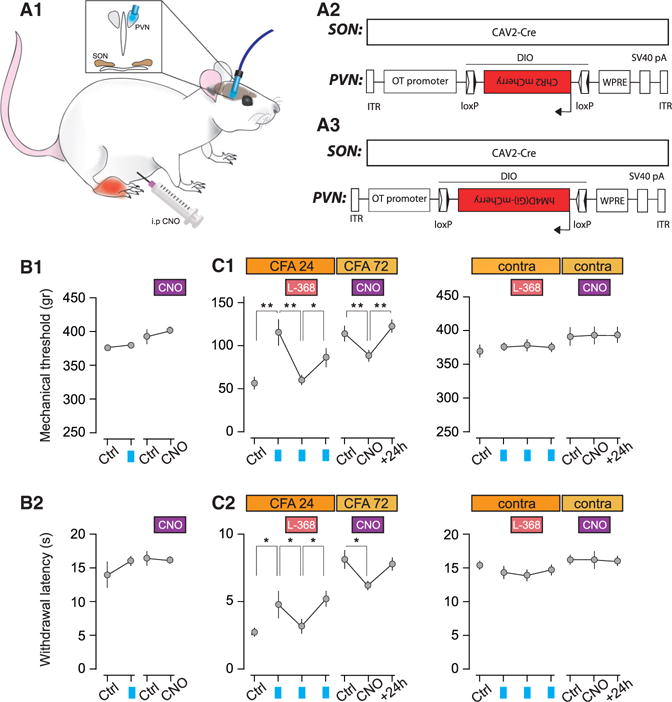
(A–A3) Scheme of the experimental procedure. The CAV2-Cre was injected in the SON and Cre-responding virus driving either (A2) ChR2 or (A3) hM4Di to achieve the expression of respective proteins in OT neurons of the PVN.
(B–B2) Mechanical thresholds and (B2) thermal hot latencies of naive animals before and after PVN-BL (ChR2, n = 6 and CNO, n = 10).
(C–C2) Mechanical thresholds and (C2) thermal hot latencies of the CFA-injected hindpaw (left graphs) and the contralateral hindpaw (right graphs). The effect of PVN-BL was assessed before, right after i.p. injection of OTR antagonist L-368,899 (1 mg/kg), and after its washout (n = 6). The effect of CNO (3 mg/kg) was measured 1 hr after i.p. injection and its 24 hr washout (n = 10). All results are expressed as average ± SEM. The statistical significance: * p < 0.05, ** p < 0.01, and one-way ANOVA followed by Tukey’s multiple comparison post hoc test.
PVN-BL stimulation significantly, but not entirely, alleviated the CFA-mediated hyperalgesia by raising the threshold of response to both the mechanical (from 56.7 ± 7.6 g to 116.6 ± 16.4 g) and thermal hot stimulation (from 2.8 ± 0.2 to 4.8 ± 0.7 s; Figures 6C1 and 6C2). In contrast, PVN-BL failed to mitigate the mechanical hyperalgesia measured in condition of the Cuff peripheral neuropathy (Figures S7B2 and S7B3). Furthermore, return of the pain symptoms occurred after PVN-BL was fully blocked by i.p. injection of the blood brain barrier (BBB)-permeable OTR antagonist L-368,899 (Figures 6C1 and 6C2).
Conversely, CNO-induced inhibition of parvOT neurons significantly increased the CFA-mediated hyperalgesia by lowering the threshold of response to both the mechanical (from 115 ± 12.1 g to 88 ± 9.8 g) and thermal hot stimulation (from 8.1 ± 0.9 s to 6.3 ± 0.3 s; Figures 6C1 and 6C2). CNO had no effect in rats with the Cuff (Figures S7C2 and S7C3). These results from gain- and loss-of-function approaches highlight the role of parvOT control of peripheral painful sensitization, supported by our in vivo electrophysiological data.
In the course of our study, we observed that both PVN-BL and CNO failed to modify mechanical and thermal hot sensitivity in the absence of any peripheral sensitization, for example, in the contralateral paw or in naive animals (Figures 6B1, 6B2, 6C1, 6C2, S7C2, and S7C3).
Taken together, these findings provide evidence that 30 parvOT neurons are able to strongly promote analgesia in a pathological condition of inflammatory, but not nerve injury-induced neuropathic pain, presumably by both central (SC-mediated) and peripheral (SON-mediated) mechanisms.
DISCUSSION
Here, we identified, by a combination of latest state of the art viral-vector based (Grinevich et al., 2016a), anatomical, optogenetic, electrophysiological, and behavioral approaches, a small (n ~30) subpopulation of parvOT neurons in the PVN, which projects to magnOT neurons in the SON and to NK1R or OTR-positive WDR neurons in the deep layers of the SC. Functionally, we demonstrated that this network can inhibit spinal pain processing in a dual manner with two distinct time courses. Thus, nociceptive transmission from Aδ- and C-type primary afferents to WDR neurons is efficiently repressed by OT release from parvOT in the deep layers of the SC and from SON magnOT in the blood. Release in the SC is directly triggered from parvOT-spinal projections and follows a fast mode of action; release in the blood is indirectly triggered from SON magnOT neurons that are activated by parvOT projections and follows a slower time course. The functional role of this subpopulation of parvOT neurons was further confirmed in two rat models of peripheral painful sensitization, indicating that activation of parvOT neurons can decrease mechanical and thermal sensitivities in inflammatory, but not nerve injury-induced neuropathic pain.
Synaptic Crosstalk between OT Neurons
The question of how OT neurons in different nuclei within the hypothalamus interact with each other is a recurrent theme in past literature, but has not been elucidated experimentally. Belin and colleagues recorded pairs of OT neurons from SON and PVN and proposed an internuclear connection serving as a basis for synchronous firing during lactation (Belin et al., 1984, Belin and Moos, 1986). The hypothesis of an OT-mediated communication was stated already in the early 80’s (Silverman et al., 1981), following observations that application of OT (or dOVT) into the third ventricle or in the SON synchronized (respectively, de-synchronized) activity of OT neurons in PVN and SON (Freund-Mercier and Richard, 1984; Lambert et al., 1993). Furthermore, the presence of synapses containing OT-immunoreactivity was demonstrated in the SON (Theodosis, 1985). Although we did not examine internuclear connectivity that underlies synchronized burst firing, our anatomical and functional data demonstrate that PVN-SON interconnectivity plays an important role in inhibiting spinal nociceptive processing and alleviation of inflammatory pain.
In an early study, lesion of the SON did not cause any loss of magnOT neurons in the PVN (Olivecrona, 1957), providing a first indication that parvOT PVN neurons might be at the basis of internuclear connection to the SON. However, as of today, the parvOT neurons in the PVN have remained much less studied than the magnOT neurons, mostly because of technical difficulties, specifically in labeling and modulating the activity of parvOT neurons. To our knowledge, the possibility to study a direct parvOT innervation of the SON by retrograde tracing techniques has seldom been discussed (e.g., Lambert et al., 1993) and any potentially involved parvocellular neurons have never been identified.
At the SON level, Bruni and Perumal (1984) have described an extensive network of small-diameter, beaded, unmyelinated fibers with no particular organizational pattern and of unknown origin that establishes functional axo-somatic and axo-dendritic contacts with magnOT neurons. At 30 years later, we reveal here a monosynaptic connection between parvOT PVN and magnOT SON neurons as respective pre- and postsynaptic components. The detection of a postsynaptic SON component was further confirmed by their stimulation through application of CCK (Renaud et al., 1987) and an increase in peripheral OT levels.
In contrast to the OT system, direct connectivity between VP-ergic neurons in rats has not been convincingly demonstrated and, accordingly, we were unable to find VP/Venus positive fibers descending the PVN in the SON and vice versa.
ParvOT Neurons Modulate NK1R Positive WDR Neurons
In addition to the control of magnOT activity, this newly described subpopulation of parvOT neurons densely projects exclusively to the deep layers (V, VI, and X) of the SC. Axonal terminals from parvOT were found in close appositions with NK1R positive WDR neurons, some of which are likely OTR-positives. However, we are not excluding projections of these parvOT neurons to non-WDR deep neurons. Nevertheless, their functional and selective inhibition of C- and Aδ- mediated discharges in WDR suggest that nociceptive C-fiber project to deep layers, accordingly with models of dorsal horn circuits that include projections to the lamina V (Cervero and Connell, 1984; Ribeiro-da-Silva and De Koninck, 2008). Functionally, this fits with our results suggesting that OT modulates the excitability of WDR shown as an inhibition of discharges mediated by fibers containing substance P.
ParvOT Neurons Coordinate Neuroendocrine and Hardwired Inhibitory Pain Control
In accordance with our anatomical data, WDR action potential discharges in response to noxious peripheral stimulation are reduced by optogenetic manipulation of the subpopulation of OT neurons in the PVN and its subsequent stimulation at the level of the SON. This reduction was selective to sensory information transmitted by Aδ- and C-fibers, which are, in their majority, nociceptive-specific.
Regarding peripherally mediated OT effects, it has recently been shown that OTR could be expressed by non-peptidergic C-type sensory neurons in DRG (Moreno-López et al., 2013) and the in vitro application of OT suppresses their activity (Gong et al., 2015). Furthermore, intravenous administration of a selective OTR agonist induces an inhibition of discharges mediated by nociceptive-specific primary afferents (Juif et al., 2013). Our present work provides an additional support for this idea by selectively activating a circuit leading to release of OT to the blood (Figure 7). The effect was fully peripheral, since inhibition of nociceptive messages was completely abolished by the addition of the OTR selective antagonist dOVT in the blood flow.
Figure 7. The Role of the Novel Type of ParvOT Neurons in Coordinating Central and Peripheral OT Release to Promote Analgesia.
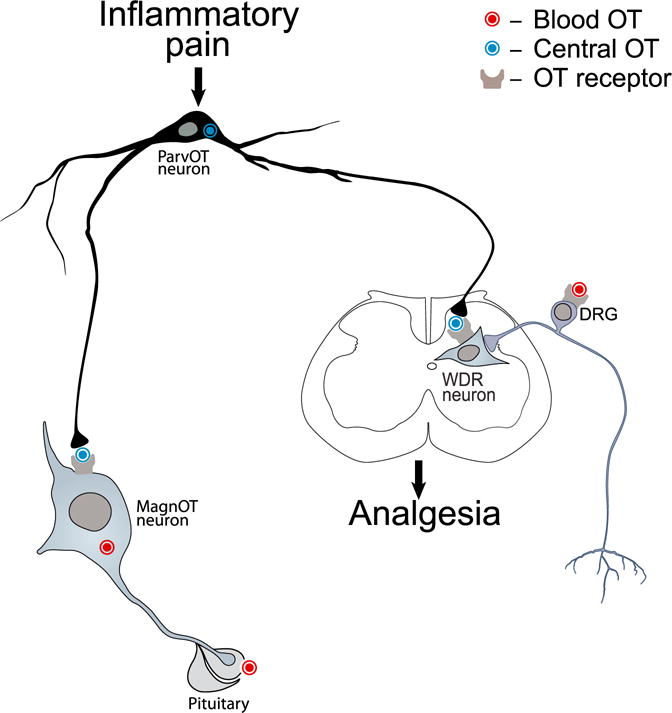
We hypothesize that pain stimulates the identified subset of parvOT PVN neurons, which simultaneously release OT in the SON and SC, exerting respectively delayed and longer lasting and immediate and shorter lasting analgesia. The peripheral analgesic effect of OT is likely mediated by its action on BBB-free sensory neurons of the DRG.
Identification of a subpopulation of parvOT neurons projecting collaterals to both the SON and deep layers of the SC gave rise to the idea that these neurons may exert both a peripheral and central control by OT which we found to take place with a dual time course. This was confirmed by optogenetically stimulating parvOT PVN axons located either in the SON or in the SC. This stimulation led to a reduction of WDR discharges in response to a peripheral noxious stimulation, which was selective for Aδ- and C-type nociceptive fibers. The effects in deep layers of the SC seemed to be mediated by the OTR, as we did not find any effects of VP V1a receptor similar to what has been reported (Qiu et al., 2014). As OT terminals on WDR-like neurons contained VGluT2, we assessed glutamate (Glu) and OT contribution to the SC-BL effect on WU intensity. This revealed that both OT and Glu participated to the inhibition of WU. These results are in accordance with our in vitro patch-clamp experiment and can be interpreted by a network effect as OT axons are likely to form en passant synapses (Knobloch et al., 2012), allowing local (micro)volume-transmission from release sites (Knobloch and Grinevich, 2014; Grinevich et al., 2016b). The combination of two processes can then explain the observed effects: (1) OT acts on OTR in WDR neurons to inhibit them via Gi intracellular pathway (Figure 3E) and (2) coreleased Glu either activates local GABA-interneurons in layers V–VI and around the cc (Schneider and Lopez, 2002; Deuchars et al., 2005), which, in turn, inhibits WDR neurons, or binds a mGluR leading to the direct inhibition of WDR neurons by a Gi/o pathway (Gerber et al., 2000; Niswender and Conn, 2010).
Surprisingly, evoked spinal OT release by this subpopulation of parvOT did not modify nociceptive processing by neurons in superficial layers. This suggested that the OT inhibition of WDR firing was not induced by OTR activation in superficial layers, but only in deep dorsal horn layers. This was in agreement with our anatomical data describing the vast majority of parvOT neurons projecting to the deep layers. We failed to reveal any functional contribution of this subpopulation of parvOT projecting to SON and SC in a nerve-induced neuropathic pain, which may be modulated by OT projections to superficial layers of the dorsal horn. In contrast, they exerted a tonic inhibitory control on WU and pain symptoms in the peripheral inflammation. We speculate that the inflammatory component in pain state regulates the excitability of this subset of parvOT neurons, as observed by c-Fos induction in parvOT neurons (data not shown). Our results indicate that the described subpopulation of parvOT neurons specifically targets NK1R and OTR positive neurons located in the deep layers of the SC to exert antinociceptive action on WDR to promote analgesia.
Described ParvOT Neurons as a New OT-Ergic Cell Type
Following pioneering works of Swanson and Kuypers (1980), Sawchenko and Swanson (1982), and Swanson and Sawchenko (1983), parvOT neurons have been considered as heterogeneous cell populations with descending projections to brainstem and/or SC regions. However, in the present study, we found a small group of parvOT neurons that forms a pathway distinct from the classical hypothalamo-neurohypophyseal axonal tract through the hypothalamus. Although we did not analyze in detail axonal collaterals of these parvOT cells in other forebrain regions, their existence in the hypothalamus adds a new feature to parvOT cells. In addition, these neurons simultaneously project collaterals to deep layers of the SC, representing a unique group of specifically located cells, which is likely distinct from the OT-immunoreactive neurons described by Jójárt et al. (2009) that massively project axons to the superficial layers of the SC. Based on the unique connectivity of the identified parvOT neurons, we speculate that they represent a new type of OT neurons, which coordinate central and peripheral inhibition of nociception and pain perception, and hence, play a role in promoting analgesia (Figure 7).
EXPERIMENTAL PROCEDURES
Animals
Anatomical, electrophysiological, optogenetic, and behavioral studies were performed with Wistar rats (for details of the experiment see the respective figure legend). If not mentioned, rats were housed under standard conditions with food and water available ad libitum. All experiments were conducted under licenses and in accordance with EU regulations.
Viruses
rAAVs (serotype 1/2) carrying conserved regions of OT and VP promoters and genes of interest in direct or “floxed” orientations were cloned and produced as reported previously (Knobloch et al., 2012). CAV2 equipped with GFP or Cre recombinase was purchased from the Institute of Molecular Genetics in Montpellier CNRS, France (Bru et al., 2010)
Neuroanatomy
To trace internuclear connections, rAAVs expressing Venus were injected into the PVN or SON to follow their axonal projections within the hypothalamus. Alternatively, CAV2-Cre was injected into the SON, while Cre-dependent floxed rAAV was injected into the PVN to identify OT PVN neurons synapsing onto SON neurons.
To trace hypothalamus-SC connections, a CAV2-Cre virus was injected into the SON and floxed rAAV, into the PVN or CAV2-Cre virus was injected in the SC, while Cre-dependent rAAV, into the PVN. This allowed us to visualize OT axon pattern in the SC and to identify projecting PVN OT neurons, respectively. After transcardial perfusion with 4% paraformaldehyde (PFA), brains and/or SC were sectioned and stained with antibodies against OT, VP, vGluT2, GFP, NeuN, NK1R, and OTR. Images for qualitative and quantitative analyses were taken on confocal microscopes Leica SP2 and SP5.
Electrophysiology Experiments
For in vitro patch-clamp recordings, 4 to 8 weeks after injection of virus in adult rats, brains were removed, the hypothalamus or lumbar SC was isolated, cut into 400 μm coronal slices, and kept in artificial cerebrospinal fluid (ACSF: 118 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 2 mM KCl, 2 mM MgCl2*6H2O, 2 mM CaCl2*2H2O, and 1.2 mM NaH2PO4) saturated 95% di-oxygen (O2), 5% carbon dioxide (CO2). Visualized neurons were patched with borosilicate glass pipette (4–9 MU) filled with 140 mM KMeSO4, 10 mM HEPES, 2 mM MgCl2, 0.1 mM CaCl2, 0.1 mM (1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid) (BAPTA), 2 mM ATP Na salt, and 0.3 mM guanosine triphosphate (GTP) Na salt (pH 7.3), adjusted to 300 mOsm, and voltage-clamped at −60 mV.
For in vivo extracellular recordings, 4 to 8 weeks after injection of virus, adult animals were anaesthetized with 4% isoflurane and placed in stereotaxic frame. Extracellular neuronal activity was recorded using a stainless electrode with 10 MU impedance (FHC; UE(FK1)).
Behavioral Experiments
Mechanical allodynia was measured using a calibrated forceps (Bioseb). Thermal allodynia/hyperalgesia was measured using the Plantar test using Hargeaves method (Ugo Basile). Peripheral painful inflammatory sensitization was obtained by a single unilateral intraplantar injection of CFA (Sigma-Aldrich, 100 μl in the right paw). Nerve injury-induced neuropathy was induced using the cuffing method.
Supplementary Material
Highlights.
Thirty parvocellular oxytocin neurons (ParvOT) alleviate acute pain
ParvOT project to WDR sensory neurons in spinal cord (SC)
ParvOT activate OT release from magnocellular OT neurons (magnOT)
Dual pain suppression by peripheral magnOT and central SC OT
Acknowledgments
This work was supported by Chica and Heinz Schaller Research Foundation; German Research Foundation (DFG) grant GR 3619/4-1; Human Frontiers Science Program RGP0019/2015; DFG within the Collaborative Research Center (SFB) 1134 (to V.G.) and 1158 (to V.G. and R.K.); PHC PROCOP program 32975SA (DAAD and Campus France) (to V.G. and A.C.); and the IASP Early Career Research grant 2012, FP7 Career Integration grant 334455, Initiative of Excellence (IDEX) Attractiveness grant 2013–15, University of Strasbourg Institute for Advanced Study (USIAS) fellowship 2014–15, and Foundation Fyssen research grant 2015 (to A.C.). The authors thank Judith Müller, Elke Lederer, and Heike Böhli for cloning and packaging viral vectors; Natalie Landeck and Ali Cetin for the selection of the VP promoter; Anna Illarionova for cloning Cre-depended rAAVs; Scott Sternson for ChR2-mCherry construct; Jonathan Fadok for canine virus; Ulrich Herget and Annemarie Scherbarth for their help with confocal and light-sheet microscopy; Marianna Leonzino for help with the HEK293 experiments; Claudia Pitzer and the Interdisciplinary Neurobehavioral Core for behavioral experiments performed there; Vincent Lelièvre and Pascal Darbon for useful inputs to physiological experiments; Thomas Splettstoesser (SciStyle; www.scistyle.com) for his help with the preparation of figures; and Anne Seller for proofreading the manuscript.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2016.01.041.
AUTHOR CONTRIBUTIONS
Conceptualization, A.C. and V.G.; Methodology, P.P., P.H.S., R.Stoop, A.C., and V.G.; Formal Analysis, M.E., M.Melchior, H.S.K.-B., J.W., and A.C.; Investigation—Neuroanatomy, M.E., H.S.K.-B., M.d.S.G., L.C.R., F.A., T.G., M.B., M.Mitre, and G.G.; Investigation—In Vivo Electrophysiology, M.Melchior, J.W., Y.T., and N.P.-D.; Investigation—In Vitro Electrophysiology J.W., A.C.C., and R.T.d.R.; Investigation—Behavior, M.Melchior, J.W., M.d.S.G., N.P.-D., and L.L.T.; Investigation—Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS), V.C. and Y.G.; Resources, B.C., R.C.F., M.V.C., R.Sprengel, and R.K.; Writing—Original Draft, M.Melchior, H.S.K.-B., J.W., P.P., P.H.S., R.Stoop, A.C., and V.G.; Writing—Review & Editing, M.E., M.Melchior, H.S.K.-B., J.W., P.P., P.H.S., R.Stoop, A.C., and V.G.; Visualization, M.E., M.Melchior, H.S.K.-B., J.W., A.C., and V.G.; Supervision, A.C. and V.G.; Project Administration, R.Stoop, A.C., and V.G.; and Funding Acquisition, A.C. and V.G.
References
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann W, Scharrer E. The site of origin of the hormones of the posterior pituitary. Am Sci. 1951;39:255–259. [PubMed] [Google Scholar]
- Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin V, Moos F, Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nuclei in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57:201–203. doi: 10.1007/BF00231147. [DOI] [PubMed] [Google Scholar]
- Breton JD, Poisbeau P, Darbon P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru T, Salinas S, Kremer EJ. An update on canine adenovirus type 2 and its vectors. Viruses. 2010;2:2134–2153. doi: 10.3390/v2092134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni JE, Perumal PM. Cytoarchitecture of the rat’s supraoptic nucleus. Anat Embryol (Berl) 1984;170:129–138. doi: 10.1007/BF00318997. [DOI] [PubMed] [Google Scholar]
- Budai D, Larson AA. Role of substance P in the modulation of C-fiber-evoked responses of spinal dorsal horn neurons. Brain Res. 1996;710:197–203. doi: 10.1016/0006-8993(95)01384-9. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Saulière A, Manning M, Bouvier M, Galés C, Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem. 2012;287:3617–3629. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;20:88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- Condés-Lara M, González NM, Martínez-Lorenzana G, Delgado OL, Freund-Mercier MJ. Actions of oxytocin and interactions with glutamate on spontaneous and evoked dorsal spinal cord neuronal activities. Brain Res. 2003;976:75–81. doi: 10.1016/s0006-8993(03)02690-8. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J Neurosci. 2005;25:1063–1070. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 2000;100:393–406. doi: 10.1016/s0306-4522(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Gong L, Gao F, Li J, Li J, Yu X, Ma X, Zheng W, Cui S, Liu K, Zhang M, et al. Oxytocin-induced membrane hyperpolarization in pain-sensitive dorsal root ganglia neurons mediated by Ca(2+)/nNOS/NO/KATP pathway. Neuroscience. 2015;289:417–428. doi: 10.1016/j.neuroscience.2014.12.058. [DOI] [PubMed] [Google Scholar]
- González-Hernández A, Rojas-Piloni G, Condés-Lara M. Oxytocin and analgesia: future trends. Trends Pharmacol Sci. 2014;35:549–551. doi: 10.1016/j.tips.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Knobloch HS, Roth LC, Althammer F, Domansky A, Vinnikov I, Stanifer M, Boulant S. In: Somatic transgenesis (Viral vectors) In Molecular Neuroendocrinology: From Genome to Physiology. First. Murphy D, Gainer H, editors. John Wiley & Sons; 2016a. pp. 243–274. [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol Psychiatry. 2016b;79:155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Jójárt J, Jójárt I, Boda K, Gálfi M, Mihály A, B-Baldauf Z, Vecsernyés M. Distribution of oxytocin-immunoreactive neuronal elements in the rat spinal cord. Acta Biol Hung. 2009;60:333–346. doi: 10.1556/ABiol.60.2009.4.1. [DOI] [PubMed] [Google Scholar]
- Juif PE, Poisbeau P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain. 2013;154:1449–1456. doi: 10.1016/j.pain.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Juif PE, Breton JD, Rajalu M, Charlet A, Goumon Y, Poisbeau P. Long-lasting spinal oxytocin analgesia is ensured by the stimulation of allopregnanolone-like neurosteroid synthesis which potentiates GABAA receptor-mediated synaptic inhibition. J Neurosci. 2013;33:16617–16626. doi: 10.1523/JNEUROSCI.3084-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Iarovici DM. Green fluorescent latex microspheres: a new retrograde tracer. Neuroscience. 1990;34:511–520. doi: 10.1016/0306-4522(90)90159-2. [DOI] [PubMed] [Google Scholar]
- Knobloch S, Grinevich V. Evolution of central oxytocin pathways in vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Moos FC, Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993;57:1027–1038. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Pagani J, Young WS., 3rd Using transgenic mouse models to study oxytocin’s role in the facilitation of species propagation. Brain Res. 2010;1364:216–224. doi: 10.1016/j.brainres.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JA, Halmos KC, Tasker JG. A slow transient potassium current expressed in a subset of neurosecretory neurons of the hypothalamic paraventricular nucleus. J Neurophysiol. 2000;84:1814–1825. doi: 10.1152/jn.2000.84.4.1814. [DOI] [PubMed] [Google Scholar]
- Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG. Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electro-physiological properties. J Neuroendocrinol. 2002;14:929–932. doi: 10.1046/j.1365-2826.2002.00867.x. [DOI] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- Moreno-López Y, Martínez-Lorenzana G, Condés-Lara M, Rojas-Piloni G. Identification of oxytocin receptor in the dorsal horn and nociceptive dorsal root ganglion neurons. Neuropeptides. 2013;47:117–123. doi: 10.1016/j.npep.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivecrona H. Paraventricular nucleus and pituitary gland. Acta Physiol Scand Suppl. 1957;40:1–178. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth. Academic Press; 1998. [Google Scholar]
- Petersson M. Cardiovascular effects of oxytocin. Prog Brain Res. 2002;139:281–288. doi: 10.1016/s0079-6123(02)39024-1. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Ritchie J, Henry JL. Nerve constriction in the rat: model of neuropathic, surgical and central pain. Pain. 1999;83:37–46. doi: 10.1016/s0304-3959(99)00085-8. [DOI] [PubMed] [Google Scholar]
- Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z, Li JD, Zhou QY, Hu WP. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171:3065–3076. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, De Koninck Y. In: Morphological and neurochemical organization of the spinal dorsal horn In Science of Pain. Basbaum AI, Bushnell MC, editors. Elsevier; 2008. pp. 279–310. [Google Scholar]
- Ritz LA, Greenspan JD. Morphological features of lamina V neurons receiving nociceptive input in cat sacrocaudal spinal cord. J Comp Neurol. 1985;238:440–452. doi: 10.1002/cne.902380408. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Scharrer E. Die Lichtempfindlichkeit blinder Elritzen (Untersuchungen über das Zwischenhirn der Fische) Z Vgl Physiol. 1928;7:1–38. [Google Scholar]
- Scharrer E, Scharrer B. Secretory cells within the hypothalamus. Res Publ Assoc Res Nerv Ment Dis. 1940;20:170–194. [Google Scholar]
- Schneider SP, Lopez M. Immunocytochemical localization of glutamic acid decarboxylase in physiologically identified interneurons of hamster spinal laminae III–V. Neuroscience. 2002;115:627–636. doi: 10.1016/s0306-4522(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015;524:88–92. doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Hoffman DL, Zimmerman EA. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN) Brain Res Bull. 1981;6:47–61. doi: 10.1016/s0361-9230(81)80068-8. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Theodosis DT. Oxytocin-immunoreactive terminals synapse on oxytocin neurones in the supraoptic nucleus. Nature. 1985;313:682–684. doi: 10.1038/313682a0. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- Zhu H, Roth BL. Silencing synapses with DREADDs. Neuron. 2014;82:723–725. doi: 10.1016/j.neuron.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


