Abstract
Objective
The prevalence of obesity continues to rise, and it is understood that regulation of white adipose tissue (WAT) function is important to systemic metabolic homeostasis. Immune cells play a central role in the maintenance of WAT and their compositions change in number and inflammatory phenotype with the progression of obesity. Because of its energy-burning capabilities, brown adipose tissue (BAT) has become a focus of obesity research. While novel studies have focused on the function of brown adipocytes in thermogenesis, the tissue as a whole has not been immunologically characterized.
Methods
BAT immune cell populations were analyzed by flow cytometry and immunohistochemistry in diet-induced obese mice (3, 8, or 16 weeks of diet) and aged mice (1, 6–7, 10–15 months).
Results
Our data confirm the presence of macrophages and eosinophils, as previously reported, and also show that 20–30% of the immune cells in BAT are B cells. The number of B cells and eosinophils increases with diet-induced obesity while macrophages decrease. There is no change in number of any immune cell quantified with age.
Conclusions
These studies reveal a novel finding of B220+ B cells in BAT, and show that BAT immune cell populations change in response to diet-induced obesity.
Keywords: aging, B cells, brown adipose tissue, immunophenotype, immunometabolism
Introduction
Properly functioning white adipose tissue (WAT) is vital for systemic metabolic homeostasis. Studies of brown adipose tissue (BAT) function in metabolism have come to the forefront because BAT is capable of producing energy through non-shivering thermogenesis (1). BAT responds to sympathetic nerve signaling through activation of UCP1. This process can be activated by the catecholamine norepinephrine, cold exposure, and adenosine (2). A goal of investigators is to manipulate white adipocytes to resemble brown adipocytes, a process termed “beiging” (1, 3). While this strategy shows obvious health benefits, there is not enough known about the physiology of BAT and how the tissue environment is involved in thermogenesis.
There are a variety of immune populations resident in WAT in lean animals and the immune repertoire in WAT changes in obesity (4, 5). Macrophages make up about 12% of WAT in lean mice and this can increase to 41% in obese mice (6). Other innate immune cells such as eosinophils, neutrophils, and dendritic cells, as well as adaptive immune cells, are also involved in regulation of WAT health (7).
Immune signaling in BAT has not been systematically studied. There is evidence of the presence of various immune cell types in BAT, such as eosinophils (8), macrophages and monocytes (9), and regulatory T cells (10), but many of these studies were carried out as supporting data to studies on WAT. Because of the lack of systematic understanding of the BAT immune cell repertoire, we used an “immunophenotyping” approach to determine which cell types are present. This information will be vital for further comparing the function of BAT in normal and diseased states.
Methods
Mouse studies
Animal procedures were performed following IACUC approval. Male and female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and aged or fed special diets in the Vanderbilt facility. Mice were maintained on chow diet for the aging studies (1, 6–7, or 10–15 months) and on 10% low fat diet (LFD) or 60% high fat diet (HFD) (Research Diets, Inc. New Brunswick, NJ) for 3, 8, or 16 weeks for the obesity studies. Mice were fed ad libitum. Upon termination of the study, BAT was harvested by cutting away connecting tissue that looked more like WAT.
Flow cytometry
Stromal vascular fraction (SVF) of the total BAT from one animal per sample was collected following collagenase digestion and centrifugal separation as previously described (11). Briefly, BAT was digested for 4 h with 2 mg/mL collagenase type 4 (Worthington, Lot #45H15909). The tissue was pressed through a 100 μm filter. The isolated SVF was incubated with Fc block for 5 min on ice, washed, and incubated with fluorophore-conjugated antibodies for 20 min at 4°C. The following antibodies were used: B220-FITC, CD3-APC/Cy7, CD45-PE/Cy7, F4/80-APC, and SiglecF-PE (BD Biosciences, Franklin Lakes. NJ). DAPI was used for viability staining. Flow cytometry was carried out on the entire volume from each sample at the Vanderbilt Flow Cytometry Shared Resource using a BD Special Order Research Product (SORP) LSR Fortessa. Please see Figure S1 for the gating scheme.
Histology & Imaging
BAT tissue was fixed in 10% formalin overnight, paraffin embedded, sectioned, and stained by the Vanderbilt Translational Pathology Shared Resource for hematoxylin and eosin, CD11b (macrophages), B220 (B cells), and Major Basic Protein (MBP – eosinophils). Images were captured and analyzed through the Vanderbilt Digital Shared Resource and Leica Digital Image Hub software.
Statistical Analysis
All statistics were performed using GraphPad Prism software. BAT mass changes and all LFD:HFD cell population comparisons were analyzed by unpaired Student’s t-test. Aging-associated differences and body weight curves were analyzed by one-way and two-way ANOVA, respectively.
Results
Metabolic parameters of mice on diet
Male and female wild type C57BL/6J mice gained weight on HFD (Figure 1A–B). In addition to WAT expansion, BAT mass was also increased and was significantly greater in mice fed HFD for 8 and 16 weeks (Figure 1C–D).
Figure 1. Metabolic parameters of mice on diet.
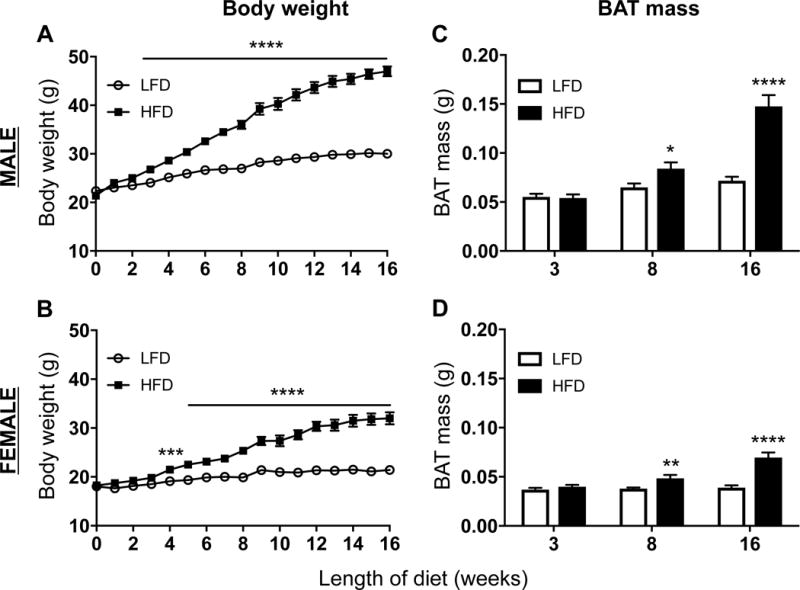
Wild type C57BL/6J mice were placed on 10% low fat diet (LFD, white bars) or 60% high fat (HFD, black bars) for 3, 8, or 16 weeks. Body weight was measured weekly (A: male, B: female), and brown adipose tissue was collected and weighed (C: male, D: female). Data are presented as mean ± SEM of 4–5 samples per group.
*P<0.05
**P<0.01
***P< 0.001
**** P< 0.0001
Flow cytometry of BAT SVF
BAT SVF was analyzed for the presence of B cells, macrophages, and eosinophils using flow cytometry (see Figure S1 for gating scheme). CD45+ leukocytes represent less than 5% of all live cells. In general, there is a higher frequency of B220+ B cells (Figure 2A – B) and a lower frequency of F4/80+ macrophages (Figure 2C – D) in HFD- compared to LFD-fed mice. Concomitantly, there is a lower frequency of SiglecF+ eosinophils in animals with diet-induced obesity (Figure S2). The data show that 13.5% ± 2.1 of live leukocytes are SiglecF+ in male mice fed HFD, while 6.9% ± 1.5 (p<0.05) are SiglecF+ in mice fed LFD for 8 weeks. The very low prevalence of B220+ B cells, CD11b+ macrophages (Figure 3), and MBP+ eosinophils (Figure S2) was confirmed by immunohistochemical staining of BAT from male wild type animals fed LFD or HFD for 8 weeks.
Figure 2. Flow cytometry of brown adipose tissue (BAT) stromal vascular fraction (SVF).
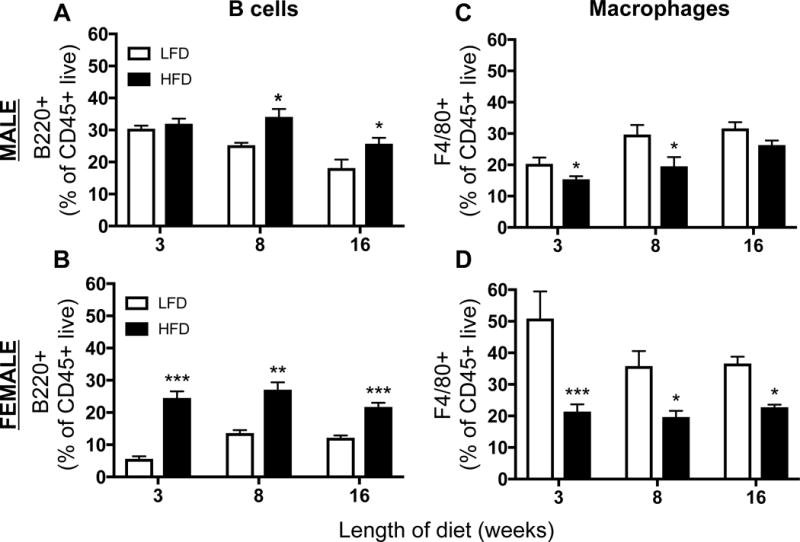
BAT was collected from wild type C57BL/6J mice after 3, 8, or 16 weeks of 10% low fat diet (LFD, white bars) or 60% high fat (HFD, black bars). SVF was processed and analyzed by flow cytometry for B220+ B cells (A: male, B: female) and F4/80+ macrophages (C: male, D: female) as a percent of CD45+ live cells. Data are presented as mean ± SEM of 4–5 samples per group.
*P<0.05
**P<0.01
***P< 0.001
****P< 0.0001
Figure 3. Brown adipose tissue (BAT) immunohistochemistry.
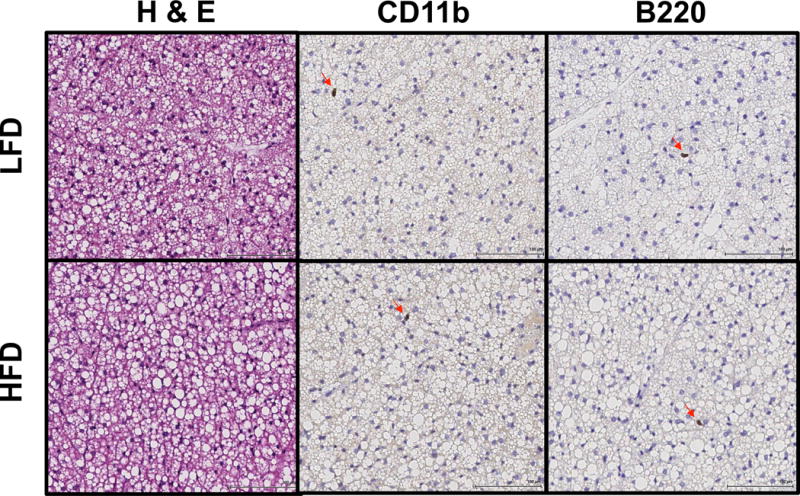
BAT was collected from wild type C57BL/6J mice fed 10% low-fat (LFD) or 60% high-fat (HFD) diet for 8 weeks, and fixed in 10% formalin before further processing and staining for hematoxylin & eosin (H & E), CD11b (macrophages), or B220 (B cells). Data presented are representative images from 3 samples per group.
Analysis of brown adipose tissue (BAT) immune cells with aging
Additionally, the effect of aging on BAT immune cell repertoire was investigated. The animals’ body weights increased with age, and mice that were 6–7 month and 10–15 months old were significantly heavier than mice that were only 1 month old (P<0.0001; Figure S3A & B). BAT mass increased with age in the male mice, but not the female mice, in a similar pattern as the body weight (P<0.01; Figure S3C & D). The SVF was analyzed by flow cytometry and there were no observed changes in B cell or macrophage repertoires in response to aging (Figure S3E – F).
Discussion
Our data show that macrophages, eosinophils, and B cells are present in BAT, cumulatively making up about 75% of the CD45+ immune cell population. While previous reports have shown that eosinophils (8) and macrophages (9) are present in BAT, to our knowledge, this is the first report of the presence of B cells. We show a higher frequency of B cells during in obese compared to lean mice, while macrophages and eosinophils show the opposite trend. These trends are present in both sexes, but the males do not appear to have as strong of a diet-driven effect.
Previous studies have assessed macrophages in BAT by flow cytometry with markers such as Cx3Cr1, F4/80, CD14, CD64, MerTK, and MHCII (12). During the preparation of our manuscript, Wolf et al. beautifully showed that during development, most BAT macrophages are CX3CR1+MCHII−, but that they progressively form 4 distinct populations based upon CX3CR1 and MCHII expression (12). While our data showed a reduction in the percent of macrophages in BAT in obese mice, we did not see changes during aging. The understanding of aging WAT is that it becomes more inflammatory, with a similar phenotype to obese AT (13). With these studies however, we cannot rule out phenotypic changes associated with aging, as we did not assess gene expression patterns of CX3CR1, MHCII levels, or polarization markers.
The underlying tone of some work on BAT macrophages has been that they exist in relatively high numbers in the BAT and that they play an important role in BAT physiology. However, in our studies, we show that macrophages make up only 30% of the BAT immune cell population, which is already less than 5% of all live cells. Published studies showing histology for macrophages in BAT also show low numbers. Buettner and colleagues reported that Mac-2-positive macrophages exist in only small numbers in BAT (14) and Czeck and colleagues did not observe any F4/80+ macrophages in BAT (15). It has also been reported that the inflammatory nature of macrophages in diet-induced obesity is lower in BAT compared to WAT, and we in fact detected a reduction in BAT macrophage percentage in obese mice. Furthermore, Dowal et al. have shown that co-culture of macrophages with brown adipocytes has little impact on their inflammatory nature (16). The function of BAT macrophages in homeostasis or in pathogenesis is not yet clear. A role for BAT macrophages in catecholamine synthesis and adaptive thermogenesis was suggested by one group (17) but was recently disputed (14). Certainly, macrophages are important cells for tissue homeostasis; however, given their very low numbers, the lack of changes in gene expression of macrophages co-cultured with brown adipocytes, the absence of change with obesity or aging, and the recent publication showing no effect of M2 macrophages on catecholamine synthesis or adaptive thermogenesis (14), the physiological function of BAT macrophages is unclear.
Interestingly, we found that 20–30% of leukocytes in BAT are B cells. While B cells have traditionally been studied for their role in antigen processing and antibody secretion, their immunoregulatory role has surfaced in the context of inflammatory disease. B cell phenotypic plasticity is influenced by both norepinephrine (18) and adenosine (19), which are also known to activate thermogenesis in BAT. Additionally, norepinephrine is released in response to antigen presentation, and is necessary for normal expression of IgG (20). Further studies are needed to characterize BAT B cells and their role in this tissue.
Due to the paucity of immune cells in BAT, we limited our analyses to macrophages, eosinophils and B cells. However, future studies could provide a more detailed analysis of their phenotype, as well as reveal other immune populations. Despite these limitations, our work adds to the field’s quantification of immune populations in BAT, especially with the addition of B cells.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Macrophages and other immune cells present in white adipose tissue (WAT) change in number and inflammatory status during development of obesity and in aging.
Proper immune cell repertoire and function are important for metabolic homeostasis in WAT.
Resident macrophages are found in brown adipose tissue (BAT).
What does your study add?
B220+ B cells are present in BAT, making up about 30% of the leukocyte population.
The ratio of macrophages and B cells in BAT changes in response to HFD feeding.
The immune cell repertoire of the BAT does not change in response to aging.
Acknowledgments
We would like to thank our lab members for their assistance with the animal studies and for critical discussions regarding our data.
Funding: KRP was supported by two NIH T-32 training grants: Training in Pharmacological Sciences (GM007628) and Immunobiology of Blood & Vascular Systems (HL069765). AHH was supported by an Established Investigator Award from the American Heart Association (12EIA827), by the Veterans Affairs (5I01BX002195), and by an Innovation Award from the American Diabetes Association (1-17-IBS-140). DKF assisted with flow cytometry experiments that were performed in the Vanderbilt Medical Center (VMC) Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (CA68485) and the Vanderbilt Digestive Disease Research Center (DDRC; DK058404), from which Dr. Hasty received a scholarship. Imaging was performed at the Vanderbilt Translational Pathology Shared Resource, which is supported by NCI/NIH Cancer Center Support Grant 2P30CA068485-14 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 5U24DK059637-13.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 2.Jhunjhunwala S, Jiang Z, Stawiski EW, Gnad F, Liu J, Mayba O, Du P, Diao J, Johnson S, Wong KF, et al. Diverse modes of genomic alteration in hepatocellular carcinoma. Genome Biol. 2014;15(8):436. doi: 10.1186/s13059-014-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27(3):234–50. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrante AW., Jr The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15(Suppl 3):34–8. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262(1):134–52. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winer S, Winer DA. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunology and cell biology. 2012;90(8):755–62. doi: 10.1038/icb.2011.110. [DOI] [PubMed] [Google Scholar]
- 8.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrikova D, Sijmonsma TP, Sowodniok K, Richards DM, Delacher M, Sticht C, Gretz N, Schafmeier T, Feuerer M, Herzig S. Brown adipose tissue harbors a distinct sub-population of regulatory T cells. PLoS One. 2015;10(2):e0118534. doi: 10.1371/journal.pone.0118534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr JS, Kennedy AJ, Hasty AH. Isolation of adipose tissue immune cells. J Vis Exp. 2013;75:e50707. doi: 10.3791/50707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf Y, Boura-Halfon S, Cortese N, Haimon Z, Shalom HS, Kuperman Y, Kalchenko V, Brandis A, David E, Segal-Hayoun Y, et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nature Immunology. 2017;18:665–74. doi: 10.1038/ni.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 2016;86:97–105. doi: 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23(5):623–30. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet- induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301(4):H1425–37. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowal L, Parameswaran P, Phat S, Akella S, Majumdar ID, Ranjan J, Shah C, Mogre S, Guntur K, Thapa K, et al. Intrinsic Properties of Brown and White Adipocytes Have Differential Effects on Macrophage Inflammatory Responses. Mediators Inflamm. 2017;2017:9067049. doi: 10.1155/2017/9067049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. Journal of leukocyte biology. 2006;79(6):1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 19.Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122(1):9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26(2):195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


