Atherosclerosis is a chronic inflammatory disease that progresses to complex, unstable arterial lesions1. Restenosis is an acute inflammatory vascular disease and a major limitation of percutaneous angioplasty procedures. Both are characterized by de-differentiation of vascular smooth muscle cells (SMCs) resulting in neointimal hyperplasia and vessel occlusion. Differentiated SMCs are highly specialized cells whose primary role is to maintain vessel homeostasis, vessel tone, blood pressure, and blood flow distribution3. This function is driven through expression of SMC-specific contractile and contractile-related proteins, including smooth muscle myosin heavy chain (SMMHC/Myh11), smooth muscle alpha actin (αSMA/Acta2), SM22α (Tagln1), and calponin (Cnn1), among others. Unlike terminally differentiated cardiac and skeletal muscle, SMCs retain a significant degree of phenotypic plasticity, exhibiting the ability to undergo extensive changes in phenotype in response to specific stimuli (i.e. dedifferentiated SMC). SMC dedifferentiation is associated with a transition to a highly proliferative, inflammatory phenotype characterized by downregulation of SMC-specific genes and increased production of multiple inflammatory and matrix-associated mediators. Thus, SMCs are major contributors to vascular disease progression and defining molecular mechanisms regulating SMC phenotypic transitions is critical to define novel therapeutics for treatment of vascular disease.
Regulation of SMC differentiation is complex, involving multiple signaling pathways and transcriptional regulators. Most SMC-specific genes are under transcriptional control by the transcription factor, SRF, and its cardiac and SMC-specific cofactor, myocardin, the SRF-myocardin axis3–5. SRF binds the serum response element or CArG box, in which one or more are present within promoter and/or intronic regions of SMC-specific genes3,4. In contrast, myocardin does not directly bind DNA, but transactivates SMC-specific genes through its interaction with SRF5. While the SRF-myocardin axis is central to transcriptional regulation of SMC genes, additional factors and mechanisms have been identified that serve to cooperate with or fine-tune SRF-myocardin activity. For instance, SRF-myocardin cooperatively interact with other cis-regulatory elements and their binding factors to maintain SMC differentiation, including, but not limited to the transcription factors, Nkx3.1 and 3.2, GATA-4 and -6, SMADs, the homeodomain protein Prx1, and the LIM proteins CRP1 and CRP23. Our group demonstrated that the tumor suppressor, PTEN, interacts with SRF in the nucleus and functions as an indispensable co-factor to maintain the differentiated SMC phenotype6. In addition, multiple signaling pathways have been identified that stimulate or maintain SMC differentiation through regulation of SMC-specific transcriptional machinery7. TGFβ-, RhoA-, and p38-dependent signaling have been implicated in promoting SMC differentiation through activation of transcriptional regulators interacting with the SRF-myocardin axis. The Notch intracellular domain (ICD) interacts with the transcription factor, RBPJ to control SMC differentiation thereby implicating the Notch signaling pathway in SMC differentiation control. Collectively, there are multiple parallel and/or intersecting transcriptional networks involved in maintaining the SMC differentiated phenotype.
In addition to direct transcriptional control, additional mechanisms are essential for modifying/regulating SMC contractile gene expression. Epigenetic regulation of chromatin structure of CArG-containing regions of SMC genes is essential for proper SMC differentiation control. The Owens group identified enrichment of several histone modifications involved in chromatin relaxation and gene induction, including acetylation and methylation marks on histones flanking CArG boxes in the Myh11 and Acta2 genes3. These histone modifications regulate SRF binding to SM gene CArG boxes. In addition, it has been shown that myocardin interacts with the p300 histone acetyltransferase, which mediates histone acetylation of SMC genes and SM gene expression. In contrast, overexpression of histone deacetylases (HDACs) decreases SM gene expression. Therefore, chromatin structure and specific SMC-restricted histone marks are important modifiers essential for SMC differentiation control. In addition, activity of short and long non-coding RNAs regulate SMC phenotype8. Several microRNAs (miRNAs) either positively or negatively regulate SMC differentiation. For instance, miR-145/143 promotes SM contractile gene expression by directly targeting transcriptional repressors of these genes (e.g. Klf4). In contrast, miR-21 and miR-221/222 promote SMC de-differentiation by targeting known growth repressors (e.g. PTEN). miRNAs that directly target SM contractile genes to promote SMC dedifferentiation have yet to be identified. While several classes of long non-coding RNAs (lncRNAs) have been described and emerging data suggest important functions for lncRNAs on SMC phenotype control, little is known of the role of the novel class of non-coding RNAs, circular (circ)RNAs, on SMC contractile protein expression.
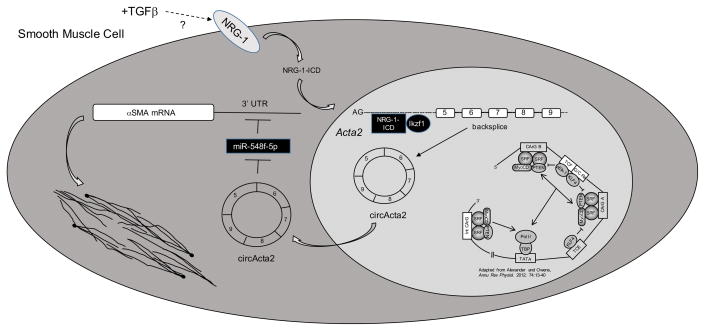
In this issue of Circulation Research, Yan Sun, et. al9. assessed the role of the intracellular domain of the epidermal growth factor family member, neuregulin-1 (NRG-1-ICD) on SMC αSMA expression. The authors demonstrated that TGFβ induces NRG-1 expression and cleavage thereby releasing NRG-1-ICD, which translocates to the nucleus and interacts with the transcription factor, Ikzf1. In the nucleus, the NRG-1-ICD:Ikzf1 complex binds to the first intron of the Acta2 gene and induces formation of the circRNA, circActa2 through circularization of exon-5 to exon-9. TGFβ-mediated induction of circActa2 promotes αSMA protein expression through direct interaction with miR-548f-5p, thereby functioning as a miRNA “sponge” to decrease miR-548f-5p repression of Acta2. Functionally this is associated with stabilization of actin filaments and enhanced contraction (Figure). Decreased expression of circActa2 and dissociation of circActa2 and miR-548f-5p in human intimal hyperplastic lesions led the authors to conclude that the NRG-1-ICD/circActa2/miR-548f axis functions to fine-tune αSMA expression and that dysregulation of circActa2 and miR-548f expression promotes intimal lesion formation. These findings are important as they reveal a new, novel mechanism of regulating αSMA expression, which is essential for SMC function. The NRG-1-ICD/circActa2/miR-548f-5p axis, therefore, may represent a novel therapeutic target to limit vascular disease progression.
Figure.
In addition to the established direct role of SRF, myocardin, and other transcriptional activators and repressors on SMC contractile gene expression (bottom right3), levels of the contractile protein, αSMA, are fine-tuned through the activity of a TGFβ / NRG-1-ICD / circActa2 / miR-548f-5p axis. TGFβ stimulates NRG1 expression and cleavage, promoting nuclear translocation of NRG-1-ICD. Nuclear NRG-1-ICD recruits Ikzf1 and forms a stable transcriptional complex that interacts with the first intron of the Acta2 gene inducing circActa2 formation. circActa2 functions as a miRNA “sponge,” interacting with and repressing miR-548f-59, which targets αSMA mRNA for degradation, thereby resulting in increased αSMA levels and enhanced SMC contractile function. NRG-1, neuregulin-1; NRG-1-ICD, neuregulin-1 intracellular domain; circActa2, circular RNA Acta2; TGFβ, transforming growth factor-β; SRF, serum response factor; MyoCD, myocardin; miR-548f-5p, microRNA-548f-5p; αSMA, smooth muscle alpha actin (Acta2).
There are several novel and significant discoveries from this manuscript. First, the authors define a novel function for NRG-1 in SMCs. NRG-1 is well known for its role in cardiovascular development, largely through its paracrine effects following cleavage of the extracellular EGF domain10. Further, released soluble NRG-1 has been shown to suppress SMC proliferation11. Less is known regarding NRG-1-ICD, with the majority of information derived from studies in neurons. Here the authors demonstrate that NRG-1-ICD functions as a transcriptional activator in SMCs through a stable complex with Ikzf1 to induce expression of a non-coding circRNA that functions to suppress miRNA-mediated repression of αSMA9. Collectively, NRG-1 modulates SMC phenotype via release of both its intracellular domain and extracellular EGF-like domain. Release and nuclear translocation of NRG-1-ICD is mediated through proteolytic cleavage, which is similar to Notch signaling in the regulation of SMC differentiation7. In the case of NRG-1, TGFβ promotes proteolytic cleavage and release of NRG-1-ICD, a novel function of TGFβ in driving SMC contractile gene expression. At the present time, however, the signaling mechanism(s) mediating the effects of TGFβ on NRG-1 cleavage and NRG-1-ICD translocation and whether this effect is mediated through canonical TGFβ signaling remain unknown.
While traditionally disregarded as rare and non-functional, emerging data demonstrate that functional circRNAs harbor conserved miRNA seed sequences supporting important roles for circRNAs as miRNA “sponges” to inhibit endogenous miRNAs12. The role of circRNAs in heart failure has been well recognized13. In contrast, little is known of circRNAs in vascular biology, although two studies demonstrated an association of circRNA expression with human thoracic aortic dissection14 and atherosclerosis15, thereby underlying the potential clinical relevance of functional circRNAs in vascular disease. The current manuscript is the first demonstration of a functional circRNA regulating SMC differentiation control and contractile function. The studies in human intimal hyperplastic tissue provide evidence that circularization of lncRNAs may impart protection from human vascular disease. Further, while miRNAs have been shown to facilitate SM gene expression or promote SMC de-differentiation, these effects are not mediated directly on SM contractile genes. This report is the first to describe direct miRNA-mediated repression of a SM contractile gene, which has important implications for maintenance of the contractile phenotype beyond direct transcriptional control.
Clinical Significance and Additional Questions
αSMA is a critical component of the SMC contractile machinery and essential for actin cytoskeletal dynamics necessary for physiological vessel wall homeostasis. Subtle changes in expression could have profound effects on normal vascular function and likely contributes to vascular disease progression. The findings presented here describe a novel pathway functioning to fine-tune αSMA levels and may have significant clinical impact that leads to new and novel therapeutic approaches to control the differentiated SMC phenotype. The data suggest that activation of NRG-1-ICD, induction of circActa2, or inhibition of miR548f-5p could represent novel strategies to target pathological vascular remodeling. Moving forward there are several unanswered questions. (a) Do similar mechanisms and unique circRNA/miRNA pairs regulate other SMC-specific contractile proteins (e.g. SMMHC/Myh11)? (b) Do other mediators promote NRG-1 induction and cleavage or is this specific to TGFβ? If specific, what mechanisms mediate NRG-1 induction? Cleavage? (c) Are circActa2 levels changed during intimal lesion formation? Hypertension? If so, how does this contribute to disease progression? (d) Induction of αSMA is observed during fibroblast-to-myofibroblast transition mediated by TGFβ. Is this pathway activated and does it play a role in chronic inflammatory, pro-fibrotic conditions? If so, targeting this axis could represent a novel therapeutic approach to limit fibroblast-to-myofibroblast transitions and myofibroblast function.
Acknowledgments
SOURCES OF FUNDING
This work was funded by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health to MCMW-E (R01 HL121877 and R01 HL123616).
Footnotes
DISCLOSURES
The author declares no competing financial interests or other conflicts of interest.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Mitra AK, Agrawal DK. In stent restenosis: Bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 4.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292(1):C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 5.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14(5):558–66. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Horita H, Wysoczynski C, Walker LA, Moulton KS, Li M, Ostriker A, Tucker R, McKinsey TA, Churchill MEA, Nemenoff RA, Weiser-Evans MCM. Nuclear PTEN functions as an essential regulator of SRF-dependent transcription to control smooth muscle differentiation. Nature Communications. 2017;7:10830. doi: 10.1038/ncomms10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31(7):1495–505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miano JM, Long X. The short and long of noncoding sequences in the control of vascular cell phenotypes. Cell Mol Life Sci. 2015;72(18):3457–88. doi: 10.1007/s00018-015-1936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Yang Z, Zhang X, Zhang M, Zhao X, Zhao H, Suzuki T, Wen J. A novel regulatory mechanism of smooth muscle α-actin expression by NRG-1/circActa2/miR-548f-5p axis. Circulation Research. 2017 Jul 11; doi: 10.1161/CIRCRESAHA.117.311441. [DOI] [PubMed] [Google Scholar]
- 10.Lenneman CG. Neuregulin-1 signaling in the pathogenesis of chemotherapy-induced heart failure. Curr Heart Fail Rep. 2014;11(2):134–8. doi: 10.1007/s11897-014-0193-9. [DOI] [PubMed] [Google Scholar]
- 11.Clement CM, Thomas LK, Mou Y, Croslan DR, Gibbons GH, Ford BD. Neuregulin-1 attenuates neointimal formation following vascular injury and inhibits the proliferation of vascular smooth muscle cells. J Vasc Res. 2007;44(4):303–12. doi: 10.1159/000101776. [DOI] [PubMed] [Google Scholar]
- 12.Hentze MW, Preiss T. Circular RNAs: splicing’s enigma variations. EMBO J. 2013;32(7):923–5. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux Y, Creemers EE, Boon RA, Werfel S, Thum T, Engelhardt S, Dimmeler S, Squire I on behalf of the Cardiolinc network. Circular RNAs in heart failure. Eur J Heart Fail. 2017;19(6):701–709. doi: 10.1002/ejhf.801. [DOI] [PubMed] [Google Scholar]
- 14.Zou M, Huang C, Li X, He X, Chen Y, Liao W, Liao Y, Sun J, Liu Z, Zhong L, Bin J. Circular RNA expression profile and potential function of hsa_circRNA_101238 in human thoracic aortic dissection. Oncotarget. 2017 Jul 5; doi: 10.18632/oncotarget.18998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gäbel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, Teupser D. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nature Communications. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]