Abstract
There is a pressing need to establish recruitment, retention, and adherence feasibility to inform clinical trials that will evaluate how exercise affects the symptoms and disease trajectory in Alzheimer's disease (AD). This paper reported the recruitment rate, retention, and adherence from a 6-month cycling study in community-dwelling older adults with mild-to-moderate AD using a single-group, repeated-measures design. Seven recruitment strategies were tested. Participants were prescribed an individualized, 15–45-min moderate intensity cycling 3 times a week for 6 months. The results showed a 1.87 recruitment rate (No. of participants recruited per month per site), 78.6% retention (No. of completers/No. of enrolled participants), and 86.4% adherence (number sessions meeting prescription dose/total number of sessions). The findings addressed a major gap in aerobic exercise studies in AD. Successful recruitment relies on community partnership, whereas strategies for ensuring participant exercise safety collectively improved retention and adherence.
Keywords: Aerobic exercise, Alzheimer's disease, Dementia, Aging, Adherence
1. Introduction
Alzheimer's disease (AD) is an epidemic that affects 5.4 million Americans currently and will inflict 14 million by 2050 if no cure is found.1 The social and financial costs of AD are tremendous. The AD triad symptoms of cognitive impairment, functional decline, and behavioral and psychological symptoms of dementia (BPSD) result in loss of independence, increased risks for institutionalization, hospitalization, and mortality, and poor quality of life.2 The cost of AD to the society is extreme which was estimated at $202.6 billion in 2010.1 Since individuals newly diagnosed with AD have an average 8–10-year life expectancy and 70% of them live in the community with family caregivers, 15 million family caregivers delivered 17 billion hours of unpaid care valued at $183 billion in 2010.1 Caregiving predisposes family caregivers to new diseases and exacerbation of pre-existing conditions, which cost another $7.9 billion. Since no treatment can yet prevent or slow down AD, there exists a pressing need to develop and test effective new interventions for treating AD.
2. Review of the literature
The potential of aerobic exercise for treating AD has become increasingly evident with the convergence of findings from 4 areas of research: (1) epidemiological studies link exercise to reduced risk for AD in old age3,4; (2) meta-analyses of randomized controlled trials (RCTs) indicate that aerobic exercise improves cognition in older adults without dementia5; (3) basic science findings suggest that aerobic exercise could improve brain structure and function and mitigate AD neuropathology via biologically sound pathways, e.g., promoting neuronal function, synaptogenesis, and neurotransmitter function, and reducing β-amyloid load in AD-transgenic mice6; and (4) emerging studies in persons with cognitive impairment and dementias have reported that aerobic exercise improves cognition, function, and BPSD.7–9 Nonetheless, exercise studies in AD are just emerging, have small sample sizes, and show conflicting results that are at least partially attributable to issues of recruitment, retention, and adherence.10–23
Recruitment of older adults with AD presents many unique challenges because AD causes multifaceted impairments in memory, language, awareness, insights, judgment, decision-making, communications, function, and behavior. Even AD drug RCTs report low recruitment rate, defined as the number of participants qualified per site per month, which ranged from 0.26 to 1.82.24 Recruitment difficulty might be one of the reasons why few exercise studies in AD have used a well-characterized AD sample.25 A meta-analysis identified 30 exercise RCTs (mainly nursing home samples) using cognitive impairment as the criterion,7 while a later meta-analysis found only 4 RCTs using AD as the criterion.25 Of the existent aerobic exercise studies in AD, seven studies used community-dwelling samples; however, they rarely reported the recruitment rates and the use and effectiveness of recruitment methods.10–23 Poor reporting of recruitment is not a singular character of AD exercise studies, but a permeating issue plaguing exercise research in older adults in general.26 In addition, some recruitment methods that worked well for older adults without AD are not applicable to those with AD. For example, the lack of AD registry within a researcher's community and recruitment costs for phone and postcard recruitments have diminished their usability in AD.27
Similarly, retention is not always reported in aerobic exercise studies of AD, and varied from 92% (12-h session over 11 weeks plus phone follow-up to teach caregivers to implement aerobic, strength, balance, and flexibility exercise daily)18 and 59% (12-week, caregiver-delivered, daily aerobics, strength, balance, and flexibility),16–72.7% (24-week supervised cycling by an exercise therapist)23 in community-dwelling samples. Similar variability in retention was also found in nursing home residents with AD (66.8%–91.5%)15,17,19,20,28 and in AD drug RCTs (59%–88%).29 Those data, however, do suggest that retention of older adults with AD is as good as that (59%–100%) from exercise studies of older adults without AD.26,27,30 One analysis showed that the retention of AD participants was heavily influenced by how the studies were designed and conducted, e.g., visit frequency, study length, target AD population, design changes after study initiation, and patient-caregiver dyads.24
Currently, study findings on the treatment effect of aerobic exercise on AD symptoms are conflicting, which is largely due to the varying doses of aerobic exercise prescribed and delivered (adherence).25,31 The differences in prescribed exercise doses are drastic among such a limited number of studies in AD: exercise frequency ranged from 1 to 5 times a week, session duration from 20 to 40 min, intensity from very low to moderate, and program duration from 5 weeks to 4 years. The majority of studies relied on caregivers to deliver exercise10–23 with only 4 studies ascertaining the delivered exercise doses in real time.10,11,13,23 On top of the wide differences in exercise prescription, adherence further varies greatly from 59% to 100% in community-dwelling older adults with AD,16,18,23 although the adherence is consistent with that in older adults without AD. Older adults without AD showed higher adherence in supervised exercise.30,32–34
Therefore, there exists a pressing need to establish the feasibility of recruitment, retention, and adherence of aerobic exercise interventions before resource-intensive RCTs can be launched to evaluate the effectiveness of aerobic exercise in AD. The purpose of this paper is to report the recruitment rate, retention, and adherence from a single-site, pilot feasibility study of a 6-month aerobic exercise intervention in community-dwelling older adults with mild-to-moderate AD to inform and advance future exercise research in AD.
3. Method
The study used a single-group, repeated-measures design to test a 6-month, standardized, and individualized cycling intervention in a well-defined community-dwelling sample of older adults with mild-to-moderate AD. Participants received the intervention 3 times a week. The study was approved by the university's Institutional Review Board.
3.1. Sample
The inclusion criteria for the study sample included: a) English-speaking; b) community-dwelling; c) resting heart rate < 100 beats/min; d) Mini-Mental State Examination (MMSE) scores between 12 and 24 (indicating mild-to-moderate AD) during screening35; e) Clinical Dementia Rating (CDR) scale scores between 1 (mild dementia) and 3 (severe dementia) during screening36; f) ≥60 years of age; g) verification of the probable AD diagnosis by healthcare providers; and h) medical clearance for participation in cycling by healthcare providers. If participants had a cardiac history, medical clearance from the cardiologists was also needed. The exclusion criteria were as follows: unstable medical conditions in the past 6 months (i.e., hip fracture, ongoing and unplanned weight loss, severe shortness of breath, deep vein thrombosis, hernia, unhealed sores, joint swelling, and pain or trouble walking); new symptoms that had not been examined by a healthcare provider; neurological and psychiatric disorders other than AD in the past 5 years; alcohol or chemical dependency in the past 5 years; and >5 on the 15-item Geriatric Depression Scale during screening.37
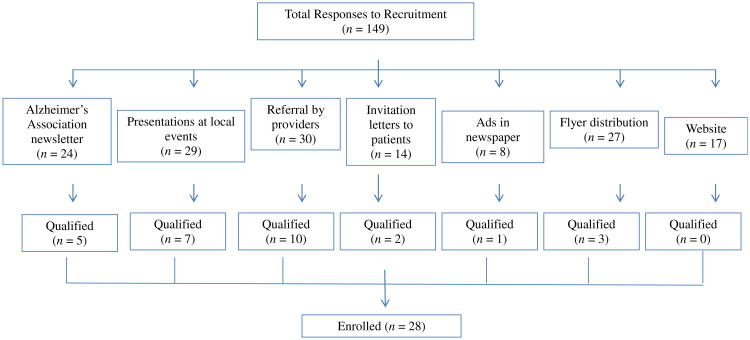
Seven recruitment strategies were used for recruiting participants: 1) Alzheimer's Association newsletters; 2) presentation of the study at local events; 3) referral by healthcare providers; 4) study invitation letters to potential participants who were identified through the university's medical center; 5) advertisements in newspapers; 6) study flyer postings; and 7) a link to the study at the principal investigator's academic website.
3.2. Setting
The exercise intervention was delivered at sites chosen to minimize costs and travel time and maximize the possibility that participants would continue to exercise after the study ended. Participants received the intervention at either a YMCA gymnasium or an assisted living facility in a major Midwestern city, depending on the convenience of the participant. Both sites were in close proximity to tertiary hospitals.
3.3. Outcomes
Three outcomes were evaluated after the implementation of recruitment, retention, and adherence strategies: recruitment rate, retention, and adherence. The recruitment rate was operationalized as the monthly participant accrual rate and calculated as the ratio of the total number of subjects qualified divided by the total number of recruitment months. Retention was operationalized as the percentage of the number of participants who completed the 6-month data collection divided by the total number of enrolled participants. Adherence was measured in 2 ways: 1) the number and percentage of exercise sessions that a participant attended; and 2) the number and percentage of exercise sessions that a participant achieved both the prescribed cycling intensity and duration dose divided by the total number of attended exercise sessions.
3.4. Study procedure
3.4.1. Staff training
All staff completed a 4-h didactic training and 1-week practice training about AD, exercise prescription, delivery, safety monitoring, and data collection until they reached 100% agreement with the protocol. The principal investigator trained the exercise therapist about exercise delivery until she was compliant with the protocol. The principal investigator randomly attended 10% of the exercise sessions and re-trained the staff as needed.
3.4.2. 3-Step screening and informed consent
3.4.2.1. Step 1 phone screen
After obtaining verbal consent, undergraduate research assistants (RAs) conducted a phone interview with the family caregiver and the potential participant (30 min) using a structured interview form to identify the presence of probable AD and contraindications to exercise such as recent heart attack or stroke.
3.4.2.2 Step 2 in-person interview
Potential participants who had a diagnosis of AD were invited to an in-person interview where the RA and investigator obtained informed consent and/or assent from the potential participant (30 min). The RA explained the study in detail such as purpose, involvement, data collection, risks, benefits, and voluntary nature, and the investigator answered any questions. Afterward, the RA administered a 10-item questionnaire to the potential participant about the study. Participants who could correctly answer 80% or more were allowed to sign their own consent. Otherwise, participants signed assent and surrogate consents were obtained from spouse, adult child, and sibling in the descending order. After obtaining the informed consent/assent, the principal investigator interviewed the family caregiver using the CDR36 (30 min) while the RA administered the MMSE38 to the potential participant (15 min). Then, the principal investigator proceeded to interview the potential participant (30 min) using the CDR while blinded to the MMSE score. After the interviews, the principal investigator scored the CDR, reviewed the MMSE with the RA, and informed the potential participant and family caregiver if further screening was warranted.
3.4.2.3 Step 3 medical verification
A letter that required the verification of AD diagnosis and exercise safety was faxed to the healthcare providers of participants whose MMSE scores were between 12 and 24 and CDR scores were between 1 and 3. Responses from healthcare providers typically took 1–2 weeks. Participants with medical clearance were enrolled in the study.
3.4.3. Receiving the individualized aerobic exercise intervention
Since exercise safety is the cornerstone for retention and adherence, nine strategies were built into the study design to ensure participant safety: (1) After completing baseline data collection, qualified participants began the intervention under the supervision of an exercise therapist. The exercise therapist supervised 2 participants (if by herself) or 3 participants (if an RA was also available). (2) Two methods were used to individualize moderate intensity: a) 65%–75% of heart rate (HR) reserve (HRR) which was the difference between the resting HR and the peak HR obtained from baseline 6-min walk39 and shuttle walk tests40; and b) a participant's subjective rating of 12–14 on the 6-20 Borg Rating of Perceived Exertion (RPE) scale.41 The RPE was used for participants whose HRR could not be accurately derived due to cardiac arrhythmia or suppressed due to HR-altering drugs such as β-adrenergic blocking agents. The proper use of the RPE was continuously reinforced. The equipment used for cycling was Pre-cor™ (Woodinville, WA) and LIVESTRONG® R1x recumbent stationary cycles (Taiwan). The use of 2 different brands did not affect exercise delivery because cycling was individualized based on HRR and RPE. (3) The “talk test,” the ability to speak a sentence without losing breath, was used every 5 min to monitor exertion. (4) Blood pressure was measured every 10–15 min before, during, and after cycling. (5) Participants were transported by RAs for each exercise session using a rented university vehicle which provided liability coverage for staff. (6) In each session, participants wore a wireless Polar™ F7 HR monitor (Polar Electro Finland Oy, Kempele, Finland) for continuous HR monitoring. HR and RPE were documented every 5 min during cycling. (7) Participants did 10-min warm-up, 15–45-min cycling at moderate intensity, and 10-min cool-down in each session. The duration of cycling at moderate intensity was progressively increased by 5 min from 15 min to 45 min a session over time (see Table 1). (8) Research staff were trained by the principal investigator to understand AD (e.g., causes, symptoms, changes in personality), establish good professional relationships (e.g., getting to know and respect your participants and family caregivers, being patient and smiling, establishing rapport, and addressing any concerns), communicate with older adults with AD (e.g., answering each question patiently as if it were a new question, validating achievements and progress), and responding to BPSD as unmet needs. (9) Exercise journals were provided to each participant by the exercise therapist to document the exercise dose achieved, the next pick-up date and time, or any important communications such as changes in schedule. The phone numbers of all study personnel were included in the exercise journals for easy reference.
Table 1.
Cycling-exercise progression.
| Week | Cycling intensity | Duration (min) | |
|---|---|---|---|
|
| |||
| % of HR reserve | RPE | ||
| 1 | 40–55 | 9–10 | 10–15 |
| 2 | 40–55 | 9–10 | 15–20 |
| 3 | 55–65 | 10–11 | 20–25 |
| 4 | 55–65 | 10–11 | 25–30 |
| 5 | 60–65 | 10–11 | 30–35 |
| 6 | 65–75 | 12–13 | 30–35 |
| 7 | 65–75 | 12–13 | 35–40 |
| 8 | 65–75 | 12–13 | 40–55 |
| 9–24 | 65–75 | 12–13 | 45 |
Note: HR, heart rate; RPE, rating of perceived exertion. Durations do not include warm-up and cool-down periods.
3.5. Data analysis
Data were first graphed to observe any outliers. Then, data were analyzed using mean, median, ranges, and standard deviation (SD) in SPSS 18 for windows separately for all participants and then only participants who completed the 6-month data collection.
4. Results
Of the 149 individuals who responded to our recruitments over 15 months, 118 were reachable by phone. More than half of the respondents (58.5%) did not have a diagnosis of AD. All 49 respondents who had a diagnosis of AD were invited for the in-person interview, and 29 met all eligibility criteria for enrollment. One participant withdrew consent before any baseline data were collected because of busy life schedule. Thus, 28 participants were qualified for enrollment over 15 months, resulting in a recruitment rate of 1.87. Recruitment strategies that qualified the most participants included referral by healthcare providers (n = 10), presentation of the study at local events (n = 7), and the Alzheimer's Association newsletters (n = 5; see Fig. 1).
Fig. 1.
Responses to recruitment methods.
The median age of the study sample was 78.0 years (n = 28). Their median baseline MMSE scores were 21.0 while their CDR scores ranged from 1 to 3. About 39% of them were male. There were no significant differences between participants who dropped out (n = 6) and those who completed the 6-month data collection (n = 22) except for their baseline CDR scores. Participants who dropped out had higher CDR scores than those who completed the study (see Table 2).
Table 2.
Sample characteristics.
| Mean ± SD Median Range Interquartile range | All enrolled participants (n = 28) | Participants who completed 6-month data collection (n = 22) | Participants who dropped out (n = 6) |
|---|---|---|---|
| Age | 78.1 ± 8.34 | 78.1 ± 8.51 | 78.2 ± 8.6 |
| 78.0 | 78.0 | 79.0 | |
| 60–91 | 60–91 | 64–89 | |
| 11 | 12 | 14 | |
| Education (years) | 16.1 ± 2.98 | 16.0 ± 3.12 | 16.3 ± 2.66 |
| 16.0 | 16.0 | 16.0 | |
| 12–24 | 12–24 | 12–20 | |
| 3.8 | 3.3 | 3.5 | |
| Baseline MMSE | 19.9 ± 4.02 | 20.3 ± 3.67 | 18.2 ± 5.11 |
| 21.0 | 21.0 | 19.0 | |
| 12–27 | 12–27 | 12–23 | |
| 6 | 3 | 10 | |
| Baseline CDR | 1 | 1 | 2 |
| 1 | 1 | 1.5 | |
| 1–3 | 1–2 | 1–3 | |
| 0 | 0 | 2.0 | |
| No. (%) male | 11 (39.3%) | 8 (36.4%) | 3 (50%) |
Note: CDR, clinical dementia rating scale; MMSE, mini-mental state examination; SD, standard deviation.
Of the 28 enrolled participants, 21.4% (n = 6) dropped out due to the following reasons: relocation (n = 2), study-unrelated fractures (n = 2), study-unrelated transient ischemic attack (n = 1), and busy schedule (n = 1). As a result, the retention was 78.6%. Of the prescribed 72 total exercise sessions, participants attended a mean 84.7% of sessions (an average 61 sessions with a range of 5–72 and SD of 19.67). The average percent of sessions that participants achieved the session cycling prescription was 86.4%, ranging from 4% to 100%. Excluding the one participant who only met the session prescription in 4% of the 72 attended sessions (age 87 years, baseline MMSE 18, CDR 2, education 14 years), the rest of the participants attended 83.3% of the prescribed sessions (60 sessions on average with a range 5–72 and SD of 19.9), and achieved the session cycling prescription on 89.4% of the attended sessions (range 70%–100%).
Among the 22 participants who completed the 6-month data collection, they attended a mean 97.2% of the sessions (70 sessions on average with a range of 55–72 and SD of 5.02). They reached the session prescription on 86.4% of the sessions (range 4%–100%). Excluding the participant who only met the session prescription in 4% of the 72 sessions, the remaining participants (n = 21) attended a mean 97.2% of the 72 sessions (an average 70 sessions with a range of 55–72 and SD of 5.12) and reached the session prescription in 90.3% of the attended sessions (range 75%–100%).
5. Discussions
The main findings of this single-site, pilot study included a 1.87 recruitment rate, 78.6% retention, and 86.4% adherence to a 6-month aerobic exercise intervention in community-dwelling older adults with mild-to-moderate AD. This study filled an existing gap by providing some cost-effective recruitment strategies for future exercise studies in older adults, a method for prescribing moderate intensity aerobic exercise to pave the way for future effectiveness and dose—response studies, and strategies to ensure exercise adherence.26 Of the seven recruitment strategies tested, referral by healthcare professionals, presentations at community events, collaboration with the local Alzheimer's Association chapter, and study flyer distributions to support groups, adult day centers, and senior residences generated the most success. The clear criteria for requiring an existing AD diagnosis further improved recruitment because 58.5% of the respondents did not have an AD diagnosis and were excluded from screening. On the other hand, newspaper advertisements, the most costly recruitment method, did not generate many responses. Neither did website recruitment through a study link on the investigator's academic website. It remains unknown if a dedicated study website or registration with the Alzheimer's Association TrialMatch™ website or www.clinicaltrials.gov will yield different results and need to be further tested. It is very important to highlight that the 1.87 recruitment rate is better than that reported in AD drug RCTs (0.26–1.82),24 indicating the willingness of older adults with AD to participate in exercise studies and the importance of an aggressive and well thought-out recruitment plan.
Since the design and conduct of a study affects retention and adherence in AD,24 particular attention was given to the design and conduct of this study which resulted in 78.6% retention and 86.4% adherence. The 78.6% retention is toward the higher end of the reported retention ranges of 59%–92% in AD exercise studies,15–20,23,28 59%–88% in AD drug RCTs,24,29 and 59%–100% in exercise studies of older adults without AD.26,27,30 This study provided important information for sample size determination in future studies. While adverse drug events, lost to follow-up, and unsatisfactory therapeutic effects are main reasons for attrition in AD drug RCTs,24,29 participants in this study dropped out due to non-study related reasons, e.g., relocation, falls and fractures (1 tripped over rug when got up in the middle of night to drink and 1 tripped over a curb while moving a box), TIA episode (during time-off from our exercise), and busy life schedule. Furthermore, in this study, the 28 participants attended 61 of the 72 prescribed sessions (84.7%) and achieved the prescribed cycling dose in 86.4% of the attended sessions. Except for one, other participants attended 60 sessions on average (83.3%) with 89.4% of those sessions meeting the prescribed doses. Those results were even better among the 22 participants who completed the 6-month data collection, attending 70 sessions on average (97.2%) and reaching the prescription dose in 90.3% of the sessions. Those results demonstrate significant improvements from previous studies which had often used low exercise doses and reported 17%–90% adherence.31
Reasons for retention success in this study are largely due to designing and conducting the study based on lessons learned from AD exercise and drug studies. Since adverse drug events were repeatedly identified as the main reason for dropout, this study was designed with participant safety as the cornerstone. Participant safety was ensured from the beginning of enrollment where participants were screened for exercise contraindications and medical clearance was obtained from healthcare providers. Participant safety was further ensured during the implementation of the study by using 9 strategies: participant supervision/guidance with a 1:2 or 2:3 (research staff:participant) ratio; individualized prescription of moderate intensity using HRR and RPE; talk test during cycling; blood pressure monitoring during cycling, providing transportation to participants; HR and RPE monitoring every 5 min during cycling; warm-up and cool-down activities before and after cycling as well as progressive dose increases over time; professional relationships with participants; and use of exercise journals as a way to show progress and facilitate communications. Other strategies that further contribute to retention and adherence included flexibility in scheduling exercise sessions, reimbursement for gym memberships, rapport with families, and communications with families regularly via phone and exercise journals.
Findings from this study are limited by its single-group design, intensive staff effort, limiting exclusion criteria, and small sample size. While the single-group design was appropriate for developing and testing the intervention, quasi-experimental designs with control groups and RCTs would have allowed the determination if attrition and adherence rates differed between groups, an important consideration for internal validity. Further, this study was designed with extensive staff support to ensure it was scientifically rigorous; hence, its clinical applicability could be limited. However, many aspects of the study protocol can be easily implemented clinically without requiring intensive staff effort, e.g., clear exercise prescription, and involving caregivers in exercise delivery. Moreover, the exclusion criteria for participants were very limiting. While the exclusion criteria helped to recruit a homogenous sample, they reduced the generalizability of the study findings. Certain criteria might prevent potential participants who were likely to benefit from participating, such as those who had pain and trouble walking in the past 6 months. Last, the sample size for this study was small, although small samples are common and appropriate for pilot studies and the insights gained are important for advancing AD exercise research.
The findings of this study have several important implications for future research of aerobic exercise in AD. First, local resources for recruitment are more effective at a relatively low cost. Developing connections with local communities, healthcare providers, support groups, and having a presence in community events had been the most successful strategies for recruitment in this study. Online recruitment such as a dedicated study website and inclusion in the Alzheimer's Association's TrialMatch website or www.clinicaltrials.gov could be successful, but not tested in this study. Second, researchers need to be thoughtful and consider participant's safety as the foremost concern in the design of future studies. Exercise safety will greatly enhance participant's and caregiver's enjoyment of being part of the study as it did for this study. Last, future studies should consider the inclusion of the 9 safety strategies tested in this study. It is difficult to attribute achieved retention and adherence to any one(s) of the strategies used for implementing the study. Overall, findings from this study provides important feasibility data for designing future studies that can test the effectiveness and the dose–response relationships of aerobic exercise in AD using scientifically rigorous RCTs.
The clinical implications of the study findings include that healthcare providers need to be cognizant of the uniqueness of older adults with AD and engage them in aerobic exercise with peers and people who understand AD and know how to communicate with older adults with AD. Many participants and caregivers commented how they felt special for being part of the study. A quick cognitive screening test such as the MMSE might be sufficient to determine the cognitive status of participants. Although we used extensive exclusion criteria that were very limiting in order to qualify a homogenous sample, older adults who meet those exclusion criteria are likely to benefit from exercise because it is more harmful for older adults not to exercise.42 Clinicians do not need to implement all of the 9 strategies designed for participant safety in an exercise program, although using all 9 strategies will likely generate the best experiences for everyone involved. However, individualizing exercise dose and dose progression over time as well as monitoring of adverse events during and after exercise should be part of any exercise programs and clinical education about exercise in AD.
6. Conclusion
This study established important feasibility data for recruiting and engaging older adults with mild-to-moderate AD in aerobic exercise. Successful recruitment relies on community partnership. Strategies for ensuring participant exercise safety collectively improved retention and adherence.
Acknowledgments
This work was jointly supported by a National Institute of Health K12 Career Advancement Award (RR023247-04) and a American Health Assistance Foundation grant (A2009344).
References
- 1.Alzheimer's Association. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Yu F, Kolanowski A, Strumpf N, Eslinger P. Improving cognition and function through exercise intervention in Alzheimer's disease. J Nurs Scholarship. 2006;38(4):358–365. doi: 10.1111/j.1547-5069.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 3.Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63(1):62–66. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith P, Blumenthal J, Hoffman B, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 10.Arkin SM. Alzheimer rehabilitation by students: interventions and outcomes. Neuropsychol Rehabil. 2001;11(3–4):273–317. [Google Scholar]
- 11.Arkin SM. Student-led exercise sessions yield significant fitness gains for Alzheimer's patients. Am J Alzheimers Dis Other Demen. 2003;18(3):159–170. doi: 10.1177/153331750301800302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCurry SM, Pike KC, Logsdon RG, Vitiello MV, Larson EB, Teri L. Predictors of short- and long-term adherence to a daily walking program in persons with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2010;25(6):505–512. doi: 10.1177/1533317510376173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palleschi L, Vetta F, De Gennaro E, et al. Effect of aerobic training on the cognitive performance of elderly patients with senile dementia of Alzheimer type. Arch Gerontol Geriatr. 1996;5:47–50. doi: 10.1016/0167-4943(96)86912-3. [DOI] [PubMed] [Google Scholar]
- 14.Rolland Y, Rival L, Pillard F, et al. Feasibility of regular physical exercise for patients with moderate to severe Alzheimer disease. J Nutr Health Aging. 2000;4(2):109–113. [PubMed] [Google Scholar]
- 15.Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 16.teinberg M, Leoutsakos JS, Podewils LJ, Lyketsos C. Evaluation of a home-based exercise program in the treatment of Alzheimer's disease: the maximizing independence in dementia(MIND) study. IntJ Geriatr Psychiatry. 2009;24(7):680–685. doi: 10.1002/gps.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tappen RM, Roach KE, Applegate EB, Stowell P. Effect of a combined walking and conversation intervention on functional mobility of nursing home residents with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(4):196–201. doi: 10.1097/00002093-200010000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 19.Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer's disease. Aging Ment Health. 2008;12(1):72–80. doi: 10.1080/13607860701529932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CL, Tappen RM. Effect of exercise on mood in nursing home residents with Alzheimer 's disease. AmJ Alzheimers Dis Other Demen. 2007;22(5):389–397. doi: 10.1177/1533317507305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu F, Kolanowski A. Facilitating aerobic exercise training in older adults with Alzheimer's disease. Geriatr Nurs. 2009;30(4):250–259. doi: 10.1016/j.gerinurse.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Yu F, Leon A, Bliss D, Dysken M, Savik K, Wyman J. Aerobic training for older men with Alzheimer's disease: individual examples of progression. Res Gerontol Nurs. 2011;4(4):243–250. doi: 10.3928/19404921-20110303-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu F, Nelson NW, Savik K, Wyman JF, Dyskin M, Bronas UG. Affecting cognition and quality of life via aerobic exercise in Alzheimer's disease. West J Nurs Res. 2012 doi: 10.1177/0193945911420174. http://dx.doi.org/10.1177/0193945911420174. [DOI] [PMC free article] [PubMed]
- 24.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimer's Res Ther. 2010;2(6):34. doi: 10.1186/alzrt58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes D, Forbes S, Morgan D, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489. doi: 10.1002/14651858.CD006489.pub2. http://dx.doi.org/10.1002/14651858.CD006489.pub2. [DOI] [PubMed]
- 26.Foster CE, Brennan G, Matthews A, McAdam C, Fitzsimons C, Mutrie N. Recruiting participants to walking intervention studies: a systematic review. Int J Behav Nutr Phys Act. 2011;8:137. doi: 10.1186/1479-5868-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jancey J, Howat P, Lee A, et al. Effective recruitment and retention of older adults in physical activity research: PALS study. AmJ Health Behav. 2006;30(6):626–635. doi: 10.5555/ajhb.2006.30.6.626. [DOI] [PubMed] [Google Scholar]
- 28.Kemoun G, Thibaud M, Roumagne N, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn. 2010;29(2):109–114. doi: 10.1159/000272435. [DOI] [PubMed] [Google Scholar]
- 29.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2009;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40-65 years: the S.W.E.A.T. study (sedentary women exercise adherence trial) Prev Med. 2003;36(1):17–29. doi: 10.1006/pmed.2002.1134. [DOI] [PubMed] [Google Scholar]
- 31.Yu F. Guiding research and practice: a conceptual model for aerobic exercise training in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2011;26(3):184–194. doi: 10.1177/1533317511402317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox KL, Burke V, Beilin LJ, et al. Short and long-term adherence to swimming and walking programs in older women—the sedentary women exercise adherence trial (SWEAT 2) Prev Med. 2008;46(6):511–517. doi: 10.1016/j.ypmed.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Kahn SR, Shrier I, Shapiro S, et al. Six-month exercise training program to treat post-thrombotic syndrome: a randomized controlled two-centre trial. CMAJ Can Med Assoc J. 2011;183(1):37–44. doi: 10.1503/cmaj.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jancey J, Lee A, Howat P, Clarke A, Wang K, Shilton T. Reducing attrition in physical activity programs for older adults. J Aging Phys Act. 2007;15(2):152–165. doi: 10.1123/japa.15.2.152. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1994;44(10):1984. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 37.Burke WJ, Roccaforte WH, Wengel SP. The short form of the geriatric depression scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR, Fanjiang G. Mini-mental State Examination: User's Guide. Odessa, FL: PAR; 2001. [Google Scholar]
- 39.Ries J, Echternach J, Nof L, Blodgett M. Test-retest reliability and minimal detectable change scores for the timed ‘up & go’ test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569–579. doi: 10.2522/ptj.20080258. [DOI] [PubMed] [Google Scholar]
- 40.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–1024. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med. 1982;3(3):153–158. doi: 10.1055/s-2008-1026080. [DOI] [PubMed] [Google Scholar]
- 42.National Institute on Aging. Exercise: A Guide from the National Institute on Aging (NIH Publication No 01-4258) Washington, DC: U.S. Governmental Printing Office; 2004. [Google Scholar]