Abstract
Background
A proportion of patients with initial presentation of ulcerative proctitis (UP) progress to more extensive colitis. We sought to characterize the natural history and identify clinical predictors of extension in UP.
Methods
We performed a retrospective cohort study of participants with a new diagnosis of UP from January 2000 to December 2015. We used cox proportional hazard modeling to identify predictors of disease extension.
Results
Through December of 2015, we identified 169 cases of UP with a median age of diagnosis of 40 years (range: 16–91 years) and a median follow up of 4.3 years (range: 3.3–15.1 years). 53 (31%) patients developed extension over the follow up time. Compared to non-extenders, the need for immunosuppressive or biologic therapy was significantly higher among extenders (34% vs. 2.6%, p < 0.001). In multivariable analyses, compared to UP cases with BMI < 25, the adjusted hazard ratios (aHRs) of extension were 1.75 (95% CI, 0.95 – 3.23) and 2.77 (95% CI, 1.07 – 7.14) among overweight and obese patients, respectively (Ptrend = 0.03). Similarly, patients with a history of appendectomy or endoscopic finding of moderate to severe disease had a higher risk of extension (aHR = 2.74, 95% CI 1.07 – 7.01 and 1.96, 95% CI 1.05–3.67, respectively).
Conclusion
In a retrospective cohort study, we show that appendectomy, BMI, and endoscopic activity at the time of diagnosis of proctitis are associated with increased risk of extension. In addition, our data suggest that extenders are more likely to require immunosuppressive or biologic therapy.
Keywords: Inflammatory Bowel Disease, Ulcerative Proctitis, Disease Extension
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disorder of the gastrointestinal tract with heterogeneous disease presentation. In nearly 20% of cases, the initial presentation is an acute severe colitis, while nearly 50% of patients may never require hospitalization related to their disease1–3. Ulcerative proctitis is a unique and often mild form of UC, characterized by inflammation limited to the rectum. In most patients with initial diagnosis of proctitis, the disease does not extend to more proximal areas of the large intestine. Specifically, prior studies suggest that only 10–30% of patients with ulcerative proctitis experience disease extension4–9.
It is expected that patients with disease extension are more likely to suffer from UC complications and are at higher risk of requiring treatment escalation. A recent study in pediatric population with new diagnosis of UC demonstrated a 13% cumulative risk of colectomy at 15 years among ulcerative proctitis patients with no difference in rate of surgery, anti-TNF use, or extension compared to other UC patients6. Nevertheless, data on clinical predictors of disease extension in ulcerative proctitis are sparse. In addition, prior studies evaluating risk factors for disease extension in ulcerative proctitis were limited by small sample size, single center experience, limited follow up time, and lack of detailed information on lifestyle factors4,6,8,10. Finally, there is a paucity of data on differences in treatment course and disease complications between proctitis patients with disease extension to those without.
We therefore sought to examine the predictors of extension in patients with initial diagnosis of ulcerative proctitis using data from a large health care network. In addition, we explored the differences in treatment patterns between extenders and non-extenders as defined by need for immunosuppressive medications or biologics, and rates of surgery.
METHODS
Study population
From January 2000 through December 2015, we identified cases of ulcerative proctitis using the Research Patient Data Registry (RPDR), which encompasses hospitals within the Partners healthcare system (i.e. Brigham and Women’s Hospital, Faulkner Hospital, Massachusetts General Hospital, Newton Wellesley Hospital, and North Shore Medical Center). RPDR is a centralized clinical data registry that gathers clinical information including notes, labs, imaging, procedures, and pathology for both inpatient and ambulatory care. An online query tool allows investigators to explore clinical data through a self-service system, while the data request wizards allow the users to ask for more detailed medical record information on the identified patient population.
Cases of ulcerative proctitis were identified by searching the RPDR for billing diagnoses of UC OR ulcerative proctitis AND by searching the pathology database among these individuals for key words “chronic active colitis” or “active chronic colitis”. Medical records were then reviewed to confirm the initial diagnosis of ulcerative proctitis by endoscopy and pathology. Patients who were diagnosed with Crohn’s disease, ischemic colitis, or indeterminate colitis during followed up were excluded.
Clinical Characteristics, Laboratory and Endoscopic Findings
At the time of diagnosis of proctitis, we collected information on age, race, body mass index (BMI), current medications, smoking status, and family history of inflammatory bowel disease through review of medical records. Similarly, laboratory data including autoimmune markers (e.g. ANA, etc), inflammatory markers (ESR or CRP), hematocrit (Hct), and platelet count (Plt) at the time of diagnosis were collected. For women, we also collected information on parity and full-term pregnancy over follow up time. We used the Simple Clinical Colitis Activity Index (SCCAI) to assess clinical activity at the time of diagnosis and Mayo clinic endoscopy score to assess endoscopic activity. When Mayo clinic endoscopy scores were not specifically reported, we (E.W., Y.W.C., S.M.C, and H.K.) reviewed endoscopic images to calculate Mayo clinic score. As cecal patch has previously been reported with distal colitis, we also collected information on presence of this finding at the initial or subsequent colonoscopies. Finally, information on histology at the time of diagnosis including severity of colitis was also obtained from pathology reports.
Ascertainment of outcomes
Information on disease extension, defined as endoscopic and histologic evidence of UC beyond the rectum, was obtained through review of medical records. Date of extension was considered to be the date of colonoscopy/sigmoidoscopy at which point extension was identified. Data on UC-related medications including aminosalicylates, steroids, thiopurines (azathioprine and 6-mercaptopurines), methotrexate, cyclosporine, anti-TNF therapy (infliximab, adalimumab, and golimumab), and vedolizumab at any time after diagnosis were also obtained through review of medical records.
Statistical Analysis
Baseline characteristics of participants at the time of proctitis diagnosis were reported in frequency and median for categorical and continuous variables, respectively. Person-time for each participant was calculated from date of diagnosis of proctitis to the date of extension, death from any cause, date of last encounter with the healthcare system, or December 31, 2015, whichever came first. We used Cox proportional hazards modeling adjusting for covariates to calculate adjusted hazard ratios (aHR) and 95% confidence interval (CIs). We developed a multivariable model using TRIPOID guidelines, incorporating age, sex, previously identified clinical factors associated with extension including appendectomy and smoking, and novel independent factors identified through backward selection modeling using a threshold p value of 0.1511. We used the conservative threshold p value of 0.15 based on simulation studies demonstrating that a higher value should be considered in automated selection models, particularly in studies of small sample size12,13. To account for secular trends in patterns of treatment over follow up time, we stratified all analyses by calendar year. We used Kaplan Meier curve to demonstrate rate of extension over time and log-rank test to compare treatment course and surgical rates according to disease extension. We used SAS version 9.4 (Cary, NC) for these analyses. All P-values were 2-sided and < 0.05 was considered statistically significant. The study was approved by the institutional review board at the Partners Healthcare.
RESULTS
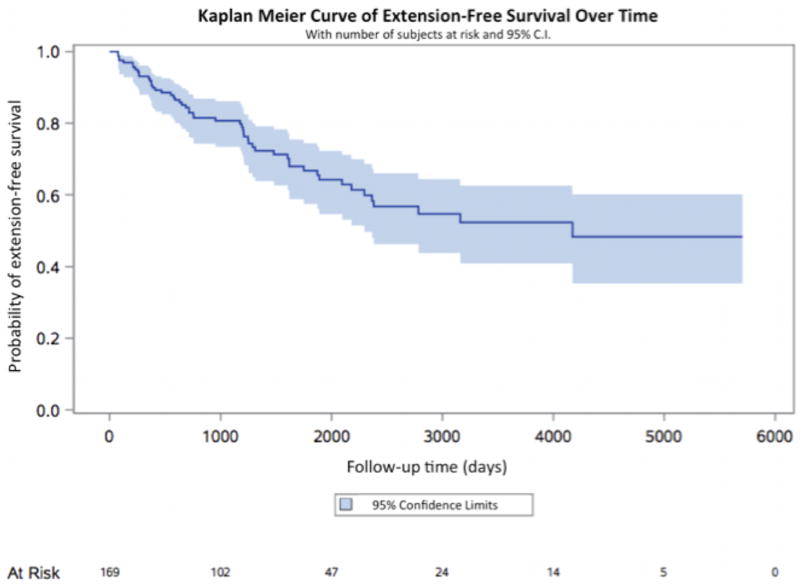
Through December 2015, we confirmed 169 ulcerative proctitis cases with a median follow up of 4.3 years (range: 3.3–15.1 years) (Table 1). The median age of diagnosis was 40 years (range: 16–91 years) and the majority of participants were female (55%) and white (82.9%). The median BMI was 24.8 kg/m2 (range: 17.0 – 53.1 kg/m2). At the time of diagnosis, nearly 64% of patients had both endoscopic and histologic evidence of moderate to severe colitis and 9.5% had a cecal patch. Through the end of follow up, 137 (81%) patients had at least one follow up colonoscopy and 53 patients (31%) developed disease extension with a median time from diagnosis to extension of 2.1 years (range: 0.3–2.9 years) (Figure 1). Among extenders, 36 (67.9%) developed left-sided UC (E2) while 17 (32.1%) developed extensive UC (E3).
Table 1.
Characteristics of study participants at the time of diagnosis (N= 169)
| Variable | Median (range) or n (%) | |
|---|---|---|
| Age (years) | 40.0 (16.0 – 91.7) | |
| Race/ethnicity (n = 164) | ||
| White | 136 (82.9%) | |
| Non-white | 28 (17.1%) | |
| Gender: male (%) / female (%) | 76 (45%) / 93 (55%) | |
| BMI (kg/m2) (n = 127) | 24.8 (17.0 – 53.1) | |
| Ever smoking | 64 (37.9%) | |
| Current smoking | 12 (7.1%) | |
| Pregnancy¶ | 14 (15.1%) | |
| Family history of IBD | 27 (16.0%) | |
| Laboratory data | ||
| Hgb (g/dl) (n = 106) | 13.9 (9.4 – 16.8) | |
| WBC (K/uL) (n = 107) | 7.1 (3.2 – 11.7) | |
| Plt (K/uL) (n = 102) | 261.5 (117 – 516) | |
| ESR (mm/h) (n = 34) | 10.5 (2.0 – 42.0) | |
| CRP (mg/L) (n = 31) | 0.5 (0 – 24.3) | |
| Total Protein (g/dl) (n = 71) | 7.3 (4 – 8.6) | |
| Albumin (g/dl) (n = 72) | 4.5 (3.2 – 5.3) | |
| Endoscopic severity at diagnosis (n = 162) | ||
| Normal or Mild | 58 (35.8%) | |
| Moderate to severe | 104 (64.2%) | |
| Pathologic severity at diagnosis (n = 146)+ | ||
| Normal or Mild | 49 (33.6%) | |
| Moderate to severe | 97 (66.4%) | |
| Cecal patch at diagnosis | 16 (9.5%) | |
| Appendectomy | 9 (5.3%) | |
| Colectomy | 3 (1.8%) | |
Expressed as percent of total women, n = 93 and represent pregnancy over follow up time.
Figure 1. Kaplan-Meier curve of extension-free survival of ulcerative proctitis over time.
Included are number of subjects at risk as well as 95% confidence intervals.
We sought to identify predictors of disease extension using automated selection models. In multivariable analyses, BMI, endoscopic disease severity, and appendectomy were the only variables that were independently associated with increased risk of extension (Table 2). Compared to proctitis cases with BMI less than 25 kg/m2, the aHRs of extension were 1.75 (95% CI, 0.95 – 3.23) and 2.77 (95% CI, 1.07 – 7.14) among overweight (BMI = 25–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) patients, respectively (Ptrend = 0.03). Similarly, patients with a history of appendectomy or moderate to severe endoscopic activity at the time of diagnosis had over a 2-fold increase in risk of extension (aHR = 2.74, 95% CI 1.07 – 7.01 and aHR = 1.96, 95% CI 1.05–3.67). We also observed an inverse association between current smoking at the time of diagnosis and risk of disease extension compared to non-smokers, though this also did not reach statistical significance (aHR = 0.23, 95% CI 0.05 – 1.13, p = 0.071). Other factors including age of diagnosis, gender, race, inflammatory markers, family history of inflammatory bowel disease, pregnancy, NSAIDs use, and histologic activity were not associated with risk of extension (Supplementary Table 1).
Table 2.
Multivariable-adjusted risk of disease extension for independent predictors*
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Moderate-severe endoscopic disease activity | 1.96 (1.05–3.67) | 0.03 |
| Overweight (BMI = 25–29.9 kg/m2) | 1.75 (0.95 – 3.23) | 0.07 |
| Obese (BMI ≥ 30 kg/m2) | 2.77 (1.07 – 7.14) | 0.03 |
| Current smoking | 0.23 (0.05 – 1.13) | 0.07 |
| Appendectomy | 2.74 (1.07 – 7.01) | 0.04 |
Final model was adjusted for age and gender.
We considered the possibility that interval censoring of patients without long-term follow up within the health network may have differentially decreased the follow up time for patients with mild disease as they would have been less likely to seek care. Thus, we performed sensitivity analysis extending follow up to December of 2015 for all patients regardless of the number encounters after diagnosis in Partners healthcare network and obtained similar risk estimates compared to our primary analysis. Compared to proctitis cases with BMI less than 25 kg/m2, the aHRs of extension were 1.73 (95% CI, 0.94–3.20) and 2.36 (95% CI, 0.95–5.88) among overweight (BMI = 25–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) patients, respectively (Ptrend = 0.04). Similarly, both appendectomy and moderate to severe endoscopic disease activity were associated with increased risk of extension (aHR = 2.99, 95% CI 1.17–7.66 and 1.92, 95% CI 1.02–3.59, respectively). Conversely, smoking at the time of diagnosis was inversely associated with risk of extension (aHR = 0.22, 95% CI 0.05 – 1.05).
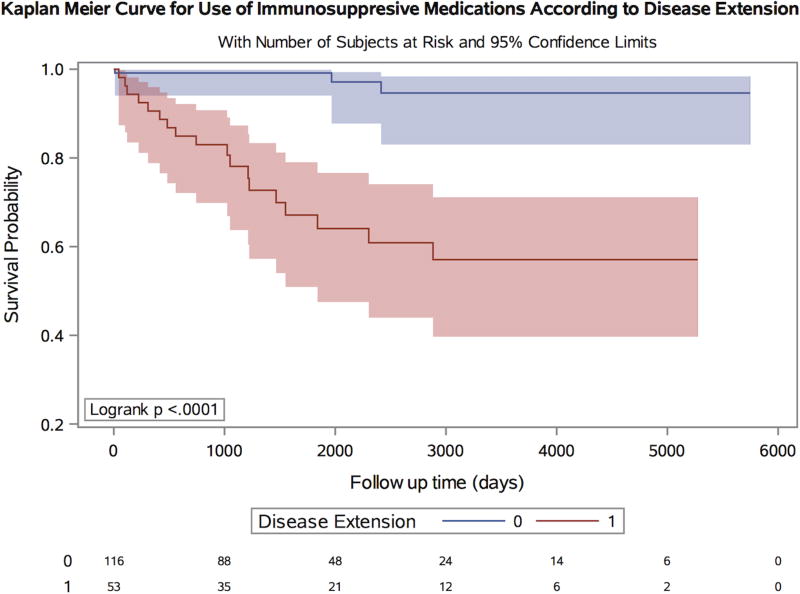
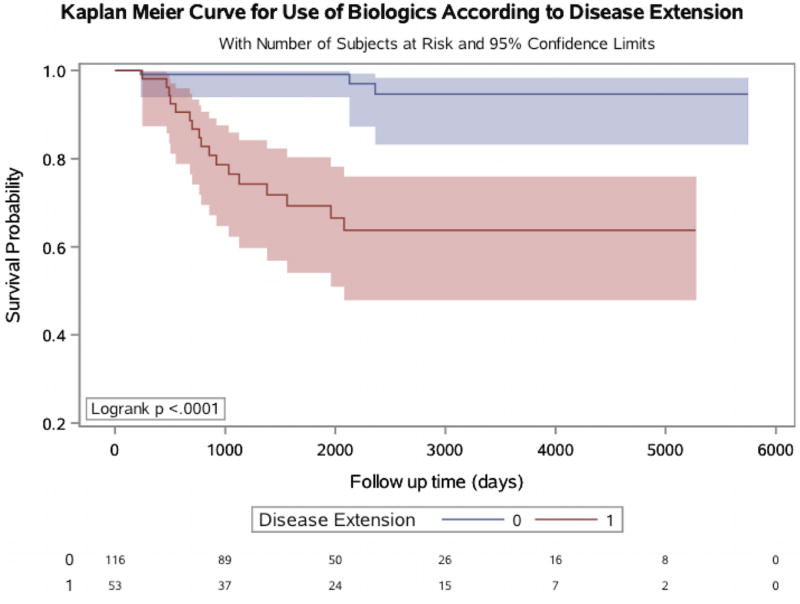
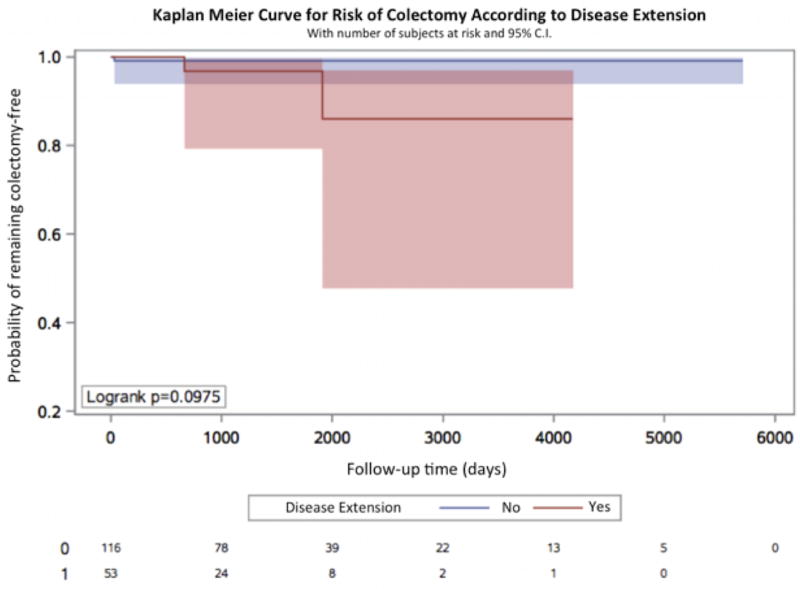
We next examined whether there were significant differences between treatment patterns of patients with ulcerative proctitis and those who developed extension over time. There were no significant differences in use of 5-aminosalicylate or steroids comparing proctitis patients who had disease extension to those without. Specifically, 37 (69.8%) of extenders were prescribed 5- aminosalicylate compared to 97 (83.6%) of non-extenders (Pcomparison = 0.06). Similarly, 37.7% of extenders and 25% of non-extenders were prescribed steroids (Pcomparison = 0.10). In contrast, there were significant differences in rates of immunosuppressive and biologic therapy use between the two groups (Figure 2). While 18 (34%) extenders were prescribed oral immunosuppressive medications during follow up, only 3 (2.6%) non-extenders received these medications (Pcomparison <0.001). Similarly, 17 (32%) extenders received biologic therapy compared to only 3 (2.6%) non-extenders (Pcomparison <0.001) (Figure 3). Finally, the rate of colectomy over follow up also appeared to be higher among extenders, although this comparison did not reach statistical significance [2 (3.8%) vs. 1 (0.9%), p = 0.0975) (Figure 4). We also considered the possibility that stratifying patients at baseline according to disease extension, future event, may have differentially biased our estimates. We therefore performed a sensitivity analysis by matching extenders to proctitis cases according to disease duration (+/− 1 year) at the time of diagnosis of extension and compared rates of immunosuppressive and biologic use and obtained similar results (Pcomparison < 0.001). There was, however, no difference in colectomy rates between the two groups (P = 0.29).
Figure 2. Kaplan-Meier curve for use of immunosuppressive medications over time according to disease extension.
Included are number of subjects at risk as well as 95% confidence intervals.
Figure 3. Kaplan-Meier curve for use of biologic medications over time according to disease extension.
Included are number of subjects at risk as well as 95% confidence intervals.
Figure 4. Kaplan-Meier curve for risk of colectomy over time according to disease extension.
Included are number of subjects at risk as well as 95% confidence intervals.
DISCUSSION
In a retrospective cohort study, we demonstrated that obesity as measured by BMI at the time of initial diagnosis of proctitis, appendectomy, and disease activity as measured at index endoscopy are associated with increased risk of extension. Additionally, there was a trend toward a decreased risk of extension in current smokers at time of diagnosis, although the risk did not reach statistical significance. Finally, our data suggest that compared to non-extenders, proctitis patients who have extension of their disease beyond the rectum over time are also more likely to be prescribed immunosuppressive and biologic therapy.
Our results are supported by several prior findings. In a retrospective cohort study of 98 patients with an average follow up of 109 months, Kim and colleagues reported a 27.6% rate of extension7. Consistent with our findings, Kim et al also found that disease severity at the time of diagnosis was associated with increased risk of extension. Similarly, Meucci and colleagues assessed the rate of extension in 272 proctitis patients with a mean follow up of 52 months and found an extension rate of 27.1%8. They also demonstrated an inverse association between smoking and risk of extension. Interestingly in both studies, BMI at the time of diagnosis was not assessed.
Our data does, however, contrast with findings from other studies. Safroneeva and colleagues did not find an association between BMI and risk of disease extension9. However, in their study, rate of disease extension was compared between individuals with BMI greater than 20 to those less than 20. Thus, obesity (defined by BMI>30) could not have been identified as a risk factor for disease extension. In support of this, when using similar categories for BMI in our analysis, we did not find an association between BMI and risk of disease extension. Additionally, in a retrospective cohort study from Japan, Anzai and colleagues studied the significance of cecal patch in patients with UC and reported that all 9 proctitis patients with cecal patch had evidence of disease extension through follow up. However, there was no comparable control group (i.e. proctitis patients without Cecal patch) to determine whether cecal patch is a predictor of extension.
Several studies have examined the relationship between appendectomy and UC. Most studies have demonstrated a potential protective benefit from appendectomy, particularly early in life on risk of development of UC10,14–17. Although the exact mechanism underlying this prior observation is largely unknown, a number of plausible mechanisms have been proposed. First, animal studies and human data suggest that appendix appears to be a priming site for innate immune cells that play a critical role in development of UC18,19. Second, in mice, appendix/cecal patch plays a critical role in the generation of immunoglobulin-A (IgA)-producing B cells that home to the colon but not the small intestine with appendectomy leading to a delayed accumulation of IgA-producing cell in the large intestine20. Third, appendix may serve as a reservoir for commensal bacteria and therefore its removal could lead to more dynamic changes in the colonic microbiota. Despite compelling evidence suggesting protective role of appendectomy on development of UC, it remains largely unclear whether appendectomy plays a role in the clinical course of patients with UC or proctitis21,22. In our study, we also demonstrated appendectomy was associated with increased risk of extension in patients with ulcerative proctitis. This finding is in contract with some prior studies highlighting potential therapeutic benefit of appendectomy in patients with ulcerative protitis. In a prospective study of 30 patients with new diagnosis of ulcerative proctitis undergoing appendecetomy as a treatment, 90% of patient had clinical improvements23.
Our study is the first to report BMI as an independent predictor of disease extension in patients with ulcerative proctitis. Although the precise pathophysiology of ulcerative colitis remains largely unknown, a likely key pathogenic mechanism is loss of immune tolerance to gut microbiota in genetically susceptible individuals24. Nevertheless, it’s unclear whether shared biologic pathways drive the pathogenesis of both proctitis and ulcerative colitis, particularly since a large proportion of patients with proctitis have mild and limited disease. Our finding that BMI at the time of diagnosis is associated with risk of extension may therefore have biologic implications. First, obesity has been previously linked to significant alterations in the structure of gut microbiota. In obese mice, there is a 50% reduction in abundance of Bacteroidetes with a proportional increase in Firmicutes25,26. Similar findings have been reported in obese human subjects27. Second, obesity as measured by BMI has been linked to elevated levels of proinflammatory markers such as tumor necrosis factor-alpha (TNF-α) and C-reactive protein (CRP)28,29. Third, obese individuals have been shown to have higher levels of inflammation in the gastrointestinal tract as measured by stool calprotectin30. Therefore, it’s possible that obesity through its effect on the gut microbiota and immune function may drive the evolution of proctitis into ulcerative colitis.
Our study has several strengths. First, we confirmed all cases of proctitis by medical records reviews using standardized criteria, representing a significant advantage over prior studies that rely on self-report or clinic/hospital discharged codes, which may not accurately reflect true diagnoses. Second, we collected detailed information on other important lifestyle factors, medications, as well as endoscopic, laboratory and histologic findings in nearly all of our cases, and were therefore able to identify novel risk factors for disease extension. Finally, our relatively long follow up allowed for a more precise estimate of the risk over time.
Our study has several limitations. First, although our samples size was similar, if not larger than most of the prior studies, we may have had limited power to detect more modest differences or associations. Second, we did not have complete data on time period before diagnosis of disease and therefore could not fully account for use of other medications that may have contributed to disease extension. Third, data on endoscopic activity was collected from review of procedural reports and images, which may introduce biases related to inter-observer differences. However, the correlation between endoscopic scoring across the study reviewers was excellent (r = 0.92). Fourth, as not all patients in the study had follow up colonoscopy, we cannot definitively exclude the possibility of outcome misclassification. Nevertheless, individuals with disease extension are more likely to be symptomatic and therefore receive endoscopic evaluation. Finally, our study is observational and therefore we cannot rule out the possibility of residual confounding. However, we collected data on all factors that have previously been associated with progression of proctitis/ulcerative colitis and considered them in all analyses.
In a retrospective cohort study, we show that appendectomy, BMI and endoscopic severity at the time of diagnosis of proctitis are associated with increased risk of extension. In addition, our data suggest that extenders are more likely to require immunosuppressive or biologic therapy over time. If validated, these results could guide the development of targeted therapeutic algorithms for those patients who may be at highest risk for disease progression.
Supplementary Material
Acknowledgments
Grant Support: Dr. Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681).
Financial Disclosures: Dr. Khalili has received consulting fee from Abbvie Inc., Samsung Bioepis, and Takeda Phacmeceuticals. Dr. Khalili receives research funding from Takeda Pharmaceuticals.
Footnotes
Conflict of Interest: None to declare
Ethical Approval: The study was approved by Partners Human Research Committee (Institutional Review Board).
Authors Contributions
EW - acquisition of data; drafting and critical revision of the manuscript
YWC - acquisition of data; critical revision of the manuscript
SMC- acquisition of data; critical revision of the manuscript
VY - acquisition of data; critical revision of the manuscript
VD- study concept, critical revision of the manuscript
JJG- study concept, acquisition of data; analysis; critical revision of the manuscript
HK- study design; acquisition of data; statistical analysis; drafting of the manuscript
References
- 1.Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis. 2013;19(9):1858–1866. doi: 10.1097/MIB.0b013e31828c84c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4(4):431–437. doi: 10.1016/j.crohns.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Edwards FC, Truelove SC. The Course and Prognosis of Ulcerative Colitis. Gut. 1963;4:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anzai H, Hata K, Kishikawa J, et al. Clinical pattern and progression of ulcerative proctitis in the Japanese population: a retrospective study of incidence and risk factors influencing progression. Colorectal Dis. 2016;18(3):O97–O102. doi: 10.1111/codi.13237. [DOI] [PubMed] [Google Scholar]
- 5.Bakman Y, Katz J, Shepela C. Clinical Significance of Isolated Peri-Appendiceal Lesions in Patients With Left Sided Ulcerative Colitis. Gastroenterology Res. 2011;4(2):58–63. doi: 10.4021/gr302w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochart A, Gower-Rousseau C, Sarter H, et al. Ulcerative proctitis is a frequent location of paediatric-onset UC and not a minor disease: a population-based study. Gut. 2016 doi: 10.1136/gutjnl-2016-311970. [DOI] [PubMed] [Google Scholar]
- 7.Kim B, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Proximal disease extension and related predicting factors in ulcerative proctitis. Scand J Gastroenterol. 2014;49(2):177–183. doi: 10.3109/00365521.2013.867360. [DOI] [PubMed] [Google Scholar]
- 8.Meucci G, Vecchi M, Astegiano M, et al. The natural history of ulcerative proctitis: a multicenter, retrospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII) Am J Gastroenterol. 2000;95(2):469–473. doi: 10.1111/j.1572-0241.2000.t01-1-01770.x. [DOI] [PubMed] [Google Scholar]
- 9.Safroneeva E, Vavricka S, Fournier N, et al. Systematic analysis of factors associated with progression and regression of ulcerative colitis in 918 patients. Aliment Pharmacol Ther. 2015;42(5):540–548. doi: 10.1111/apt.13307. [DOI] [PubMed] [Google Scholar]
- 10.Anzai H, Hata K, Kishikawa J, et al. Appendiceal orifice inflammation is associated with proximal extension of disease in patients with ulcerative colitis. Colorectal Dis. 2016;18(8):O278–282. doi: 10.1111/codi.13435. [DOI] [PubMed] [Google Scholar]
- 11.Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 12.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21(1):45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 13.Binder H, Sauerbrei W, Royston P. Comparison between splines and fractional polynomials for multivariable model building with continuous covariates: a simulation study with continuous response. Stat Med. 2013;32(13):2262–2277. doi: 10.1002/sim.5639. [DOI] [PubMed] [Google Scholar]
- 14.Andersson RE, Olaison G, Tysk C, Ekbom A. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;344(11):808–814. doi: 10.1056/NEJM200103153441104. [DOI] [PubMed] [Google Scholar]
- 15.Koutroubakis IE, Vlachonikolis IG, Kouroumalis EA. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: a critical review. Inflamm Bowel Dis. 2002;8(4):277–286. doi: 10.1097/00054725-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Naganuma M, Iizuka B, Torii A, et al. Appendectomy protects against the development of ulcerative colitis and reduces its recurrence: results of a multicenter case-controlled study in Japan. Am J Gastroenterol. 2001;96(4):1123–1126. doi: 10.1111/j.1572-0241.2001.03757.x. [DOI] [PubMed] [Google Scholar]
- 17.Selby WS, Griffin S, Abraham N, Solomon MJ. Appendectomy protects against the development of ulcerative colitis but does not affect its course. Am J Gastroenterol. 2002;97(11):2834–2838. doi: 10.1111/j.1572-0241.2002.07049.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita M, Takakuwa H, Matsubayashi Y, Nishio A, Ikehara S, Okazaki K. Appendix is a priming site in the development of ulcerative colitis. World J Gastroenterol. 2005;11(31):4869–4874. doi: 10.3748/wjg.v11.i31.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med. 1996;184(2):707–715. doi: 10.1084/jem.184.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masahata K, Umemoto E, Kayama H, et al. Generation of colonic IgA-secreting cells in the caecal patch. Nat Commun. 2014;5:3704. doi: 10.1038/ncomms4704. [DOI] [PubMed] [Google Scholar]
- 21.Sahami S, Kooij IA, Meijer SL, Van den Brink GR, Buskens CJ, Te Velde AA. The Link between the Appendix and Ulcerative Colitis: Clinical Relevance and Potential Immunological Mechanisms. Am J Gastroenterol. 2016;111(2):163–169. doi: 10.1038/ajg.2015.301. [DOI] [PubMed] [Google Scholar]
- 22.Parian A, Limketkai B, Koh J, et al. Appendectomy does not decrease the risk of future colectomy in UC: results from a large cohort and meta-analysis. Gut. 2016 doi: 10.1136/gutjnl-2016-311550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolin TD, Wong S, Crouch R, Engelman JL, Riordan SM. Appendicectomy as a therapy for ulcerative proctitis. Am J Gastroenterol. 2009;104(10):2476–2482. doi: 10.1038/ajg.2009.388. [DOI] [PubMed] [Google Scholar]
- 24.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 27.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109(24):3022–3028. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 30.Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13(2):279–284. doi: 10.1158/1055-9965.epi-03-0160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.