There is widespread agreement across the American Academy of Pediatrics (AAP)1, 2, expert panels3, 4, parents and autism advocacy organizations5, as well as the U.S. Department of Health and Human Services Interagency Autism Coordinating Committee6 that early identification and intervention for toddlers with autism spectrum disorder (ASD) is a very high public health priority and that universal early screening in pediatric populations is an essential tool for early ASD risk detection. The AAP guidelines to implement universal early screening for autism1 as standard of care is one of the most positive and successful public health policies ever created for children affected by autism. Indeed, such a policy has led to the regular detection and treatment of autism by the 2nd birthday in cities with systematic screening programs7–11. Early screening by pediatricians is becoming commonplace in the US and is objectively successful3. Studies show that using standardized screening tools is the most accurate approach to early at-risk autism detection, even as compared to pediatrician judgment and surveillance12, 13. Private and public resources and research have together resulted in the development of early screening approaches that, when implemented, can detect ASD 2–3 years sooner7–11 than the national average of 4 years of age14. Implementing ASD screening as standard-of-care is particularly important for children from low SES and minority backgrounds who are consistently overlooked and under-detected and as a result have a later age of first diagnosis and delayed access to services relative to other children15. Early detection importantly allows for intervention to begin earlier, which is considered essential to achieving the best outcomes16–18. Research suggests that individuals with positive outcomes, including gains in IQ, adaptive skills, and reduction in ASD core symptoms, as well as those with optimal outcomes who no longer meet criteria for ASD over time, are more likely to have been identified and treated before age 3 years19.
In the midst of this major accomplishment and advance over the past when ASD children commonly went undetected and untreated for years across childhood, the US Preventive Services Task Force (or Task Force) released its own views about early universal screening for ASD.
The US Preventive Services Task Force Recommendation Statement
Earlier this year, the USPSTF, referred to here as “Task Force”, released a report on ASD screening that stated: “Current evidence is insufficient to assess the balance of benefits and harms of screening for autism spectrum disorder (ASD) in young children for whom no concerns of ASD have been raised by their parents or a clinician20.” In essence, the Task Force failed to recommend universal screening for ASD in the general pediatric population, because it claims there is insufficient evidence of its benefits (but later admits its “harms” are minimal). Additional details regarding the Task Force report are summarized in Figure 1.
Figure 1.
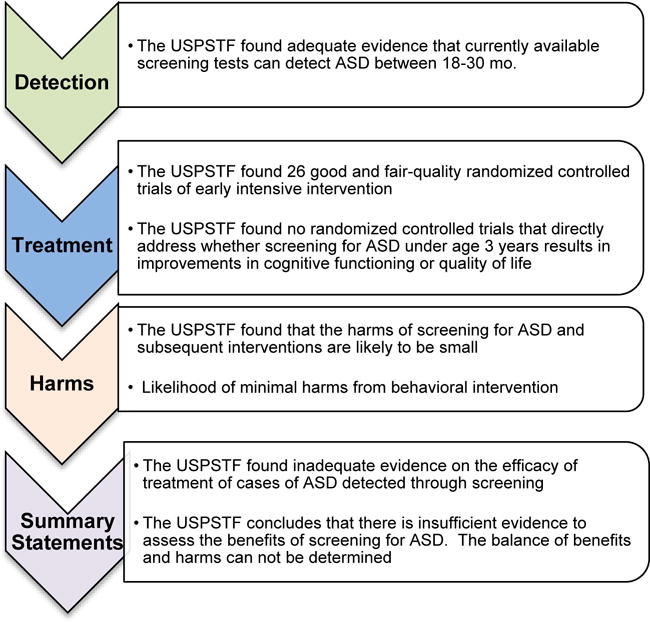
Overview of key features of USPSTF ASD Screening Recommendation Statement20.
The Task Force statement about “harms”
The statement carries the sense that universal early screening may actually do harm since the noted benefits do not outweigh “harm.” Yet, at the same time, the Task Force notes that early universal screening has been repeatedly demonstrated to be effective in detecting ASD (see3) and in fact does little to no harm. The report states: “The USPSTF found that the harms of screening for ASD and subsequent interventions are likely to be small based on evidence about the prevalence, accuracy of screening, and likelihood of minimal harms from behavioral interventions.” Surprisingly, the potential benefits of universal ASD screening, as we illustrate in Figure 2, was not fully considered in the report.
Figure 2.
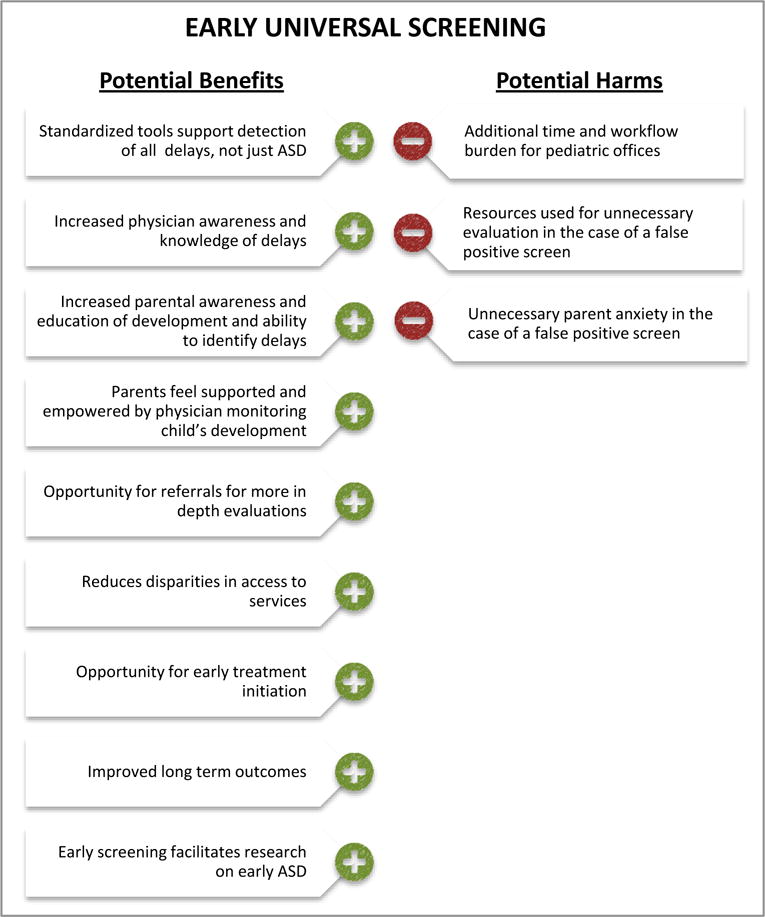
Illustration of the balance of potential benefits and harms of engaging in universal early screening for ASD. As illustrated in the figure, the potential benefits outweigh potential harm.
The Task Force statement about “benefits”
The Task Force did not clearly define what it meant by benefits, improvement, and outcomes. The Task Force suggested only vague metrics of changes in educational, behavioral, functional or IQ measures in order for them to pronounce that the “evidence is sufficient.” In reality, the potential benefits of ASD early identification and treatment range from tangible and easier to document impacts such as changes in IQ or social behavior, to more systemic changes that may impact the health, functioning, and well being of the entire family unit (see Figure 3). Truly understanding treatment response and outcome in ASD is thus a complex issue because, beyond the treatment itself, outcome is related to a range of factors including a child’s underlying genetics as well as family, educational, social, economic and cultural environmental factors. Precision medicine that takes such information into account is only beginning to be considered in ASD21. Improvements can happen both proximally and distally: in social engagement, school preparedness, language expression, language reception, and in the child’s everyday life experiences at home and in public places and school. All such improvements are legitimate changes in response to treatment. Changes are also moderated by changes in stress levels for both the child and parent that change as the result of success (or failure) in response to treatment. It is through this complex lens that the benefits of early detection and subsequent treatment should be considered along with the acknowledgement that many of the potential impacts of early treatment are not easily or quickly captured.
Figure 3.
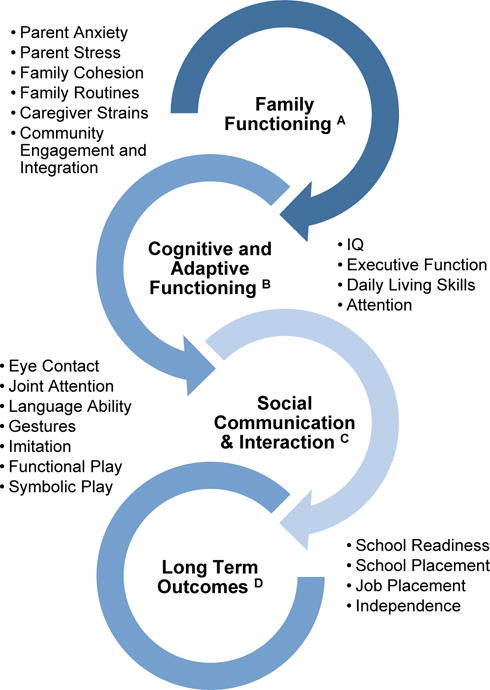
Areas of known (B,C) and theoretical (A, D) improvement following early intervention. Early detection enables services to begin sooner which in turn leads to many areas of change for a child with ASD and their family. Early treatment can impact family functioning, the child’s cognitive and adaptive functioning, social communication and interaction, and ultimately long-term outcome.
Furthermore, ASD is a complex, heterogeneous disorder and the potential benefits of early detection and subsequent treatment are equally complex and heterogeneous. For example, the report does not delineate which autism subgroups would suffice as evidence that early identification and treatment has benefit. Those with low IQ or high IQ; those with known genetic abnormalities or those without genetic abnormalities; those with only verbal deficits; or those with verbal and nonverbal deficits; early onset or later onset autism, or all individuals with autism? ASD biological and phenotypic heterogeneity is considerable22–26. Some individuals possess good verbal skills and may go to college, while others may remain nonverbal their entire lives27. Some have rare genetic mutations with strong biological impact, while others do not28. Some children with ASD may show normal visual attention levels to social images, while other may show highly abnormal attention levels29. It is thus not surprising that in response to treatment, different biological, genetic, and behavioral subgroups show different outcomes. In one recent study only toddlers with low levels of object interest at baseline showed changes in communication ability post treatment30.
The Task Force statement about “children for whom no concerns have been raised by their parents or clinical provider”
The Task Force report, as per its mission, focuses on “children for whom no concerns have been raised by their parents or clinical provider.” Whether intended or not, this phrasing may lead to the impression that universal screening may wrongly identify autism before it exists, begins, or has caused a visible challenge for the child or family. Just because a toddler has not yet been understood by parents and pediatricians to have autism, does not mean the child does not already have autism. In general population based screening research31 as well as research by others that utilize baby sibling cohorts32, ~80–100% of toddlers identified as ASD at very early ages (12–24 months) retain that diagnosis across longitudinal follow-up.
The selective focus on children for whom no concerns have been raised also implies that toddlers identified as having ASD via universal screening are in some way different from toddlers identified via parent and pediatrician concern. Currently, aside from the fact that they are generally younger33, there is no scientific evidence to suggest that ASD toddlers detected via universal screening are clinically different than other ASD toddlers. For example, in a universal screening study7, toddlers identified as ASD in the general population around age 17 months were found to have a mean total score of 16.8 on the Autism Diagnostic Observation Schedule (ADOS), which signifies fairly symptomatic levels of ASD. This high ADOS score is in alignment with scores from other studies wherein toddlers were identified not based on a general population screen, but on parent or physician concern (i.e., “clinic referral sample” see34). Another screening study directly compared toddlers that were referred for evaluation based on detection through universal screening to toddlers referred based on clinician concern. Results indicated equivalent ADOS scores at the time of intake between both groups of toddlers35. Thus, current research illustrates that children identified through universal early screening are not more or less symptomatic than children identified by parent or clinician concern.
It is also important to keep in mind that screening tools were designed to aide non-expert clinicians in quickly determining if there is a developmental concern, and by definition, should identify delays prior to parent or clinician concern. As we mention elsewhere in this article, pediatricians are not consistently able to detect ASD when it is in fact present12, 13. Ignoring this fact creates confusion regarding the purpose and utility of screening tools and undermines the interpretation of research regarding screening tools. The purpose of screening is to increase the efficiency of early identification in clinical practice.
The Task Force research recommendations
Research recommendations focus on determining whether or not the very early identification that is achieved via universal screening is associated with improved health outcomes. Several designs are proposed such as randomized clinical trials (RCTs) comparing outcomes in early screened and identified children to outcomes in wait-listed, less intensely treated, or “alternatively” treated children. Another suggestion includes using quasi experimental designs (e.g., step wedge design) in regions with low screening rates to compare early screening at 12 to 18 months with later screening or case-finding on educational, IQ, and other outcome measures at age 6 years. However, we contend that it would be unethical to randomly withhold early screening (which is accurate, easy, quick, inexpensive and can be done by anyone anywhere) or normal levels and types of treatment from some toddlers in a community while others in that same community get early screening, early identification, early services, and high quality early intervention. Furthermore, the simple and brief research suggestions in the report do not address the multiple complicating services and intervention issues discussed above having to do with heterogeneity, subgroups, different underlying biologies, and different family and environmental factors.
The Task Force missed a key clinical issue
A key concept in this discussion is that while universal screening is an essential step in the health care process for ASD, it merely creates the opportunity for early treatment and services. Screening in and of itself does not determine the quality and benefits of such treatment. Some early screened and identified children get into treatment quickly, while parents of others are reluctant to act; some early screened and identified children opt for high quality, evidence-based ABA treatment, while the parents of others opt for alternative therapies with unknown impact. Many parents, regardless of how their child was first identified, find the services and intervention landscape daunting and often somewhat confusing; parents follow diverse paths. The most important issue, therefore, is not screening, but what happens next. Thus, the Task Force is sending the country in the wrong direction down a path of experiments of screen vs non-screen, or early vs late screened, instead of the right direction which is research identifying clinically useful and practical services and interventions tailored to the diverse and yet specific needs of early identified toddlers based on biological subtypes; behavioral, cognitive, social and language symptom level and abilities; and a child’s family, community, and cultural beliefs. In short, missing in the report and the field at large is clinically translatable research on services and interventions that aim to maximize the outcome benefits of early identification for the heterogeneous ASD population.
Conclusions about the Task Force report
The overall tenor of the report implies that health care policy on early universal screening should be held in question until extensive research resources are expended that compare outcomes of screened and non-screened (or late screened) children. However, the proverbial horse is out of the barn: Early screening is commonplace in the US (approximately 50% of pediatricians use standardized screening tools at well-baby visits to detect ASD36), and is objectively successful3. Early screening is largely accurate, easy, quick, inexpensive and can be done by anyone anywhere; important help and services can begin early for identified toddlers and young children. The Task Force’s non-endorsement of universal early screening could unintentionally have a chilling effect on this major accomplishment. Withholding or delaying universal early screening from toddlers in order to satisfy vague research ideals is considered by many to be untenable37.
An Expanded View of the Benefits of Universal ASD Screening
Results from molecular and postmortem studies provide striking evidence that the biology of autism begins in prenatal and early postnatal life38–42. Numerous genetic43–48, genomic49, and animal model50–53 studies also underscore this early biological onset of autism. After birth, pediatricians and family medicine practitioners are commonly the first professionals to encounter autism as the symptoms slowly reveal themselves with increasing clarity across the next 2 years of life54, 55. Prospective and retrospective studies find that in some cases warning signs are present by or even before the 1st birthday56–59. Universal screening therefore provides the opportunity to detect a disorder that is undeniably present and observable across the first 2 years of life.
In decades past, however, pediatricians and family medicine practitioners did not have access to reliable screening tools for recognizing autism risk in infants and toddlers, leaving many children undetected and untreated often for years. For example, the California Department of Developmental Services released a report noting that children with ASD born in 1987 started receiving treatment services around age 7 years60. Another study published 10 years ago noted that only 8% of pediatricians systematically screened for autism61, citing a lack of access to reliable screening tools as a primary reason61. Parents were often frustrated and confused by the lack of answers and support from professionals, and had few resources to help their child62. Moreover, without systematic early identification, researchers could not easily study early development and devise novel early treatment approaches, dramatically slowing the pace of early treatment research. Indeed, in a review of published early intervention studies across a 9-year period from 1998 to 2006 for children ages 5 years and younger, researchers found only a meager 22 studies, averaging approximately 2.4 studies per year63.
Fortunately this bleak situation changed considerably in the past decade as remarkable progress has been made in early screening and detection of infants and toddlers in the general population3, 7, 9–11, 64. Research has demonstrated that ASD can be detected as young as 12 months7, 65, and routinely by 18–24 months9, 10, 66 using parent report screening tools at well baby check-ups. In fact, one cohort of toddlers with ASD detected using the Communication and Symbolic Scale Developmental Profile Infant/Toddler Checklist (CSBS DP IT Checklist)67, an early developmental screen (i.e., a “broadband screen”) that detects delays in social communication, expressive and receptive language and symbolic functioning, began receiving behavioral treatment by age 17 months7.
Routine Screening for ASD Facilitates Detection of All Delays
Although developed in the spirit of ASD detection, most screening tools are also highly accurate at detecting general developmental delays. For example, when the Modified Checklist of Autism in Toddlers Revised (M-CHAT-R) is used with a follow-up interview further probing parents about responses, the positive predictive value is ~0.45 for ASD specifically, but >0.90 for all developmental delays including ASD9, 10, 68. In other words, a failed score is a valid cause for a concern of developmental delay. Within this context, the risk of false positives is low, resulting in relatively few unnecessary evaluations or undue parent stress, as some type of early intervention service is likely warranted and beneficial. Recent research has also suggested the use of general developmental screening (e.g. PEDS or ASQ) to assess for a range of developmental delays, with the use of an autism specific instrument as a second tier screen for children that fail the initial general development screen may be a useful approach to accurately target autism screening within busy pediatric settings69, 70.
Screening is Rapid and Simple
Most screening tools are easy, relatively quick, and cost-effective to implement in ordinary community settings. For example, both the CSBS DP IT Checklist and the M-CHAT-R can be completed by parents in approximately 5–10 minutes7, 9, 11. Additional time (~10 minutes) could be required to further probe failed positive screens (about 8% of all cases screened), as is currently recommended as best practice with the M-CHAT-R10. These additional interview minutes are an important step in the M-CHAT process in particular because such follow-up conversations considerably reduce false positives and open the opportunity for parent-clinician dialogue. Most parent report screening tools are available in multiple languages and easily implementable in underserved communities71. Their clinical value has been demonstrated and is currently billable. Thus, a child with autism can be screen-detected even by those who are not experts in autism and who might otherwise not recognize the presence of symptoms for months or years. This is extremely important as the prevalence of autism is now reported to be 1 in 6814 and screening is the easiest, quickest and most cost-effective means of identifying such large numbers by non-autism experts in pediatric and community health settings throughout the US.
Screening Improves Clinician Awareness and Knowledge
Following induction into a screening program, 87% of pediatricians reported that using a screening tool ultimately heighted their awareness of autism and developmental delays7. In that same study 96% of pediatricians reported that using a screening tool overall enhanced their clinical practice7. It is thus not surprising that standardized screening has been shown to improve detection relative to clinical judgment alone. One study showed that when pediatricians rely on clinical judgment rather than formal screening to identify children at risk for ASD, as many as 50% of children with ASD who could have been identified on a positive screen at 18 or 24 months were missed. Thus, when only clinical judgment is used, most true toddlers with ASD will experience a potential delay in access to treatment13. In an experimental study in the Netherlands an early detection screening program was implemented and compared to a control site that relied on general surveillance and referral methods and found that the early screening program led to toddlers with ASD being identified 21 months earlier than cases from the control sites33. In another study utilizing focus groups to understand what physicians need to do to improve well child care, physicians stated that formal screening with standardized questionnaires aids them in knowing when to prioritize developmental concerns72.
Screening Reduces Disparities in Access to Services
Unfortunately, in the absence of systematic screening, families from underserved populations are more likely to be evaluated and identified with autism at later ages than other children14, 15 resulting in a disparity in access to services. A survey of pediatricians in California found that most pediatricians felt Latino and African American families were less informed about ASDs than White families73. Many pediatricians also admitted having more difficultly assessing children from primarily Spanish speaking families due to language and cultural differences73. These findings are consistent with a recent study that conducted ASD screening in state licensed daycares serving low-income populations that led to the identification of ASD in 3% of the children (mean age of 50 months)74. Importantly, none of these children had been previously identified. Fortunately, such disparities can be reduced by universal screening. For example, a recent study found that using one of several versions of the M-CHAT reduced the usual disparities observed in the age of first evaluations for underrepresented populations71. These data reinforce the idea that relying on parents to raise concerns or simple surveillance by physicians is not an effective method of identification and may have the most adverse consequences for the most vulnerable populations.
Screening May Strengthen Parent-Pediatrician Relationships
Using a standardized screening tool gives physicians a starting point for difficult conversations about delays in development and for responding to parent concerns. Proactive response to parent concerns by physicians can result in a 1 year reduction in age of treatment initiation according to a study of parental concern for autism75. Often parents with concerns about their child’s development experience stress when uncertainty and questions remain unaddressed by professionals76. Most parents are greatly relieved to have an explanation for their child’s behavior and it is not surprising that more rapid diagnoses are associated with higher levels of satisfaction in parents77. In short, early screening improves standard of care for parents as well as children.
Screening Enables Interventions To Begin Far Sooner
The primary purpose of early screening is autism risk detection and referral for follow-up evaluation and rapid treatment. Screening and detection are independent of what happens next for the child and family. Thus, screening enables further steps to be taken but does not determine what they will be. It is essential to separate screening success and treatment success. It is misleading for the Task Force to entangle screening and screening-related policy with the separate question of what happens next to the child with ASD and how successful different services and treatments may be.
Early screening does enable help, services and interventions to begin far sooner than was the case a decade ago. In fact, early screening studies in combination with increased autism awareness have ushered in a new era for services research aimed at improving a wide range of services and treatment approaches for ASD toddlers and their families that did not exist a decade or two ago78, 79. Early identification of ASD has opened doors to resources and research aimed at developing novel early social and communication treatments80–82, developing age-appropriate curriculum and assessments83, 84, as well as developing early preschool peer inclusion programs85. Such treatments have been demonstrated to improve cognitive and behavioral capacities in many individuals when started at early ages78, 79. Early detection also has the capacity to impact family functioning by providing information that equips families to seek early support and parent training, raise community awareness, and ultimately increase understanding of how best to help toddlers and young children with autism and their families to improve long-term outcomes.
Thus, the rapid progress in research detecting and diagnosing ASD at earlier and earlier ages has opened up a wide range of important areas for progress and improvement in “what happens next,” ranging from improvements in the daily lives and experiences of affected toddlers and services for the child and parents to improvements in multiple ability areas and reductions in negative experiences and behaviors (see Figure 3).
Early Screening Improves our Ability to Understand Early Development in ASD
Evidence of ASD as a disorder with very early, likely prenatal (biological) onset, highlights the first few years of life as critical periods of development. Despite the crucial importance of this period in ASD, historically, scientists knew very little about the behavioral and biological development during the first years of life due to the absence of early screening and detection. In the past decade, however, early screening and detection approaches, such as the 1-Year Well-Baby Check-Up Approach7, have transformed early developmental research opportunities and provided a rich array of scientific and clinical information about early development in ASD86–89. Many studies of very young toddlers with ASD have found unusual trajectories of brain growth87, 90–100 and axonal 101–106 and functional connectivity107, 108 in multiple regions including the cerebral cortex, amygdala, cerebellum and striatum. Greater overgrowth of the amygdala in particular is related to worse social symptoms95, 109 and associated with circulating immune markers in toddlers with ASD110. Diffusion tensor imaging (DTI) studies106 find evidence of excess axon connectivity and electroencephalogram (EEG) studies108 excess functional over-connectivity very early, by the first year and second years of life; after that early age, there appears to be an abnormally slow rate of further axon development and greater connectivity abnormality tends to be associated with worse later ASD social symptom outcome. One new functional magnetic resonance imaging (fMRI) study of early language in ASD found strikingly reduced brain activation to speech in 1 and 2 year olds with ASD who later had a poor language outcome by 3–4 years, but very strong brain activation to language at 1–2 years in infants with ASD with a later good language clinical outcome22. Such studies, and our now deepened understanding of the neural heterogeneity of ASD, its relation to clinical phenotype, and the promise of prognostic biomarkers, would not have been possible without the early screening program employed7.
In sum, the success of systematic early screening for ASD is clear, and the numerous benefits of early screening tools far outweigh the minimal harms (see Figure 2). This sound foundation of research and widespread professional and public acknowledgement of the importance and success of early screening has established early screening for autism as a public health priority and a standard of care for pediatricians.
Very Early Treatment Has The Greatest Potential To Alter Brain Function And Improve Outcome
One of the fundamental motivations for the early screening and treatment of autism is the knowledge that the human brain undergoes a profound period of establishing and refining neural connections during prenatal life and the first three postnatal years111. These first three years, when autism can be detected and treatment started, are the single most transformative period in all of human postnatal brain development. Neural circuits grow from simple ones supporting only the most basic functions to astonishingly complex ones that rapidly enable higher-order cognitive, social, behavior, learning, and language abilities (see Figure 4). Ideally, detection and treatment should begin by or before this time. For example, synaptic density in the human prefrontal cortex, the brain region centrally involved in higher-order social behavior, doubles between birth and 1–2 years in age112. Synaptic density in language areas, such as Wernicke’s and Broca’s areas, also doubles and peaks shortly thereafter by age 3 years113. The time period following a peak in synapse number is characterized by a phase of refinement where effective connections are strengthened, and weak ones die away. This important developmental step, namely the construction of specific adaptive neural circuits and the pruning of excess (unused or maladaptive) synapses, is thought to be largely dependent on input from the environment114, 115. If an at-risk toddler is identified and behavioral treatment begins either before or while brain connections are being established, it is likely that brain function for that toddler stands the best chance of being optimized, rather than if treatment begins after mature but faulty circuitry is already established. It is for this very reason that the early identification and treatment of autism is essential.
Figure 4.
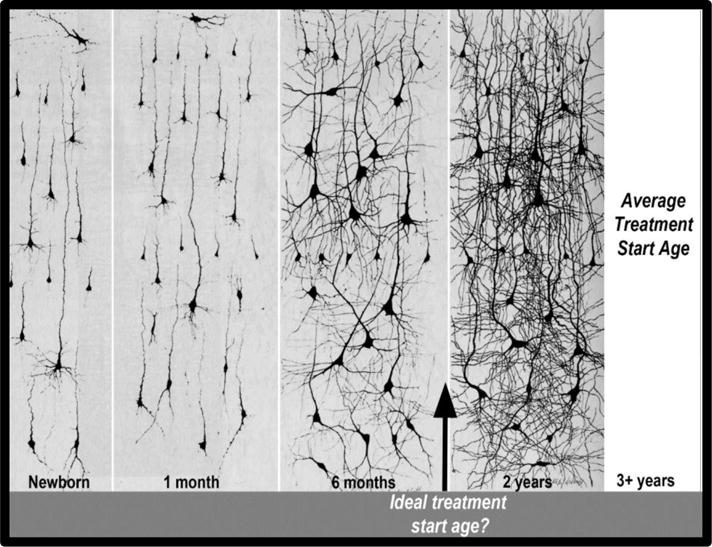
Golgi-stained sections highlighting the size and dendtric arborization of brain cells in the anterior portion of the middle frontal gyrus at different ages. As illustrated in the figure, the normal newborn has sparse neural circuitry, and then, with increasing age, there is a tremendous increase in the complexity of neural circuitry that is illustrated by the great increase of dendritic arbors from birth to 2 years. The figure highlights the ideal age for intervention might be prior to 2 years. The figure also highlights that most ASD children start treatment well after much circuitry is already formed. Adapted from Courchesne and Pierce (2005a) which was previously adapted from Nolte (1993), whose figure combined panels from Conel, 1939; 1941, 1951 and 1959.
Additional evidence highlights the importance of the first years of life as an optimal time window for early brain plasticity in human infants and toddlers. In a rare example, Nelson and colleagues (2007)116 studied the outcomes of infants reared in an impoverished institution in Bucharest Romania, some of whom were placed in foster care after various periods of time. Results indicated that institutionalized infants placed into foster care at an early age (by 18 months) had a mean developmental quotient that was 14 points higher than infants placed in foster care at later ages (after 30 months). The infants who were selected for either institutionalization or foster care placement were randomly selected and the main difference between the infant groups was the depth and complexity of environmental stimulation (institution, low stimulation vs foster care, higher stimulation). Although ethical considerations prevent parallel studies in the field of autism, it is at least theoretically clear that treatment could have a massive, perhaps even a preventative impact if started at or prior to 18 months. However, treatment started at this early age can only be achieved if clear and standardized methods of early identification are systematically followed across the nation. Fortunately, as the Task Force statement acknowledges, there are scores of studies documenting that ASD can be detected reliably between 18–30 months in the general population9, 10, 117, and new studies show it is even possible at 12 months7, 8, 11, 65, 118.
Finally, the animal model literature clearly demonstrates that the mammalian brain undergoes enormous changes in response to environmental enrichment during early development119. New neurons can appear in the dentate gyrus in the hippocampus, a brain region critical for learning and memory120–122. Synapse123, 124 and dendrite numbers can be increased125, as can myelin density126 and capillary volume124, 127. In addition to significant changes in brain structure and morphology, social environmental enrichment has been shown to change social communication behaviors in animals as well128.
Why is treatment started around the 1st to 2nd birthday ideal?
Starting treatment for ASD between 1–2 years is ideal for several reasons. First, as articulated above, new circuits, particularly in frontal cortex, are being formed rapidly during that time, generating the opportunity to shape effective connections while they are actually forming, rather than after many circuits are already formed such as at three years or later (see right side of Figure 4). Second, interventions for ASD prior to 1 year in age have not been empirically tested in large, randomly assigned samples. Thus 1 year is the youngest possible age that validated interventions could theoretically be implemented. Third, around the 1st birthday, an explosion of new skills emerge in toddlers: walking129, talking130, bi-manual manipulation of objects131, and engaging in shared attention with others132. In short, children become highly interactive with their environment and the people in it at this age, making them ready to engage in treatment, even those toddlers that have not mastered the aforementioned skills. Fourth, naturalistic, developmental behavioral interventions (NDBIs) such as Pivotal Response Training133, 134, ESDM83 and JASPER135 are available for use, and ideally suited for the early treatment of toddlers with ASD. Such treatment approaches have demonstrated efficacy in young populations79, are highly interactive, easily implemented in naturalistic settings such as the home or playground, utilize highly natural contingencies (e.g., offering access to a doll if a child says the word “doll”), and utilize shared control between treatment provider and child which enhances a toddler’s interest in engaging in activities. Such play-based naturalistic treatments are enjoyable for both parents and professionals alike - for more information about NDBIs, see136.
Ongoing Treatment Studies Utilizing Toddlers with ASD Detected by General Population Screening
Randomized control trials provide evidence of improved outcomes of toddlers detected from the general population through autism screening137, 138. For example, in a unique RCT pilot study, Baranek and colleagues followed sixteen families with a toddler at-risk for ASD detected around the 1st birthday using a general population screening tool named the 1st Year Inventory137. Toddlers were randomly assigned to a parent intervention named Adapted Responsive Teaching (ART), or referred to general community early intervention services. Level of community service uptake, changes in language and parent interaction styles were tracked. Results indicated greater utilization of community services in the control group, but greater change in child language and parent interaction style and improved social abilities in the ART treatment group137.
In another RCT study, Wetherby and colleagues (2014) tracked treatment outcomes in a combined cohort: approximately half of the toddlers were those who failed a screening tool at their pediatrician’s office, the CSBS IT Checklist, while the other half were referred due to parental or physician concern. The combined cohort received a diagnosis of ASD at a mean age of 19 months and subsequently randomly assigned to either an individualized or group based parent administered treatment138. Results indicated significant positive change in social communication, receptive language, and other skills in toddlers who received the individualized treatment. Other RCT studies that contained toddlers under age 2 years, although not detected via screening programs per se, report encouraging positive changes following early identification treatment tailored to meet the learning styles and needs of the ASD population. One such study utilized the Early Denver Start Model (ESDM) that focuses on enhancing social interaction and language, among other skills, noted that not only were significant gains in developmental quotient and language skills found following treatment83, but gains were also associated with normalized patterns of brain activity16. Specifically, toddlers with ASD that received ESDM treatment showed increased cortical activation when viewing faces relative to the toddlers with ASD that did not16. For a review of recent treatment studies see78, 79.
Overall, current research continues to bolster the fact that standardized screening tools can detect ASD at very young ages, and that toddlers make substantial gains in early intervention. Again, it is important to stress that future research may be misguided in continuing to evaluate if early screening is beneficial relative to surveillance alone as recommended by the Task Force. Rather, the field is already in a prime position to discover ways to refine and individualize early intervention and further improve outcomes for early-detected toddlers with ASD.
Summary and Conclusions
The Task Force report concludes by “advocating for more research”, which is laudable. It also agrees with the field at large that early screening tests can detect ASD among children ages 18 to 30 months for whom there is no prior concern. The report also urges randomized clinical trials in order to more rigorously establish treatment effects, noting there are many types and modalities of treatment. Public opinion was acknowledged, although its impact on the report appears modest. It also acknowledged that recommendations of other organizations were largely in support of early screening, but noted that its United Kingdom counterpart, the UK National Screening Committee, explicitly does not recommend universal screening for ASD in children under age 5 years139. The direct impact of such a myopic recommendation is unclear, but it is worth noting that the median age of ASD diagnosis in the UK is 55 months, with only ~10% of children being identified prior to age 3 years - a statistic that largely hasn’t changed across the past decade140.
By contrast, the widespread concern in the U.S. for the health and well being of children with ASD and the recognition that it begins in the first years of life has led to a concerted effort to identify and help children with ASD and their families at early ages. There has been a sea of change: The past lack of awareness for and benign neglect of children with ASD and their families is gone and replaced by early screening, identification, help and treatment. Because of early detection and treatment a significant proportion of children with autism are now able to integrate within normal settings of school, grocery stores, and playgrounds.
The lack of Task Force support for this nearly national consensus effort should not diminish the effort or progress towards early detection via screening or other methods in general pediatric populations. Reducing the consistency of universal early screening will do far more harm than good for children with autism, parents and families, teachers, and pediatricians and other developmental health care providers. The Task Force report incorrectly implies that where there is no concern, there is no autism, and disregards the idea that screening tools are used to establish whether or not there may be developmental concerns. Such tools have been embraced by the medical community as evidenced by endorsement from the AAP1. Simply relying on parent or pediatrician concern, places an alarming amount of responsibility on individuals that are not always experts in the area of autism. Adopting the use of a standardized screening tool allows for a routine procedure to assess for delays and start a discussion between the parent and provider about potential delays. The benefits and utility of standardized screening tools clearly outweigh the potential harms of waiting to identify ASD through non-standardized methods of surveillance.
If we apply the same yardstick of examining the ratio of benefits/harms to the Task Force that they have applied to universal autism screening, then it is incumbent on them to demonstrate that, on balance, waiting to identify ASD when it eventually comes to the attention of parents and practicioners is more beneficial than harmful as a standard of care. We assert that it is not. In fact, it could unintentionally encourage a lack of monitoring in the clinic that may ultimately do harm and offers little to no benefits.
We do agree, however, with the Task Force that RCTs have a valuable place in science and can be leveraged to understand the factors that lead to the best outcome in ASD. However, the right direction for ASD research is to conduct RCTs to compare different approaches for providing help, services, and treatments for autism at all early ages and regardless of how the child is identified –whether via an early screen, public awareness or a concerned pediatrician, parent or teacher. There is a critical need to enhance interventions (help, services, treatments) so they are tailored to varying age of initial clinical detection and autism subtype.
In sum, early screening is an effective clinical tool for early detection of ASD or risk for ASD. The benefits of early detection —whether via screening or other paths such as parental or professional concern— are many (Figures 2 and 3) and, contrary to the Task Force opinion, far outweigh harms. Early detection, regardless of method: (a) leads to early ASD identification, treatment, and services, (b) increases physicians’ awareness and knowledge of delays, (c) reduces disparities in access to services between low and high SES families, (d) facilitates treatment during a crucial and singular time of life when intervention could have the greatest impact on brain development, (e) can lead to important non-treatment ancillary services for parents and toddlers with ASD following early identification that they would not occur if early screening were to once again disappear, (f) is ethically required since the disorder is already in progress, can be detected, and effective treatments are available, (g) makes possible the RCT research we recommend on early interventions following early detection, (h) makes possible studies that elucidate the heterogeneous biological and behavioral development of ASD during arguably the single most important period in early postnatal human life, (i) makes possible discovery of early biomarkers of the disorder, prognosis, and treatment responsiveness22, 25, 29, 141, and (j) through such biology and biomarker studies, may one day lead to identification of powerful biotherapeutics that target specific neural subtypes and biological mechanisms and/or identification of etiological factors and means of prevention.
Given the wealth of benefits of early detection, and as a result, access to early intervention, the lack of support for early ASD universal screening by the Task Force is incomprehensible. There is an obvious disconnect between the current research and the Task Force conclusion despite their acknowledgement that early screening can detect cases of ASD and that early intervention does lead to clinical improvement. The Task Force’s narrow interpretation of the available literature because toddlers that participated in treatment studies were not necessarily detected as the result of screening, should be interpreted as just that: namely, narrow. Instead, it is imperative to continue and expand early screening in all communities, especially in those that currently have too few early services for toddlers with ASD, and provide all families with access to the best possible care.
Acknowledgments
Thank you to Cynthia Carter Barnes1,2, Adrienne Moore1,2, Kathleen Campbell3, Debra Cha1,2 and Lindsley Pence1,2 for helpful comments on final drafts of this manuscript.
1. Department of Neurosciences, School of Medicine, University of California San Diego, La Jolla, CA
2. Autism Center of Excellence, School of Medicine, University of California San Diego, La Jolla, CA
3. Duke Center for Autism and Brain Development, Duke University, Durham, NC
Financial Support: All phases of writing this paper were supported by NIH grants R01-MH104446 and R01-MH080134 (awarded to Karen Pierce).
Sources of Financial Assistance: NIMH
Footnotes
Conflicts of Interest: There are no conflicts of interest, real or perceived, for any of the authors. The funding agency had no role in the conception or writing of this manuscript.
Author Draft Statement: Karen Pierce, along with Eric Courchesne, wrote the first draft of this manuscript. Elizabeth Bacon assisted in subsequent manuscript drafts. No payment was given to anyone to write this manuscript.
References
- 1.Johnson CP, Myers SM. Disabilities Council on Children with. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 2.Myers SM, Johnson CP. Management of children with autism spectrum disorders. Pediatrics. 2007;120:1162–1182. doi: 10.1542/peds.2007-2362. [DOI] [PubMed] [Google Scholar]
- 3.Zwaigenbaum L, Bauman ML, Fein D, Pierce K, Buie T, Davis PA, et al. Early Screening of Autism Spectrum Disorder: Recommendations for Practice and Research. Pediatrics. 2015;136(Suppl 1):S41–59. doi: 10.1542/peds.2014-3667D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Jr, Dawson G, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 5.Group Cure Autism Now Foundation (CAN) Consensus. Autism Screening and Diagnostic Evaluation: CAN Consensus Statement CNS Spectrums Journal. 1998:3. [Google Scholar]
- 6.(IACC) Interagency Autism Coordinating Committee. IACC Strategic Plan for Autism Spectrum Disorder (ASD) Research —2013 Update. 2014 Retrieved from the US Department of Health and Human Services Interagency Autism Coordinating Committee website: http://iacchhsgov/strategic-plan/2013/indexshtml.
- 7.Pierce K, Carter C, Weinfeld M, Desmond J, Hazin R, Bjork R, et al. Detecting, Studying, and Treating Autism Early: The One-Year Well-Baby Check-Up Approach. Journal of Pediatrics. 2011;159:458–465. doi: 10.1016/j.jpeds.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson LR, Baranek GT, Crais ER, Steven Reznick J, Dykstra J, Perryman T. The first year inventory: retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. J Autism Dev Disord. 2007;37:49–61. doi: 10.1007/s10803-006-0334-4. [DOI] [PubMed] [Google Scholar]
- 9.Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics. 2013;131:e1121–1127. doi: 10.1542/peds.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robins DL, Casagrande K, Barton M, Chen CM, Dumont-Mathieu T, Fein D. Validation of the modified checklist for Autism in toddlers, revised with follow-up (M-CHAT-R/F) Pediatrics. 2014;133:37–45. doi: 10.1542/peds.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetherby AM, Brosnan-Maddox S, Peace V, Newton L. Validation of the Infant-Toddler Checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism. 2008;12:487–511. doi: 10.1177/1362361308094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrielsen TP, Farley M, Speer L, Villalobos M, Baker CN, Miller J. Identifying autism in a brief observation. Pediatrics. 2015;135:e330–338. doi: 10.1542/peds.2014-1428. [DOI] [PubMed] [Google Scholar]
- 13.Miller JS, Gabrielsen T, Villalobos M, Alleman R, Wahmhoff N, Carbone PS, et al. The each child study: systematic screening for autism spectrum disorders in a pediatric setting. Pediatrics. 2011;127:866–871. doi: 10.1542/peds.2010-0136. [DOI] [PubMed] [Google Scholar]
- 14.Network Autism and Developmental Disabilities Monitoring. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 15.Fountain C, King MD, Bearman PS. Age of diagnosis for autism: individual and community factors across 10 birth cohorts. J Epidemiol Community Health. 2011;65:503–510. doi: 10.1136/jech.2009.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan K, Stone WL, Dawson G. Potential neural mechanisms underlying the effectiveness of early intervention for children with autism spectrum disorder. Res Dev Disabil. 2014;35:2921–2932. doi: 10.1016/j.ridd.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55:485–494. doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, et al. Screening for Autism Spectrum Disorder in Young Children: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:691–696. doi: 10.1001/jama.2016.0018. [DOI] [PubMed] [Google Scholar]
- 21.Geschwind DH, State MW. Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 2015;14:1109–1120. doi: 10.1016/S1474-4422(15)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C, Solso S, et al. Different functional neural substrates for good and poor language outcome in autism. Neuron. 2015;86:567–577. doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventola P, Yang DY, Friedman HE, Oosting D, Wolf J, Sukhodolsky DG, et al. Heterogeneity of neural mechanisms of response to pivotal response treatment. Brain Imaging Behav. 2015;9:74–88. doi: 10.1007/s11682-014-9331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early Autism Spectrum Disorder: subtypes and short-term outcomes. J Child Psychol Psychiatry. 2015 doi: 10.1111/jcpp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pramparo T, Pierce K, Lombardo MV, Carter Barnes C, Marinero S, Ahrens-Barbeau C, et al. Prediction of autism by translation and immune/inflammation coexpressed genes in toddlers from pediatric community practices. JAMA Psychiatry. 2015;72:386–394. doi: 10.1001/jamapsychiatry.2014.3008. [DOI] [PubMed] [Google Scholar]
- 26.Kuhl PK, Coffey-Corina S, Padden D, Munson J, Estes A, Dawson G. Brain responses to words in 2-year-olds with autism predict developmental outcomes at age 6. PLoS One. 2013;8:e64967. doi: 10.1371/journal.pone.0064967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickles A, Anderson DK, Lord C. Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J Child Psychol Psychiatry. 2014 doi: 10.1111/jcpp.12269. [DOI] [PubMed] [Google Scholar]
- 28.de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22:345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, Malige A. Eye Tracking Reveals Abnormal Visual Preference for Geometric Images as an Early Biomarker of an Autism Spectrum Disorder Subtype Associated with Increased Symptom Severity. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter AS, Messinger DS, Stone WL, Celimli S, Nahmias AS, Yoder P. A randomized controlled trial of Hanen’s ‘More Than Words’ in toddlers with early autism symptoms. J Child Psychol Psychiatry. 2011;52:741–752. doi: 10.1111/j.1469-7610.2011.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. J Child Psychol Psychiatry. 2013;54:582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, et al. Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. J Child Psychol Psychiatry. 2015;56:988–998. doi: 10.1111/jcpp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oosterling IJ, Wensing M, Swinkels SH, van der Gaag RJ, Visser JC, Woudenberg T, et al. Advancing early detection of autism spectrum disorder by applying an integrated two-stage screening approach. J Child Psychol Psychiatry. 2010;51:250–258. doi: 10.1111/j.1469-7610.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 34.Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. J Child Psychol Psychiatry. 2009;50:1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleinman JM, Robins DL, Ventola PE, Pandey J, Boorstein HC, Esser EL, et al. The modified checklist for autism in toddlers: a follow-up study investigating the early detection of autism spectrum disorders. J Autism Dev Disord. 2008;38:827–839. doi: 10.1007/s10803-007-0450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arunyanart W, Fenick A, Ukritchon S, Imjaijtt W, Northrup V, Weitzman C. Developmental and Autism Screening: A Survey Across Six States. Infants and Young Children. 2012;25:175–187. [Google Scholar]
- 37.Fernell E, Wilson P, Hadjikhani N, Bourgeron T, Neville B, Taylor D, et al. Screening, intervention and outcome in autism and other developmental disorders: the role of randomized controlled trials. J Autism Dev Disord. 2014;44:2074–2076. doi: 10.1007/s10803-014-2070-5. [DOI] [PubMed] [Google Scholar]
- 38.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 39.Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Ercument Cicek A, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158:263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prontera P, Ottaviani V, Toccaceli D, Rogaia D, Ardisia C, Romani R, et al. Recurrent approximately 100 Kb microdeletion in the chromosomal region 14q11.2, involving CHD8 gene, is associated with autism and macrocephaly. American journal of medical genetics Part A. 2014;164A:3137–3141. doi: 10.1002/ajmg.a.36741. [DOI] [PubMed] [Google Scholar]
- 46.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugathan A, Biagioli M, Golzio C, Erdin S, Blumenthal I, Manavalan P, et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4468–4477. doi: 10.1073/pnas.1405266111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun. 2015;6:6404. doi: 10.1038/ncomms7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pramparo T, Lombardo MV, Campbell K, Carter Barnes C, Marinero S, Solso S, et al. Cell cycle networks link gene expression dysregulation, mutation and brain maldevelopment in autistic toddlers. Molecular Systems Biology. 2015 doi: 10.15252/msb.20156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orosco LA, Ross AP, Cates SL, Scott SE, Wu D, Sohn J, et al. Loss of Wdfy3 in mice alters cerebral cortical neurogenesis reflecting aspects of the autism pathology. Nature communications. 2014;5:4692. doi: 10.1038/ncomms5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang WQ, Chen WW, Jiang L, Liu K, Yung WH, Fu AK, et al. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. Cell reports. 2014;9:1635–1643. doi: 10.1016/j.celrep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Le Belle JE, Sperry J, Ngo A, Ghochani Y, Laks DR, Lopez-Aranda M, et al. Maternal inflammation contributes to brain overgrowth and autism-associated behaviors through altered redox signaling in stem and progenitor cells. Stem cell reports. 2014;3:725–734. doi: 10.1016/j.stemcr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- 55.Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. J Autism Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- 56.Maestro S, Muratori F, Cesari A, Cavallaro MC, Paziente A, Pecini C, et al. Course of autism signs in the first year of life. Psychopathology. 2005;38:26–31. doi: 10.1159/000083967. [DOI] [PubMed] [Google Scholar]
- 57.Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord. 2014;44:2981–2995. doi: 10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sacrey LA, Zwaigenbaum L, Bryson S, Brian J, Smith IM, Roberts W, et al. Can parents' concerns predict autism spectrum disorder? A prospective study of high-risk siblings from 6 to 36 months of age. J Am Acad Child Adolesc Psychiatry. 2015;54:470–478. doi: 10.1016/j.jaac.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Services California Department of Developmental. California Health and Human Services Agency, Department of Developmental Services. Sacramento, California: 1999. Changes in the population of persons with autism and pervasive developmental disorders in California's developmental services system: 1987 through 1988: a report to the legislature. [Google Scholar]
- 61.Dosreis S, Weiner CL, Johnson L, Newschaffer CJ. Autism spectrum disorder screening and management practices among general pediatric providers. J Dev Behav Pediatr. 2006;27:S88–94. doi: 10.1097/00004703-200604002-00006. [DOI] [PubMed] [Google Scholar]
- 62.Smith B, Chung MC, Vostanis P. The path to care in autism: is it better now? J Autism Dev Disord. 1994;24:551–563. doi: 10.1007/BF02172137. [DOI] [PubMed] [Google Scholar]
- 63.Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dietz C, Swinkels S, van Daalen E, van Engeland H, Buitelaar JK. Screening for Autistic Spectrum Disorder in Children Aged 14–15 Months. II: Population Screening with the Early Screening of Autistic Traits Questionnaire (ESAT). Design and General Findings. J Autism Dev Disord. 2006 doi: 10.1007/s10803-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 65.Turner-Brown LM, Baranek GT, Reznick JS, Watson LR, Crais ER. The First Year Inventory: a longitudinal follow-up of 12-month-old to 3-year-old children. Autism. 2012 doi: 10.1177/1362361312439633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone WL, Coonrod EE, Turner LM, Pozdol SL. Psychometric properties of the STAT for early autism screening. J Autism Dev Disord. 2004;34:691–701. doi: 10.1007/s10803-004-5289-8. [DOI] [PubMed] [Google Scholar]
- 67.Wetherby A, Prizant B. Communication and symbolic behavior scales developmental profile - first normed edition. Baltimore, MD: Paul H. Brookes; 2002. [Google Scholar]
- 68.Robins DL. Screening for autism spectrum disorders in primary care settings. Autism. 2008;12:537–556. doi: 10.1177/1362361308094502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glascoe FP, Macias MM, Wegner LM, Robertshaw NS. Can a broadband developmental-behavioral screening test identify children likely to have autism spectrum disorder? Clin Pediatr (Phila) 2007;46:801–805. doi: 10.1177/0009922807303928. [DOI] [PubMed] [Google Scholar]
- 70.Hardy S, Haisley L, Manning C, Fein D. Can Screening with the Ages and Stages Questionnaire Detect Autism? J Dev Behav Pediatr. 2015;36:536–543. doi: 10.1097/DBP.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herlihy LE, Brooks B, Dumont-Mathieu T, Barton ML, Fein D, Chen CM, et al. Standardized screening facilitates timely diagnosis of autism spectrum disorders in a diverse sample of low-risk toddlers. J Dev Behav Pediatr. 2014;35:85–92. doi: 10.1097/DBP.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanner JL, Stein MT, Olson LM, Frintner MP, Radecki L. Reflections on well-child care practice: a national study of pediatric clinicians. Pediatrics. 2009;124:849–857. doi: 10.1542/peds.2008-2351. [DOI] [PubMed] [Google Scholar]
- 73.Zuckerman KE, Mattox K, Donelan K, Batbayar O, Baghaee A, Bethell C. Pediatrician identification of Latino children at risk for autism spectrum disorder. Pediatrics. 2013;132:445–453. doi: 10.1542/peds.2013-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janvier YM, Harris JF, Coffield CN, Louis B, Xie M, Cidav Z, et al. Screening for autism spectrum disorder in underserved communities: Early childcare providers as reporters. Autism. 2015 doi: 10.1177/1362361315585055. [DOI] [PubMed] [Google Scholar]
- 75.Zuckerman KE, Lindly OJ, Sinche BK. Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. J Pediatr. 2015;166:1431–1439 e1431. doi: 10.1016/j.jpeds.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carbone PS, Behl DD, Azor V, Murphy NA. The medical home for children with autism spectrum disorders: parent and pediatrician perspectives. J Autism Dev Disord. 2010;40:317–324. doi: 10.1007/s10803-009-0874-5. [DOI] [PubMed] [Google Scholar]
- 77.Howlin P, Moore A. Diagnosis in autism: A survey of over 1200 patients in the UK. Autism. 1997;1:135–162. [Google Scholar]
- 78.Bradshaw J, Steiner AM, Gengoux G, Koegel LK. Feasibility and effectiveness of very early intervention for infants at-risk for autism spectrum disorder: a systematic review. J Autism Dev Disord. 2015;45:778–794. doi: 10.1007/s10803-014-2235-2. [DOI] [PubMed] [Google Scholar]
- 79.Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, et al. Early Intervention for Children With Autism Spectrum Disorder Under 3 Years of Age: Recommendations for Practice and Research. Pediatrics. 2015;136(Suppl 1):S60–81. doi: 10.1542/peds.2014-3667E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ingersoll B, Wainer A. Initial efficacy of project ImPACT: a parent-mediated social communication intervention for young children with ASD. J Autism Dev Disord. 2013;43:2943–2952. doi: 10.1007/s10803-013-1840-9. [DOI] [PubMed] [Google Scholar]
- 81.Kasari C, Gulsrud AC, Wong C, Kwon S, Locke J. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J Autism Dev Disord. 2010;40:1045–1056. doi: 10.1007/s10803-010-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landa RJ, Holman KC, O’Neill AH, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. J Child Psychol Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bacon EC, Dufek S, Schreibman L, Stahmer AC, Pierce K, Courchesne E. Measuring outcome in an early intervention program for toddlers with autism spectrum disorder: use of a curriculum-based assessment. Autism Res Treat. 2014;2014:964704. doi: 10.1155/2014/964704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahmer AC, Akshoomoff N, Cunningham AB. Inclusion for toddlers with autism spectrum disorders: the first ten years of a community program. Autism. 2011;15:625–641. doi: 10.1177/1362361310392253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Courchesne E, Webb SJ, Schumann CM. From toddlers to adults: The changing landscape of the brain in autism. USA: Oxford University Press; 2011. [Google Scholar]
- 88.Lainhart JE. Brain imaging research in autism spectrum disorders: in search of neuropathology and health across the lifespan. Curr Opin Psychiatry. 2015;28:76–82. doi: 10.1097/YCO.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pierce K. Early functional brain development in autism and the promise of sleep fMRI. Brain Res. 2011;1380:162–174. doi: 10.1016/j.brainres.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Courchesne E, Karns C, Davis HR, Ziccardi R, Carper R, Tigue Z, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 91.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 92.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 93.Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, Toth K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Webb SJ, Nalty T, Munson J, Brock C, Abbott R, Dawson G. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. J Child Neurol. 2007;22:1182–1190. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Archives of general psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chawarska K, Campbell D, Chen L, Shic F, Klin A, Chang J. Early generalized overgrowth in boys with autism. Arch Gen Psychiatry. 2011;68:1021–1031. doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain : a journal of neurology. 2013;136:2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 102.Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, et al. Abnormal white matter integrity in young children with autism. Human brain mapping. 2011;32:534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism research : official journal of the International Society for Autism Research. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Travers BG, Tromp do PM, Adluru N, Lange N, Destiche D, Ennis C, et al. Atypical development of white matter microstructure of the corpus callosum in males with autism: a longitudinal investigation. Mol Autism. 2015;6:15. doi: 10.1186/s13229-015-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Solso S, Xu R, Proudfoot J, Hagler DJ, Campbell K, Venkatraman V, Carter Barnes C, Ahrens-Barbeau C, Pierce K, Dale A, Eyler L, Courchesne E. DTI Provides Evidence Of Possible Axonal Over-Connectivity In Frontal Lobes In ASD Toddlers. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orekhova EV, Elsabbagh M, Jones EJ, Dawson G, Charman T, Johnson MH, et al. EEG hyper-connectivity in high-risk infants is associated with later autism. J Neurodev Disord. 2014;6:40. doi: 10.1186/1866-1955-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Breece E, Paciotti B, Nordahl CW, Ozonoff S, Van de Water JA, Rogers SJ, et al. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain Behav Immun. 2013;31:69–75. doi: 10.1016/j.bbi.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huttenlocher P. Neural plasticity: The effects of environment on the development of cerebral cortex Cambridge. Massachusetts: Harvard University Press; 2002. [Google Scholar]
- 112.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 113.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 114.Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. Behav Brain Sci. 1997;20:537–556. doi: 10.1017/s0140525x97001581. discussion 556–596. [DOI] [PubMed] [Google Scholar]
- 115.Katz IC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 116.Nelson CA, 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 117.Kruizinga I, Visser JC, van Batenburg-Eddes T, Carter AS, Jansen W, Raat H. Screening for autism spectrum disorders with the brief infant-toddler social and emotional assessment. PLoS One. 2014;9:e97630. doi: 10.1371/journal.pone.0097630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben-Sasson A, Carter AS. The application of the first year inventory for ASD screening in Israel. J Autism Dev Disord. 2012;42:1906–1916. doi: 10.1007/s10803-011-1436-1. [DOI] [PubMed] [Google Scholar]
- 119.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 120.Garthe A, Roeder I, Kempermann G. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus. 2015 doi: 10.1002/hipo.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 123.Kondo M, Takei Y, Hirokawa N. Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron. 2012;73:743–757. doi: 10.1016/j.neuron.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neuroscience Letters. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- 125.Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- 126.Toritsuka M, Makinodan M, Kishimoto T. Social Experience-Dependent Myelination: An Implication for Psychiatric Disorders. Neural Plast. 2015;2015:465345. doi: 10.1155/2015/465345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Research. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- 128.Brenes JC, Lackinger M, Hoglinger GU, Schratt G, Schwarting RK, Wohr M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J Comp Neurol. 2015 doi: 10.1002/cne.23842. [DOI] [PubMed] [Google Scholar]
- 129.Cratty BJ. Perceptual and Motor Development in Infants and Children. Second. New Jersey: Prentice-Hall, Inc; 1979. [Google Scholar]
- 130.Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. Cognition and communication from nine to thirteen months: Correlational findings. In: Bates E, editor. The emergence of symbols: Cognition and communication in infancy. New York: Academic; 1979. [Google Scholar]
- 131.Babik I, Michel GF. Development of role-differentiated bimanual manipulation in infancy: Part 1. The emergence of the skill. Dev Psychobiol. 2015 doi: 10.1002/dev.21382. [DOI] [PubMed] [Google Scholar]
- 132.Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr Soc Res Child Dev. 1998;63(i–vi):1–143. [PubMed] [Google Scholar]
- 133.Steiner AM, Gengoux GW, Klin A, Chawarska K. Pivotal response treatment for infants at-risk for autism spectrum disorders: a pilot study. J Autism Dev Disord. 2013;43:91–102. doi: 10.1007/s10803-012-1542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koegel RL, Schreibman L, Good A, Cerniglia L, Murphy C, Koegel LK. How to teach pivotal behaviors to children with autism: A training manual. Santa Barbara: University of California; 1989. Santa Barbara. [Google Scholar]
- 135.Kasari C, Gulsrud A, Paparella T, Hellemann G, Berry K. Randomized comparative efficacy study of parent-mediated interventions for toddlers with autism. J Consult Clin Psychol. 2015;83:554–563. doi: 10.1037/a0039080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schreibman L, Dawson G, Stahmer AC, Landa R, Rogers SJ, McGee GG, et al. Naturalistic Developmental Behavioral Interventions: Empirically Validated Treatments for Autism Spectrum Disorder. J Autism Dev Disord. 2015;45:2411–2428. doi: 10.1007/s10803-015-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baranek GT, Watson LR, Turner-Brown L, Field SH, Crais ER, Wakeford L, et al. Preliminary efficacy of adapted responsive teaching for infants at risk of autism spectrum disorder in a community sample. Autism Res Treat. 2015;2015:386951. doi: 10.1155/2015/386951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wetherby AM, Guthrie W, Woods J, Schatschneider C, Holland RD, Morgan L, et al. Parent-implemented social intervention for toddlers with autism: an RCT. Pediatrics. 2014;134:1084–1093. doi: 10.1542/peds.2014-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allaby M, Sharma M. Screening for autism spectrum disorders in children below the age of 5 years. A draft report for the UK National Screening Committee. Solutions for Public Health. 2011 [Google Scholar]
- 140.Brett D, Warnell F, McConachie H, Parr JR. Factors Affecting Age at ASD Diagnosis in UK: No Evidence that Diagnosis Age has Decreased Between 2004 and 2014. J Autism Dev Disord. 2016 doi: 10.1007/s10803-016-2716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9:18. doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


