Abstract
T cell specificity emerges from a myriad of processes, ranging from the biological pathways that control T cell signaling to the structural and physical mechanisms that influence how TCRs bind antigen/MHC. Of these processes, the binding specificity of the TCR is a key component. However, TCR specificity is enigmatic: TCRs are at once specific but also cross-reactive. Although long-appreciated, this duality continues to puzzle immunologists and has implications for the development of TCR-based therapeutics. Here we review TCR specificity, emphasizing results that have emerged from structural and physical studies of TCR binding. We show how the TCR specificity/cross-reactivity duality can be rationalized from structural and biophysical principles. There is excellent agreement between predictions from these principles and classic predictions about the scope of TCR cross-reactivity. We demonstrate how these same principles can also explain amino acid preferences in immunogenic epitopes and highlight opportunities for structural considerations in predictive immunology.
Keywords: TCR, specificity, cross-reactivity, structure, prediction
T cell specificity is a hallmark of cellular immunity. Specificity results from a myriad of processes, ranging from the biological mechanisms that control the composition of the T cell repertoire and its reactivity, to the physiochemical mechanisms that influence the interactions between T cell receptors (TCRs), major histocompatibility (MHC) proteins, and antigenic peptides. In between are numerous other mechanisms that influence T cell responsiveness, antigen presentation and density, and the efficiency and outcome of T cell signaling. Despite this complexity, TCR binding specificity is a foundational component of T cell specificity. Here, we review recent progress in understanding TCR specificity to help place it in the context of other processes that make up the equation of T cell specificity.
Binding specificity arises from the structural and physicochemical “fit” between a receptor and its ligand. In theory, structure can be used to rationalize, or even predict TCR binding specificity. The concept of structural fit, however, is elusive and not easily quantified from structures. Just as tissue microenvironments influence cellular states, structural environments impact interatomic interactions, both attractive and repulsive. Some interactions operate at long ranges, outside of what might traditionally be viewed as a receptor-ligand interface. Motion, which can strongly influence how two molecules interact, is poorly gauged from structures. Structures themselves are the results of experiments with noise and error. These realities explain why predicting protein-ligand affinities from structure remains challenging even after decades of improvements (1–3). However, there is considerable knowledge about the factors that influence binding, and there has been much progress towards interpreting binding data from structures and using this to make qualitative predictions about specificity. These advances can readily be applied to TCRs and are discussed below. Our discussion is largely from the perspective of TCR recognition in class I MHC systems, due largely to available data, but the general themes are easily extendable to TCR recognition in class II and other antigen presentation systems in cellular immunity.
Caution ahead: TCR specificity necessarily invokes binding affinity, but is only a component of T cell specificity
Specificity is precisely defined in biochemical interactions that involve two molecules, such as enzyme-substrate or antibody-antigen interactions. An affinity matured antibody that binds its target 1000-fold more tightly than unrelated antigens is considered highly specific. Solution binding affinities are therefore implicit in discussions of specificity. But as reviewed recently (4), TCRs and MHC proteins are embedded in membranes, which greatly influences the biophysics of protein interactions (5). Thus, the concept of a solution KD (sometimes referred to in immunology as a 3D affinity), on which the biochemical definition of binding specificity is based, cannot truly apply to T cell biology.
Considering TCRs and their ligands in their biological contexts not only brings up the physical influences of membrane confinement, but also calls attention to the fact that TCR binding and specificity are often evaluated with experiments that measure biological outcomes dependent on T cell signaling processes. In addition to TCR binding strength, T cell signaling incorporates a large variety of physical and biological complexities, incorporating everything from peptide binding to MHC proteins, T cell membrane composition, kinase and coreceptor expression levels, and more (4, 6–16). One complexity receiving current attention is the supramolecular architecture of the TCR signaling complex. Unusual TCR binding topologies have been associated with altered immunological outcomes (17, 18), potentially by hindering coreceptor or CD3 engagement and possibly the formation of higher order clusters (6, 19, 20). Supramolecular architectural differences can in principle occur independently of TCR affinity for peptide/MHC (pMHC). T cell mechanics and the biology of the CD4/CD8 coreceptors are two other complexities of notable interest. The former is an enigmatic process where different peptides alter the force dependence of membrane-bound TCR-pMHC interactions (4, 14, 15). The latter relates T cell responses to the levels of coreceptor directly associated with the Lck kinase (13).
Supramolecular architectures, force dependencies, and coreceptor scanning provide examples of how T cell specificity can be influenced independently of classical biochemical parameters that determine the number of ligated receptors (i.e. affinity and receptor/ligand concentrations). Because these complexities superimpose on receptor binding in determining T cell function, functional outcomes scale imperfectly with TCR binding affinity measured in solution. Indeed, many outliers have been noted over the years, and both high and low thresholds are believed to exist (21–23). Nonetheless, experimental binding affinities and their ratios are the lens through which structural interpretations of binding and specificity are viewed. Binding affinities give access to the binding free energy (ΔG°), or the “glue” within an interface. When we discuss van der Waals interactions, hydrogen bonds, burial of hydrophobic surface area, etc., we implicitly consider their contributions to ΔG°.
Fortunately, when we consider ratios of affinities (or equivalently, differences in binding free energies or ΔΔG values), we can often discount many of the aspects that distinguish binding affinity from other contributions to T cell function. This has been borne out by numerous experiments in which changes within a TCR-pMHC interface lead to changes in binding affinity and corresponding changes in functional readouts (again, sometimes nonlinearly and with occasional outliers) (21–23). This is a good thing: without the ability to rely on relationships between ΔG° and structure, structural immunology would be of questionable value for interpreting specificity. Some healthy skepticism, however, and an awareness of the distinction between what we might refer to as TCR binding specificity (based upon solution affinities and interpretable in the context of structural information) and T cell functional specificity (variations in biological T cell responses) is nonetheless important.
Rules are made to be broken and roles are not easily defined
Through efforts in structural immunology, we now have several dozen structures of different TCRs bound to various pMHC ligands (and more than 160 structures if we include the “redundant” structures with altered peptides, mutants, high affinity variants, etc). This structural database has been reviewed extensively, recently by Rossjohn, Gras, and colleagues (24, 25). Although there are common themes and trends within the collection of structures, exceptions exist for nearly every “rule” that emerged in the early days of structural immunology. For example, hypervariable complementarity determining region (CDR) loops contact peptides, but also frequently contact the MHC protein. Germline loops, while commonly aligned alongside the α helices of MHCs, often contact the peptide. A variety of angles make up the TCR “diagonal binding mode,” and TCRs that bind with reversed binding modes have now been described (17, 26). There are biological implications for the trends and their exceptions – for example, as noted above, TCRs that bind pMHC with outlier geometries seem to signal weaker or not at all, possibly due to supramolecular architectural limits (17, 18). A key lesson is that many of our simplifying assumptions about the rules and roles in TCR binding have turned out to be limiting. Indeed, partly because of the inadequacies of simplifying assumptions, there is still much to be learned from new structures of TCRs and their complexes.
One of the common assumptions of TCR specificity is that it emerges from hypervariable CDR3α and CDR3β loops. After all, in the majority of TCR-pMHC structures the hypervariable loops most closely align with the peptide. Hypervariable loops defining specificity makes biological sense: T cell repertoires consist of millions of different receptors sharing a few dozen genetically-encoded germline loops but possessing (almost) randomly generated hypervariable loops. In fact, hypervariable loop composition has very recently been shown to allow predictions of TCR specificity (27). But because of their proximity, hypervariable loops cannot at the atomic level act independently of their neighboring germline loops nor the MHC protein.
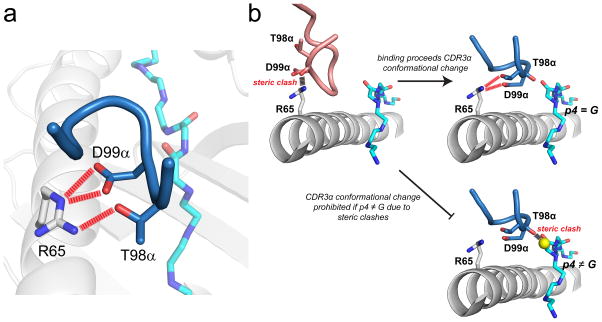
An illustrative example is the A6 TCR, which recognizes the HTLV-1 Tax11-19 peptide presented by the class I MHC protein HLA-A*0201 (HLA-A2). The A6-Tax11-19/HLA-A2 complex was the first TCR-pMHC structure to be solved at high resolution (28). The structure showed that although CDR3α helped accommodate the peptide, the loop also made a series of electrostatic interactions with the HLA-A2 α1 helix (Fig. 1A). A deconstruction of the strengths of individual interactions in the interface showed that these interactions between CDR3α and HLA-A2 were the strongest in the entire TCR-pMHC interface (29). At first glance this was a puzzling finding: if the strongest interactions in the interface are between a hypervariable loop and the MHC protein, how can the A6 TCR show peptide specificity? Indeed, the A6 TCR shows typical specificity and is not a “degenerate” binder as shown with positional scanning and peptide libraries (30).
Figure 1.
High peptide specificity emerging from how a TCR interfaces with the MHC protein. A) In the structure of the A6 TCR bound to Tax11-19/HLA-A2, Thr98 and Asp99 of CDR3α form strong electrostatic interactions with Ar65 on the HLA-A2 α1 helix (29). B) In order to interact with Arg65, CDR3α must undergo a conformational change upon binding (31). In the absence of the loop conformational change, steric clashes would occur between the CDR3α backbone and Arg65. Upon making the conformational change, the backbone of CDR3α is tightly packed against position 4 of the peptide. If position 4 is anything other than glycine, steric clashes would exist, preventing the loop from adopting its needed conformation, as shown in bottom right for an alanine at position 4.
Two other pieces come together to tell the full story. First, in binding Tax11-19/HLA-A2 the A6 CDR3α loop must adjust its position to avoid steric clashes with the HLA-A2 α1 helix and form the key electrostatic interactions (31). In doing so, it aligns against Gly4 of the peptide (Fig. 1B). Second, the A6 TCR shows exquisite specificity for a glycine at position 4 of the peptide – no other residues are tolerated (30). Structural analyses showed that if anything other than glycine is present at P4, the loop cannot adopt the proper conformation to interact with the MHC protein due to steric clashes. Thus, the way the CDR3α loop interfaces with both peptide and MHC contributes to peptide specificity.
Although the A6-Tax11-19/HLA-A2 interaction is one of the best-studied, numerous other TCR complexes provide other examples of how traditional “roles” for the various interface components break down at an atomic level. Some of these examples are summarized in a recent analysis of several structures (32). In some cases, germline-encoded CDR1 loops interface with the N-terminal or C-terminal halves of peptides, contributing to peptide specificity (33–35). Conformations of neighboring CDR loops can influence one another (36–38). In other cases, TCR-peptide perturbations have been compensated by new TCR-MHC interactions without any apparent losses in peptide specificity (31).
More recently, in a deconstruction of a variant of the A6 TCR whose specificity was switched from the Tax11-19 peptide to the MART-126-35 peptide via molecular evolution (39), it was found that specificity determinants were distributed throughout the germline and hypervariable CDR loops, including amino acids distal from the binding site that influenced loop architecture (38, 40). The overall message is that when considering the determinants of TCR binding specificity we need to consider the interface in its entirety, including the structural and physical relationships between the various CDR loops - hypervariable or not - and the composite peptide/MHC surface.
Rationalizing the specificity/cross-reactivity duality of TCRs
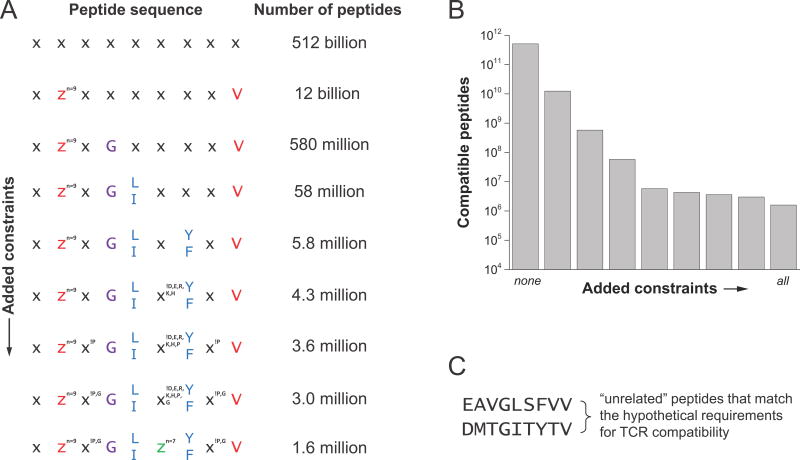
As pointed out in Mason’s seminal 1998 review and then later by Sewell, the universe of potential antigens is orders of magnitude larger than the number of unique TCRs in an individual, necessitating a highly cross-reactive TCR repertoire (41, 42). Experimentally, a single TCR has been shown to recognize more than a million peptides (43). But how do we square such high cross-reactivity with observations of high specificity, such as the requirement of a glycine at P4 for binding of the A6 TCR to Tax11-19/HLA-A2? Absolute specificity for an amino acid at one position still permits millions of other peptides. Consider a 9-mer with at least one ideal class I MHC anchor residue at P9. Many peptides are immunogenic with only one ideal anchor (44), so imagine the second anchor residue can be substituted by one of the nine smaller/uncharged amino acids without inducing a structural change in the peptide (45, 46). Now consider a hypothetical TCR with an absolute requirement for a glycine at P4. With these generous stipulations, there are 580 million matching peptides, as shown in Fig. 2. But, simply replacing Gly4 with an alanine would abolish recognition of any.
Figure 2.
Structural and physiochemical constraints on which peptides a TCR can recognize permit a high degree of cross-reactivity but also high specificity. In the example shown here for a class I system, there are 512 billion possible 9-mer peptides. Constraining one primary anchor and limiting the second reduces this to 12 billion. Further restraints designed to mimic principles of TCR specificity progressively reduce the number of possible peptides. This is illustrated for a hypothetical case where “compatibility” with a TCR is introduced in step-wise fashion. Compatibility adds a requirement for a glycine at P4, a requirement for a hydrophobic leucine or isoleucine at P5, an aromatic tyrosine or phenylalanine at P7, removal of charges from the center, and exclusion of glycine and proline from all remaining positions. Under all these constraints, there are still 1.6 million compatible peptides. Two, seemingly unrelated peptides are shown in the lower right. Note that “compatibility” as defined indicates a peptide which permits a TCR to bind with an affinity strong enough to productively signal, implying that all compatible peptides need not be recognized with the same affinity.
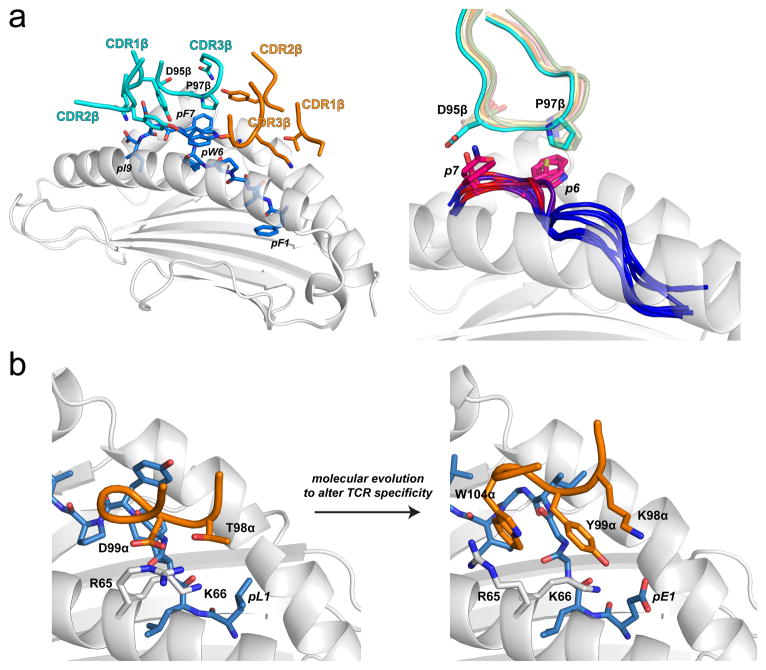
Recent studies of TCR cross-reactivity shed further light on structural aspects of specificity. Using yeast display, Garcia and colleagues screened libraries encoded by genes for single chain peptide/H-2Ld complexes with randomized peptide sequences. Sequences encoding proteins which bound strongly to the 42F3 TCR were identified by flow cytometry using TCR tetramers and used to generate what can be termed a “sequence fitness landscape” for peptides compatible with the 42F3 TCR (47). Structural analyses of some of these complexes showed a TCR focus on a “hot spot” – a region of the peptide that was structurally and chemically similar between different agonist ligands, forming similar interactions with the TCR (Fig. 3A). Outside of the hot spot more sequence diversity was permitted.
Figure 3.
Hot spots demonstrated by sequence landscapes in TCR-pMHC interfaces. A) Illustration of a structurally conserved hot spot within the interface between the 42F3 TCR and peptides presented by H2-Ld (47). The left panel shows the interface between 42F3 and the QL9 (QLSPFPFDL) mimotope FLSPFWFDI/Ld. The hot spot region is localized to the peptide bulge and engaged primarily by residues of CDR3β. The right panel shows the hot spot as found in eight different agonist 42F3-agonist/Ld structures. Peptide backbones at position 6 through 9 are colored by contact frequency with the TCR, with red indicating the greatest number of contacts. The side chains of the key amino acids at positions 7 and 8 are shown, as is the conformation of the CDR3β loop. Pro97β remains in position to interact with peptide position 6, whereas Asp95β adjusts its conformation to optimize charge complementarity with peptide position 7. B) In altering peptide specificity, the molecular evolution process acted upon a hot spot in the interface between A6 TCR and Tax11-19/HLA-A2 (see also Fig. 1) (38). By changing Thr98α to a lysine and Asp99α to a tyrosine, the complex electrostatic interactions between the TCR and the HLA-A2 α1 helix were disrupted, forcing Arg65 of HLA-A2 to adopt a new conformation, permitting Trp104 of CDR3α to sandwich between the arginine and the peptide backbone and forming a new hot spot in the interface the modified TCR forms with the MART-126-35/HLA-A2 ligand.
Hot spots are found in almost every protein-protein interaction, and indeed have been described many times in TCR-pMHC complexes (48–56). They can be discerned via sequence landscapes as noted above (47), but have been more traditionally defined as regions where mutations have the greatest impact on binding (57). TCRs where hot spots have been explored through point mutations are listed in a recently developed online database (https://zlab.umassmed.edu/atlas/web/) (58). But point mutants are almost always to alanine, a rather limited exploration of chemical space (51, 59). Extending on point mutations is the recent deployment of deep mutational scanning to TCRs, which can rapidly and exhaustively assess the impact and importance of multiple mutations throughout the CDR loops, generating a sequence fitness landscape for the receptor (38, 40). Using deep mutational scanning, it was recently shown that for a variant of the A6 TCR, although specificity emerged from the action of numerous sites as noted above, molecular evolution altered specificity by modifying the interactions between the CDR3β loop and the charges on the HLA-A2 α1 helix. Essentially, the yeast display process “converted” a hot spot that drove compatibility with Tax11-19/HLA-A2 to another that drove compatibility with MART-126-35/HLA-A2 (Fig. 3B).
Although not solely responsible for binding specificity, the occurrence of hot spots within TCR-pMHC interfaces can explain the simultaneous observation of both high and low specificity in TCR binding: subtle perturbations in hot spot regions of a peptide will have profound impacts on binding, whereas changes outside a hot spot can be more easily tolerated. In fact, the discovery and consequences of hot spots in peptides were foreshadowed: returning to the A6 structure published in 1996, Wiley and colleagues noted “…the observation that although substantial contacts are made to peptide residues Y5 and Y8, only a few atoms of peptide residues 1, 2, 4, 6 and 7 are in contact, places physical limits on TCR specificity for peptide” (28).
Due to structural and chemical variability, not every TCR-pMHC interface will share similar hot spots. This variability is highlighted by comparisons of different TCRs binding the same pMHC (Table 1): in some instances very different structural/physical solutions have been seen, indicating different mechanisms of obtaining binding free energy (e.g., refs. (60–64). In some interfaces, hot spots will be direct interactions, as is frequently envisioned. In other cases, hot spots may be cryptic, such as alignments to avoid charge repulsion or steric clashes (31, 47). Engineering TCRs can alter the locations and overall contributions of hot spots. Indeed, it has recently been shown that improvements in TCR affinity can be found by changing not only the amino acids that contact peptide or MHC, but also those in the “second shell” away from the contact surface (38, 40).
Table 1.
Structures of different TCRs bound to the same peptide/MHC complex a
| TCR | peptide | MHC | PDB | References |
|---|---|---|---|---|
| class I systems | ||||
| DMF5, DMF4 | AAGIGILTV | HLA-A2 | 3QDJ, 3QEQ | 60 |
| DMF5, DMF4, Mel5 | ELAGIGILTV | HLA-A2 | 3QDG, 3QDM, 3HG1 | 34, 60 |
| LC13, CF34, RL42 | FLRGRAYGL | HLA-B8 | 1MI5, 3FFC, 3SJV | 85, 102, 103 |
| LS01, LS10 | GILGFVFTL | HLA-A2 | 5ISZ, 5JHD | 61 |
| A6, B7 | LLFGYPVYV | HLA-A2 | 1AO7, 1BD2 | 28, 104 |
| NP2-B17, NP1-B17 | ASNENMETM | H-2Db | 5SWS, 5SWZ | 17 |
| SB27 b, CA5, SB47 | LPEPLPQGQLTAY | HLA-B35 | 2AK4, 4JRX, 4JRY | 105, 106 |
| RA14, C7, C25 | NLVPMVATV | HLA-A2 | 3GSN, 5D2L, 5D2N | 49, 63 |
| 2C c, 42F3 | QLSPFPFDL | H-2Ld | 2E7L, 3TF7 | 18, 107 |
| C1-28, T36-5 | RFPLTFGWCF | HLA-A24 | 3VXM, 3VXU | 108 |
| class II systems | ||||
| 2B4, 226 | ADLIAYLKQATKG | I-Ek | 3QIB, 3QIU | 64 |
| JR5.1, D2, S16 | APQPELPYPQPGS | HLA-DQ2 | 4OZF, 4OZG, 4OZH | 109 |
| S13, L3-12, T316, Bel502 | APSGEGSFQPSQENPQGS | HLA-DQ8 | 4Z7U, 4Z7V, 4Z7W, 5KS9 | 109, 110 |
| B3K506, YAe62, 2W20, 14.C6 | FEAQKAKANKAVD | I-Ab | 3C5Z, 3C60, 3C6L, 4P5T | 37, 111 |
| E8, G4 | GELIGILNAAKVPAD | HLA-DR1 | 2IAM, 4E41 | 112 |
| T15 d, Bel602 | GPQQSFPEQEA | HLA-DQ8 | 5KSB, 5KSA | 110 |
| FS18, FS17 | GSLQPLALEGSLQKRGIV | HLA-DR4 | 4Y19, 4Y1A | 26 |
The table includes parental molecules only, excluding mutants, variants, etc. except as noted in the footnotes below.
SB27 is crystallized with HLA-B*3508, whereas CA5 and SB47 are with B*3505
The 2C variant crystallized is a high affinity mutant.
The peptides in complex with T15 and Bel602 differ at the P1 residue.
Following the themes above, when peptide hot spots do exist, they should not be expected to be engaged solely by hypervariable loops – again, rules are made to be broken. Returning to the example of A6 TCR, the germline CDR1β loop forms a very strong hydrogen bond with the C-terminal end of the Tax11-19 peptide, resulting in high specificity for a tyrosine at P8 (29, 30). In another case, the Mel5 TCR forms a specificity-determining salt-bridge with Glu1 of the MART-126-35 peptide using the germline CDR1α loop (33, 34). The HCV1406 TCR which recognizes the HCV NS3 epitope requires a lysine at P1, which is also engaged by CDR1α (35). Lastly, the flexibility inherent to some TCRs (65) may permit different hot spots with different ligands, as is believed to occur the well-studied TCR 2C (66, 67).
Importantly, hot spots are not necessarily amino acid specific – the key feature is compatible ligands share structural and chemical similarity. Thus, there may be similar specificity for a large hydrophobic residue, a charge, or a hydrogen bond donor, etc., depending on the structural details (30). Return to the imaginary example of the 9-mer in Fig. 2, and add a requirement for a leucine or isoleucine hot spot to make hydrophobic interactions with the TCR. Now the number of “compatible” peptides that meet the criteria for TCR recognition is reduced from 580 million to 58 million. Add another constraint for a tyrosine or phenylalanine at another position, and the number of compatible peptides is 5.8 million (note that “compatibility” as defined in this example requires TCR binding with sufficient strength to productively signal. Compatible affinities will cover a wide range, which might influence signal strength and functional outcome, but a response will still be elicited).
Although instructional, this is admittedly a simplified argument: the implicit assumption that any random amino acid will work in the non-specified positions and still yield a compatible peptide is certainly wrong. As discussed below, charges are less frequently observed in the centers of immunogenic peptides (68). Take this into account for our 9-mer example and the number of compatible peptides becomes 4.3 million. Again, other constraints could be envisioned – some amino acids will alter peptide conformation. To crudely mimic this, we can exclude proline and then glycine from non-terminal positions; now, the numbers are 3.6 and 3.0 million. Restrict P6 to the smallest seven amino acids to limit peptide-MHC steric clashes and the number is 1.6 million. This is a large number of peptides, but consider there are 12 billion that only match the anchor residue requirements, and 512 billion of random nonamers – 1.6 × 106 out of these much larger numbers of peptides meets the biochemical definition of high specificity. Two hypothetical, unrelated but compatible peptides are shown in Fig. 2C. The two peptides differ substantially in sequence, yet have positions where subtle perturbations would abolish TCR recognition.
While illustrative, the examples above do not address all possibilities. Adding more realism, there will be cooperative influences at different positions, leading to positional correlations in amino acid preferences and reducing the number of compatible peptides (69). But although the example is shown for 9-mers, longer peptides are processed, presented, and recognized, which will increase the number of compatible peptides (70). For our hypothetical TCR, we easily settle on a number of compatible peptides in the millions, with regions of both low and high homology, reconciling how TCRs can be at once specific but also cross-reactive (71). Twenty years later, Mason’s remarkably prescient prediction that a single TCR should be able to recognize at least a million peptides is fully consistent with and can be fully rationalized by structural and biophysical principles (41).
Using structural information to help guide the search for cross-reactive epitopes
Based on the previous discussion, any one TCR will productively engage with what at first glance might appear to be unrelated ligands, yet also show high specificity towards subtle peptide changes – a duality that as shown above can be rationalized from structural and physical principles. This duality and our ability to rationalize it is instructional as we enter the age of TCR-based molecular and cellular therapeutics. Recently, an engineered, high affinity TCR targeting the MAGE-A3 tumor antigen presented by HLA-A1 was used in a clinical trial testing gene-engineered T cells for melanoma. Unbeknownst at the time, and despite substantial preclinical testing, the receptor also recognized a peptide from a protein expressed in cardiac tissue, leading to severe off-target autoimmunity and patient fatalities (72). The cross-reactive peptide was subsequently identified as an epitope from the protein Titin (73). The sequence of the Titin peptide is ESDPIVAQY. The sequence of the MAGE-A3 peptide is EVDPIGHLY. Consider these peptides in light of Fig. 2 and the surrounding discussion above: the residues at positions 1, 3, 4, 5, and 9 are identical. That the same high affinity receptor recognized both peptides is, in hindsight, not surprising.
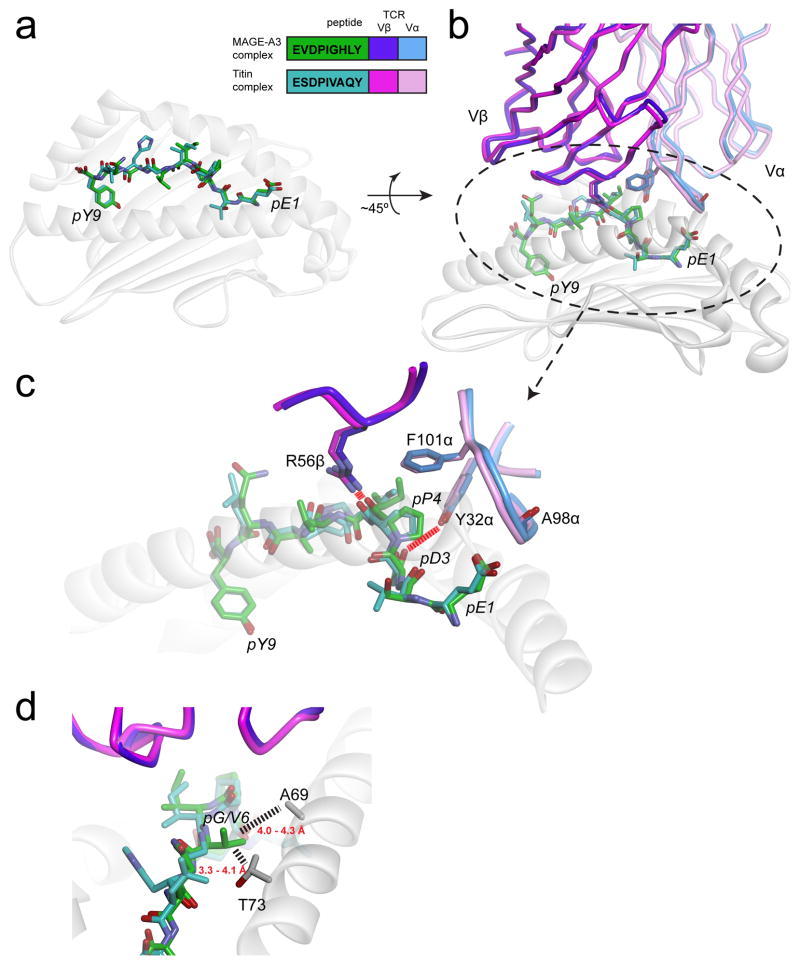
Crucially though, two viral and bacterial peptides that showed similar degrees of homology with the MAGE-A3 epitope were not recognized by the TCR used in the clinical trial (73). Can the variety of outcomes with Titin, MAGE-A3, and these other epitopes be rationalized? The answer is yes, as the structures of a variant of the same TCR (MAG-IC3) bound to the MAGE-A3 and Titin peptides presented by HLA-A1 were recently published (74). In the MAG-IC3 structures with MAGE-A3 and Titin, the peptides are presented almost identically, which is not unusual given their similarities and the fact that nonamers bound to class I MHC proteins often adopt similar conformations (75, 76). The TCR in turn engages these two peptides very similarly (Fig. 4A). In both structures, pGlu1 of the peptide is capped by the backbone of CDR3α, with the carbonyl of Ala98α rotated away to optimize charge complementarity. Despite being buried in the HLA-A1 binding groove, pAsp3 hydrogen bonds with Tyr32 of CDR1α. The hydrophobic pPro4 is capped by Phe101 of CDR3α, and Arg56 of CDR2β hydrogen bonds to the pPro4 backbone. The side chain of pIle5 fills a gap between the TCR and HLA-A2. The TCR mostly ignores differences in the C-terminal halves of the two peptides due to its tilt towards the peptide N-termini.
Figure 4.
The MAGE-A3 and Titin epitopes are presented and recognized almost identically by the MAG-IC3 TCR (74). A) Near-identical presentation of the two epitopes in the HLA-A1 peptide binding groove. The inset shows the peptide sequences and the color scheme used for all panels. B) Overview of how the two ligands are engaged by the MAG-IC3 TCR. C) Details of identical, key interactions in the two interfaces. Charge complementarity with pGlu1 is optimized by the positioning of the carbonyl oxygen of Ala98 of CDR3α away from the glutamate side chain (not shown is a salt-bridge from Arg170 of HLA-A1 that helps fix the glutamate). Tyr32 of CDR1α hydrogen bonds with pAsp3. Phe101 of CDR3α “caps” the hydrophobic pPro4, and Arg56 of CDR2β hydrogen bonds with the pPro4 backbone. Not evident in the figure is how pIle5 packs between the TCR and the HLA-A2 α2 helix. D) The Titin and MAGE-A3 peptides have a valine and a glycine at P6, respectively. Substitution with larger amino acids would result in clashes with Ala69 and Thr73 of the HLA-A1 α1 helix, explaining why pathogen-derived peptides similar to the Titin and MAGE-A3 peptides would not be recognized. Dashed lines show distances between the side chains of Val6 of the Titin peptide and residues of HLA-A1 (note that in generating this figure, the CDR1α loop in the two structures was optimized to better fit the experimental electron density and amino acid geometry, yielding coordinates slightly altered from those deposited in the PDB).
The structures also suggest why the viral and bacterial peptides similar to MAGE-A3 and Titin were not recognized: these peptides contain a tyrosine and lysine, respectively, at position 6, neither of which would fit in the tight constraints between the peptide and MHC (Fig. 4B). Despite being very similar to Titin/MAGE-A3 elsewhere, the bacterial and viral peptides therefore likely have an altered peptide conformation which would change how the receptor sees the peptide. Overall, the structures and related physical considerations can fully rationalize the outcomes of TCR specificity in this instance.
Such hindsight, however, is not helpful if the goal is to assess risks of TCR cross-reactivity in advance. Could MAGE-A3 and Titin cross-reactivity be predicted in advance? Given the complexities surrounding the processing and tissue distribution of the two antigens, the answer to the question posed at the in vivo functional level is almost certainly no (73). However, a more focused question is appropriate: can hot spots and other specificity determinants be predicted in ways that can guide searches for potential cross-reactive epitopes? Structural information could be enormously helpful here, as demonstrated in recent efforts at predicting TCR specificity (27). Starting with the structure of a TCR-pMHC interface, modeling and energetic scoring of TCR-pMHC interfaces could be used to predict regions of “focus” (or hot spots) that could be used to narrow down motifs which drive specific binding. To be effective, advances will need to occur in protein modeling methodologies, particularly to account for flexibility in the TCR, peptide, and MHC proteins. Substantial increments in speed and computational power will also be required, perhaps aided by advances in distributed computing (77). These challenges are surmountable though, and structure-based prediction methods are doubtless on the horizon as a way to help assess TCR cross-reactivity. New approaches, such as deep mutational scanning, can greatly complement structural information and thus structure-based prediction methods (38, 40). Screening for ligands using yeast display, peptide libraries, or other combinatorial approaches will be similarly helpful (43, 47, 78). Together, structural information, modeling, and screening could prove enormously powerful in this aspect of predictive immunology. Moving beyond this, identifying those epitopes which are correctly processed and presented remains an additional task, but advances in appropriate prediction methods could also be leveraged here (79–83).
Demonstrating key principles via the paradox of specificity in allorecognition
Alloreactivity emerges when tissues are transplanted across MHC barriers. Owing to the high frequency of alloreactive T cells, alloreactivity has often been presumed to be relatively non-specific, attributable to TCR focus on mis-matched MHC polymorphisms, or degenerate recognition of allopeptides. Yet paradoxically, alloreactivity has been shown in many instances to proceed with specificity for both peptide and MHC (84). Recent work on alloreactivity has shown how such specificity can emerge in alloreactivity. These findings reinforce the themes noted above: the importance of the composite TCR-pMHC interface, the breakdown of once-traditional rules, and the utility of structural information in rationalizing and predicting TCR specificity and cross-reactivity.
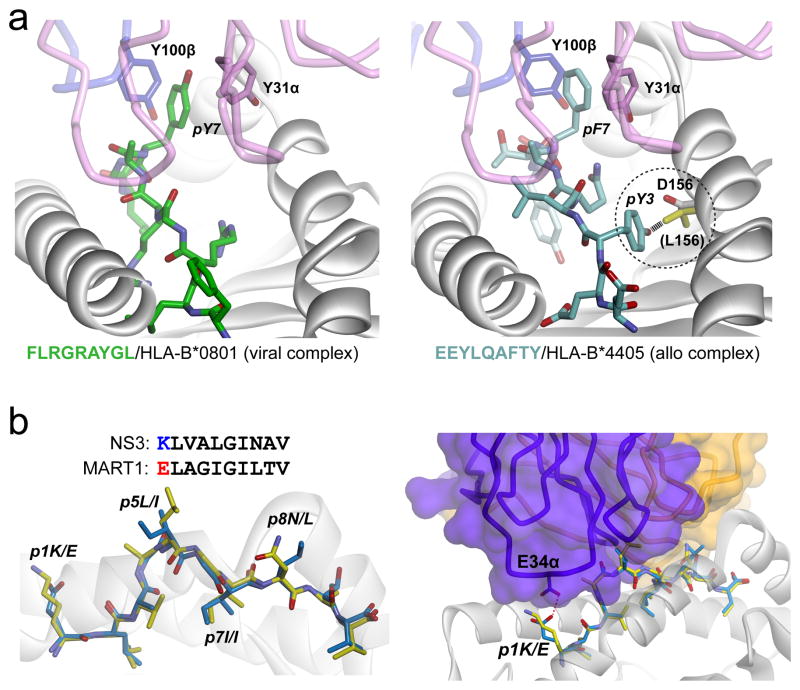
The LC13 TCR was first studied structurally as an example of a “public” anti-viral TCR, with its structure solved in complex with an EBV epitope presented by the “self” MHC HLA-B*0801 (85). LC13 was later studied in complex with an unrelated human allopeptide presented by the “foreign” MHC HLA-B*4405 (86). In the structure, the peptide mimics the viral epitope, and despite the sequence differences, it is engaged very similarly by the TCR with no indications of non-specific, degenerate recognition (Fig. 5A). Moreover, the LC13 TCR was found to discriminate between HLA-B*4405 and HLA-B*4403, which differ by only two amino acids, while HLA-B*4405 and HLA-B*0801 differ by 25. Discrimination between the B44 subtypes was attributable to a single amino acid difference in the MHC α2 helix, which in the case of B*4403 prevented the movement of the peptide into a compatible conformation. This highly specific engagement of both allopeptide and foreign MHC by LC13 shows that even allorecognition cannot escape the consequences that emerge from the need for TCRs to engage a composite peptide/MHC ligand.
Figure 5.
Structural studies of alloreactivity illustrate core principles. A) The LC13 TCR cross-reacts between the syngeneic viral-peptide/HLA-B*0801 complex (left panel) and the allogeneic self-peptide/HLA-B*4405 complex (right panel). Despite considerable sequence differences, the peptides adopt very similar conformations in the ternary complexes, with key interactions between the TCR and the protruding aromatic P7 side chain maintained (86). The TCR also discriminates between closely-related B44 subtypes due to a single amino acid difference that prohibits the peptide from adopting a compatible conformation in B*4403 (right panel, circled detail). In B*4405, the aspartic acid at position 156 forms a hydrogen bond with pTyr3. In B*4403, position 156 is a leucine (yellow) and would clash with pTyr3 as shown. B) In the structure of the HCV1406-NS3/HLA-A2 complex, the conformation and chemistry of the NS3 peptide are similar to those of the MART-126-36 peptide, except for the residue at P1 (left panel). Structurally, the MART-126-35 peptide fits within the complex without any steric clashes or chemical incompatibility, save for the P1 residue, which would experience charge repulsion with Glu134 of CDR1α (right panel). Replacing pGlu1 with lysine resulted in a MART-126-35 variant that was recognized by the HCV1406 TCR (35).
More recently, the properties of the alloreactive TCR HCV1406 were studied (35). The HCV1406 TCR was identified from T cells that expanded when a HLA-A2+ liver was transplanted into a HCV-infected HLA-A2- host (87). T cells expressing HCV1406 mediated anti-HCV immunity, specifically targeting the HCV NS3 antigen presented by HLA-A2 (35). Again, TCR binding was found to be dependent on features unique to both the peptide and the foreign MHC. In this case, TCR binding to HLA-A2 required polymorphic amino acids on the α1 helix that distinguished HLA-A2 from all class I, class II, and non-classical MHC proteins in the transplant recipient. Peptide specificity was also dependent in part on a hot spot present at the P1 residue, which was engaged by the CDR1α loop.
Thus, in alloreactivity, LC13 and HCV1406 demonstrate the general principles discussed above. We suggest that in addition to explaining specificity in alloreactivity, these same principles can help explain the high frequencies of alloreactive T cells. The combination of unique peptides, unique modes of presentation, and different features on MHC α helices provides for composite recognition surfaces very distinct from those TCRs encounter syngeneically (35). In alloreactivity, TCRs indeed see something new – but it is not only peptide or MHC, but their synergistic combination that results in significant T cell reactivity.
Lastly, the structural and biophysical data with the HCV1406 TCR allowed the identification of a novel cross-reactive peptide (Fig. 5B). Identification of this novel epitope is a straightforward demonstration of how structural information can be of use in predicting TCR cross-reactivity.
Amino acid preferences in immunogenic epitopes are readily explained through physical principles
Recent analyses of immunogenic and nonimmunogenic epitopes have led to the discovery that, at least for class I systems, immunogenic epitopes are enriched in hydrophobic/aromatic amino acids in the peptide centers (68). This has led to the development of immunogenicity prediction tools, such as those found at the Immune Epitope Database (88). A related structural analysis showed that immunogenic epitopes are enriched in hydrophobic TCR contact residues (89). These findings have potential to significantly impact epitope discovery and the development of therapeutics such as those based on cancer neoepitopes.
What is the rationale for preferential use of hydrophobic and aromatic amino acids in immunogenic epitopes? A widely believed pre-requisite for immunogenicity is a TCR binding affinity above some minimal threshold, and as described above immunogenicity often scales with binding affinity (21–23). We suggest that differences in how hydrophobic/aromatic surfaces and how charged/polar surfaces can contribute to binding affinity underlies the observed preferences.
Burial of hydrophobic surface is a key driving force in biomolecular recognition (90). But, electrostatic interactions such as those contributed by polar and charged amino acids are also important. What is the distinction? When charged or polar groups are buried within an interface, they are removed from the bulk water that solvates the unbound protein. Removal of charges from water is energetically expensive, and is referred to as the “desolvation penalty” (91, 92). The desolvation penalty is compensated by whatever new electrostatic interactions (e.g., hydrogen bonds and salt-bridges) are formed in the protein-protein interface. However, these new interactions do not always offset the desolvation penalty. This is because the energies of electrostatic interactions are highly dependent on geometrical parameters such as angles and distances, and precise alignment is required to fully offset desolvation. Indeed, studies of protein electrostatics have shown that salt bridges and hydrogen bonds within proteins and their interfaces are often unfavorable, as the desolvation penalty is not always compensated (91–94). The influence of the desolvation penalty effect has been demonstrated in TCR recognition (51) and is believed to underlie the restricted positioning of TCRs over HLA-A2 (95).
Unlike burial of charged or polar groups, barring the formation of a hole or cavity, removal of hydrophobic surface from bulk water is always favorable (96). This is simply the hydrophobic effect at work. The major geometrical requirements that influence the magnitude of the hydrophobic effect involve curvature and whether removal of hydrophobic surface creates a cavity (or hole) in the interface (97–99). Barring the latter, there are no special geometries needed to obtain favorable free energy from burial of hydrophobic surface.
Extrapolating to TCR recognition of pMHC, recognition of a peptide with charged or polar amino acids in the center requires higher structural precision in the loops of an engaging TCR in order to overcome desolvation (engaging charges near the peptide termini is easier, as the cost of desolvation is reduced if the charged group remains solvent exposed). Recognition of a peptide with a more hydrophobic center is correspondingly easier, requiring less structural precision to obtain the same binding affinity. In an individual’s TCR repertoire, there will be fewer TCRs whose CDR loops match the precise geometry needed to engage a polar peptide to bind strong enough to signal. This is not to say they do not exist - there are complexes with interfacial salt-bridges in the TCR- pMHC structural database (e.g., refs. 34, 35, 100, 101), and indeed many have been discussed above. However, two predictions from the proceeding discussion are that 1) they will be of lower frequency for strongly immunogenic complexes, as supported by amino acid preferences in immunogenic epitopes, and 2) when they do occur, interfacial salt-bridges will more likely involve peptide termini and be located at the periphery of the interface, where desolvation penalties will be reduced.
The considerations above suggest how peptide/MHC structural information could be used to improve immunogenicity predictions. Building from models based on amino acid composition, accurate structural modeling of peptides within MHC binding grooves could be used to refine predictions and possibly generate immunogenicity “scores” based on structure and energies, making allowances for conformation and the solvent exposure of hydrophobic and hydrophilic groups. Advances in structural modeling (or even high throughput crystallography) could again prove advantageous, and a deeper understanding of how best to use structural models to energetically score a peptide/MHC complex will be needed.
Conclusions
The binding specificity of the TCR is one of the key factors that contribute to specificity in T cell mediated immunity. Structural and biochemical investigations have profoundly influenced our understanding of the determinants of TCR specificity. A key finding is the inability to ascribe specificity to TCR hypervariable loops or the peptide alone: rather, the composite peptide/MHC surface and the juxtaposition of various loops of the TCR force us to consider the interface in its entirety. This includes examining the connectedness between the various components, such as peptide and MHC or hypervariable and germline loops.
Importantly, TCRs are not highly specific: as has been recognized for some time, TCR cross-reactivity is fundamental to the immune system. Yet, cross-reactivity is not random, but driven by the fact that for any one TCR, many peptides will be compatible and able to achieve an optimal “fit” with the receptor. Achieving such a fit – or a structural/energetic alignment – will not always be fully obvious or predictable from sequence comparisons, as fit is determined by structural and chemical similarities and influenced by motion. Moreover, small regions of the ligand, i.e. hot spots, may dominate the binding determinaants. The latter, together with structural and physical considerations, can explain long-standing observations that TCRs can be sensitive to small perturbations in one region of the peptide, while tolerating more dramatic changes elsewhere.
Structural biology and our knowledge of the relationships between structure and binding can also explain amino acid preferences in immunogenic peptides. Combining structural biology and modeling with various screening techniques such as yeast display, deep scanning mutagenesis, and combinatorial peptide libraries has the potential to yield new approaches for predicting and productively modulating TCR binding properties. With further advances in understanding the myriad of other physical constraints and biological complexities that contribute to the overall specificity of cell responses, we will be closer to solving and manipulating the entire equation that describes functional T cell specificity.
Acknowledgments
We thank Dr. David Cole for critical comments on the manuscript.
Footnotes
Supported by NIH grants R35GM118166, R01AI129543, and R01GM103773.
References
- 1.Mobley DL, Gilson MK. Predicting Binding Free Energies. Annual Review of Biophysics. 2017:46. doi: 10.1146/annurev-biophys-070816-033654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erijman A, Rosenthal E, Shifman JM. How Structure Defines Affinity in Protein-Protein Interactions. PLOS ONE. 2014;9:e110085. doi: 10.1371/journal.pone.0110085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vangone A, Bonvin AMJJ. Contacts-based prediction of binding affinity in protein–protein complexes. eLife. 2015;4:e07454. doi: 10.7554/eLife.07454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu C, Jiang N, Huang J, Zarnitsyna VI, Evavold BD. Insights from in situ analysis of TCR–pMHC recognition: response of an interaction network. Immunological Reviews. 2013;251:49–64. doi: 10.1111/imr.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasberger B, Minton AP, DeLisi C, Metzger H. Interaction between proteins localized in membranes. Proc Natl Acad Sci U S A. 1986;83:6258–6262. doi: 10.1073/pnas.83.17.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Mariuzza R. Structural and Biophysical Insights into the Role of CD4 and CD8 in T Cell Activation. Frontiers in Immunology. 2013:4. doi: 10.3389/fimmu.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rock KL, Farfán-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. Journal of immunology (Baltimore, Md : 1950) 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Z, Theoret MR, Touloukian CE, Surman DR, Garman SC, Feigenbaum L, Baxter TK, Baker BM, Restifo NP. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J Clin Invest. 2004;114:551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridgeman JS, Sewell AK, Miles JJ, Price DA, Cole DK. Structural and biophysical determinants of αβ T-cell antigen recognition. Immunology. 2012;135:9–18. doi: 10.1111/j.1365-2567.2011.03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu W, Shi X, Xu C. Regulation of T cell signalling by membrane lipids. Nat Rev Immunol. 2016;16:690–701. doi: 10.1038/nri.2016.103. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 12.Acuto O, V, Bartolo D, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 13.Stepanek O, Prabhakar Arvind S, Osswald C, King Carolyn G, Bulek A, Naeher D, Beaufils-Hugot M, Abanto Michael L, Galati V, Hausmann B, Lang R, Cole David K, Huseby Eric S, Sewell Andrew K, Chakraborty Arup K, Palmer E. Coreceptor Scanning by the T Cell Receptor Provides a Mechanism for T Cell Tolerance. Cell. 2014;159:333–345. doi: 10.1016/j.cell.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Zhu C. Mechanical regulation of T-cell functions. Immunological reviews. 2013;256 doi: 10.1111/imr.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brazin KN, Mallis RJ, Das DK, Feng Y, Hwang W, Wang J-h, Wagner G, Lang MJ, Reinherz EL. Structural Features of the αβTCR Mechanotransduction Apparatus That Promote pMHC Discrimination. Frontiers in Immunology. 2015:6. doi: 10.3389/fimmu.2015.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowerman NA, Colf LA, Garcia KC, Kranz DM. Different Strategies Adopted by Kb and Ld to Generate T Cell Specificity Directed against Their Respective Bound Peptides. Journal of Biological Chemistry. 2009;284:32551–32561. doi: 10.1074/jbc.M109.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gras S, Chadderton J, Del Campo Claudia M, Farenc C, Wiede F, Josephs Tracy M, Sng Xavier YX, Mirams M, Watson Katherine A, Tiganis T, Quinn Kylie M, Rossjohn J, La Gruta Nicole L. Reversed T Cell Receptor Docking on a Major Histocompatibility Class I Complex Limits Involvement in the Immune Response. Immunity. 2016;45:749–760. doi: 10.1016/j.immuni.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Adams Jarrett J, Narayanan S, Liu B, Birnbaum Michael E, Kruse Andrew C, Bowerman Natalie A, Chen W, Levin Aron M, Connolly Janet M, Zhu C, Kranz David M, Garcia KC. T Cell Receptor Signaling Is Limited by Docking Geometry to Peptide-Major Histocompatibility Complex. Immunity. 2011;35:681–693. doi: 10.1016/j.immuni.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133–139. doi: 10.1016/j.immuni.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Kuhns MS, Girvin AT, Klein LO, Chen R, Jensen KDC, Newell EW, Huppa JB, Lillemeier BF, Huse M, Chien Y-h, Garcia KC, Davis MM. Evidence for a functional sidedness to the αβTCR. Proceedings of the National Academy of Sciences. 2010;107:5094–5099. doi: 10.1073/pnas.1000925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone JD, Kranz D. Role of T cell receptor affinity in the efficacy and specificity of adoptive T cell therapies. Frontiers in Immunology. 2013:4. doi: 10.3389/fimmu.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chervin AS, Stone JD, Holler PD, Bai A, Chen J, Eisen HN, Kranz DM. The Impact of TCR-Binding Properties and Antigen Presentation Format on T Cell Responsiveness. J Immunol. 2009;183:1166–1178. doi: 10.4049/jimmunol.0900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebeisen M, Oberle S, Presotto D, Speiser D, Zehn D, Rufer N. Molecular Insights for Optimizing T Cell Receptor Specificity Against Cancer. Frontiers in Immunology. 2013:4. doi: 10.3389/fimmu.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T Cell Antigen Receptor Recognition of Antigen-Presenting Molecules. Annual Review of Immunology. 2015;33:169–200. doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- 25.Miles JJ, McCluskey J, Rossjohn J, Gras S. Understanding the complexity and malleability of T-cell recognition. Immunol Cell Biol. 2015;93:433–441. doi: 10.1038/icb.2014.112. [DOI] [PubMed] [Google Scholar]
- 26.Beringer DX, Kleijwegt FS, Wiede F, van der Slik AR, Loh KL, Petersen J, Dudek NL, Duinkerken G, Laban S, Joosten A, Vivian JP, Chen Z, Uldrich AP, Godfrey DI, McCluskey J, Price DA, Radford KJ, Purcell AW, Nikolic T, Reid HH, Tiganis T, Roep BO, Rossjohn J. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat Immunol. 2015;16:1153–1161. doi: 10.1038/ni.3271. [DOI] [PubMed] [Google Scholar]
- 27.Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, Ji X, Han A, Krams SM, Pettus C, Haas N, Arlehamn CSL, Sette A, Boyd SD, Scriba TJ, Martinez OM, Davis MM. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017 doi: 10.1038/nature22976. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 29.Piepenbrink KH, Blevins SJ, Scott DR, Baker BM. The basis for limited specificity and MHC restriction in a T cell receptor interface. Nat Commun. 2013:4. doi: 10.1038/ncomms2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausmann S, Biddison WE, Smith KJ, Ding YH, Garboczi DN, Utz U, Wiley DC, Wucherpfennig KW. Peptide recognition by two HLA-A2/Tax11-19-specific T cell clones in relationship to their MHC/peptide/TCR crystal structures. J Immunol. 1999;162:5389–5397. [PubMed] [Google Scholar]
- 31.Scott DR, Borbulevych OY, Piepenbrink KH, Corcelli SA, Baker BM. Disparate Degrees of Hypervariable Loop Flexibility Control T-Cell Receptor Cross-Reactivity, Specificity, and Binding Mechanism. Journal of Molecular Biology. 2011;414:385–400. doi: 10.1016/j.jmb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrows SR, Chen Z, Archbold JK, Tynan FE, Beddoe T, Kjer-Nielsen L, Miles JJ, Khanna R, Moss DJ, Liu YC, Gras S, Kostenko L, Brennan RM, Clements CS, Brooks AG, Purcell AW, McCluskey J, Rossjohn J. Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proceedings of the National Academy of Sciences. 2010;107:10608–10613. doi: 10.1073/pnas.1004926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madura F, Rizkallah PJ, Miles KM, Holland CJ, Bulek AM, Fuller A, Schauenburg AJA, Miles JJ, Liddy N, Sami M, Li Y, Hossain M, Baker BM, Jakobsen BK, Sewell AK, Cole DK. T-cell Receptor Specificity Maintained by Altered Thermodynamics. Journal of Biological Chemistry. 2013;288:18766–18775. doi: 10.1074/jbc.M113.464560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole DK, Yuan F, Rizkallah PJ, Miles JJ, Gostick E, Price DA, Gao GF, Jakobsen BK, Sewell AK. Germline-governed recognition of a cancer epitope by an immunodominant human T-cell receptor. Journal of Biological Chemistry. 2009;284:27281–27289. doi: 10.1074/jbc.M109.022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Singh NK, Spear TT, Hellman LM, Piepenbrink KH, McMahan RH, Rosen HR, Vander Kooi CW, Nishimura MI, Baker BM. How an alloreactive T-cell receptor achieves peptide and MHC specificity. Proceedings of the National Academy of Sciences. 2017;114:E4792–E4801. doi: 10.1073/pnas.1700459114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng L, Langley RJ, Wang Q, Topalian SL, Mariuzza RA. Structural insights into the editing of germ-line–encoded interactions between T-cell receptor and MHC class II by Vα CDR3. Proceedings of the National Academy of Sciences. 2012;109:14960–14965. doi: 10.1073/pnas.1207186109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stadinski BD, Trenh P, Duke B, Huseby PG, Li G, Stern LJ, Huseby ES. Effect of CDR3 Sequences and Distal V Gene Residues in Regulating TCR–MHC Contacts and Ligand Specificity. The Journal of Immunology. 2014;192:6071–6082. doi: 10.4049/jimmunol.1303209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris Daniel T, Singh Nishant K, Cai Q, Smith Sheena N, Vander Kooi Craig W, Procko E, Kranz David M, Baker Brian M. An Engineered Switch in T Cell Receptor Specificity Leads to an Unusual but Functional Binding Geometry. Structure. 2016;24:1142–1154. doi: 10.1016/j.str.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SN, Wang Y, Baylon JL, Singh NK, Baker BM, Tajkhorshid E, Kranz DM. Changing the peptide specificity of a human T-cell receptor by directed evolution. Nat Commun. 2014:5. doi: 10.1038/ncomms6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris DT, Wang N, Riley TP, Anderson SD, Singh NK, Procko E, Baker BM, Kranz DM. Deep Mutational Scans as a Guide to Engineering High Affinity T Cell Receptor Interactions with Peptide-bound Major Histocompatibility Complex. Journal of Biological Chemistry. 2016;291:24566–24578. doi: 10.1074/jbc.M116.748681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunology Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 42.Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol. 2012;12:669–677. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, Dolton G, Clement M, Llewellyn-Lacey S, Price DA, Peakman M, Sewell AK. A Single Autoimmune T Cell Receptor Recognizes More Than a Million Different Peptides. Journal of Biological Chemistry. 2012;287:1168–1177. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, Baker BM, Mandoiu II, Srivastava PK. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. The Journal of Experimental Medicine. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borbulevych OY, Insaidoo FK, Baxter TK, Powell DJ, Jr, Johnson LA, Restifo NP, Baker BM. Structures of MART-1(26/27–35) Peptide/HLA-A2 Complexes Reveal a Remarkable Disconnect between Antigen Structural Homology and T Cell Recognition. J Mol Biol. 2007;372:1123–1136. doi: 10.1016/j.jmb.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. Increased Immunogenicity of an Anchor-Modified Tumor-Associated Antigen Is Due to the Enhanced Stability of the Peptide/MHC Complex: Implications for Vaccine Design. J Immunol. 2005;174:4812–4820. doi: 10.4049/jimmunol.174.8.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams JJ, Narayanan S, Birnbaum ME, Sidhu SS, Blevins SJ, Gee MH, Sibener LV, Baker BM, Kranz DM, Garcia KC. Structural interplay between germline interactions and adaptive recognition determines the bandwidth of TCR-peptide-MHC cross-reactivity. Nat Immunol. 2016;17:87–94. doi: 10.1038/ni.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harkiolaki M, Holmes SL, Svendsen P, Gregersen JW, Jensen LT, McMahon R, Friese MA, van Boxel G, Etzensperger R, Tzartos JS, Kranc K, Sainsbury S, Harlos K, Mellins ED, Palace J, Esiri MM, van der Merwe PA, Jones EY, Fugger L. T cell-mediated autoimmune disease due to low-affinity crossreactivity to common microbial peptides. Immunity. 2009;30:348–357. doi: 10.1016/j.immuni.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Gras S, Saulquin X, Reiser JB, Debeaupuis E, Echasserieau K, Kissenpfennig A, Legoux F, Chouquet A, Le Gorrec M, Machillot P, Neveu B, Thielens N, Malissen B, Bonneville M, Housset D. Structural Bases for the Affinity-Driven Selection of a Public TCR against a Dominant Human Cytomegalovirus Epitope. J Immunol. 2009;183:430–437. doi: 10.4049/jimmunol.0900556. [DOI] [PubMed] [Google Scholar]
- 50.Ishizuka J, Stewart-Jones GBE, van der Merwe A, Bell JI, McMichael AJ, Jones EY. The Structural Dynamics and Energetics of an Immunodominant T Cell Receptor Are Programmed by Its V[beta] Domain. Immunity. 2008;28:171–182. doi: 10.1016/j.immuni.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 51.Gagnon SJ, Borbulevych OY, Davis-Harrison RL, Baxter TK, Clemens JR, Armstrong KM, Turner RV, Damirjian M, Biddison WE, Baker BM. Unraveling a Hotspot for TCR Recognition on HLA-A2: Evidence Against the Existence of Peptide-independent TCR Binding Determinants. Journal of Molecular Biology. 2005;353:556. doi: 10.1016/j.jmb.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Moza B, Buonpane RA, Zhu P, Herfst CA, Rahman AK, McCormick JK, Kranz DM, Sundberg EJ. Long-range cooperative binding effects in a T cell receptor variable domain. Proc Natl Acad Sci U S A. 2006;103:9867–9872. doi: 10.1073/pnas.0600220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borg NA, Ely LK, Beddoe T, Macdonald WA, Reid HH, Clements CS, Purcell AW, Kjer-Nielsen L, Miles JJ, Burrows SR, McCluskey J, Rossjohn J. The CDR3 regions of an immunodominant T cell receptor dictate the ‘energetic landscape’ of peptide-MHC recognition. Nat Immunol. 2005;6:171–180. doi: 10.1038/ni1155. [DOI] [PubMed] [Google Scholar]
- 54.Degano M, Garcia KC, Apostolopoulos V, Rudolph MG, Teyton L, Wilson IA. A functional hot spot for antigen recognition in a superagonist TCR/MHC complex. Immunity. 2000;12:251–261. doi: 10.1016/s1074-7613(00)80178-8. [DOI] [PubMed] [Google Scholar]
- 55.Cole DK, Bulek AM, Dolton G, Schauenberg AJ, Szomolay B, Rittase W, Trimby A, Jothikumar P, Fuller A, Skowera A, Rossjohn J, Zhu C, Miles JJ, Peakman M, Wooldridge L, Rizkallah PJ, Sewell AK. Hotspot autoimmune T cell receptor binding underlies pathogen and insulin peptide cross-reactivity. The Journal of Clinical Investigation. 2016;126:2191–2204. doi: 10.1172/JCI85679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee PUY, Churchill HRO, Daniels M, Jameson SC, Kranz DM. Role of 2C T Cell Receptor Residues in the Binding of Self- and Allo-Major Histocompatibility Complexes. J Exp Med. 2000;191:1355–1364. doi: 10.1084/jem.191.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keskin O, Ma BY, Nussinov R. Hot regions in protein-protein interactions: The organization and contribution of structurally conserved hot spot residues. Journal of Molecular Biology. 2005;345:1281–1294. doi: 10.1016/j.jmb.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 58.Borrman T, Cimons J, Cosiano M, Purcaro M, Pierce BG, Baker BM, Weng Z. ATLAS: A database linking binding affinities with structures for wild-type and mutant TCR-pMHC complexes. Proteins: Structure, Function, and Bioinformatics. 2017;85:908–916. doi: 10.1002/prot.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeLano WL. Unraveling hot spots in binding interfaces: progress and challenges. Curr Opin Struct Biol. 2002;12:14–20. doi: 10.1016/s0959-440x(02)00283-x. [DOI] [PubMed] [Google Scholar]
- 60.Borbulevych OY, Santhanagopolan SM, Hossain M, Baker BM. TCRs Used in Cancer Gene Therapy Cross-React with MART-1/Melan-A Tumor Antigens via Distinct Mechanisms. J Immunol. 2011;187:2453–2463. doi: 10.4049/jimmunol.1101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song I, Gil A, Mishra R, Ghersi D, Selin LK, Stern LJ. Broad TCR repertoire and diverse structural solutions for recognition of an immunodominant CD8+ T cell epitope. Nat Struct Mol Biol. 2017;24:395–406. doi: 10.1038/nsmb.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G, Yang X, Ko A, Sun X, Gao M, Zhang Y, Shi A, Mariuzza RA, Weng N-p. Sequence and Structural Analyses Reveal Distinct and Highly Diverse Human CD8+ TCR Repertoires to Immunodominant Viral Antigens. Cell Reports. 2017;19:569–583. doi: 10.1016/j.celrep.2017.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X, Gao M, Chen G, Pierce BG, Lu J, Weng N-p, Mariuzza RA. tructural Basis for Clonal Diversity of the Public T Cell Response to a Dominant Human Cytomegalovirus Epitope. Journal of Biological Chemistry. 2015 doi: 10.1074/jbc.M115.691311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newell EW, Ely LK, Kruse AC, Reay PA, Rodriguez SN, Lin AE, Kuhns MS, Garcia KC, Davis MM. Structural Basis of Specificity and Cross-Reactivity in T Cell Receptors Specific for Cytochrome c-I-E. Journal of immunology. 2011;186:5823–5832. doi: 10.4049/jimmunol.1100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayres CM, Scott DR, Corcelli SA, Baker BM. Differential utilization of binding loop flexibility in T cell receptor ligand selection and cross-reactivity. Scientific Reports. 2016;6:25070. doi: 10.1038/srep25070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones LL, Colf LA, Stone JD, Garcia KC, Kranz DM. Distinct CDR3 Conformations in TCRs Determine the Level of Cross-Reactivity for Diverse Antigens, but Not the Docking Orientation. J Immunol. 2008;181:6255–6264. doi: 10.4049/jimmunol.181.9.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia KC, Degano M, Pease LR, Huang M, Peterson PA, Teyton L, Wilson IA. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 68.Calis JJA, Maybeno M, Greenbaum JA, Weiskopf D, De Silva AD, Sette A, Keşmir C, Peters B. Properties of MHC Class I Presented Peptides That Enhance Immunogenicity. PLOS Computational Biology. 2013;9:e1003266. doi: 10.1371/journal.pcbi.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bremel RD, Homan EJ. Recognition of Higher Order Patterns in Proteins: Immunologic Kernels. PLOS ONE. 2013;8:e70115. doi: 10.1371/journal.pone.0070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassan C, Chabrol E, Jahn L, Kester MGD, de Ru AH, Drijfhout JW, Rossjohn J, Falkenburg JHF, Heemskerk MHM, Gras S, van Veelen PA. Naturally Processed Non-canonical HLA-A*02:01 Presented Peptides. Journal of Biological Chemistry. 2015;290:2593–2603. doi: 10.1074/jbc.M114.607028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wucherpfennig KW, Allen PM, Celada F, Cohen IR, De Boer R, Garcia KC, Goldstein B, Greenspan R, Hafler D, Hodgkin P, Huseby ES, Krakauer DC, Nemazee D, Perelson AS, Pinilla C, Strong RK, Sercarz EE. Polyspecificity of T cell and B cell receptor recognition. Seminars in Immunology. 2007;19:216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a Titin-Derived HLA-A1–Presented Peptide as a Cross-Reactive Target for Engineered MAGE A3–Directed T Cells. Science Translational Medicine. 2013;5:197ra103–197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raman MCC, Rizkallah PJ, Simmons R, Donnellan Z, Dukes J, Bossi G, Le Provost GS, Todorov P, Baston E, Hickman E, Mahon T, Hassan N, Vuidepot A, Sami M, Cole DK, Jakobsen BK. Direct molecular mimicry enables off-target cardiovascular toxicity by an enhanced affinity TCR designed for cancer immunotherapy. Scientific Reports. 2016;6:18851. doi: 10.1038/srep18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schueler-Furman O, Elber R, Margalit H. Knowledge-based structure prediction of MHC class I bound peptides: a study of 23 complexes. Folding and Design. 1998;3:549–564. doi: 10.1016/S1359-0278(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 76.Tong JC, Tan TW, Ranganathan S. Modeling the structure of bound peptide ligands to major histocompatibility complex. Protein Science : A Publication of the Protein Society. 2004;13:2523–2532. doi: 10.1110/ps.04631204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belden OS, Baker SC, Baker BM. Citizens unite for computational immunology! Trends in Immunology. 36:385–387. doi: 10.1016/j.it.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birnbaum Michael E, Mendoza Juan L, Sethi Dhruv K, Dong S, Glanville J, Dobbins J, Özkan E, Davis Mark M, Wucherpfennig Kai W, Garcia KC. Deconstructing the Peptide-MHC Specificity of T Cell Recognition. Cell. 2014;157:1073–1087. doi: 10.1016/j.cell.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tenzer S, Peters B, Bulik S, Schoor O, Lemmel C, Schatz MM, Kloetzel PM, Rammensee HG, Schild H, Holzhutter HG. Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cell Mol Life Sci. 2005:62. doi: 10.1007/s00018-005-4528-2. [DOI] [PubMed] [Google Scholar]
- 80.Peters B, Bulik S, Tampe R, van Endert PM, Holzhütter HG. Identifying MHC Class I Epitopes by Predicting the TAP Transport Efficiency of Epitope Precursors. The Journal of Immunology. 2003;171:1741–1749. doi: 10.4049/jimmunol.171.4.1741. [DOI] [PubMed] [Google Scholar]
- 81.Lundegaard C, Lund O, Buus S, Nielsen M. Major histocompatibility complex class I binding predictions as a tool in epitope discovery. Immunology. 2010;130:309–318. doi: 10.1111/j.1365-2567.2010.03300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang P, Sidney J, Dow C, Mothé B, Sette A, Peters B. A Systematic Assessment of MHC Class II Peptide Binding Predictions and Evaluation of a Consensus Approach. PLOS Computational Biology. 2008;4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stranzl T, Larsen MV, Lundegaard C, Nielsen M. NetCTLpan: pan-specific MHC class I pathway epitope predictions. Immunogenetics. 2010;62:357–368. doi: 10.1007/s00251-010-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- 85.Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 86.Macdonald WA, Chen Z, Gras S, Archbold JK, Tynan FE, Clements CS, Bharadwaj M, Kjer-Nielsen L, Saunders PM, Wilce MCJ, Crawford F, Stadinsky B, Jackson D, Brooks AG, Purcell AW, Kappler JW, Burrows SR, Rossjohn J, McCluskey J. T Cell Allorecognition via Molecular Mimicry. Immunity. 2009;31:897–908. doi: 10.1016/j.immuni.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 87.Rosen HR, Hinrichs DJ, Leistikow RL, Callender G, Wertheimer AM, Nishimura MI, Lewinsohn DM. Cutting edge: identification of hepatitis C virus-specific CD8+ T cells restricted by donor HLA alleles following liver transplantation. J Immunol. 2004;173:5355–5359. doi: 10.4049/jimmunol.173.9.5355. [DOI] [PubMed] [Google Scholar]
- 88.Fleri W, Paul S, Dhanda SK, Mahajan S, Xu X, Peters B, Sette A. The Immune Epitope Database and Analysis Resource in Epitope Discovery and Synthetic Vaccine Design. Frontiers in Immunology. 2017:8. doi: 10.3389/fimmu.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chowell D, Krishna S, Becker PD, Cocita C, Shu J, Tan X, Greenberg PD, Klavinskis LS, Blattman JN, Anderson KS. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proceedings of the National Academy of Sciences. 2015;112:E1754–E1762. doi: 10.1073/pnas.1500973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dill KA. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 91.Bosshard HR, Marti DN, Jelesarov I. Protein stabilization by salt bridges: concepts, experimental approaches and clarification of some misunderstandings. J Mol Recognit. 2004;17:1–16. doi: 10.1002/jmr.657. [DOI] [PubMed] [Google Scholar]
- 92.Sheinerman FB, Honig B. On the role of electrostatic interactions in the design of protein-protein interfaces. J Mol Biol. 2002;318:161–177. doi: 10.1016/S0022-2836(02)00030-X. [DOI] [PubMed] [Google Scholar]
- 93.Karanicolas J, Kuhlman B. Computational Design of Affinity and Specificity at Protein-Protein Interfaces. Current opinion in structural biology. 2009;19:458–463. doi: 10.1016/j.sbi.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.lee L-P, Tidor B. Optimization of binding electrostatics: Charge complementarity in the barnase-barstar protein complex. Protein Sci. 2001;10:362–377. doi: 10.1110/ps.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blevins SJ, Pierce BG, Singh NK, Riley TP, Wang Y, Spear TT, Nishimura MI, Weng Z, Baker BM. How structural adaptability exists alongside HLA-A2 bias in the human αβ TCR repertoire. Proceedings of the National Academy of Sciences. 2016;113:E1276–E1285. doi: 10.1073/pnas.1522069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tanford C. The hydrophobic effect and the organization of living matter. Science. 1978;200:1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- 97.Nicholls A, Sharp KA, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 98.Tanford C. Interfacial free energy and the hydrophobic effect. Proceedings of the National Academy of Sciences. 1979;76:4175–4176. doi: 10.1073/pnas.76.9.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eriksson AE, Baase WA, Zhang XJ, Heinz DW, Blaber M, Baldwin EP, Matthews BW. Response of a Protein-Structure to Cavity-Creating Mutations and Its Relation to the Hydrophobic Effect. Science. 1992;255:178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- 100.Ding YH, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- 101.Yin L, Crawford F, Marrack P, Kappler JW, Dai S. T-cell receptor (TCR) interaction with peptides that mimic nickel offers insight into nickel contact allergy. Proceedings of the National Academy of Sciences. 2012;109:18517–18522. doi: 10.1073/pnas.1215928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gras S, Burrows SR, Kjer-Nielsen L, Clements CS, Liu YC, Sullivan LC, Bell MJ, Brooks AG, Purcell AW, McCluskey J, Rossjohn J. The Shaping of T Cell Receptor Recognition by Self-Tolerance. Immunity. 2009;30:193–203. doi: 10.1016/j.immuni.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Gras S, Wilmann PG, Chen Z, Halim H, Liu YC, Kjer-Nielsen L, Purcell AW, Burrows SR, McCluskey J, Rossjohn J. A Structural Basis for Varied αβ TCR Usage against an Immunodominant EBV Antigen Restricted to a HLA-B8 Molecule. The Journal of Immunology. 2012;188:311–321. doi: 10.4049/jimmunol.1102686. [DOI] [PubMed] [Google Scholar]
- 104.Ding YH, Smith KJ, Garboczi DN, Utz U, Biddison WE, Wiley DC. Two human T cell receptors bind in a similar diagonal mode to the HLA-A2/Tax peptide complex using different TCR amino acids. Immunity. 1998;8:403–411. doi: 10.1016/s1074-7613(00)80546-4. [DOI] [PubMed] [Google Scholar]
- 105.Tynan FE, Burrows SR, Buckle AM, Clements CS, Borg NA, Miles JJ, Beddoe T, Whisstock JC, Wilce MC, Silins SL, Burrows JM, Kjer-Nielsen L, Kostenko L, Purcell AW, McCluskey J, Rossjohn J. T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat Immunol. 2005;6:1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 106.Liu YC, Miles JJ, Neller MA, Gostick E, Price DA, Purcell AW, McCluskey J, Burrows SR, Rossjohn J, Gras S. Highly Divergent T-cell Receptor Binding Modes Underlie Specific Recognition of a Bulged Viral Peptide bound to a Human Leukocyte Antigen Class I Molecule. Journal of Biological Chemistry. 2013;288:15442–15454. doi: 10.1074/jbc.M112.447185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a Single T Cell Receptor Recognizes Both Self and Foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 108.Shimizu A, Kawana-Tachikawa A, Yamagata A, Han C, Zhu D, Sato Y, Nakamura H, Koibuchi T, Carlson J, Martin E, Brumme CJ, Shi Y, Gao GF, Brumme ZL, Fukai S, Iwamoto A. Structure of TCR and antigen complexes at an immunodominant CTL epitope in HIV-1 infection. Scientific Reports. 2013;3:3097. doi: 10.1038/srep03097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petersen J, Montserrat V, Mujico JR, Loh KL, Beringer DX, van Lummel M, Thompson A, Mearin ML, Schweizer J, Kooy-Winkelaar Y, van Bergen J, Drijfhout JW, Kan W-T, La Gruta NL, Anderson RP, Reid HH, Koning F, Rossjohn J. T-cell receptor recognition of HLA-DQ2–gliadin complexes associated with celiac disease. Nat Struct Mol Biol. 2014;21:480–488. doi: 10.1038/nsmb.2817. [DOI] [PubMed] [Google Scholar]
- 110.Petersen J, Kooy-Winkelaar Y, Loh Khai L, Tran M, van Bergen J, Koning F, Rossjohn J, Reid Hugh H. Diverse T Cell Receptor Gene Usage in HLA-DQ8-Associated Celiac Disease Converges into a Consensus Binding Solution. Structure. 2016;24:1643–1657. doi: 10.1016/j.str.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells Spotlight the Germline Rules for [alpha][beta] T Cell-Receptor Interactions with MHC Molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deng L, Langley RJ, Brown PH, Xu G, Teng L, Wang Q, Gonzales MI, Callender GG, Nishimura MI, Topalian SL, Mariuzza RA. Structural basis for the recognition of mutant self by a tumor-specific, MHC class II-restricted T cell receptor. Nat Immunol. 2007;8:398–408. doi: 10.1038/ni1447. [DOI] [PubMed] [Google Scholar]