Abstract
Objectives
Many patients with small bowel neuroendocrine tumors (SBNETs) have multifocal tumors (MFTs), but the frequency of MFTs has varied widely across SBNET studies. It is also unclear whether patients with MFTs have more advanced disease or worse clinical course than those with unifocal SBNETs (UFTs). We set out to determine the frequency of multifocal and unifocal SBNETs, compare clinicopathologic factors, somatostatin receptor (SSTR2) expression, and survival.
Methods
Clinicopathologic variables from 179 patients with surgically managed SBNETs were collected. Statistical comparisons were made using Welch’s t-test, Wilcoxon test and Fisher’s exact test. Survival was assessed using the Kaplan-Meier method. SSTR2 expression was analyzed by qPC, and Ki-67 expression by immunohistochemistry.
Results
MFTs were found in 45% of patients with SBNETs. Clinicopathologic factors such as grade, TNM stage, presence of distant metastases, mean SSTR2 expression, success of imaging modalities, preoperative and postoperative hormone levels were not significantly different between multifocal and unifocal groups. Progression-free and overall survival were also not significantly affected by multifocality.
Conclusions
Clinicopathologic features and survival of patients with MFTs and UFTs are remarkably similar. Although the etiology of MFTs is unclear, patients with MFTs do not have a more aggressive clinical course than patients with unifocal SBNETs.
Keywords: Carcinoid, small bowel neuroendocrine tumors, multifocality, SSTR2, Ki-67, survival
INTRODUCTION
Small bowel neuroendocrine tumors (SBNETs) are generally small, indolent neoplasms most commonly found in the jejunum and ileum.1 Their incidence has increased greatly since the 1970s and they are now the most common small bowel malignancy in the United States.2 They arise from enterochromaffin cells of the small intestine, which comprise approximately 1% of the mucosal cells.3 Due to their relative rarity and nonspecific clinical symptoms, the average length of time between symptom onset and diagnosis is 9.2 years, and patients are often diagnosed after metastasis to the liver.4 Despite metastatic disease, patients with SBNETs can still have long-term survival.5,6
The clinical presentation of patients with SBNETs may include signs or symptoms of carcinoid syndrome, bowel obstruction, GI bleeding or non-specific abdominal pain. Unfortunately, few SBNETs are detected when they are at an early stage and potentially curable by resection, and in these cases, tumors are often discovered incidentally while the patient is being evaluated for something else.7 Primary SBNETs may be small, yet still present with bulky mesenteric nodal disease and extensive liver metastases. Moertel et al. observed that <2% of patients with SBNETs <1 cm had metastases, which increased to 50% for those with 1–2 cm tumors, and 80% for those >2 cm.8 Soga et al reported an even higher rate of metastasis in GI carcinoids <1 cm in size of 30%.9
On careful exploration and palpation of the small bowel, many patients with SBNETs are found to have more than one tumor, although how this influences metastasis and prognosis in patients is unclear. The frequency of multifocal SBNETs has been reported to range between 13–45%.8,10–13 The high variance in reported rates of multifocality may be due to differences in patient populations, and whether this was determined intraoperatively or at autopsy.8 The etiology of multiple tumors remains unsettled, with some authors suggesting that these arise from dissemination through submucosal lymphatics,14 and others by the development of oligoclonal tumors through field cancerization.15 Large retrospective studies in breast and lung cancer have shown that tumor multifocality is associated with poorer prognosis,16,17 and smaller studies in SBNETs have also suggested that multifocality may be associated with increased tumor aggressiveness.12,13 We set out to ascertain the rate of multifocal tumors in patients with SBNETs at a single center, and determine whether patients with multifocal tumors (MFTs) had worse outcomes than patients with solitary SBNETs.
MATERIALS AND METHODS
Patients with SBNETs who had surgical management of their primary and/or metastatic tumors at the University of Iowa between 1999 and 2016 were enrolled into an IRB approved Neuroendocrine Tumor Registry. Clinical information, including operative notes, radiology, pathology, and laboratory testing were retrieved from the electronic medical record. Demographic information, date of diagnosis, date of first NET surgery, last follow-up, and death were recorded. Clinicopathologic information, including primary tumor size, liver metastases, lymph node metastases, other metastases, Tumor Node Metastasis (TNM) staging, grade, and number of tumors were collected. Intraoperatively, careful palpation of the small bowel from the ligament of Treitz to the ileocecal valve was carried out, and the location and number of tumors recorded. Patients were excluded if information was missing regarding inspecting the entire small bowel. If a patient’s tumors were multifocal, the largest tumor size was recorded as the primary tumor size. Preoperative and postoperative symptoms such as flushing, diarrhea, abdominal pain, and bronchial symptoms were also documented, as well as preoperative and postoperative biochemical marker levels for serotonin, chromogranin A, and pancreastatin.
Ki-67 Expression and Grading
The majority of tumors were stained for Ki-67 using the MIB-1 antibody, although this was not routinely performed for tumors resected prior to 2010. Areas of high tumor density were identified on the corresponding H&E stained slide, and within these areas, a 400× field containing at least 500 tumor cells with as many Ki-67 positive cells as possible were selected. For samples with fewer tumor cells, the threshold was lowered to 350 cells. All Ki-67 positive and negative tumor cells were counted. Any cells with MIB-1 activity were counted as positive and cells without staining were considered negative for Ki-67 expression. The Ki-67 index was calculated by dividing the total number of positive cells by the sum of the numbers of positive and negative cells. All samples were graded using the highest Ki-67 index with a modified WHO classification system (G1 Ki-67=0–2%; G2 >2–20%; G3 >20%).18,19 When Ki-67 had not been performed, the grade assigned by the Pathologist using morphology or mitotic index was recorded.
Somatostatin Receptor 2 (SSTR2) Expression
SSTR2 expression was measured by quantitative (qPCR). Total RNA was isolated from RNAlater-preserved tumor tissue using the Trizol method (Life Technologies, Carlsbad, Calif) and reverse transcribed to cDNA. Each sample was amplified in triplicate using Taqman reagents (Life Technologies) on StepOne-Plus and 7900 HT-Fast RT-PCR platforms (Life Technologies). GAPDH and POLR2A served as internal controls and the mean threshold cycle (Ct) of these genes were used to calculate normalized values for the mean SSTR2 threshold cycle (dCt).
Data Analysis
Data were exported to the software program R (v3.3.1; Vienna, Austria) for analysis. Overall survival and progression-free survival were determined using the Kaplan Meier method. Clinicopathologic factors were compared between groups using Welch’s t-test, Wilcoxon test, and Fisher’s exact test.
RESULTS
Clinical Characteristics of Patients With Multifocal and Unifocal Tumors
There were 179 patients who underwent surgical exploration for primary and/or metastatic SBNETs at our institution over this period where details from full exploration of the small bowel were available. Of these, 81 (45.3%) had MFTs, and 98 (54.7%) patients had unifocal tumors (UFTs; Table 1). The number of primary tumors found during surgical exploration ranged from 2–129 tumors in those with MFTs. Forty-three patients had 2–5 tumors, twenty had 6–10 tumors, eleven had 11–20 tumors, and seven had greater than 20 tumors (21, 28, 29, 38, 42, 47, and 129 tumors, respectively). The median number of multifocal primary tumors was five and the mean was 9.5 tumors. Overall, 57.0% of SBNET patients were male, with the multifocal group being slightly more male-predominant (63.0% vs 52.0% for UFTs), but this difference was not significant (P = 0.17). Patients with MFTs were diagnosed between ages 30.0–83.3 years with a median age of diagnosis of 58.7 years, whereas the age of diagnosis for UFTs ranged from 15.4–85.2 years with a median of 59.4 years (P = 0.69).
TABLE 1.
Clinical Characteristics of Patients With Multifocal and Unifocal Tumors
| MFT (n = 81) | UFT (n = 98) | P | |
|---|---|---|---|
| Sex, male, n (%) | 51 (63.0) | 51 (52.0) | 0.17 |
| Age at diagnosis, median (range), y | 58.7 (30.0–83.3) | 59.4 (15.4–85.2) | 0.69 |
| Diagnosed before age 60, n (%) | 44 (53.0) | 52 (53.1) | 1.00 |
MFT indicates multifocal tumors; UFT, unifocal tumors.
Survival
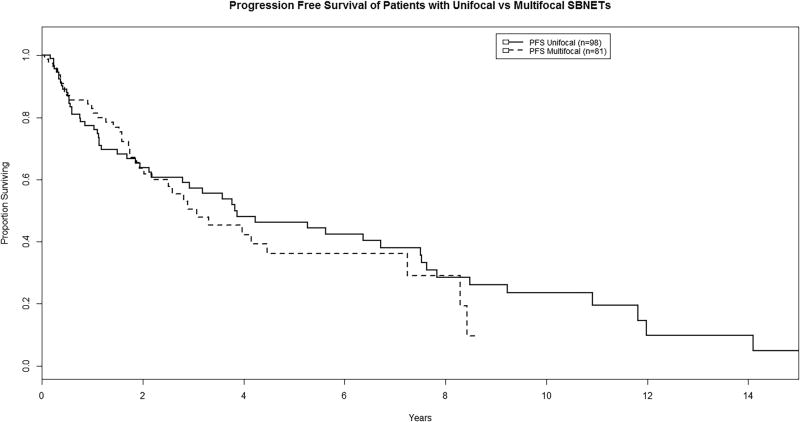
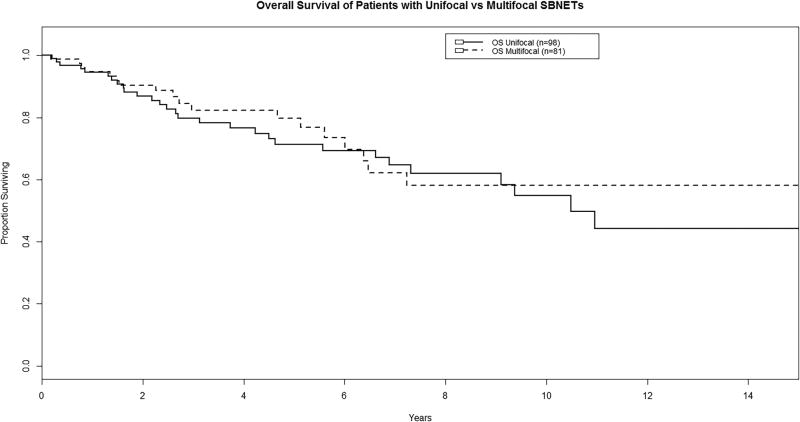
Median progression-free survival (PFS) was 3.07 years for patients with MFTs and 3.83 years for UFTs, and there was no significant difference between groups (P = 0.57; Figure 1). Median overall survival (OS) was 10.5 years for patients with UFTs, and was not reached for those with MFTs. At five and ten years, OS was very similar for those with MFTs as UFTs (P = 0.63; Figure 2).
FIGURE 1.
Progression-free survival of patients with multifocal vs. unifocal tumors (P = 0.57).
FIGURE 2.
Overall survival of patients with multifocal vs. unifocal tumors (P = 0.63).
Tumor Pathology
Median primary tumor size was not significantly different between MFTs and UFTs (1.7 cm and 2.0 cm respectively, P = 0.09; Table 2). There were also no significant differences in TNM staging. T3/T4 tumors were found in 79.7% of MFT patients and in 78.1% with UFTs (P = 0.85). Nodal metastases were seen in 91.8% of patients with MFTs and 89.1% with UFTs (P = 0.79). Distant metastases were seen in 78.8% of patients with MFTs and 79.8% of those with UFTs (P = 1.00), and hepatic metastases were also equally likely in patients with MFTs vs. UFTs (69.1% vs. 75.5%, P = 0.40). Other distant, non-hepatic, non-nodal metastases were less common, and included lung, peritoneum, ovary, and bone. There was no significant difference in the frequency of these types of metastases for those with MFTs and UFTs (43.2% vs. 49.0%, P = 0.46). In terms of tumor grade, both groups were nearly identical (Table 3). Each group contained one patient with a grade 3 primary tumor. The remainder were grade 1 or 2, and there was no significant difference in the incidence of grade 1 (P = 0.42) or grade 2 tumors (P = 0.51) between both groups. Ki-67 expression was used to determine WHO grade in about half of MFT and UFT patients, and mean Ki-67 expression was found to be similar for both groups (P = 0.46). Finally, the level of SSTR2 expression as determined by qPCR was not significantly different in MFTs vs. UFTs (P = 0.59).
TABLE 2.
Tumor Pathology, Tumor Size, and TNM Staging
| MFT (n = 81) |
UFT (n = 98) |
P | |
|---|---|---|---|
| Median primary tumor size | 1.7 cm | 2.0 cm | 0.09 |
| Tumor stage, n (%) | |||
| T1 | 2 (2.5) | 4 (4.1) | 0.69 |
| T2 | 14 (17.7) | 17 (17.7) | 1.00 |
| T3 | 34 (43.0) | 42 (43.8) | 1.00 |
| T4 | 29 (36.7) | 33 (34.4) | 0.75 |
| Total | 79 (100) | 96 (100) | |
| Nodal stage, n (%) | |||
| N0 | 6 (8.2) | 10 (10.9) | 0.79 |
| N1 | 67 (91.8) | 82 (89.1) | 0.79 |
| Total | 73 (100) | 92 (100) | |
| Metastasis stage, n (%) | |||
| M0 | 17 (21.2) | 19 (20.2) | 1.00 |
| M1 | 63 (78.8) | 75 (79.8) | 1.00 |
| Total | 80 (100) | 94 (100) | |
| Distant metastases (non-liver, non-nodal), n (%) | 35 (43.2) | 48 (49.0) | 0.46 |
| Liver metastases, n (%) | 56 (69.1) | 74 (75.5) | 0.40 |
MFT indicates multifocal tumors; UFT, unifocal tumors.
TABLE 3.
Tumor Grading and Ki-67, SSTR2 Expression
| MFT (n = 81) | UFT (n = 98) | P | |
|---|---|---|---|
| Grade 1 tumors, n (%) | 46 (59.7) | 58 (65.9) | 0.42 |
| Grade 2 tumors, n (%) | 30 (39.0) | 30 (33.0) | 0.51 |
| Grade 3 tumors, n (%) | 1 (1.3) | 1 (1.1) | 0.51 |
| Total | 77 (100) | 89 (100) | |
| Number of patients with Ki-67 expression measurements, n (%) | 47 (58.0) | 42 (42.9) | 0.05 |
| Mean Ki-67 index | 2.5% | 2.0% | 0.46 |
| qPCR: SSTR2 expression | |||
| Number of patients with SSTR2 expression quantification, n (%) | 28 (34.6) | 29 (29.6) | 0.63 |
| Mean SSTR2 expression (dCt) | 3.10 | 2.84 | 0.59 |
MFT indicates multifocal tumors; UFT, unifocal tumors.
Tumor Functionality and Preoperative Lab Values
To determine if there were any differences in tumor functionality and tumor secretory product levels in patients with MFTs and UFTs, preoperative symptoms and biochemical markers were analyzed. There was no significant difference in the frequency of functional tumors (P = 0.77), defined as whether patients had symptoms of flushing, or diarrhea, and elevation of chromogranin A or pancreastatin above the normal range or two-fold elevation of serotonin (>400ng/ml, due to increased variability of this marker). There were also no significant differences in the rate of preoperative elevation of biochemical markers above the normal range (or twice normal for serotonin) in MFTs vs. UFTs. Pancreastatin was elevated in 75.3% of MFTs vs. 79.8% of UFTs (P = 0.56), chromogranin A in 64.3% of MFTs vs. 64.2% of UFTs (P = 1.00), and two-fold elevation of serotonin in 78.7% of MFTs vs. 72.7% of UFTs (P = 0.47; Table 4).
TABLE 4.
Tumor Functionality and Elevation of Preoperative Laboratory Values
| MFT (n = 81) | UFT (n = 98) | P | |
|---|---|---|---|
| Functional tumors, n (%) | 42 (51.9%) | 48 (49.0) | 0.77 |
| Two-fold elevation of serotonin, n (%) | 59/75 (78.7) | 64/88 (72.7) | 0.47 |
| Elevated chromogranin A*, n (%) | 45/70 (64.3) | 52/81 (64.2) | 1.00 |
| Elevated pancreastatin†, n (%) | 55/73 (75.3) | 67/84 (79.8) | 0.56 |
Reference ranges changed over time: before 2008 this was <375 ng/ml; between 2008–2011 it was 0–50 ng/ml; after 2012 it was <95 ng/ml
Pancreastatin reference range before 2007 was 10–95 pg/ml; after 2007 it was 10–135 pg/ml
MFT indicates multifocal tumors; UFT, unifocal tumors.
Preoperative Imaging of Multifocal and Unifocal Patients
Two imaging modalities were investigated to determine if there was any difference in primary tumor detection for MFTs and UFTs. Computerized tomography (CT) scan was the most commonly performed imaging modality, and 49/64 (76.6%) patients with MFTs had their tumor(s) localized (as defined by bowel wall thickening, mass, or mesenteric adenopathy20), as compared to 55/78 (70.5%) UFT patients (P = 0.45). Patients were equally likely to have their disease detected by somatostatin receptor scintigraphy (SRS) whether they had MFTs (27/53, 50.9%) or UFTs (29/58, 50.0%; P = 1.00). Endoscopy was less commonly performed and less useful for finding tumors than either CT or SRS. The primary tumor was visualized by endoscopy in 10/36 (27.8%) MFT patients and 15/33 (45.5%; P = 0.14) UFT patients (Table 5).
TABLE 5.
Preoperative Imaging of Patients With Multifocal and Unifocal SBNETs
| MFT (n = 81) | UFT (n = 98) | P | |
|---|---|---|---|
| CT detects primary, n (%) | 49/64 (76.6) | 55/78 (70.5) | 0.45 |
| Octreoscan detects primary, n (%) | 27/53 (50.9) | 29/58 (50.0) | 1.00 |
| Endoscopy detects primary, n (%) | 10/36 (27.8) | 15/33 (45.5) | 0.14 |
MFT indicates multifocal tumors; UFT, unifocal tumors.
CONCLUSIONS
In one of the earliest and largest studies of carcinoids of the small intestine, Moertel et al reported that 60 of their 207 (29%) patients with SBNETs diagnosed pathologically at surgery (72 patients) or autopsy (137 patients) at the Mayo Clinic had MFTs. The majority had 3 or more tumors, and some had dozens of SBNETs.8 Burke et al reported on the experience from the Armed Forces Institute of Pathology, where they found that 43 of 167 (29%) SBNET patients had MFTs, ranging from 2–100 tumors.16 In an autopsy series from Malmo, Sweden, the incidence of all carcinoid tumors in the population was found to be 1.22%, and 50 of 152 (33%) SBNET cases were multifocal.10 In a study pooling data from 3 Boston hospitals, Yantiss et al reported 18 of 68 (26%) patients had MFTs, the most numerous having 30 tumors.15 In Northern Ireland, Watson et al found a lower incidence of MFTs, which were seen in only 7 of 55 (12.7%) SBNET patients, which ranged from 3 to 10 in number.11 In the current study, we found a higher frequency of MFTs of 45.3% in 179 patients from a single institution, operated upon by a single surgeon. This higher rate of detection may be due to a more systematic intraoperative exploration for these tumors, but details on the conduct and consistency of surgical exploration or postmortem examination are limited in these previous studies. Given the high incidence of multifocality and effectiveness of surgical treatment, we believe that careful bimanual palpation of the small bowel during surgery is critical to detect these tumors so that all can be resected. We prefer open exploration with direct bimanual palpation over laparoscopic exploration, because tumors can easily be missed by the latter method when the bowel is palpated only using graspers. Laparoscopy may succeed in identifying larger tumors or those with obvious trans-serosal extension, but will miss small tumors, which may be only a few mm in size. However, a hybrid approach may be reasonable, with laparoscopic exploration but careful digital palpation of the entire jejunum and ileum through a small incision.
While the existence of multifocality has been well established in SBNETs,8,10–13 its effects on tumor biology has not been extensively studied. Burke et al found that having multiple SBNETs was negatively correlated with survival on univariate analysis, but that this was not an independent factor for survival on multivariate analysis.13 Yantiss et al evaluated numerous clinicopathologic factors and found that SBNET patients with MFTs were significantly younger, more commonly female, more likely to suffer from carcinoid syndrome, had more advanced tumor stage, and had shorter PFS and OS.12 The current study was much larger, and examined additional clinicopathologic factors, including SSTR2 and Ki-67 expression. We did not find any significant differences between these clinicopathologic factors in the groups with MFTs versus UFTs, and there was also no difference in PFS or OS. We conclude that the tumor characteristics and clinical course of SBNET patients with MFTs is not more aggressive than that seen in patients with UFTs.
In other cancers, however, tumor multifocality has been associated with more aggressive disease. Vera-Badillo et al compared 67,557 women from twenty-two studies and found that women with multifocal breast tumors have a greater probability of nodal metastasis and relapse, resulting in worse survival than women with unifocal tumors.16 Similarly, Arslan et al compared 168 patients with locally advanced non-small cell lung cancer and found that OS in patients with multifocal lung cancer was significantly shorter as compared to those with unifocal tumors.17 Despite the poorer prognoses for multifocal tumors from these other sites, current TNM staging for SBNETs does not change based upon multifocality. The European Neuroendocrine Tumor Society (ENETS) Tumor Node Metastasis (TNM) staging classification of midgut and hindgut NETs acknowledges multifocality by adding an “m” to the T stage (T1m, T2m, T3m, T4m). However, only the greatest dimension of the largest tumor is considered, and the “m” designation has no impact on the overall staging category.19 This seems appropriate given the lack of survival differences between patients with MFTs versus UFTs in this study.
Many patients with SBNETs are treated with somatostatin analogues, either by monthly injection5,21 or peptide receptor radionuclide therapy (PRRT).22 We found similar levels of expression of SSTR2 in the MFT and UFT groups, and therefore treatment with somatostatin analogues is likely equally useful in patients with MFTs and UFTs. SSTR2 is also an excellent target for imaging. This includes both somatostatin receptor scintigraphy (SRS) with 111Indium-labeled somatostatin analog and single photon emission computed tomography,23 and 68Gallium-labeled somatostatin analog with positron emission tomography.24,25 A study by Maxwell et al found that SBNET tumor size ≤2 cm led to significantly reduced tumor detection by SRS.23 In the current study, we found that half of the patients in both groups had primary tumors that were two centimeters or less in diameter, and overall, only 27/53 (50.9%) of the multifocal group and 29/58 (50.0%) of unifocal group had positive SRS.
The mechanisms responsible for the high frequency of multifocality in SBNET remain undefined. Unlike the multifocal pancreatic neuroendocrine tumors seen in patients with MEN1 or VHL, familial cases of SBNETs are rare. However, the National Institutes of Health has followed 33 families with multiple members having SBNETs, and the overall incidence of multifocality was 67% (36 of 54 patients, with 2–50 tumors). One large SBNET family was found to have a 4 base pair germline deletion in the inositol polyphosphate multikinase gene (IPMK), but mutations of IPMK were not found in their other families.26 Besides a familial predisposition due to an inherited mutation, another explanation for the origin of MFTs in SBNET patients is that tumor growth into lymphatic channels redirects flow and causes submucosal spread of tumor cells along the small bowel. Wang et al felt that this was the explanation for why they saw multiple tumors in patients with more extensive mesenteric nodal disease.14 Supporting the hypothesis of MFTs arising from a single primary, Guo et al. found evidence for monoclonality in separate tumors derived from four patients by the finding of non-random X chromosome inactivation.27 Another possibility for the origin of MFTs is that an exogenous growth factor influences specific stem cells to undergo malignant transformation into SBNETs. The development of gastric carcinoids are an example of this type of transformation, where the release of trophic factors (hypergastrinemia) induces the formation of multiple tumors.15 This could be one mechanism of field cancerization, where both genetic and epigenetic changes accumulate in the small bowel over time, due to environmental exposure and/or even genetic predisposition, resulting in multiple independent tumors. By studying loss of heterozygosity and X chromosome inactivation, Katona et al found evidence for the independent origin of multifocal tumors in 11 of 13 (85%) of midgut carcinoid patients.15 We conclude that the available evidence has not firmly established whether MFTs originate from a single tumor or from multiple, independent primary sites in patients with sporadic SBNETs, but the latter would be the most likely mechanism in cases of familial SBNETs.
In summary, multifocality of SBNETs was higher in this study than previously reported in other large series, being found in almost half of patients having careful surgical exploration. Given this high frequency, we prefer open surgery to allow for thorough bimanual palpation of the small bowel over laparoscopic methods, although the latter may be acceptable provided the entire small bowel is palpated through a small incision. SBNET multifocality was not associated with more aggressive tumor characteristics or reduced patient survival, and therefore multifocal and unifocal tumors can be treated in a similar fashion. Surgical resection remains the optimal treatment of SBNETs,28–34 but a more extensive resection may be required for MFTs than UFTs. Comparable rates of SSTR2 expression were found in MFTs and UFTs, suggesting that treatment with somatostatin analogues may be similarly effective. In conclusion, surgeons should have a high index of suspicion for the presence of MFTs at exploration in SBNET patients, and these tumors should be carefully sought out so that appropriate resection of all tumors is performed.
Acknowledgments
Financial Support
This work was supported by the Surgical Oncology grant T32 CA148062-01 (JEM, KJK), University of Iowa College of Medicine Summer Research fellowship (ABC), and Iowa NET SPORE 1 P50 CA174521-01 (AJB, TMO, JRH).
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Jensen RT. Endocrine Tumors of the Gastrointestinal Tract and Pancreas. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 19. New York, NY: McGraw-Hill; 2015. [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 3.Eroschenko VP. diFiore’s Atlas of Histology with Functional Correlations. 8. Media, PA: Williams & Wilkins; 1996. [Google Scholar]
- 4.Vinik AI, Silva MP, Woltering EA, et al. Biochemical Testing for Neuroendocrine Tumors. Pancreas. 2009;38:876–889. doi: 10.1097/MPA.0b013e3181bc0e77. [DOI] [PubMed] [Google Scholar]
- 5.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 6.Korse CM, Taal BG, van Velthuysen ML, et al. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer. 2013;49:1975–1983. doi: 10.1016/j.ejca.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Shebani KO, Souba WW, Finkelstein DM, et al. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815–821. doi: 10.1097/00000658-199906000-00008. discussion 822–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moertel CG, Sauer WG, Dockerty MB, et al. Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901–912. doi: 10.1002/1097-0142(196109/10)14:5<901::aid-cncr2820140502>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587–1595. doi: 10.1002/cncr.20939. [DOI] [PubMed] [Google Scholar]
- 10.Berge T, Linell F. Carcinoid tumours. Frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand A. 1976;84:322–330. [PubMed] [Google Scholar]
- 11.Watson RG, Johnston CF, O'Hare MM, et al. The frequency of gastrointestinal endocrine tumours in a well-defined population--Northern Ireland 1970–1985. Q J Med. 1989;72:647–657. [PubMed] [Google Scholar]
- 12.Yantiss RK, Odze RD, Farrave FA, et al. Solitary versus multiple carcinoid tumors of the ileum. Am J Surg Pathol. 2003;27:811–817. doi: 10.1097/00000478-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Burke AP, Thomas RM, Elsayed AM, et al. Carcinoids of the Jejunum and Ileum: An Immunohistochemical and Clinicopathologic Study of 167 Cases. Cancer. 1997;79:1086–1093. [PubMed] [Google Scholar]
- 14.Wang YZ, Joseph S, Lindholm E, et al. Lymphatic mapping helps to define resection margins for midgut carcinoids. Surgery. 2009;146:993–997. doi: 10.1016/j.surg.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Katona TM, Jones TD, Wang M, et al. Molecular Evidence for Independent Origin of Multifocal Neuroendocrine Tumors of the Enteropancreatic Axis. Cancer Res. 2006;66:4936–4942. doi: 10.1158/0008-5472.CAN-05-4184. [DOI] [PubMed] [Google Scholar]
- 16.Vera-Bradillo FE, Napoleone M, Ocana A, et al. Effect of multifocality and multicentricity on outcome in early stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;146:235–244. doi: 10.1007/s10549-014-3018-3. [DOI] [PubMed] [Google Scholar]
- 17.Arslan D, Tural D, Koca T, et al. Prognostic factors in clinical stage T4N2 locally advanced non-small cell lung cancer. J BUON. 2015;20:573–579. [PubMed] [Google Scholar]
- 18.Rindi G, Kloeppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–762. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 19.Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman TF, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4. Lyon, France: IARC Press; 2010. p. 13. [Google Scholar]
- 20.Keck KJ, Maxwell JE, Menda Y, et al. Identification of primary tumors in patients presenting with metastastic gastroenteropancreatic neuroendocrine tumors. Surgery. 2017;161:272–279. doi: 10.1016/j.surg.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. New Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 22.Vinjamuri S, Gilbert TM, Banks M, et al. Peptide receptor radionuclide therapy with 90Y-DOTATATE/90Y-DOTATOC in patients with progressive metastatic neuroendocrine tumours: assessment of response, survival and toxicity. Br J Cancer. 2013;108:1440–1448. doi: 10.1038/bjc.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxwell JE, Sherman SK, Menda Y, et al. Limitations of Somatostatin Scintigraphy in Primary Small Bowel NETs. J Surg Res. 2014;190:548–553. doi: 10.1016/j.jss.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68GA-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-Octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 26.Sei Y, Zhao X, Forbes J, et al. A hereditary form of small intestinal carcinoid associated with a germline mutation in inositol polyphosphate multikinase. Gastroenterology. 2015;149:67–78. doi: 10.1053/j.gastro.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Z, Li Q, Wilander E, Pontén J. Clonality analysis of multifocal carcinoid tumours of the small intestine by X-chromosome inactivation analysis. J Pathol. 2000;190:76–79. doi: 10.1002/(SICI)1096-9896(200001)190:1<76::AID-PATH499>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Strosberg J. Neuroendocrine tumours of the small intestine. Best Pract Res Clin Gastroenterol. 2012;26:755–773. doi: 10.1016/j.bpg.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140:891–897. doi: 10.1016/j.surg.2006.07.033. discussion 897–898. [DOI] [PubMed] [Google Scholar]
- 30.Huang LC, Poultsides GA, Norton JA. Surgical management of neuroendocrine tumors of the gastrointestinal tract. Oncology (Williston Park) 2011;25:794–803. [PubMed] [Google Scholar]
- 31.Knigge U, Hansen CP. Surgery for GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26:819–831. doi: 10.1016/j.bpg.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Zappa M, Abdel-Rehim M, Hentic O, et al. Liver-directed therapies in liver metastases from neuroendocrine tumors of the gastrointestinal tract. Target Oncol. 2012;7:107–116. doi: 10.1007/s11523-012-0219-8. [DOI] [PubMed] [Google Scholar]
- 33.Landry CS, Scoggins CR, McMasters KM, et al. Management of hepatic metastasis of gastrointestinal carcinoid tumors. J Surg Oncol. 2008;97:253–258. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 34.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1063. doi: 10.1016/j.surg.2003.07.025. discussion 1063–1065. [DOI] [PubMed] [Google Scholar]