Abstract
Background
Manganese (Mn) is an essential element required for growth and development, but higher body burdens have been associated with neurobehavioral decrements in children.
Objectives
We examined whether prenatal or postnatal Mn measured in deciduous teeth was associated with scores on a test of visuospatial learning and memory.
Methods
Deciduous teeth were collected from 142 participants (ages 10–14 years) residing near varied ferro-manganese industry in Italy. Mn concentrations were measured in prenatal and postnatal tooth regions by laser ablation inductively coupled plasma mass spectrometry (ICP-MS). The Virtual Radial Arm Maze (VRAM), an animal-human analogue task, was used to assess visuospatial learning and memory. We used generalized additive, linear and zero-inflated Poisson mixed regression models to estimate associations between prenatal or postnatal Mn concentrations and repeated measures of all four VRAM outcomes: time, distance, working and reference memory errors. Effect measure modification by sex was examined in stratified models.
Results
U-shaped associations between prenatal Mn and VRAM outcomes were observed among girls only (pGAMM =0.001 to 0.02 in stratified models). Compared to the mid-tertile of prenatal Mn, girls in the highest tertile took 7.7 seconds [95% CI: −6.1, 21.5] longer to complete the task, traveled 2.3 maze units [0.1, 4.4] farther, and committed more working and reference memory errors (β for count ratio=1.33 [1.01, 1.83]; 1.10 [0.98, 1.24], respectively). This association was not observed among boys. In contrast, for postnatal Mn, no significant associations were found, and patterns were similar for boys and girls.
Conclusions
The prenatal period may be a critical window for the impact of environmental Mn on visuospatial ability and executive function, especially for females.
Keywords: environmental epidemiology, manganese, children, neurobehavior, teeth
1. Introduction
Evidence for manganese (Mn) as a neurodevelopmental toxicant is mounting; yet our understanding of Mn susceptibility remains limited (Bellinger 2013). Pediatric epidemiologic studies on health effects of environmental Mn exposure have reported adverse associations with cognition, memory, behavior and motor function (Bouchard et al. 2011; Khan et al. 2011; Lucchini et al. 2012a; Mora et al. 2015; Rahman et al. 2016). However, results have varied across studies, partly due to differences in exposure timing, as specific neurodevelopmental periods may be uniquely sensitive to Mn exposure (Grandjean and Landrigan 2006). In particular, the prenatal and early postnatal periods may be unique windows of susceptibility for Mn overexposure (Sanders et al. 2015). During pregnancy, Mn absorption is up-regulated, and Mn is actively transported across the placenta from mother to fetus, as well as across the developing blood brain barrier (Aschner et al. 2005). In the postnatal period, infant homeostatic mechanisms of Mn absorption and excretion are underdeveloped to manage excess Mn exposure (Erikson et al. 2007). Although there is an increased nutritional need for Mn in early life (Mistry and Williams 2011; National Academy of Science 2001), an ideal exposure range has not been defined and it is unclear at what level Mn becomes toxic rather than beneficial during this dynamic developmental period (Ljung and Vahter 2007).
Several prospective studies have reported inverse or “U” shaped associations between Mn exposure in the prenatal or early postnatal period and performance on neurobehavioral tests in early childhood (Chung et al. 2015; Claus Henn et al., 2010; Lin et al. 2013; Yang et al. 2014; Yu et al. 2014). However, most studies assessing effects of prenatal Mn estimated fetal exposure using maternal biomarkers or umbilical cord blood (Gunier et al. 2014; Smargiassi et al. 2002; Zota et al. 2009); yet a single blood Mn spot measurement does not comprehensively capture fetal Mn exposure across the prenatal and postnatal periods. Dentin Mn is a non-invasive, validated biomarker that has been correlated with cord blood Mn (Arora et al. 2011, 2012; Gunier et al. 2014) and allows for retrospective exposure assessment in a cumulative manner. Because teeth accumulate metals and their mineralization forms a daily pattern similar to growth rings, measurement of dentin Mn provides precise exposure information from the beginning of the second trimester when deciduous teeth begin formation until approximately 1 year of age (Arora etal. 2012; Hillson 1996).
Regions in the central nervous system such as the frontal cortex and extrapyramidal motor regions that subserve a number of cognitive processes, including executive function, visuospatial learning, and memory, are particularly sensitive to Mn homeostasis. With one exception (Mora et al. 2015), most epidemiologic studies that assessed childhood Mn exposure and learning and memory were cross-sectional in design and contained no prenatal or postnatal Mn measurements (Haynes et al. 2015; Hernández-Bonilla et al. 2016; Nascimento et al. 2016; Oulhote et al. 2014a; Torres-Agustín et al. 2013; Wasserman et al. 2011; Wright et al. 2006).
We examined early-life windows of Mn exposure measured in deciduous teeth in relation to a novel neurobehavioral task administered later in childhood among male and female adolescents living near varied ferro-manganese alloy industry. We used the Virtual Radial Arm Maze (VRAM) test, a neurobehavioral animal-human analogue test, to evaluate visuospatial learning and memory. Given that previous studies of associations between Mn and neurobehavior found interactions by sex (Gunier et al. 2015; Bouchard et al. 2011; Riojas-Rodríguez et al. 2010; Menezes-Filho et al. 2014; Torres-Agustín et al. 2013), we hypothesized that Mn associations would differ for girls and boys.
2. Methods
2.1. Study Participants
Adolescent participants of the PHIME (Public Health Impact of Manganese Exposure in susceptible populations) study were the subjects for this analysis. Located in northern Italy, this study was designed to investigate associations between Mn exposure from anthropogenic emissions and neurodevelopmental outcomes. Details of the study have been described elsewhere (Lucas et al. 2015; Lucchini et al. 2012b, 2012a). Briefly, PHIME participants include children ages 10–14 years residing in three geographically distinct, but demographically similar, regions in the Province of Brescia, Italy: Bagnolo Mella (BM), an area with currently active ferro-manganese industry that has been in operation since 1970; Valcamonica (VC), where ferro-manganese plants were operating for approximately a century until 2001; and Garda Lake (GL), a tourist area with no history of ferroalloy industry.
We enrolled 720 children from these three regions via informational community and public school session recruitment. For funding reasons, participants were enrolled in two phases: 312 participants were enrolled in the first phase (2007–2010) and 408 were enrolled in the second phase (2010–2014). The inclusion criteria for participants of both enrollment phases were: (1) born to families residing in the study area since 1970; (2) living in the study area since birth; and (3) ages 10–14 years old at time of enrollment. Potential participants were excluded from the study if they (1) had any diagnosed neurological, metabolic, hepatic or endocrine diseases, or clinically evident hand/finger motor deficits; (2) were currently taking any prescription psychoactive drugs or had psychiatric disturbances; (3) had received total parenteral nutrition; or (4) had inadequately corrected visual deficits.
The Virtual Radial Arm Maze (VRAM) was incorporated into the neurobehavioral test battery as part of the second phase only. From among the 408 participants enrolled during the second phase, 402 (99%) completed the VRAM. A convenience sample of naturally shed teeth was collected from 142 (35%) of the 402 participants. Complete exposure and outcome data were available for 142 participants, which comprise the final sample for this analysis. Study protocols were approved by the Institutional Review Boards at the Ethical Committee of the Public Health Agency of Brescia, the University of California, Santa Cruz, and the Icahn School of Medicine at Mount Sinai.
2.2. Manganese Exposure Measurements
Prenatal and early postnatal Mn exposures in children were estimated using naturally shed deciduous teeth. We collected canines, incisors and molars (first and second) that were shed 6 months prior to or during the study. Analysis methods have been validated and described previously in detail (Arora et al. 2011, 2012; Gunier et al. 2014). Briefly, teeth were washed in an ultrasonic bath of ultrapure Milli-Q water (18.2 Mohm-cm2) and dried before and after sectioning. Teeth were sectioned on a vertical plane using a fitted diamond-tipped stainless-steel blade. The neonatal line, a histological line delineating pre- and postnatally formed regions of enamel and dentin (Sabel et al. 2008), was identified using confocal scanning laser microscopy (CSLM). Using laser ablation inductively coupled mass spectrometry (LA-ICP-MS; Agilent QQQ 8800 coupled with ESI 193nm laser ablation unit), thirty Mn measurements were taken throughout the pre and postnatal regions for each tooth and used to calculate an area under the curve (AUC) in order to estimate cumulative Mn exposure during the prenatal and postnatal periods, separately. Because mineral density varies within and between teeth, Mn concentrations were normalized to Ca concentrations (55Mn:43Ca ratio). We used Mn:Ca AUC for the prenatal and postnatal periods separately as our final exposure measurements. The limit of detection was 0.02 μg/g. Laboratory measurements were made by technicians who were blinded to the participants’ outcome and demographic information.
2.3. VRAM Test Procedure
The Virtual Radial Arm Maze (VRAM), a computerized maze task, was administered to assess visuospatial learning, working memory and reference memory (Astur et al. 2004). The VRAM is an adapted version of the Radial Arm Maze (RAM), a task that has previously been successful in assessing Mn, lead (Pb) and chlorpyrifos effects on visuospatial learning and memory in rodents (Astur et al. 2004; Haider et al. 2005; Kern et al. 2010; Levin et al. 2001). This virtual task was recently developed, and to our knowledge, has thus far been used only in adults (Astur et al. 2004, 2005; Goodrich-Hunsaker and Hopkins 2010). The VRAM and its administration in our population have been described previously (Braun et al. 2012). The prior analysis, conducted on a subset of participants in the present study, reported associations between better VRAM performance and higher scores on IQ subtests (Block Design, arithmetic, digit span and verbal comprehension) and on the California Verbal Learning Test (Braun et al. 2012). The VRAM was administered in a quiet room located in the participant’s school using a laptop and a Microsoft Sidewinder joystick (Microsoft Corp, Seattle, WA). The task involves using the joystick to navigate a maze, which is pictured on the screen as a 3D maze situated in a room (Supplemental Figure S1). The maze consists of eight arms of equal distance, four of which are baited with a visual auditory reward. Using visual cues in the room image, the participant learns (and must subsequently recall) which arms are baited, and navigates to retrieve rewards. Similar to the rodent task, the participant is able to move the joystick forward, right and left, but may not move backward. The participant is asked to complete eight consecutive trials in a maximum of 180 seconds per trial, where the four baited arms remain the same. If a participant travels down the same arm twice within a given trial, the response is considered a working memory error. Traveling down an un-baited arm between two trials is considered a reference memory error. VRAM performance measures include: 1) time (seconds) to complete the task; 2) distance traveled (maze units); 3) number of working memory errors; and 4) number of reference memory errors.
2.4. Covariates
Sociodemographic factors were assessed using a standardized questionnaire either at the in-person visit or over the telephone. Information included area of residence (Bagnolo Mella, Valcamonica, Garda Lake); birth order (first, second, third or higher); visual deficits (yes, no); and alcohol consumption (yes, no). Home Observation Measurement of the Environment (HOME) score (1–9) was assessed using 10 items selected from the HOME Short Form Scales of the National Longitudinal Survey of Youth: Children and Young Adults 1979 (National Longitudinal Surveys). Socioeconomic status (low, medium, high) was calculated using methodology developed in Italy that combines occupation and parental education (Cesana et al. 1995; Lucchini et al. 2012b). Information was also collected on videogame use (doesn’t play, rarely plays, 1 hour/day, 2 or more hours/day), because it was previously found to be a strong predictor of VRAM performance in our study (Braun et al. 2012). Information on nausea or dizziness experienced during the VRAM test was also gathered.
Venous whole blood samples were collected within a two-week period around neurodevelopmental assessment (when children were 10–14 years old) for measurement of Pb and iron status. Blood samples (4 mL) were collected using a 19-gauge butterfly catheter into a Li-Heparin Sarstedt Monovette Vacutainer. Measurement of blood metals was performed at the University of California, Santa Cruz using previously described methods (Lucchini et al. 2012b). Iron status was estimated by measuring ferritin and hematocrit levels. At the time of tooth collection, we also assessed the quality of each tooth sample: tooth attrition was rated on a scale of 0 to 3 (where 0 denotes no tooth loss from wear, 3 denotes more than two-thirds tooth loss).
2.5. Statistical Analysis
We carried out univariate and bivariate analyses for each variable. Tooth Mn concentrations were natural log (In) transformed to reduce influence from extreme individual measurements. In bivariate analyses, time and distance were modeled as continuous outcome variables, while working and reference memory errors were modeled as count ratios. Measurements below the limit of detection were included in the analyses as half of the lowest detectable measurement (n=2; postnatal tooth concentration=0.015).
We estimated associations between prenatal and postnatal tooth Mn and four VRAM outcomes: time (seconds), distance (maze units), working memory errors (count) and reference memory errors (count). Because Mn is both an essential nutrient and a toxicant, a potential nonlinear association was allowed by modeling Mn as a smoothed term using generalized additive mixed models (GAMMs) with penalized splines to predict VRAM performance measures. A random intercept to account for within-person correlation over the eight trials was included. In the absence of an established clinical cut-off for high or low tooth Mn, we categorized prenatal and postnatal tooth Mn into tertiles of their respective distributions, to model both low and high categories as compared to mid-range concentrations. Given the distribution of VRAM performance measures, multivariable linear mixed models were used to estimate the adjusted associations of Mn exposure and time or distance measures. Because count data (working and reference memory errors) did not meet the Poisson model assumption of the mean equaling the variance, and given inflated zeros in the distribution of each outcome, we used zero-inflated Poisson mixed regression for tertile analyses and report associations as count ratios. For GAMMs with penalized splines, we used quasi-Poisson mixed models because zero-inflated Poisson was not available for GAMMs. We assumed data were missing completely at random and performed complete case analyses.
Potential confounders were chosen based on prior literature (Braun et al. 2012; Claus Henn et al. 2010), biological plausibility, and results from bivariate analyses (selected if variable was associated with both exposure and outcome at p<0.10). Models were built for all four VRAM measures using regression modeling in a step-wise approach, retaining covariates that changed the Mn effect estimate by at least 10%. Final models included sex; age at time of VRAM testing (years; continuous); socioeconomic status (low, medium, vs. high); natural log-transformed blood Pb (continuous); VRAM trial number (1–8); videogame use (rarely plays, 1 hour per day, 2+hours per day vs. doesn’t play); and tooth attrition (less than one-third and more than one-third vs. none). To examine possible confounding of the postnatal Mn-VRAM associations by prenatal Mn, we also fit models of postnatal Mn adjusted for a smoothed term of prenatal Mn.
Mn biomarker levels have been shown to vary by sex (Oulhote et al. 2014b), and several previous studies have reported sex-specific Mn associations between exposure and neurobehavioral outcomes (Gunier et al. 2015; Torres-Agustin et al. 2013; Bouchard et al. 2011). Therefore, we explored effect measure modification by 1) stratifying models by sex and 2) including an interaction term (sex*Mn) in regression models. We considered interactions to be significant at p<0.1.
In secondary analyses we assessed the robustness of our observed associations by excluding participants who experienced motion sickness (i.e., dizziness or nausea) during the test. All statistical analyses were conducted using R version 3.2.2 (The R Foundation for Statistical Computing, www.r-project.org)(R Development Core Team 2008).
3. Results
Summary statistics on sociodemographic characteristics, tooth Mn levels and VRAM scores are presented in Table 1, stratified by sex. Among the 142 study participants, 44.4% (n=63) were male and 55.6% (n=79) were female. The distribution of prenatal tooth Mn was different in males and females, with more females than males in the second tertile, and more males than females in the third tertile (p=0.06). However, geometric mean (GM) prenatal tooth Mn concentrations were not significantly different between males and females (0.42 55Mn:43Ca AUC × 104 in males vs. 0.41 in females, p=0.62). Compared to males, females had lower SES (e.g. 29.1% of females in low SES vs. 9.7% of males; p=0.01), played videogames less frequently (e.g. 56.9% of females played >=1 hr/day vs. 78.6% of males; p=0.05), took more time to complete the VRAM tasks (87.5 seconds for females vs. 74.0 for males, p<0.01), and traveled more distance in the maze (14.7 maze units for females vs. 13.8 for males, p=0.05)(Table 1). Prenatal and postnatal tooth Mn concentrations did not vary by site (GM [GSD], prenatal Mn: BM=0.40 [1.5] μg/g; GL=0.41 [1.5] μg/g; VC=0.46 [1.3] μ/g; ANOVA F-test p=0.24; postnatal Mn: BM=0.12 [1.7] μ/g; GL=0.13 [1.6] μ/g; VC=0.13 [1.6] μ/g; ANOVA F-test p=0.27).
Table 1.
Characteristics of participants by sex
| Characteristic | All participantsa (n=142) n (%) or Mean ± SD |
Range | Females (n=79) n (%) or Mean ± SD |
Males (n=63) n (%) or Mean ± SD |
P-valuec |
|---|---|---|---|---|---|
| Age (years) | 12.4 ± 0.9 | 10.9 – 14.2 | 12.5 ± 0.9 | 12.3 ± 0.8 | 0.26 |
| Site | |||||
| Bagnolo Mella | 80 (56.3%) | 44 (55.7%) | 36 (57.1%) | 0.60 | |
| Valcamonica | 30(21.1%) | 15 (19.0%) | 15 (23.8%) | ||
| Garda Lake | 32 (22.5%) | 20 (25.3%) | 12 (19.0%) | ||
| Socioeconomic status | |||||
| Low | 29 (20.6%) | 23 (29.1%) | 6 (9.7%) | 0.01* | |
| Medium | 81 (57.4%) | 38 (48.1%) | 43 (69.4%) | ||
| High | 31 (22.0%) | 18 (22.8%) | 13 (21.0%) | ||
| Home score | 6.3 ± 1.5 | 3 – 9 | 6.2 ± 1.6 | 6.3 ± 1.4 | 0.70 |
| Birth order | |||||
| First | 72 (50.7%) | 40 (50.6%) | 32 (50.8%) | 0.90 | |
| Second | 50 (35.2%) | 27 (34.2%) | 23 (36.5%) | ||
| Third or higher | 20 (14.1%) | 12 (15.2%) | 8 (12.7%) | ||
| Videogame use | |||||
| Doesn’t play | 14 (10.0%) | 9 (11.4%) | 5 (8.2%) | 0.05* | |
| Rarely plays | 33 (23.6%) | 25 (31.6%) | 8 (13.1%) | ||
| 1 hr/day | 57 (40.7%) | 28 (35.4%) | 29 (47.5%) | ||
| 2+ hrs/day | 36 (25.7%) | 17 (21.5%) | 19 (31.1%) | ||
| Visual deficits | |||||
| Yes | 36 (33.3%) | 21 (36.8%) | 15 (29.4%) | 0.41 | |
| No | 72 (66.7%) | 36 (63.2%) | 36 (70.6%) | ||
| Nausea/dizziness during test | |||||
| Yes | 23 (17.3%) | 15 (20.8%) | 8 (13.1%) | 0.24 | |
| No | 110 (82.7%) | 57 (79.2%) | 53 (86.9%) | ||
| Alcohol consumption | |||||
| Yes | 2 (1.4%) | 0 (0.0%) | 2 (3.2%) | -- | |
| No | 140 (98.6%) | 72 (100%) | 61 (96.8%) | ||
| Tooth type | |||||
| Incisor | 16 (36.4%) | 11 (37.9%) | 5 (33.3%) | 0.91 | |
| Canine | 7 (15.9%) | 5 (17.2%) | 2 (13.3%) | ||
| Molar | 21 (47.7%) | 13 (44.8%) | 8 (53.3%) | ||
| Level of tooth lost to attrition | |||||
| None | 74 (52.1%) | 47 (59.5%) | 27 (42.9%) | 0.08* | |
| Less than one-third | 56 (39.4%) | 28 (35.4%) | 28 (44.4%) | ||
| More than one-third | 12 (8.5%) | 4 (5.1%) | 8(12.7%) | ||
| Blood lead (μg/dL) GM ± GSD | 1.4 ± 1.7 | 0.4 – 8.8 | 1.3 ± 1.7 | 1.5 ± 1.7 | 0.14 |
| Ferritin (ng/mL) GM ± GSD | 30.3 ± 1.8 | 6.0 – 174.0 | 28.9 ± 1.9 | 32.3 ± 1.7 | 0.27 |
| Hemoglobin (g/dL) GM ± GSD | 13.7 ± 1.1 | 11.3 – 16.7 | 13.6 ± 1.1 | 13.8 ± 1.1 | 0.23 |
|
| |||||
| Prenatal Tooth Mn GM ± GSD | 0.42 ± 1.5 | 0.11 – 0.89 | 0.41 ± 1.4 | 0.42 ± 1.6 | 0.62 |
| Low | 47 (33.1%) | 0.11 – 0.36 | 26 (32.9%) | 21 (33.3%) | |
| Middle | 49 (34.5%) | 0.36 – 0.50 | 33 (41.8%) | 16 (25.4%) | 0.06* |
| High | 46 (32.4%) | 0.50 – 0.89 | 20 (25.3%) | 26 (41.3%) | |
| Postnatal Tooth Mn GM ± GSD | 0.12 ± 1.7 | 0.01 – 0.40 | 0.13 ± 1.4 | 0.11 ± 1.9 | 0.02** |
| Low | 47 (33.1%) | 0.01 – 0.11 | 12 (26.6%) | 26 (41.3%) | |
| Middle | 49 (34.5%) | 0.11 – 0.15 | 28 (35.4%) | 21 (33.3%) | 0.13 |
| High | 46 (32.4%) | 0.15 – 0.40 | 30 (38.0%) | 16 (25.4%) | |
| VRAM time | 81.5 ±45.1 | 4 – 181 | 87.5 ± 46.3 | 74.0 ± 42.3 | <0.01*** |
| VRAM distance | 14.3 ±7.3 | 1.5 – 40.6 | 14.7 ± 7.4 | 13.8 ± 7.2 | 0.05* |
| VRAM working memory errors | 2.4 ± 3.2 | 0 – 22 | 2.4 ± 3.3 | 2.3 ± 3.1 | 0.34 |
| VRAM reference memory errors | 2.4 ± 1.5 | 0 – 4 | 2.4 ± 1.4 | 2.3 ± 1.6 | 0.13 |
Of participants, data were missing for SES (n=1), home score (n=9), videogame use (n=2), visual deficits (n=34), nausea/dizziness during test (n=9) tooth type (n=98), blood lead (n=3), ferritin (n=4), and hemoglobin (n=5).
Units for prenatal and postnatal tooth Mn (AUC 55Mn:43 Ca)
P-value for difference between females and males
Significance levels are indicated as follows:
for p<0.10,
for p<0.05,
for p<0.01
Compared to participants excluded from analyses due to missing VRAM or tooth data (n=575), those included in this analysis (n=142) were similar on most characteristics except age (mean 12.0 years among included vs. 12.4 years among excluded, p<0.01), sex (included: 55.6% female, excluded: 46.1% female, p=0.04), site location (included: 56.3% and 21.1% from Bagnolo Mella and Valcamonica, respectively; excluded: 23.0% and 39.8%, p<0.01), and serum ferritin concentrations (included: 30.3 ng/mL, excluded: 24.4 ng/mL, p<0.01) (Supplemental Table S1).
To understand predictors of prenatal and postnatal tooth Mn concentrations, we modeled bivariate associations between In-transformed tooth Mn concentrations and potential confounders (Table 2). Third or higher birth order was associated with 27.4% lower prenatal Mn tooth concentrations (95% CI: 13.1%, 39.3%) than first-born children [β=−0.32 (95% CI: −0.50, −0.14), percent change calculated as (exp(beta)−1)*100%]. Compared to canine teeth, incisors and molars had 41.7% (95% CI: 2.0%, 97.4%) and 41.7% (95% CI: 3.0%, 93.5%) higher concentrations of prenatal Mn, respectively. Compared to no tooth attrition, increasing tooth attrition was associated with 9.3% (95% CI: 2.7%, 19.9%) and 39.1% (95% CI: 24.2%, 51.0%) lower prenatal Mn concentrations for those with less than one-third lost, and more than one-third lost, respectively. This is expected because Mn levels are higher earlier in pregnancy and attrition removes the tooth forming earlier in the second trimester, thus causing a decrease in the prenatal Mn AUC. Female sex was associated with 22.1% higher postnatal Mn concentrations than males (95% CI: 4.1%, 44.8%).
Table 2.
Crude Associations of Covariates with Log-Transformed Prenatal and Postnatal Mn Tooth Concentrations (AUC 55Mn:43 Ca)a (n=142)
| Covariate | Prenatal Mn Tooth Concentrations
|
Postnatal Mn Tooth Concentrations
|
||
|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | |
|
|
|
|
||
| Age (years) | 0.01 | −0.06, 0.09 | 0.03 | −0.07, 0.13 |
| Sex (female vs. male) | −0.03 | −0.16, 0.09 | 0.20 | 0.04, 0.37** |
| Site | ||||
| Valcamonica (vs. GL) | 0.10 | −0.09, 0.29 | 0.02 | −0.23, 0.27 |
| Bagnolo Mella (vs. GL) | −0.03 | −0.19, 0.12 | −0.13 | −0.34, 0.08 |
| Socioeconomic status | ||||
| Low (vs. high) | 0.03 | −0.16, 0.23 | 0.19 | −0.06, 0.45 |
| Medium (vs. high) | 0.09 | −0.06, 0.25 | 0.14 | −0.07, 0.35 |
| Home score | −0.02 | −0.06, 0.03 | −0.05 | −0.11, 0.01 |
| Birth order | ||||
| Second (vs. first) | −0.06 | −0.20, 0.07 | −0.15 | −0.33, 0.04 |
| Third or higher (vs. first) | −0.32 | −0.50, −0.14*** | −0.13 | −0.38, 0.12 |
| Videogame use | ||||
| Doesn’t play (vs. 2+ hrs/day) | 0.09 | −0.14, 0.32 | 0.07 | −0.25, 0.38 |
| Rarely plays (vs. 2+ hrs/day) | −0.02 | −0.19, 0.16 | 0.12 | −0.12, 0.36 |
| 1 hr/day (vs. 2+ hrs/day) | 0.03 | −0.13, 0.19 | 0.09 | −0.12, 0.30 |
| Visual deficits (yes vs. no) | 0.04 | −0.11, 0.19 | −0.06 | −0.27, 0.16 |
| Nausea/dizziness during test | ||||
| Yes (vs. no) | −0.11 | −0.28, 0.06 | −0.02 | −0.21, 0.25 |
| Tooth type | ||||
| Incisor (vs. canine) | 0.35 | 0.02, 0.68** | −0.06 | −0.36, 0.25 |
| Molar (vs. canine) | 0.35 | 0.03, 0.66** | 0.15 | −0.15, 0.44 |
| Level of tooth lost due to attrition | ||||
| Less than one-third (vs. none) | −0.10 | −0.22, 0.03 | −0.23 | −0.40, −0.05** |
| More than one-third | −0.50 | −0.71, −0.28*** | −0.19 | −0.49, 0.12 |
| Blood lead (μg/dL) | 0.02 | −0.03, 0.07 | 0.03 | −0.04, 0.10 |
| Ferritin (ng/mL) | 0.001 | −0.01, 0.01 | 0.001 | −0.01, 0.00 |
| Hemoglobin (g/dL) | 0.001 | −0.06, 0.06 | −0.03 | −0.11, 0.06 |
Of participants, data were missing for SES (n=1), videogame use (n=2), visual deficits (n=34), nausea/dizziness during test (n=9) tooth type (n=98), blood lead (n=3), ferritin (n=4), hemoglobin (n=5), home score (n=9).
Significance levels are indicated as follows:
for p<0.10,
for p<0.05,
for p<0.01
3.1. Associations of Prenatal Mn with VRAM
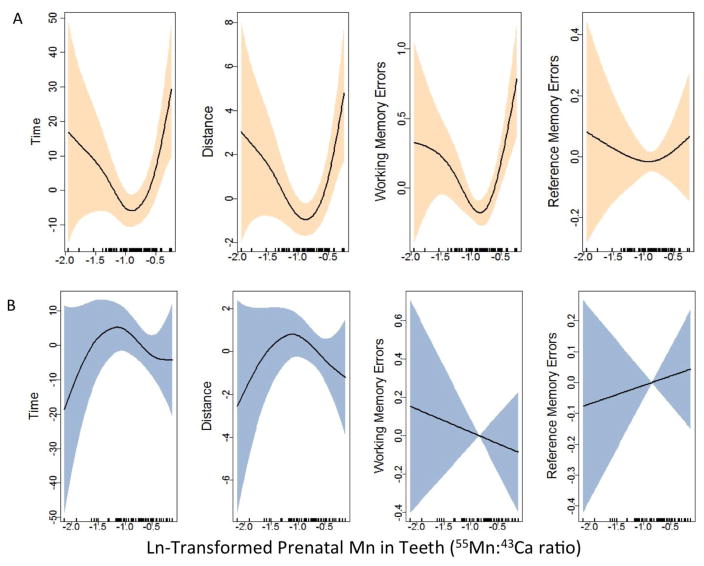
Associations of prenatal Mn and VRAM measures were examined using generalized additive mixed models with penalized splines for Mn. In models with Mn-sex cross-product terms, significant interactions were observed for time (p<0.001), distance (p=0.02), and working memory errors (p=0.03). In models stratified by sex, Mn associations differed between girls and boys on all VRAM measures (Figure 1). Among female participants, worse VRAM scores were observed at low and high concentrations of prenatal tooth Mn, compared to mid-range concentrations (pGAMM <0.01 for time, distance and working memory errors; p= 0.69 for reference memory errors). In multivariate mixed effect models with Mn parameterized as tertiles, both low (tertile 1) and high (tertile 3) Mn were associated with poorer VRAM performance, compared to the middle tertile of prenatal Mn, among females [time: low, (β=6.7 (−5.5, 18.9); high, (β=7.7 (−6.1, 21.5); distance: low, (β=1.5 (−0.4, 3.4); high, (β=2.3 (0.1, 4.4); working memory errors count ratio: low, (β=1.27 (0.95, 1.70); high, (β=1.33 (1.01, 1.83); reference memory errors count ratio: low, (β=1.12(1.00, 1.24); high, (β=1.10 (0.98, 1.24)] (Table 3). In contrast, among male participants, this pattern was not observed (Figure 1B). Performance on most VRAM outcomes was better among male participants with high (tertile 3) Mn levels compared to those with mid-range Mn, although associations were weak and not statistically significant (Table 3).
Figure 1.
Adjusted associations between continuous prenatal tooth Mn concentrations and VRAM measures for girls (A) and boys (B). Results from sex-stratified generalized additive mixed models of VRAM measures of time (T), distance (D), working memory errors (WME), and reference memory errors (RME) as predicted by natural log transformed prenatal tooth manganese concentrations (smoothed; AUC 55Mn:43 Ca). Models are adjusted for age, socioeconomic status, Ln transformed blood Pb, videogame use, trial and tooth attrition. Shaded region represents 95% confidence interval. (A) Among girls (n=79): pGAMM <0.01 for T, D and WME; p= 0.69 for RME; (B) Among boys (n=63); T: p=0.37; D: p=0.30; WME: p= 0.58; RME: p=0.66. Tertiles correspond to the following In Mn concentrations: T1: −2.2 to −1.0; T2: −1.0 to −0.7; T3: −0.7 to −0.1 (AUC 55Mn:43 Ca).
Table 3.
Change in VRAM performance measures for tertiles of tooth Mn levels
| Prenatal Mnc | All subjects (n=136, obs=1048)a | Girls (n=79, obs=606)b | Boys (n=57, obs=442) | P-INTf | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | ||
|
|
|
|
||||||||
| β1 (95% CI) | β2 | β3(95% CI) | β1 (95% CI) | β2 | β3(95% CI) | β1 (95% CI) | β2 | β3(95% CI) | ||
|
|
|
|
||||||||
| VRAM component | ||||||||||
| Time (seconds) | 4.6 (−5.0, 14.2) | ref | −2.1 (−11.7, 7.6) | 6.8 (−5.5, 19.3) | ref | 7.9 (−6.1, 21.8) | 2.7 (−13.3, 18.8) | ref | −9.2 (−24.8, 6.4) | 0.09 |
| Distance (maze units) | 1.2 (−0.4, 2.7) | ref | 0.5 (−1.0, 2.1) | 1.5 (−0.4, 3.4) | ref | 2.3 (0.1, 4.4)**g | 0.9 (−2.0, 3.7) | ref | −1.2 (−4.0, 1.5) | 0.05 |
| WMEh (count ratio) | 1.18 (0.96, 1.42) | ref | 1.08 (0.85, 1.30) | 1.27 (0.95, 1.70) | ref | 1.33 (1.01, 1.83)** | 1.07 (0.74, 1.49) | ref | 0.84 (0.57, 1.22) | 0.15 |
| RMEi (count ratio) | 1.06 (0.97, 1.16) | ref | 0.99 (0.92, 1.09) | 1.12 (1.00,1.24)* | ref | 1.10 (0.98, 1.24) | 0.93 (0.82, 1.06) | ref | 0.97 (0.82, 1.12) | 0.97 |
|
|
||||||||||
| Postnatal Mnd | All subjects (n=137, obs=1056) | Girls (n=80, obs=614) | Boys (n=57, obs=442) | |||||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | ||
|
|
|
|
||||||||
| β1 (95% CI) | β2 | β3 (95% CI) | β1 (95% CI) | β2 | β3 (95% CI) | β1 (95% CI) | β2 | β3 (95% CI) | ||
|
|
|
|
||||||||
| VRAM component | ||||||||||
| Time (seconds) | −6.5 (−16.1, 3.1) | ref | −6.6 (−16.1, 2.8) | −9.8 (−23.1, 3.4) | ref | −9.6 (−21.7, 2.4) | 2.0 (−12.9, 17.0) | ref | −1.8 (−18.5, 14.9) | 0.59 |
| Distance (maze units) | −0.6 (−2.1, 1.0) | ref | −0.6 (−2.1, 0.9) | −0.8 (−2.9, 1.4) | ref | −0.9 (−2.9, 1.0) | 0.02 (−2.6, 2.7) | ref | 0.3 (−2.7, 3.2) | 0.60 |
| WME (count ratio) | 0.95 (0.77, 1.17) | ref | 1.04 (0.82, 1.28) | 0.81 (0.59, 1.10) | ref | 0.99 (0.75, 1.30) | 1.24 (0.89, 1.68) | ref | 1.33 (0.89, 1.87) | 0.57 |
| RME (count ratio) | 0.91 (0.84, 1.02) | ref | 0.92 (0.83, 1.00) | 0.91 (0.80, 1.05) | ref | 0.89 (0.77, 1.01) | 0.97 (0.84, 1.17) | ref | 0.99 (0.88, 1.14) | 0.81 |
Models with all subjects included are adjusted for age, sex, socioeconomic status, videogame use, blood lead, trial, and tooth attrition.
Models stratified by sex are adjusted for age, socioeconomic status, videogame use, blood lead, trial, and tooth attrition.
Prenatal Mn tertile concentration ranges: T1: 0.11 – 0.36; T2: 0.36 – 0.50; T3: 0.50 – 0.89 (AUC 55Mn:43 Ca).
Postnatal Mn tertile concentration ranges: T1: 0.015 – 0.11; T2: 0.11 – 0.15; T3: 0.15 – 0.40 (AUC 55Mn:43 Ca).
Tertile 2 is referent.
P-INT is the p-value for the interaction term between sex and Mn tertile 3 (sex*Mn T3) in models with all subjects.
Significance levels are indicated as follows:
for p<0.10,
for p<0.05,
for p<0.01
Working memory errors
Reference memory errors
3.2. Associations of Postnatal Mn with VRAM
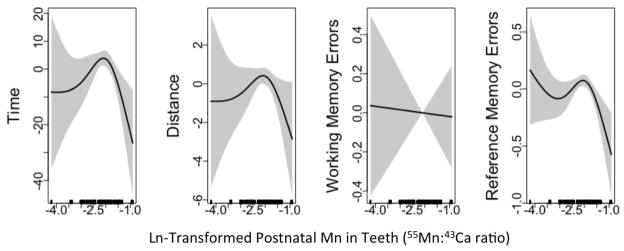
Postnatal Mn associations with VRAM measures were similarly shaped for girls and boys (Supplemental Figure S2), although a significant interaction between sex and smoothed postnatal Mn was observed in the model for time (p<0.01). Given the similar trends for girls and boys, we plotted associations of postnatal Mn for all participants together (Figure 2) as well as stratified by sex (Supplemental Figure 2). Adjusted generalized additive mixed models for postnatal Mn and VRAM performance including all participants appeared non-linear for time, distance, and RME, and best performance for all four outcomes was observed at higher Mn concentrations (Figure 2). In multivariate mixed effects models with postnatal Mn tooth concentrations modeled as tertiles, patterns of associations were less consistent and not statistically significant (Table 3). No clear distinction was observed between males and females. Additionally, when we adjusted for prenatal Mn (as a penalized spline term) in models of postnatal Mn, results were similar [e.g. for distance, without prenatal adjustment: low, (β=−0.6 (−2.1, 1.0); high, (β=−0.6 (−2.1, 0.9); with adjustment: low, β=−0.4 (−2.0, 1.1); high, β=−0.7 (−2.2, 0.9)] (Supplemental Table S2).
Figure 2.
Adjusted associations between continuous postnatal tooth Mn concentrations and VRAM measures. Results from generalized additive mixed models of VRAM measures of time (T), distance (D), working memory errors (WME), and reference memory errors (RME) as predicted by natural log transformed prenatal tooth manganese concentrations (smoothed; AUC 55Mn:43Ca). Models are adjusted for age, socioeconomic status, Ln transformed blood Pb, videogame use, trial and tooth attrition. Shaded region represents 95% confidence interval. (n=137); pGAMMs: T: p=0.09; D: p = 0.33; WME: p=0.88; RME: p=0.06. Tertiles correspond to the following In Mn concentrations: T1: −4.2 to −2.2; T2: −2.2 to −1.9; T3: −1.9 to −0.9 (AUC 55Mn:43 Ca).
3.3. Sensitivity Analyses
The robustness of our findings was evaluated by excluding participants who experienced dizziness or nausea during the test (n=23) and whose information was missing for that covariate (n=7). Results from these models were similar to those including all participants (Table 3), for both prenatal and postnatal Mn associations, although the coefficient of prenatal Mn tertiles predicting VRAM distance and working memory errors count ratio among girls was attenuated and no longer statistically significant (Supplemental Table S3; Figures S3, S4).
4. Discussion
We observed a “U” shaped association between prenatal Mn and VRAM performance measures among girls only, suggesting that both low and high prenatal Mn may adversely affect visuospatial learning and working memory. These results are consistent with previous reports on associations between Mn exposure and infant development and with the known biological properties of Mn as both a nutrient and toxicant (Chung et al. 2015; Claus Henn et al. 2010). This “U” shaped association was not observed among boys, and was not observed for exposure in the postnatal period in either sex. Instead, associations of postnatal Mn with VRAM performance during adolescence did not vary by sex and were null. The magnitude and precision of our effect estimates were similar in crude and adjusted models, as well as in sensitivity analyses, demonstrating the robustness of our findings (Supplemental Table 4). Our findings highlight two important aspects of Mn-associated neurodevelopment: first, there appears to be a critical window in the prenatal period for the outcomes studied here; and second, our data support sex differences with girls being more strongly affected than boys.
Few studies of child neurodevelopment have measured prenatal and postnatal Mn using the validated tooth-matrix biomarker of Mn exposure (Gunier et al. 2015; Mora et al. 2015). Mn levels in prenatal and postnatal tooth dentine in our study were similar to levels reported in the CHAMACOS cohort, which is located in an agricultural area of California with heavy use of Mn-containing pesticides (geometric mean prenatal Mn: 0.46 μg/g [females and males] vs. in our study: 0.41 μg/g [females]; 0.42 μg/g [males]; postnatal Mn: 0.15 μg/g [females]; 0.13 μg/g [males] vs. our study: 0.13 μg/g [females]; 0.11 μg/g [males])(Gunier et al. 2015; Mora et al. 2015). We cannot directly compare our findings with those of Ericson et al. 2007, the only other study of tooth Mn and neurobehavior, because Mn concentrations were measured in enamel (vs. dentine in our study).
Sex-specific prenatal and early postnatal Mn associations have been reported in the aforementioned studies using the tooth-matrix biomarker of Mn exposure, but findings have been inconsistent (Gunier et al. 2015; Mora et al. 2015). Adverse associations were reported between postnatal tooth Mn concentrations and 6-month Mental and Psychomotor Development Indices of the Bayley Scales of Infant Development (BSID) among 102 girls, but not boys (Gunier et al. 2015). The study also observed negative associations with prenatal Mn and 6-month BSID scores among girls, but only in girls with low maternal hemoglobin concentrations during pregnancy, an indicator of iron deficiency (Gunier et al. 2015). Another analysis from the same cohort examining later childhood neurobehavioral outcomes in boys and girls (Mora et al. 2015) reported associations between higher prenatal tooth Mn and hyperactivity, internalizing and externalizing problems at ages 7 to 10 years, as well as poorer visuospatial memory abilities at 9 years among children with prenatal lead levels ≥0.8 μg/dL. However, among boys, higher prenatal and postnatal Mn was associated with improved visual memory performance (NEPSY-II Memory design) and motor abilities (Luria-Nebraska Motor scale and finger tap)(Mora et al. 2015). Our results in boys were inconsistent and non-significant, possibly due to lower postnatal levels relative to prenatal levels in our population.
Other cross-sectional epidemiologic studies have also observed sex-specific differences in associations between Mn levels measured in childhood and neurological outcomes (Bouchard et al. 2011; Lucchini et al. 2012a; Menezes-Filho et al. 2014; Riojas-Rodriguez et al. 2010; Torres-Agustín et al. 2013), while some have not (Bouchard et al. 2007; Chung et al. 2015; Hernández-Bonilla et al. 2011; Lucchini et al. 2012b; Oulhote et al. 2014a; Riojas-Rodriguez et al. 2010). In 7–12 year-old girls living near Mn mining or ferro-manganese industry, hair Mn concentrations were negatively associated with scores on the Wechsler Intelligence Scale for Children (n=172) (Riojas-Rodríguez et al. 2010), verbal learning and memory (Torres-Agustín et al. 2013), and inattention and externalizing behavior (n=70)(Menezes-Filho et al. 2014). Similarly, a study in Quebec (n=363) found higher hair and water Mn in girls to be associated with poorer performance on full scale IQ assessment; however the sex-hair Mn interaction term was non-significant (p=0.55)(Bouchard et al. 2011). In contrast, few studies observed poorer neurological performance among boys with high Mn exposure compared to mid-range concentrations (Lucchini et al. 2012a; Rahman et al. 2016). Rahman et al., 2016 reported that higher prenatal water Mn concentrations were associated with worse parental reports of conduct in boys. Within our PHIME study cohort, boys exposed to higher soil Mn and with higher hair and blood Mn had increased tremor intensity and decreased odor identification relative to girls (Lucchini et al. 2012a). Most studies, including ours, were not specifically designed to evaluate sex-interactions; therefore, low statistical power may in part explain some of the inconsistency between studies.
New evidence suggests that genetic variation in the SLC30A10 locus plays a role in Mn transport and the neuro-toxicological vulnerability to Mn insult (Wahlberg et al. 2016; Chen et al. 2015b). Mutations on the solute carrier family 30 member 10 (SLC30A10) locus on chromosome 1 are thought to increase Mn retention, and are the only known hereditary risk factors for Mn-induced parkinsonism (Tuschl et al. 2012; Quadri et al. 2012; Chen et al. 2015b). Although Mn concentrations did not vary by sex in our sample, one possible explanation for the sex-specific Mn associations we estimated is that the girls in our study may have more SLC30A10 mutations than boys, leading to increased susceptibility to Mn neurotoxicity.
The biologic mechanism for Mn neurotoxicity involves production of reactive oxygen species that either damage central nervous system (CNS) structures or alter their developmental trajectory. Notably, dopaminergic (DA) neurons in the CNS including frontal cortex and extrapyramidal motor regions are sensitive to Mn homeostasis (Aschner et al. 2007; Dorman 2006). Dopaminergic expression (DA concentrations, receptors and transporters) in the brain plays an important role in successful completion of neurobehavioral tasks related to learning, memory, mood and motivation/reward pathways (Sotomayor-Zarate et al. 2014). Mn, but also sex hormones, act on dopamine receptors and transporters (Blecharz-Klin et al. 2012; Kern et al. 2010; Llop et al. 2013; Sotomayor-Zarate et al. 2014). Further, mounting evidence now suggests the structure and functioning of many DA-rich cerebral environments are sexually dimorphic (Gillies et al. 2014a). Male and female brains may rely on different strategies to achieve the same goal, likely due to the occurrence of different neural mechanisms and information processing (Cahill 2006; Gillies et al. 2014b). It is plausible that Mn may affect DA expression across brain regions, and thus neurobehavior, in a sex-dependent manner. In terms of visuospatial learning and memory, it is plausible that pathways and information processing in female brains are more susceptible to Mn insult than in male brains. We assessed only the VRAM task in our analyses, but it is possible that Mn-associated insult occurs through a different mechanism and manifests itself in a different manner among males.
There is a paucity of animal studies investigating sex-specific effects of Mn exposure on neurobehavior. The majority of studies examining the effect of Mn on neurobehavior have used rodents or non-human primates of male sex only, or have not stratified by sex (Beaudin et al. 2016; Blecharz-Klin et al. 2012; O’Neal et al. 2014; Pappas et al. 1997; Schneider et al. 2009, 2013, 2015). In one recent study, female, but not male, Sprague-Dawley rats exposed to Mn during development had increased dopamine and norepinephrine in neostriatal tissue relative to controls (Vorhees et al. 2014). However, another study found Mn-induced persistent neuronal morphologic changes in male mice striata, but not in females (Madison et al. 2011).
A strength of our study is the use of dentine-Mn as an exposure biomarker, allowing us to retrospectively, but objectively, capture exposure during multiple developmental stages (prenatal and early postnatal) and identify which stages, if any, were critical windows for Mn-associated neurodevelopment. As with any exposure measurement, there is the potential for measurement error; however, given that this method is cumulative over time, rather than a spot measure in urine/blood/hair/plasma, measurement error should be reduced. A newer methodology for measuring tooth Mn concentrations allows for hundreds of measurements across several months capturing exposure in short time windows repeatedly (Modabbernia et al. 2016). In our study, however, the biomarker was an earlier technology that averaged the area under the curve over longer time points (30 total spot measurements) to derive a single cumulative prenatal or postnatal measurement. For that reason, we may have missed time dependent effects of discrete susceptibility windows (weeks to months during neurodevelopment) that the newer approach would capture. The ability to cumulatively assess prenatal and early postnatal exposure is nonetheless a major technological advance.
Another strength of our study was that we performed neurobehavioral testing at a sensitive time point (late childhood and adolescence) that has not yet been frequently studied in the context of fetal Mn exposure. While sex hormones begin to influence the brain in utero during mid-pregnancy, early puberty is another critical time point for brain development. Because increased concentrations of sex hormones likely activate developing neural pathways in a sexually dimorphic manner, perinatal Mn-associated neurobehavioral decrements may be observed differently before and after this time point (Ahmed et al. 2008; Sisk and Zehr 2005). Additionally, studies using neurobehavioral assessments performed in later childhood or adolescence are more sensitive and reliably related to neurobehavioral abilities than assessments performed in the first few years of life (White et al. 2009). Only two other studies of perinatal Mn have investigated neurobehavioral outcomes in mid to late childhood (Ericson et al. 2007; Mora et al. 2015). These studies both found adverse associations of increased prenatal Mn with internalizing and externalizing scores among children aged 6 to 10.5 years. Similar to our study, postnatal Mn associations were inconsistent: higher postnatal Mn was beneficial or harmful depending on the outcome, co-exposures, and sex of participants (Mora et al. 2015; Ericson et al. 2007). These data reflect a latency period of more than a decade from exposure to effect and demonstrate the potentially powerful influence of chemicals on fetal brain development.
Our study is the first to use VRAM, a sensitive and novel outcome, to assess Mn neurotoxicity in humans. Using the VRAM provides an animal-human analogue to bridge the gap between animal and human endpoints of Mn neurotoxicity, and supports interpretation of results from epidemiologic and toxicological studies. Evidence further suggests that damage to analogous brain regions in humans and rodents is associated with similar outcomes on the respective visual maze tasks. Humans with damage to the hippocampus, a brain region involved in visuospatial ability, performed worse on VRAM than normal controls (Goodrich-Hunsaker and Hopkins 2010). Similarly, rats with hippocampal lesions performed worse on RAM (Goodrich-Hunsaker and Hopkins 2010). Furthermore, increased hippocampal and frontal cortex activity were seen on functional imaging when human participants carried out the VRAM task (Astur et al. 2005). These brain regions are critical for memory, learning and executive functioning.
There were notable similarities in the findings of our study compared to findings of a comparable rodent study (Kern et al. 2010). Kern et al. (2010) exposed neonate Sprague-Dawley rats to MnCl2 by oral gavage during postnatal day (PND) 1 through 21 and evaluated visuospatial learning and memory using the RAM on PND 33–46 in male rats only. Mn-exposed rats committed more working memory errors as well as reference memory errors compared to controls. Similarly, we found that higher prenatal Mn exposure was significantly associated with more working memory errors in adjusted non-linear and multivariable models, though only among girls. Further, in brain tissue analysis, compared to respective controls, Mn-exposed rats had altered expression of dopamine receptor D1, D2 and DA transporter in sampled tissue from Mn-relevant brain regions including the prefrontal cortex, dorsal striatum and nucleus accumbens. The finding of Mn-induced alterations in dopaminergic receptors and transporters is consistent with our results for girls in the prenatal period. These similarities in findings were seen despite several differences in study design. First, only male rats were used for RAM testing. However, female and male rats’ blood and brain tissue were analyzed for Mn at the time of RAM testing and no differences between sexes were found. Second, routes of Mn exposure affect the absorption of manganese in the body and its distribution in brain regions (O’Neal and Zheng 2015); the route of Mn exposure in our study is mostly inhalation, while in the animal study, rats were orally exposed. Third, although the sequence of events in brain development for rat and human brains is similar, developmental timing is different: the first 7 to 10 postnatal days in the rat correspond to the third trimester of pregnancy in humans (Semple et al. 2013). Therefore the exposure period assessed by Kern et al. (2010) is synonymous to a combination of prenatal and postnatal exposure periods in humans.
There are limitations to our study. The small sample size limited statistical power to detect associations, particularly in stratified models. As with any epidemiologic study fitting multiple models, chance is one possible explanation for our findings. However, our findings have biologic plausibility and are supported by previous literature. Future work should use a larger sample size to validate these findings. There may be unmeasured confounding or effect modification from maternal iron status, in which low maternal iron increases Mn absorption and negatively impacts neurodevelopment (Gunier et al. 2015; Finley 1999). We lack data on maternal iron status, but any potential differences in maternal iron status are not likely to be related to sex of the fetus and therefore we would not expect this to explain our sex-specific findings. Selection bias may be possible, if those selected into the study differ by a factor that is related to both exposure and outcome. However, subjects included in our analysis were similar to the full cohort on the majority of measured characteristics, thereby reducing concerns of selection bias (Table S1). Differences in participation based on study site are an artifact of the study design, in which the second phase of the study targeted recruitment in a new site with active ferro-manganese activity (Bagnolo Mella). Individuals were recruited without knowledge of their tooth Mn levels from earlier in life and without knowledge about their neurobehavioral performance. Furthermore, study site was not related to prenatal or postnatal dentine Mn levels (Table 2), nor was it related to VRAM performance. It is unclear why tooth Mn levels were similar across the three study sites, when we expected to see higher Mn in areas with ferroalloy industry (Bagnolo Mella and Valcamonica). One possible explanation may be that, while Garda Lake has no ferroalloy industry, there may be other sources of environmental Mn exposure, such as from application of Mn-containing fungicide to vineyards(Paronetto, Lanfranco 2011).
Conclusions
Our study is the first to investigate the association of sensitive windows of Mn exposure with performance on an animal-human analogue task that captures Mn-specific neurotoxicity. Findings from our data suggest that girls may be particularly vulnerable to prenatal Mn overexposure in relation to visuospatial development. Future research should emphasize sex-specific effects of Mn, domain-specific neurobehavioral assessments and time-specific exposure windows of neurodevelopment. Our findings are important to public health, especially for communities that may be more exposed to Mn in their environment, which include those with naturally elevated Mn in drinking water (Bouchard et al. 2011; Wasserman et al. 2006) and those who live near Mn-related industries such as steel-alloy production (Haynes et al. 2015; Lucchini et al. 2012a; Menezes-Filho et al. 2014) or agricultural areas where Mn-containing fungicides are applied (Gunier et al. 2015).
Supplementary Material
Highlights.
Children are vulnerable to Mn toxicity, yet susceptibility factors are understudied.
We measured Mn in teeth to estimate exposure during critical developmental windows.
Complex visuospatial ability was measured using an animal task adapted for humans.
The prenatal period may be important for Mn effects on girls’ visuospatial ability.
Acknowledgments
Funding sources
This work was funded by research from the National Institutes Health: T32ES014562, R00ES022986, R01ES019222, R01ES026033, DP2ES025453, P30ES000002, the European Union Sixth Framework Program: FOODCT-2006-016253, and University of Brescia, Italy: UNBSCLE 9015. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
We thank the PHIME staff, participants and families.
Footnotes
Conflict of interest statement
The authors claim no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–7. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining Fetal Manganese Exposure from Mantle Dentine of Deciduous Teeth. Env Sci Technol. 2012 May;1:5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–1319. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman Aschner DC. Manganese Dosimetry: Species Differences and Implications for Neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–47. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astur RS, Germain SAS, Baker EK, Calhoun V, Pearlson GD, Todd Constable R. fMRI Hippocampal Activity During a Virtual Radial Arm Maze. Appl Psychophysiol Biofeedback. 2005:30. doi: 10.1007/s10484-005-6385-z. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Beaudin SA, Strupp BJ, Strawderman M, Smith DR. Early Postnatal Manganese Exposure Causes Lasting Impairment of Selective and Focused Attention and Arousal Regulation in Adult Rats. Environ Health Perspect. 2016 doi: 10.1289/EHP258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Saf Health Work. 2013;4:1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecharz-Klin K, Piechal A, Joniec-Maciejak I, Pyrzanowska J, Widy-Tyszkiewicz E. Effect of intranasal manganese administration on neurotransmission and spatial learning in rats. Toxicol Appl Pharmacol. 2012;265:1–9. doi: 10.1016/j.taap.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur M, Bouffard T, et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Lucchini R, Bellinger DC, Hoffman E, Nazzaro M, Smith DR, et al. Predictors of virtual radial arm maze performance in adolescent Italian children. Neurotoxicology. 2012;33:1203–1211. doi: 10.1016/j.neuro.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cesana GC, Ferrario M, De Vito G, Sega R, Grieco A. Evaluation of the socioeconomic status in epidemiological surveys: hypotheses of research in the Brianza area MONICA project. Med Lav. 1995;86:16–26. [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MMB, Bowman AB, et al. Manganese homeostasis in the nervous system. J Neurochem. 2015;134:601–610. doi: 10.1111/jnc.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SE, Cheong H-K, Ha E-H, Kim B-N, Ha M, Kim Y, et al. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ Health Perspect. 2015;123:717–22. doi: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Bellinger D, Hopkins M, Coull B, et al. Prenatal manganese exposure and early childhood neurodevelopment among residents near a mining-impacted Superfund site. Environ Health Perspect. doi: 10.1289/EHP925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Schwartz J, Téllez-Rojo MM, Lamadrid-Figueroa H, Hernández-Avila M, et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–9. doi: 10.1097/EDE.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC. Tissue Manganese Concentrations in Young Male Rhesus Monkeys following Subchronic Manganese Sulfate Inhalation. Toxicol Sci. 2006;92:201–210. doi: 10.1093/toxsci/kfj206. [DOI] [PubMed] [Google Scholar]
- Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113:369–77. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am J Clin Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, McArthur S, Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience. 2014a;282:69–85. doi: 10.1016/j.neuroscience.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, McArthur S, Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience. 2014b;282:69–85. doi: 10.1016/j.neuroscience.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hopkins RO. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav Neurosci. 2010;124:405–413. doi: 10.1037/a0019193. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan P. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, et al. Manganese in teeth and neurodevelopment in young Mexican-American children. Environ Res. 2015;142:688–695. doi: 10.1016/j.envres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Mora AM, Smith D, Arora M, Austin C, Eskenazi B, et al. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Environ Sci Technol. 2014;48:14695–702. doi: 10.1021/es503866a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Shameem S, Ahmed SP, Perveen T, Haleem DJ. Repeated administration of lead decreases brain 5-HT metabolism and produces memory deficits in rats. Cell Mol Biol Lett. 2005;10:669–676. [PubMed] [Google Scholar]
- Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, et al. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA) Environ Health Perspect. 2015;123:1066–71. doi: 10.1289/ehp.1408993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Bonilla D, Escamilla-Núñez C, Mergler D, Rodríguez-Dozal S, Cortez-Lugo M, Montes S, et al. Effects of manganese exposure on visuoperception and visual memory in schoolchildren. Neurotoxicology. 2016;57:230–240. doi: 10.1016/j.neuro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Hernández-Bonilla D, Schilmann A, Montes S, Rodríguez-Agudelo Y, Rodríguez-Dozal S, Solís-Vivanco R, et al. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology. 2011;32:615–621. doi: 10.1016/j.neuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Hillson S. Dental anthropology 1996 [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–78. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F, et al. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect. 2011;119:1501–6. doi: 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/S0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Lin C-C, Chen Y-C, Su F-C, Lin C-M, Liao H-F, Hwang Y-H, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–7. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Ljung K, Vahter M. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect. 2007;115:1533–8. doi: 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311:3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Lucas EL, Bertrand P, Guazzetti S, Donna F, Peli M, Jursa TP, et al. Impact of ferromanganese alloy plants on household dust manganese levels: implications for childhood exposure. Environ Res. 2015;138:279–90. doi: 10.1016/j.envres.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012a;33:687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Zoni S, Guazzetti S, Bontempi E, Micheletti S, Broberg K, et al. Inverse association of intellectual function with very low blood lead but not with manganese exposure in italian adolescents. Environ Res. 2012b;118:65–71. doi: 10.1016/j.envres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32:896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, De Carvalho-Vivas CF, Viana GFS, Ferreira JRD, Nunes LS, Mergler D, et al. Elevated manganese exposure and school-aged children’s behavior: A gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011;2011:841749. doi: 10.1155/2011/841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Velthorst E, Gennings C, De Haan L, Austin C, Sutterland A, et al. Early-life metal exposure and schizophrenia: A proof-of-concept study using novel tooth-matrix biomarkers. Eur Psychiatry. 2016;36:1–6. doi: 10.1016/j.eurpsy.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernández-Bonilla D, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento S, Baierle M, Göethel G, Barth A, Brucker N, Charão M, et al. Associations among environmental exposure to manganese, neuropsychological performance, oxidative damage and kidney biomarkers in children. Environ Res. 2016;147:32–43. doi: 10.1016/j.envres.2016.01.035. [DOI] [PubMed] [Google Scholar]
- National Academy of Science. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press; Washington, D.C: 2001. [PubMed] [Google Scholar]
- National Longitudinal Surveys. Appendix A: HOME-SF Scales (NLSY79 Child) National Longitudinal Surveys; [accessed 15 December 2016]. Available: https://www.nlsinfo.org/content/cohorts/nlsy79-children/other-documentation/codebook-supplement/appendix-home-sf-scales/page/0/0/#AppendixA1. [Google Scholar]
- O’Neal SL, Lee J-W, Zheng W, Cannon JR. Subacute manganese exposure in rats is a neurochemical model of early manganese toxicity. Neurotoxicology. 2014;44:303–13. doi: 10.1016/j.neuro.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Curr Environ Heal reports. 2015;2:315–28. doi: 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur M-È, et al. Neurobehavioral Function in School-Age Children Exposed to Manganese in Drinking Water. Environ Health Perspect. 2014a;122:1343–1350. doi: 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Bouchard MF. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environ Heal. 2014b:13. doi: 10.1186/1476-069X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas BA, Zhang D, Davidson CM, Crowder T, Park GA, Fortin T. Perinatal manganese exposure: behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol Teratol. 1997;19:17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Paronetto Lanfranco, FD . Advances in food and nutrition research. 9 Amarone: A Modern Wine Coming from an Ancient Production Technology; 2011. [DOI] [PubMed] [Google Scholar]
- Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90:467–77. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SM, Kippler M, Tofail F, Bölte S, Hamadani JD, Vahter M. Manganese in Drinking Water and Cognitive Abilities and Behavior at 10 Years of Age: A Prospective Cohort Study. Environ Health Perspect. 2016 doi: 10.1289/EHP631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, et al. Intellectual Function in Mexican Children Living in a Mining Area and Environmentally Exposed to Manganese. Env Heal Perspect. 2010;118:1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, et al. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118:1465–70. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel N, Johansson C, Kühnisch J, Robertson A, Steiniger F, Norén JG, et al. Neonatal lines in the enamel of primary teeth--a morphological and scanning electron microscopic investigation. Arch Oral Biol. 2008;53:954–63. doi: 10.1016/j.archoralbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr Environ Heal reports. 2015;2:284–94. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of chronic manganese exposure on working memory in non-human primates. Brain Res. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Williams C, Ault M, Guilarte TR. Chronic manganese exposure impairs visuospatial associative learning in non-human primates. Toxicol Lett. 2013;221:146–51. doi: 10.1016/j.toxlet.2013.06.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Williams C, Ault M, Guilarte TR. Effects of chronic manganese exposure on attention and working memory in non-human primates. Neurotoxicology. 2015;48:217–22. doi: 10.1016/j.neuro.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Takser L, Masse A, Sergerie M, Mergler D, St-Amour G, et al. A comparative study of manganese and lead levels in human umbilical cords and maternal blood from two urban centers exposed to different gasoline additives. Sci Total Environ. 2002;290:157–64. doi: 10.1016/s0048-9697(01)01071-3. [DOI] [PubMed] [Google Scholar]
- Sotomayor-Zarate R, Cruz G, Renard GM, Espinosa P, Ramirez VD. Sex hormones and brain dopamine functions. Cent Nerv Syst Agents Med Chem. 2014;14:62–71. doi: 10.2174/1871524914666141226105137. [DOI] [PubMed] [Google Scholar]
- Torres-Agustín R, Rodríguez-Agudelo Y, Schilmann A, Solís-Vivanco R, Montes S, Riojas-Rodríguez H, et al. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res. 2013;121:39–44. doi: 10.1016/j.envres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Clayton PT, Gospe SM, Gulab S, Ibrahim S, Singhi P, et al. Syndrome of Hepatic Cirrhosis, Dystonia, Polycythemia, and Hypermanganesemia Caused by Mutations in SLC30A10, a Manganese Transporter in Man. Am J Hum Genet. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Amos-Kroohs RM, Braun AA, Grace CE, Schaefer TL, et al. Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone. Toxicol reports. 2014;1:1046–1061. doi: 10.1016/j.toxrep.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg K, Kippler M, Alhamdow A, Rahman SM, Smith DR, Vahter M, et al. Common Polymorphisms in the Solute Carrier SLC30A10 are Associated With Blood Manganese and Neurological Function. Toxicol Sci. 2016;149:473–83. doi: 10.1093/toxsci/kfv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, et al. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology. 2011;32:450–457. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Campbell R, Echeverria D, Knox S, Janulewicz P. Assessment of neuropsychological trajectories in longitudinal population-based studies of children. J Epidemiol Community Health. 2009;63(Suppl 1):i15–26. doi: 10.1136/jech.2007.071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school - age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Yang X, Bao Y, Fu H, Li L, Ren T, Yu X. Selenium protects neonates against neurotoxicity from prenatal exposure to manganese. PLoS One. 2014;9:e86611. doi: 10.1371/journal.pone.0086611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X-D, Zhang J, Yan C-H, Shen X-M. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–238. doi: 10.1016/j.envres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Zoni S, Lucchini RG. Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Curr Opin Pediatr. 2013;25:255–60. doi: 10.1097/MOP.0b013e32835e906b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20:367–73. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.