Opioids
Opioids represent an efficacious therapeutic modality for some, but not all pain states. Singular reliance on opioid therapy for pain management, has limitations and abuse potential has deleterious consequences for patient and society. Our understanding of pain biology has yielded insights and opportunities for alternatives to conventional opioid agonists. The aim is to have efficacious therapies, with acceptable side effect profiles and minimal abuse potential, which is to say an absence of re-enforcing activity in the absence of a pain state. The present work provides a non-exclusive overview of current drug targets and potential future directions of research and development. We discuss channels activators and blockers, including: sodium channel blockers, potassium channel activators and calcium channel blockers; glutamate receptor targeted agents, including: NMDA, AMPA and metabotropic receptors). Further, we discuss therapeutics targeted at GABA, alpha2 adrenergic and opioid receptors. We also considered antagonists of angiotensin 2 and Toll receptors, and agonists/antagonists of adenosine, purine receptors. And cannabinoids. Novel targets considered are those focusing on lipid mediators and anti-inflammatory cytokines. Of interest is development of novel targeting strategies which produce long-term alterations in pain signaling, including viral transfection and toxins. We consider issues in the development of drugable molecules, including preclinical screening. While there are examples of successful translation, mechanistically promising pre-clinical candidates may unexpectedly fail during clinical trials because the preclinical models may not recapitulate the particular human pain condition being addressed. Molecular target characterization can diminish the disconnect between preclinical and humans’ targets, which should assist in developing non-addictive analgesics.
INTRODUCTION
The management of pain is a clinical imperative. Aside from humanistic concerns, failure to adequately control pain has negative consequences in terms of system biology. Opioids, through their potent modulatory effect mediated via canonical receptors on pain processing, have been, and remain, an essential component of pain management. Nevertheless, reliance on this therapeutic approach has limitations and deleterious consequences to the patient and society. Opioid misuse is an expanding crisis with over 36,000 deaths due to opioid overdose in 2015 alone.1,2 Pharmaceutical companies have pursued abuse-deterrent opioid formulations.3,4 While these formulations reduce the possibility of the content of the pills being extracted, the underlying properties of the pharmaceutical agent (i.e. opioids) remain the same and extraction deterrent systems are subject to being overcome.5–8
Our understanding of systems that mediate and regulate nociceptive processing has yet to produce a recognized alternative to opioids. Advances in pain biology has, however, yielded remarkable insights and opportunities. We will provide an overview of salient areas of research that focus on current advances in pharmacological target. Meaningful advances in drug therapy must consider not only i) analgesic efficacy, but as well ii) therapeutic ratio (separation of pain relief from side effects); iii) constancy of response over extended use (e.g. tolerance); iv) lack of positive reinforcing properties in the absence of a pain state. Due to space restriction, this review must be considered a non-exclusive overview of advances in terms analgesic targets.
PAIN PHENOTYPES
Pain is an aversive state that reflects the perceptual covariates of events that arise from stimuli of sufficient intensity to induce tissue damage or which otherwise mimic the activity induced by such stimuli, as in nerve injury. It is heuristically useful to think of mechanisms generating the aversive condition associated with afferent stimulation as having 4 elements.
Acute nociception in which an acute, non-injuring, high intensity stimulus activates small unmyelinated and myelinated afferents, driving intensity linked excitation of second order dorsal horn projection neurons, leading to a stimulus-linked pain report/escape.
Following tissue injury and inflammation, hyperalgesia occurs at the injury site, causing an enhanced response to moderate stimuli, and an enlarging receptive field, including areas not injured, resulting in a 2nd hyperalgesia/allodynia. This phenotype reflects a peripheral sensitization (development of ongoing activity, and a left shift in the intensity response relationship at the terminal) and a central /spinal sensitization (heightened excitability of the primary afferent terminal and second order neurons causing an enhanced discharge to a given afferent input).
Injury to the peripheral nerve resulting in ongoing dysesthesias and enhanced sensitivity to light touch and modest changes in temperatures (allodynia), associated with reactive changes in the afferent axon, DRG and dorsal horn (typically reflecting a loss of inhibitory regulation).
Following persistent inflammation and tissue injury, the evolving pain state displays characteristics suggesting the development of a nerve injury phenotype, e.g. an acute to chronic pain transition.
The biology of these above states has been reviewed in detail elsewhere.9–11 These comments importantly emphasize that a pain condition may represent multiple mechanistic phenotypes. Accordingly, the regulation of the encoding and trafficking of the nociceptive stimulus to higher centers may reflect a role for engaging multiple targets.
ISSUES IN ANALGESIC DRUG DEVELOPMENT
Demonstration of target analgesic efficacy
Development of analgesic drugs with known targets and mechanisms of action can employ models of target engagement, such as in silico and in vitro modeling (e. g. opioids and COX inhibitors) which can move a drug with some predictability into a behavioral assessment. Novel targets often arise based on association of the target with specific systems, but their efficacy in regulating the pain state requires a sense of what role that target plays in mediating the behaviorally defined pain construct. Preclinical behavioral models provide such insights. Detailed reviews of preclinical models that focus on events secondary to inflammation (acute and chronic) and nerve injury (mono and poly neuropathies) with their strengths and shortcomings have been provided elsewhere.12,13 While instances of failure of the predictive models have been discussed (as is true for virtually every translational system in biology), mechanistic studies have made a number of valid predictions of clinical efficacy ranging from COX inhibitors to antimigraine drugs.9 Several issues regarding preclinical models are noted.
Each behavioral model has mechanistic components particular to that system. Convergent results from multiple models and comparable dose effect relationships increase the likelihood of assessing mechanisms relevant to the human state.
Preclinical models have long examined a single sex, for several reasons including economy and the belief there is little difference between the sexes. Numerous instances at the behavioral and mechanistic level can now be cited to dispute this assertion.14,15
Many models’ threshold measurements. Alternative models employ “spontaneous behaviors”, including general activity, rearing, weight bearing and gait as markers of an aversive condition.16 There is also an understanding that if there is an aversive condition generated by an injury, a drug that has no intrinsic rewarding property but which serves to diminish that pain state will in fact acquire a positive reinforcing property in the presence of the pain state. Such “conditioned place preference” models have an important place in current drug evaluations.16,17
While preclinical analgesic drug evaluation has been largely successful in rodents, characterization of issues of analgesic efficacy and tolerability may also be achieved through naturally occurring pathologies in companion animals, notably dogs. The incidence of canine osteoarthritis and osteosarcoma provides an important way station in defining efficacy in controlled trials using validated inventories and neurological assessments.18 While safety-toxicology studies in such animals are routinely part of an IND package during drug development, there may be an advantage to pursuing efficacy studies as well. Such information is pivotal in the development of veterinary analgesic products and their approval by the US-FDA-veterinary division to manage the pain states in this patient population. The predicted spending on analgesics for pets alone was predicted to be ~$335 million in 2011, so there is a secondary market that can incentivize additional testing in the veterinary patient.19
Human experimental models initiating a local injury (e.g. ultraviolet B irradiation, thermode burn) or afferent stimulation (capsaicin) are increasingly used to determine efficacy of both new and existing analgesics. Their apparent ability to demonstrate efficacy with known analgesics provides some validation of their sensitivity,20 and to define a drug effect and corresponding side effects at the effective dose.
The following commentary considers a variety of targets and comments on systemic and neuraxial routes of delivery, reflecting the fact that drug effects upon pain processing frequently reflect an action at the first order synapse. It must be stressed that these discussions do not raise issues of safety. This commentary is particularly relevant for neuraxial drugs where appropriate assessment of neuraxial safety must be undertaken prior to such drug implementation.21
Finally, it is challenging to find a drug target that can alter a pain state with a favorable therapeutic ratio (e.g. little or no effects upon mentation, arousal or motor function). An important concern we must now consider is that the drug target is not a mediator of positive reinforcement.
Assessment of abuse liability
An important issue in developing novel analgesics is to overcome the potential for abuse. If the drug acts upon components of systems associated with positive re-enforcement or possessive of stimulant and/or sedative effects, suspicion of its potential for abuse must be elevated. Such examples, at present, include drugs interacting with opioid receptors, CNS depressants (GABAA receptors); CNS stimulants (increased dopamine release/block re-uptake; nicotine), hallucinogens, glutamate antagonists (ketamine) and cannabinoids.22,23 It seems reasonable that a molecule lacking CNS bioavailability would show a reduced likelihood of having a reinforcing property (e.g. loperamide24), but now even large molecules are considered not to be excluded as having potential liability.25 To this end, locomotor, reinforcing and dependence-producing effects of the agent must be routinely assessed. A variety of strategies are considered relevant and have been effective in predicting human-drug behavior including self-administration, drug discrimination and conditioned place preference paradigms.26, 27,28
SURVEY OF CURRENT TARGETS OF PAIN THERAPEUTICS
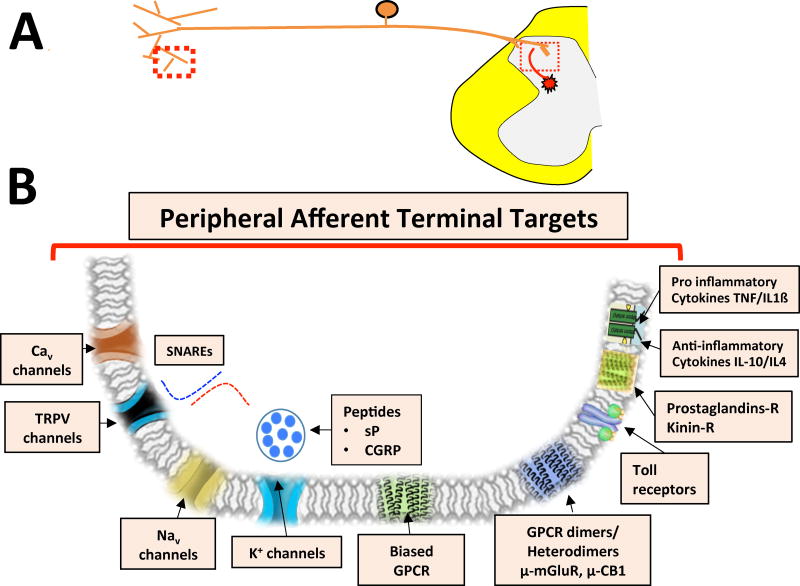
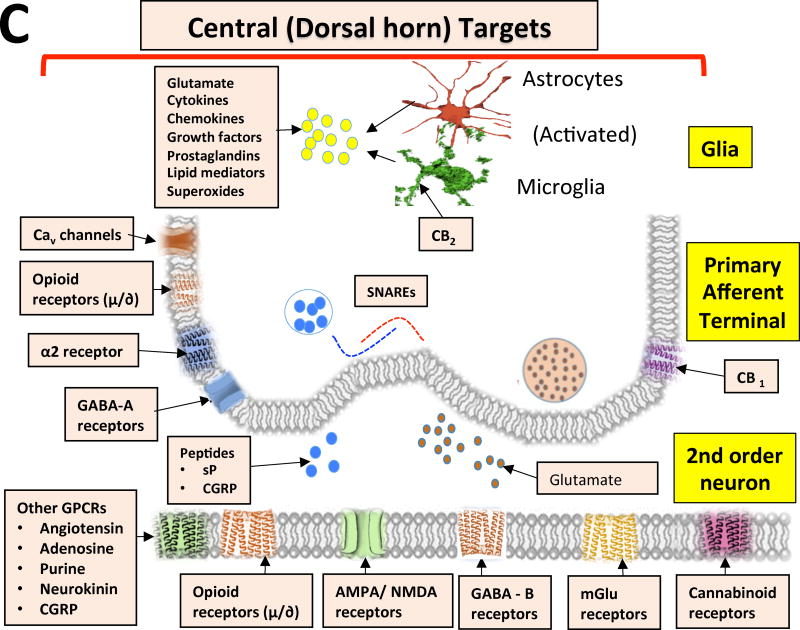
In the following sections, we will consider several current drug targets and potential future directions of research and development. Figure 1 summarizes these targets as they reflect upon actions at the level of the peripheral terminal and central sites. Table 1 summarizes those agents that moved into clinical trials.
Figure 1. Potential Opioid and Non-Opioid Targeted Drugs.
Figure showing some of the key drug targets that act as peripheral (peripheral afferent neuron – peripheral terminal) and central (dorsal horn) targets. Cav - voltage-dependent calcium channel; TRPV - transient receptor potential cation channel; Nav – voltage-gated sodium channel; K - potassium channel; GPCRs - G-protein-coupled receptors; NMDA - N-methyl-d-aspartate; AMPA - α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; GABA - γ-aminobutyric acid; α2 – adrenergic alpha 2 receptor; CB1 - cannabinoid receptor 1; mGlu - metabotropic glutamate receptor
Table 1.
Studies registered on Clinical Trials.gov
| Name of the drug |
Target | Registration Number |
Testing | Indication | Phase of Clinical Trial |
Status of the Study |
|---|---|---|---|---|---|---|
| Eslicarbazepine acetate (ESL) | Voltage-Gated Sodium Channel (VGSC) antagonist | NCT00980746 | assess the efficacy of ESL | painful diabetic neuropathy | Phase 2 | completed |
| Eslicarbazepine acetate (ESL) | Voltage-Gated Sodium Channel (VGSC) antagonist | NCT00981227 | assess the efficacy of ESL | postherpetic neuralgia | Phase 2 | completed |
| Eslicarbazepine acetate (ESL) | Voltage-Gated Sodium Channel (VGSC) antagonist | NCT01820585 | 3 different doses of ESL vs. placebo | fibromyalgia | Phase 2 | completed |
| Eslicarbazepine acetate (ESL) | Voltage-Gated Sodium Channel (VGSC) antagonist | NCT01124097 | assess the efficacy of ESL | postherpetic neuralgia | Phase 3 | terminated |
| Eslicarbazepine acetate (ESL) | Voltage-Gated Sodium Channel (VGSC) antagonist | NCT01129960 | assess the efficacy of ESL | painful diabetic neuropathy | Phase 3 | terminated |
| Neosaxitoxin (NeoSTX) | Voltage Gated Sodium Channels (VGSC) antagonist | NCT01786655 | Neosaxitoxin (NeoSTX) alone or in combination with bupivacaine with or without epinephrine | safety in healthy volunteers | Phase 1 | completed |
| Tetrodotoxin (TTX) | Voltage gated sodium channels (VGSC) antagonist | NCT01655823 | multiple dose levels of tetrodotoxin (TTX) vs. placebo | neuropathic pain | Phase 2 | terminated |
| Z160 | Selective N-type Calcium Channel (Cav 2.2) Blocker | NCT01757873 | compare Z160 and placebo | postherpetic neuralgia | Phase 2 | completed |
| Z160 | Selective N-type Calcium Channel (Cav 2.2) Blocker | NCT01655849 | compare Z160 and placebo | lumbosacral radiculopathy | Phase 2 | completed |
| Ifenprodil Tartrate | NMDA receptor antagonist | NCT01896388 | confirm whether ifenprodil tartrate is effective in the treatment of adolescents PTSD patients | posttraumatic stress disorder | Phase 1/2 | currently recruiting participants |
| Etifoxine | GABAA receptor agonist Agonist at β2 and β3 subunit of the GABAA receptor complex | NCT02147548 | Confirm the effect of etifoxine and lorazepam on vigilance and cognitive functions in the elderly | healthy volunteers | Phase 3 | completed |
| ADL5859 | delta-opioid agonist | NCT00993863 | assess the efficacy and safety of ADL5859 compared with placebo and active control (ibuprofen) | acute dental pain | Phase 2 | completed |
| ADL5859 ADL5747 | delta-opioid agonist | NCT00979953 | assess the efficacy of ADL5859 vs. placebo and ADL5747 vs. placebo | osteoarthritis of the knee | Phase 2 | Completed |
| TRV130 (olicerdine) | Opioid mu receptor agonist Beta arrestin inhibitor | NCT02335294 NCT02820324 | analgesic efficacy IV oliceridine compared with placebo | acute pain after abdominoplasty | Phase 2 Phase 3 | completed |
| TRV130 (olicerdine) | Opioid mu receptor agonist Beta arrestin inhibitor | NCT02100748 NCT02815709 | analgesic efficacy of IV TRV130 vs placebo | bunionectomy | Phase 2 Phase 3 | completed |
| TRV130 (olicerdine) | Opioid mu receptor agonist Beta arrestin inhibitor | NCT02656875 | safety evaluation of TRV130 in patients with acute pain | moderate to severe pain caused by medical conditions or surgery | Phase 3 | currently recruiting participants |
| TRV130 (olicerdine) | Opioid mu receptor agonist Beta arrestin inhibitor | NCT02520297 | analgesic efficacy of TRV130 for moderate to severe acute pain | fracture pain | Phase 2 | terminated |
| EMA401 | Angiotensin 2 receptor (AT2R) antagonist | NCT02435199 | compare EMA401 300 mg and placebo | diabetic neuropathies | Phase 2 | withdrawn |
| EMA401 | Angiotensin 2 receptor (AT2R) antagonist | NCT02426411 | compare two different doses of EMA401 and placebo | postherpetic neuralgia | Phase 2 | withdrawn |
| EMA401 | Angiotensin 2 receptor (AT2R) antagonist | NCT03094195 | 3 different doses vs. placebo | postherpetic neuralgia | Phase 2 | not yet open for recruitment |
| AF219 (gefapixant) | P2X3 receptor antagonist | NCT02349425 | crossover, dose escalation study of gefapixant (AF-219) | refractory chronic cough | Phase 2 | completed |
| AF219 (gefapixant) | P2X3 receptor antagonist | NCT01432730 | effectiveness of gefapixant in reducing daytime objective cough frequency | idiopathic chronic cough | Phase 2 | completed |
| AF219 (gefapixant) | P2X3 receptor antagonist | NCT02397460 | crossover study in healthy and chronic cough subjects | chronic cough | Phase 2 | completed |
| AF219 (gefapixant) | P2X3 receptor antagonist | NCT02476890 | crossover study in healthy and chronic cough subjects | chronic cough | Phase 2 | completed |
| Resiniferatoxin (RTX) | Transient Receptor Potential Vanilloid 1 (TRPV1) receptor agonist | NCT00804154 | safety of intrathecal RTX | cancer pain | Phase 1 | suspended participant recruitment |
| Substance P-Saporin | Neurokinin 1 receptor (NK1R) antagonist | NCT02036281 | 8 different doses intrathecally | terminally ill patients, histologically-confirmed advanced cancer, for intractable pain | Phase 1 | currently recruiting participants |
Sodium channel blockers/Potassium channel activators
Axon excitability depends directly upon voltage gated sodium channel while activation of potassium channels produces hyperpolarization reducing membrane excitability. These represent potential peripheral targets for altering afferent transmission.
Sodium channels
Voltage-gated sodium channel (Nav are the target of all clinical "local anesthetics"29 Nine Nav isoforms with distinct activation properties and tissue distributions have been identified: Nav1.1 and Nav 1. (large DRG/axons), Nav1.4 and Nav 1.5 (skeletal and cardiac muscle), and Nav 1.7,- 1.(small sensory DRGs / afferents).30 Following inflammation and nerve injury, increases in small afferent Nav (Nav1.3, 1.7, 1.8 and 1.9) expression are believed to underlie ectopic afferent traffic and increased responsiveness.30, 31,31,32
Specific confirmation of the role of Nav 1.7 in human pain processing is based on the phenotype of naturally occurring gain and loss of function mutations in Nav1.7 channels wherein those expressing these mutations respectively show pronounced increased and decreased pain states.33,34
While local anesthetics given perineurally and neuraxially produce conduction block anesthesia, systemic anesthetics such as IV lidocaine have surprisingly selective anti-hyperpathic effects in a variety of preclinical models and human pain states at concentrations that do not produce a general conduction block, suggesting a differential sensitivity of systems related to facilitated states after tissue and nerve injury.35,36
Future development of the sodium channel blocking drugs focuses on the role of selective blockers of channels expressed on nociceptive linkages. Clinically used local anesthetics (amide and ester) do not selectively block these channels,37 although several isoforms are sensitive to puffer fish toxin, tetrodotoxin (TTX) (Nav1.1–7), with the remainder resistant to TTX.37,38 Toxin-based sodium channel blockers, neosaxitoxin and tetrodotoxin, after perineural and intrathecal delivery demonstrated long-lasting nerve blocks and surprisingly after systemic delivery in human and animal models.39,40,38,41 (Table 1). As regards selective channel antagonists, intrathecally-delivered, toxin-based Nav1.742 and 1.843 inhibitors have shown preclinical efficacy in models of inflammation and nerve injury, with a favorable therapeutic ratio. Development of systemically bioavailable, small molecule, channel selective antagonists as analgesics have faced challenges.44,45 Clinical work with oral targeted, sodium channel selective blockade was negative,46 although promising results from multi-center studies in post-herpetic neuralgia and primary erythromelalgia have been reported.47 Loss of response to local anesthetics (e.g. tolerance or tachyphylaxis) has been reported after neural blocks, but the phenomena does not appear to be robust.48
An interesting application of the specific association of TRPV1 with pain afferents has been the use of protonated local anesthetics such as QX314, which are able to enter the otherwise impermeant axon membrane through TRPV1 channels upon their activation by capsaicin, and result in function block of the sodium channel in the TRPV1 (+) afferent axon.49 It is now understood that lidocaine by itself is a TRPV1 agonist and can promote passage of the protonated form,50 allowing quaternary lidocaine (QX314) to enter the TRPV1-bearing axon and selectively block the Nav channel, resulting in specific block of TRPV1 (+) primary afferents.50
Potassium channels
There are 4 major families of K channels (Voltage-gated (Kv), Calcium-activated (K Ca); Inwardly rectifying (Kir); Two P domain (K2P) potassium channels, which, when activated lead to membrane hyperpolarization through increased potassium conductance. Genetic analyses illustrate that variations in several K+ channel genes are relevant to the risk for persistent pain after injury (KCNS1-Kv9.1, GIRKs-Gir, TRESK-K2P18.1), increased pain sensitivity (KCNS1, GIRKs), and analgesic efficacy of G protein coupled receptors (GIRK2).51 Inwardly rectifying, ATP sensitive potassium (K-ATP) channels are widely expressed in numerous cell types including neurons and are linked to anti-allodynic and anti-hyperalgesic activity. ATP sensitive potassium channel agonist mediated antinociceptive effects are reversed with pre-treatment with ATP-sensitive K+ channel blockers52,53
Interestingly, autoantibodies targeting Kv channels can lead to neuronal hyperexcitability and a pain state.54 Increasing potassium channel expression and potassium conductance via receptor channel agonists assists in hyperpolarizing (normalizing) otherwise enhanced axon, DRG and terminal excitability, resulting in anti-hyperalgesic actions.55,56
Calcium channel blockers
Movement of calcium into the cell represents a significant source of charge leading to membrane depolarization, while increased intracellular calcium leads to activation of a variety of kinases that phosphorylate: enzymes, channels (lower threshold for activation and increasing ion permeability) and receptors, resulting in hyperalgesic states.57–60 One source of this intracellular calcium is a variety of high and low voltage gated calcium channels (VGCC): high-VGCCs include: L-(CaV1.1–4), P/Q-(CaV2.1), N-(CaV2.2) and R-(CaV2.3) type channels; low-VGCCs include T-type (CaV3.1–3)61,62 These are transmembrane channels, composed of multiple subunits endowing members of each family with distinguishing properties of voltage gating and antagonist pharmacologies. They are located on primary afferents and postsynaptic membranes in spinal dorsal horn..63
N-type channel (Cav 2.2)
The N-type calcium channel is present on presynaptic nerve terminals in the superficial dorsal horn and dorsal root ganglia. Upregulation occurs following peripheral nerve injury.64 Ziconotide, is an N-type VGCC blocker,65 possessing potent anti-hyperpathic properties in rodents and humans when administered intrathecally as a bolus or an infusion66, 67 and is without tachyphylaxis (tolerance).68 Although ziconotide remains the only approved N type channel blocker, there are efforts to develop new peptides and small molecules65,69 and to alter nociceptive properties of N-type VGCC function by hindering its membrane trafficking.70, 71 In humans, a systematically active N-type calcium channel blocker (Z160) failed in Phase 2 clinical trials in treatment of post-herpetic neuralgia and lumbosacral radiculopathy.72 (Table 1.)
L-type channel (Cav 1)
Channels are largely present post synaptically and are considered to play a possible role in maintaining facilitated states. Intrathecal delivery preclinically of channel blockers (nifedipine, verapamil and benzothiazepines) have shown efficiency in altering injury induced hyperpathia.73
T-type channel (Cav3.2)
T-type calcium channels are present in the dorsal horn and channel blockers, such as ethosuximide and mibefradil, have anti hyperalgesic effects in rodents.74
Glutamate receptor targeted agents
Glutamate released from primary afferents, interneurons and from sequestrated stores in astrocytes may interact with a variety of receptor gated ionophores and receptors with G-protein coupling.
NMDA receptor
The NMDA-R is a calcium ionophore composed of three subunits (NR1, NR2 and NR3), each with multiple distinguishable subunits.75 This channel is expressed on primary afferents in the dorsal horn, on second order neurons and on non-neuronal cells (oligodendroglia and astrocytes). Glutamate is released from afferents and interneurons, and binds to the NMDA receptor. At the spinal dorsal horn, high frequency C-fiber stimulation leads to post synaptic depolarization, removal of a Mg2+ ion blocking the pore, and, if the allosterically coupled channel binding sites for glycine and polyamines are occupied,76 there is a channel influx of sodium and calcium76 leading to a cascade known as wind-up.77 NMDA-R blockade inhibits this phenomenon.
Block of NMDA receptor function is achieved by competitive glutamate binding site blockers, noncompetitive channel blockers and agents blocking associated allosteric binding sites.78 While NMDA receptor function may be prevented by blocking any of these sites, the side effect profile (learning, memory, excitability) for these different agents vary considerably and impact upon clinical tolerability.79
Preclinical work has demonstrated the anti-hyperpathic effects in inflammatory and nerve injury models of a variety of intrathecally and/or systemically administered competitive glutamate blockers (2 amino 5 phosphonovalorate), non-competitive NMDA channel blockers (ketamine, MK-801 and memantine, Conantokin-G; agmatine), and glycine site blocker (7 Chlorokynurenic acid, Ifenprodil).80–82 Ifenprodil administered into the rostral cingulate cortex alleviated bone cancer pain in rats.83 While there are surprisingly few high quality clinical trials, ketamine has a long clinical history of use alone and in combination with opioids in diverse pain states characterized by hyperalgesia and allodynia, including neuropathic pain, surgery, and fibromyalgia.84–88 Ifenprodil, an inhibitor of the NMDA glycine-binding site, is currently being tested for the treatment of posttraumatic stress disorder in Phase1/2 study (Table 1).
The abuse potential of NMDA antagonists is controversial and complex.89 Channel blockers such as ketamine have identified abuse potential. This effect may be mediated by channels associated with specific subunit constituents.90 The role of other antagonism motifs in contributing to abuse potential is not known.
AMPA receptor
The AMPA receptor is a glutamate activated sodium selective ionophore, composed of 4 subunits (GluR1-GluR4) which plays a pivotal role in acute dorsal horn evoked excitation.91 In preclinical studies, Tezampanil (LY-293558, NGX-424) displayed efficacy in postoperative pain and spasticity.92,93 In humans, oral administration showed efficacy upon capsaicin evoked hyperalgesia.94 and in post-operative pain.95
With peripheral injury, the AMPA subunit composition changes leading to a calcium permeable channel. Joro spider toxin, selective for calcium permeable AMPA site, decreases secondary mechanical allodynia development evoked in tissue injury models.96 The abuse potential of these agents is not known.
Metabotropic receptors
Eight mGluRs (mGluR1–8) have been identified and are divided into three groups97: Group I (mGluR1 and mGluR5) stimulates phospholipase C (PLC); Group II (mGluR2 and mGluR3) and Group III (mGluR4 – mGluR8) inhibit adenylate cyclase.97,98 mGluRs have been localized on the primary afferents, neurons and glia within the brain and spinal cord.99,100 Group I resides post-synaptically, and Group II and Group III are dominantly located on presynaptic terminal.101
Activation of Group I mGluRs is linked to central sensitization and persistent nociception, while the activation of Group II mGluRs suppresses facilitated states.102 Group I mGluR antagonists have an analgesic action by an effect upon peripheral terminal, spinally and at supraspinal sites.103 Group II mGluR agonists regulate neurotransmitter release and depress pain transmission by acting at different levels of pain neuraxis, including nociceptors, dorsal horn and supraspinal regions such as the amygdala and PAG.104 Group III mGluR agonists are also involved in the control of hyperalgesia following inflammation. As with the other metabotrophic receptors, agonist injections into a peripherally inflamed site or into the spinal dorsal horn regulates glutamatergic transmission in inflammatory and neuropathic pain.103 Group I mGluRs antagonists and Groups II and III mGluRs agonists exhibited analgesic properties in neuropathic or inflammatory pain states105–112 and may serve as a basis to develop future spinally-targeted agents.113,114 Importantly, antagonists affecting Group I mGluRs, have minimal impact on fast synaptic transmission and minimal cognitive effects as compared to ionotropic glutamate antagonists.115
Blocking a glutamate transporter (EAAT-3), reduces intracellular glutamate, attenuates pain and decreases cellular activation. In addition to their cytoplasmic location, mGluR5 are nuclear and may mediate these effects of intracellular glutamate. Accordingly, cell permeable mGluR5 antagonists may show increased efficacy in attenuating neuropathic pain.116
As regards abuse potential, central Group I mGluRs appear to be substrates for stimulants.115 Importantly, antagonists at Group I mGluRs reduce self-administration with no alteration in motor function or the reward value of natural rewards, while agonists at Group II mGluRs prevent reinstatement of drug-seeking after abstinence.117
GABA receptors
GABA, a principal inhibitor transmitter, is expressed in neurons throughout brain and spinal cord. GABAergic spinal interneurons presynaptically regulate large mechanosensitive afferents and postsynaptic excitation input by a potential interaction with two GABA receptors: the GABA-A ionophore and the GABA-B metabotrophic receptor.118,119
GABA-A
The GABA-A receptor is a GABA-gated chloride ionophore, and is composed of five subunits, each with four transmembrane spanning domains. The specific subunits define the binding of a number of molecules at the ionophore. Drugs can activate the channel (GABA. Muscimol), while others (benzodiazepines, neurosteroids, alcohol, many anesthetics) act as positive allosteric modulators at channels having specific subunit composition, stabilizing an open conformation in the presence of the agonist and at greater concentrations to directly activate the chloride channel.120 Studies show dense and variable staining for GABA-A subunits in brain,121 and spinal dorsal horn and on primary afferent terminals,118,122 regulating their excitability.123, 124 Non-spinal GABA-A ionophore activation leads to sedative, anxiolytic and amnestic effects, whereas at the spinal level increased GABA-A activity alters motor function.125 The high degree of GABA-A receptor structure/subtype heterogeneity raises expectations for determining specific structures to target these subtypes.126, 127–129 Subtype specificity may exhibit different effects upon neuronal inhibition in various systems.130
GABA-A agonists, such as muscimol or isoguavacine, display preclinical efficacy in neuropathic pain models.131–135 Intrathecal benzodiazepines depressed nociceptive reflexes in dogs,136 while bolus intrathecal midazolam has displayed efficacy in postoperative, low back and labor pain in humans.137–140 At allosteric binding sites, neurosteroids, such as allopregnanolone, have shown efficacy in preclinical models of tissue and nerve injury.141 Of note, etifoxine promotes production of 3alpha-reduced neurosteroids and has efficacy in reducing mechanical and thermal pain symptoms in vincristine-induced neuropathic pain.142 Further, etifoxine by binding to GABA-A receptor subunits has shown to be effective in different pain disorders followed by anxiety.143 Etifoxine was clinically tested in combination with lorazepam for cognitive improvement in elderly patients (Table 1).
The abuse potential of GABA-A targeted drugs is clearly suggested by role of the GABA-A receptor in reward circuitry. It is clear however that the potential abuse for any GABA-A targeted drug must be interpreted in terms of the subunits with which the drug interacts and the systems with which the subunits are associated.144
GABA- B
Two GABA-B receptors have been cloned and are metabotrophic receptors serving to block the opening of voltage-gated Ca channel and activate inwardly rectifying K channels. These receptors are expressed peripherally and centrally, including thalamus, brain stem nuclei and spinal cord. While positive antinociceptive actions have been reported, they tend to be minimal. An important element is the potent effect upon motor neuron excitability leading to a clinically useful effect on elevated motor tone underlying spasticity occurring with neuraxial injury.119 Lioresal, typically employed by PO or intrathecal delivery in spasticity is not a controlled agent, but significant withdrawal can be seen with drug termination.
Opioid Receptor Targeted Drugs
Mu opioid receptor targeted agonists represent the gold standard for modifying acute nociceptive processing. This action reflects the association of these receptors i) with small afferent input that encode nociceptive processing at the spinal dorsal horn and, ii) at supraspinal levels, regulating spinal processing through descending pathways, altering perceptual processing and initiating reinforcing/reward circuit function.145 Apart from their analgesic efficacy, the classic opioids display tolerance, physical dependence, respiratory depression and a high propensity for abuse.
Receptor targeting
There are three identified gene products that yield three families of opioid receptors (mu/MOR, delta /DOR and kappa/KOR)146 that, when activated, alter pain processing in a naloxone-reversible fashion. More recently, identification of a receptor for the neuropeptide nociceptin has led to designation of a fourth receptor family (NOP), which is typically naloxone insensitive. While subtypes have been proposed, it appears likely that differences in pharmacology within a class may reflect on properties endowed by receptor organization and post-translational processing versus a distinctive receptor protein. These receptors are widely distributed in the brain and spinal cord and are characterized by comparable transmembrane spanning motifs and intracellular G protein coupled receptor (GPCR) signaling. At the membrane level, opioid receptors have typically been shown to be coupled, so that there is a presynaptic action reducing terminal release through a block of calcium-mediated exocytosis and membrane hyperpolarization through an increased potassium conductance.147 At the spinal level, the distribution of opiate receptors on C-fiber terminals and second order neurons is consistent with the analgesic actions being mediated by a block of excitatory transmitter release from C-fibers and inhibition of 2nd order neuron excitability.145 A peripheral opiate action manifested on sensitized afferent nerve terminals is observed reflecting in part the presence of opiate receptors on the peripheral terminals of the afferent.148 Supraspinal opiate actions have been identified wherein the classic descending pathways are considered to be activated by the effects of the opiate receptor on GABA interneurons in the mesencephalon removing a tonic modulation of downstream descending projections.149 Higher order action on forebrain structures have additionally been identified145 and likely reflect upon the effects of opioids on distress.150 Recent work has suggested possible efficacy of kappa opioid antagonists as a migraine therapeutic.302 Preclinical actions of opioids and their effects mediated through the several opioid receptors on pain behavior after systemic and spinal delivery have been reviewed extensively.145,151–153
As noted, the common opiate target for the clinically used agents is typically the mu receptor. The possibility that amongst these receptors there may be subtypes appears likely to reflect other aspects of signaling included ligand bias and the role of heteromers (see below). Delta opioid receptors clearly exert a regulatory role.152 Intrathecal delta preferring agonist such as DADL has analgesic efficacy in humans after intrathecal delivery.154 Two non-peptide molecules ADL5747 and ADL5859 were two orally bioavailable compounds155 tested for acute (NCT00993863) and chronic (NCT00979953) pain management in Phase 2 clinical trials but were not more effective than placebo in osteoarthritic patients.
Kappa opioid agonists which are peripherally restricted have shown minimum abuse potential and efficacy in inflammatory and visceral pain. This along with the potential of a reduced side effect profile and lower abuse potential suggests such agonists as promising candidates for treating pain.156,157
Interestingly while there is a typical aim to seek selective agonists, some have argued that effective improvements in efficacy side effect profiles may be achieved though ligands targeting multiple opioid receptors.158,159
Biased ligands
One of the major strategies that is gaining interest is that GPCRs can associate with multiple second messengers (such as G alpha proteins, β-arrestin, etc.) and ligands can modulate GPCR response via one of those functional pathways, thereby exhibiting “biased agonism.”160,161 Such biased agonists at the mu opioid receptors produce analgesia with limited side effects.162 Currently, a biased ligand (TRV-130) shows analgesia with reduced respiratory depression in phase II clinical trials.163 (Table 1) Recently, an in silico screening approach has identified PZM21, as a mu opioid biased agonist that shows promising analgesic data with reduced side effects.164
Heteromeric Receptors
Many G protein-coupled receptors couple to yield homo- and heteromers.165–167 Such oligomerized receptors serve as targets for developing novel analgesics. For instance, a bivalent ligand containing mu agonist and delta antagonist pharmacophores linked via a spacer (MDAN-21) effectively bridges mu-delta opioid receptor heteromers and exhibits enhanced efficacy and a reduced tendency for tolerance.168,169 Better understanding of mu and delta opioid receptor heteromers will help in understanding peripheral pain as well as development of tolerance as it has been shown that several clinically used opioids are also selective for these heteromers.170–172 A combination of mu-receptor agonists and cannabinoid receptor agonists in rhesus monkey models, showed significant antinociception.173 Mu opioid receptor and CB1 (cannabinoid) receptor heterodimers174 and mu-mGluR5175,176 heteromers with opioid and non-opioid binding sites, expressed strong antinociceptive effects in a range of models. In addition, a small molecule agonist for the mu-kappa opioid receptor heteromer, NNTA, is a potent antinociceptive agent with no propensity to display physical dependence or drug-seeking behavior.177
Tissue target selective opioids
Inflamed tissues display an acidic environment as compared to a healthy tissue. NFEPP is a mu opioid agonist that displays pH-sensitive binding and is thus limited in its activity to a peripheral action at injured/inflamed tissues inflammatory, and is reported to be absent CNS effects or display addiction potential.178
Abuse liability of the classical analgesic opiate agonists reflecting an effect upon higher order neuraxial function is clear. To the degree that a pain state reflects upon activity generated by a peripheral stimulus (e.g. tissue injury, inflammation, neuroma, etc.) opioids with a peripherally- restricted action acting upon systems outside the blood brain barrier offers a potential way forward. As reviewed above, there is anticipation that NOP agonists or opioid agonists restricted to a peripheral action do not have intrinsic reinforcing effects.153 Additional work on the biased ligands and heterodimer systems is required.
Alpha2 adrenergic receptor targeted drugs
Alpha 2 adrenergic agonists have a potent analgesic action that is accompanied by sedation. The analgesic effects are mediated in large part by spinal alpha 2 receptors of which there are three subtypes (Alpha 2A,B,C).179 These are G protein-coupled receptors that regulate dorsal horn excitation produced by small primary afferent input. Studies with mutations, antisense, and antagonists suggest an important role for the alpha2A subtype.180–182 Alpha 2 agonists delivered systemically or intrathecally have significant effects upon acute, inflammatory and nerve injury hyperpathias.183,184 In humans neuraxial alpha2 agonist (clonidine) and systemic (clonidine, tizanidine dexmedetomidine) have analgesic properties with sedation being a common sequelae of the actions of these agents. Dexmedetomidine is not a controlled substance. While the dependence potential of dexmedetomidine has not been studied in human, preclinical studies have shown, as with clonidine, withdrawal upon discontinuation.185
Cannabinoids
Cannabinoids can produce strong antinociceptive results in various animal models of acute, tissue injury, and nerve injury-induced nociception.186 Cannabinoid receptors (CB1 and CB2) are G-protein-bound receptors that negatively bind via Gi/o proteins.187 CB1 receptors are found in spinal neurons, particularly in the dorsal root ganglia,188 and its agonists decrease excitatory transmitter release, whereas CB2 receptors reside in spinal microglia and attenuate microglial activation.189,190 Cannabinoids mediate their psychotropic effects through CB1, not CB2.191,192 Ligands that interact with CB1 and CB2 demonstrated the ability to regulate nociceptive processing.193,194 Agents that block the metabolism of CB1 endogenous agonists consequentially increase its concentration and may be used to activate cannabinoid receptor function.195 CB1 and CB2 selective agents intrathecally-delivered decreased facilitated states such formalin model, hyperpathia in neuropathy models and in tumor bone pain in rodents.196–198 Cannabinoid role in pain processing is based on spinal and nociceptive neuron inhibition, although peripheral sites of action have also been identified. The use of spinal cord stimulation (SCS) in a rodent neuropathic pain model revealed long-lasting and incremental reduction of hyperalgesia mediated by endocannabinoids. The effect was amplified by co-administration of LY2183240, an endocannabinoid reuptake/breakdown inhibitor, and inhibited by a CB1 R antagonist, AM251, but not by a CB2 R antagonist, AM630.199 CB2 receptor antagonists may be a potential target in treating chronic pain of several etiologies by modifying cytokine profile when blocking peripheral immune tissue receptors, and by blocking receptors in neurons and glial cells.200
The anti-nociceptive effect of eslicarbazepine acetate (ESL), an anti-epileptic drug derived from carbamazepine/oxcarbazepine, has been shown to be mediated by serotonergic 5-HT1B/1D and cannabinoid CB1/CB2 receptors. ESL showed beneficial effect in different neuropathic and visceral pain models.201 ESL has been tested clinically in different pain conditions (diabetic neuropathy, PHN, fibromyalgia, etc. (Table 1).
Abuse potential associated with CB1 receptor agonists has been well documented.202 The CB2 receptors has been shown to modulate ventral tegmental dopamine neuron activity, circuitry considered pivotal in the addictive process.203
Angiotensin 2 receptor antagonist
Angiotensin may reside in primary afferents and can activate facilitatory cascades mediated through AT1 and AT2 receptors.204,205 An AT2 antagonist, EMA401 has been tested in Phase 2 clinical trials for the treatment of post-herpetic neuralgia (PHN), and preliminary data showed that it is well tolerated and it exhibited a primary analgesic efficacy endpoint.206 Two phase 2b studies with EMA401 for PHN and painful diabetic neuropathy were put on hold.(207) However, recently, the new Phase 2 study for PHN was registered at clinicaltrials.gov. (Table 1)
Adenosine agonists/antagonists
In models of acute nociceptive processing,208 neuropathy208–212 and inflammatory pain213 administration of adenosine and related ligands yielded significant anti-hyperalgesic effects. Intrathecally administered adenosine lowered allodynia in experimental pain models,214,215 and in patients suffering from neuropathic pain,216,217 although negative results have also been reported.218 Adenosine activates four G-protein-bound receptors: A1, A2A, A2B, A3.219 A1 receptors, which pre-synaptically inhibit neurotransmitter release and post-synoptically inhibit excitatory transmission,220,221 are found on dorsal horn neurons and on small-to-medium-sized neurons of the DRG.220, 222–225 A2A receptor agonists were reportedly able to cause long-term reversal of allodynia in mononeuropathies,226 while the possible explanation was the role of A2A agonists as potential glial inhibitors. In addition, A2A receptors may enhance glutamate release, and A2A antagonists may behave protectively by reducing such excitatory effect.227 A2A receptor knock-out mice showed a significant reduction of the mechanical allodynia and a suppression of thermal hyperalgesia and allodynia, and well as attenuated expression of microglia and astrocytes, confirming potential beneficial role of A2A receptor antagonists in the treatment of neuropathic pain.228 Activation of the A3 adenosine receptor (A3AR) blocked hyperalgesia in mono and polyneuropathies.229 The abuse potential of agonists at these A1–3 receptors is not known.
Purine agonist/antagonists
Adenosine triphosphate, widely present in the CNS,230 reacts with P2 receptor family with several subtypes. The P2X ligand-gated ionotropic receptors (consisting of 7 subtypes) and P2Y\G-protein-coupled receptors (divided into 8 subtypes). P2Y receptors may signal either via Gq/G11 to initiate the phospholipase C/inositol triphosphate (InsP3) endoplasmic reticulum Ca2+-release pathway (the P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors) or via Gi/o, blocking adenylate cyclase and modulating ion channel function.231 Both P2X and P2Y receptors reside in dorsal root ganglia, spinal neurons and glia.232These receptors as glia activators, lead to spinal release of proinflammatory proteins and cytokines, associated to commencing facilitated pain states.233–237 Transient reversal of hyperpathia after nerve injury was achieved via intrathecal administration of P2X and P2Y inhibitors.238–241 The P2X3 subtype is predominantly on C- and Aδ-fiber primary afferent neurons. P2X3 antagonists have shown efficacy in inflammatory and in mono and poly neuropathic pain states.242 P2X4 subtype is important in spinal facilitation that originated from tissue and nerve injury.243 P2X4R antisense oligodeoxynucleotide intrathecal delivery prevented P2X4R protein expression and restrained mechanical allodynia development.238 P2X4R by modulating neuroimmune interactions in the spinal cord and DRG could have an important role development of neuropathic pain, signifying potential therapeutic effects of P2X4 receptor antagonists.244 Electroacupuncture showed beneficial effect in neuropathic pain models by attenuating IFN-γ release and reduced expression of P2X4R in microglia.245 Furthermore, duloxetine, a serotonin and noradrenaline reuptake inhibitor, showed results in neuropathic pain models by inhibition of P2X4 receptors.246 AF-219, a P2X3 antagonist is in clinical development as an antitussive. The abuse potential of purine receptor agonists and antagonists is unknown.247 (Table 1)
Innate immune signaling
Toll-like receptors (TLRs), a key sensory component in innate immune function are found on neuronal and non-neuronal cells in the spinal cord and function by recognizing injury-associated molecular structures, while being strongly associated with proalgesic/inflammatory cytokines (DRG).248,249 Intrathecal TLR4 antagonist administration resulted in improved effects within inflammatory and neuropathic pain states250 and was associated with opiate-induced hyperalgesia phenomenon.251 Another perspective on the role of TLR4 signaling was noted when it was found that the spinal delivery of a TLR4 antagonist (LPS-RS) would prevent the transition from an acute inflammatory state to chronic post inflammatory state with neuropathic pain phenotype252 while a small molecule TLR4 antagonist (TAK242) would prevent the onset of late phase allodynia after intraplantar formalin.253 Repeated intrathecal administration of LPS-RS (TLR2 and TLR4 antagonist) and LPS-RS Ultrapure (TLR4 antagonist) attenuated allodynia and hyperalgesia and potentiated the effect of buprenorphine but not morphine254 Effort has been put into developing new structures to block TLR activation by interacting with the TLR4 ligand or downstream signaling255–257 as shown by the antihyperpathic effects achieved by inhibition of MyD88 signaling.258
Lipid mediators
Prostaglandins
The role of lipid mediators, such as the omega 6 derived prostaglandins, which produce a sensitized primary afferent and is centrally facilitated and mediated by eponymous receptors, has been long appreciated. Discovery of cyclooxygenase isoforms led to the rational development of prostanoid receptor antagonists and isoform specific inhibitors, which were shown to have both a peripheral anti-inflammatory and a central action on spinal facilitatory processing.259 Non selective and COX-2 inhibitors have been shown to have significant anti-hyperpathic effects in a variety of tissue injury pain states in animal models259 and in humans.260 Unfortunately, typical limiting issues involve target related actions on cyclooxygenase (GI, platelet function and cardiovascular) side effects.261 An interesting parallel to the NSAIDs is the actions of acetaminophen.262 This molecule has been shown to be efficacious in a variety of preclinical models and in clinical pain states associated with inflammation and tissue injury and in mono and polyneuropathies, with dose dependent effects upon hyperalgesia and allodynia.263–265 Available as an OTC, this agent, in us for more than 100 years, has revealed no abuse liability. In spite of its utility, its mechanism of action is at best controversial.264
Soluble epoxide hydrolases
The epoxidized metabolites obtained from omega-3 long chain fatty acids show anti-inflammatory and an anti-hyperpathic effect in a variety of preclinical models. However, they are being rapidly metabolized by enzymatic hydrolysis by soluble epoxide hydrolases (SHI). Of interest inhibitors of SHI have been shown to have significant anti-hyperalgesic actions in a variety of preclinical models.266
Proresolvins
Inflammatory cascades are typically self-limited leading to the healing phase of an injury. One of the mechanisms of this resolution has been a variety of lipid mediators referred to as proresolvins. These endogenous mediators include omega 3 (resolvins, protectins, and maresins) and omega 6 derived lipoxins. It is increasingly recognized that anti-inflammation and proresolution cascades represent distinct mechanisms for controlling the inflammatory response.267 Delivery of a variety of these proresolvin molecules has shown to have significant anti-hyperpathic actions in a variety of inflammatory, mono and polyneuropathic models.268,269 The abuse potential of these lipid mediators is not known.
Anti-inflammatory Cytokines
Upon activation of various glial signaling cascades, numerous cytokines (including activation of NF-κB) influence the proinflammatory mediators’ production (e.g. TNF, IL-6, and IL-1β), which, in turn, activate pro-algesic cascades.270 Additionally, such cascades can aid in the release of anti-inflammatory products (e.g. IL-4, IL-6, IL-10, IL-11, IL-13, TGF-β) and soluble cytokine receptors,271 which control the inflammatory cascade.272
IL-10
It has been shown that IL-10 is one of the most powerful endogenous anti-inflammatory cytokines in nervous system.273 In animal models, IL-10 intrathecal delivery demonstrated therapeutic efficacy in various chronic pain models, primarily in treating different types of neuropathic pain.273 In different animal models, viral vector-mediated expression of IL-10 in DRGs prevented development of painful diabetic neuropathy274 and helped in treatment of HIV-induced neuropathy.275
IL4
Another anti-inflammatory cytokine IL-4 showed beneficial role in treating different types of neuropathic pain in different animal models.276–278 An interesting variation is the intrathecal transfection of an IL4/IL10 fusion protein leading to a potent and persistent antihyperalgesia.279
Toxins
There is an increasing interest in potential of producing long term changes in neuraxial pain processing by the peripheral or spinal delivery of agents which target the functionality of systems processing pain information. Here the consideration is for the treatment of persisting pain states. The role of these therapeutic approaches is not clear at present time. For those approaches leading to permanent loss of cells such as the saporin conjugates or the TRPV1 agonists, it appears less likely that they would be employed outside of the terminal patient (as in cancer). Toxins that result in long lasting but irreversible effects such as the botulinum toxins might betherepeutic approach for persistent pain states in a non-terminal patient.
TRPV1 receptors
TRPV1 channels are with few exceptions located on the central and peripheral terminals of high threshold primary afferents. Topical280 and spinal delivery281 of TRPV1 agonists such as capsaicin or analogues such as resiniferatoxin (RTX) desensitize the TRPV1 (+) afferent and destroy the DRG terminal282 by calcium cytotoxicity.283 and analgesia. The effects after topical delivery has led to the approval of transdermal capsaicin.284 Neuraxial delivery of TRPV1 agonists has been shown to result in robust anti-nociception in dogs.285, 286 Intrathecal RTX showed potent and persistent anti-hyperalgesic effects refractory bone cancer pain in canines, without evidence of deafferentation sequelae.287 One clinical trial testing the use of intrathecal resiniferatoxin for intractable cancer pain was begun and is currently on hold. (Table 1)
Saporin conjugates
G-protein-coupled receptors undergo internalization when occupied by their respective agonists.288 Appropriate linking of a G-protein targeted agonist such as substance P (SP) and a toxin such as saporin (plant product from Saponaria officinalis which is not otherwise taken up by the cell) will result after agonist binding to the neurokinin 1 receptor (NK1r), internalization of the agonist and toxin complex into the cell expressing that receptor.289 Saporin blocks riboslyation and protein synthesis, resulting in cell death. The neurokinin 1 (NK1) receptor, a GPCR, found on postsynaptic second order dorsal horn nociceptive neurons290 is taken up into the neuron and the neuron dies. Intrathecally administered sP-saporin, but not saporin, robustly destroys NK1(+) dorsal horn neurons and attenuates pain states in rodents and bone cancer pain in dogs291, 292,293 Intrathecal administration of sP-Saporin is currently in Phase 1 clinical trial for the treatment of intractable cancer pain (Table 1). Importantly, this functional coupling of a ligand to saporin is effective for any ligand for any G protein coupled receptor that displays internalization.289
Botulinum toxin
These toxins are composed of a heavy chain (HC) and a light chain (LC). The HC portion enables the toxin to be taken into the cell. Once inside, the complex is cleaved, freeing LC, which serves as enzyme cleaving SNARES.294, 295 SNAREs mobilize vesicles for transmitter release and aid in the transport of GLUA1 AMPA receptor subunits to the membrane.296 In case of SNARE cleavage, transmitter release is blocked. Preclinically, intrathecally administered BoNTs produced anti-hyperalgesic effects in various inflammatory and neuropathic hyperpathia.297–300 The BoNT uptake is ubiquitous and the potent effects upon transmitter release may include inhibitory interneurons and motor neurons.301,302 Several BoNT serotypes have been shown after topical application to be taken up and to block both local (peripheral) release from a nociceptor and to be transported centrally to inhibit downstream nociceptive processing, with indications of a possible pre- and post-synaptic effect.303 Intravesical injections of onabotulinumtoxin-A (BoNT-A) showed significant pain reduction in patients with interstitial cystitis/bladder pain syndrome (IC/BPS) refractory to other treatments suggesting a local effect upon the urothelium.304 Coupling of the light chain of BoNT-A with substance P showed a beneficial role in treating chronic pain after intrathecal delivery.305
Transfection targets
The use of viral transection at the spinal level represents an exciting approach to modify spinal function. Intrathecal delivery of various transfection systems has been used to increase the expression of cytokines,302,303 knock down of pivotal targets with shRNAs,(304,305) expression of transcription factor decoy proteins,306 over-expression of microRNAs are among many spinal targets that have been successfully manipulated through transfection approaches. Technically, it is clear that while intrathecal delivery of AAV may transfect ganglion neurons, parenchymal transfection may be limited, in part by the diffusion barrier presented by the pia and transfection enhanced by subpial delivery.307 Intraganglionic injections have also been suggested as an efficient tool to alter afferent function.308 The ability to produce long-term, regulated changes in processing offer a potential to modify the pathological expression of pain by modifying system function.
Conclusions
The FDA has been mandated to address the national epidemic of opioid abuse with policies aimed at reversing the epidemic. One element of this plan, recognizing the pivotal role opiate receptors play in pain management, is to stimulate development of more effective pain medications with abuse-deterrent (AD) properties and abuse-deterrent formulations (ADFs) of opioids. The rational way forward is to develop analgesics that minimize abuse potential. In order to improve the translations, the FDA launched the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) that issued recommendations to improve the reproducibility of research pertaining to pain studies.(306)
In the last three decades, by virtue of funding from the national funding agencies and by pharma, we have obtained an increased understanding of pain mechanisms and accordingly an abundance of relevant targets for which we must develop drugable molecules. The development of novel targets and the implementation of approaches that can alter processing for extended periods (transfection and toxins) represent exciting advances in managing the chronic condition.
An important issue in analgesic drug discovery is that apparently promising pre-clinical candidates can fail during clinical trials. Several reasons for this may be entertained. It is straightforward to model human conditions for which the initiating mechanisms are likely known, as for example in chemotherapy-induced neuropathy. Conversely, it is difficult, if not impossible, to rationally define surrogate models for a pain state such as fibromyalgia, where the mechanisms of hyperpathia observed in the human conditions are not known.307 Thus, preclinical models may fail to recapitulate the human pain condition being studied. Research into mechanisms and the appreciation of the role played by innate and adaptive immunity are likely to shed light on these complex problems, revealing novel, mechanistically-defined targets that lack congruence of the clinical and preclinical target, where minor species differences in a receptor sequence may yield a drug that does not engage the human target. Modern molecular techniques and target sequencing makes this disconnect less likely. Further research into biological biomarkers (exosomes) and genetic characterization will provide an important link to define human and animal covariates.308,309
It is interesting to note, as outlined in Figure 1, that a preponderance of the targets producing therapeutic efficacy as analgesics (vs. anesthetics) display a robust effect upon primary afferent and dorsal horn processing that leads to surprisingly specific changes in pain behavior, denoting the role played by the content of the ascending message in characterizing components of the aversive nature of the stimulus event. This emphasis does not exclude the likelihood that many agents, notably opioids, can exert a potent effect upon pain behavior after supraspinal action with such actions accounting for changes in the affective-motivational component of the pain state. While it appears likely that specific supraspinal systems may be found that possess a pharmacology specifically targeting the pain state, current research has provided little evidence that what affects pain processing/behavior at supraspinal sites does not also have pronounced effects upon behavior and perception, aspects of which are associated with positive reward and the addictive potential. These results suggesting the interdigitation of these affective components are in parallel with the early work involving surgical resection of limbic and forebrain structures. While such interventions were reported to lead to a loss of the affective components of the pain state, they also led to profound changes in personality and judgement.310
Finally, the translational development of analgesics for the clinic must increasingly consider the issues of drug abuse. The aim is to address the specific management of pain and suffering. Clearly, agents minimizing the psychological underpinnings of suffering may well display a positive reinforcing component in the absence of pain. This conflation emphasizes the complexity of the problem and the challenges to selectively modify one of the most basic cognitive elements, the pain experience.
Acknowledgments
Disclosures of Funding: The authors acknowledge generous funding support from the following NIH grants: A. S. Y. – DA041912 (NIDA), T.LY: DA15353; NS 099338.
Footnotes
Nebojsa Nick Knezevic
Conflicts of Interest: None
Ajay Yekkirala
Conflicts of Interest: Dr. Ajay Yekkirala holds a patent on an analgesic agent and is co-founder, Chief Scientific Officer and shareholder of Blue Therapeutics, Inc., a biotechnology company developing novel analgesics.
Tony L. Yaksh
Conflicts of Interest: The author has performed contract work with Sorrento on TRPV1 agonists, Advanced targeting on substance P Saporin, Vistagen with glycine site antagonism, Johnson and Johnson on Nav 1.7 antagonists, in particular, and others that were disclosed online.
Nebojsa Nick Knezevic
Contribution: This author contributed to the writing and editing of the manuscript, and approved the final version
Ajay Yekkirala
Contribution: This author contributed to the writing and editing of the manuscript, and approved the final version
Tony L. Yaksh
Contribution: This author contributed to the writing and editing of the manuscript, and approved the final version
Contributor Information
Nebojsa Nick Knezevic, Department of Anesthesiology, Advocate Illinois Masonic Medical Center; Departments of Anesthesiology and Surgery, University of Illinois, Chicago, IL.
Ajay Yekkirala, Department of Neurobiology, Harvard Medical School, and Boston Children's Hospital, Boston, MA; Blue Therapeutics, Harvard Innovation Launch Lab, Allston, MA.
Tony L. Yaksh, Departments of Anesthesiology and Pharmacology, University of California, San Diego, CA; La Jolla, CA.
References
- 1.CDC. Opioid overdose deaths Opioid overdose deaths: Centers for Disease Control and Prevention. 2015 [Google Scholar]
- 2.Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain--Misconceptions and Mitigation Strategies. N Engl J Med. 2016;374:1253–63. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 3.Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med. 2014;15:440–51. doi: 10.1111/pme.12295. [DOI] [PubMed] [Google Scholar]
- 4.Kunins HV. Abuse-deterrent opioid formulations: part of a public health strategy to reverse the opioid epidemic. JAMA Intern Med. 2015;175:987–8. doi: 10.1001/jamainternmed.2015.0939. [DOI] [PubMed] [Google Scholar]
- 5.Chilcoat HD, Coplan PM, Harikrishnan V, Alexander L. Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin) Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Coplan PM, Chilcoat HD, Butler SF, Sellers EM, Kadakia A, Harikrishnan V, Haddox JD, Dart RC. The Effect of an Abuse-Deterrent Opioid Formulation on Opioid Abuse-Related Outcomes in the Post-Marketing Setting. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicero TJ, Ellis MS, Kasper ZA. A tale of 2 ADFs: differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. Pain. 2016;157:1232–8. doi: 10.1097/j.pain.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 8.Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012;367:187–9. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- 9.Yaksh TL, Woller SA, Ramachandran R, Sorkin LS. The search for novel analgesics: targets and mechanisms. F1000Prime Rep. 2015;7:56. doi: 10.12703/P7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren K, Dubner R. Activity-triggered tetrapartite neuron-glial interactions following peripheral injury. Curr Opin Pharmacol. 2016;26:16–25. doi: 10.1016/j.coph.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker SM, Beggs S, Baccei ML. Persistent changes in peripheral and spinal nociceptive processing after early tissue injury. Exp Neurol. 2016;275(Pt 2):253–60. doi: 10.1016/j.expneurol.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaplan S, Eckert W, III, Carruthers N. Drug Discovery and Development for Pain. In: KLaL AR, editor. Translational Pain Research: From Mouse to Man Frontiers in Neuroscience. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. [Google Scholar]
- 13.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–7. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–66. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 15.Doyle HH, Murphy AZ. Sex differences in innate immunity and its impact on opioid pharmacology. J Neurosci Res. 2017;95:487–99. doi: 10.1002/jnr.23852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Ann N Y Acad Sci. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roughan JV, Coulter CA, Flecknell PA, Thomas HD, Sufka KJ. The conditioned place preference test for assessing welfare consequences and potential refinements in a mouse bladder cancer model. PLoS One. 2014;9:e103362. doi: 10.1371/journal.pone.0103362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DC, Bell M, Rhodes L. Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. Am J Vet Res. 2013;74:1467–73. doi: 10.2460/ajvr.74.12.1467. [DOI] [PubMed] [Google Scholar]
- 19.FTC. Competition in the pet medications industry Federal Trade Commission Staff Report: Federal Trade Commission Staff Report. 2015 [Google Scholar]
- 20.van Amerongen G, de Boer MW, Groeneveld GJ, Hay JL. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br J Clin Pharmacol. 2016;82:903–22. doi: 10.1111/bcp.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaksh TL, Fisher CJ, Hockman TM, Wiese AJ. Current and Future Issues in the Development of Spinal Agents for the Management of Pain. Curr Neuropharmacol. 2017;15:232–59. doi: 10.2174/1570159X14666160307145542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson KA, Lovinger DM. Presynaptic G Protein-Coupled Receptors: Gatekeepers of Addiction? Front Cell Neurosci. 2016;10:264. doi: 10.3389/fncel.2016.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Nong Z, Li Y, Huang J, Chen C, Huang L. Role of Dopamine Signaling in Drug Addiction. Curr Top Med Chem. 2017 doi: 10.2174/1568026617666170504100642. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe JH, Kanzler M, Green J. Abuse potential of loperamide. Clin Pharmacol Ther. 1980;28:812–9. doi: 10.1038/clpt.1980.239. [DOI] [PubMed] [Google Scholar]
- 25.Gauvin DV, Zimmermann ZJ, Baird TJ. Preclinical assessment of abuse liability of biologics: In defense of current regulatory control policies. Regul Toxicol Pharmacol. 2015;73:43–54. doi: 10.1016/j.yrtph.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Mansbach RS, Feltner DE, Gold LH, Schnoll SH. Incorporating the assessment of abuse liability into the drug discovery and development process. Drug Alcohol Depend. 2003;70:S73–85. doi: 10.1016/s0376-8716(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 27.Kallman MJ. Preclinical Abuse Potential Assessment. Handb Exp Pharmacol. 2015;229:115–30. doi: 10.1007/978-3-662-46943-9_5. [DOI] [PubMed] [Google Scholar]
- 28.Swedberg MD. Drug discrimination: A versatile tool for characterization of CNS safety pharmacology and potential for drug abuse. J Pharmacol Toxicol Methods. 2016;81:295–305. doi: 10.1016/j.vascn.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Wood JN. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;12(Suppl 3):S93–9. doi: 10.1111/j.1526-4637.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 32.Kharatmal SB, Singh JN, Sharma SS. Voltage-Gated Sodium Channels as Therapeutic Targets for Treatment of Painful Diabetic Neuropathy. Mini Rev Med Chem. 2015;15:1134–47. doi: 10.2174/1389557515666150722112621. [DOI] [PubMed] [Google Scholar]
- 33.Waxman SG. Painful Na-channelopathies: an expanding universe. Trends Mol Med. 2013;19:406–9. doi: 10.1016/j.molmed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst. 2014;19:53–65. doi: 10.1111/jns5.12071. [DOI] [PubMed] [Google Scholar]
- 35.Araujo MC, Sinnott CJ, Strichartz GR. Multiple phases of relief from experimental mechanical allodynia by systemic lidocaine: responses to early and late infusions. Pain. 2003;103:21–9. doi: 10.1016/s0304-3959(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 36.Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003345.pub2. CD003345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Docherty RJ, Farmer CE. The pharmacology of voltage-gated sodium channels in sensory neurones. Handb Exp Pharmacol. 2009:519–61. doi: 10.1007/978-3-540-79090-7_15. [DOI] [PubMed] [Google Scholar]
- 38.Nieto FR, Cobos EJ, Tejada MA, Sanchez-Fernandez C, Gonzalez-Cano R, Cendan CM. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar Drugs. 2012;10:281–305. doi: 10.3390/md10020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwamoto T, Takasugi Y, Higashino H, Ito H, Koga Y, Nakao S. Antinociceptive action of carbamazepine on thermal hypersensitive pain at spinal level in a rat model of adjuvant-induced chronic inflammation. J Anesth. 2011;25:78–86. doi: 10.1007/s00540-010-1046-7. [DOI] [PubMed] [Google Scholar]
- 40.Kohane DS, Lu NT, Gokgol-Kline AC, Shubina M, Kuang Y, Hall S, Strichartz GR, Berde CB. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg Anesth Pain Med. 2000;25:52–9. doi: 10.1016/s1098-7339(00)80011-5. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Navarro AJ, Berde CB, Wiedmaier G, Mercado A, Garcia C, Iglesias V, Zurakowski D. Comparison of neosaxitoxin versus bupivacaine via port infiltration for postoperative analgesia following laparoscopic cholecystectomy: a randomized, double-blind trial. Reg Anesth Pain Med. 2011;36:103–9. doi: 10.1097/aap.0b013e3182030662. [DOI] [PubMed] [Google Scholar]
- 42.Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, Kaczorowski GJ, Garcia ML, Koltzenburg M, Priest BT. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74:1476–84. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- 43.Moon JY, Song S, Yoon SY, Roh DH, Kang SY, Park JH, Beitz AJ, Lee JH. The differential effect of intrathecal Nav1.8 blockers on the induction and maintenance of capsaicin- and peripheral ischemia-induced mechanical allodynia and thermal hyperalgesia. Anesth Analg. 2012;114:215–23. doi: 10.1213/ANE.0b013e318238002e. [DOI] [PubMed] [Google Scholar]
- 44.Vetter I, Deuis JR, Mueller A, Israel MR, Starobova H, Zhang A, Rash LD, Mobli M. NaV1.7 as a pain target - From gene to pharmacology. Pharmacol Ther. 2017;172:73–100. doi: 10.1016/j.pharmthera.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Deuis JR, Dekan Z, Wingerd JS, Smith JJ, Munasinghe NR, Bhola RF, Imlach WL, Herzig V, Armstrong DA, Rosengren KJ, Bosmans F, Waxman SG, Dib-Hajj SD, Escoubas P, Minett MS, Christie MJ, King GF, Alewood PF, Lewis RJ, Wood JN, Vetter I. Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Sci Rep. 2017;7:40883. doi: 10.1038/srep40883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace MS, Rowbotham M, Bennett GJ, Jensen TS, Pladna R, Quessy S. A multicenter, double-blind, randomized, placebo-controlled crossover evaluation of a short course of 4030W92 in patients with chronic neuropathic pain. J Pain. 2002;3:227–33. doi: 10.1054/jpai.2002.123650. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg YP, Price N, Namdari R, Cohen CJ, Lamers MH, Winters C, Price J, Young CE, Verschoof H, Sherrington R, Pimstone SN, Hayden MR. Treatment of Na(v)1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker. Pain. 2012;153:80–5. doi: 10.1016/j.pain.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Kongsgaard UE, Werner MU. Tachyphylaxis to local anaesthetics. What is the clinical evidence? A systematic review. Acta Anaesthesiol Scand. 2016;60:6–14. doi: 10.1111/aas.12631. [DOI] [PubMed] [Google Scholar]
- 49.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–10. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 50.Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol. 2011;164:48–58. doi: 10.1111/j.1476-5381.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsantoulas C. Emerging potassium channel targets for the treatment of pain. Curr Opin Support Palliat Care. 2015;9:147–54. doi: 10.1097/SPC.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 52.Perimal EK, Akhtar MN, Mohamad AS, Khalid MH, Ming OH, Khalid S, Tatt LM, Kamaldin MN, Zakaria ZA, Israf DA, Lajis N, Sulaiman MR. Zerumbone-induced antinociception: involvement of the L-arginine-nitric oxide-cGMP -PKC-K+ ATP channel pathways. Basic Clin Pharmacol Toxicol. 2011;108:155–62. doi: 10.1111/j.1742-7843.2010.00635.x. [DOI] [PubMed] [Google Scholar]
- 53.Zulazmi NA, Gopalsamy B, Min JC, Farouk AA, Sulaiman MR, Bharatham BH, Perimal EK. Zerumbone Alleviates Neuropathic Pain through the Involvement of l-Arginine-Nitric Oxide-cGMP-K(+) ATP Channel Pathways in Chronic Constriction Injury in Mice Model. Molecules. 2017;22 doi: 10.3390/molecules22040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein CJ, Lennon VA, Aston PA, McKeon A, Pittock SJ. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology. 2012;79:1136–44. doi: 10.1212/WNL.0b013e3182698cab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du X, Gamper N. Potassium channels in peripheral pain pathways: expression, function and therapeutic potential. Curr Neuropharmacol. 2013;11:621–40. doi: 10.2174/1570159X113119990042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsantoulas C, McMahon SB. Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci. 2014;37:146–58. doi: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price TJ, Ghosh S. ZIPping to pain relief: the role (or not) of PKMzeta in chronic pain. Mol Pain. 2013;9:6. doi: 10.1186/1744-8069-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edelmayer RM, Brederson JD, Jarvis MF, Bitner RS. Biochemical and pharmacological assessment of MAP-kinase signaling along pain pathways in experimental rodent models: a potential tool for the discovery of novel antinociceptive therapeutics. Biochem Pharmacol. 2014;87:390–8. doi: 10.1016/j.bcp.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Chahine M, O'Leary ME. Regulation/modulation of sensory neuron sodium channels. Handb Exp Pharmacol. 2014;221:111–35. doi: 10.1007/978-3-642-41588-3_6. [DOI] [PubMed] [Google Scholar]
- 60.Luo XQ, Cai QY, Chen Y, Guo LX, Chen AQ, Wu ZQ, Lin C. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord contributes to chronic visceral pain in rats. Brain Res. 2014;1542:167–75. doi: 10.1016/j.brainres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Yaksh TL. Calcium channels as therapeutic targets in neuropathic pain. J Pain. 2006;7:S13–30. doi: 10.1016/j.jpain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol Rev. 2015;67:821–70. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin) 2010;4:510–7. doi: 10.4161/chan.4.6.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroeder CI, Doering CJ, Zamponi GW, Lewis RJ. N-type calcium channel blockers: novel therapeutics for the treatment of pain. Med Chem. 2006;2:535–43. doi: 10.2174/157340606778250216. [DOI] [PubMed] [Google Scholar]
- 65.Adams DJ, Callaghan B, Berecki G. Analgesic conotoxins: block and G protein-coupled receptor modulation of N-type (Ca(V) 2.2) calcium channels. Br J Pharmacol. 2012;166:486–500. doi: 10.1111/j.1476-5381.2011.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel R, Montagut-Bordas C, Dickenson AH. Calcium channel modulation as a target in chronic pain control. Br J Pharmacol. 2017 doi: 10.1111/bph.13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brookes ME, Eldabe S, Batterham A. Ziconotide Monotherapy: A Systematic Review of Randomised Controlled Trials. Curr Neuropharmacol. 2017;15:217–31. doi: 10.2174/1570159X14666160210142056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of omega-conopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain. 1995;60:83–90. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- 69.Chu YX, Zhang Y, Zhang YQ, Zhao ZQ. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun. 2010;24:1176–89. doi: 10.1016/j.bbi.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Feldman P, Khanna R. Challenging the catechism of therapeutics for chronic neuropathic pain: Targeting CaV2.2 interactions with CRMP2 peptides. Neurosci Lett. 2013;557(Pt A):27–36. doi: 10.1016/j.neulet.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie JY, Chew LA, Yang X, Wang Y, Qu C, Wang Y, Federici LM, Fitz SD, Ripsch MS, Due MR, Moutal A, Khanna M, White FA, Vanderah TW, Johnson PL, Porreca F, Khanna R. Sustained relief of ongoing experimental neuropathic pain by a CRMP2 peptide aptamer with low abuse potential. Pain. 2016;157:2124–40. doi: 10.1097/j.pain.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salat K, Kowalczyk P, Gryzlo B, Jakubowska A, Kulig K. New investigational drugs for the treatment of neuropathic pain. Expert Opin Investig Drugs. 2014;23:1093–104. doi: 10.1517/13543784.2014.916688. [DOI] [PubMed] [Google Scholar]
- 73.Roca-Lapirot O, Radwani H, Aby F, Nagy F, Landry M, Fossat P. Calcium signalling through L-type calcium channels: role in pathophysiology of spinal nociceptive transmission. Br J Pharmacol. 2017 doi: 10.1111/bph.13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snutch TP, Zamponi GW. Recent Advances in the Development of T-type Calcium Channel Blockers for Pain Intervention. Br J Pharmacol. 2017 doi: 10.1111/bph.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol. 2011;4:379–88. doi: 10.1586/ecp.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glasgow NG, Siegler Retchless B, Johnson JW. Molecular bases of NMDA receptor subtype-dependent properties. J Physiol. 2015;593:83–95. doi: 10.1113/jphysiol.2014.273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–32. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 78.Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol Sci. 2011;32:726–33. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 80.Yaksh TL, Schwarcz R, Snodgrass HR. Characterization of the effects of L-4-chlorokynurenine on nociception in rodents. J Pain. 2017 doi: 10.1016/j.jpain.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–38. [PubMed] [Google Scholar]
- 82.Yamamoto T, Yaksh TL. Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve. Excitatory amino acid antagonists. Pain. 1992;49:121–8. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]
- 83.Feng H, Chen Z, Wang G, Zhao X, Liu Z. Effect of the ifenprodil administered into rostral anterior cingulate cortex on pain-related aversion in rats with bone cancer pain. BMC Anesthesiol. 2016;16:117. doi: 10.1186/s12871-016-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartzman RJ, Alexander GM, Grothusen JR. The use of ketamine in complex regional pain syndrome: possible mechanisms. Expert Rev Neurother. 2011;11:719–34. doi: 10.1586/ern.11.31. [DOI] [PubMed] [Google Scholar]
- 85.Sheehy KA, Muller EA, Lippold C, Nouraie M, Finkel JC, Quezado ZM. Subanesthetic ketamine infusions for the treatment of children and adolescents with chronic pain: a longitudinal study. BMC Pediatr. 2015;15:198. doi: 10.1186/s12887-015-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iacobucci GJ, Visnjevac O, Pourafkari L, Nader ND. Ketamine: An Update on Cellular and Subcellular Mechanisms with Implications for Clinical Practice. Pain Physician. 2017;20:E285–E301. [PubMed] [Google Scholar]
- 87.Littlejohn G, Guymer E. Modulation of NMDA Receptor Activity in Fibromyalgia. Biomedicines. 2017;5 doi: 10.3390/biomedicines5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bennett M, Bonanno L, Kuhn W. Effectiveness of ketamine as an adjuvant to opioid-based therapy in decreasing pain associated with opioid tolerance in adults undergoing orthopedic surgery: a systematic review protocol. JBI Database System Rev Implement Rep. 2016;14:22–8. doi: 10.11124/JBISRIR-2016-003160. [DOI] [PubMed] [Google Scholar]
- 89.Hopf FW. Do specific NMDA receptor subunits act as gateways for addictive behaviors? Genes Brain Behav. 2017;16:118–38. doi: 10.1111/gbb.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bergeron S, Rompre PP. Blockade of ventral midbrain NMDA receptors enhances brain stimulation reward: a preferential role for GluN2A subunits. Eur Neuropsychopharmacol. 2013;23:1623–35. doi: 10.1016/j.euroneuro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 91.Tong CK, MacDermott AB. Both Ca2+-permeable and -impermeable AMPA receptors contribute to primary synaptic drive onto rat dorsal horn neurons. J Physiol. 2006;575:133–44. doi: 10.1113/jphysiol.2006.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin HC, Keller AJ, Jung JK, Subieta A, Brennan TJ. Epidural tezampanel, an AMPA/kainate receptor antagonist, produces postoperative analgesia in rats. Anesth Analg. 2007;105:1152–9. doi: 10.1213/01.ane.0000281435.58012.e3. table of contents. [DOI] [PubMed] [Google Scholar]
- 93.Oshiro M, Hefferan MP, Kakinohana O, Lukacova N, Sugahara K, Yaksh TL, Marsala M. Suppression of stretch reflex activity after spinal or systemic treatment with AMPA receptor antagonist NGX424 in rats with developed baclofen tolerance. Br J Pharmacol. 2010;161:976–85. doi: 10.1111/j.1476-5381.2010.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sang CN, Hostetter MP, Gracely RH, Chappell AS, Schoepp DD, Lee G, Whitcup S, Caruso R, Max MB. AMPA/kainate antagonist LY293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans. Anesthesiology. 1998;89:1060–7. doi: 10.1097/00000542-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Gilron I, Max MB, Lee G, Booher SL, Sang CN, Chappell AS, Dionne RA. Effects of the 2-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid/kainate antagonist LY293558 on spontaneous and evoked postoperative pain. Clin Pharmacol Ther. 2000;68:320–7. doi: 10.1067/mcp.2000.108677. [DOI] [PubMed] [Google Scholar]
- 96.Sorkin LS, Yaksh TL, Doom CM. Mechanical allodynia in rats is blocked by a Ca2+ permeable AMPA receptor antagonist. Neuroreport. 1999;10:3523–6. doi: 10.1097/00001756-199911260-00011. [DOI] [PubMed] [Google Scholar]
- 97.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 98.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–30. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osikowicz M, Mika J, Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol. 2013;98:372–84. doi: 10.1113/expphysiol.2012.069922. [DOI] [PubMed] [Google Scholar]
- 100.Boye Larsen D, Ingemann Kristensen G, Panchalingam V, Laursen JC, Norgaard Poulsen J, Skallerup Andersen M, Kandiah A, Gazerani P. Investigating the expression of metabotropic glutamate receptors in trigeminal ganglion neurons and satellite glial cells: implications for craniofacial pain. J Recept Signal Transduct Res. 2014;34:261–9. doi: 10.3109/10799893.2014.885049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–22. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palazzo E, Marabese I, de Novellis V, Rossi F, Maione S. Supraspinal metabotropic glutamate receptors: a target for pain relief and beyond. Eur J Neurosci. 2014;39:444–54. doi: 10.1111/ejn.12398. [DOI] [PubMed] [Google Scholar]
- 103.Chiechio S. Modulation of Chronic Pain by Metabotropic Glutamate Receptors. Adv Pharmacol. 2016;75:63–89. doi: 10.1016/bs.apha.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 104.Chiechio S, Nicoletti F. Metabotropic glutamate receptors and the control of chronic pain. Curr Opin Pharmacol. 2012;12:28–34. doi: 10.1016/j.coph.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Mills CD, Johnson KM, Hulsebosch CE. Group I metabotropic glutamate receptors in spinal cord injury: roles in neuroprotection and the development of chronic central pain. J Neurotrauma. 2002;19:23–42. doi: 10.1089/089771502753460213. [DOI] [PubMed] [Google Scholar]
- 106.Mills CD, Johnson KM, Hulsebosch CE. Role of group II and group III metabotropic glutamate receptors in spinal cord injury. Exp Neurol. 2002;173:153–67. doi: 10.1006/exnr.2001.7828. [DOI] [PubMed] [Google Scholar]
- 107.Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139:117–26. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Fisher K, Coderre TJ. Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. Neuroreport. 1996;7:2743–7. doi: 10.1097/00001756-199611040-00067. [DOI] [PubMed] [Google Scholar]
- 109.Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain. 1996;68:255–63. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- 110.Zeilhofer HU. Loss of glycinergic and GABAergic inhibition in chronic pain--contributions of inflammation and microglia. Int Immunopharmacol. 2008;8:182–7. doi: 10.1016/j.intimp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 111.Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain. 2008;137:112–24. doi: 10.1016/j.pain.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 112.Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology. 2012;116:122–32. doi: 10.1097/ALN.0b013e31823de68d. [DOI] [PubMed] [Google Scholar]
- 113.Acher F, Goudet C. Therapeutic potential of group III metabotropic glutamate receptor ligands in pain. Curr Opin Pharmacol. 2015;20:64–72. doi: 10.1016/j.coph.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Montana MC, Gereau RW. Metabotropic glutamate receptors as targets for analgesia: antagonism, activation, and allosteric modulation. Curr Pharm Biotechnol. 2011;12:1681–8. doi: 10.2174/138920111798357438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vincent K, Wang SF, Laferriere A, Kumar N, Coderre TJ. Spinal intracellular metabotropic glutamate receptor 5 (mGluR5) contributes to pain and c-fos expression in a rat model of inflammatory pain. Pain. 2017;158:705–16. doi: 10.1097/j.pain.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 117.Markou A. The role of metabotropic glutamate receptors in drug reward, motivation and dependence. Drug News Perspect. 2007;20:103–8. doi: 10.1358/dnp.2007.20.2.1083435. [DOI] [PubMed] [Google Scholar]
- 118.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 119.Malcangio M. GABAB receptors and pain. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 120.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 122.Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–97. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Labrakakis C, Tong CK, Weissman T, Torsney C, MacDermott AB. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol. 2003;549:131–42. doi: 10.1113/jphysiol.2002.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31:8134–42. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yaksh TL, Allen JW. The use of intrathecal midazolam in humans: a case study of process. Anesth Analg. 2004;98:1536–45. doi: 10.1213/01.ANE.0000122638.41130.BF. table of contents. [DOI] [PubMed] [Google Scholar]
- 126.Krall J, Balle T, Krogsgaard-Larsen N, Sorensen TE, Krogsgaard-Larsen P, Kristiansen U, Frolund B. GABAA receptor partial agonists and antagonists: structure, binding mode, and pharmacology. Adv Pharmacol. 2015;72:201–27. doi: 10.1016/bs.apha.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–9. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 128.Ferando I, Mody I. Interneuronal GABAA receptors inside and outside of synapses. Curr Opin Neurobiol. 2014;26:57–63. doi: 10.1016/j.conb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Petersen JG, Bergmann R, Krogsgaard-Larsen P, Balle T, Frolund B. Probing the orthosteric binding site of GABAA receptors with heterocyclic GABA carboxylic acid bioisosteres. Neurochem Res. 2014;39:1005–15. doi: 10.1007/s11064-013-1226-6. [DOI] [PubMed] [Google Scholar]
- 130.Hoestgaard-Jensen K, Dalby NO, Krall J, Hammer H, Krogsgaard-Larsen P, Frolund B, Jensen AA. Probing alpha4betadelta GABAA receptor heterogeneity: differential regional effects of a functionally selective alpha4beta1delta/alpha4beta3delta receptor agonist on tonic and phasic inhibition in rat brain. J Neurosci. 2014;34:16256–72. doi: 10.1523/JNEUROSCI.1495-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dirig DM, Yaksh TL. Intrathecal baclofen and muscimol, but not midazolam, are antinociceptive using the rat-formalin model. J Pharmacol Exp Ther. 1995;275:219–27. [PubMed] [Google Scholar]
- 132.Nadeson R, Guo Z, Porter V, Gent JP, Goodchild CS. gamma-Aminobutyric acidA receptors and spinally mediated antinociception in rats. J Pharmacol Exp Ther. 1996;278:620–6. [PubMed] [Google Scholar]
- 133.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 134.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–7. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 135.Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 2006;23:1111–24. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- 136.Whitwam JG, Niv D, Loh L, Jack RD. Depression of nociceptive reflexes by intrathecal benzodiazepine in dogs. Lancet. 1982;2:1465. doi: 10.1016/s0140-6736(82)91368-x. [DOI] [PubMed] [Google Scholar]
- 137.Serrao JM, Marks RL, Morley SJ, Goodchild CS. Intrathecal midazolam for the treatment of chronic mechanical low back pain: a controlled comparison with epidural steroid in a pilot study. Pain. 1992;48:5–12. doi: 10.1016/0304-3959(92)90125-U. [DOI] [PubMed] [Google Scholar]
- 138.Tucker AP, Lai C, Nadeson R, Goodchild CS. Intrathecal midazolam I: a cohort study investigating safety. Anesth Analg. 2004;98:1512–20. doi: 10.1213/01.ANE.0000087075.14589.F5. table of contents. [DOI] [PubMed] [Google Scholar]
- 139.Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam II: combination with intrathecal fentanyl for labor pain. Anesth Analg. 2004;98:1521–7. doi: 10.1213/01.ANE.0000112434.68702.E4. table of contents. [DOI] [PubMed] [Google Scholar]
- 140.Ho KM, Ismail H. Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis. Anaesth Intensive Care. 2008;36:365–73. doi: 10.1177/0310057X0803600307. [DOI] [PubMed] [Google Scholar]
- 141.Coronel MF, Labombarda F, Gonzalez SL. Neuroactive steroids, nociception and neuropathic pain: A flashback to go forward. Steroids. 2016;110:77–87. doi: 10.1016/j.steroids.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 142.Aouad M, Charlet A, Rodeau JL, Poisbeau P. Reduction and prevention of vincristine-induced neuropathic pain symptoms by the non-benzodiazepine anxiolytic etifoxine are mediated by 3alpha-reduced neurosteroids. Pain. 2009;147:54–9. doi: 10.1016/j.pain.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Choi YM, Kim KH. Etifoxine for pain patients with anxiety. Korean J Pain. 2015;28:4–10. doi: 10.3344/kjp.2015.28.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stephens DN, King SL, Lambert JJ, Belelli D, Duka T. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav. 2017;16:149–84. doi: 10.1111/gbb.12321. [DOI] [PubMed] [Google Scholar]
- 145.Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997;41:94–111. doi: 10.1111/j.1399-6576.1997.tb04623.x. [DOI] [PubMed] [Google Scholar]
- 146.Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol. 2015;172:317–23. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reisine T, Law SF, Blake A, Tallent M. Molecular mechanisms of opiate receptor coupling to G proteins and effector systems. Ann N Y Acad Sci. 1996;780:168–75. doi: 10.1111/j.1749-6632.1996.tb15121.x. [DOI] [PubMed] [Google Scholar]
- 148.Stein C, Baerwald C. Opioids for the treatment of arthritis pain. Expert Opin Pharmacother. 2014;15:193–202. doi: 10.1517/14656566.2014.861818. [DOI] [PubMed] [Google Scholar]
- 149.Ingram SL. Cellular and molecular mechanisms of opioid action. Prog Brain Res. 2000;129:483–92. doi: 10.1016/S0079-6123(00)29035-3. [DOI] [PubMed] [Google Scholar]
- 150.Kim J, Ham S, Hong H, Moon C, Im HI. Brain Reward Circuits in Morphine Addiction. Mol Cells. 2016;39:645–53. doi: 10.14348/molcells.2016.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chavkin C. The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36:369–70. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abdallah K, Gendron L. The Delta Opioid Receptor in Pain Control. Handb Exp Pharmacol. 2017 doi: 10.1007/164_2017_32. [DOI] [PubMed] [Google Scholar]
- 153.Kiguchi N, Ding H, Ko MC. Central N/OFQ-NOP Receptor System in Pain Modulation. Adv Pharmacol. 2016;75:217–43. doi: 10.1016/bs.apha.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Onofrio BM, Yaksh TL. Intrathecal delta-receptor ligand produces analgesia in man. Lancet. 1983;1:1386–7. doi: 10.1016/s0140-6736(83)92170-0. [DOI] [PubMed] [Google Scholar]
- 155.Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD, Graczyk TM, Belanger S, Cassel JA, Feschenko MS, Brogdon BL, Smith SA, Christ DD, Derelanko MJ, Kutz S, Little PJ, DeHaven RN, DeHaven-Hudkins DL, Dolle RE. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4'-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–6. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- 156.Kivell B, Prisinzano TE. Kappa opioids and the modulation of pain. Psychopharmacology (Berl) 2010;210:109–19. doi: 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- 157.Jones MR, Kaye AD, Kaye AJ, Urman RD. The emerging therapeutic roles of kappa-opioid agonists. J Opioid Manag. 2016;12:101–7. doi: 10.5055/jom.2016.0321. [DOI] [PubMed] [Google Scholar]
- 158.Gunther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S. Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br J Pharmacol. 2017 doi: 10.1111/bph.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pasternak GW. Opioids and their receptors: Are we there yet? Neuropharmacology. 2014;76(Pt B):198–203. doi: 10.1016/j.neuropharm.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–39. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, Skobieranda F, Violin JD, Webster LR. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155:1829–35. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 163.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–72. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 164.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–90. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yekkirala AS. Two to tango: GPCR oligomers and GPCR-TRP channel interactions in nociception. Life Sci. 2012;92:438–45. doi: 10.1016/j.lfs.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 166.Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66:413–34. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alfaras-Melainis K, Gomes I, Rozenfeld R, Zachariou V, Devi L. Modulation of opioid receptor function by protein-protein interactions. Front Biosci (Landmark Ed) 2009;14:3594–607. doi: 10.2741/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–13. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem Biol. 2013;8:1412–6. doi: 10.1021/cb400113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yekkirala AS, Kalyuzhny AE, Portoghese PS. Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [D-Ala2-MePhe4-Glyol5]enkephalin as selective µ-∂ agonists. ACS Chemical Neuroscience. 2010;1:146–54. doi: 10.1021/cn9000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yekkirala AS, Banks ML, Lunzer MM, Negus SS, Rice KC, Portoghese PS. Clinically employed opioid analgesics produce antinociception via mu-delta opioid receptor heteromers in Rhesus monkeys. ACS Chem Neurosci. 2012;3:720–7. doi: 10.1021/cn300049m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maguire DR, France CP. Impact of efficacy at the mu-opioid receptor on antinociceptive effects of combinations of mu-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther. 2014;351:383–9. doi: 10.1124/jpet.114.216648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Le Naour M, Akgun E, Yekkirala A, Lunzer MM, Powers MD, Kalyuzhny AE, Portoghese PS. Bivalent ligands that target mu opioid (MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance. J Med Chem. 2013;56:5505–13. doi: 10.1021/jm4005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smeester BA, Lunzer MM, Akgun E, Beitz AJ, Portoghese PS. Targeting putative mu opioid/metabotropic glutamate receptor-5 heteromers produces potent antinociception in a chronic murine bone cancer model. Eur J Pharmacol. 2014;743:48–52. doi: 10.1016/j.ejphar.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Akgun E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS. Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proc Natl Acad Sci U S A. 2013;110:11595–9. doi: 10.1073/pnas.1305461110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese PS. N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc Natl Acad Sci U S A. 2011;108:5098–103. doi: 10.1073/pnas.1016277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, Durmaz V, Sabri P, Reidelbach M, Machelska H, Weber M, Stein C. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science. 2017;355:966–9. doi: 10.1126/science.aai8636. [DOI] [PubMed] [Google Scholar]
- 179.Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–95. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- 180.Takano Y, Yaksh TL. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J Pharmacol Exp Ther. 1992;261:764–72. [PubMed] [Google Scholar]
- 181.Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–65. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mizobe T, Maghsoudi K, Sitwala K, Tianzhi G, Ou J, Maze M. Antisense technology reveals the alpha2A adrenoceptor to be the subtype mediating the hypnotic response to the highly selective agonist, dexmedetomidine, in the locus coeruleus of the rat. J Clin Invest. 1996;98:1076–80. doi: 10.1172/JCI118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yaksh TL. Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol Biochem Behav. 1985;22:845–58. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 184.Giovannoni MP, Ghelardini C, Vergelli C, Dal Piaz V. Alpha2-agonists as analgesic agents. Med Res Rev. 2009;29:339–68. doi: 10.1002/med.20134. [DOI] [PubMed] [Google Scholar]
- 185.Nguyen V, Tiemann D, Park E, Salehi A. Alpha-2 Agonists. Anesthesiol Clin. 2017;35:233–45. doi: 10.1016/j.anclin.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 186.Dhopeshwarkar A, Mackie K. CB2 Cannabinoid receptors as a therapeutic target-what does the future hold? Mol Pharmacol. 2014;86:430–7. doi: 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vasileiou I, Fotopoulou G, Matzourani M, Patsouris E, Theocharis S. Evidence for the involvement of cannabinoid receptors' polymorphisms in the pathophysiology of human diseases. Expert Opin Ther Targets. 2013;17:363–77. doi: 10.1517/14728222.2013.754426. [DOI] [PubMed] [Google Scholar]
- 188.Morisset V, Ahluwalia J, Nagy I, Urban L. Possible mechanisms of cannabinoid-induced antinociception in the spinal cord. Eur J Pharmacol. 2001;429:93–100. doi: 10.1016/s0014-2999(01)01309-7. [DOI] [PubMed] [Google Scholar]
- 189.Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60:255–66. doi: 10.1016/j.brainresrev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Starowicz K, Przewlocka B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos Trans R Soc Lond B Biol Sci. 2012;367:3286–99. doi: 10.1098/rstb.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–27. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 2015;77:475–87. doi: 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–21. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Davis MP. Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert Opin Investig Drugs. 2014;23:1123–40. doi: 10.1517/13543784.2014.918603. [DOI] [PubMed] [Google Scholar]
- 195.Piomelli D. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology. 2014;76(Pt B):228–34. doi: 10.1016/j.neuropharm.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cui JH, Kim WM, Lee HG, Kim YO, Kim CM, Yoon MH. Antinociceptive effect of intrathecal cannabinoid receptor agonist WIN 55,212-2 in a rat bone tumor pain model. Neurosci Lett. 2011;493:67–71. doi: 10.1016/j.neulet.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 197.Cui JH, Ju J, Yoon MH. Pharmacology of cannabinoid receptor agonists and a cyclooxygenase-2 inhibitor in rat bone tumor pain. Pharmacology. 2013;92:150–7. doi: 10.1159/000354296. [DOI] [PubMed] [Google Scholar]
- 198.Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Kuhn MN, Zvonok AM, Thakur GA, Makriyannis A, Milligan ED. Immunofluorescent spectral analysis reveals the intrathecal cannabinoid agonist, AM1241, produces spinal anti-inflammatory cytokine responses in neuropathic rats exhibiting relief from allodynia. Brain Behav. 2012;2:155–77. doi: 10.1002/brb3.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sun L, Tai L, Qiu Q, Mitchell R, Fleetwood-Walker S, Joosten EA, Cheung CW. Endocannabinoid activation of CB1 receptors contributes to long-lasting reversal of neuropathic pain by repetitive spinal cord stimulation. Eur J Pain. 2017;21:804–14. doi: 10.1002/ejp.983. [DOI] [PubMed] [Google Scholar]
- 200.Shang Y, Tang Y. The central cannabinoid receptor type-2 (CB2) and chronic pain. Int J Neurosci. 2016:1–12. doi: 10.1080/00207454.2016.1257992. [DOI] [PubMed] [Google Scholar]
- 201.Tomic MA, Pecikoza UB, Micov AM, Stepanovic-Petrovic RM. The Efficacy of Eslicarbazepine Acetate in Models of Trigeminal, Neuropathic, and Visceral Pain: The Involvement of 5-HT1B/1D Serotonergic and CB1/CB2 Cannabinoid Receptors. Anesth Analg. 2015;121:1632–9. doi: 10.1213/ANE.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 202.Panagis G, Mackey B, Vlachou S. Cannabinoid Regulation of Brain Reward Processing with an Emphasis on the Role of CB1 Receptors: A Step Back into the Future. Front Psychiatry. 2014;5:92. doi: 10.3389/fpsyt.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A. 2014;111:E5007–15. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Smith MT, Woodruff TM, Wyse BD, Muralidharan A, Walther T. A small molecule angiotensin II type 2 receptor (AT(2)R) antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen-activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. Pain Med. 2013;14:1557–68. doi: 10.1111/pme.12157. [DOI] [PubMed] [Google Scholar]
- 205.Smith MT, Wyse BD, Edwards SR. Small molecule angiotensin II type 2 receptor (AT(2)R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding, and efficacy in rats. Pain Med. 2013;14:692–705. doi: 10.1111/pme.12063. [DOI] [PubMed] [Google Scholar]
- 206.Rice AS, Dworkin RH, McCarthy TD, Anand P, Bountra C, McCloud PI, Hill J, Cutter G, Kitson G, Desem N, Raff M, group EMAs EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–47. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- 207.Keppel Hesselink JM, Schatman ME. EMA401: an old antagonist of the AT2R for a new indication in neuropathic pain. J Pain Res. 2017;10:439–43. doi: 10.2147/JPR.S128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sosnowski M, Stevens CW, Yaksh TL. Assessment of the role of A1/A2 adenosine receptors mediating the purine antinociception, motor and autonomic function in the rat spinal cord. J Pharmacol Exp Ther. 1989;250:915–22. [PubMed] [Google Scholar]
- 209.Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996;277:1642–8. [PubMed] [Google Scholar]
- 210.Cui JG, Sollevi A, Linderoth B, Meyerson BA. Adenosine receptor activation suppresses tactile hypersensitivity and potentiates spinal cord stimulation in mononeuropathic rats. Neurosci Lett. 1997;223:173–6. doi: 10.1016/s0304-3940(97)13435-8. [DOI] [PubMed] [Google Scholar]
- 211.Yamaguchi D, Terayama R, Omura S, Tsuchiya H, Sato T, Ichikawa H, Sugimoto T. Effect of adenosine A1 receptor agonist on the enhanced excitability of spinal dorsal horn neurons after peripheral nerve injury. Int J Neurosci. 2014;124:213–22. doi: 10.3109/00207454.2013.842566. [DOI] [PubMed] [Google Scholar]
- 212.Katz NK, Ryals JM, Wright DE. Central or peripheral delivery of an adenosine A1 receptor agonist improves mechanical allodynia in a mouse model of painful diabetic neuropathy. Neuroscience. 2015;285:312–23. doi: 10.1016/j.neuroscience.2014.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zahn PK, Straub H, Wenk M, Pogatzki-Zahn EM. Adenosine A1 but not A2a receptor agonist reduces hyperalgesia caused by a surgical incision in rats: a pertussis toxin-sensitive G protein-dependent process. Anesthesiology. 2007;107:797–806. doi: 10.1097/01.anes.0000286982.36342.3f. [DOI] [PubMed] [Google Scholar]
- 214.Rane K, Karlsten R, Sollevi A, Gordh T, Jr, Svensson BA. Spinal cord morphology after chronic intrathecal administration of adenosine in the rat. Acta Anaesthesiol Scand. 1999;43:1035–40. doi: 10.1034/j.1399-6576.1999.431011.x. [DOI] [PubMed] [Google Scholar]
- 215.Eisenach JC, Curry R, Hood DD. Dose response of intrathecal adenosine in experimental pain and allodynia. Anesthesiology. 2002;97:938–42. doi: 10.1097/00000542-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 216.Belfrage M, Segerdahl M, Arner S, Sollevi A. The safety and efficacy of intrathecal adenosine in patients with chronic neuropathic pain. Anesth Analg. 1999;89:136–42. doi: 10.1097/00000539-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 217.Eisenach JC, Rauck RL, Curry R. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 218.Sharma M, Mohta M, Chawla R. Efficacy of intrathecal adenosine for postoperative pain relief. Eur J Anaesthesiol. 2006;23:449–53. doi: 10.1017/S0265021506000342. [DOI] [PubMed] [Google Scholar]
- 219.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 220.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 221.Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994;72:1611–21. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- 222.Choca JI, Green RD, Proudfit HK. Adenosine A1 and A2 receptors of the substantia gelatinosa are located predominantly on intrinsic neurons: an autoradiography study. J Pharmacol Exp Ther. 1988;247:757–64. [PubMed] [Google Scholar]
- 223.Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–40. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 224.Roberts JC, Grocholski BM, Kitto KF, Fairbanks CA. Pharmacodynamic and pharmacokinetic studies of agmatine after spinal administration in the mouse. J Pharmacol Exp Ther. 2005;314:1226–33. doi: 10.1124/jpet.105.086173. [DOI] [PubMed] [Google Scholar]
- 225.Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience. 2003;121:907–16. doi: 10.1016/s0306-4522(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 226.Loram LC, Harrison JA, Sloane EM, Hutchinson MR, Sholar P, Taylor FR, Berkelhammer D, Coats BD, Poole S, Milligan ED, Maier SF, Rieger J, Watkins LR. Enduring reversal of neuropathic pain by a single intrathecal injection of adenosine 2A receptor agonists: a novel therapy for neuropathic pain. J Neurosci. 2009;29:14015–25. doi: 10.1523/JNEUROSCI.3447-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev Neurosci. 2011;22:231–9. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- 228.Bura SA, Nadal X, Ledent C, Maldonado R, Valverde O. A 2A adenosine receptor regulates glia proliferation and pain after peripheral nerve injury. Pain. 2008;140:95–103. doi: 10.1016/j.pain.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 229.Chen Z, Janes K, Chen C, Doyle T, Bryant L, Tosh DK, Jacobson KA, Salvemini D. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J. 2012;26:1855–65. doi: 10.1096/fj.11-201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 231.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 232.Kobayashi K, Yamanaka H, Noguchi K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int. 2013;88:10–6. doi: 10.1007/s12565-012-0163-9. [DOI] [PubMed] [Google Scholar]
- 233.Bianco F, Fumagalli M, Pravettoni E, D'Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48:144–56. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 234.Inoue K, Tsuda M. [The role of microglia and ATP receptors in a mechanism of neuropathic pain] Nihon Yakurigaku Zasshi. 2006;127:14–7. doi: 10.1254/fpj.127.14. [DOI] [PubMed] [Google Scholar]
- 235.Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 236.Clark AK, Wodarski R, Guida F, Sasso O, Malcangio M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia. 2010;58:1710–26. doi: 10.1002/glia.21042. [DOI] [PubMed] [Google Scholar]
- 237.Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–15. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- 238.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 239.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–96. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 240.Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60:1529–39. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- 241.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–56. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jung YH, Kim YO, Lin H, Cho JH, Park JH, Lee SD, Bae J, Kang KM, Kim YG, Pae AN, Ko H, Park CS, Yoon MH, Kim YC. Discovery of Potent Antiallodynic Agents for Neuropathic Pain Targeting P2X3 Receptors. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.6b00401. [DOI] [PubMed] [Google Scholar]
- 243.Trang T, Salter MW. P2X4 purinoceptor signaling in chronic pain. Purinergic Signal. 2012;8:621–8. doi: 10.1007/s11302-012-9306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jurga AM, Piotrowska A, Makuch W, Przewlocka B, Mika J. Blockade of P2X4 Receptors Inhibits Neuropathic Pain-Related Behavior by Preventing MMP-9 Activation and, Consequently, Pronociceptive Interleukin Release in a Rat Model. Front Pharmacol. 2017;8:48. doi: 10.3389/fphar.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chen XM, Xu J, Song JG, Zheng BJ, Wang XR. Electroacupuncture inhibits excessive interferon-gamma evoked up-regulation of P2X4 receptor in spinal microglia in a CCI rat model for neuropathic pain. Br J Anaesth. 2015;114:150–7. doi: 10.1093/bja/aeu199. [DOI] [PubMed] [Google Scholar]
- 246.Yamashita T, Yamamoto S, Zhang J, Kometani M, Tomiyama D, Kohno K, Tozaki-Saitoh H, Inoue K, Tsuda M. Duloxetine Inhibits Microglial P2X4 Receptor Function and Alleviates Neuropathic Pain after Peripheral Nerve Injury. PLoS One. 2016;11:e0165189. doi: 10.1371/journal.pone.0165189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385:1198–205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 248.Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, Schaloske RH, Deems RA, Dennis EA, Yaksh TL. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol. 2010;160:1754–64. doi: 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–44. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–91. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jacobsen JH, Watkins LR, Hutchinson MR. Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. Int Rev Neurobiol. 2014;118:129–63. doi: 10.1016/B978-0-12-801284-0.00006-3. [DOI] [PubMed] [Google Scholar]
- 252.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, Yaksh TL. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:2881–91. doi: 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: The role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun. 2016;56:271–80. doi: 10.1016/j.bbi.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J. Blockade of Toll-Like Receptors (TLR2, TLR4) Attenuates Pain and Potentiates Buprenorphine Analgesia in a Rat Neuropathic Pain Model. Neural Plast. 2016;2016:5238730. doi: 10.1155/2016/5238730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Connolly DJ, O'Neill LA. New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol. 2012;12:510–8. doi: 10.1016/j.coph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 256.Lucas K, Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang X, Smith C, Yin H. Targeting Toll-like receptors with small molecule agents. Chem Soc Rev. 2013;42:4859–66. doi: 10.1039/c3cs60039d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu F, Wang Z, Qiu Y, Wei M, Li C, Xie Y, Shen L, Huang Y, Ma C. Suppression of MyD88-dependent signaling alleviates neuropathic pain induced by peripheral nerve injury in the rat. J Neuroinflammation. 2017;14:70. doi: 10.1186/s12974-017-0822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–83. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- 260.Jones P, Dalziel SR, Lamdin R, Miles-Chan JL, Frampton C. Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007789.pub2. CD007789. [DOI] [PubMed] [Google Scholar]
- 261.Sanghi S, MacLaughlin EJ, Jewell CW, Chaffer S, Naus PJ, Watson LE, Dostal DE. Cyclooxygenase-2 inhibitors: a painful lesson. Cardiovasc Hematol Disord Drug Targets. 2006;6:85–100. doi: 10.2174/187152906777441803. [DOI] [PubMed] [Google Scholar]
- 262.Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21:201–32. doi: 10.1007/s10787-013-0172-x. [DOI] [PubMed] [Google Scholar]
- 263.Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–31. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 264.Toussaint K, Yang XC, Zielinski MA, Reigle KL, Sacavage SD, Nagar S, Raffa RB. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. 2010;35:617–38. doi: 10.1111/j.1365-2710.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 265.Candido KD, Perozo OJ, Knezevic NN. Pharmacology of Acetaminophen, Nonsteroidal Antiinflammatory Drugs, and Steroid Medications: Implications for Anesthesia or Unique Associated Risks. Anesthesiol Clin. 2017;35:e145–e62. doi: 10.1016/j.anclin.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 266.Wagner K, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins Other Lipid Mediat. 2011;96:76–83. doi: 10.1016/j.prostaglandins.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–8. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lim JY, Park CK, Hwang SW. Biological Roles of Resolvins and Related Substances in the Resolution of Pain. Biomed Res Int. 2015;2015:830930. doi: 10.1155/2015/830930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sacerdote P, Franchi S, Moretti S, Castelli M, Procacci P, Magnaghi V, Panerai AE. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J Neuroimmune Pharmacol. 2013;8:202–11. doi: 10.1007/s11481-012-9428-2. [DOI] [PubMed] [Google Scholar]
- 271.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 272.Tayal V, Kalra BS. Cytokines and anti-cytokines as therapeutics--an update. Eur J Pharmacol. 2008;579:1–12. doi: 10.1016/j.ejphar.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 273.Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation. 2012;15:520–6. doi: 10.1111/j.1525-1403.2012.00462.x. discussion 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Thakur V, Gonzalez M, Pennington K, Chattopadhyay M. Viral vector mediated continuous expression of interleukin-10 in DRG alleviates pain in type 1 diabetic animals. Mol Cell Neurosci. 2016;72:46–53. doi: 10.1016/j.mcn.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 275.Zheng W, Huang W, Liu S, Levitt RC, Candiotti KA, Lubarsky DA, Hao S. Interleukin 10 mediated by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 in rats. Anesth Analg. 2014;119:693–701. doi: 10.1213/ANE.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kiguchi N, Kobayashi Y, Saika F, Sakaguchi H, Maeda T, Kishioka S. Peripheral interleukin-4 ameliorates inflammatory macrophage-dependent neuropathic pain. Pain. 2015;156:684–93. doi: 10.1097/j.pain.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 278.Sun S, Chen D, Lin F, Chen M, Yu H, Hou L, Li C. Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol Immunol. 2016;77:184–92. doi: 10.1016/j.molimm.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 279.Eijkelkamp N, Steen-Louws C, Hartgring SA, Willemen HL, Prado J, Lafeber FP, Heijnen CJ, Hack CE, van Roon JA, Kavelaars A. IL4-10 Fusion Protein Is a Novel Drug to Treat Persistent Inflammatory Pain. J Neurosci. 2016;36:7353–63. doi: 10.1523/JNEUROSCI.0092-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vyklicky L, Novakova-Tousova K, Benedikt J, Samad A, Touska F, Vlachova V. Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol Res. 2008;57(Suppl 3):S59–68. doi: 10.33549/physiolres.931478. [DOI] [PubMed] [Google Scholar]
- 281.Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Szolcsanyi J. Capsaicin and sensory neurones: a historical perspective. Prog Drug Res. 2014;68:1–37. doi: 10.1007/978-3-0348-0828-6_1. [DOI] [PubMed] [Google Scholar]
- 283.Brown DC. Resiniferatoxin: The Evolution of the "Molecular Scalpel" for Chronic Pain Relief. Pharmaceuticals (Basel) 2016;9 doi: 10.3390/ph9030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kissin I, Szallasi A. Therapeutic targeting of TRPV1 by resiniferatoxin, from preclinical studies to clinical trials. Curr Top Med Chem. 2011;11:2159–70. doi: 10.2174/156802611796904924. [DOI] [PubMed] [Google Scholar]
- 285.Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–9. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 286.Bevan S, Quallo T, Andersson DA. Trpv1. Handb Exp Pharmacol. 2014;222:207–45. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 287.Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015;156:1018–24. doi: 10.1097/j.pain.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Latapy C, Beaulieu JM. beta-Arrestins in the central nervous system. Prog Mol Biol Transl Sci. 2013;118:267–95. doi: 10.1016/B978-0-12-394440-5.00011-5. [DOI] [PubMed] [Google Scholar]
- 289.Wiley RG, Lappi DA. Targeted toxins in pain. Adv Drug Deliv Rev. 2003;55:1043–54. doi: 10.1016/s0169-409x(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 290.Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–9. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- 291.Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–98. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Brown DC, Agnello K. Intrathecal substance P-saporin in the dog: efficacy in bone cancer pain. Anesthesiology. 2013;119:1178–85. doi: 10.1097/ALN.0b013e3182a95188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wiese AJ, Rathbun M, Butt MT, Malkmus SA, Richter PJ, Osborn KG, Xu Q, Veesart SL, Steinauer JJ, Higgins D, Lappi DA, Russell B, Yaksh TL. Intrathecal substance P-saporin in the dog: distribution, safety, and spinal neurokinin-1 receptor ablation. Anesthesiology. 2013;119:1163–77. doi: 10.1097/ALN.0b013e3182a95164. [DOI] [PubMed] [Google Scholar]
- 294.Pellett S. Learning from the past: historical aspects of bacterial toxins as pharmaceuticals. Curr Opin Microbiol. 2012;15:292–9. doi: 10.1016/j.mib.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 295.Pellett S, Yaksh TL, Ramachandran R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 2015;7:4519–63. doi: 10.3390/toxins7114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kakegawa W, Yuzaki M. A mechanism underlying AMPA receptor trafficking during cerebellar long-term potentiation. Proc Natl Acad Sci U S A. 2005;102:17846–51. doi: 10.1073/pnas.0508910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Marinelli S, Luvisetto S, Cobianchi S, Makuch W, Obara I, Mezzaroma E, Caruso M, Straface E, Przewlocka B, Pavone F. Botulinum neurotoxin type A counteracts neuropathic pain and facilitates functional recovery after peripheral nerve injury in animal models. Neuroscience. 2010;171:316–28. doi: 10.1016/j.neuroscience.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 298.Huang PP, Khan I, Suhail MS, Malkmus S, Yaksh TL. Spinal botulinum neurotoxin B: effects on afferent transmitter release and nociceptive processing. PLoS One. 2011;6:e19126. doi: 10.1371/journal.pone.0019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lee WH, Shin TJ, Kim HJ, Lee JK, Suh HW, Lee SC, Seo K. Intrathecal administration of botulinum neurotoxin type A attenuates formalin-induced nociceptive responses in mice. Anesth Analg. 2011;112:228–35. doi: 10.1213/ANE.0b013e3181ffa1d7. [DOI] [PubMed] [Google Scholar]
- 300.Coelho A, Oliveira R, Rossetto O, Cruz CD, Cruz F, Avelino A. Intrathecal administration of botulinum toxin type A improves urinary bladder function and reduces pain in rats with cystitis. Eur J Pain. 2014;18:1480–9. doi: 10.1002/ejp.513. [DOI] [PubMed] [Google Scholar]
- 301.Ramachandran R, Yaksh TL. Therapeutic use of botulinum toxin in migraine: mechanisms of action. Br J Pharmacol. 2014;171:4177–92. doi: 10.1111/bph.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Matak I, Lackovic Z. Botulinum toxin A, brain and pain. Prog Neurobiol. 2014;119–120:39–59. doi: 10.1016/j.pneurobio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 303.Marino MJ, Terashima T, Steinauer JJ, Eddinger KA, Yaksh TL, Xu Q. Botulinum toxin B in the sensory afferent: transmitter release, spinal activation, and pain behavior. Pain. 2014;155:674–84. doi: 10.1016/j.pain.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sidaway P. Pain: BoNT-A reduces pain in patients with treatment refractory IC/BPS. Nat Rev Urol. 2015;12:300. doi: 10.1038/nrurol.2015.101. [DOI] [PubMed] [Google Scholar]
- 305.Mustafa G, Anderson EM, Bokrand-Donatelli Y, Neubert JK, Caudle RM. Anti-nociceptive effect of a conjugate of substance P and light chain of botulinum neurotoxin type A. Pain. 2013;154:2547–53. doi: 10.1016/j.pain.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Andrews NA, Latremoliere A, Basbaum AI, Mogil JS, Porreca F, Rice AS, Woolf CJ, Currie GL, Dworkin RH, Eisenach JC, Evans S, Gewandter JS, Gover TD, Handwerker H, Huang W, Iyengar S, Jensen MP, Kennedy JD, Lee N, Levine J, Lidster K, Machin I, McDermott MP, McMahon SB, Price TJ, Ross SE, Scherrer G, Seal RP, Sena ES, Silva E, Stone L, Svensson CI, Turk DC, Whiteside G. Ensuring transparency and minimization of methodologic bias in preclinical pain research: PPRECISE considerations. Pain. 2016;157:901–9. doi: 10.1097/j.pain.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov. 2017 doi: 10.1038/nrd.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chizh BA, Greenspan JD, Casey KL, Nemenov MI, Treede RD. Identifying biological markers of activity in human nociceptive pathways to facilitate analgesic drug development. Pain. 2008;140:249–53. doi: 10.1016/j.pain.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Latremoliere A, Costigan M. Combining Human and Rodent Genetics to Identify New Analgesics. Neurosci Bull. 2017 doi: 10.1007/s12264-017-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.White J, Sweet WH. Pain and the Neurosurgeon. Springfield, IL: Charles Thomas; 1969. [Google Scholar]