The molecular mechanism governing affinity-based B cell selection for germinal center colonization is unclear. Zaretsky et al. show that B cell ICAMs promote efficient B cell selection for clonal expansion by supporting sustained interactions with T follicular helper cells.
Abstract
The germinal center (GC) reaction begins with a diverse and expanded group of B cell clones bearing a wide range of antibody affinities. During GC colonization, B cells engage in long-lasting interactions with T follicular helper (Tfh) cells, a process that depends on antigen uptake and antigen presentation to the Tfh cells. How long-lasting T–B interactions and B cell clonal expansion are regulated by antigen presentation remains unclear. Here, we use in vivo B cell competition models and intravital imaging to examine the adhesive mechanisms governing B cell selection for GC colonization. We find that intercellular adhesion molecule 1 (ICAM-1) and ICAM-2 on B cells are essential for long-lasting cognate Tfh–B cell interactions and efficient selection of low-affinity B cell clones for proliferative clonal expansion. Thus, B cell ICAMs promote efficient antibody immune response by enhancement of T cell help to cognate B cells.
Introduction
Germinal centers (GCs) are microanatomic sites that emerge within secondary lymphoid organs in response to an immunogenic challenge. Within the GC, B cells undergo extensive cell division, somatic hypermutation (SHM), and affinity-based selection by T follicular helper (Tfh) cells (Allen et al., 2007; Victora and Nussenzweig, 2012). These specialized CD4+ T effector cells preferentially select B cells that present high levels of peptide-MHCII (pMHCII) for extensive proliferation or differentiation to antibody-forming cells (Victora et al., 2010). Iterative cycles of cell division and SHM followed by selection by Tfh cells in the GC results in a progressive increase in serum antibody affinity (Kepler and Perelson, 1993), a process known as antibody affinity maturation (Eisen and Siskind, 1964). Formation of protective antibodies is greatly dependent on an initial selection stage of antigen-specific B cells from the germline repertoire for GC colonization (Schmidt et al., 2015).
Numerous antigen-specific B cells expressing B cell receptors (BCRs) of various affinities have an intrinsic potential to respond to their cognate antigen and clonally expand, before GC formation (Dal Porto et al., 2002; Shih et al., 2002; Schwickert et al., 2011). However, only B cells that express the highest-affinity BCRs are selected by Tfh cells to undergo clonal expansion and differentiation into either early plasmablasts or GC cells (Phan et al., 2003; Schwickert et al., 2011). This selection process among the responding B cells takes place at the border between the B cell follicle and the T zone where antigen-specific B cells congregate after initial priming (Garside et al., 1998; Reif et al., 2002; Okada et al., 2005; Schwickert et al., 2011). In similarity to the GC response, B cell clonal selection is thought to depend on stringent T cell–dependent selection that promotes GC colonization by B cells bearing relatively higher-affinity BCRs (Schwickert et al., 2011). Several studies found that the early GC reaction that emerges in response to immunization with a complex antigen is composed of many different clones bearing BCRs of various affinities, including low-affinity clones (Kuraoka et al., 2016; Tas et al., 2016). Furthermore, the germline variants of mutated broadly neutralizing antibodies to influenza virus and HIV show surprisingly low binding affinities to their cognate antigens. Nevertheless, germline clones such as these must be selected during the earliest stages of the B cell response for optimal protection from these pathogens (Lingwood et al., 2012; Klein et al., 2013; Bannard and Cyster, 2017). How B cell clones bearing BCRs of various affinities are selected for clonal expansion and GC colonization remains unclear.
Intravital imaging experiments have demonstrated that B cell competition for T cell help at the earliest stages of the immune response is highly dynamic, involving B cells interacting with multiple T cells (Okada et al., 2005; Qi et al., 2008; Schwickert et al., 2011). Long-lasting T–B contacts are essential for GC seeding (Qi et al., 2008) and are thought to promote selection of the highest-affinity B cell clones for proliferative expansion by facilitating delivery of essential T cell–derived help signals for B cells (Qi et al., 2008; Schwickert et al., 2011; Qi, 2016). Optimal T–B interactions depend in part on signaling lymphocytic activation molecules (SLAMs) and their intracellular adaptor SLAM-associated protein (Qi et al., 2008; Cannons et al., 2011). These molecules are thought to support adhesive contacts between T and B cells; however, they lack the typical characteristics of adhesion molecules such as TCR-triggered clustering and conformational changes. In addition to SLAMs, Tfh cells express high levels of the integrin lymphocyte function–associated antigen 1 (LFA-1; Meli et al., 2016), and B cells express variable levels of the LFA-1 ligands intercellular adhesion molecule 1 (ICAM-1) and ICAM-2 (Dennig et al., 1994; Montoya et al., 2002; Sánchez-Madrid and Serrador, 2009). TCR triggering by pMHCII induces LFA-1 conformational changes and clustering that are required for multivalent associations with ICAM molecules, leading to stable T cell–APC synapses (Evans et al., 2009; Feigelson et al., 2010). Furthermore, it was shown that LFA-1 binding to ICAMs is required for optimal TCR signaling and T cell effector functions (Brunmark and O’Rourke, 1997; Wülfing et al., 1998; Scholer et al., 2008). Whether these molecules play a role in T–B interactions in vivo and B cell selection for clonal expansion by Tfh cells is unknown.
High levels of pMHCII are required for both long-lasting T–B interactions and B cell selection for clonal expansion by Tfh cells (Victora et al., 2010; Schwickert et al., 2011; Shulman et al., 2014). However, cognate pMHCII:TCR interactions are insufficient for establishing optimal T–B adhesion and TCR signaling in vitro (Brunmark and O’Rourke, 1997; Wülfing et al., 1998; Liu et al., 2016). Thus, additional adhesive mechanisms and costimulatory counterreceptors, which are potentially regulated by TCR binding of pMHCII, are likely to contribute to optimization of cognate T–B interactions and subsequent B cell clonal expansion. In the present study, we examined the potential additive roles of ICAM-1 and ICAM-2 on individual B cells in the selection of B cell clones by Tfh cells. We found that B cell ICAMs are essential for long-lasting pMHCII-driven Tfh–B cell interactions that promote B cell selection for proliferative clonal expansion during GC colonization.
Results
ICAM-1 and ICAM-2 are highly expressed on activated B cells, and their integrin receptor LFA-1 is elevated on Tfh cells
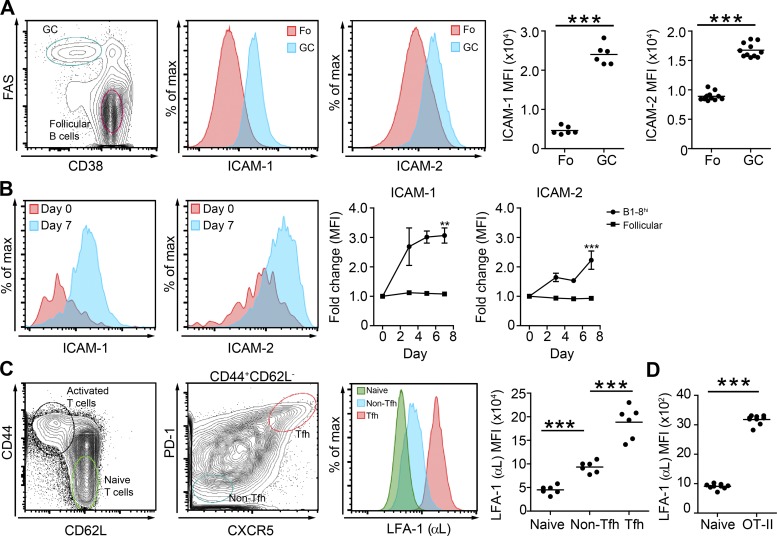
The LFA-1 integrin ligands, ICAM-1 and ICAM-2 are highly expressed on activated lymphocytes and are important for T cell–APC interactions (Dustin, 2008; Scholer et al., 2008; Sánchez-Madrid and Serrador, 2009). To examine whether B cell ICAMs play a role in B cell immune responses, we first examined whether these molecules are up-regulated on the surface of GC B cells. To this end, we immunized WT mice with 4-hydroxy-3-nitrophenyl acetyl (NP) conjugated to OVA s.c. and analyzed follicular (FAS− CD38+) and GC (FAS+ CD38low) B cells by flow cytometry 7 d after immunogenic challenge. We found that GC B cells express fivefold more ICAM-1 and 1.9-fold more ICAM-2 compared with follicular B cells in popliteal LNs (Fig. 1 A). To examine the kinetics of ICAM-1 and ICAM-2 expression, we tracked ICAM levels over time on antigen-specific B cells. For this purpose, we used B1-8hi B cells that carry a knock-in Ig heavy chain that, when combined with an Igλ light chain, produces a high-affinity BCR specific for NP (Shih et al., 2002). Flow cytometric analysis of adoptively transferred B1-8hi B cells revealed that NP-specific Igλ+ B1-8hi B cells exhibited 2.7-fold more ICAM-1 at day 3 after immunization compared with day 0, and these elevated levels remained stably high within the GC compartment at day 7 (Fig. 1 B). ICAM-2 levels were 1.6-fold elevated at day 3 and 2.2-fold elevated during GC formation through day 7 (Fig. 1 B). We did not detect changes in ICAM expression on follicular B cells that are largely not specific for NP (Fig. 1 B). For analysis of LFA-1 expression on Tfh cells, we immunized WT mice with NP-OVA and compared integrin expression in the naive, activated non-Tfh, and Tfh subpopulations. Consistent with previous findings (Meli et al., 2016), Tfh cells were found to express the highest levels of LFA-1 among the responding T cells 7 d after an immune challenge (Fig. 1 C). To examine whether LFA-1 up-regulation takes place early during the immune response, we adoptively transferred OVA-specific OT-II T cells into WT mice and measured LFA-1 expression on day 3 after immunization with NP-OVA. We found that antigen-specific T cells express threefold higher levels of LFA-1 compared with their naive counterparts (Fig. 1 D). We conclude that ICAMs and LFA-1 are up-regulated early during the antibody immune response on antigen-specific B and T cells, respectively.
Figure 1.
Expression of ICAM-1 and ICAM-2 on B cells and LFA-1 on T cells in response to immunization. (A) Flow cytometric plot and histograms showing gating strategy and ICAM expression on follicular (Fo) and germinal center (GC) B cells in popliteal LNs of mice, 7 d after immunization with NP-OVA. Graph shows quantification of mean fluorescence intensity (MFI) of ICAM-1 and ICAM-2. Data are pooled from two independent experiments with a total of six to 12 mice per group. (B) Representative histograms depicting ICAM-1 and ICAM-2 expression on adoptively transferred B1-8hi B cells (day 0) and NP-specific Igλ+ B1-8hi B cells, 7 d after immunization. Graphs show fold increase in ICAM-1 and ICAM-2 expression over time of NP-specific B cells (Igλ+) and follicular polyclonal B cells. Data are pooled from two independent experiments with a total of six mice per group. (C) Flow cytometric plots and histograms depicting the gating strategy for T cell subsets and their LFA-1 (αL) expression 7 d after immunization with NP-OVA. Data are pooled from two independent experiments with a total of six mice per group. (D) LFA-1 expression on endogenous naive T cells and on transferred TdTomato+ OT-II T cells 3 d after immunization. Data are pooled from two independent experiments with a total of eight mice per group. **, P = 0.01; ***, P < 0.001; two-tailed Student’s t test. Each dot in the graphs represents a single mouse; lines represent the mean; error bars represent SEM.
B cell ICAMs provide a selective advantage in T cell–dependent immune responses
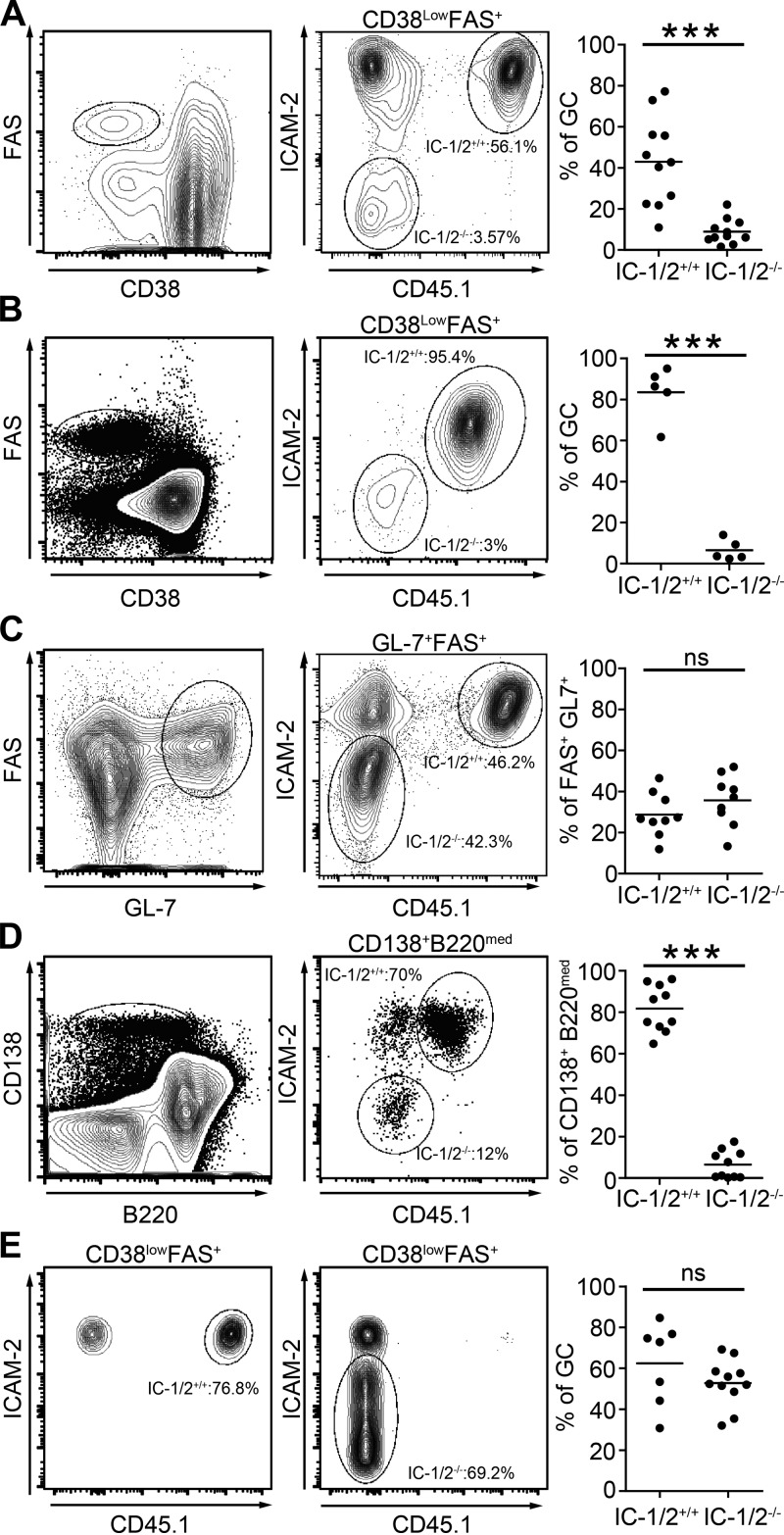
Integrins are important for T cell interactions with ICAM-decorated APCs, including B cells (Wülfing et al., 1998). However, ICAM-1–, ICAM-2–, and LFA-1–deficient mice are not suitable for analysis of the antibody response, as these molecules are expressed on many cell types and are critical for immune cell trafficking (Berlin-Rufenach et al., 1999; Ley et al., 2007). Moreover, the functions of ICAM-1 and ICAM-2 are partially redundant. Thus, to study the role of these molecules specifically on antigen-specific B cells, we interbred ICAM-1– and ICAM-2–deficient mice (ICAM-1/2−/−; Gorina et al., 2014) with B1-8hi transgenic mice to produce ICAM-1/2 double-knockout B1-8hi mice. To test the role of B cell ICAMs independent of their role in Tfh formation and maintenance (Meli et al., 2016), we adoptively transferred ICAM-1/2+/+ and ICAM-1/2−/− B cells to the same WT hosts and examined their competence to form GCs. Equal numbers of B cells derived from CD45.1+ ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi mice were adoptively transferred into WT hosts before s.c. immunization with NP-OVA. After 14 d, we found that the GC compartment in popliteal LNs contained 4.8-fold more ICAM-1/2+/+ than ICAM-1/2−/− B1-8hi B cells (Fig. 2 A). Similar results were obtained using the MD4 BCR transgenic mice as hosts, in which endogenous B cells do not recognize NP and do not participate in the immune response (Fig. 2 B). Analysis of the distribution of adoptively transferred cells between the GC light and dark zone revealed that the few ICAM-1/2−/− B1-8hi B cells that entered the GC response were normally distributed between these two compartments (Fig. S1). The defect of the ICAM-deficient B cells was not a result of inefficient homing to LNs, because the proportion of the two types of transferred cells within the naive compartment (FAS− CD38+) was similar (Fig. S2). To understand whether ICAMs play a role specifically in T cell–dependent immune responses, we examined their role in the T cell–independent immune response wherein B cells are primed by nonprotein antigen and undergo extensive proliferation in the absence of cognate T cell help. To elicit such a response, WT hosts that were adoptively transferred with a mixture of CD45.1+ ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi B cells were immunized with a nonprotein antigen NP-Ficoll. By analysis of the B cell activation markers, FAS and GL-7, we found that under these conditions both types of B cells responded to the antigen to an equal extent (Fig. 2 C). Collectively, these results demonstrate that the T cell–dependent B cell immune response depends on B cell ICAMs, whereas the T cell–independent immune response does not require ICAMs. During the first stages of the emerging T cell–dependent immune response, antigen-specific B cells are also selected by Tfh to differentiate into plasmablasts. Adoptively transferred ICAM-1/2−/− B1-8hi B cells were outcompeted by ICAM-1/2+/+ B1-8hi B cells for entry into the plasmablast compartment, as assessed by expression of the plasma cell marker CD138 (Fig. 2 D). To investigate whether the defective participation of ICAM-1/2−/− B cells in GCs is a result of ineffective competition, we next examined GC formation in LNs in the absence of high-affinity competitors. We found that when ICAM-1/2+/+ or ICAM-1/2−/− B1-8hi B cells were transferred separately into WT hosts, their proportion in the GC compartment was comparable (Fig. 2 E). We conclude that B cell ICAMs provide a competitive advantage to B cells, specifically in a T cell–dependent antibody response.
Figure 2.
B cell ICAMs are required for competitive GC and plasmablast formation in a T cell–dependent immune response. (A) Representative flow cytometric analysis and quantification of IC-1/2+/+ and IC-1/2−/− B1-8hi B cells in GCs of recipient mice 14 d after immunization with NP-OVA. Data are pooled from two independent experiments with a total of 11 mice per group. (B) Analysis of the GC compartment as in A, in MD4 host mice, 7 d after immunization. Data are pooled from two independent experiments with a total of five mice per group. (C) Representative flow cytometric analysis of activated B cells in WT hosts 3 d after adoptive cell transfer and immunization with NP-Ficoll. Data are pooled from two independent experiments with a total of nine mice per group. (D) Flow cytometric analysis of adoptively transferred B cells in the CD138+ B220med plasmablast compartment. Data are pooled from two independent experiments with a total of 10 mice per group. (E) Percentages of B cell subsets within the GC compartment in NP-OVA immunized mice to which either IC-1/2+/+ or IC-1/2−/− B1-8hi B cells were transferred. Data are pooled from two independent experiments with a total of seven to 11 mice per group. Each dot in the graphs represents a single mouse; lines represent the mean. ***, P < 0.0004; ns, not significant; two-tailed Student’s t test.
ICAMs provide a competitive advantage in B cell polyclonal immune responses
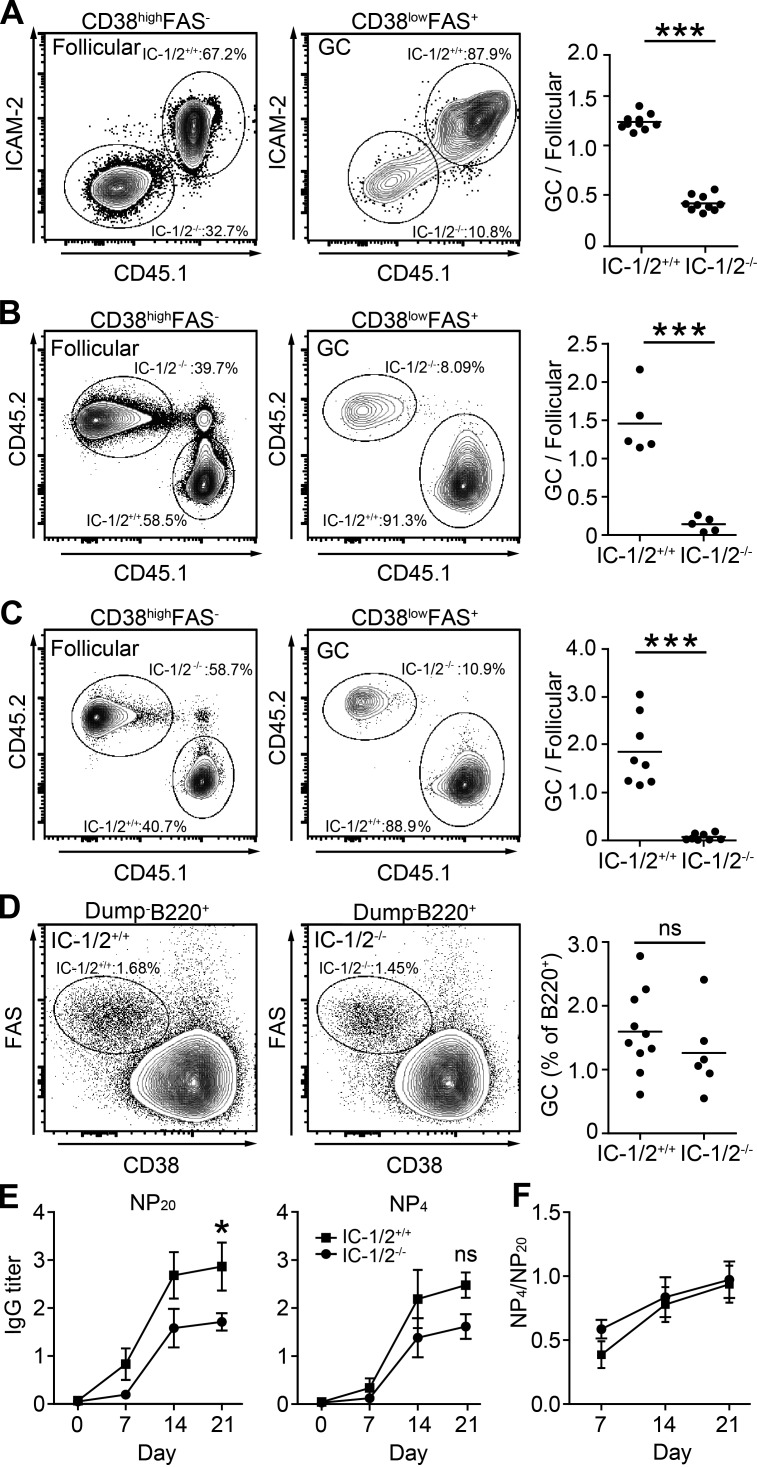
We found that ICAM-1/2 promote B cell selection in competition among B cells bearing BCRs of equal affinity; however, B cell competition takes place among many B cell clones bearing BCRs of varying affinities and specificities. To determine whether our findings would extend to the polyclonal immune response in which multiple, distinct B cell clones compete, we next generated mixed BM chimeras in which ∼50% of the B cells were CD45.1+ ICAM-1/2+/+ and ∼50% were ICAM-1/2−/−. To elicit a highly polyclonal immune response, chimeric mice were infected subcutaneously in the hind foot pads with vesicular stomatitis virus (VSV; Sammicheli et al., 2016). After 14 d, the GC compartment within draining LNs was analyzed by flow cytometry. Because the reconstitution of each type of BM cells varied among mice, we normalized the percentage of each B cell subpopulation in the GC to their follicular counterparts. Consistent with the adoptive transfer model, we found that the proportion of ICAM-1/2+/+ B cells in the GC compartment was significantly higher than that of ICAM-1/2−/− B cells (Fig. 3 A). Similar results were obtained by immunizing chimeric mice with NP-OVA in alum (Fig. 3 B). Peyer’s patches contain GCs that are driven by various intestinal-derived antigens during homeostasis. In agreement with the immunization experiments, we found that ICAM-1/2+/+ polyclonal B cells dominated within the Peyer’s patch GCs of chimeric mice (Fig. 3 C). In agreement with the adoptive cell transfer model (Fig. 2 E), GC formation in response to immunization with NP-OVA was intact in noncompetitive experiments, in which chimeric mice were reconstituted with a mixture of cells that consist of 80% BM cells derived from B cell–deficient mice (JHT) and 20% BM cells derived from either ICAM-1/2+/+ or ICAM-1/2−/− mice (Fig. 3 D). Collectively, we conclude that B cell ICAMs are required for effective polyclonal B cell competition during a T cell–dependent humoral immune response.
Figure 3.
B cell ICAMs are required for effective B cell competition in polyclonal immune responses. (A) Representative flow cytometric plots of GC and follicular compartments composition in popliteal LNs of chimeric hosts (∼50% IC-1/2+/+ and ∼50% IC-1/2−/− B cells) 14 d after infection with VSV. IC-1/2+/+ B1-8hi B cells were gated as CD45.1+, and IC-1/2−/− B1-8hi B cells were gated as ICAM-2− and CD45.1−. The graph shows the percentage of B cells in the GC (FAS+ CD38low) normalized to the follicular B cell compartment (FAS− CD38high). Data are pooled from two independent experiments with a total of 10 mice per group. (B) Analysis of chimeric mice as in A that were immunized with NP-OVA. IC-1/2+/+ B1-8hi B cells were gated as CD45.1+, and IC-1/2−/− B1-8hi B cells were gated as CD45.2+. Data are pooled from two independent experiments with a total of five mice per group. (C) Analysis of the proportion of each cell type in GCs in Peyer’s patches of chimeric mice. Data are pooled from two independent experiments with a total of eight mice per group. (D) Analysis of the GC composition in chimeric mice hosting either IC-1/2+/+ or IC-1/2−/− B cells. Gated as CD8−, CD4−, F4/80−, GR-1− (dump) and B220+. Data are pooled from two independent experiments with a total of six to eight mice per group. Each dot in the graphs represents a single mouse; lines represent the mean. (E) Titers of total (NP20) and high-affinity (NP4) anti-NP IgG in serum of NP-OVA immunized chimeric mice. (F) Ratios of high-affinity/total anti-NP antibodies. Pooled data from two experiments with total of four to five mice in each group. *, P = 0.05; ***, P < 0.0001; ns, not significant; two-tailed Student’s t test.
Next we examined whether ICAMs play a role in antibody formation. To this end, we immunized chimeric mice as in Fig. 3 D and evaluated the presence of antigen-specific antibodies in the serum over time. We found that in chimeric mice in which B cells lack ICAMs, the titer of IgG antibodies that bind NP20 (total binding antibodies) in ELISA was significantly lower compared with control mice (Fig. 3 E). However, analysis of higher-affinity antibodies (anti-NP4), showed smaller differences among this fraction of antibodies (Fig. 3 E). Furthermore, analysis of high-affinity and total anti-NP antibody ratios (anti-NP4/anti-NP20) showed an increase in antibody affinity at days 7–21 in both types of chimeric mice (Fig. 3 F). Collectively, these results suggest that ICAMs are required for optimal antibody secretion during the peak of the GC reaction. Nevertheless, the increase in antibody affinity is B cell ICAM independent.
B cell ICAMs promote B cell selection for GC colonization during early clonal competition for T cell help
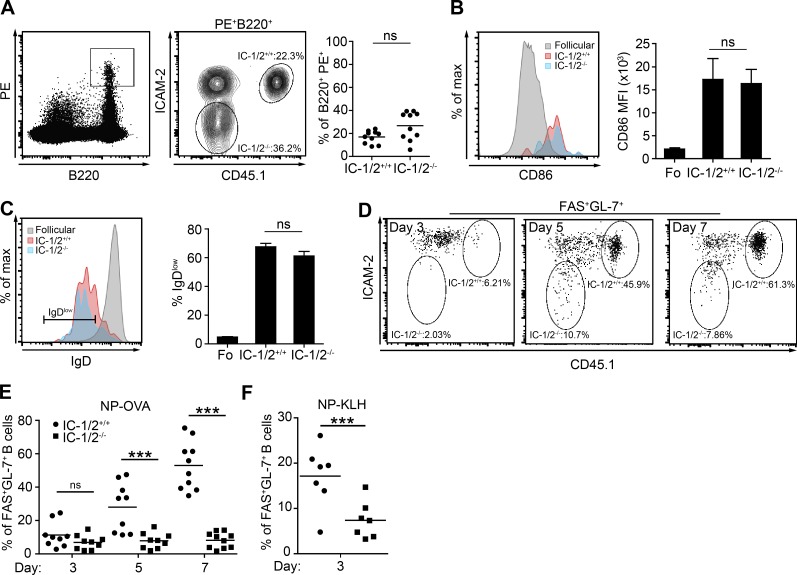
We next examined at which stage of the T cell–dependent immune response B cell ICAMs play a role. The antibody response begins when B cells interact with a cognate antigen followed by internalization of the BCR:antigen complex for processing and presentation to T cells as pMHCII complexes. We examined whether ICAMs are required for antigen capture by antigen-specific B cells and for up-regulation of the early activation marker CD86 and down-regulation of IgD. To this end, host mice were adoptively transferred with a mixture of CD45.1+ ICAM-1/2+/+ and ICAM-1/2−/− B-1-8hi B cells and immunized with a fluorescent antigen, NP-PE. After 9 h, the percentage of transferred cells that captured the antigen were examined by flow cytometry. We found that the fraction of PE+ B cells was equivalent in ICAM-1/2+/+ and ICAM-1/2−/− B cells (Fig. 4 A). We conclude that the initial interaction of B cells with cognate antigen, followed by antigen capture and internalization, does not depend on B cell ICAMs. To examine whether ICAMs are required for antigen-driven B cell activation, we analyzed expression of the early activation marker CD86 on PE+ B cells that captured the antigen. We found that PE+ ICAM-1/2+/+ and ICAM-1/2−/− B cells expressed similar levels of CD86 (Fig. 4 B). Furthermore, the percentage of IgDlow B cells among PE+ ICAM-1/2+/+ and ICAM-1/2−/− B-1-8hi B cells was similar (Fig. 4 C). Collectively, these results demonstrate that B cell ICAMs do not play a role in the initial activation of B cells in vivo.
Figure 4.
B cell ICAMs are necessary for clonal competition but not for early B cell activation. (A) Flow cytometric plots and graphs showing the percentage of PE+ B cells among transferred IC-1/2+/+ and IC-1/2−/− B1-8hi B cells 9 h after immunization with NP-PE. Data are pooled from two independent experiments with a total of 10 mice per group. (B) CD86 expression on follicular and PE+ IC-1/2+/+ and IC-1/2−/− B1-8hi B cells. Data are pooled from two independent experiments with a total of eight to 10 mice per group. (C) Percentage of follicular and transferred PE+ B cells expressing low levels of IgD 16 h after immunization with NP-PE. Data are pooled from two independent experiments with a total of eight to 10 mice per group. Bars and error bars represent the mean and SEM, respectively. (D–F) Percentage of IC-1/2+/+ and IC-1/2−/− B1-8hi B cells expressing FAS and GL-7 in host mice on the indicated days after NP-OVA (D, E) or NP-KLH (F) immunization. Data are pooled from two independent experiments with seven to 10 mice total for each condition or time point. Each dot in the graphs represents a single mouse; lines represent the mean. ***, P < 0.007; ns, not significant; two-tailed Student’s t test.
B cell clones compete for GC seeding at the T–B border during the early stages of the antibody response, as well as at later stages, in the GC reaction (Victora and Nussenzweig, 2012). Thus, we next examined whether ICAMs play a role specifically in clonal competition during GC colonization. To identify the earliest stages at which B cell ICAMs play a role in B cell competition, the proportions of transferred ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi B cells were examined over the course of the emerging immune response. We found that on day 5 after immunization with NP-OVA, the fraction of ICAM-1/2+/+ B1-8hi B cells was significantly higher than ICAM-1/2−/− B1-8hi B cells among cells expressing the activation markers, FAS and GL-7 (Fig. 4, D and E). Because the response to NP-OVA was relatively weak at day 3, we repeated the experiment by immunizing mice with a more potent NP carrier, NP-keyhole limpet hemocyanin (KLH), and found that ICAM-1/2−/− B1-8hi B cells were already outcompeted by day 3, a time point that precedes GC formation (Fig. 4 F). Taking these results together, we conclude that B cell ICAMs play a role in B cell immune response primarily after initial priming, when B cells are subjected to selection by Tfh cells for GC colonization.
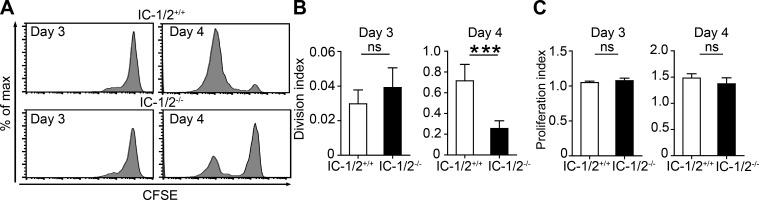
Proliferative clonal expansion during GC formation depends on B cell ICAMs
Our results show that B cell ICAMs provide a selective advantage during the initial stages of GC formation when the earliest competition for T cell help takes place. Next, we examined how B cell ICAMs promote B cell selection and clonal expansion. Outcompeted B cells at the T–B boundary proliferate significantly less than selected B cells (Schwickert et al., 2011). We examined whether the defect of ICAM-deficient B cells lies in ineffective proliferation during GC colonization. To this end, equal numbers of ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi B cells were stained with CFSE and transferred into host mice. By assessing the mean number of cell divisions of the adoptively transferred B cells (division index), we did not find a defect in proliferation of ICAM-1/2−/− compared with ICAM-1/2+/+ B1-8hi B cells 3 d after immunization with NP-OVA (Fig. 5, A and B). This suggests that early proliferation events are B cell ICAM independent. However, on day 4 after NP-OVA immunization and during GC colonization, the adoptively transferred ICAM-1/2−/− B cells proliferated threefold less than ICAM-1/2+/+ B cells (Fig. 5, A and B). In contrast, quantification of cell proliferation among cells that divided at least once (proliferation index) revealed that all of the dividing cells of the ICAM-1/2−/− B cells proliferated to a similar extent as the ICAM-1/2+/+ B cells (Fig. 5 C). Thus, we conclude that B cell ICAMs are required for entry into the cell cycle rather than controlling the extent and quality of B cell proliferation rate.
Figure 5.
Efficient B cell proliferation during GC colonization depends on B cell ICAMs. (A) Flow cytometric histograms of CFSE-labeled IC-1/2+/+ and IC-1/2−/− B1-8hi B cells recovered from popliteal LNs 3 and 4 d after immunization with NP-OVA. (B) Quantification of IC-1/2+/+ and IC-1/2−/− B cell proliferation among the initially transferred cells (division index) in the experiment shown in A. (C) Quantification of B cell proliferation of cells that divide at least once (proliferation index). Bar graphs represent the mean + SEM of two independent experiments with 10 mice total for each condition or time point. ***, P < 0.001; ns, not significant; two-tailed Student’s t test.
Long-lasting T–B interactions depend on B cell ICAMs
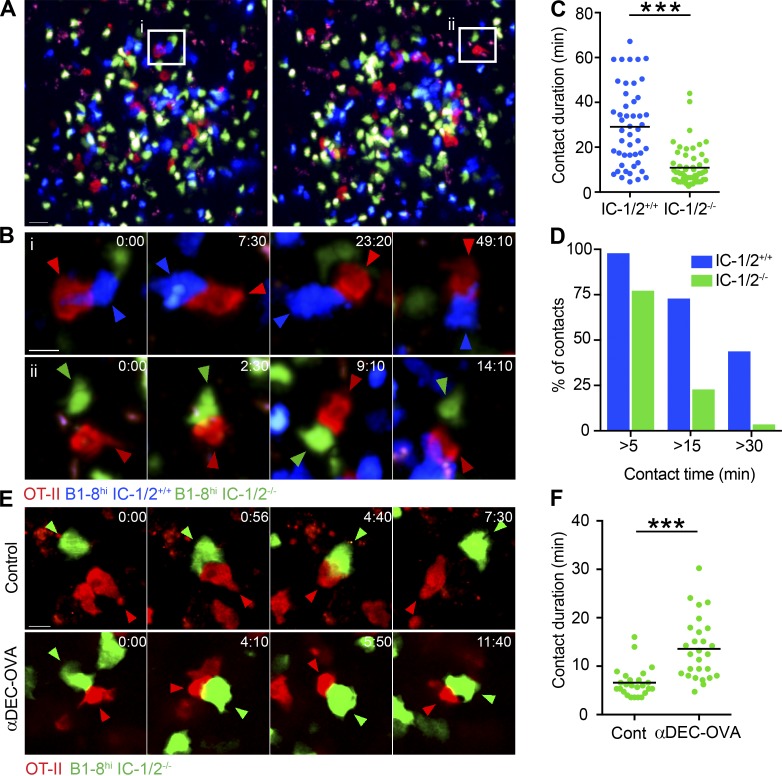
ICAMs are adhesive molecules that support T cell interactions with APCs (Dustin, 2008). Given the role of ICAM-1/2 in the T cell–dependent immune response, we speculated that B cell ICAMs promote B cell selection by supporting T–B interactions. To this end, we sought to visualize antigen-specific T and B cell interactions in vivo during early B cell competition for T cell help. A mixture of cells including DsRed+ OVA-specific T cells (OT-II) and ICAM-1/2+/+ CFP+ B1-8hi and ICAM-1/2−/− CFSE-labeled B1-8hi B cells was transferred into WT hosts before s.c. immunization with NP-OVA. After 1.5–2.5 d, popliteal LNs were exposed, and competition among transferred cells was visualized by intravital two-photon laser scanning microscopy (TPLSM). Consistent with previous reports (Okada et al., 2005), transferred cells formed clusters deep within the LN and far from the capsule in an ICAM-independent manner (Fig. 6 A). Both T and B cells within these clusters showed typical blast-like morphology, suggesting they had recently encountered antigen (Fig. 6 A and Video 1). Automated analysis revealed no difference in the velocity of ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi B cells, demonstrating that B cell ICAMs are not required for motility (Fig. S3). In agreement with previous findings (Okada et al., 2005), tracking individual T and B cell pairs showed that these cell conjugates were motile, with B cells often dragging T cells on the trailing edge (Fig. 6 B and Video 2). However, although T cell interactions with ICAM-1/2+/+ B1-8hi B cells lasted a mean of 29 min, T cell interactions with ICAM-1/2−/− B1-8hi B cells were 2.7-fold shorter (Fig. 6, B and C; and Videos 2 and 3). B cell ICAM molecules were largely dispensable for forming short-term contacts with cognate T cells; however, contacts lasting more than 15 min were ICAM-1/2 dependent (Fig. 6 D). We conclude that B cell ICAMs are essential for sustained Tfh–B cell interactions, but not for short-lived engagements.
Figure 6.
Long-lasting and pMHCII-dependent Tfh–B cell interactions depend on B cell ICAMs. (A) Images show clusters of DsRed+ OT-II T cells (red), CFP+ IC-1/2+/+ B1-8hi B cells (blue), and CFSE-labeled IC-1/2−/− B1-8hi B cells (green) within popliteal LNs 2 d after immunization with NP-OVA. Representative T–B interactions shown in B are marked by white rectangles. Bar, 20 µm. Images correspond to Video 1. (B) Dynamics of T–B interactions. Red, blue, and green arrowheads indicate T cells, IC-1/2+/+, and IC-1/2−/− B cells, respectively. Bar, 5 µm. Images correspond to Videos 2 and 3. (C and D) Analysis of contact duration of OT-II T cells with either IC-1/2+/+ or IC-1/2−/− B1-8hi B cells. Total contacts (C) and interactions binned according to duration (D) are shown. Data represent two to three independent experiments with two to three mice total for each group. (E) Dynamic interactions of DsRed+ OT-II T cells and GFP+ IC-1/2−/− B1-8hi B cells 4–8 h after injection of anti–DEC205-OVA or PBS (control). Red and green arrowheads indicate T cells and IC-1/2−/− B cells, respectively. Bar, 10 µm. Images correspond to Videos 4 and 5. (F) Analysis of contact duration of OT-II T cells with IC-1/2−/− B1-8hi B cells. Data represent two independent experiments with two to three mice total for each condition. In C and F, each dot in the graphs represents a single T–B interaction; lines represent the mean. ***, P < 0.0001; two-tailed Student’s t test.
High levels of pMHCII promote long-lasting T–B interactions during early clonal competition as well as in the GC reaction (Schwickert et al., 2011; Shulman et al., 2014), but whether these selective adhesive interactions depend on B cell ICAMs is not known. To address this, we combined antigen targeting to antigen-specific B cells with intravital TPLSM imaging. To increase the levels of pMHCII, we used an antibody that targets DEC205, an endocytic receptor that can deliver proteins to MHCII processing compartments and is fused at its C terminus to the carrier protein OVA (anti–DEC205-OVA; Victora et al., 2010). A mixture of cells including DsRed+ OT-II T cells and ICAM-1/2−/− GFP+ B1-8hi B cells was transferred into WT hosts before s.c. immunization with NP-OVA. After 2 d, anti–DEC205-OVA or PBS was administered to the hind footpads 4–8 h before TPLSM imaging. As shown in Fig. 6 C, Tfh interactions with ICAM-1/2−/− B cells were also short under these noncompetitive conditions in mice injected with PBS (mean of 7 min; Fig. 6, E and F; and Video 4). Targeting antigen by anti–DEC205-OVA injection to the host mice increased the duration of the contacts by twofold (mean of 14 min; Fig. 6, E and F; and Video 5); however, the duration of the T–B interactions was still significantly shorter than those observed between T cells and ICAM-1/2+/+ B cells, and long-lasting interactions (>30 min) did not form at all (Fig. 6, C–F). We conclude that presentation of high levels of cognate pMHCII can support cognate T–B cell interactions in ICAM-independent manner; however, optimal long-lasting interactions are strictly ICAM dependent.
B cell ICAMs promote selection of low-affinity B cell clones for GC colonization
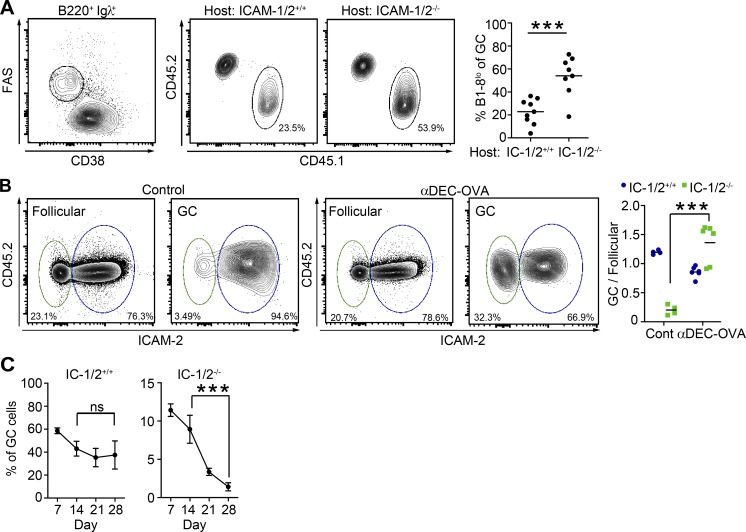
B cell clones present various levels of pMHCII on their surface depending on their BCR affinity (Schwickert et al., 2011). Our intravital imaging experiments demonstrate that B cells presenting lower levels of pMHCII to T cells depend more on ICAMs for formation of long-lasting interactions with T cells than B cells that present higher levels of pMHCII. This finding suggests that B cell clones bearing low-affinity BCR and presenting low levels of pMHCII depend on ICAMs for their selection for GC colonization. To address this possibility, we competed B cells expressing an NP-specific low-affinity BCR (ICAM-1/2+/+ B1-8lo B cells) with the higher-affinity NP-specific B cells found in the endogenous repertoire of naive C57BL/6 WT mice (Cumano and Rajewsky, 1986). In this WT strain, endogenous NP-specific B cells frequently use the germline VH186.2 gene, which has ∼4-fold higher affinity BCR than the adoptively transferred B1-8lo B cells (Allen et al., 1988). B1-8lo B cells were transferred into ICAM-1/2+/+ or ICAM-1/2−/− BM chimeric mice and analyzed by flow cytometry 7 d after immunization. As expected (Schwickert et al., 2011), B1-8lo B cells did not effectively enter the GC compartment in ICAM-1/2+/+ chimeric mice (mean 22.8% of GC cells; Fig. 7 A). However, B1-8lo B cells were 2.4-fold more effective in entering the GC compartment, when competing with endogenous NP-specific B cells in ICAM-1/2−/− chimeras (Fig. 7 A). These results suggest that ICAM molecules are beneficial for selection of low-affinity B cell clones for clonal expansion and GC colonization.
Figure 7.
B cell ICAMs are required for selection of low-affinity B cell clones but not for B cells presenting high levels of pMHCII. (A) Flow cytometric plots and graph showing the fraction of adoptively transferred Igλ+ B1-8lo CD45.1+ B cells in the GC compartment of IC-1/2+/+ or IC-1/2−/− chimeric mice, 7 d after NP-OVA immunization. Data are pooled from two independent experiments with a total of eight to nine mice per group. (B) Flow cytometric analysis of IC-1/2+/+ and IC-1/2−/− B cells in NP-OVA immunized chimeric mice (∼20% IC-1/2−/− DEC205+/+, ∼80% IC-1/2+/+ DEC205−/−) treated with either anti–DEC205-OVA or anti–DEC205-CS or PBS (controls) on day 5 after immunization with NP-OVA. The percentage of each B cell subset in the GC compartment 8 d after immunization was normalized to the percentage in the follicular compartment. Data are pooled from two independent experiments with a total of four to six mice per group. Each dot in the graphs represents a single mouse; lines represent the mean. (C) Analysis of the proportion of adoptively transferred IC-1/2+/+ and IC-1/2−/− B1-8hi B cells within the GC compartment (CD38low FAS+) over time in mice immunized with NP-OVA. Each dot in the graphs represents the mean ± SEM. Data represent one to two experiments with 3–11 mice total for each time point. ***, P < 0.0004; ns, not significant; two-tailed Student’s t test. Host mice in which the GC contained more than 70% endogenous B cells were excluded from the analysis.
Next, we examined whether ICAM-1 and -2 are required for selective expansion of high-affinity clones presenting high levels of pMHCII. For this, we used the DEC205 antigen targeting approach. Irradiated mice were reconstituted with a mixture of cells comprising ∼80% ICAM-1/2+/+ DEC205−/− and ∼20% ICAM-1/2−/− DEC205+/+ BM cells. After immunization with NP-OVA, chimeric mice were treated with anti–DEC205-OVA, anti–DEC205-CS (a noncognate antigen), or PBS (the latter two conditions serving as controls). Because DEC205 itself does not affect B cell competition (Schwickert et al., 2011), ICAM-1/2−/− DEC205+/+ B cells were outcompeted by the ICAM-1/2+/+ DEC205−/− B cells because of their ICAM-1/2 deficiency (Fig. 7 B), consistent with our data (Fig. 3 B). Nevertheless, in mice that received the anti–DEC205-OVA fusion antibody, the proportion of ICAM-1/2−/− B cells that entered the GC compartment was significantly increased compared with control treatment (Fig. 7 B). Collectively, these results show that B cells presenting high levels of pMHCII elicit selective T cell help signals that increase their clonal expansion independent of B cell ICAMs, whereas B cells expressing low-affinity BCRs depend on ICAMs for their selection.
Next, we examined whether B cell ICAMs are important for participation in the immune response during the peak of the GC reaction. The proportion of B cells that enter the GC and do not efficiently compete in the GC reaction are expected to gradually decrease over time. Thus, we measured the percentage of each type of adoptively transferred B cells (ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi B cells) within the GC compartment for up to 28 d after immunization, as in Fig. 2 A. We found that the few ICAM-1/2−/− B1-8hi B cells that entered the GC response declined gradually over time and were nearly undetectable on day 28 after immunization, whereas the proportion of ICAM-1/2+/+ B1-8hi B cells did not significantly change from day 14 to 28 (Fig. 7 C). We conclude that in addition to GC colonization, B cell ICAMs are required for continued participation of B cells in the GC reaction.
Discussion
Dynamic cellular interactions are crucial for selection of antigen-specific B cells by Tfh cells for GC colonization (Okada et al., 2005; Qi et al., 2008; Qi, 2016). High levels of pMHC complexes presented by B cells during this early competition increase the frequency and duration of T–B interactions and thereby facilitate B cell selection for expansion during GC colonization (Schwickert et al., 2011). Within the GC, high levels of pMHCII promote the formation of stable T–B interactions required for delivery of T cell–derived signals that select B cells to undergo extensive cell proliferation or leave the GC reaction (Allen et al., 2007; Victora et al., 2010; Gitlin et al., 2014, 2015; Shulman et al., 2014). Because TCR and pMHCII interactions do not form optimal adhesive contacts, our findings provide an explanation for how high levels of pMHCII support long-lived T–B adhesiveness. The current study adds ICAMs as critical molecular players that support cognate T–B interactions and pMHCII-mediated selection of B cells for proliferative clonal expansion.
Our findings demonstrate that B cell ICAMs play a major role in B cell selection for clonal expansion rather than B cell priming. We base this conclusion on the following findings. (a) Early events such as antigen capture and B cell activation do not appear to depend on B cell ICAMs, indicating that the initial priming events are B cell ICAM independent. (b) Early proliferation of antigen-specific B cells is ICAM independent, whereas clonal expansion at later time points is ICAM dependent. (c) T cell–independent immune responses that involve B cell priming as well as extensive B cell proliferation do not depend on B cell ICAMs. (d) T–B interactions are defective in the absence of B cell ICAMs. Collectively, although we cannot entirely exclude that B cell ICAMs play a role in T cell–independent functions of B cells, we demonstrate that the crucial adhesive step during B cell clonal selection for proliferative expansion by Tfh cells (Schwickert et al., 2011) requires these molecules.
During clonal competition, T–B conjugates are highly motile, with the B cells dragging the T cells on their trailing edge (Okada et al., 2005). By directly visualizing clonal competition in vivo, we demonstrate that B cell ICAMs are key for pMHCII-driven Tfh interactions with the B cell trailing edge and for preventing detachment of T–B conjugates. In migrating leukocytes, ICAMs are enriched at the trailing edge, where they form a high-avidity platform for integrin counterreceptors expressed by various cell types (Sánchez-Madrid and Serrador, 2009). Long-lasting T–B contacts were thought to be essential for B cell selection for GC seeding (Okada et al., 2005; Qi et al., 2008); however, our intravital imaging models demonstrate that suboptimal interactions are sufficient for selection of B cells bearing high levels of pMHCII. Nevertheless, because both pMHCII complexes and ICAMs are present on all normal B cells, we conclude that B cell selection must involve long-lasting and ICAM-dependent Tfh–B cell interactions.
Our findings suggest an answer to how low-affinity clones are selected for GC colonization even in the presence of higher-affinity B cell clones (Kuraoka et al., 2016; Tas et al., 2016). In responding T cells, TCR triggering induces inside-out LFA-1 activation (Evans et al., 2009), and the level of antigen presentation on the B cell surface likely controls the ability of the in situ activated LFA-1 to avidly bind B cell ICAMs. As these ICAM-mediated T–B adhesions can amplify the magnitude of TCR signals, they lower the threshold levels of pMHCII required for optimal T cell activation (Brunmark and O’Rourke, 1997; Wülfing et al., 1998) and thereby facilitate the selection of B cells for clonal expansion. Based on our antigen targeting experiments as well as serum antibody analysis, we suggest that such ICAM-dependent signal amplification is less important for high-affinity B cell clones presenting high levels of pMHCII; however, B cells bearing low-affinity BCRs and presenting lower pMHCII depend on ICAMs and long-lasting T–B interactions for their selection for clonal expansion and GC colonization. T–B contacts before GC formation as well as during the GC reaction deliver critical selection signals to B cells in the form of costimulatory molecules, such as ICOS and CD40L, essential for B cell clonal expansion (Liu et al., 2015). Interestingly, the surface expression of these membrane-bound signaling molecules is regulated by dopamine delivery from Tfh to B cells, a process that depends on TCR triggering by cognate pMHCII on B cells as well as on LFA-1 and ICAM interactions (Papa et al., 2017). Taking these findings together, we suggest that B cell ICAMs stabilize cognate T–B contacts and thereby facilitate the feedforward loop of molecular interactions that determine the fate of the B cells.
ICAM-mediated selection of B cells for clonal expansion confers an advantage during early stages of the response, before GC competition and affinity maturation. Extensive proliferation of a selected clone increases the probability that it will assume multiple fates early on during the immune response, including differentiation into plasmablast, GC-independent memory cell, and GC cell lineages (Taylor et al., 2015). Because these clones are very rare within a polyclonal repertoire (Taylor et al., 2015), ICAMs may amplify T cell–derived signals required for selection of these clones for entry into the cell cycle. Consistent with this notion, we found that B cell ICAMs promote selection of B cells for clonal expansion and formation of both GC cells and early plasmablasts. Furthermore, during the early GC reaction, clones are gradually counterselected as a result of clonal competition as well as loss-of-function mutations in their immunoglobulin genes (Victora and Nussenzweig, 2012). ICAM-dependent clonal expansion of a particular clone increases the probability that some of its members will be retained in the GC for an extended period of time and acquire affinity-enhancing mutations in their immunoglobulin genes. In addition, B cell ICAMs prolonged B cell participation in the GC reaction under competitive conditions, but as opposed to the sharp drop in the number of ICAM-deficient B cells during the early phases of the response, these cells were gradually diluted out from the GC compartment over a period of 4 wks. Furthermore, ICAMs were not required for normal B cell distribution between the light and dark zones in GCs and antibody affinity maturation. These findings suggest that B cell ICAMs are less important for the short T–B engagements at the GC light zone than for the long-lasting T–B contacts that take place during early clonal selection (Okada et al., 2005; Shulman et al., 2014; Liu et al., 2015).
Antibody responses are composed of a diverse collection of B cell clones, which is essential for targeting different components of pathogens and preventing immune escape through antigenic variation or genetic drift (Kaur et al., 2011). Expansion of an optimal clonal repertoire during the antibody response increases the probability of emergence of antibodies with sustained protective potential. Understanding the molecular mechanism of this process can help improve the design of effective vaccination strategies that target clones with the capacity to mutate their immunoglobulins and form protective neutralizing antibodies regardless of their initial BCR affinity.
Materials and methods
Mice
ICAM-1/2−/− mice (Gorina et al., 2014) were provided by B. Engelhardt. NP-specific B1-8hi and B1-8lo mice (Shih et al., 2002) and DEC205−/− mice (Guo et al., 2000) were a gift from M. Nussenzweig (The Rockefeller University, New York, NY). OT-II, SJL CD45.1+, GFP+, DsRed+, CFP+, GFP+, and MD4 transgenic mice were purchased from the Jackson Laboratory. B cell–deficient mice (JHT) were previously described (Gu et al., 1993). WT mice (C57BL/6) were provided by Harlan. All experiments with mice were approved by the Weizmann Institute Animal Care and Use Committee.
Adoptive cell transfers
B cells and T cells were purified by forcing spleen tissue through mesh into PBS or RPMI containing 2% serum and 1 mM EDTA. Resting B cells and T cells were purified using anti-CD43 magnetic beads or CD4+ T cell–negative isolation kits (Miltenyi), respectively. After purification, 2–6 × 106 B1-8hi or B1-8lo B cells (comprising 15% and 3%, respectively, Igλ+ NP-specific B cells) or OT-II T cells were transferred intravenously into host mice before immunization. In competition experiments, the ratio of adoptively transferred cells (ICAM-1/2+/+ and ICAM-1/2−/− B1-8hi B cells) was 50:50. For plasmablast analysis, 0.5 × 106 OT-II T cells were cotransferred with the B1-8hi B cells (Fig. 2 D).
Chimeric mice
For generation of chimeric mice, WT hosts were irradiated with 850 rad and injected intravenously with BM cells. To generate B cell–specific ICAM-1/2 knockout mice, hosts were reconstituted with a mixture of BM cells containing 80% BM cells derived from B cell–deficient mice (JHT) and 20% BM cells derived from either ICAM-1/2+/+ or ICAM-1/2−/− mice. Chimeric mice in which 50% of the B cell compartment was derived from WT mice and 50% was from ICAM-1/2−/− mice were reconstituted with 80% JHT, 10% ICAM-1/2+/+ and 10% ICAM-1/2−/− BM cells. In Fig. 3 A, mice were reconstituted with 50% ICAM-1/2+/+ and 50% ICAM-1/2−/− BM cells. In Fig. 7 B, mice were reconstituted with 80% ICAM-1/2+/+ DEC205−/− BM cells and 20% ICAM-1/2−/− DEC205+/+ BM cells.
Immunizations and treatments
Mice were injected with 25 µl PBS containing 10 µg NP16-OVA precipitated in alum (2:1) into the hind footpads. In Fig. 4 F, mice were immunized with 10 µg NP31-KLH in alum. For analysis of antigen uptake and CD86 and IgD expression, mice were immunized with 10 µg soluble NP-PE and analyzed after 9 h for PE uptake and CD86 expression or after 16 h for IgD expression. For antigen targeting, mice were injected with 3 µg anti–DEC205-OVA, anti–DEC205-CS, or PBS (controls) into the hind footpads at the indicated time points. For virus infection, mice were injected with 105 plaque forming units of VSV into the hind footpad as described (Sammicheli et al., 2016).
Flow cytometry
Single-cell suspensions were obtained by forcing popliteal lymph nodes through mesh into PBS or RPMI containing 2% serum and 1 mM EDTA. Cells were incubated with 2 µg/ml anti-16/32 (clone 93) for blockage of FC receptors for 5–10 min. Cell suspensions were washed and incubated with fluorescently labeled antibodies (Table S1) for 20–40 min. GC cells were gated as live/single, B220+ CD38low FAS+. Early activated B cells were defined as GL-7+ FAS+ on days 3–7 after immunization. Plasmablasts were defined as B220med CD138+. Transferred cells within GCs and early activated B cells, were detected by staining cell suspensions with GC markers, as indicated, along with CD45.1 and ICAM-2 antibodies for detection of ICAM-1/2+/+ B1-8hi B cells (CD45.1+, ICAM-2+), ICAM-1/2−/− B1-8hi B cells (CD45.1−, ICAM-2−) and host B cells (CD45.1−, ICAM-2+). For detection of ICAM-1/2+/+ and ICAM-1/2−/− B cells in chimeric mice, cell suspensions were stained for GCs (CD38low FAS+), CD45.1 and CD45.2 or ICAM-2 antibodies. In experiments involving chimeric mice, B cells were gated as B220+ and dump− (CD4, CD8, GR-1, and F4/80). For proliferation experiments, cells were labeled with CFSE at 5 µM for 20 min at 37°C before cell transfer. Stained cell suspensions were analyzed by using CytoFlex flow cytometer (Beckman Coulter). Division index was calculated as the mean number of cell divisions that a cell in the original adoptively transferred cell population has undergone. The initial number of cells was estimated according to the generation (Gx) of the cells; G0+G1/2+G2/4+G3/8 and total number of divisions: G1/2 × 1+G2/4 × 2+G3/8 × 3. Division index = total number of divisions/initial number of cells. Proliferation index was calculated as the total number of divisions divided by the number of cells that divided at least once and the number of cells that entered divisions: (Initial cell number) – G0. Proliferation index: total number of divisions/number of cells that entered division. In some mice, more than 70% of the GC cells were endogenous B cells. These mice were excluded from the experiment analysis.
ELISA
Chimeric mice hosting IC-1/2+/+ or IC-1/2−/− B cells were immunized intraperitoneally with 100 µg NP-OVA in alum. Serum was collected from mice every 7 d. NP-specific antibodies in serum were quantified by ELISA with NP4-BSA or NP20-BSA that were coated on 96-well plates. NP-specific IgG in serum of immunized mice was detected using anti-mouse IgG-horseradish peroxidase (Victora et al., 2010).
Intravital imaging
Mice were transferred with B and T cells expressing DsRed, CFP, or GFP or with B cells stained with CFSE and immunized with NP-OVA in the hind foot pads. After 1.5–2.5 d, host mice were anesthetized with a mixture of 50 mg ketamine, 15 mg xylazine, and 2.5 mg acepromazine per kg of body weight and were maintained under anesthesia by inhalation of 1% isofluorane. Hind legs were shaved and placed on a stage heated to 37°C. Popliteal LNs were surgically exposed, immobilized, covered with a coverslip and placed under the microscope objective (Zeiss 20× 1.05-NA plan objective). In Fig. 6 (E and F), immunized mice were injected with anti–DEC205-OVA or PBS and subjected to intravital TPLSM after 4–8 h.
Image acquisition
A Zeiss LSM 880 upright microscope fitted with Coherent Chameleon Vision laser was used for intravital imaging experiments. Images were acquired with a femtosecond-pulsed two-photon laser tuned to 900 or 940 nm. The microscope was fitted with a filter cube containing 565 LPXR to split the emission to a PMT detector (with a 579–631-nm filter for DsRed fluorescence) and to an additional 505 LPXR mirror to split the emission to 2 GaAsp detectors (with a 500–550-nm filter for CFSE and GFP fluorescence and a 460–480-nm filter for CFP fluorescence). Images were acquired once per 50–60 s as 50–100 µm z-stacks with 5-µm steps between each z-plane. The zoom was set to 1.4, and pictures were acquired at 512 × 512 x-y resolution.
Image analysis
CFSE-labeled cells and CFP+ B cells were tracked over time by using the surface tool in Imaris software (Bitplane). Small or large surfaces that were unlikely to represent cells were removed. To analyze contact duration, T–B conjugates were tracked manually by using Imaris and ImageJ. A contact was defined as an interaction between a T and a B cell that moved together and lasted longer than 4 min.
Online supplemental material
Fig. S1 shows light and dark zone distributions of ICAM-1/2+/+ and ICAM-1/2−/− B cells. Fig. S2 shows homing of follicular ICAM-1/2+/+ and ICAM-1/2−/− B cells to lymph nodes. Fig. S3 shows the velocity of ICAM-1/2+/+ and ICAM-1/2−/− B cells during clonal selection. Videos 1, 2, and 3 show T cell interactions with ICAM-1/2+/+ and ICAM-1/2−/− B cells. Videos 4 and 5 show T cell interactions with ICAM-1/2−/− B cells after control or anti–DEC205-OVA treatments. Table S1 lists antibodies used for flow cytometry.
Supplementary Material
Acknowledgments
We thank M.C. Nussenzweig (Rockefeller University) for discussions and mice and Urban Deutsch (University of Bern) for ICAM-1/2−/− BM cells. We thank Matteo Iannacone (San Raffaele Institute) for providing VSV.
This project has received funding from the European Research Council under the European Union’s Horizon 2020 Framework Programme (grant agreement 677713). Supported by research grants from the Morris Kahn Institute for Human Immunology and Human Frontier Science Program (CDA-00023/2016), Azrieli Foundation, and Rising Tide Foundation. Supported by grants from the Benoziyo Endowment Fund for the Advancement of Science, the Sir Charles Clore Research Prize, Comisaroff Family Trust, the Irma and Jacques Ber-Lehmsdorf Foundation, the Gerald O. Mann Charitable Foundation, and the David M. Polen Charitable Trust. The authors declare no competing financial interests.
Author contributions: I. Zaretsky, O. Atrakchi, R.D. Mazor, L. Stoler-Barak, and A. Biram planned and performed experiments. S.W. Feigelson provided assistance with mouse husbandry and experiments. A.D. Gitlin provided assistance with experiments and manuscript writing. B. Engelhardt provided mouse models. Z. Shulman planned experiments and wrote the manuscript.
Footnotes
Abbreviations used:
- BCR
- B cell receptor
- GC
- germinal center
- ICAM
- intercellular adhesion molecule
- NP
- 4-hydroxy-3-nitrophenyl acetyl
- pMHCII
- peptide–MHCII
- Tfh
- T follicular helper
- TPLSM
- two-photon laser scanning microscopy
References
- Allen C.D.C., Okada T., and Cyster J.G.. 2007. Germinal-center organization and cellular dynamics. Immunity. 27:190–202. 10.1016/j.immuni.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D., Simon T., Fred S., Rajewsky K., and Cumani A.. 1988. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J. 7:1995–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O., and Cyster J.G.. 2017. Germinal centers: programmed for affinity maturation and antibody diversification. Curr. Opin. Immunol. 45:21–30. 10.1016/j.coi.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Berlin-Rufenach B.C., Otto F., Mathies M., Westermann J., Owen M.J., Hamann A., and Hogg N.. 1999. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med. 189:1467–1478. 10.1084/jem.189.9.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunmark A., and O’Rourke A.M.. 1997. Augmentation of mature CD4+ T cell responses to isolated antigenic class II proteins by fibronectin and intercellular adhesion molecule-1. J. Immunol. 159:1676–1685. [PubMed] [Google Scholar]
- Cannons J.L., Tangye S.G., and Schwartzberg P.L.. 2011. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29:665–705. 10.1146/annurev-immunol-030409-101302 [DOI] [PubMed] [Google Scholar]
- Cumano A., and Rajewsky K.. 1986. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 5:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Porto J.M., Haberman A.M., Kelsoe G., and Shlomchik M.J.. 2002. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 195:1215–1221. 10.1084/jem.20011550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennig D., Lacerda J., Ying Y., Gasparetto C., and O’Reilly R.J.. 1994. ICAM-1 (CD54) Expression on B lymphocytes is associated with their costimulatory function and can be increased by coactivation with IL-1 and IL-7. Cell. Immunol. 156:414–423. 10.1006/cimm.1994.1186 [DOI] [PubMed] [Google Scholar]
- Dustin M.L. 2008. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 221:77–89. 10.1111/j.1600-065X.2008.00589.x [DOI] [PubMed] [Google Scholar]
- Eisen H.N., and Siskind G.W.. 1964. Variations in affinities of antibodies during the immune response. Biochemistry. 3:996–1008. 10.1021/bi00895a027 [DOI] [PubMed] [Google Scholar]
- Evans R., Patzak I., Svensson L., De Filippo K., Jones K., McDowall A., and Hogg N.. 2009. Integrins in immunity. J. Cell Sci. 122:215–225. 10.1242/jcs.019117 [DOI] [PubMed] [Google Scholar]
- Feigelson S.W., Pasvolsky R., Cemerski S., Shulman Z., Grabovsky V., Ilani T., Sagiv A., Lemaitre F., Laudanna C., Shaw A.S., and Alon R.. 2010. Occupancy of lymphocyte LFA-1 by surface-immobilized ICAM-1 is critical for TCR- but not for chemokine-triggered LFA-1 conversion to an open headpiece high-affinity state. J. Immunol. 185:7394–7404. 10.4049/jimmunol.1002246 [DOI] [PubMed] [Google Scholar]
- Garside P., Ingulli E., Merica R.R., Johnson J.G., Noelle R.J., and Jenkins M.K.. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 281:96–99. 10.1126/science.281.5373.96 [DOI] [PubMed] [Google Scholar]
- Gitlin A.D., Shulman Z., and Nussenzweig M.C.. 2014. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 509:637–640. 10.1038/nature13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin A.D., Mayer C.T., Oliveira T.Y., Shulman Z., Jones M.J.K., Koren A., and Nussenzweig M.C.. 2015. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 349:643–646. 10.1126/science.aac4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina R., Lyck R., Vestweber D., and Engelhardt B.. 2014. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 192:324–337. 10.4049/jimmunol.1300858 [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y.R., and Rajewsky K.. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 73:1155–1164. 10.1016/0092-8674(93)90644-6 [DOI] [PubMed] [Google Scholar]
- Guo M., Gong S., Maric S., Misulovin Z., Pack M., Mahnke K., Nussenzweig M.C., and Steinman R.M.. 2000. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum. Immunol. 61:729–738. 10.1016/S0198-8859(00)00144-0 [DOI] [PubMed] [Google Scholar]
- Kaur K., Sullivan M., and Wilson P.C.. 2011. Targeting B cell responses in universal influenza vaccine design. Trends Immunol. 32:524–531. 10.1016/j.it.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler T.B., and Perelson A.S.. 1993. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol. Today. 14:412–415. 10.1016/0167-5699(93)90145-B [DOI] [PubMed] [Google Scholar]
- Klein F., Mouquet H., Dosenovic P., Scheid J.F., Scharf L., and Nussenzweig M.C.. 2013. Antibodies in HIV-1 vaccine development and therapy. Science. 341:1199–1204. 10.1126/science.1241144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka M., Schmidt A.G., Nojima T., Feng F., Watanabe A., Kitamura D., Harrison S.C., Kepler T.B., and Kelsoe G.. 2016. Complex antigens drive permissive clonal selection in germinal centers. Immunity. 44:542–552. 10.1016/j.immuni.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., and Nourshargh S.. 2007. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–689. 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- Lingwood D., McTamney P.M., Yassine H.M., Whittle J.R.R., Guo X., Boyington J.C., Wei C.-J., and Nabel G.J.. 2012. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature. 489:566–570. 10.1038/nature11371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Xu H., Shih C., Wan Z., Ma X., Ma W., Luo D., and Qi H.. 2015. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 517:214–218. 10.1038/nature13803 [DOI] [PubMed] [Google Scholar]
- Liu Y., Blanchfield L., Ma V.P.-Y., Andargachew R., Galior K., Liu Z., Evavold B., and Salaita K.. 2016. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc. Natl. Acad. Sci. USA. 113:5610–5615. 10.1073/pnas.1600163113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli A.P., Fontés G., Avery D.T., Leddon S.A., Tam M., Elliot M., Ballesteros-Tato A., Miller J., Stevenson M.M., Fowell D.J., et al. 2016. The integrin LFA-1 controls T follicular helper cell generation and maintenance. Immunity. 45:831–846. 10.1016/j.immuni.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya M.C., Sancho D., Vicente-Manzanares M., and Sánchez-Madrid F.. 2002. Cell adhesion and polarity during immune interactions. Immunol. Rev. 186:68–82. 10.1034/j.1600-065X.2002.18607.x [DOI] [PubMed] [Google Scholar]
- Okada T., Miller M.J., Parker I., Krummel M.F., Neighbors M., Hartley S.B., O’Garra A., Cahalan M.D., and Cyster J.G.. 2005. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3:e150 10.1371/journal.pbio.0030150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa I., Saliba D., Ponzoni M., Bustamante S., Canete P.F., Gonzalez-Figueroa P., McNamara H.A., Valvo S., Grimbaldeston M., Sweet R.A., et al. 2017. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature. 547:318–323. 10.1038/nature23013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Amesbury M., Gardam S., Crosbie J., Hasbold J., Hodgkin P.D., Basten A., and Brink R.. 2003. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J. Exp. Med. 197:845–860. 10.1084/jem.20022144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H. 2016. T follicular helper cells in space-time. Nature 16:612–625. 10.1038/nri.2016.94 [DOI] [PubMed] [Google Scholar]
- Qi H., Cannons J.L., Klauschen F., Schwartzberg P.L., and Germain R.N.. 2008. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 455:764–769. 10.1038/nature07345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif K., Ekland E.H., Ohl L., Nakano H., Lipp M., Förster R., and Cyster J.G.. 2002. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 416:94–99. 10.1038/416094a [DOI] [PubMed] [Google Scholar]
- Sammicheli S., Kuka M., Di Lucia P., de Oya N.J., De Giovanni M., Fioravanti J., Cristofani C., Maganuco C.G., Fallet B., Ganzer L., et al. 2016. Inflammatory monocytes hinder antiviral B cell responses. Sci. Immunol. 1:1–11. 10.1126/sciimmunol.aah6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., and Serrador J.M.. 2009. Bringing up the rear: defining the roles of the uropod. Nat. Rev. Mol. Cell Biol. 10:353–359. 10.1038/nrm2680 [DOI] [PubMed] [Google Scholar]
- Schmidt A.G., Therkelsen M.D., Stewart S., Kepler T.B., Liao H.X., Moody M.A., Haynes B.F., and Harrison S.C.. 2015. Viral receptor-binding site antibodies with diverse germline origins. Cell. 161:1026–1034. 10.1016/j.cell.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer A., Hugues S., Boissonnas A., Fetler L., and Amigorena S.. 2008. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 28:258–270. 10.1016/j.immuni.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Schwickert T.A., Victora G.D., Fooksman D.R., Kamphorst A.O., Mugnier M.R., Gitlin A.D., Dustin M.L., and Nussenzweig M.C.. 2011. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 208:1243–1252. 10.1084/jem.20102477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T.A., Meffre E., Roederer M., and Nussenzweig M.C.. 2002. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat. Immunol. 3:570–575. 10.1038/ni803 [DOI] [PubMed] [Google Scholar]
- Shulman Z., Gitlin A.D., Weinstein J.S., Lainez B., Esplugues E., Flavell R.A., Craft J.E., and Nussenzweig M.C.. 2014. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 345:1058–1062. 10.1126/science.1257861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas J.M.J., Mesin L., Pasqual G., Targ S., Jacobsen J.T., Mano Y.M., Chen C.S., Weill J.-C., Raynaud C.-A., Browne E.P., et al. 2016. Visualizing antibody affinity maturation in germinal centers. Science. 351:1048–1054. 10.1126/science.aad3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.J., Pape K.A., Steach H.R., and Jenkins M.K.. 2015. Apoptosis and antigen affinity limit effector cell differentiation of a single naive B cell. Science. 347:784–787. 10.1126/science.aaa1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., and Nussenzweig M.C.. 2012. Germinal centers. Annu. Rev. Immunol. 30:429–457. 10.1146/annurev-immunol-020711-075032 [DOI] [PubMed] [Google Scholar]
- Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., and Nussenzweig M.C.. 2010. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 143:592–605. 10.1016/j.cell.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wülfing C., Sjaastad M.D., and Davis M.M.. 1998. Visualizing the dynamics of T cell activation: Intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl. Acad. Sci. USA. 95:6302–6307. 10.1073/pnas.95.11.6302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.