Abstract
Functional annotation of mutations that cause human limb anomalies is enabled by basic developmental studies. Here we focus on the prepatterning stage of limb development and discuss a recent model that proposes anterior and posterior domains of the early limb bud generate two halves of the future skeleton. By comparing phenotypes in humans with those in model organisms, we evaluate whether this prepatterning concept helps to annotate human disease alleles.
Graphical Abstract
Two early limb bud regulatory domains generate two halves of the limb skeleton.
Overview
Congenital limb anomalies reflect altered development. Although most human anomalies can be classified clinically as failed or mispatterned formation of skeletal elements, their embryonic origins are often not clear. By linking human mutations with embryonic processes that are discovered in model organisms, we have the potential to more precisely unravel the aetiology of congenital anomalies and exciting advances in both arenas are facilitating progress in that direction.
The prepatterning stage of limb development is critical for polarising early limb bud mesenchyme and generating signalling centres that drive further development. Since recent reviews have expertly discussed contemporary concepts that address limb pattern formation1–4 and malformation5, our goal here is to focus on human mutations that potentially affect the prepatterning stage of limb development.
Let’s consider how far we can link anomalies to developmental processes. Formation of the limb can be considered in stages during which key processes take place. These include initiation and early budding, formation of signalling centres called the apical ectodermal ridge (AER) and zone of polarising activity (ZPA), expansion and patterning of progenitor cells, and differentiation of skeletal and other tissue elements. Twentieth century embryologists made remarkable progress by discovering signalling centres and establishing key principles that underlie these processes. Over the past thirty years, important molecular mediators of limb development were identified, many of which were later shown to play critical roles in other organ systems. Can we now ascribe precise functions to mutations in human patients?
The developmental relevance of human mutations is muddled by two general problems. One is that a handful of signalling pathways regulate multiple processes that are relevant during different stages of development. Therefore mutation of, say, a Wnt pathway component could affect early outgrowth or later elongation of differentiating skeletal anlage – two key functions of the pathway. Another problem is that pattern, defined by the spatial distribution of skeletal elements that is most obviously altered in congenital anomalies, is likely specified, determined (committed) and refined during different stages of development. Mutations could therefore disrupt pattern at different times.
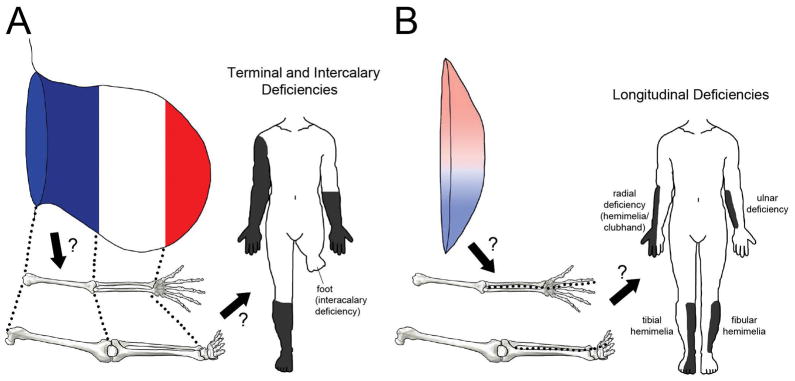
Human limb anomalies have long been referred to as failures of formation and classified according to the position and orientation of missing segments. Longitudinal deficiencies (hemimelia in which embryonically anterior eg. radius/tibia/digit 1 or posterior eg. ulna/fibula/digit 5 elements are lacking) are more common than transverse terminal deficiencies (congenital amputation), and transverse intercalary deficiencies (phocomelia in which a central portion of the limb such as the forearm/lower leg is absent and the hand/foot arises from the upper arm/thigh) are least common6. Since traditional models of limb development have treated anteroposterior (AP) and proximodistal (PD) pattern formation separately, it might seem that longitudinal and transverse deficiencies could be attributable to AP and PD patterning problems, respectively (Fig. 1). Given that the limb skeleton differentiates in a proximal to distal sequence, it also seems that more proximal deficiencies should reflect earlier developmental problems. In this review we examine data from model organisms and contemporary models of pattern formation with a focus on early development of the limb bud to evaluate whether these concepts are valid.
Figure 1. Do models of embryonic pattern formation explain congenital limb deficiencies?
A Do problems with (early or progressive) PD pattern specification result in limb truncation (terminal deficiency) or loss of central elements (intercalary deficiency)? B Do problems with AP pattern formation result in anterior or posterior longitudinal limb deficiency (hemimelia)?
Models of limb pattern formation
Since the mid-twentieth century, theoretical models have served as useful platforms to integrate observational and experimental data obtained from model organisms and potentially help to annotate pathogenic mutations identified in humans. Alan Turing described reaction-diffusion, a theoretical model to explain how developmental pattern arises. In Turing’s proposal, interaction between morphogens coupled with their diffusion is sufficient to disrupt the equilibrium of a homogenous field of cells causing them to form some patterns7. We now have genetic and molecular evidence that supports this model with regard to limb skeletal pattern, especially within the autopod8,9. The classical morphogen gradient model was proposed well after Turing10–12. Combined with modern molecular data, this model proposes that a posterior high to anterior low ratio of GLI transcriptional activator (GLIA that is promoted by Sonic hedgehog (SHH)) to GLI transcriptional repressor (primarily GLI3R) provides AP positional information12–14. These two models are likely simultaneously valid since positional information can fine tune reaction-diffusion15.
The progress zone model which was formulated in 1970s proposed that PD segments are specified gradually due to cell-intrinsic timers10,16. An alternative view that was proposed in the same era17 and elaborated upon in the early 2000s proposes that PD segments are specified together at an early stage and subsequently expanded18. A two signal model also invokes progressive proximal to distal specification under the control of opposing proximal retinoic acid (RA) and distal fibroblast growth factor (FGF)19. Elegant chick embryo experiments provided experimental evidence for this concept20–22. However, the role of RA in this model remains controversial because in the mouse embryo, studies employing mutations of the Raldh2 and Rdh10 genes which encode enzymes for RA synthesis indicated that RA acts to prepare the forelimb field for initiation rather than to specify proximal pattern23,24. Contemporary updates of AP and PD patterning concepts that weigh the relative importance of cell-extrinsic secreted positional cues and cell-intrinsic fate timers are increasingly integrating pattern formation with tissue growth over time. For example, SHH specifies the condensation pattern and promotes expansion of the same group of skeletal progenitors, likely in a two-step biphasic manner25–27. Common to many of these modern concepts is an early ground state in which the prospective limb field exhibits AP polarity.
AP prepattern
The limb field exhibits AP polarity well before the ZPA that expresses Shh is established, and even before growth of the limb bud from the flank is initiated. Surgical rotation of the chick presumptive limb field by 180° about its central axis results in reversal of AP limb polarity, indicating that the axis is fixed prior to outgrowth28,29. Moreover, mice completely lacking Shh exhibit AP polarity of the stylopod segment that is regarded as a manifestation of prepattern established in the early limb field30.
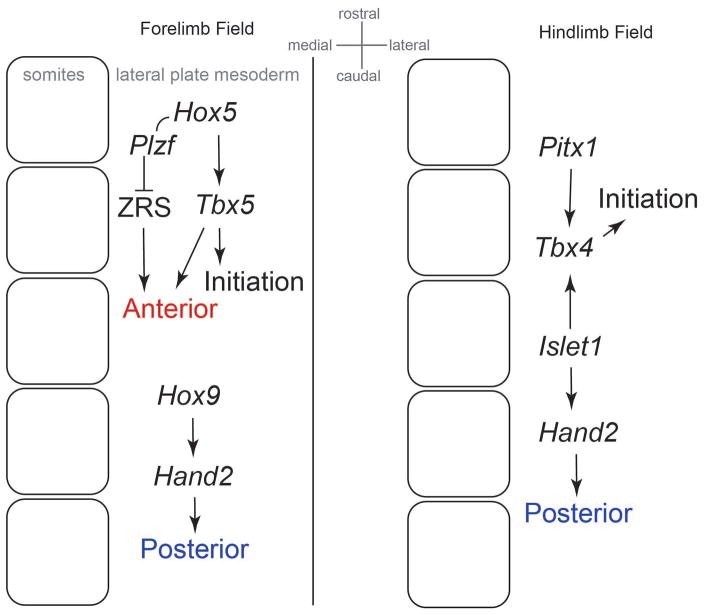
Both the rostrocaudal location and early AP polarity of the forelimb field are attributable to appropriate combinations of Hox genes that are expressed in overlapping domains along the long embryo axis. Hox4 and Hox5 paralogs induce expression of Tbx531 that is required for early forelimb bud growth32. Hox5 and Hox9 paralogs are required for development of anterior and posterior regions of the forelimb, respectively33,34. In the hindlimb field, Pitx1 and Islet1, rather than Hox genes, promote Tbx4 that drives early outgrowth35–38, and Islet1 also promotes posterior identity36. Relative to the hindlimb therefore, AP polarity of the forelimb bud is more clearly defined by the same family of Hox genes that pattern the rostrocaudal embryo axis. Nonetheless, both Hox9 and Islet1 promote posterior expression of Hand2, a key regulator of posterior prepattern in both the forelimb and hindlimb (Fig. 2).
Figure 2. Factors that promote limb field initiation and initial AP polarity.
Initial forelimb AP polarity is related to the colinear expression of Hox genes along the rostrocaudal embryo axis (Hox5-9 paralogues). In contrast, initial hindlimb AP polarity is related to other factors expressed in the caudal region of the embryo such as Pitx1 and Islet1.
GLI3/HAND2 mark AP polarity
Two critical upstream factors of Shh are HAND2 and HOX proteins. HAND2 is a transcription factor that promotes posterior skeletal identity by positively regulating Shh via a cis-regulatory element known as the ZPA regulatory sequence (ZRS)39. The products of some 5′ Hox paralogues, HOXA/D10-13, also upregulate Shh by acting on the ZRS40 and may act synergistically with HAND241. HOXD activation of the ZRS requires control of the spatial distribution of HOX transcription by TALE homeodomain proteins PBX1 and PBX242. In contrast, HOX5 promotes anterior identity by interacting with PLZF to suppress activity of the ZRS enhancer33 (Fig. 2). As such, these HOX proteins affect Shh directly without influencing prepattern.
During the prepatterning stage, HAND2 is counterbalanced anteriorly and mutually antagonised by GLI343,44. TBX3 likely mediates HAND2 repression of GLI3 in posterior forelimb cells45. GLI3 interferes with activation of the ZRS enhancer41 and also restricts SHH activity in anterior limb bud cells to perpetuate AP polarity during later stages. When both Hand2 and Gli3 are removed from the limb field, the limb develops with a fairly symmetrical pattern of skeletal elements, indicating a lack of AP polarity41.
Twist1 genetically interacts with Gli346 and its product promotes anterior identity by forming heterodimers with HAND2 to antagonise Shh expression in anterior mesenchyme47,48. Loss of Twist1 very early in the mouse arrests limb initiation while later loss (and presumably hypomorphic human mutation) causes posteriorisation of anterior structures such as the radius and thumb49,50. Ectopic expression of Hand2 phenocopies loss of Twist151, indicating that precise balance between these transcription factors is essential to establish appropriate AP polarity.
In addition to promoting early anterior identity, Gli3 also restrains mesenchymal cell proliferation in the limb bud52, a function that helps to explain the preaxial polydactyly observed in Gli3 mutants53. During early stages therefore, mutual antagonism between GLI3 and HAND2 is regarded as the molecular manifestation of prepattern and represents the central foundation of the two domain hypothesis (Fig. 3A).
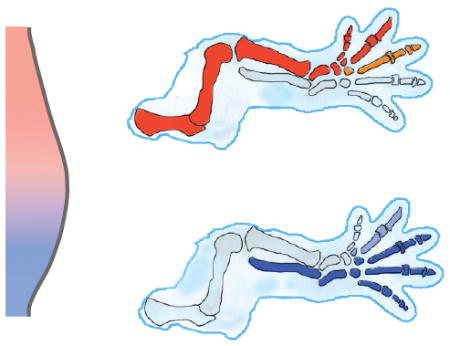
Figure 3. Two domain hypothesis.
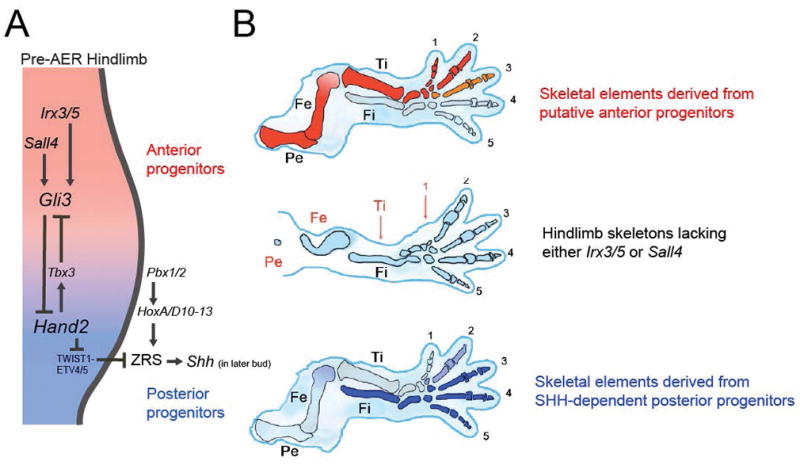
A In the pre-AER hindlimb, AP limb bud polarity is perpetuated by mutual antagonism between anterior and posterior domains that are defined by Irx3/5/Sall4/Gli3 and Hand2, respectively. B Anterior and posterior domains may generate proximal-anterior and distal-posterior skeletal elements, respectively.
Two domain hypothesis
The two domain hypothesis expands upon the AP prepattern concept and posits that progenitor cells within early limb field mesoderm are prepatterned into anterior and posterior groups whose descendants populate the entire limb skeleton54–56 (Fig. 3B). Recently, upstream anterior regulators of Gli3 have been identified that expand upon the prepattern concept. IRX3 and IRX5 (IRX3/5) are TALE class homeodomain proteins that directly promote expression of Gli3 in the hindlimb55. SALL4 is a spalt family zinc finger transcription factor that also upregulates Gli3 in the hindlimb and to a lesser extent in the forelimb56. Consistent with their action in a common anterior identity pathway, Sall4 is downstream of Tbx5 in the mouse forelimb bud57 and its Drosophila homologue spalt regulates irx58.
In the absence of Irx3/5 or Sall4, the hindlimb exhibits a small stylopod (femur) and loss of the anterior zeugopod element (tibia) and anterior digits (primarily one and two). These deficient skeletal elements are those that remain intact in Shh mutants59,60. In other words, these genetic studies support the idea that Irx3/5/Sall4/Gli3 and Hand2/Shh are required to generate complementary anterior/proximal and posterior/distal skeletal elements, respectively. Moreover, genetic lineage tracing of Shh-expressing descendents and of cells that respond to Shh is consistent with expansion of an early posterior population of progenitor cells that gives rise to the radius/tibia and posterior digits61,62. Therefore based mostly upon genetic data, it seems that early AP limb bud prepattern becomes elaborated into two sets of obliquely oriented skeletal descendants (Fig. 3).
It is tempting to speculate whether the apparent oblique orientation of final skeletal pattern relative to initial AP prepattern hints at how the two initial domains function. One possibility is that anterior and posterior cell progenitors are committed halves of the future skeleton whose relative positions shift obliquely during development. This concept is supported by the results of surgical removal of anterior or posterior halves of the early (72–96 h) chick limb bud which closely resemble Irx3/5/Sall4 and Shh mutant skeletal phenotypes, respectively63. Those experiments suggest that anterior and posterior cell fates are ‘determinate’ rather than ‘regulatory’ by that stage, suggesting that two distinct cell populations underlie AP domains. However, random clonal analyses64,65 and prospective fate mapping66 have revealed no evidence of an AP compartment boundary in the limb bud. On the contrary, the initial limb bud is characterised by some degree of AP cell mixing due to cell movements as seen by live imaging67. Mesodermal cells cross gene expression boundaries66 without disrupting those boundaries presumably due to plasticity of cell fate during early stages68. Therefore, a more likely alternative is that anterior and posterior domains specify but do not fix the initial fate of mesodermal cells. Future genetic lineage tracing of early anterior and posterior population cells that express Irx3/5, Sall4, or Hand2, for example, will clarify the degree to which early progenitors presage mature skeletal pattern.
During the prepatterning stage, anterior and posterior regions of the limb field have distinct signalling requirements to establish and maintain their identity. In particular, GLI3 and KIF7 together establish a hedgehog signal-free zone replete with GLI3R in the anterior limb field 54,55. This early state is essential for bud outgrowth since ectopic anterior hedgehog pathway activation is deleterious and aborts the formation of the AER and ZPA54. After the prepatterning stage when the AER and ZPA are established, markers of SHH pathway activation progressively expand from the posterior aspect of the limb bud55. During this process, overlap may occur between anterior GLI3R-rich and posterior GLIA-rich domains. However, any SHH signal that may be present in the anterior-most digit progenitor region remains insufficient to transcribe detectable levels of markers of pathway activation and ectopic anterior pathway activation at those stages results in preaxial polydactyly, a manifestation of anteriorly expanded posterior identity14,69. Therefore, balance between anterior and posterior signal interactions defines normal digit pattern and is also essential to permit appropriate regeneration of the axolotl limb70. By suppressing early anterior SHH pathway activation, IRX3/5 and SALL4 safeguard early establishment of signalling centres and anterior skeletal identity.
Despite abnormal expression of at least some genes, forelimbs of Irx3/5 and Sall4 mutant mice are not mispatterned55,56. However, phenotypic abnormalities of the forelimb that mirror the proximal/anterior skeletal deficiency of the hindlimb appear in an Irx3/5 mutant background when Kif7 is also deleted. Taken together with the greater hedgehog signalling-free anterior area in the forelimb compared to the hindlimb, that implies forelimb/hindlimb differences in sensitivity to deletion of Irx3/5, and possibly of Sall4, are due to the relative extent of the anterior hedgehog-free zone. This sensitivity could help to explain the hindlimb or forelimb propensity of certain human mutations.
Relation of the two domain hypothesis to other models
Irx3/5 and Sall4 regulate prepattern because they are required prior to or during limb initiation, well before signalling centres are established. Conditional and tamoxifen inducible deletion of Irx3/5 at progressively earlier times prior to limb initiation resulted in progressively more severe deficiency of anterior skeletal elements along the PD axis55. Unexpectedly, progressively later deletion of Irx3/5 ‘restored’ skeletal elements in a distal to proximal sequence, implying that Irx3/5 are required earliest for digit one and latest for the stylopod. Although this temporal requirement for Irx3/5 is not intuitive and requires temporal lineage tracing for verification, it does indicate that skeletal identities are not specified simultaneously then simply expanded during outgrowth. Similarly, conditional deletion of Sall4 using different Cre lines that cause recombination at different times before limb bud outgrowth revealed that progressive specification of PD cell fates characterises the anterior domain at an early stage56. Therefore, the two domain hypothesis supports early specification of the anterior region yet incorporates a temporal component that is a feature of the progress zone model.
A possible explanation for the progressive nature of early specification concerns the intimate relationship between morphogenesis, growth and pattern that has been brought to the fore by work linking patterning genes with cell proliferation in the limb bud27 and other contexts71. Morphogenetic cell movements from proximal to distal and from anterior to posterior partly underlie early outgrowth of the limb bud67,72–74. In light of those movements, future lineage tracing may help to explain whether the duration of cell transit through an Irx3/5/Sall4 expression domain affects PD cell fate.
Interestingly, removal of one Shh allele in an Irx3/5 mutant background rescued anterior skeletal pattern55. Given that Shh expression starts ~12 hours after the onset of limb bud outgrowth, this rescue lends support to the idea that, although PD fate may be specified, it is not fixed until later in development68. The experiment also implies that Irx3/5 are not required to generate pattern per se, but rather balance between Irx and Shh influence is most important as it is between Gli3 and Shh75. One possibility is that a basal mechanism of pattern formation, such as reaction-diffusion, is fine tuned by these factors at an early stage. It has been shown that interaction between two adjacent cell populations can itself generate pattern according to principles of reaction-diffusion76, a concept that may apply to the early AP polarised limb bud. By experimentally evaluating the importance of extrinsic signals such as FGF, WNT and BMP that are central to autopod reaction-diffusion, it is conceivable that early events controlled by Irx3/5, Sall4, Gli3 and Hand2 can be linked to existing frameworks to explain complete limb pattern.
Does the prepattern concept help to annotate human mutations?
Mutations in limb development genes are associated with syndromic and nonsyndromic limb deficiency. By attempting to cross reference human mutations that cause congenital limb anomalies with functional studies that were performed in model organisms, one is reminded how interrelated growth and polarity are during development of the early limb bud. Because a previous review effectively summarised congenital human limb malformations and genes that regulate limb development in model animals5, here we focus on mutations that regulate early outgrowth and polarity of the limb bud.
Genetic screens that focused on candidate limb development genes and nearby noncoding regions among patients with congenital limb deficiencies identified point mutations and single nucleotide polymorphisms (SNPs) that were associated with a large variety of phenotypes77,78. Many of the mutations identified involve genes that regulate early AP polarity in the mouse (Table 1).
Table 1.
Human limb deficiency genes grouped according to their early limb bud function and corresponding anterior, posterior, segmental or transverse skeletal pattern phenotype.
| Anterior genes | Phenotype |
|---|---|
| GLI3 | preaxial polydactyly, tibial hemimelia |
| SALL4 | short humerus, radial and thumb deficiency |
| TBX5 | radial and thumb deficiency |
| TWIST1 | syndactyly, partial duplication of first ray |
| Posterior genes | |
| HAND2 excess | radial and thumb anomalies |
| NIPBL (regulates HAND2) | ulnar and tibial deficiency |
| ZRS | polydactyly, tibial hemimelia, ulnar/fibular dimelia |
| TBX3 | ulnar deficiency |
| Early outgrowth genes | |
| FGF10 | radial and thumb deficiency |
| FGFR1 | syndactyly, fused elbow, broad thumb |
| FGFR2 | syndactyly, fused elbow |
| WNT3 | tetra-amelia |
Anterior genes
Several, but not all, human phenotypes arising from mutations of anterior skeletal identity genes fit with their patterning functions in model organisms. Mutations of GLI3, SALL479,80 (Duane-radial ray syndrome, OMIM 607323) and TBX581 (Holt-Oram syndrome, 142900) are associated with defects of anterior limb elements. Phenotypes associated with hypomorphic alleles or haploinsufficiency of these genes often involve deficiency of anterior skeletal elements such as the radius and thumb and may also exhibit a short humerus (the upper limb equivalent to Irx3/5 mutation in the mouse hindlimb). Altered TWIST1-HAND2 dimerisation underlies first (anterior) digit anomalies seen in Saethre-Chotzen syndrome51 (OMIM 101400). In the case of GLI3, some mutant alleles indeed affect anterior skeletal formation resulting in tibial hemimelia as a consequence of ineffective anterior SHH pathway repression82. However, most anomalies associated with GLI3 mutations include anterior (preaxial) polydactyly83, likely due to the proliferation effects in later stages of limb development52. Although the anterior-most autopod is affected by these more common GLI3 alleles, the anterior skeleton remains largely unaffected possibly because of the hypomorphic nature of the mutation in contrast with the relatively severe protein truncation associated with tibial hemimelia. IRX5 mutation has been described in a syndrome that includes craniofacial anomalies and poor long bone quality84, although limb pattern defects have not been described probably due to an intact IRX3 gene since Irx3 and Irx5 are largely redundant in the mouse limb55. Allelic differences and redundancy therefore help to explain of the phenotypic variation due to anterior identity gene mutations.
Posterior genes
Some human mutations of posterior limb genes result in posterior skeletal deficiency while others affect anterior or posterior skeletal identity or character in a manner that is not always intuitive. The first ‘deficiency’ type includes mutations of the ZRS enhancer andTBX3, both of which are downstream of Hand2 and regulate Shh in the mouse. Heterozygous TBX3 mutation results in posterior skeletal deficiency78,85 (ulnar-mammary syndrome, OMIM 181450) while some ZRS mutations are associated with tibial (anterior) longitudinal deficiency (hemimelia)86–88. HAND2 overdose in partial trisomy distal 4q causes anterior zeugopod and autopod skeletal defects89, although it is not clear why a presumably posteriorised limb would exhibit these deficiencies. In Cornelia de Lange Syndrome (OMIM 122470), mutation of NIPBL which regulates Hand2 in zebrafish90 can be associated with ulnar (posterior) and tibial (anterior) deficiency91–93 suggesting that cohesinopathy affects early limb polarity differently in the forelimb and the hindlimb. The second ‘mispattern’ type of mutations include other single base pair substitutions or microduplications of the ZRS sequence that cause preaxial polydactyly94 and Laurin-Sandrow syndrome (OMIM 135750)95 in which the anterior (radial/tibial) aspect of the hand/foot exhibits posterior character resembling mirror duplication of the posterior (ulnar/fibular) aspect of the upper and lower limbs. These phenotypes are presumably due to ectopic anterior SHH pathway activation signalling although the precise mechanism remains unclear. This variety of AP pattern phenotypes underscores the importance of balance between multiple regulators of AP pattern and suggests that we do not fully understand basic questions about how skeletal formation and identity are coregulated.
Limb initiation and early outgrowth genes
By trying here to highlight human deficiencies that result from mutations in genes that promote limb initiation in model organisms, we find that outgrowth is almost inseparable from AP polarity. For example, Tbx5 and Fgf10 are required for forelimb initiation in the mouse32,96,97, in part by promoting epithelial to mesenchymal transition of coelomic epithelium into lateral plate mesoderm cells in the forelimb field72. In the mouse forelimb bud, Tbx5 regulates Sall457 which is required for expression of the anterior pattern regulator Gli356. Therefore as expected, haploinsufficiency of Tbx5/TBX5 causes anterior (radius and thumb) forelimb deficiency in mouse and Holt-Oram syndrome98 (OMIM 142900) in human. Point mutations and single nucleotide polymorphisms (SNPs) of TBX5 are also associated with nonsyndromic radius and thumb deficiency77,78. Although a polarity effect of hypomorphic FGF10 is less clear, common SNPs and mutations of FGF10 as well as of other FGF ligands and receptors (FGFR1, FGFR2) predispose to a variety of nonsyndromic and syndromic transverse and longitudinal defects78,99 including lacrimoauriculardentaldigital (OMIM 149730), Pfeiffer (OMIM 101600) and Crouzon (OMIM 176943) syndromes. Another pathway that converges upon Fgf10 to promote limb initiation in the chick and mouse is canonical Wnt signalling. Accordingly, loss of WNT3 function in humans causes tetra-amelia100, presumably due to failure of initiation. The combination of transverse and longitudinal defects caused by this group of alleles underscores the interrelated nature of growth and pattern formation.
Predictive power of model organisms?
The examples above show that human phenotypes largely correspond to the expected function of mutant genes based on work in model organisms. Some of these correlations, as with human TBX5 mutations, underscore the intimate relationship between limb polarity and early growth. Early limb field polarity that establishes an anterior region free of SHH pathway activation is likely a feature in common to both mouse and human embryos. However, we do not yet have sufficient basic information to neatly predict AP phenotypes.
It may be interesting to mark our current state of progress by attempting to predict unknown genetic etiologies of human limb anomalies based on the phenotype. One would expect nonsyndromic ulnar dimelia101,102, or mirror duplication of the posterior aspect of the upper (or lower) limb, for example, to be due to loss of anterior identity. This loss could be due to mutation of an anterior identity gene such as a regulator of GLI3 or expansion of a posterior identity factor such as a regulator of HAND2. However, ectopic anterior SHH pathway activation prior to establishment of signalling centres is unlikely to be causal since that should result in severe loss of skeletal elements rather than simply posteriorised character54. Mutation that causes ulnar duplication would be functionally distinct to that which causes ulnar deficiency95 since skeletal formation is largely unaffected in the former, possibly because the causal mutation affects a basal patterning mechanism without affecting proliferation or morphogenesis.
Proximal femoral focal deficiency is another interesting example of a usually sporadic skeletal deficiency for which a mutation has not been identified103–105. A curious feature of this deficiency is that it can be associated with either posterior (fibular) or anterior (tibial) longitudinal deficiency (hemimelia). When femoral deficiency is associated with tibial hemimelia (Fig. 4), the affected skeletal elements match the proximal-anterior domain described above and therefore might be due to a mutation affecting IRX3/5 or SALL4. Consistent with the loss of anterior skeletal elements in this condition, there is evidence that a Drosophila Irx homologue called caupolican regulates proliferation in addition to pattern specification71. In contrast, associated fibular hemimelia is more difficult to assign neatly to one half of the skeleton according to the two domain model. Possible mutations could affect the posterior/distal portion of the skeleton according to the two domain model since a small portion of the femur is dependent on Shh in mouse30. An alternative possibility is that an upstream regulator of the ZRS such as HOXA10 or HOXD10 affects both stylopod development and AP limb bud identity. A variant deficiency called congenital short femur could be attributable to a different allele that affects segmental stylopod development but not AP pattern. Isolated fibular hemimelia might be due to a regulator of posterior identity similar to TBX3 that is upstream of the ZRS and regulates skeletal formation as in the ulnar deficiency syndrome mentioned above. Like the Gli3 and caupolican examples, genes that regulate pattern likely also regulate proliferation or morphogenesis to a greater extent than is appreciated currently. Of course, continued exploration of basic developmental mechanisms combined with annotation of disease alleles in model organisms will facilitate deeper understanding of the intimate relationship between formation and pattern of specialised tissues that is relevant to human anomalies.
Figure 4. Longitudinal deficiency.
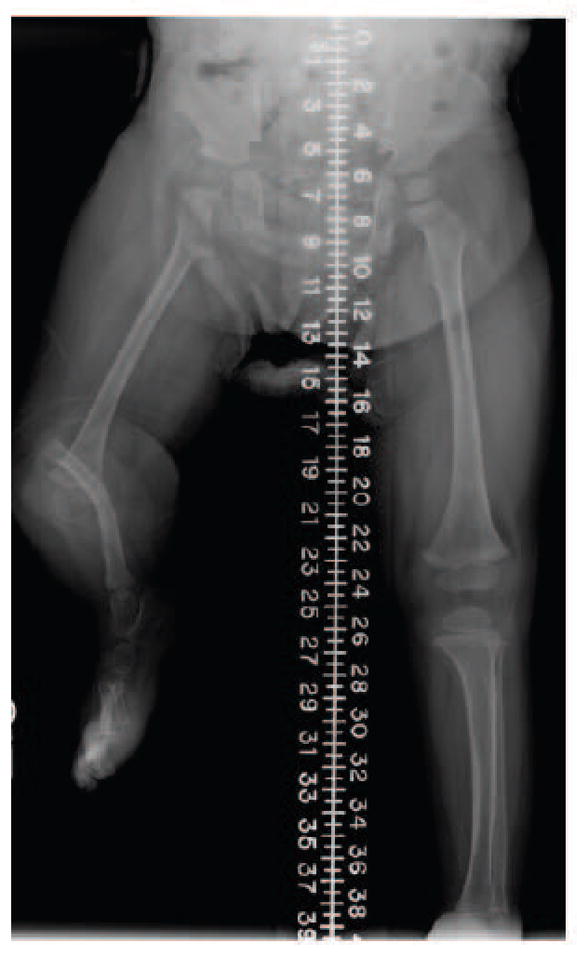
Femoral deficiency associated with an absent tibia is potentially attributable to misregulation of the anterior domain.
Acknowledgments
This work was supported by a CIHR grant to S.H. and C-c.H (137092) and an NIH grant to Y.K. (AR064195).
References
- 1.Tickle C. How the embryo makes a limb: determination, polarity and identity. J Anat. 2015;227:418–430. doi: 10.1111/joa.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuniga A. Next generation limb development and evolution: old questions, new perspectives. Development. 2015;142:3810–3820. doi: 10.1242/dev.125757. [DOI] [PubMed] [Google Scholar]
- 3.Delgado I, Torres M. Gradients, waves and timers, an overview of limb patterning models. Semin Cell Dev Biol. 2016;49:109–115. doi: 10.1016/j.semcdb.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuniga A, Zeller R, Probst S. The molecular basis of human congenital limb malformations. Wiley Interdiscip Rev Dev Biol. 2012;1:803–822. doi: 10.1002/wdev.59. [DOI] [PubMed] [Google Scholar]
- 6.Gold NB, Westgate MN, Holmes LB. Anatomic and etiological classification of congenital limb deficiencies. Am J Med Genet A. 2011;155A:1225–1235. doi: 10.1002/ajmg.a.33999. [DOI] [PubMed] [Google Scholar]
- 7.Turing AM. The chemical basis of morphogenesis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1952;237:37–72. doi: 10.1098/rstb.1952.0012. [DOI] [Google Scholar]
- 8.Sheth R, et al. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science. 2012;338:1476–1480. doi: 10.1126/science.1226804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raspopovic J, Marcon L, Russo L, Sharpe J. Modeling digits. Digit patterning is controlled by a Bmp-Sox9-Wnt Turing network modulated by morphogen gradients. Science. 2014;345:566–570. doi: 10.1126/science.1252960. [DOI] [PubMed] [Google Scholar]
- 10.Summerbell D, Lewis JH, Wolpert L. Positional information in chick limb morphogenesis. Nature. 1973;244:492–496. doi: 10.1038/244492a0. [DOI] [PubMed] [Google Scholar]
- 11.Tickle C, Summerbell D, Wolpert L. Positional signalling and specification of digits in chick limb morphogenesis. Nature. 1975;254:199–202. doi: 10.1038/254199a0. [DOI] [PubMed] [Google Scholar]
- 12.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 13.Towers M, Wolpert L, Tickle C. Gradients of signalling in the developing limb. Curr Opin Cell Biol. 2012;24:181–187. doi: 10.1016/j.ceb.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 14.te Welscher P, et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- 15.Green JB, Sharpe J. Positional information and reaction-diffusion: two big ideas in developmental biology combine. Development. 2015;142:1203–1211. doi: 10.1242/dev.114991. [DOI] [PubMed] [Google Scholar]
- 16.Wolpert L, Tickle C, Sampford M. The effect of cell killing by x-irradiation on pattern formation in the chick limb. J Embryol Exp Morphol. 1979;50:175–193. [PubMed] [Google Scholar]
- 17.Stocum DL. Outgrowth and pattern formation during limb ontogeny and regeneration. Differentiation. 1975;3:167–182. doi: 10.1111/j.1432-0436.1975.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 18.Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- 19.Mercader N, et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- 20.Cooper KL, et al. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332:1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosello-Diez A, Arques CG, Delgado I, Giovinazzo G, Torres M. Diffusible signals and epigenetic timing cooperate in late proximo-distal limb patterning. Development. 2014;141:1534–1543. doi: 10.1242/dev.106831. [DOI] [PubMed] [Google Scholar]
- 22.Rosello-Diez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham TJ, et al. Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Rep. 2013;3:1503–1511. doi: 10.1016/j.celrep.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, et al. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr Biol. 2009;19:1050–1057. doi: 10.1016/j.cub.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, et al. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Mackem S. Analysis of mutants with altered shh activity and posterior digit loss supports a biphasic model for shh function as a morphogen and mitogen. Dev Dyn. 2011;240:1303–1310. doi: 10.1002/dvdy.22637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towers M, Mahood R, Yin Y, Tickle C. Integration of growth and specification in chick wing digit-patterning. Nature. 2008;452:882–886. doi: 10.1038/nature06718. [DOI] [PubMed] [Google Scholar]
- 28.Hamburger V. Morphogenetic and axial self-differentiation of transplanted limb primordia of 2-day chick embryos. Journal of Experimental Zoology. 1938;77:379–399. [Google Scholar]
- 29.Chaube S. On axiation and symmetry in transplanted wing of the chick. J Exp Zool. 1959;140:29–77. doi: 10.1002/jez.1401400104. [DOI] [PubMed] [Google Scholar]
- 30.Chiang C, et al. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 31.Minguillon C, et al. Hox genes regulate the onset of Tbx5 expression in the forelimb. Development. 2012;139:3180–3188. doi: 10.1242/dev.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal P, et al. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- 33.Xu B, et al. Hox5 interacts with Plzf to restrict Shh expression in the developing forelimb. Proc Natl Acad Sci U S A. 2013;110:19438–19443. doi: 10.1073/pnas.1315075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu B, Wellik DM. Axial Hox9 activity establishes the posterior field in the developing forelimb. Proc Natl Acad Sci U S A. 2011;108:4888–4891. doi: 10.1073/pnas.1018161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanctot C, Moreau A, Chamberland M, Tremblay ML, Drouin J. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–1810. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- 36.Itou J, et al. Islet1 regulates establishment of the posterior hindlimb field upstream of the Hand2-Shh morphoregulatory gene network in mouse embryos. Development. 2012;139:1620–1629. doi: 10.1242/dev.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szeto DP, et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13:484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logan M, Tabin CJ. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science. 1999;283:1736–1739. doi: 10.1126/science.283.5408.1736. [DOI] [PubMed] [Google Scholar]
- 39.Lettice LA, et al. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc Natl Acad Sci U S A. 2002;99:7548–7553. doi: 10.1073/pnas.112212199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kmita M, et al. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435:1113–1116. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- 41.Galli A, et al. Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. 2010;6:e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capellini TD, et al. Pbx1/Pbx2 requirement for distal limb patterning is mediated by the hierarchical control of Hox gene spatial distribution and Shh expression. Development. 2006;133:2263–2273. doi: 10.1242/dev.02395. [DOI] [PubMed] [Google Scholar]
- 43.Panman L, Zeller R. Patterning the limb before and after SHH signalling. J Anat. 2003;202:3–12. doi: 10.1046/j.1469-7580.2003.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osterwalder M, et al. HAND2 targets define a network of transcriptional regulators that compartmentalize the early limb bud mesenchyme. Dev Cell. 2014;31:345–357. doi: 10.1016/j.devcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Rourke MP, Soo K, Behringer RR, Hui CC, Tam PP. Twist plays an essential role in FGF and SHH signal transduction during mouse limb development. Dev Biol. 2002;248:143–156. doi: 10.1006/dbio.2002.0730. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, et al. Preaxial polydactyly: interactions among ETV, TWIST1 and HAND2 control anterior-posterior patterning of the limb. Development. 2010;137:3417–3426. doi: 10.1242/dev.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krawchuk D, et al. Twist1 activity thresholds define multiple functions in limb development. Dev Biol. 2010;347:133–146. doi: 10.1016/j.ydbio.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loebel DA, Hor AC, Bildsoe HK, Tam PP. Timed deletion of Twist1 in the limb bud reveals age-specific impacts on autopod and zeugopod patterning. PLoS One. 2014;9:e98945. doi: 10.1371/journal.pone.0098945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loebel DA, et al. Regionalized Twist1 activity in the forelimb bud drives the morphogenesis of the proximal and preaxial skeleton. Dev Biol. 2012;362:132–140. doi: 10.1016/j.ydbio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Firulli BA, et al. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Rios J, et al. GLI3 constrains digit number by controlling both progenitor proliferation and BMP-dependent exit to chondrogenesis. Dev Cell. 2012;22:837–848. doi: 10.1016/j.devcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- 54.Zhulyn O, et al. A switch from low to high Shh activity regulates establishment of limb progenitors and signaling centers. Dev Cell. 2014;29:241–249. doi: 10.1016/j.devcel.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Li D, et al. Formation of proximal and anterior limb skeleton requires early function of Irx3 and Irx5 and is negatively regulated by Shh signaling. Dev Cell. 2014;29:233–240. doi: 10.1016/j.devcel.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Akiyama R, et al. Sall4-Gli3 system in early limb progenitors is essential for the development of limb skeletal elements. Proc Natl Acad Sci U S A. 2015;112:5075–5080. doi: 10.1073/pnas.1421949112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koshiba-Takeuchi K, et al. Cooperative and antagonistic interactions between Sall4 and Tbx5 pattern the mouse limb and heart. Nat Genet. 2006;38:175–183. doi: 10.1038/ng1707. [DOI] [PubMed] [Google Scholar]
- 58.de Celis JF, Barrio R. Function of the spalt/spalt-related gene complex in positioning the veins in the Drosophila wing. Mech Dev. 2000;91:31–41. doi: 10.1016/s0925-4773(99)00261-0. [DOI] [PubMed] [Google Scholar]
- 59.Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 60.Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 61.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 63.Warren AE. Experimental studies on the development of the wing in the embryo of gallus domesticus. The American Journal of Anatomy. 1934;54:449–485. [Google Scholar]
- 64.Arques CG, Doohan R, Sharpe J, Torres M. Cell tracing reveals a dorsoventral lineage restriction plane in the mouse limb bud mesenchyme. Development. 2007;134:3713–3722. doi: 10.1242/dev.02873. [DOI] [PubMed] [Google Scholar]
- 65.Marcon L, Arques CG, Torres MS, Sharpe J. A computational clonal analysis of the developing mouse limb bud. PLoS Comput Biol. 2011;7:e1001071. doi: 10.1371/journal.pcbi.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vargesson N, et al. Cell fate in the chick limb bud and relationship to gene expression. Development. 1997;124:1909–1918. doi: 10.1242/dev.124.10.1909. [DOI] [PubMed] [Google Scholar]
- 67.Wyngaarden LA, et al. Oriented cell motility and division underlie early limb bud morphogenesis. Development. 2010;137:2551–2558. doi: 10.1242/dev.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyngaarden LA, Hopyan S. Plasticity of proximal-distal cell fate in the mammalian limb bud. Dev Biol. 2008;313:225–233. doi: 10.1016/j.ydbio.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 69.Masuya H, Sagai T, Wakana S, Moriwaki K, Shiroishi T. A duplicated zone of polarizing activity in polydactylous mouse mutants. Genes Dev. 1995;9:1645–1653. doi: 10.1101/gad.9.13.1645. [DOI] [PubMed] [Google Scholar]
- 70.Nacu E, Gromberg E, Oliveira CR, Drechsel D, Tanaka EM. FGF8 and SHH substitute for anterior-posterior tissue interactions to induce limb regeneration. Nature. 2016;533:407–410. doi: 10.1038/nature17972. [DOI] [PubMed] [Google Scholar]
- 71.Barrios N, Gonzalez-Perez E, Hernandez R, Campuzano S. The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields. PLoS Genet. 2015;11:e1005463. doi: 10.1371/journal.pgen.1005463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gros J, Tabin CJ. Vertebrate limb bud formation is initiated by localized epithelial-to-mesenchymal transition. Science. 2014;343:1253–1256. doi: 10.1126/science.1248228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gros J, et al. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol. 2010;20:1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao Q, Stinnett HK, Ho RK. Asymmetric cell convergence-driven zebrafish fin bud initiation and pre-pattern requires Tbx5a control of a mesenchymal Fgf signal. Development. 2015;142:4329–4339. doi: 10.1242/dev.124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- 76.Nakamasu A, Takahashi G, Kanbe A, Kondo S. Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proc Natl Acad Sci U S A. 2009;106:8429–8434. doi: 10.1073/pnas.0808622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furniss D, et al. Genetic screening of 202 individuals with congenital limb malformations and requiring reconstructive surgery. J Med Genet. 2009;46:730–735. doi: 10.1136/jmg.2009.066027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Browne ML, et al. Evaluation of genes involved in limb development, angiogenesis, and coagulation as risk factors for congenital limb deficiencies. Am J Med Genet A. 2012;158A:2463–2472. doi: 10.1002/ajmg.a.35565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kohlhase J, et al. Okihiro syndrome is caused by SALL4 mutations. Hum Mol Genet. 2002;11:2979–2987. doi: 10.1093/hmg/11.23.2979. [DOI] [PubMed] [Google Scholar]
- 80.Kohlhase J, et al. Mutations at the SALL4 locus on chromosome 20 result in a range of clinically overlapping phenotypes, including Okihiro syndrome, Holt-Oram syndrome, acro-renal-ocular syndrome, and patients previously reported to represent thalidomide embryopathy. J Med Genet. 2003;40:473–478. doi: 10.1136/jmg.40.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15:30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 82.Deimling S, et al. Tibial hemimelia associated with GLI3 truncation. J Hum Genet. 2016;61:443–446. doi: 10.1038/jhg.2015.161. [DOI] [PubMed] [Google Scholar]
- 83.Malik S. Polydactyly: phenotypes, genetics and classification. Clin Genet. 2014;85:203–212. doi: 10.1111/cge.12276. [DOI] [PubMed] [Google Scholar]
- 84.Bonnard C, et al. Mutations in IRX5 impair craniofacial development and germ cell migration via SDF1. Nat Genet. 2012;44:709–713. doi: 10.1038/ng.2259. [DOI] [PubMed] [Google Scholar]
- 85.Bamshad M, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 86.Cho TJ, et al. Tibial hemimelia-polydactyly-five-fingered hand syndrome associated with a 404 G>A mutation in a distant sonic hedgehog cis-regulator (ZRS): a case report. J Pediatr Orthop B. 2013;22:219–221. doi: 10.1097/BPB.0b013e32835106b2. [DOI] [PubMed] [Google Scholar]
- 87.VanderMeer JE, et al. A novel ZRS mutation leads to preaxial polydactyly type 2 in a heterozygous form and Werner mesomelic syndrome in a homozygous form. Hum Mutat. 2014;35:945–948. doi: 10.1002/humu.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wieczorek D, et al. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum Mutat. 2010;31:81–89. doi: 10.1002/humu.21142. [DOI] [PubMed] [Google Scholar]
- 89.Tamura M, et al. Overdosage of Hand2 causes limb and heart defects in the human chromosomal disorder partial trisomy distal 4q. Hum Mol Genet. 2013;22:2471–2481. doi: 10.1093/hmg/ddt099. [DOI] [PubMed] [Google Scholar]
- 90.Muto A, et al. Nipbl and mediator cooperatively regulate gene expression to control limb development. PLoS Genet. 2014;10:e1004671. doi: 10.1371/journal.pgen.1004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pfeiffer RA, Correll J. Hemimelia in Brachmann-de Lange syndrome (BDLS): a patient with severe deficiency of the upper and lower limbs. Am J Med Genet. 1993;47:1014–1017. doi: 10.1002/ajmg.1320470715. [DOI] [PubMed] [Google Scholar]
- 92.Pankau R, Johannson W, Meinecke P. Brachmann-de Lange syndrome in 16 of our patients. Monatsschr Kinderheilkd. 1990;138:72–76. [PubMed] [Google Scholar]
- 93.Meinecke P, Hayek H. Brief historical note on the Brachmann-de Lange syndrome: a patient closely resembling the case described by Brachmann in 1916. Am J Med Genet. 1990;35:449–450. doi: 10.1002/ajmg.1320350328. [DOI] [PubMed] [Google Scholar]
- 94.Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15:294–300. doi: 10.1016/j.gde.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Lohan S, et al. Microduplications encompassing the Sonic hedgehog limb enhancer ZRS are associated with Haas-type polysyndactyly and Laurin-Sandrow syndrome. Clin Genet. 2014;86:318–325. doi: 10.1111/cge.12352. [DOI] [PubMed] [Google Scholar]
- 96.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 97.Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bruneau BG, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 99.Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC. FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am J Med Genet. 2002;112:266–278. doi: 10.1002/ajmg.10775. [DOI] [PubMed] [Google Scholar]
- 100.Niemann S, et al. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74:558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gorriz G. Ulnar dimelia--a limb without anteroposterior differentiation. J Hand Surg Am. 1982;7:466–469. doi: 10.1016/s0363-5023(82)80041-5. [DOI] [PubMed] [Google Scholar]
- 102.Harrison RG, Pearson MA, Roaf R. Ulnar dimelia. J Bone Joint Surg Br. 1960;42-B:549–555. doi: 10.1302/0301-620X.42B3.549. [DOI] [PubMed] [Google Scholar]
- 103.Lenz W, Zygulska M, Horst J. FFU complex: an analysis of 491 cases. Hum Genet. 1993;91:347–356. doi: 10.1007/BF00217355. [DOI] [PubMed] [Google Scholar]
- 104.Gillespie R, Torode IP. Classification and management of congenital abnormalities of the femur. J Bone Joint Surg Br. 1983;65:557–568. doi: 10.1302/0301-620X.65B5.6643558. [DOI] [PubMed] [Google Scholar]
- 105.Pappas AM. Congenital abnormalities of the femur and related lower extremity malformations: classification and treatment. J Pediatr Orthop. 1983;3:45–60. doi: 10.1097/01241398-198302000-00009. [DOI] [PubMed] [Google Scholar]