Abstract
We assessed trends, predictors and outcomes of resource utilization in hospital inpatient discharges with a principal diagnosis of Alzheimer's disease (AD) with at least one procedure. Using Nationwide Inpatient Sample data (NIS, 2002–2012), discharges primarily diagnosed with AD, aged ≥60 y and with ≥1 procedure, were selected (Weighted N= 92,300). Hospital resource utilization were assessed using ICD-9-CM codes, while hospitalization outcomes included total charges (TC, 2012$), length of stay (LOS, days), and mortality risk (MR, %). Brain and respiratory/gastrointestinal procedure utilization both dropped annually by 3–7%, while cardiovascular procedures/evaluations, blood evaluations, blood transfusion, and resuscitation (“CVD/Blood”) as well as neurophysiological and psychological evaluation and treatment (“Neuro”) procedures increased by 5–8%. Total charges, length of stay, and mortality risk were all markedly higher with use of respiratory/gastrointestinal procedures as opposed to being reduced with use of “Brain” procedures. Procedure count was positively associated with all three hospitalization outcomes. In sum, patterns of hospital resources that were used among AD inpatients changed over-time, and were associated with hospitalization outcomes such as total charges, length of stay, and mortality risk.
Keywords: Alzheimer's disease, healthcare resource utilization, hospital inpatients, hospitalization outcomes, hospital procedures
Introduction
The world's older adult population is projected to double in the coming three decades and many will develop serious chronic diseases such as Alzheimer's disease (AD) as incident cases continue to emerge since multi-modal treatments for AD remain unsuccessful [1–3]. Recently, AD cases were projected to double by 2050 [4, 5], with the incidence rates increasing by 40% by 2025 [4]. Various health services are needed to assist in maintaining quality of life for older adults which creates a significant burden at all levels of the health care spectrum [4]. Consequently, health care expenditure ascribed to AD is expected to increase from $203 billion in 2013 to $1.2 trillion in 2050 [4]. Notwithstanding greater use of primary care services, psychiatrists, neurologists, and psychotropic medications [6, 7], evidence also shows that hospitalization risk among community-dwelling dementia patients in general is higher than non-dementia cases [8, 9], with a large portion of AD-related healthcare cost accounted for by hospital care costs [10, 11]. Evidence also points to a link between AD severity and health care costs, [12–19] driven by an elongated hospital stay, [17] or more frequent home care [18]. In fact, in order to obtain a true overview of the impact of AD, reliance on cognitive assessment is seldom sufficient. Additional aspects such as activities of daily living (ADLs), care-giver burden, behavioral symptoms, quality of life, and resource utilization need to be assessed [20].
A looming increase in hospital care costs triggered by higher AD rates both in the community and in inpatient setting [21–23] requires more in-depth research as to the trends, predictors (e.g., patient-level and hospital-level characteristics), and outcomes (e.g., total charges/admission, length of stay, and in-hospital mortality) of various types of procedures and resources that are utilized by AD inpatients. Specifically, it is unclear what proportion of AD admissions have utilized procedures in the span of a decade, which 10 procedures are the most common among those utilizing at least one procedure, what proportion of all procedures are directly related to the diagnosis and treatment of AD, and how are different types and groups of procedures related to key predictors and outcomes.
Our key study objectives were: (A) To compare procedure groups (AD versus non-AD related) by patient-level and hospital-level characteristics; (B) To describe over-time trends in procedure utilization among an inpatient sample of US older adults with principal diagnosis of AD who had utilized at least one hospital procedure [B.1. Top 10 most common procedures; B.2. Major procedure types; B.3. Procedure groups (AD-related versus non-AD related); B.4. Number of procedures]; (C) To examine trends in hospitalization outcome overall and within procedure groups; (D) To compare hospitalization outcomes among older adults with principal diagnosis of AD and with at least one procedure [D.1. Top 10 most common procedures; D.2. Major procedure types; D.3. Procedure groups (AD-related versus non-AD related); D.4. Number of procedures], after adjustment for patient-level and hospital-level characteristics. Other secondary objectives included: (E) To compare principally diagnosed AD inpatients by healthcare resource utilization status (yes versus no) in terms of patient-level and hospital-level characteristics as well as co-morbidities and outcomes of hospitalization; (F) To examine trends in healthcare resource utilization among principally diagnosed AD patients (2002–2012).
Materials And Methods
Database and study participants
Sponsored by the Agency for Healthcare Research and Quality (AHRQ), the Nationwide Inpatient Sample (NIS) is part of the Healthcare Cost and Utilization Project (HCUP) database and software tools family, with partnerships across federal, state, and industry agencies. The largest of its kind, NIS collects annual data on 7-8 million hospital stays, reflecting all discharges from around 1,000 hospitals, a probability sample from the HCUP State Inpatient Databases (SID) data. With a sampling probability of ∼20%, NIS's design is stratified covering U.S. non-rehabilitation, community hospitals, and targeting all acute care hospital discharges in the U.S. The NIS provides nationwide information on hospital utilization, charges, and quality of care. In 2012, NIS selected all discharges from the hospital universe (n = 4,840), around a 20% sample of hospitals in this target universe [24].
To select a representative sample of hospitals, five hospital characteristics found in the AHA hospital files were used to specify sampling strata: (1) Geographic Region – Northeast, Midwest, West, and South; (2) Control – public, private not-for-profit, and proprietary; (3) Location – urban or rural; (4) Teaching Status – teaching or non-teaching, (5) Bed Size – small, medium, and large.
The NIS collects data on clinical and resource-use information from a typical discharge abstract, while protecting the privacy of patients, physicians, and hospitals (See Supplementary Material 1 for details).
Using the United Nations definition of the older population, (http://www.who.int/healthinfo/survey/ageingdefnolder/en/), we selected the age group 60 y or older. Of 87,039,711 patient admissions (2002–2012 NIS), 35,258,031 were aged 60 y or more (weighted mean age±SE: 75.37±0.03, and weighted proportion female ± SE: 56.0% ± 0.1), with total weighted sample of 166,871,086 nationwide. In our study, we only included AD patients hospitalized with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 331.0 as the principal diagnosis (Supplementary Material II). Among this sub-set of admissions (n = 126,284, Weighted N = 604,642), we further excluded those with no procedures reported, yielding an unweighted n = 19,209 (weighted N = 92,300), consisting of older adult admissions principally diagnosed with AD having at least one reported procedure. The study protocol was reviewed and approved by the National Institute on Environmental Health Sciences Institutional Review Board of the National Institutes of Health.
Reported procedure algorithms
Top 10 ranking procedures
Using data on admissions of older adults aged 60 y or older with principal diagnosis of AD and at least one procedure, we tabulated frequencies of ICD-9-CM codes for the principal procedure done as well as the remaining 14 procedures. We created a second database (PR database) for the reported ICD-9-CM codes including their frequencies per procedure. The sum of the frequencies of procedure use across procedure numbers was computed for each ICD-9-CM code which allowed us to obtain the top-ranking procedures utilized in our study sample. Only the most common 10 procedures were examined, and ICD-9-CM Vol 3 codes and labels were obtained from: http://www.findacode.com/search/search.php (Supplementary Material III).
Procedure types
Using the PR database, we grouped ICD-9-CM codes into meaningful sub-types (n = 36), (Supplementary Material II), based on ascendingly sorted codes, which were then classified into 9 major types, namely: (1) cardiovascular procedures/evaluations, blood evaluations, blood transfusion, and resuscitation, labelled as “CVD/Blood”; (2) “Brain” procedures, spinal tap, head/brain imaging; (3) Neurophysiological and psychological evaluation and treatment (“Neuro”); (4) Eye/ear/nose/throat/mouth/teeth (“sensory/dental”); (5) Bone, soft tissue, muscles, amputation, breast, skin, (“Skin/muscle/bone”); (6) Respiratory, gastrointestinal, liver, pancreas, abdomen (“Respiratory/gastrointestinal”); (7) Genito-urinary procedures and evaluation (“GU”) (8) Injection, shunt, implant, insert, device removal/replacement (“Injection/device”); and (9) “Other” procedures.
Procedure groups
Using procedure types, we further grouped discharges into those with “non-AD related only” procedures and those with at least one “AD-related” procedure. AD-related procedures were those categorized as either one of two types, which are known to be procedures used for the diagnosis of AD and treatment of related psychiatric/cognitive disorders, namely neurophysiological/neuropsychological/neuropsychiatric or “Brain procedure, including brain imaging”. All other procedures were considered non-AD related.
Co-morbidity measures
The AHRQ comorbidity measures identify coexisting medical conditions not directly associated with the principal diagnosis or the main reason for admission, and likely diagnosed before the hospital stay. Developed as one of the HCUP tools, the AHRQ comorbidity measures are described in detail at http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. In the present study, we included 27 of 29 co-morbidities, excluding AIDS due to rare occurrence and neurological co-morbidity for likely redundancies with AD diagnosis (http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/Table2-FY12-V37.pdf). The sum of those co-morbidities was used as an index for total co-morbidity burden in the admission and was considered as a potential confounder in multiple regression models. Similarly, another widely used measure, the Charlson co-morbidity index was also computed using 15 possible diagnoses and included among potential confounding factors [25].
Outcome measures
Three outcome measures of hospitalization were considered, mortality (MR) upon discharge, length of stay (LOS) in days, and total charges (TC) in USD. We specifically compared study sample hospitalization outcomes across having reported a specific procedure, type, or groups of procedures versus not. Moreover, trends in MR, LOS, and TC were also examined overall and within procedure groups (AD versus non-AD related procedure). TC values were adjusted for inflation using the 2012 consumer price index [26]. Detailed information on total charges are available in: https://www.hcup-us.ahrq.gov/db/vars/totchg/nisnote.jsp. Generally, total charges do not include professional fees and non-covered charges. If the source provides total charges with professional fees, then the professional fees are removed from the charge during HCUP processing. In a small number of HCUP databases, professional fees cannot be removed from total charges because the data source cannot provide the information, depending on the State. Emergency department charges incurred prior to admission to the hospital may be included in total charges. Medicare requires a bundled bill for Medicare patients admitted to the hospital through the emergency department. Other payers may or may not have similar requirements.
Covariates
Patient-level characteristics
Among patient-level characteristics, we included age (y), sex, race (White, Black, Hispanic, Asian/Pacific Islander, Native American, and Other), median household income for zip code of patient (expressed as quartiles), insurance status (Medicare, Medicaid, Private insurance, self-pay, no charge, and other) and admission day (weekday versus weekend), total co-morbidities, and the Charlson co-morbidity index.
Hospital-level characteristics
Hospital-level characteristics included bed size (Small, Medium, Large), location/teaching status of the hospital (rural, urban, non-teaching, urban teaching), and region of the hospital (Northeast, Midwest, South, and West).
Statistical analysis
Using Stata 14.0 (StataCorp, College Station, TX) [27], our analyses took into account survey design complexity by following the analytic guidelines outlined by HCUP NIS [24] and incorporating sampling weights, primary sampling units, and strata. This allowed us to estimate population proportions, means, and regression coefficients using svy commands [27]. Standard errors were computed using Taylor series linearization. Multiple linear and logistic regression models were conducted, mainly to examine trends in procedure types and outcomes of hospitalization over time as well as test of effects of procedures on outcomes of hospitalization. Based on our key objectives, (A) utilization rates of hospital procedures, types/groups were explored among selected discharges. A trend test was conducted for the 2002 to 2012 period using a bivariate logistic regression, with year as the sole predictor of the binary procedure utilization variable; (B) AD and non-AD procedure groups in the selected sample were then compared by patient-level and hospital-level characteristics using design-based F-tests; (C) Trends in outcomes of hospitalization were examined using linear regression for TC and LOS and binary logistic regression for MR (estimating the odds ratio of mortality per year, OR with 95% CI), with year as the sole predictor. This analysis was carried out overall and stratified by use of AD-related procedures versus not; (D) To compare hospitalization outcomes by use of top 10 procedures, procedure types and groups, we conducted a series of multiple linear and logistic regression models that adjusted for patient-level, hospital-level characteristics, co-morbidities and survey year. The procedure parameter in this model can be interpreted as the net effect of using a procedure, procedure type or group of procedures on TC, LOS, and MR, after adjustment for potential confounding factors. Covariates included in all models were: survey year, age, sex, race, income group, insurance status, admission on a weekend versus weekday, number of co-morbid conditions, the Charlson co-morbidity index, and several hospital-level characteristics (See Covariates section).
Results
In selecting our study population, we observed that ∼85% of patients hospitalized with a principle diagnosis of AD (60+ years of age) had no procedure performed. Supplementary Table 1 compares principally diagnosed AD inpatients by healthcare resource (i.e., procedure) utilization status (yes versus no) in terms of patient-level and hospital-level characteristics as well as co-morbidities and outcomes of hospitalization. (Objective E) Selected individuals with principal diagnosis as AD aged ≥60 y with any procedure when compared to those without a procedure performed had consistently higher TC, LOS, and MR. Moreover, they were younger, less likely to be women and/or Whites, more likely to belong to the uppermost income quartile, and to be a weekend admission. Moreover, they had higher total co-morbidities and Charlson co-morbidity index, and were more likely to be located in the Northeast and in an urban teaching hospital setting.
Table 1 compares procedure groups (AD versus non-AD related) by patient-level and hospital-level characteristics (Objective A). Notably, hospitalized patients with an AD-related procedure compared to those with only non-AD related procedures were younger, had reduced likelihoods of being White, having Medicare insurance as the primary payer, a weekend admission, and had a lower number of co-morbidities based on the two indices. Moreover, patients in the AD-related procedure group were more likely to be admitted in the Northeast region of the US, with a significantly higher proportion admitted to an urban teaching hospital compared to those in the non-AD related procedure group.
Table 1. Characteristics of study sample of discharges ≥60 y with principal diagnosis as AD and at least one procedure performed by procedure group; NIS 2002–2012.
| All (2002–2012) | Non-AD only | AD | p-value* | |
|---|---|---|---|---|
| Weighted N | 92,300 | 47,850 | 44,449 | |
| Patient-level characteristics | ||||
| Age, y (Mean±SE) | 81.1 ± 0.1 | 81.7 ± 0.1 | 80.4 ± 0.1 | <0.001 |
| Female, (%±SE) | 57.6 ± 0.5 | 57.0 ± 0.5 | 58.2 ± 0.8 | 0.20 |
| Race, (%±SE)† | 0.006 | |||
| White | 73.9 ± 1.3 | 75.4 ± 1.0 | 72.3 ± 2.2 | |
| Black | 14.4 ± 0.7 | 14.6 ± 0.8 | 14.2 ± 1.0 | |
| Hispanic | 6.4 ± 0.6 | 6.1 ± 0.6 | 6.6 ± 0.8 | |
| Asian | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.2 | |
| Native American | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.3 ± 0.1 | |
| Other | 4.0 ± 1.0 | 2.4 ± 0.3 | 5.6 ± 0.2 | |
| Income quartiles, (%±SE) | ||||
| I | 27.1 ± 1.3 | 27.5 ± 1.0 | 26.6 ± 2.3 | 0.11 |
| II | 23.5 ± 0.7 | 24.3 ± 0.7 | 22.6 ± 1.1 | |
| III | 23.2 ± 0.7 | 24.1 ± 0.7 | 22.2 ± 1.1 | |
| IV | 26.3 ± 0.1 | 24.2 ± 1.1 | 28.6 ± 2.0 | |
| Insurance status, (%±SE) | ||||
| Medicare | 90.9 ± 0.6 | 92.0 ± 0.4 | 89.8 ± 1.2 | 0.042 |
| Medicaid | 2.0 ± 0.3 | 1.4 ± 0.2 | 2.6 ± 0.5 | |
| Private insurance | 5.5 ± 0.2 | 5.3 ± 0.3 | 5.8 ± 0.5 | |
| Self-pay | 0.8 0.2 | 0.6 0.1 | 0.9 0.4 | |
| No charge | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Other | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.2 | |
| Admission day | ||||
| Weekday | 81.6 ± 0.4 | 80.7 ± 0.4 | 82.6 ± 0.7 | 0.018 |
| Weekend | 18.4 ± 0.4 | 19.3 ± 0.4 | 17.4 ± 0.7 | |
| Total co-morbidities (0–27) | 2.45 ± 0.02 | 2.63 ± 0.02 | 2.25 ± 0.04 | <0.001 |
| Charlson co-morbidity index | <0.001 | |||
| 0 | 44.5 ± 0.5 | 41.8 ± 0.6 | 47.5 ± 0.9 | |
| 1 | 27.9 ± 0.4 | 27.1 ± 0.5 | 28.9 ± 0.5 | |
| 2 | 27.5 ± 0.5 | 31.1 ± 0.5 | 24.0 ± 0.9 | |
| Hospital-level characteristics | ||||
| Region of Hospital | ||||
| Northeast | 32.0 1.9 | 27.3 1.7 | 37.0 3.2 | 0.004 |
| Midwest | 20.7 ± 1.7 | 22.5 ± 1.2 | 18.6±3.2 | |
| South | 34.5 ± 1.8 | 39.1 ± 1.6 | 29.6 ±3.0 | |
| West | 12.9 1.3 | 11.1 0.8 | 14.8 2.3 | |
| Bed Size | ||||
| Small | 13.5 ± 1.5 | 12.9 ± 1.0 | 14.1 ± 2.6 | 0.84 |
| Medium | 29.5 ± 1.7 | 29.2 ± 1.7 | 29.8 ± 2.9 | |
| Large | 57.0 ± 2.0 | 57.8 ± 1.7 | 56.1 ± 3.4 | |
| Location/teaching status | ||||
| Rural | 14.1 ± 1.7 | 14.9 ± 1.1 | 13.3 ± 3.1 | 0.039 |
| Urban, non-teaching | 41.7 ± 1.9 | 45.9 ± 1.7 | 37.3 ± 3.2 | |
| Urban, teaching | 44.1 ± 2.0 | 39.2 ± 1.6 | 49.4 ± 3.4 | |
Based on design-based F-test from linear regression models for continuous characteristics with procedure group as the predictor or design-based F-test from a cross-tabulation of two categorical variables (categorical characteristic and procedure group).
Data was available on a Weighted N of 75,839 of the 92,300 for the race variable. All other variables had <5% missing data.
Supplementary Figure 1 illustrates over-time trends in procedure use (at least one procedure versus no procedure), overall and for each group of procedure [non-AD only (yes versus no procedure) and AD (yes versus no procedure)]. While the overall prevalence of procedure use over the years was 15.3%, this prevalence tended to be reduced by 1% annually for the “non-AD only”, while remaining relatively stable for the “AD” group of procedures (Objective F).
Table 2 describes over-time trends in procedure utilization among an inpatient sample of U.S. older adults with principal diagnosis of AD who had utilized at least one hospital procedure [Objective B; n = 19,209 (weighted N = 92,300)]. Notably, CT scan, brain MRI and spinal tap utilization dropped annually by 6%, 5%, and 3%, respectively. Similarly, this annual drop was estimated at 7% for gastrostomy. In terms of major procedures, there was a 5% and an 8% annual increase in the CVD/Blood and “Neuro” types of procedures, respectively, coupled with an annual decrease by 6% and 7% in Brain and Respiratory/gastrointestinal procedures, respectively. No time trends were noted in terms of AD versus non-AD only procedure group or number of procedures (one versus two or more).
Table 2. Trends in procedure use and type by year among AD patients with at least one procedure, NIS 2002–2012.
| Survey Year | Odds Ratio† | 95 % CI | p-trend | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | ||||
| Weighted N | 6,865 | 8,386 | 9,122 | 8,217 | 9,811 | 9,154 | 8,430 | 8,911 | 7,810 | 8,478 | 7,115 | |||
| Top 10 procedures, %* | ||||||||||||||
| CT scan | 16.0 | 19.1 | 18.2 | 16.2 | 17.8 | 15.7 | 16.2 | 10.3 | 13.6 | 13.6 | 9.0 | 0.94 | (0.90; 0.98) | 0.005 |
| Other psychiatric drug | 4.8 | 3.6 | 7.3 | 6.1 | 12.2 | 7.9 | 1.5 | 6.3 | 8.1 | 11.8 | 10.2 | 1.07 | (0.96; 1.19) | 0.22 |
| Spinal tap | 8.3 | 5.7 | 5.7 | 7.0 | 6.1 | 4.7 | 6.7 | 5.5 | 5.8 | 5.1 | 5.1 | 0.97 | (0.94; 1.00) | 0.029 |
| Nail debridement | 5.3 | 4.9 | 5.8 | 4.7 | 6.6 | 6.4 | 6.4 | 7.3 | 5.9 | 2.7 | 6.2 | 1.00 | (0.96; 1.04) | 0.97 |
| Other group therapy | 1.3 | 7.5 | 5.2 | 2.6 | 2.9 | 4.8 | 3.0 | 12.7 | 7.9 | 9.2 | 8.9 | 1.13 | (0.99; 1.29) | 0.08 |
| Gastrostomy | 8.4 | 6.6 | 5.7 | 7.3 | 5.5 | 5.2 | 5.3 | 5.1 | 5.0 | 3.9 | 3.7 | 0.93 | (0.91; 0.96) | <0.001 |
| Brain MRI | 6.0 | 6.2 | 5.1 | 8.1 | 5.7 | 6.3 | 6.4 | 3.0 | 3.8 | 5.9 | 3.4 | 0.95 | (0.92; 0.99) | 0.021 |
| Transfusion of packed cells | 3.3 | 4.3 | 4.3 | 5.0 | 5.1 | 5.4 | 5.2 | 4.9 | 6.7 | 4.7 | 3.9 | 1.02 | (0.99; 1.05) | 0.11 |
| Other physical therapy | 8.5 | 4.5 | 3.2 | 4.4 | 6.7 | 3.4 | 3.9 | 5.0 | 3.8 | 2.5 | 3.0 | 0.93 | (0.84; 1.04) | 0.20 |
| Heart ultrasound | 3.6 | 5.7 | 4.3 | 4.6 | 3.6 | 3.8 | 3.8 | 6.4 | 4.1 | 3.7 | 4.0 | 0.99 | (0.96; 1.03) | 0.75 |
| Procedure type, %* | ||||||||||||||
| CVD/Blood | 21.6 | 22.0 | 22.0 | 25.0 | 24.6 | 30.7 | 27.1 | 24.8 | 32.2 | 31.3 | 29.9 | 1.05 | (1.02; 1.08) | <0.001 |
| Brain | 28.7 | 29.5 | 27.6 | 29.6 | 28.7 | 24.8 | 28.0 | 18.3 | 22.0 | 22.0 | 16.9 | 0.94 | (0.91; 0.97) | <0.001 |
| Neuro | 15.0 | 20.7 | 18.5 | 20.1 | 23.6 | 19.2 | 17.0 | 30.7 | 22.4 | 26.7 | 33.2 | 1.08 | (1.02; 1.14) | 0.010 |
| Sensory/dental | 1.3 | 1.2 | 1.0 | 1.2 | 0.6 | 1.7 | 1.4 | 1.4 | 1.0 | 0.5 | 0.8 | 0.96 | (0.91; 1.00) | 0.07 |
| Skin/muscle/bone | 14.7 | 14.0 | 13.8 | 12.7 | 13.9 | 14.1 | 14.8 | 14.5 | 13.8 | 9.7 | 12.9 | 0.98 | (0.96; 1.10) | 0.22 |
| Respiratory/gastrointestinal | 19.1 | 17.4 | 15.8 | 15.4 | 12.7 | 12.6 | 13.6 | 12.0 | 11.4 | 9.8 | 9.1 | 0.93 | (0.90; 0.95) | <0.001 |
| GU | 4.4 | 3.3 | 4.7 | 4.7 | 3.5 | 5.0 | 6.0 | 5.0 | 6.9 | 4.0 | 4.4 | 1.03 | (0.99; 1.06) | 0.12 |
| Injection/device | 1.2 | 1.2 | 1.2 | 0.5 | 1.0 | 1.0 | 1.1 | 0.5 | 1.3 | 1.3 | 1.1 | 1.00 | (0.95; 1.06) | 0.91 |
| Other | 13.1 | 10.0 | 11.6 | 8.9 | 8.9 | 9.0 | 9.5 | 7.3 | 7.7 | 9.7 | 6.3 | 0.95 | (0.89; 1.01) | 0.09 |
| Procedure group, %*,¥ | ||||||||||||||
| Non-AD only | 51.2 | 51.7 | 52.8 | 51.6 | 46.7 | 54.6 | 54.6 | 50.6 | 57.4 | 50.0 | 49.5 | 1.00 | (0.96; 1.03) | 0.91 |
| AD | 48.8 | 48.3 | 47.2 | 48.4 | 53.3 | 45.4 | 45.4 | 49.4 | 42.6 | 50.0 | 50.5 | |||
| Number of procedures, %*,¥ | ||||||||||||||
| One | 57.2 | 60.5 | 62.4 | 64.4 | 64.9 | 61.8 | 63.8 | 60.6 | 61.7 | 63.5 | 62.2 | 0.99 | (0.96; 1.02) | 0.56 |
| Two or more | 42.8 | 39.6 | 37.6 | 35.6 | 35.1 | 35.1 | 36.2 | 39.4 | 38.3 | 36.4 | 37.9 | |||
AD, Alzheimer's disease; CT, computerized axial tomography of head; GU, genito-urinary procedures; MRI, magnetic resonance imaging.
Percent of all discharges >60 y of age with principal diagnosis as AD and at least one procedure performed. Top 10 procedures (top to bottom) are listed in descending order of overall prevalence in the selected population (2002–2012).
From bivariate logistic regression with outcome being “procedure type or group” (0 = no, 1 =yes) and continuous year as the only predictor.
Categories for “procedure group” and “number of procedures” (unlike “top 10 procedures” and “procedure type”) are mutually exclusive.
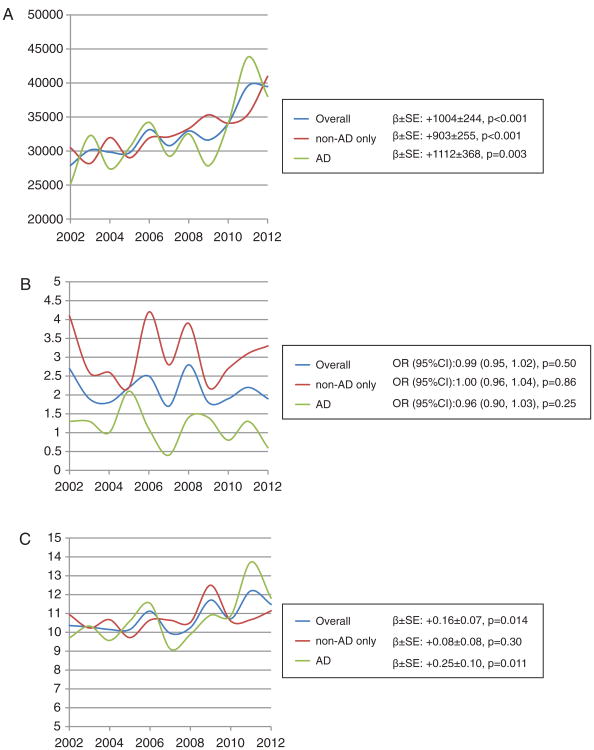
Among hospitalization outcomes (Fig. 1A-C), TC increased dramatically over the years, with the mean annual rate of change ranging between $903 (non-AD related) and $1112 (AD-related). While MR remained stable, hospital stays were becoming increasingly longer among patients with an AD-related procedure.
Fig. 1.
A) Trends in mean total charges (TC, $) across years for study population with principally diagnosed AD and at least one procedure, overall and by procedure group; NIS, 2002 to 2012. B) Trends in mortality risk (MR, %) across years for study population with principally diagnosed AD and at least one procedure, overall and by procedure group; NIS, 2002 to 2012. C) Trends in mean length of stay (LOS, days) across years for study population with principally diagnosed AD and at least one procedure, overall and by procedure group; NIS, 2002 to 2012.
Following Objective D, we compared hospitalization outcomes in our study population by top 10 procedures, procedure type, procedure group, and number of procedure categories, adjusting for patient- and hospital-level covariates. Findings from Table 3 indicate a reduced TC ascribed to the use of CT scans versus not using them (i.e., using other types of procedures), with the same pattern observed for the “Brain” procedure type and the “Other” types of procedure category, as well as the AD versus non-AD only contrast. Conversely, TC was increased markedly with use of gastrostomy tubes (+$17,798), transfusion of packed cells (+$12,706), and spinal tap ($5,272). In terms of procedure types, “CVD/Blood” procedures were linked to a $4,542 average increase in TC, bone/muscle/skin with a $6,036 increase, and respiratory/gastrointestinal procedures (including gastrostomy tubes) with an $18,652 increase. As expected, having 2+ procedures was associated with a significantly higher TC compared to only one procedure by $10,565 on average. The latter contrast (i.e., 2+ versus 1 procedure) was also associated with a longer LOS and a higher MR. Hospital stays were also elongated by the utilization of “Other psychiatric drugs” (+4.71 days), nail debridement (+8.02 days), gastrostomy (+1.22 days), “Neuro” procedure types (+3.47 days), bone/muscle/skin (+4.55 days), Respiratory/gastrointestinal (+1.53 days), and injection/device (+3.18 days). Among top 10 procedures and procedure types/groups, those that were associated with increased MR included gas-trostomy (OR = 1.87, 95% CI: 1.28–2.71, p = 0.001), CVD/blood (OR=3.68, 95% CI: 2.86–4.72, p < 0.001), and respiratory/gastro (OR = 4.14, 95% CI: 3.21–5.35, p < 0.001). In contrast, the use of a CT scan, other psychiatric drugs, other group therapy, brain, and “Neuro” types of procedures, as well as AD versus non-AD only procedures were all associated with a lower MR. In addition, “Brain” types of procedures, including CT scan, spinal tap, and brain MRI were generally associated with a shorter LOS.
Table 3. Hospitalization outcomes as predicted by top 10 procedures, procedure types, procedure groups, and number of procedures among discharges >60 y with principal AD diagnosis and at least one procedure, NIS 2002–2012.
| Total Charges (TC, $) | Length of Stay (LOS, days) | Mortality Risk (MR: 1 = discharged dead, 0 = discharged alive) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Weighted N = 71,364 | Weighted N = 72,476 | Weighted N = 72,445 | |||||||
|
|
|
|
|||||||
| β | (SE) | p- value | β | (SE) | p- value | OR | 95% CI | p- value | |
| Top 10 procedures (Model 1A-1L)* | |||||||||
| CT scan | − 6,483 | (1,700) | <0.001 | −3.99 | (0.61) | <0.001 | 0.56 | (0.38; 0.81) | 0.002 |
| Other psychiatric drug | +5,599 | (3,473) | 0.11 | +4.71 | (1.07) | <0.001 | 0.10 | (0.02; 0.69) | 0.019 |
| Spinal tap | +5,272 | (1,765) | 0.003 | −2.08 | (0.41) | <0.001 | 1.02 | (0.60; 1.75) | 0.93 |
| Nail debridement | +8,767 | (3,207) | 0.006 | +8.02 | (1.15) | <0.001 | 0.58 | (0.30; 1.13) | 0.11 |
| Other group therapy | + 1,021 | (2,398) | 0.67 | +3.73 | (0.90) | <0.001 | 0.22 | (0.09; 0.55) | 0.001 |
| Gastrostomy | +17,798 | (2,367) | <0.001 | +1.22 | (0.53) | 0.023 | 1.87 | (1.28; 2.71) | 0.001 |
| Brain MRI | + 1,380 | (2,024) | 0.50 | −2.42 | (0.53) | <0.001 | 0.54 | (0.27,1.07) | 0.08 |
| Transfusion of packed cells | +12,706 | (2,900) | <0.001 | +0.41 | (0.55) | 0.46 | 1.03 | (0.62; 1.69) | 0.92 |
| Other physical therapy | −343 | (2,676) | 0.90 | +0.16 | (0.78) | 0.84 | 0.65 | (0.31; 1.40) | 0.28 |
| Heart ultrasound | +2,650 | (2,362) | 0.26 | −2.04 | (0.53) | <0.001 | 0.87 | (0.52; 1.45) | 0.59 |
| Procedure type (Model 2A-2I)* | |||||||||
| CVD/Blood | +4,542 | (1,155) | <0.001 | −0.48 | (0.48) | 0.33 | 3.68 | (2.86; 4.72) | <0.001 |
| Brain | −3,603 | (1,300) | 0.006 | −3.93 | (0.46) | <0.001 | 0.62 | (0.46; 0.83) | 0.002 |
| Neuro | + 1,238 | (1,600) | 0.44 | +3.47 | (0.52) | <0.001 | 0.26 | (0.17; 0.41) | <0.001 |
| Sensory/dental | +2,025 | (3,438) | 0.56 | 0.83 | (1.25) | 0.51 | 0.31 | (0.04; 2.25) | 0.25 |
| Skin/muscle/bone | +6,036 | (1,638) | <0.001 | +4.55 | (0.66) | <0.001 | 0.91 | (0.63; 1.32) | 0.63 |
| Respiratory/gastrointestinal | +18,652 | (1,733) | <0.001 | +1.53 | (0.43) | <0.001 | 4.14 | (3.21; 5.35) | <0.001 |
| GU | + 1,581 | (2,078) | 0.45 | −1.00 | (0.59) | 0.09 | 1.08 | (0.64; 1.81) | 0.77 |
| Injection/Device | +13,644 | (7,455) | 0.07 | +3.18 | (1.54) | 0.039 | 1.90 | (0.85; 4.28) | 0.12 |
| Other | −4,147 | (1,167) | <0.001 | −0.85 | (0.51) | 0.10 | 0.99 | (0.64; 1.55) | 0.97 |
| Procedure group (Model 3)*,¥ | |||||||||
| AD versus non-AD only | −2,497 | (1,112) | 0.025 | −0.45 | (0.44) | 0.31 | 0.39 | (0.30; 0.51) | <0.001 |
| Number of procedures (Model 4)*,¥ | |||||||||
| 2+ versus 1 | +10,565 | (966) | <0.001 | +1.77 | (0.32) | <0.001 | 1.84 | (1.44; 2.34) | <0.001 |
AD, Alzheimer's disease; CT, computerized axial tomography of head; GU, genito-urinary procedures; MRI, magnetic resonance imaging.
Outcome is utilized the procedure or procedure type/group: “yes = 1 versus no = 0” for discharges ≥60 y of age with principal diagnosis as AD and at least one procedure performed.
From multiple linear (TC and LOS outcomes) or logistic regression (MR outcome), with predictor being in each model alternatively each of the top 10 procedures (yes = 1 versus no = 0); (Models 1A-1L), each of the procedure major types (Models 2A-2L), procedure group: AD = 1 versus non-AD only = 0 (Model 3) and number of procedures: 2+ = 1 versus 1 = 1 (Model 4). All models adjusted for survey year, patient-level, hospital-level covariates and co-morbidity indices (See covariate section for details).
Categories for “procedure group” and “number of procedures” (unlike “top 10 procedures” and “procedure type”) are mutually exclusive.
Discussion
To our knowledge, this is the first study to systematically examine trends, predictors, and outcomes of hospital resource utilization in patients hospitalized with a principle diagnosis of Alzheimer's dementia using a nationwide inpatient sample of older adults. Our study indicated that ∼85% of patients hospitalized with a principle diagnosis of AD (60+ years of age) had no procedure performed. Among the remaining 15%, Brain procedure utilization (e.g., CT scan, brain MRI, spinal tap) and respiratory/gastrointestinal procedures dropped annually by 3–7%, while CVD/blood and “Neuro” procedures increased by 5–8%. The “AD-related” procedure group was significantly younger than the “non-AD related only” procedure group. With MR remaining stable, hospital stays have become increasingly longer in the “AD-related” procedure group. TC increased annually by $903 (non-AD related) and $1112 (AD-related). TC, LOS, and MR were all markedly higher with use of respiratory/gastro procedures as contrasted with a reduced TC and MR and a shorter LOS associated with “Brain” procedures, including CT scan, Brain MRI, and spinal tap. The number of procedures was positively associated with all three hospitalization outcomes.
Based on our study findings, the type of resources that were utilized by patients hospitalized with a principle diagnosis of Alzheimer's dementia were a reflection of their co-morbidities and the severity of their other illnesses. In fact, the use of “Brain” procedures was primarily observed among younger AD inpatients with lower MR. In contrast, a higher MR, accompanied with higher TC and LOS, was the profile of AD patients who utilized “Respiratory/Gastro” types of procedures which are often used by inpatients with more severe illness. A recent review indicated that cognitive and functional status measures were positively linked to direct costs, including inpatient medical costs, whereas functional and behavioral measures were related to indirect costs and caregiver burden, [28] a finding that was corroborated by other more recent studies [12, 29]. Our study adds to this observation by showing that the use of specific types of resources that are directly related to AD diagnosis, such as “Brain” procedures, are linked to a lower direct inpatient cost and a shorter length of hospitalization in addition to a lower MR, given a less severe stage of the illness and lower number of co-morbidities.
Moreover, an important observation is the increase in the neuropsychological/neurophysiological procedures over time, including the use of psychiatric drugs and various diagnostic tools. In fact, despite the lack of a cure for AD, the psychiatrist has a major role in differential diagnosis, evaluation, and treatment of psychiatric symptoms accompanying AD, prescribing and monitoring medications, assessing competency, and educating families as well as working with caregivers to monitor/prevent burnout and depression [30]. This applies to both the inpatient and outpatient settings. An earlier cost-effectiveness study of using donepezil in mild-moderate AD suggested that the cost was strongly related to mental status scores, and that over 60% of the direct cost was ascribed to long-term institutional care [31]. Cost-saving was also shown to be in the mild stage of AD in another study of high dose rivastigmine [32]. The type of cost-effective studies were discussed at length in the context of AD in at least two reviews [33, 34].
In a large longitudinal study conducted in the UK, AD diagnosis was associated with a significant increase in primary and secondary care resource utilization, continuing beyond diagnosis, applying equally to secondary care referrals, drug prescription (e.g., acetylcholinesterase inhibitors) and hospitalizations [6]. In an earlier case-control study also conducted in the UK, whereby cost of healthcare was compared between AD cases with varying severity levels and healthy controls, it was observed that over a period of 3 months, the average total health-related cost per control (£387 or ∼$639 in January, 1999: https://fred.stlouisfed.org/data/EXUSUK.txt) was insignificant compared to the direct cost incurred by AD patients (£6616 for mild AD (∼$10,907), £10,250 for moderate AD (∼$16,913), and £13,593 for severe AD (∼$22,428). It is worth noting that indirect costs including caregiver time lost, account for ∼68% of total cost, followed by the direct medical costs (24.7%) which include hospitalizations [17]. Similar estimates of cost patterns among dementia patients paired with their caregivers were obtained in an intervention study conducted in Denmark [35] and another multi-country large cross-sectional study (France, Germany, and UK) [36]. In the latter study, while >50% of total societal costs resulted from caregiver informal care, those were increased by co-morbidities and physical limitations, while lower total societal costs were associated with caregiver not working for pay and patient living alone or living with spouse. Moreover, higher total societal costs was unanimously linked with increase in caregiver burden [36].
Similarly, in a 3-year prospective study of Canadian dementia patients living in the community (84% were AD), it was estimated that adult day care and home help were the largest cost factors (mean monthly costs of $65 and $64, respectively) [37]. In another European cohort of AD and vascular dementia patients, 84–85% of total costs were indirect costs ascribed to caregiver burden, while the remaining direct cost was mostly medical, [15] a finding replicated by other studies whereby cost was driven by physical disability [13–16, 19]. In fact, there is a case for promoting home care for as long as possible for AD patients and their family with pharmacological treatment which would reduce not only caregiver burden, but incidence of hospitalizations and overall healthcare costs [38]. Given the greater burden of indirect costs at the societal level, less attention was devoted to the medical costs, specifically to hospitalizations among AD patients. Moreover, in a pooled analysis of 16 European studies, the median value for total annual care costs for AD was estimated at € 28,000 (range € 6,614–€ 64,426) [year 2005 values: 1 € = $1.36 in January of 2005: https://www.ecb.europa.eu/stats/exchange/eurofxref/html/eurofxref-graph-usd.en.html]. The study suggested a lack of patient-level information on resource use which would determine care costs and predict the impact of therapeutic interventions [16].
More recently, a cohort study of older adults (50–85 y) with mild to moderate AD recruited from multiple European countries revealed that total costs increased over time, with direct medical costs increasing at the most rapid rate over time and informal care costs accounting for 40.3% of total costs at follow-up [12]. Our study is the first to delve deeper into the hospitalized AD inpatient population to examine the different types of procedures that were used during the hospital stay. Our findings highlight the heterogeneity of principally diagnosed AD patients in terms of co-morbid conditions and severity of hospitalization which may affect their survival, types of procedures, number of procedures, TC, and LOS. In fact, the use of non-AD procedures only is a marker of higher MR and possibly a prolonged hospital stay with multiple procedures that might yield a costly hospitalization. In contrast, the use of AD procedures, mostly diagnostic, may indicate that the inpatient has a better prognosis than average coupled with lower MR and TC, though with elongated LOS in few procedure types. It also is worth noting that many of the procedures that are key to the diagnosis of AD such as brain procedures (e.g., CT scan, brain MRI, spinal tap) may have increasingly become available and accessible in outpatient settings which explains the drop in their utilization within the inpatient setting as shown in our study.
Our study has several notable strengths including national representativeness, large sample size, and availability of extensive healthcare data that allow for trends analyses. However, this study is not without limitations, which include its reliance on an administrative database using ICD-9-CM codes, which may cause diagnostic misclassification. This is remediated partially by the AHRQ which periodically ensures quality checks with internal and external validation. Moreover, longitudinal analysis is impossible due to the de-identification of discharge abstracts. In fact, multiple discharges from the same condition per patient cannot be ascertained given the NIS structure. Moreover, detailed patient data are lacking including medication regimens and laboratory findings, precluding examining important related research questions. Given prior evidence of an association between AD severity and costs, our study may be limited by the lack of information on the stage of AD and the severity of other co-morbid conditions. Nevertheless, a code of AD was given to overt dementia of probable AD type, as opposed to mild cognitive impairment (ICD9-CM code: 331.83), which was given as a separate diagnosis for mild cases or very early AD. Moreover, while the NIS data provides TC as well as cost-charge-ratio at the hospital level to compute actual cost, those costs are not sub-divided LOS-related versus procedure-related. Nevertheless, both LOS and number of procedures are positively associated with TC. A final limitation is the potential for missing data, though censoring is unlikely to be informative due to the large sample size.
In sum, patterns of hospital resources that were used among AD inpatients changed over-time, and were associated over the years with hospitalization outcomes such as total charges, length of stay and mortality risk. Future longitudinal studies should examine the impact of utilizing specific inpatient and outpatient resources on healthcare cost and other outcomes among AD patients living in the community.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute on Aging, NIA/NIH/IRP in collaboration with the Johns Hopkins School of Medicine.
Footnotes
Authors' disclosures available online (http://j-alz.com/manuscript-disclosures/16-1225r2).
Supplementary Material: The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-161225.
References
- 1.Vincent GK, Velkof VA. The next four decades: The older population in the United States: 2010 to 2050. U.S. Census Bureau; Washington, DC: 2010. [Google Scholar]
- 2.U.S. Federal Interagency Forum on Aging Related Statistics (FIFARS) (2012) Older Americans. Key indicators of wellbeing. 2012 http://www.agingstats.gov/agingstatsdotnet/MainSite/Data/2012Documents/Docs/EntireChartbook.pdf.
- 3.Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer's disease: Lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14:926–944. doi: 10.1016/S1474-4422(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Reed C, Happich M, Nyhuis A, Lenox-Smith A. Health care resource utilisation in primary care prior to and after a diagnosis of Alzheimer's disease: A retrospective, matched case-control study in the United Kingdom. BMC Geriat. 2014;14:76. doi: 10.1186/1471-2318-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalowsky B, Eichler T, Thyrian JR, Hertel J, Wucherer D, Hoffmann W, Flessa S. Healthcare resource utilization and cost in dementia: Are there differences between patients screened positive for dementia with and those without a formal diagnosis of dementia in primary care in Germany? Int Psychogeriatr. 2016;28:359–369. doi: 10.1017/S1041610215001453. [DOI] [PubMed] [Google Scholar]
- 8.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagger C, Andersen K, Breteler MM, Copeland JR, Helmer C, Baldereschi M, Fratiglioni L, Lobo A, Soininen H, Hofman A, Launer LJ. Prognosis with dementia in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S16–S20. [PubMed] [Google Scholar]
- 10.Rudolph JL, Zanin NM, Jones RN, Marcantonio ER, Fong TG, Yang FM, Yap L, Inouye SK. Hospitalization in community-dwelling persons with Alzheimer's disease: Frequency and causes. J Am Geriatr Soc. 2010;58:1542–1548. doi: 10.1111/j.1532-5415.2010.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe EG, Lettieri CJ. Health care rationing in the aged: Ethical and clinical perspectives. Drugs Aging. 1999;15:37–47. doi: 10.2165/00002512-199915010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lacey LA, Niecko T, Leibman C, Liu E, GrundmanM ELN-AIP-901 Investigator Group. Association between illness progression measures and total cost in Alzheimer's disease. J Nutr Health Aging. 2013;17:745–750. doi: 10.1007/s12603-013-0368-1. [DOI] [PubMed] [Google Scholar]
- 13.Gustavsson A, Cattelin F, Jonsson L. Costs of care in a mild-to-moderate Alzheimer clinical trial sample: Key resources and their determinants. Alzheimers Dement. 2011;7:466–473. doi: 10.1016/j.jalz.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Gustavsson A, Brinck P, Bergvall N, Kolasa K, Wimo A, Winblad B, Jonsson L. Predictors of costs of care in Alzheimer's disease: A multinational sample of 1222 patients. Alzheimers Dement. 2011;7:318–327. doi: 10.1016/j.jalz.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Sicras A, Rejas J, Arco S, Flores E, Ortega G, Esparcia A, Suarez A, Gordillo MJ. Prevalence, resource utilization and costs of vascular dementia compared to Alzheimer's dementia in a population setting. Dement Geriatr Cogn Disord. 2005;19:305–315. doi: 10.1159/000084556. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson L, Wimo A. The cost of dementia in Europe: A review of the evidence, and methodological considerations. Pharmacoeconomics. 2009;27:391–403. doi: 10.2165/00019053-200927050-00004. [DOI] [PubMed] [Google Scholar]
- 17.Souetre E, Thwaites RM, Yeardley HL. Economic impact of Alzheimer's disease in the United Kingdom. Cost of care and disease severity for non-institutionalised patients with Alzheimer's disease. Br J Psychiatry. 1999;174:51–55. doi: 10.1192/bjp.174.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Trabucchi M. An economic perspective on Alzheimer's disease. J Geriatr Psychiatry Neurol. 1999;12:29–38. doi: 10.1177/089198879901200107. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie P, O'Shea E, Cullinan J, Buchanan J, Bobula J, Lacey L, Gallagher D, Mhaolain AN, Lawlor B Enhancing Care in Alzheimer's Disease (ECAD) Study Team. Longitudinal costs of caring for people with Alzheimer's disease. Int Psychogeriatr. 2015;27:847–856. doi: 10.1017/S1041610214002063. [DOI] [PubMed] [Google Scholar]
- 20.Winblad B, Wimo A, Almkvist O. Outcome measures in Alzheimer's disease: Do they go far enough? Dement Geriatr Cogn Disord. 2000;11(Suppl 1):3–10. doi: 10.1159/000051226. [DOI] [PubMed] [Google Scholar]
- 21.Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D'Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 23.Beydoun MA, Beydoun HA, Gamaldo AA, Rostant OS, Dore GA, Zonderman AB, Eid SM. Nationwide inpatient prevalence, predictors, and outcomes of Alzheimer's disease among older adults in the United States, 2002-2012. J Alzheimers Dis. 2015;48:361–375. doi: 10.3233/JAD-150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Healthcare Cost and Utilization Project (HCUP), Nationwide Inpatient Sample (NIS) Documentation. [Accessed Feb. 5th]; http://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Bureau of Labor Statistics. CPI inflation calculator. 2017 https://www.bls.gov/data/inflationcalculator.htm.
- 27.STATA. Stata Corporation; Texas: 2015. [Google Scholar]
- 28.Mauskopf J, Racketa J, Sherrill E. Alzheimer's disease: The strength of association of costs with different measures of disease severity. J Nutr Health Aging. 2010;14:655–663. doi: 10.1007/s12603-010-0312-6. [DOI] [PubMed] [Google Scholar]
- 29.Rapp T, Andrieu S, Molinier L, Grand A, Cantet C, Mullins CD, Vellas B. Exploring the relationship between Alzheimer's disease severity and longitudinal costs. Value Health. 2012;15:412–419. doi: 10.1016/j.jval.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Grossberg GT, Lake JT. The role of the psychiatrist in Alzheimer's disease. J Clin Psychiatry. 1998;59(Suppl 9):3–6. [PubMed] [Google Scholar]
- 31.Whitehouse PJ, Winblad B, Shostak D, Bhattacharjya A, Brod M, Brodaty H, Dor A, Feldman H, Forette F, Gau-thier S, Hay J, Henke C, Hill S, Mastey V, Neumann P, O'Brien B, Pugner K, Sano M, Sawada T, Stone R, Wimo A. First international pharmacoeconomic conference on Alzheimer's disease: Report and summary. Alzheimer Dis Assoc Disord. 1998;12:266–280. doi: 10.1097/00002093-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Fenn P, Gray A. Estimating long-term cost savings from treatment of Alzheimer's disease. A modelling approach. Pharmacoeconomics. 1999;16:165–174. doi: 10.2165/00019053-199916020-00005. [DOI] [PubMed] [Google Scholar]
- 33.Lebowitz BD. A public health approach to clinical therapeutics in psychiatry: Directions for new research. Dialogues Clin Neurosci. 2000;2:309–314. doi: 10.31887/DCNS.2000.2.3/blebowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21:587–611. doi: 10.1146/annurev.publhealth.21.1.587. [DOI] [PubMed] [Google Scholar]
- 35.Sogaard R, Sorensen J, Waldorff FB, Eckermann A, Buss DV, Waldemar G. Cost analysis of early psychosocial intervention in Alzheimer's disease. Dement Geriatr Cogn Disord. 2014;37:141–153. doi: 10.1159/000355368. [DOI] [PubMed] [Google Scholar]
- 36.Dodel R, Belger M, Reed C, Wimo A, Jones RW, Happich M, Argimon JM, Bruno G, Vellas B, Haro JM. Determinants of societal costs in Alzheimer's disease: GERAS study baseline results. Alzheimers Dement. 2015;11:933–945. doi: 10.1016/j.jalz.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook R, Herrmann N, Hebert R, McCracken P, Robillard A, Luong D, Yu A. Canadian Outcomes Study in Dementia: Study methods and patient characteristics. Can J Psychiatry. 2004;49:417–427. doi: 10.1177/070674370404900702. [DOI] [PubMed] [Google Scholar]
- 38.Zhu CW, Sano M. Economic considerations in the management of Alzheimer's disease. Clin Interv Aging. 2006;1:143–154. doi: 10.2147/ciia.2006.1.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.