Abstract
The glomerular filtration barrier is known as a “size cut-off” slit to retain nanoparticles or proteins larger than 6~8 nm in the body, and to rapidly excrete the smaller ones through the kidneys. However, in a sub-nm size regime, we found that this barrier behaved as an atomically precise “bandpass” filter to significantly slow down renal clearance of few-atom gold nanoclusters (AuNCs) with the same surface ligands but different sizes (Au18, Au15 and Au10–11). Compared to Au25 (~1.0 nm), just few-atom decreases in the size resulted in 4~9 times reductions in the renal clearance efficiency in the early elimination stage because the smaller AuNCs were more readily trapped by the glomerular glycocalyx than the larger ones. This unique in vivo nano-bio interaction in the sub-nm regime also slows down the extravasation of sub-nm AuNCs from normal blood vessels and enhances their passive targeting to cancerous tissues through enhanced permeability and retention effect. This discovery highlights the size precision in the body’s response to nanoparticles and opens a new pathway to develop nanomedicines for many diseases associated with glycocalyx dysfunction.
Among many factors involved in the retention and elimination of engineered nanoparticles (NPs)1–3, the size is known to play a key role4–6. Upon the particle size, engineered NPs interact with a variety of physiological barriers differently, are retained in the body for different periods of time and follow distinct clearance pathways7–10. For example, engineered NPs can end up in the spleen if their sizes are comparable to inter-endothelial cell slits of the spleen (200~500 nm)9 or accumulate in the liver once their sizes are close to vascular fenestration of the liver (50~100 nm)9. This reticuloendothelial system (RES) (liver, spleen, etc.) uptake is the most common pathway for the body to remove engineered NPs with sizes above 6 nm out of blood stream, but it often takes much longer time for the body to completely eliminate them if they are not biodegradable11. On the other hand, for engineered NPs with sizes below 6 nm, they can be readily eliminated through the kidneys by crossing a unique multiple-layer structure of glomeruli12, 13. For instance, Choi et al. developed a class of zwitterionic quantum dots (Qdots) and found that Qdots with hydrodynamic diameters (HDs) below 6 nm cleared out of the body through the urinary system much more efficiently than the larger ones: the renal clearance efficiency of Qdots increased from 45 %ID to 75 %ID (4 h post injection) with a just slight decrease of HDs from 5.64 nm to 4.64 nm14. A similar trend was also observed in renal clearable silica NPs15: a decrease of HD from 6 nm to 3.3 nm resulted in a significant increase of renal clearance efficiency from 55 %ID to 67.5 %ID (24 h post injection). We also developed renal clearable (AuNPs) with HDs of 3.0 nm16 and 2.2 nm17, which were eliminated out of the body at an efficiency of 43 and 53 %ID (24 h post injection), respectively. These studies are also consistent with a general observation of protein filtration through the glomeruli, where the smaller proteins such as 5.3 nm ScFv can be eliminated out of the body at an efficiency 7 times higher than those larger ones14. Moreover, the smaller 3.0 nm inulin is nearly 30% more efficient than 3.4 nm myoglobin in renal clearance14. With these findings, it has been generally accepted that the glomeruli serve as a one-directional “size cut-off” slit, where engineered NPs or proteins with the sizes smaller than 6 nm can pass through the kidneys and the smaller ones more efficiently clear out of the body than the larger ones.
Since size-dependent glomerular filtration is mainly observed from engineered NPs or proteins with sizes ranging from 2 nm to 6 nm. A fundamental question emerges: is this size-dependency in the glomerular filtration still valid at an even smaller size scale? Investigation of how the glomeruli filtrate out engineered NPs in the sub-nanometer (sub-nm) regime not only helps gain more comprehensive understandings of the glomerular filtration and kidney diseases in general but is also fundamental importance of precise control of nanomedicines that can break into much smaller fragments in vivo. Here, we reported that in a sub-nm size range (~1 nm), the glomerulus is no longer a “size cut-off” slit18 but can become an atomically precise “bandpass” barrier to significantly slow down renal clearance of sub-nm gold nanoclusters (sub-nm AuNCs). In the sub-nm regime, renal clearance of AuNCs exponentially increases with an increase of the number of atoms in the particle. Unlike the size-dependency observed in the glomerular filtration of engineered NPs larger than 2 nm, which was mainly dictated by the pore size of glomerular basement membrane (GBM) and podocyte19, the inverse size-dependency in the glomerular filtration in the sub-nm regime originated from a discovery that the smaller sub-nm AuNPs are more easily and physically retained by the endothelial glycocalyx of the glomerulus
Atomically precise glutathione-coated gold nanoclusters
The observation of size-dependency in the glomerular filtration in the sub-nm size regime was made possible by a recent breakthrough in the precise control of the number of gold atoms in sub-nm AuNCs20–23. Atomically precise AuNCs coated with glutathione (GSH) were synthesized by controlling reduction kinetics of gold ions in the presence of glutathione in aqueous solutions based on reported method 24 (Detailed procedures were described in supplementary information (SI) and specific literatures from ref 24 were cited in SI). Electrospray synthesis of Au25SG18, Au18SG14, Au15SG13 and Au10–11SG10–11 (Supplementary Fig. 1). While these AuNCs are slightly different in the numbers of glutathione ligands on the particles, our recent studies have shown a 50% difference in the number of glutathione ligands on 2.5 nm AuNPs have little effect on their renal clearance efficiencies25. Moreover, a pharmacokinetics study of Au22SG18 and Au22SG16 further shows that a slight difference in the number of GS ligand induces little differences in blood retention and clearance of sub-nm AuNCs (Supplementary Fig. 2 and 3). All these results are consistent with the previous observation of renal clearance of a class of cysteine coated Qdots, where their sizes rather than the number of cysteine ligands govern their renal clearance efficiencies14. We specifically used glutathione to stabilize ultrasmall AuNCs because zwitterionic glutathione can effectively minimize adsorption of the AuNPs to serum protein 17, 26. Similarly, these ultrasmall glutathione coated AuNCs are also highly resistant to the adsorption of serum protein and blood cells (Supplementary Fig. 4 and 5); thus they can retain their original sizes in native physiological environment and allow us to quantitatively correlate renal clearance, pharmacokinetics as well as tumour targeting with their original sizes without interference from serum protein and blood cells during the circulation. In addition, among these ultrasmall clusters, Au25 and Au18 give near-infrared (NIR) emissions (Supplementary Fig. 6), which allows us to in-situ fluorescently image the interactions of Au25 and Au18 with the endothelium of normal blood vessels.
Renal clearance of gold nanoclusters
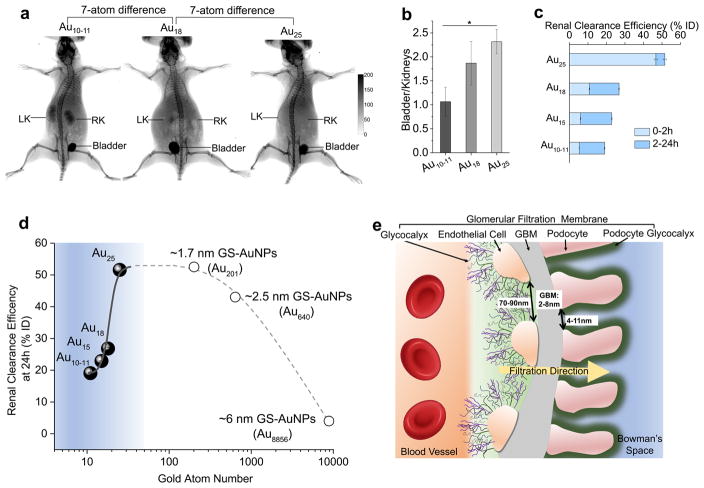
Strong X-ray absorption cross section of gold atom offers a unique opportunity to noninvasively image renal clearance of these ultrasmall AuNCs. We selected three AuNCs, Au10–11, Au18 and Au25, with a 7-atom difference in size, for X-ray imaging studies. As shown in Supplementary Movie 1, Au25 was rapidly eliminated through the kidneys, renal elimination of Au18 was slow and renal clearance of Au10–11 was even slower. At 40 min post injection (p.i.), much more Au10–11 and Au18 were retained in the kidneys than Au25 (Fig. 1a) and the ratio of bladder to kidney derived from X-ray imaging was 2.32±0.25, 1.87±0.46 and 1.06±0.30 for Au25, Au18 and Au10–11 (Fig. 1b), respectively. Since these AuNCs are highly physiologically stable and resistant to serum protein adsorption for both short (30 min.) and long period (24 hrs) (Supplementary Fig. 4 and 7), the observed differences in bladder/kidney ratios clearly indicate that the kidney filtration is highly sensitive to the cluster size in the sub-nm regime. In order to more quantitatively understand renal clearance of these atomically precise AuNCs, BALB/C mice were intravenously injected with 100 μL phosphate buffered saline (PBS) containing Au25, Au18, Au15 and Au10–11, respectively at the same molar concentration of 100 μmol/L, followed by quantification of the Au amount in urine with inductively coupled plasma-mass spectrometry (ICP-MS) at 2, 5, 8, 12 and 24 h post injection (p.i.). Consistent with X-ray imaging studies, renal clearance of sub-nm AuNCs was highly sensitive to the number of Au atoms (Fig. 1c, Supplementary Table 2). A significant differences in renal clearance between Au25 (46.71 %ID, 2 h p.i.) to Au18 (to 10.79 %ID, 2 h p.i.) indicates that a 7-Au atom decrease in size can lead to a 4 times decrease in renal clearance efficiency at 2 h p.i.. Furthermore, the renal clearance efficiency declined to 6.03 and 5.29 %ID at 2 h p.i. once the cluster size was further reduced to Au15 and Au10–11, respectively. At 24 h p.i., the urinary excretion of these AuNCs also decreased as the decrease of the number of Au atoms in the clusters (51.57, 26.82, 22.90 and 19.07 %ID for Au25, Au18, Au15 and Au10–11, respectively). Comparing those results with our previously reported renal clearance efficiencies of ~1.7 nm17, ~2.5 nm16 and ~6 nm GS-AuNPs17(Fig. 1d), we found that Au25 (~1.0 nm) was almost identical to ~1.7 nm GS-AuNPs (Au201)17 in renal clearance efficiency 24 hr p.i. (~52 %ID for Au25 and 53 %ID for 1.7 nm AuNPs), despite there is an 8-time difference in the number of Au atoms. But ~2.5 nm GS-AuNPs16 (Au640, ~43 %ID, 24 hr p.i.) cleared out of the body slowly than the smaller Au25 and Au201. In addition, only 4 %ID of ~6 nm GS-AuNPs17 (Au8856, 24 hr p.i.) were renally cleared. Combining these results clearly shows an inverse size-dependency in renal clearance of AuNCs in the sub-nm region, distinct from the prevailing understanding that the smaller NPs always more rapidly clear through the urinary system than larger NPs 14, 15. This indicates that the glomerulus (Fig. 1e), composed of glycocalyx, endothelial cells, glomerular basement membrane and podocyte,12, 27 is no longer a one-directional “size-cut-off” slit18 but becomes an atomically precise “bandpass” barrier to significantly slow down renal clearance of ultrasmall AuNPs with sizes in sub-nm regime (Fig. 1d).
Figure 1. Renal clearance of different sized gold nanoclusters and schematic diagram of the glomerular filtration membrane.
a, Whole-body X-ray images of the mice after being intravenously injected with Au10–11, Au18 and Au25, respectively at 40 min post injection. While all three different AuNCs were cleared through the kidneys into the bladder, the smallest Au10–11 has much longer kidney retention than Au18, which in turn has longer kidney retention than Au25 even though there is only a 7-atom difference among these three AuNCs. b, The X-ray intensity bladder to kidney ratios of Au10–11, Au18 and Au25 at 40 min post injection, clearly showing that more Au10–11 and Au18 were retained in the kidneys than Au25. * means P<0.05 based on One-way ANOVA. (n=3 for Au10–11, Au25, n=4 for Au18) c, Renal clearance efficiency of Au10–11, Au15, Au18 and Au25 in 0–2 h and 2–24 h after intravenous injection (n=3 for Au10–11, Au15, Au25, n=6 for Au18). d, The renal clearance efficiencies of Au10–11, Au15, Au18 and Au25, 1.7 nm (Au201), 2.5 nm (Au640) and 6 nm (Au8856) GS-AuNPs 24h post injection over the number of gold atoms. Below Au25, the renal clearance efficiency exponentially decreased with the decrease of the number of gold atoms in the NPs. e, Glomerulus12, an important component of renal filtration, is composed of kidney blood vessel, glomerular filtration membrane and Bowman’s space. The glomerular filtration membrane is composed of multiple layers: endothelial glycocalyx, endothelial cell, glomerular basement membrane (GBM) and podocyte. Podocytes are covered by 200 nm glycocalyx. Generally, the fenestration between endothelial cells is 70–90 nm; GBM junction is 2–8 nm; the sizes of podocyte slits are in the range of 4–11 nm (These numbers are measured in rat kidney but very similar to those found in human kidneys, Supplementary Table 1). Combination of these layers, the size threshold for kidney filtration is ~6 nm: NPs or proteins with a hydrodynamic diameter (HD) <6 nm can pass through glomerular filtration membrane readily while it is difficult for the large ones to cross through it.
Glomerular filtration of Au18 and Au25
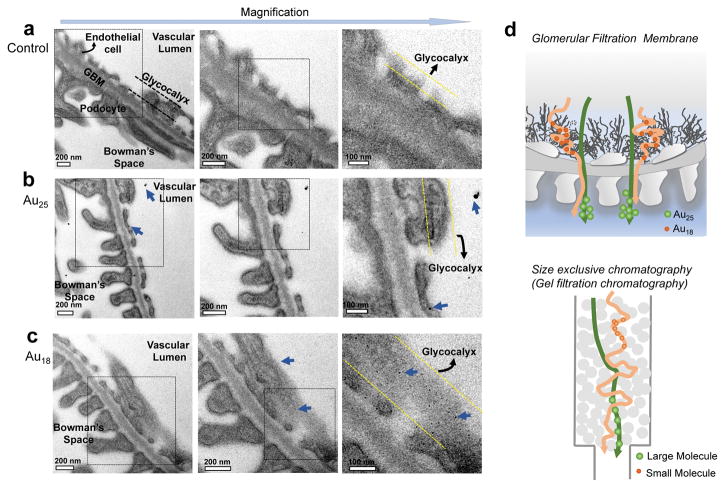
Since renal clearance is fundamentally governed by the glomerular filtration barrier, in order to unravel the origin of the observed size-dependent clearance in sub-nm regime, we investigated interactions of two representative AuNCs, Au18 and Au25, with the glomeruli using electron microscope (EM) because these two AuNCs only have a 7-atom difference in size but exhibit a 4-time difference in renal clearance 2 hr p.i.. Since these AuNCs were too small to be directly revealed with EM, we applied silver staining28 to increase their sizes and contrast. As shown in Supplementary Fig. 8, both Au18 and Au25 can catalyse the deposition of metallic silver on their surface, resulting in formation of larger hybrid silver-gold NPs in the glomerular arterioles and peritubular capillaries (yellow line) while no silver NPs were observed from the silver-stained glomerular tissues of the mouse without injection of AuNCs (Control). To unravel the differences of the nano-bio interactions between Au18 and Au25 in vivo, and avoid the interference from the AuNCs in the blood, we perfused the glomeruli with saline to remove those clusters that have weak interactions with the glomeruli in the blood, followed by tissue fixation with 4% paraformaldehyde/PBS. As shown in Fig. 2, all the fine structures of the glomeruli were observed, including endothelium (endothelial glycocalyx and endothelial cell), glomerular basement membrane, podocytes and glycocalyx layers. While no any silver NPs were observed from the control glomeruli after silver staining (Fig. 2a) and only few large silver-enhanced Au25 were found in the glomeruli of the mice injected with Au25 (Fig. 2b), a large number of monodispersed silver-enhanced Au18 were retained on the glycocalyx of endothelium and podocytes (Fig. 2c and Supplementary Fig. 9).
Figure 2. Electron microscopic images of ultrastructure of glomerular filtration membrane (GFM) from the mice after intravenous injection of PBS, Au25 and Au18, respectively.
a, The GFM of the mouse injected with no particle, only PBS. No any NPs were observed from control glomeruli. b, The GFM of the mice at 10 min post injection of Au25, only few large silver-enhanced Au25 were found in the glomeruli of the mouse. c, The GFM of the mouse at 10 min post injection of Au18, a large number of monodispersed silver-enhanced Au18 were observed, which mainly bound to the glycocalyx of endothelial cells, glomerular basement membrane (GBM) as well as podocytes. d, Comparison between the glomerular filtration of these sub-nm AuNCs and size exclusive/gel filtration chromatography. Au18 was more easily retained by the glycocalyx than Au25, as a result, Au25 was filtrated much faster than Au18 during the same time of period, very similar to the separation in the size exclusive/gel filtration chromatography, where larger molecules/particles will be filtrated faster than smaller ones because the smaller ones are physically retained by the stationary phase for a longer period.
To unravel whether the long retention of Au18 on the glycocalyx is involved in any chemical interactions or not, we incubated Au18 with heparan sulfate proteoglycan, a major component of the glycocalyx layer12, 29 for more than 24 hrs. As control, Au25 clusters were also investigated under the same condition. As shown in Supplementary Fig. 10, very little binding of Au18 and Au25 to heparan sulfate proteoglycan were observed, implying that the retention of smaller Au18 on the glycocalyx was not due to any specific chemical interactions. In addition, very few Au18 and Au25 were found in the glomeruli 7 days p.i. at the tissue-level (Supplementary Fig. 11), further suggesting that both clusters were eventually filtered through the glomerular compartments without involvement of any specific cellular uptake. Therefore, the slow renal clearance of Au18 over Au25 is very likely because Au18 was physically retained on the glycocalyx for a longer period than Au25, very similar to the inverse size-dependency observed in gel filtration or size-exclusion chromatography30: larger molecules/particles will be filtrated faster than smaller ones because the smaller ones are physically retained by stationary materials for a longer period. (Fig. 2d). These results also imply that potential pore sizes on the glycocalyx can be smaller than 1nm; as a result, they can retain sub-nm AuNCs for long periods of time.
Normal vessel extravasation and pharmacokinetics
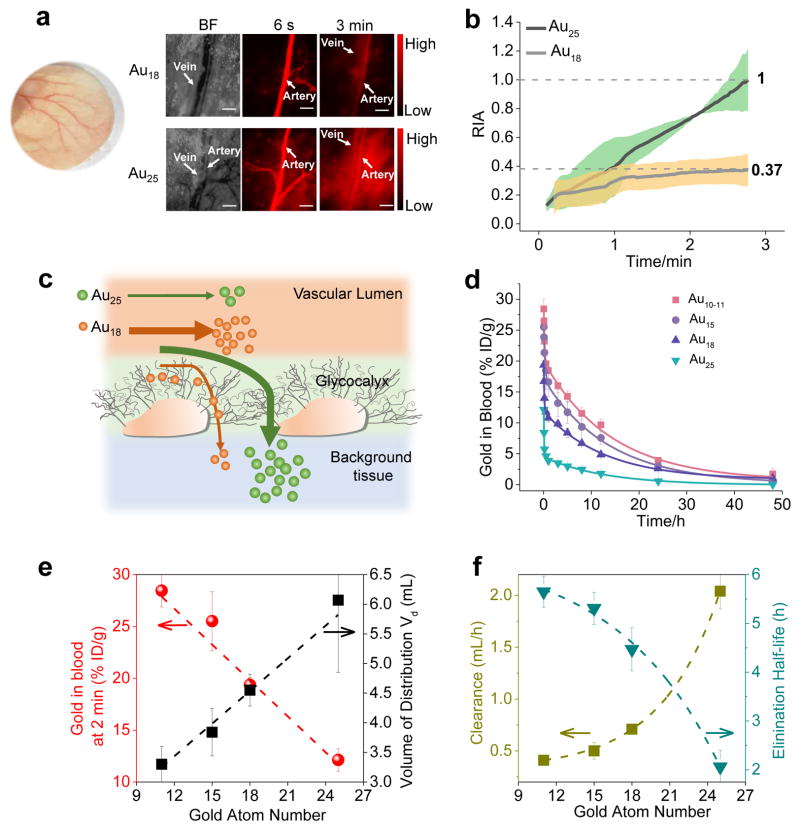
As a major component of the glomerulus, the glycocalyx also lines normal blood vessels31, 32; thus, the long retention of Au18 on the endothelial glycocalyx observed in the glomeruli might also affect their extravasation from the normal blood vessels. By taking advantages of NIR emission from both Au18 and Au25, we noninvasively imaged the extravasation of these two AuNCs from normal blood vessels to the interstitial tissue space in ears. As shown in Fig. 3a and Supplementary Movie 2 and 3, right after the intravenous injection, both of Au18 and Au25 were immediately transported to the artery, followed by a gradual increase in fluorescence intensities of the tissue interstitial space. We used the ratio of (interstitial intensity-background intensity) / (artery intensity-background intensity) to quantify the differences in extravasation between these two different AuNCs (Au18 and Au25). As shown in Fig. 3b, it took 2.7 min for Au25 to reach an equivalent intensity between the tissue interstitial space and artery while the intensity of Au18 in the tissue interstitial space is only 37% of that in artery during the same period. This difference clearly indicates that it is more difficult for Au18 to cross the blood vessel walls than Au25 (Fig. 3c), consistent with the observation of the slow glomerular filtration of Au18 over Au25.
Figure 3. In vivo behaviors of few-atom gold nanoclusters.
a, Noninvasive bright-field and fluorescence images of mouse ear blood vessels after intravenous injection of Au18 and Au25 at 6 s and 3 min, respectively, showing that Au25 crossed the endothelium more rapidly than Au18. Scale bar: 150 μm. BF: bright field. Color picture of mouse ear was inserted. b, The ratio of (interstitial intensity-background intensity)/(artery intensity- background intensity) (RIA) of Au18 and Au25, gray shadow represents standard deviation from different mouse (n=3 per group). This result showed that Au25 is much more permeable than Au18. c, Schematic diagram of Au18 and Au25 crossing blood vessel walls. The junction between endothelial cells is about 5 nm. The smaller Au18 has higher affinity with the glycocalyx of the endothelium of the blood vessels than Au25; as a result, few of Au18 was extravasated into interstitial space of tissues than Au25 during the same time period. d, Blood pharmacokinetics of Au10–11, Au15, Au18 and Au25. Although all the clusters show two-compartment pharmacokinetics, they exhibit distinct distribution and elimination half-lives even though there are only few-atom differences among these clusters (n=3, mean ± s.d.). e, Correlation of the number of gold atoms in the clusters with their retention in the plasma at 2 min. p.i., and their volume distribution (Vd) derived from pharmacokinetics measurements, showing that the blood retention was linearly decreased but the Vd of AuNCs linearly increased with the increase of the number of gold atoms. f, Correlation of elimination half-life and clearance (CL) of few-atom AuNCs with the number of gold atoms, showing that clearance exponentially increases but elimination half-life exponentially decreased with the increase of the number of gold atoms in the clusters.
While other smaller sized AuNCs are not luminescent and their interactions with the blood vessels were hardly imaged with fluorescence microscope like Au18 and Au25, combination of the observed size-dependent renal clearance and the slow extravasation of smaller Au18 imply that other smaller AuNCs might also have longer blood retention and elimination half-lives. By quantifying their blood pharmacokinetics (Fig. 3d, Supplementary Table 3), we were able to obtain more quantitative nano-bio interactions in vivo in this sub-nm regime. As shown in Fig. 3d, the smallest Au10–11 exhibited the longest blood retention, followed by Au15, Au18 and Au25. At 2 min. p.i., the blood concentration of AuNCs linearly decreased from 28.45, to 25.51, 19.37, 12.13 %ID/g while the volume of distribution (Vd33, a parameter that reflects the extent of NPs in extravascular tissues) linearly increased from 3.30 to 3.84, 4.55 and 6.07 mL with the number of atoms increased from 11 to 15, 18 to 25, respectively (Fig. 3e, Supplementary Table 4), consistent with the different extravasation kinetics of Au18 and Au25 from the fluorescence imaging studies. On the other hand, clearance (CL)33, a parameter to measure the efficiency of the body in eliminating the particles through the urinary system, exponentially increased from 0.41, 0.50, 0.71 to 2.04 mL/h and the elimination half-life (t1/2β) of AuNCs exponentially decreased from 5.65, 5.31, 4.47 to 2.09 hr with an increase of the number of atoms from 11, 15, 18 to 25, respectively (Fig. 3f, Supplementary Table 4). Consistency among these studies of the urine excretion, glycocalyx retention, extravasation and pharmacokinetics of these sub-nm AuNCs clearly points out that in vivo behaviours of sub-nm AuNCs are highly size-dependent: the smaller sized AuNCs have longer the blood retention and slower extravasation and glomerular filtration.
Passive tumour targeting of gold nanoclusters
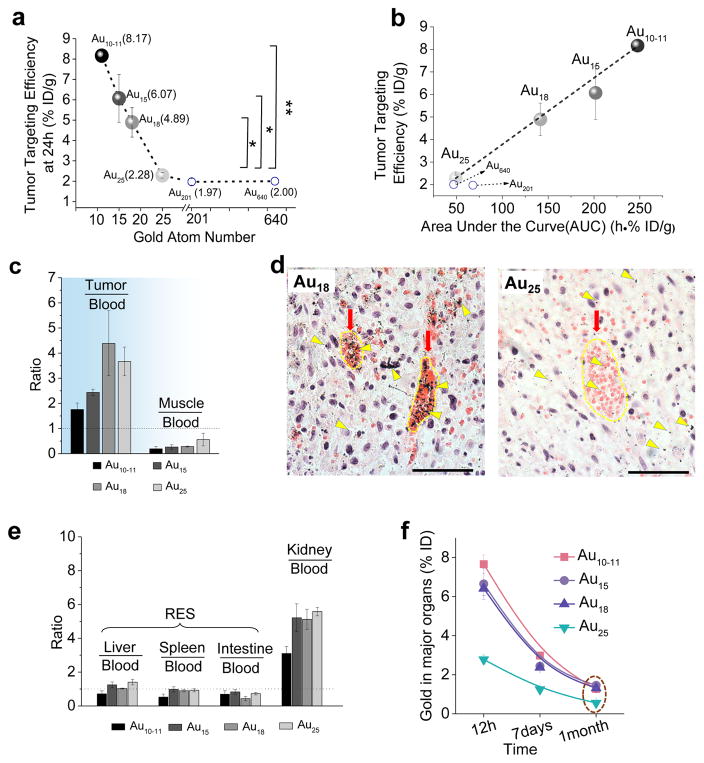
Not limited to glomerular filtration and extravasation from normal tissues, the passive tumour targeting of sub-nm AuNCs is also size-dependent. Using the nude mice bearing MCF-7 breast xenografts as model, we quantified the tumour targeting efficiencies of these AuNCs 24 h p.i., which were 8.17 ± 0.23 %ID/g, 6.07 ± 1.18 %ID/g, 5.27 ± 0.23 %ID/g and 2.28 ± 0.13 %ID/g, for Au10–11, Au15, Au18 and Au25, respectively (Fig. 4a, other organs accumulation in Supplementary Fig. 12). These results clearly indicate that the efficiency of passive tumour targeting in the sub-nm regime linearly decreased with the increase of the number of Au atoms in the clusters. Once the sizes of AuNPs became larger than Au25, this linear relationship vanished; instead, the tumour uptake of ~1.7 nm GS-AuNPs (Au201, ~1.97 %ID/g) and ~2.5 nm GS-AuNPs (Au640, ~2.00 %ID/g) became comparable to that of Au25 (2.28 %ID/g). To unravel the origin of the observed size-dependent tumour targeting of sub-nm AuNCs, we correlated tumour targeting efficiency with blood retention (area under pharmacokinetics curve, AUC). The linear relationship between the targeting efficiency and AUC (Fig. 4b) indicates that the size-dependent tumour targeting in the sub-nm regime is fundamentally due to size-dependent blood retention of sub-nm AuNCs: the smallest AuNCs, Au10–11, has the longest retention in the blood; as a result, they have the highest accumulation in tumours. Since tumour/blood ratios of all the sub-nm AuNCs are all larger than 1 and much higher than muscle/blood ratios at 24 hr p.i. (Fig. 4c), the selective accumulation of sub-nm AuNCs is fundamentally because of the enhanced permeability and retention (EPR) effect34: leaky and dense vasculature leading to long-retention of nano sized particles in tumours. Further investigation of distribution of Au18 and Au25 in tumours 24 hr p.i. (Fig. 4d) confirms that both AuNCs crossed the leaky tumour vasculature and entered tumour microenvironment. More importantly, because Au18 has much longer blood retention than Au25, more Au18 clusters were retained in tumour microenvironment than Au25 clusters, consistent with the observed size-dependent tumour targeting in the sub-nm regime. Combination of these results with our previous observation of passive tumour targeting of 2.5 nm renal clearable AuNPs26, 35 confirms that the EPR effect is indeed retained in renal clearable AuNPs with size down to few atoms, which could further broaden EPR effect, a major strategy used by large non-renal clearable NPs in the tumour targeting5.
Figure 4. Tumour targeting efficiency and long-term accumulation in body.
a, The relationship between tumour accumulation efficiency of Au10–11, Au15, Au18 and Au25 (n=3, mean ± s.d.) and ~1.7 nm AuNPs (Au201) and ~2.5 nm AuNPs (Au640) at 24 h post injection (p.i.). Below Au25, tumour targeting efficiency increased with the decrease of the number of gold atoms. Above Au25, tumour targeting efficiency are comparable. * P<0.05, ** P<0.005, Student’s t-test. b, The linear relationship between tumour targeting efficiencies 24 h p.i. and area under the pharmacokinetics curve (AUC) (n=3, mean ± s.d.), indicating that the tumour targeting of these AuNCs originated from EPR effect. c, Ratios of tumour/blood and muscle/blood of Au10–11, Au15, Au18 and Au25 at 24 h p.i. (n=3, mean ± s.d.). These tumour/blood ratios of all the sub-nm AuNCs are all larger than 1 and much higher than muscle/blood ratios, showing that the selective accumulation of sub-nm AuNCs in tumour is fundamentally due to enhanced permeability and retention (EPR) effect. d, The distribution of Au18 and Au25 in MCF-7 tumour at 24 h p.i. Major tumour vasculatures were indicated with red arrows. Silver-stained Au18 and Au25 were indicated with yellow triangles. Nucleus and cytoplasm were stained by H&E. Scale bar is 50 μm. These images clearly showed that more Au18 were accumulated in tumour microenvironment and vasculatures than Au25. e, Ratios of liver/blood, spleen/blood, intestine/blood and kidney/blood for Au10–11, Au15, Au18 and Au25 at 24 h p.i (n=3, mean ± s.d.). f, The accumulation of Au10–11, Au15, Au18 and Au25 in the major organs in the body includes: brain, stomach, intestine, heart, spleen, lung, liver and kidney at 12 h, 7 days and 1 month p.i. (n=3, mean ± s.d.).
Different from tumour/blood ratios, the ratios of the liver, spleen and intestine to the blood at 24 hrs. p.i. were all close to 1 (Fig. 4e), suggesting that these ultrasmall AuNCs have little interactions with those RES organs. On the other hand, the ratio of the kidney to the blood was much larger than 1 (Fig. 4e) and the kidney accumulation of these AuNCs continually decreased over time (Supplementary Fig. 13), clearly indicating that the kidneys remain the major organ for eliminating these ultrasmall AuNCs. After one month, the major organs accumulation of different-sized AuNCs decreased to 1.5 %ID (Fig. 4f, Supplementary Fig. 14), suggesting that all these AuNCs were gradually eliminated from the body through the urinary system.
In summary, by investigating in vivo behaviours of sub-nm AuNCs with high resistance to the binding of serum protein and blood cells, we discovered that the glomeruli can serve as an atomically precise barrier to slow down the renal clearance of the clusters with size below 1 nm: a few-atom decrease in the cluster size results in a nearly one order reduction in renal clearance. This “surprising” observation fundamentally originates from the fact that the smaller AuNCs are more easily and physically retained by the glycocalyx of the glomeruli, similar to the separation principle used in gel filtration or size-exclusion chromatography. Such size-dependent interaction between sub-nm AuNCs and the glycocalyx also dictates the extravasation of sub-nm AuNCs from normal blood vessels and significantly impacts their accumulation in cancerous tissues through EPR effect. This discovery highlights how precisely the glomerulus and the body could response to ultrasmall AuNPs, which might be generalized to many other renal clearable nanosystems14, 15, 36 and further improve our comprehensive understandings of the glomerular filtration in the sub-nm regime. Considering the similarity of rodents and human in the pore sizes of glomerular filtration membrane (Supplementary Table S1), these findings will also open a new pathway to design clinically translatable precision nanomedicines that can target many endothelial-dysfunction associated diseases such as strokes, atherosclerosis, hypertension, chronic renal failure once the biocompatibility of these renal clearable smaller AuNCs is fully investigated.
Method
Materials and equipment
Hydrogen tetrachloroaurate used for the synthesis of gold nanoclusters was obtained from Fisher Scientific (U.S.). All the other chemicals were obtained from Sigma-Aldrich and used as received unless specified. The gold in urine, blood and each organ was detected by Agilent 7900 inductively coupled plasma mass spectrometry (ICP-MS). The luminescence spectra of Au18SG14, Au25SG18 and Au22SG16, Au22SG18 were collected with a PTI QuantaMaster™ 30 Fluorescence Spectrophotometer (Birmingham, NJ). The blood-vessel images were taken with IX-71 microscope (Olympus). Optical images of kidneys and tumours were taken with IX-71 microscope (Olympus). Electron microscopic (EM) images of the kidney were recorded with a 120 kV Tecnai G2 spirit transmission electron microscope (FEI) equipped with a LaB6 source. The animal studies were performed according to the guidelines of the University of Texas System Institutional Animal Care and Use Committee. The female BALB/c mice (BALB/cAnNCr, strain code is 047) of 6–8 weeks old, weighing 20~25 g, were purchased from Envigo. The female nude mice (Athymic NCr-nu/nu, strain code is 069) of 6–8 weeks old, weighing 20–25 g, were also purchased from Envigo for tumour targeting study. All of these mice were randomly allocated and four-group-housed per ventilated cage under standard environmental conditions (23±1°C, 50±5% humidity and a 12/12 h light/dark cycle) with free access to water and standard laboratory food.
Synthesis of glutathione-coated gold nanoclusters
The gold nanoclusters were synthesized according to previously reported procedures with slight modifications37–42. The Au10–11SG10–11 and Au15SG13 nanoclusters were synthesized using carbon monoxide (CO) as a reducing agent under a controlled pH condition.37 Briefly, aqueous solutions of HAuCl4·3H2O (6.25 mL, 20 mM) and glutathione (GSH, 5 mL, 50 mM) were added to 114 mL water. This reaction mixture was stirred vigorously for 2 minutes, followed by adjusting pH to 7 and 9 with 1 M sodium hydroxide for the synthesis of Au10–11SG10–11 and Au15SG13, respectively. CO gas was bubbled through the mixture at one atmospheric pressure to reduce Au(I)-SG for 3–4 minutes. The reaction flasks were then sealed and the solutions were stirred for 24 h at room temperature.
Synthesis of Au18SG1438: aqueous solutions of HAuCl4·3H2O (2 mL, 10 mM) and glutathione (3 mL, 20 mM) were added to 15 mL water and stirred vigorously for 5 minutes. Then, a solution of borane tert–butylamine in toluene (20 mL, 10 mM) was added to the above mixture and the reaction mixture was stirred overnight (~16 h). After completion of the reaction, the aqueous phase was concentrated under a reduced pressure, followed by precipitation of the crude cluster product with excess methanol. The as-obtained precipitate was washed repeatedly with methanol and finally dried under vacuum to yield Au18SG14 clusters in a powder form.
Synthesis of Au25SG1839, 40: In the first step, HAuCl4·3H2O (0.090 g, 0.228 mmol) was first dissolved in 5 mL water, then added to a toluene solution of tetraoctylammonium bromide (10 mL, 0.145 g, 0.265 mmol). The two-phase solution was vigorously stirred for 15 min for phase transfer of Au(III) salt from aqueous to toluene phase. The aqueous layer was then removed, and triphenylphosphine (PPh3) (0.180 g, 0.686 mmol) was added under vigorous stirring. A freshly prepared ethanol solution of sodium borohydride (NaBH4) (0.026 g, 0.684 mmol, 5 mL) was rapidly added to the whitish suspension to reduce Au(I)-(PPh3)X (X=Cl or Br) to the clusters. After 6 h, toluene was removed via rotary evaporation. The resulting reddish brown product was first washed with water followed by repeated hexane:dichloromethane (6:1) washings to remove excess reactants. The Au:PPh3 clusters were finally extracted with dichloromethane, to which an aqueous solution of GSH (5 mL, 0.400 g, 1.3 mmol) was added. This reaction was stirred at 55 °C for 36 h. Then excess acetone was added to the aqueous phase to precipitate out the crude Au25SG18 cluster product. The as-obtained precipitate was then repeatedly washed with excess methanol and dried under vacuum to yield Au25SG18 clusters in powder form.
Synthesis of Au22SG16 and Au22SG1841, 42: 1.0 mmol of glutathione was added to the methanol solution (50 mL) of HAuCl4 (0.25 mmol). The mixture was cooled to 0°C for 30 min. The aqueous solution of NaBH4 (0.2 M, 12.5 mL) was added into the mixture, after which this mixture was stirred at 1600 rpm for 60 min. The resulting precipitate was washed with methanol repeatedly and purified with NAP-5 column in water to remove the impurities. The as-synthesized Au nanoclusters were separated using polyacrylamide gel electrophoresis.
Stability test of the AuNCs in the physiological environment
To test whether these AuNCs bind to serum protein or not, the Au10–11SG10–11, Au15SG13, Au18SG14 and Au25SG18 were incubated with either phosphate-buffered saline (PBS) or PBS supplemented with 10% (v/v) fetal bovine serum (FBS) at 37 °C for 30 min. In addition, Au10–11SG10–11, Au18SG14 and Au25SG18 were incubated with PBS or PBS supplemented with 10% (v/v) FBS at 37 °C for 24 h to test whether these AuNCs bind to serum protein after long-term (24 h) incubation with FBS. In order to identify the colorless protein band, FBS incubated-Au10–11SG10–11, Au15SG13, Au18SG14, Au25SG18 and pure FBS were stained by 10% (v/v) Coomassie Brilliant Blue 250 (CBB 250). All these samples were analyzed using 2% agarose gel electrophoresis (Mini-sub cell GT Gel electrophoresis system from Bio-Rad Laboratories INc.). The amount of gold in each band was quantified using ICP-MS.
To quantify the long-term physiological stability of these AuNCs, Au10–11SG10–11, Au18SG14 and Au25SG18 were incubated with PBS solution supplemented with 10% (v/v) FBS for 24 h incubation at 37 °C. Their UV-vis absorption spectra were collected at different incubation time points (0, 1, 12 and 24 h).
Blood-cell binding test
The whole blood was collected from female BALB/c mice (6–8 weeks, 20–25 g) and then stored in an anti-coagulation BD Vacutainer. This blood was divided into 4×3 groups for incubation with Au10–11SG10–11, Au15SG13, Au18SG14, Au25SG18 at a 10% v/v ratio at room temperature for 30 min, followed by being centrifuged at room temperature at 500g to separate the plasma and blood cells. Finally, the amounts of gold in plasma and blood cells were quantified using ICP-MS.
X-ray kidney images of the mice after intravenous injection of Au10–11, Au18 and Au25
All X-ray images of kidneys were taken using Bruker In-vivo Xtreme Imaging. Parameters: X-ray: 0.8 mm, KVP:45, Exposure type: standard, Mode: high speed, Exposure time: 15 sec, Bin: 1×1, FOV:12, fStop: 5.6, Focal plane: 0 mm. BALB/c mice were imaged under above parameters after intravenously injected with Au10–11SG10–11, Au18SG14 and Au25SG18 for 40 min. (Au10–11SG10–11: n=3; Au18SG14: n=4; Au25SG18: n=3)
In vivo renal clearance kinetics study
BALB/c mice were intravenously injected with Au10–11SG10–11 (n=3), Au15SG13 (n=3), Au18SG14 (n=6) and Au25SG18 (n=3), respectively (~100 μmol/L, 100 μL) and then placed in metabolism cages. Mouse urine was collected at 2 h, 5 h, 8 h, 12 h, and 24 h post injection. Then, the urine was completely lysed in freshly made aqua regia in plastic centrifuge tube (15 mL) for 2 days, then diluted to 10 mL using ultrapure water. All of above samples were centrifuged at 4500 rpm, 5 min and analyzed by ICP-MS.
Optical kidney images of the mice after injection of Au18SG14 and Au25SG18
Au18SG14 and Au25SG18 of 300 μL (~0.7 mmol/L) as well as 300 μL PBS (control) were iv injected into the mice, respectively. The mice were sacrificed at 10 min and 7 days p.i., and kidneys were then harvested and fixed by 4% formalin, followed by dehydration and paraffin embedding. The fixed kidneys were then sectioned into 4 μm slices. To image these ultrasmall gold clusters under bright-field optical microscopes, the silver staining was used to enhance their sizes and contrast. Before silver staining, these slices were dewaxed using xylene and then dried in lab oven at 65 °C. Each kidney slice was incubated in the silver staining solution containing 40 μL 0.1 M silver nitrate, 40 μL 0.2 g/L hydroquinone for 30 min. These silver-stained slices were then washed with ultrapure water and dried in lab oven at 65 °C again. These final samples were imaged with an IX71 optical microscope in a bright-field mode.
Electron microscopic images of the glomerulus of the mice after intravenous injection of Au18SG14 and Au25SG18
Firstly, the mice were sacrificed and fixed via transcardial perfusion at 10-min time point after i.v. injection of Au25SG18 (~0.7 mmol/L, 300 μL), Au18SG14 (~0.7 mmol/L, 300 μL) and PBS (300 μL, control), respectively. Briefly, 10 mL heparizinzed normal saline as initial perfusant was used to remove the whole-body blood under the perfusion speed of 75 mL/h, then 10 mL 4% paraformaldehyde/PBS fixative as second perfusant was injected to fix the whole animals at the same perfusion speed. After fixation perfusion, the kidneys were collected and cut into pieces of 1 mm3, which then were immersed in 4% paraformaldehyde/PBS in scintillation vials. Fixation was continued by immersion with agitation at 4 °C for 24–72 h. Secondly, these kidneys were then rinsed with 0.1 M sodium cacodylate buffer and post-fixed with 1% osmium tetroxide and 0.8 % potassium ferricyanide in 0.1 M sodium cacodylate buffer for one and a half hours at room temperature, followed by being rinsed with water and en bloc stained with 4% uranyl acetate in 50% ethanol for two hours. The kidney tissues were then dehydrated with increasing concentrations of ethanol, transitioned into resin with propylene oxide, infiltrated with Embed-812 resin and polymerized in a 60 °C oven overnight. Thirdly, the blocks were sectioned with a diamond knife (Diatome) on a Leica Ultracut 6 ultramicrotome (Leica Microsystems) and collected onto grids, followed by the silver-staining with the same method described above. Finally, EM images were taken using a Tecnai G2 spirit transmission electron microscope.
Glycocalyx-binding test of Au18SG14 and Au25SG18
To test whether these AuNCs chemically bind to the glycocalyx, we incubated Au18SG14 and Au25SG18 with 10% (v/v) major component of glycocalyx-heparan sulfate (HS) proteoglycan from Sigma-Aldrich in PBS at 37 °C for 30 min and 24 h, respectively. In order to identify the colorless HS proteoglycan band, HS proteoglycan incubated-Au18SG14, Au25SG18 were stained by 10% (v/v) Coomassie brilliant blue 250 (CBB 250). All these samples were analyzed by 2% agarose gel electrophoresis using the Mini-sub cell GT Gel electrophoresis system (Bio-Rad Laboratories INc.). The amount of gold in each band was quantified using ICP-MS.
In-situ blood-vessel imaging
The BALB/c mice were placed on the imaging station of an IX-71 microscope (Olympus) under isoflurane anesthesia after the removal of ear hair. The bright field images of blood vessels in ear were taken with a 10 x objective and Photon Max 512 CCD camera (Princeton Instruments). After the breath rate of mice was controlled at ~16–20/min, these mice were injected with Au18SG14 (~0.7 mmol/L, 300μL) and Au25SG18 (~0.7 mmol/L, 300μL) through the tail vein respectively (n=3 for Au18SG14, n=3 for Au25SG18). The fluorescence images were taken with 10 x objective under Hg-lamp excitation (Ex: 480/40 nm; Em: 700 Lang Path; Dichroic mirror: 560 nm; Exposure time: 2 s) right after the injection.
Pharmacokinetics study
The BALB/c mice (n=3 for each nanocluster) were intravenously injected with Au10–11SG10–11, Au15SG13, Au18SG14 and Au25SG18, Au22SG16, Au22SG18 (~100 μmol/L, 100 μL), respectively, followed by collection of ~30 μL blood samples from retro-orbital at 2, 5, 10, 30 min, 1, 3, 5, 8, 12, and 24 h and 48 h p.i.. The blood samples were weighted and completely lysed in 1 mL freshly made aqua regia in screw capped glass bottles (10 mL) for 3 days. They were then diluted into 10 mL using ultrapure water and transferred into 15 mL plastic centrifuge tube, centrifuged at 4500 rpm for 5 min to remove insoluble components. The final samples were analyzed with ICP-MS.
Tumour-targeting efficiency study
Tumour implantation: The human breast cancer cell line MCF-7 (purchased from ATCC company) was cultured in Minimum Essential Medium (MEM) with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin at 37 °C in humidified atmosphere containing 5% CO2. The cell suspension (in MEM with 10% (v/v) FBS) was then mixed 2:1 (v/v) with matrix gel and injected subcutaneously upper near the mammary fat pad (MFP) area of the nude mouse with a volume of 100 μL dense suspension (containing about 1×106 cells) for each mouse. The tumour was allowed to grow ~2 weeks and reach a ~6–8 mm size before the study. Au10–11SG10–11, Au15SG13, Au18SG14 and Au25SG18 (~100 μmol/L, 100 μL) were intravenously injected into MCF-7 tumour bearing nude mice (n=3), respectively. These tumours were collected and weighed after 24 h post injection. The amount of gold in the tumour tissues (% injection dose/g, % ID/g) were determined using ICP-MS.
Biodistribution study
To quantify the distributions of Au10–11SG10–11, Au15SG13, Au18SG14 and Au25SG18 in the tissues and organs over time, the mice were sacrificed 12 h p.i. (the whole blood was perfused before the mice sacrificed), 7 days and 1 month after intravenous injection of Au10–11SG10–11, Au15SG13, Au18SG14 and Au25SG18 (~100 μmol/L, 100 μL) respectively (n=3). Then major organs and tissues were collected and measured by ICP-MS to detect gold concentration (% injection dose/g, % ID/g).
Statistical analysis
Error bars are reported as mean ± s.d. The differences between groups were compared by analysis of Student’s t-test and One-way ANOVA with the result shown in Fig. 4, Supplementary Fig. 3, Fig. 5. P-value <0.05 was considered to be statistically significant. ns (no significant), *P<0.05 (significant), **P<0.005 (highly significant). Investigators conducting the experiments were not blinded. Dixon’s Q test is used for identification and rejection of outliers in data analysis.
Data availability
The data that support the plots within this paper and other findings of the study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
This study was in part supported by the NIH (1R01DK103363), CPRIT (RP140544), and a start-up fund from the University of Texas at Dallas (J.Z).
Footnotes
Author contribution
J.Z. conceived the idea and designed experiments with B.D. and R.J.; B.D. performed in vivo experiments and analysed data with J.Z.; X.J., Q.Z. and A.D. synthesized AuNCs; X.J. and M.Y. assisted with in vivo experiment; J.Z. and B.D. wrote the manuscript; J.Z. supervised the whole project. All authors discussed the results and commented on the manuscript.
References
- 1.Ohta S, Glancy D, Chan WC. DNA-controlled dynamic colloidal nanoparticle systems for mediating cellular interaction. Science. 2016;351:841–845. doi: 10.1126/science.aad4925. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer. 2016 doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annual review of biomedical engineering. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 4.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano letters. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 5.Sykes EA, Chen J, Zheng G, Chan WC. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS nano. 2014;8:5696–5706. doi: 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- 6.Huang K, et al. Size-Dependent Localization and Penetration of Ultrasmall Gold Nanoparticles in Cancer Cells, Multicellular Spheroids, and Tumors in Vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, He X, Zhang Z, Zhao Y, Feng W. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Accounts of chemical research. 2012;46:761–769. doi: 10.1021/ar2003336. [DOI] [PubMed] [Google Scholar]
- 8.Yu M, Zheng J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS nano. 2015 doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature biotechnology. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. 2008 doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye L, et al. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nature Nanotechnology. 2012;7:453–458. doi: 10.1038/nnano.2012.74. [DOI] [PubMed] [Google Scholar]
- 12.Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiological reviews. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE. Guyton and Hall textbook of medical physiology. Elsevier Health Sciences; 2015. [Google Scholar]
- 14.Choi HS, et al. Renal clearance of quantum dots. Nature biotechnology. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns AA, et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano letters. 2008;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C, et al. Near - Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angewandte Chemie. 2012;124:10265–10269. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Long M, Qin Y, Sun X, Zheng J. Luminescent gold nanoparticles with efficient renal clearance. Angewandte Chemie. 2011;123:3226–3230. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venturoli D, Rippe B. Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. American Journal of Physiology-Renal Physiology. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- 19.Rennke HG, Cotran RS, Venkatachalam MA. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. The Journal of cell biology. 1975;67:638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azubel M, et al. Electron microscopy of gold nanoparticles at atomic resolution. Science. 2014;345:909–912. doi: 10.1126/science.1251959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Jiang D-e, Kumar S, Chen Y, Jin R. Size Dependence of Atomically Precise Gold Nanoclusters in Chemoselective Hydrogenation and Active Site Structure. ACS Catalysis. 2014;4:2463–2469. [Google Scholar]
- 22.Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science. 2007;318:430–433. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
- 23.Zheng J, Petty JT, Dickson RM. High quantum yield blue emission from water-soluble Au8 nanodots. Journal of the American Chemical Society. 2003;125:7780–7781. doi: 10.1021/ja035473v. [DOI] [PubMed] [Google Scholar]
- 24.Jin R, Zeng C, Zhou M, Chen Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chemical Reviews. 2016;116:10346–10413. doi: 10.1021/acs.chemrev.5b00703. [DOI] [PubMed] [Google Scholar]
- 25.Tang S, et al. Tailoring Renal Clearance and Tumor Targeting of Ultrasmall Metal Nanoparticles with Particle Density. Angewandte Chemie. 2016 doi: 10.1002/anie.201609043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, et al. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: long tumor retention and fast normal tissue clearance. Journal of the American Chemical Society. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A, Satchell SC. Microalbuminuria: causes and implications. Pediatric Nephrology. 2011;26:1957–1965. doi: 10.1007/s00467-011-1777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou LY, Fischer HC, Perrault SD, Chan WC. Visualizing quantum dots in biological samples using silver staining. Analytical chemistry. 2009;81:4560–4565. doi: 10.1021/ac900344a. [DOI] [PubMed] [Google Scholar]
- 29.Vernier RL, Steffes MW, Sisson-Ross S, Mauer SM. Heparan sulfate proteoglycan in the glomerular basement membrane in type 1 diabetes mellitus. Kidney international. 1992;41:1070–1080. doi: 10.1038/ki.1992.163. [DOI] [PubMed] [Google Scholar]
- 30.Rubinson KA, Rubinson JF. Contemporary instrumental analysis. Prentice Hall; Upper Saddle River, NJ: 2000. [Google Scholar]
- 31.Uhl B, et al. The Endothelial Glycocalyx Controls Interactions of Quantum Dots with the Endothelium and Their Translocation Across the Blood-Tissue Border. ACS nano. 2017 doi: 10.1021/acsnano.6b06812. [DOI] [PubMed] [Google Scholar]
- 32.Nieuwdorp M, et al. The endothelial glycocalyx: a potential barrier between health and vascular disease. Current opinion in lipidology. 2005;16:507–511. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 33.Greenblatt DJ. Elimination half-life of drugs: value and limitations. Annual review of medicine. 1985;36:421–427. doi: 10.1146/annurev.me.36.020185.002225. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced drug delivery reviews. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, et al. PEGylation and Zwitterionization: Pros and Cons in the Renal Clearance and Tumor Targeting of Near - IR - Emitting Gold Nanoparticles. Angewandte Chemie. 2013;125:12804–12808. doi: 10.1002/anie.201304465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang H, et al. Renal Clearable Organic Nanocarriers for Bioimaging and Drug Delivery. Advanced Materials. 2016;28:8162–8168. doi: 10.1002/adma.201601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, et al. Scalable and precise synthesis of thiolated Au10–12, Au15, Au18, and Au25 nanoclusters via pH controlled CO reduction. Chemistry of Materials. 2013;25:946–952. [Google Scholar]
- 38.Yao Q, et al. Two - Phase Synthesis of Small Thiolate - Protected Au15 and Au18 Nanoclusters. Small. 2013;9:2696–2701. doi: 10.1002/smll.201203112. [DOI] [PubMed] [Google Scholar]
- 39.Shichibu Y, Negishi Y, Tsukuda T, Teranishi T. Large-scale synthesis of thiolated Au25 clusters via ligand exchange reactions of phosphine-stabilized Au11 clusters. Journal of the American Chemical Society. 2005;127:13464–13465. doi: 10.1021/ja053915s. [DOI] [PubMed] [Google Scholar]
- 40.Das A, et al. Total structure and optical properties of a phosphine/thiolate-protected Au24 nanocluster. Journal of the American Chemical Society. 2012;134:20286–20289. doi: 10.1021/ja3101566. [DOI] [PubMed] [Google Scholar]
- 41.Negishi Y, Nobusada K, Tsukuda T. Glutathione-protected gold clusters revisited: Bridging the gap between gold (I)− thiolate complexes and thiolate-protected gold nanocrystals. Journal of the American Chemical Society. 2005;127:5261–5270. doi: 10.1021/ja042218h. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, et al. Identification of a highly luminescent Au22 (SG) 18 nanocluster. Journal of the American Chemical Society. 2014;136:1246–1249. doi: 10.1021/ja411643u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other findings of the study are available from the corresponding author upon reasonable request.