SUMMARY
In vivo electrophysiological recordings are widely used in neuroscience research and video-electroencephalography (vEEG) has become a mainstay of preclinical neuroscience research, including studies of epilepsy and cognition. Studies utilizing vEEG typically involve comparison of measurements obtained from different experimental groups, or from the same experimental group at different times, in which one set of measurements serves as “control”, and the others as “test” of the variables of interest. Thus, controls provide mainly a reference measurement for the experimental test. Control rodents represent an undiagnosed population, and cannot be assumed to be “normal” in the sense of being “healthy.” Certain physiological EEG patterns seen in humans are also seen in control rodents. However, interpretation of rodent vEEG studies relies on documented differences in frequency, morphology, type, location, behavioral state dependence, reactivity and functional or structural correlates of specific EEG patterns and features between control and test groups.
This paper will focus on the vEEG of standard laboratory rodent strains with the aim of developing a small set of practical guidelines that can assist researchers in the design, reporting, and interpretation of future vEEG studies. To this end, we will: 1) discuss advantages and pitfalls of common vEEG techniques in rodents and propose a set of recommended practices and 2) present EEG patterns and associated behaviors recorded from adult rats of a variety of strains. We will describe the defining features of selected vEEG patterns (brain-generated or artifactual) and note similarities to vEEG patterns seen in adult humans. We will note similarities to normal variants or pathological human EEG patterns and defer their interpretation to a future report focusing on rodent seizure patterns.
Keywords: electroencephalography (EEG), Naïve control, video-EEG (vEEG), electromyography (EMG), rodents
INTRODUCTION
In vivo electrophysiological observations and recordings are widely used for many types of neurobiological investigation. This is especially true for investigations into the underlying causes, potential treatments, and strategies for prevention of epilepsy. This paper proposes recommendations for the electrophysiological investigation and monitoring studies [mainly video-EEG (vEEG)] of rodents used in epilepsy and epileptogenesis preclinical research. In order to maximize the validity and reproducibility of experiments performed in different laboratories, often in different parts of the world, it is important to attempt to standardize the experimental conditions under which the neurophysiological recordings are performed. Given that all these experiments are invasive to at least some degree, it is also important to recognize that the experimental methodology and design itself may introduce potentially variable results.
Control rodents will usually – but not always – be of the same sex, age, strain, and breeding protocol as the test animals, and they may be naïve (i.e., as provided by the vendors), handled, or undergo an incomplete version of the test treatment (sham). Ideally, control animals should be identical to the test subjects in all but the feature or intervention under investigation. The main purpose of controls is to provide reference measurements against which differences of interest to the investigator can be assessed, and it is important to understand that measurements obtained from control subjects cannot be considered to be necessarily “normal”.
This paper will focus both on the vEEG of control rodents and methods for acquiring it. Control vEEG in rodents includes physiological patterns, such as those associated with sleep or alert wakefulness, as well as patterns that are not so reliably observed. Even in humans, the interpretation of certain EEG variants has changed over the course of years. In rodents, it is more challenging to establish a physiological origin or disprove a pathologic correlation for unclear EEG patterns, due to the above limitations (e.g., communication barriers, technological limitations), the absence of reliable vEEG data from truly “wild-type” (i.e., phenotype of the strain in its natural habitat without experimental selection for specific genetic or other features or subjecting it to generations of selective breeding) rodents, and the lack of prospective studies to address these questions. In genetic terms, “wild-type” is an animal that possesses the common (>1%) polymorphism or allele at a specific gene locus, as compared to a rare spontaneous or induced variant allele when studied on a particular inbred strain. Therefore, in this manuscript, we will report patterns that have been described in the literature in experimental controls and present published interpretations. Judgments concerning the pathological nature of certain patterns will be deferred to a subsequent manuscript in which a systematic analysis of the pathological (epileptic and non-epileptic) vEEG patterns and behaviors will be discussed. Here, we will comment only on the prevalence and frequency of such patterns, and how they vary with rodent species, strain, sex, and age. We will also describe artifacts that commonly appear in rodent vEEG recordings.
As in humans, vEEG recordings in animals are obtained from electrodes placed on the surface of the scalp or skull, on or beneath the dura, or in deeper brain structures. EEG in animals is not a standardized technique as it is for the evaluation of humans, and it has been implemented in a variety of ways. Varying numbers and types of electrodes have been used to monitor brain electrical activity at various locations across the neocortex or in deep brain structures. In addition, many experiments involve monitoring of animals implanted with cannulae or microdialysis probes, which generate a focal insertional lesion, typically associated with minimal structural damage of the brain but that potentially may affect the EEG. Data may be acquired using telemetry systems or hard-wired tethered systems. Recorded signals will vary based on number, type, and location of the electrodes, acquisition rate, amplification, and filter settings. Video data can be acquired at varying temporal and spatial resolution, and one or many animals may be monitored with a single camera. Thus, the character and quality of preclinical EEG recordings depend critically upon their implementation. While there is no single implementation of vEEG recording that will be optimal, or even suitable, for all investigations, some practical guidelines and “best practices” can be suggested to obtain high quality recordings and to facilitate cross-laboratory comparison of data.
To our knowledge, the vEEG of naive rodents of standard laboratory strains has never been systematically surveyed, but is described piecemeal in the context of myriad investigations. Because the particular features of the control EEG that are described in any particular study depend on the object of investigation, a very general search strategy was employed to identify manuscripts that included control rodent EEG recordings for inclusion in this white paper. PubMed searches for “(EEG OR electroencephalography OR ECoG OR electrocorticography) AND (rat OR mouse OR guinea pig OR gerbil)” yielded over 15,000 reports. Rats, mice, guinea pigs and gerbils accounted respectively for 83%, 20%, 4% and 1% of the total. Rats were the subject of more than 12,000 reports. Because only a small fraction of these reports included control EEG tracings suitable for review and incorporation in this paper, we began our survey of the vEEG in standard laboratory rodents by relying on the expertise and knowledge of the authors to build a foundation on which to expand with literature searches.
METHODOLOGICAL CONSIDERATIONS
1. Anesthesia, antibiotics and analgesic use for electrode placement surgeries
Electrode placement for EEG recording in rodents requires surgically invasive and non-invasive procedures. Ideally these procedures are done under anesthesia and sometimes using a stereotaxic frame. The procedural goal is always to minimize pain and discomfort for the animal during and immediately following the placement of the electrodes as well as during the period of chronic vEEG recording. A long-acting analgesic may be administered during surgery to control for post-operative pain and discomfort of the animal. However, both anesthetic and analgesic agents are known to have effects on the physiology of the rodents and possibly seizure thresholds, which also may be modulated by genetic and environmental factors (Table 1).1–14
Table 1.
Effects of anesthetics, analgesics, anti-inflammatory drugs and antibiotics commonly used in rodent EEG electrode placement upon EEG.
| Type | Drug | Species / strain / age | Dose / route / timing of administration | Effects on EEG | Reference |
|---|---|---|---|---|---|
| General anesthetic | Urethane (for terminal procedures) | Rat Wistar (263 to 463g) | 1.25–1.35 g/kg (i.p.) | Delta waves | Detari et al., 19971 |
| Isoflurane | Rat, Sprague-Dawley | 1.8% (i.e., equivalent to maintenance anesthesia). Duration of exposure plays a critical role in eliciting adverse effects, including seizures | Burst suppression pattern | Hudetz, 20022 | |
| Isoflurane | Rat, Sprague-Dawley, adult | >1.5 MAC | Burst suppression pattern | Sleigh et al., 20093 | |
| Enflurane | Rat, Sprague-Dawley, adult | <0.7 MAC >0.7 MAC >1.5 MAC |
Slowing, increased amplitude Suppression with PEDS Silence with PEDs |
Sleigh et al., 20093 | |
| Halothane | Rat, Sprague-Dawley | 0.3% 1.8% |
Desynchronization Large amplitude, slow delta waves | Hudetz, 20022 | |
| Ketamine | Rat, Sprague-Dawley (3- to 5-month-old) | 150 mg/kg (i.p.) | Decrease in high gamma | Pal et al., 20154 | |
| At low doses can act as proconvulsant. Proconvulsant effects in humans reported. |
Modica et al., 1990a; Modica et al., 1990b; Myslobodsky et al., 19815,6,7 | ||||
| N2O | Withdrawal seizures in mice | Vaughn and Pruhs, 19958 | |||
| Local anesthetic agents (Nerve block agents) | Lidocaine/Procaine | Male Wistar rats | 80–140 / 130–190 mg/kg (i.p.) | Induced seizures, brain contact with local anesthetics seeping through burr holes can induce focal seizures | Sawaki et al., 20009 |
| Bupivacaine | Wistar rats | 2 mg/kg/min IV infusion | Seizures/isoelectric EEG | Zavisca et al., 199110 | |
| Antibiotic | Penicillin/beta-lactam antibiotics | Review (rodents, rabbits) | Neurotoxic and seizure inducing; high levels (toxic) of benzylpenicillin were required to induce neurotoxicity and seizures | Chow et al., 200511 | |
| Ceftriaxone | WKY rats, male | 200 mg/kg/day i.p., 8 days | Delayed and transient reduction in theta power, altered motor activity | Bellesi et al, 201212 | |
| Analgesics-Opioids | Meperidine | Mice | 15, 30, 60, or 120 mg/kg given orally or subcutaneously | Significant dose-dependent increase of convulsive threshold of maximal electroshock seizure. | Yillar et al., 200913 |
| Anti-inflammatory drugs | Non-steroidal analgesics (NSAIDs) | Rats and mice | Modulate epileptogenesis | Vezzani, 201514 |
MAC: minimum alveolar anesthetic concentration; PED: paroxysmal epileptiform discharges.
Any EEG recordings done under generalized anesthesia obviously are influenced by the mechanism of action of the anesthetic agent.15 Cortical exposure to local anesthetics can cause seizures and analgesic drugs can affect seizure susceptibility in control rodents (refer to Table 1). Requests for exemption from administering analgesics should be justified by the experimental goals or the expected effects on specific outcomes, while providing sufficient monitoring of the well-being of the animal. A behavioral scale to assess pain in mice, i.e., a mouse grimace scale (MGS), is available at16; a recommendation has been made to enhance the utility of the scale by establishing baseline MGS scores for specific strains and sexes of mice.17 Special attention should be paid to accurate reporting of all anesthetic and analgesic dosing and that all peri-operative drug exposures remain similar between experimental and control animals. Detailed reporting of drug injection sites, pre-injection acclimation to handling, if any, drug delivery vehicles, concentrations of the stock solutions and dilutions before injections, protocols for fresh drug preparations, and pH of final injected solutions improve the clarity of experimental methods and facilitate successful replication. Post-operative healing and acclimation periods before EEG acquisition must also be described. These periods vary dramatically throughout the literature, and may affect the EEG results.
Recommendations:
Report the doses, route, timing, and type of anesthetics, analgesics, antibiotics, and anti-inflammatory drugs used peri-operatively and during post-operative care in the methods.
All peri-operative drug treatments should remain similar for experimental and control animals, e.g., duration of anesthesia between experimental and sham-surgeries.
2. Types of EEG Recordings and electrodes
Scalp- and skull-attached electrode placements (Table 2) in adult rodents are usually restricted to the dorsal surface of the skull due to the large lateral temporalis muscles on either side of the skull. These include subdermal and epicranial recordings. Scalp recordings are less invasive than epidural recordings and can be used for short-lasting (hours) EEG recordings, as in EEG for functional magnetic resonance imaging (fMRI) studies, or for recording in very small neonatal rodents, but offer less localizing value, lower signal to noise ratio, more movement artifact, and are impractical for long-term monitoring.
Table 2.
Methodology of EEG recordings: location vs. type of electrode.
| Location of recordings | Scalp/Epicranial (Skull intact) |
Epidural (over the dura, dura intact) |
Subdural (Subdural space) |
|---|---|---|---|
| Type of electrodes used | Wire-gold-plated/silver/silver-chloride/tin/platinum/stainless steel helix Contact – C-shaped loops/flat ends |
Wire/Stainless steel screws/stainless steel helix | |
| Dos for type of electrode | Gold electrodes are better for polysomnography because they accentuate the slower frequencies. Silver electrodes are better for EEG and epilepsy studies. Chlorinated silver electrodes record EEGs better than unchlorinated silver electrodes. Tin conducts more efficiently and is less costly. |
Self-tapping screws with blunted tips | |
| Don’ts for type of electrode | Mix different metal electrodes for any given recording or use wires with different diameters. | Mix different metal electrodes for any given recording or use wires with different diameters. | |
| Pros for location | Lower risk of injury to CNS/infection | Low impedance/lower contamination with movement artifacts | Lower impedance/lower contamination with movement artifacts |
| Cons for location | Higher impedance, movement artifacts, difficulty in associating recording to any specific brain region. | Higher risk of injury to CNS/infection/scar tissue formation in chronic recording that increases impedance | |
Epidural recordings (Table 2) typically are done using either wire electrodes fixed on the skull or, most commonly, screw electrodes placed in burr holes in the skull. Screw electrodes are the most commonly used for long-term epidural recordings from rats and mice. They enable bilateral and anteroposterior coverage of brain activities, good signal to noise ratio, and stable recordings for several months. When the dura is broken during the insertion of a screw electrode, recordings are considered subdural. Because epidural screw electrodes are the most widely used in EEG studies, most of our discussion will focus on these recordings. Flexible multielectrode arrays can also be used for recording epidurally from the brain’s surface.18 Depth electrodes will be the focus of a different manuscript in this Supplement.19
The limitations of skull-attached recordings in rodents include the short inter-electrode distances due to anatomical spatial constraints and the volume conduction (see Supplementary List of terms and definitions).20 Selection of electrode derivations that are farther apart can aid in the identification of the source localization of particular EEG rhythms/seizure initiation zones.20 Although well performed placement of electrodes can result in stable recordings for extended periods of time in several experimental models, stability of long-term recordings may differ considerably between rats and mice. For example, it may be difficult to maintain stable extended recordings in knockout mice that have weight loss and thin skulls compared to controls.21
Recommendation: Report the types and locations of recording and reference electrodes.
3. Electrode placement–Considerations on surgical technique
When well-constructed, recording headsets can provide high-quality recordings over a period of several months. The methods detailed below incorporate measures to minimize damage to the underlying brain during implantation surgery and to very securely adhere the recording headset to the skull to ensure reliable, low‐noise EEG recording over a period of months. All epidural EEG recordings in awake, freely moving animals involve construction of a headset that protects the recording electrodes and any telemetric circuitry, and secures them to the rodent skull. As detailed below, electrode implantation and headset construction involve a risk of brain injury that could affect the EEG (Figure 1A and 1B).22 Holes or other lesions made on the brain post-mortem during harvest can be minimized by perfusion and fixation prior to brain removal. Care is needed to differentiate these artifacts from the screw lesions. In addition, inadequately secured headsets can both generate and amplify EEG artifacts and limit the duration of recordings. Well‐secured headsets are particularly important for long-term recordings to avoid loosening or loss of headsets and early termination of recordings. In tethered recordings, the wires leading to the amplifier may put additional strain on the headset.
Figure 1.
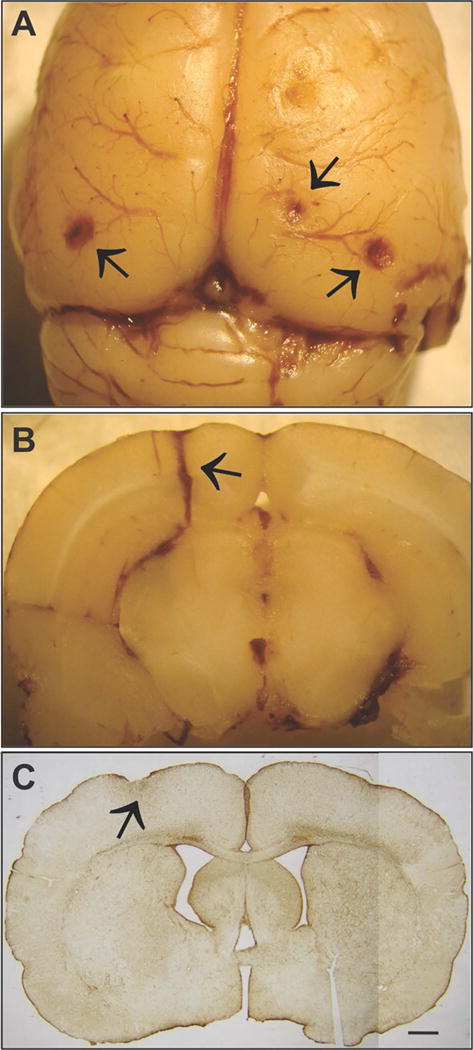
Electrode implantation surgery can result in unintended brain damage. A) When placed without the aid of a stereomicroscope, epidural screw electrodes can be advanced beyond the inner surface of the skull, compressing the underlying brain. Note indentations and hematomas (arrows) on the surface of the neocortex. Epidural electrodes: flat tip 1mm-diameter screws. B) Insertion of cannulae or depth-electrodes may occasionally damage blood vessels and result in bleeding into the brain parenchyma proximal to the electrode track (black arrow); depth electrode 280 μm diameter. A–B) courtesy of R. D’Ambrosio. C) GFAP immunostained coronal section from a rat that was excluded from analysis. Sections from beneath epidural electrodes displayed different degrees of compression and astroglial reactivity (arrow). Scale bar=1mm. From22.
a. Protecting the brain during epidural electrode implantation
Heat, trauma, and chronic compression of underlying brain tissue are the main promoters of unintended brain injury during electrode implantation. Heat can be produced during drilling and during the highly exothermic curing of the dental acrylic from which the headset is constructed.23,24,25 To dissipate the frictional heat generated during drilling, the skull and drill bit can be cooled continuously with sterile saline maintained at room temperature. To dissipate the heat associated with curing, a continuous stream of cool air may be directed onto the hardening acrylic and/or alcohol can be used for evaporative cooling. Non-exothermic dental cement is now readily available and is a recommended choice.25,26,27 The use of a stereoscopic microscope is the key to minimizing mechanical trauma and brain compression. It is much easier to avoid damaging the dura when drilling is monitored under magnification. Similarly, magnification facilitates uniform implantation of electrode anchoring screws at a depth that does not locally compress the underlying cortex. It is important to note here that skull thickness differs significantly between adult rats compared to adult mice. The age of the rodent at the time of electrode placement may play a significant role in providing stable long-term recording electrodes. Skull thickness, which increases with age,28 is important when addressing compression injury as well as epidural screw implantation and long-term viability of electrode headsets. Age may contribute to complications (compression injury) during tethering, particularly with young rodents. In addition, certain mutant mice may have additional skull thinning or fragility associated with the systemic effects of the mutation on bone growth and density in general. For example, this has been observed in Mecp2 mutant mice generated for Rett syndrome research. MECP2 is known to play a significant role in bone physiology, and patients with Rett syndrome frequently sustain low-energy fractures.29 The quality of the electrode implantation surgery can be assessed post‐mortem by neuropathological examination of coronal sections taken from beneath the electrodes. For this purpose, Nissl staining can provide only crude detection of gross damage, particularly in the neocortex. However, immunohistochemical staining for glial-fibrillary-acidic-protein (GFAP) can reveal the astroglial reaction to neuronal injury and necrosis, and provides a sensitive indicator of unintended, potentially epileptogenic neocortical damage (Figure 1C). Astroglial reaction is a very sensitive indicator of neuropathology30 and many inflammatory mediators may directly or indirectly alter neuronal excitability.31, 32,33,34
b. Securing the headset to the skull
Headsets (i.e., implanted screw electrodes and their wires, in some cases soldered/connected to preamplifiers or transmitters) can be adhered to the skull very securely with the use of dental acrylic and anchoring screws. While preparing the skull for electrode implantation it is advisable to remove as much soft tissue from the skull surface as possible because regrowth of this tissue under the headset is a major source of headset separation. A scrub with hydrogen peroxide (3%) greatly reduces this risk and creates a better surface for adhesion using non-exothermic dental acrylics.35 After the electrodes are implanted and connected by insulated wire to gold‐plated pins in a plastic pedestal, a thin ring of surgical glue (e.g., Vetbond, 3M, St Paul, MN) is applied around the circumference of the exposed skull. The surface of the exposed skull is covered with a thin layer of dental acrylic resin powder to the top of the exposed shafts of the anchor screws and screw electrodes. Acrylic resin liquid is applied dropwise, using only enough to be immediately absorbed by the powder, and the curing acrylic is cooled, as described above, by a gentle stream of air. Note that cooling is not necessary when headsets are constructed using non-exothermic cement (see Supplementary List of terms and definitions). When the initial layer of acrylic has hardened, another ring of Vetbond is applied around the circumference of the acrylic to adhere the scalp to the acrylic. This helps close the wound so that nothing gets between the acrylic and the scalp to weaken the adhesion of the headset to the skull. The remainder of the headset is built on this base and the resulting recording headsets are durable and secure, permitting reliable low‐noise tethered EEG recording over the course of prolonged experiments. In most cases, vigorous scratching of the headset will produce little to no EEG artifact, and headsets will deliver reliable recording for months.
c. Connecting the headset to the recorder (tethered electrodes versus telemetry connectors, cables, swivels, cages)
Tethered recordings are currently more economical and more scalable than telemetry. The scalability permits the use of many electrodes to localize epileptic foci and more flexibility in their placement. The selected connectors, cables, headsets, and swivels for tethered recordings should not interfere with the mobility and typical behavior (normal exploratory behavior, locomotion, grooming, feeding, or lying posture) of the animal, are not too heavy or too short for the animal and cage space, and are well protected from damage due to chewing. In general, swivels are not necessary for mice unless they are unusually active or display abnormal circling behavior. Considerations related to the selection and use of cages for monitoring include: 1) regular cage or Plexiglas cylinder; 2) potential use of a wire-mesh floor and the special care needed for the animal when it is used; and 3) required space and positioning of video cameras and their connections and synchronization to the EEG recording system.
Recommendations:
Surgical protocols for electrode implantation that minimize acute or long-term brain injury are preferred.
Laboratories should assess the neuropathological changes induced by EEG electrode implantation technique.
Ideally, experiments should have controls with age- and sex-matched EEG recordings for durations similar to those of the experimental animal groups.
4. Amplifiers and filters
a. Tethered systems
Tethered systems with pre-amplifiers at the head provide signal amplification at the source of the EEG, which minimizes motion artifacts. Signal amplification is generally not possible with standard telemetry; however, pre-amplification may be available with newer telemetry systems. Tethered systems allow for more channels, longer recording life, and higher sampling rates. However, tethered systems result in a degree of physical restraint of the animal due to the headset socket being plugged into the tether, and require care that the weight and size of the tether are not excessive for the animal and do not cause stress.
b. Telemetry systems
Telemetry eliminates cable-generated artifact and, in theory, should allow greater freedom of animal movement. An advantage of telemetry EEG compared with EEGs obtained from tethered animals is that telemetry allows a relatively unimpeded recording of the animal’s behavior.
Digital EEG systems convert the recorded waveforms into a series of numerical values, a process known as analog-to-digital conversion. The rate at which waveform data are sampled is known as the sampling rate and, as a rule, should be at least twice the highest frequency of interest, e.g., for a sampling rate of 250 Hz, frequency reconstruction (i.e., power spectrum analysis) is possible up to 125 Hz (see Nyquist frequency in Supplementary List of terms and definitions and in the report of the TASK1-WG5 group).36
Standard filters used to read routine clinical EEG are a low frequency filter of 0.5–1.0 Hz and a high frequency filter of 70 Hz. However, slower (e.g., infraslow waves <0.2Hz) or higher frequency rhythms may be of interest in specific research studies. EEG filtering by the acquisition system is particularly critical to manage because it affects the raw data. This filtering cannot be removed a posteriori. It is thus preferable to decide which signal frequency range is necessary to properly represent the phenomenon of interest and then set the sampling rate and data acquisition filtering to capture it fully. Further filtering by the acquisition system risks loss of other potentially important aspects of the signal.37 Therefore, any additional filtering to remove artifacts, or for other purposes, is best-applied offline during the analysis of the data. For example, some laboratory rats and mice have the uncanny ability to scratch their EEG headsets with a 6–8 Hz rhythmic pattern, which creates an artifactual signal in the theta range, a frequency band that may resemble other physiological or pathophysiological activities of the rodent brain. Filtering out this type of scratching artifact during acquisition is therefore a problem and is one of the reasons video recording is helpful, if not necessary, during analysis of the monitoring session. For this reason, it is preferable to develop very robust headsets that do not generate motion artifacts when the animal scratches them and to filter the data offline.
Recommendation: Set sampling frequency according to the highest frequency of interest using the Nyquist theorem.
5. Montages
Bipolar and referential montages
EEG waveforms are generated by bioelectric signals of the brain that undergo filtering, amplification, and subsequent display by a variety of either bipolar or referential montages, which are implemented by different combinations of “derivations.” A derivation is the selected inputs 1 and 2 for a particular differential amplifier. Following common mode rejection (see Supplementary List of terms and definitions) and amplification, the resultant output is a single EEG waveform or “channel” representing the difference in voltage between the two electrodes. Bipolar montages are generated by placing a series of sequential derivations consisting of pairs of electrodes without common reference (e.g., adjacent pairs of electrodes in the 10–20 system of electrode placement; see Supplementary List of terms and definitions). Bipolar montages are of greatest advantage when analyzing waveforms that are highly localized. A referential montage consists of a series of derivations in which the same electrode, i.e., the “reference,” is used in input 2 of each amplifier. Ideally, the input 2 reference electrodes is at a distance from the source of activity of interest, in a relatively neutral or “quiet” location that is not contaminated by the activity of the input 1 electrode. There is no standard position for the reference electrode when recording from rodents. Also commonly used in clinical neurophysiology is the average montage, in which the average signal of all electrodes in the montage is subtracted (input 2) from each individual channel. The presence of a common reference in referential montages permits signal localization through amplitude comparisons. In contrast, amplitude comparisons in bipolar montages are not meaningful and signal localization is done by phase reversal.
Additional details for the advantages and disadvantages of referential and bipolar montages are available in the TASK1-WG5 paper.36
Recommendation: In small animals such as rodents, use of referential montages (e.g., Figure 2A) is generally preferable to bipolar montages during data acquisition to permit the localization of the waveform of interest. Bipolar montages may help signal localization if the reference electrode is close to the waveform of interest.
Figure 2.
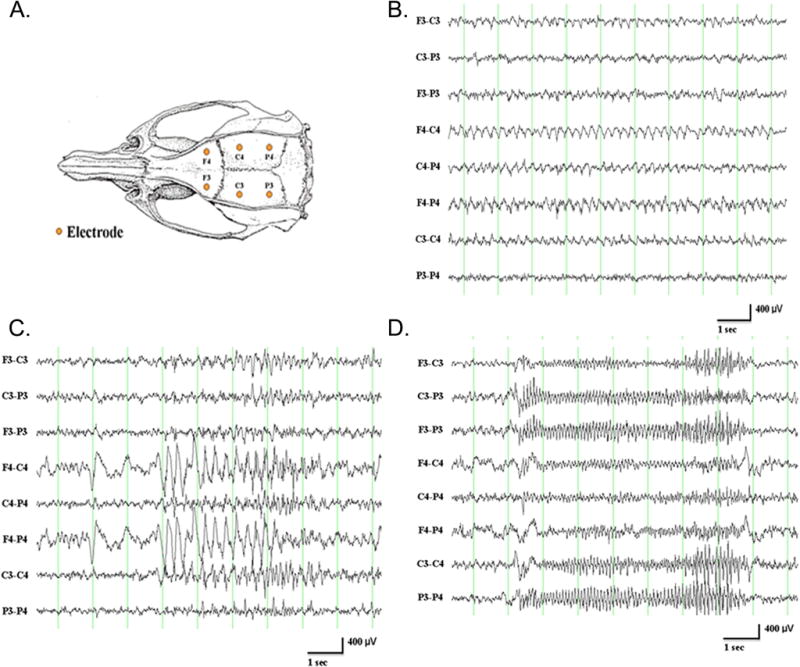
A. Schematic of skull screw electrode placement for EEG recording in a rat. B. Chew artifact in a control 4-month-old male F344 rat with epidural (skull screw) electrodes reflected by the 4 Hz rhythmic waveforms in most of the channels. F=frontal, C=central, P=parietal, 3=left, 4=right. LFF=1 Hz; HFF=70 Hz. 200 Hz sampling rate. C. Grooming artifact (face washing with forepaws) in a control 4-month-old male F344 rat reflected by rhythmic waveforms, without electrical fields, in the middle of the recording. D. Scratching artifact in a control 4-month-old male F344 rat reflected by 10–11 Hz waveforms in all channels. To view higher resolution EEG traces depicted in panel C & D please refer to Suppl. Figure 1. Please refer to Figure 2A for recording details. Courtesy of K. Kelly.
6. Electrical, electrode, muscle, and movement artifacts
Electrical artifacts classically occur at a specific frequency band: 50 Hz in Europe, 60 Hz in the US. Common electrode artifacts are generated by movements of loose wires and loose headsets. These artifacts can originate from a variety of electrical sources, power cables, or weak cable connections. During recording sessions, animals are freely moving and display normal behaviors such as exploration/locomotion, grooming, drinking, and chewing. These behaviors can modify the overall quality of the EEG recording. Chewing or teeth grinding can produce increased activity of specific frequencies such as the theta frequency band (chewing frequency, Figure 2B). These types of activities might lead to a misinterpretation of events occurring on the cortical EEG and be defined as epileptic events. Similarly, electrocardiographic artifact can be recorded at times via cortical electrodes and results in rhythmic nonspecific activities.38 Grooming (Figure 2C) can produce two types of EEG-like activities: 1) high amplitude waveforms when the animals are touching the headset, which can be confirmed by the video analysis, and 2) an increase in delta and low frequency theta power.39 Scratching produces high amplitude rhythmic activity on the EEG recording, which can be recognized easily by the video record (Figure 2D). These issues also highlight the importance of standardization of video methodology and synchronization with the recorded EEG, which is covered by the TASK1-WG5 paper.36 Whenever possible, the recording system can be used with specific filters to remove the “movement-associated noise” of these behaviors. The researcher needs to be careful that off-line, digital filtering typically will not distort the EEG signals of interest.
Recommendation: Accurate synchronization of the video and EEG data in a vEEG study is critical to facilitate identification of artifacts that depend on specific EEG montages and that might resemble pathological patterns in EEG recordings.
7. Control EEG traces in rodents
Adult rodents exhibit several of the EEG physiological patterns and background organization features seen in human EEGs of individuals with no known brain pathology. The EEG background and organization change as a function of sleep-wake and behavioral state. The main EEG characteristics are described below.
a. Behavioral state
EEG characteristics depend on the behavioral state of the animals. At rest, the rodent EEG is composed of several different frequency bands. While the frequency bands are standardized in humans, it is not the case in rodent studies. In humans, the frequency bands are categorized as delta, theta, alpha, beta, gamma.40 In rodents, similar frequency bands are recognized; however, the boundaries are not standardized. Therefore, with the goal to match pre-clinical frequencies with those commonly used in clinical EEGs for modelling human physiological function it would be appropriate to standardize future pre-clinical frequencies to delta as 1–4(3.9) Hz, theta as 4–8(7.9) Hz, alpha as 8–13(12.9) Hz and beta for 13–30(29.9) Hz.41 Higher EEG frequencies are described as gamma (30–80 Hz). High frequency oscillations (HFOs) with frequencies higher than 35 Hz have been reported in EEG recordings from animals or humans with epilepsy (typically from depth recordings, less frequently from scalp recordings) and will therefore not be discussed here. During changes in the behavioral state of the animal, the proportion of specific frequency bands in the recorded EEG can undergo change. For example, during exploratory behavior (Figure 3A) an increase of specific frequency bands (theta and gamma oscillations) was observed.42 In addition, hippocampal theta power was decreased in rats when placed in a novel environment.43 If the behavioral state of the animal changes but the EEG does not, it is worth considering the possibility that there is a recording problem.
Figure 3.
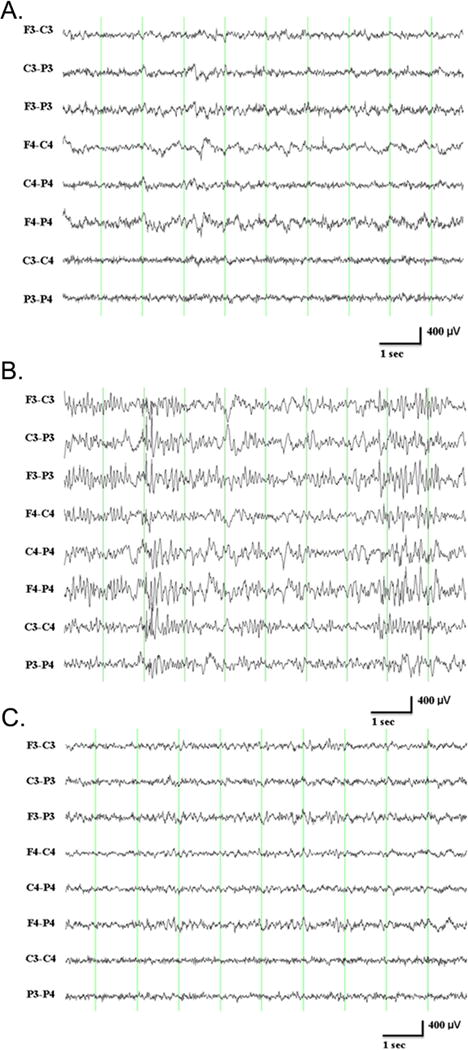
A. Mixed frequency waveforms during exploratory behavior in a control 4-month-old male F344 rat. B. Non-REM sleep (delta/spindles) in a control 4-month-old male F344 rat. Note generalized 12–14 Hz spindles prominent at the beginning and end of the page, and delta waveforms in the middle of the page. Please refer to Figure 2 for recording details. C. REM sleep (theta) in a control 4-month-old male F344 rat. Note rhythmic 7 Hz waveforms most prominent in longitudinal derivations (first 6 channels) throughout most of the page. To view higher resolution EEG traces depicted in panel B & C please refer to Suppl. Figure 2. Please refer to Figure 2A for recording details. Courtesy of K. Kelly.
In the context of the sleep-wake cycle, changes in EEG waveforms occur during transition to and within sleep states. In rodents, sleep is recorded best by doing 24-hour EEG monitoring with electrodes placed near the fronto-parietal cortex and with simultaneous recording of the muscular tone of the nuchal muscles, using electromyography (EMG) recordings.21 Tethered recording results in a slight restriction of movement due to the connection of the cable in a monitoring chamber, whereas telemetric recording enables an EEG recording to be performed without a cable in the animal’s home-cage; telemetric recordings may be preferable for recording EEG activities associated with the sleep-wake cycle. It is desirable that handling and acclimation protocols for all rats and mice introduced into tethered recording chambers (i.e., a novel environment from their home cage) remain similar for any given study. Standard continuous EEG recordings are usually done in 12 h light/dark cycles that should be the same as those of the vivarium where the animals are housed long-term.
Based on the EEG characteristics, rodent behaviors can be classified as wakefulness, slow-wave sleep (SWS) or paradoxical sleep (PS). The two different sleeping states are identified by specific EEG features: non-rapid eye movement (NREM) sleep corresponding to SWS (Figure 3B) and rapid eye movement (REM) sleep corresponding to PS (Figure 3C). NREM sleep is characterized by high amplitude slow waves, predominantly in the delta range. During the transition to the REM sleep state, sleep spindles (7–15Hz) occur and associated EMG powers are low.21 During REM sleep, EEG activities are similar to those seen during wakefulness and are composed of theta activity and faster rhythms accompanied by a loss of muscle tone.44 Wakefulness is the least homogeneous behavioral state and encompasses a combination of many behaviors, including inactivity, eating, drinking, grooming, nesting and exploratory behaviors, all of which can be associated with different EEG patterns as well as artifacts. Synchronous EMGs with continuous EEG recordings (i.e., ≥24 h) complement the analysis of sleep stages during circadian and ultradian (i.e., recurrent cycles within a 24-h period)21 sleep-wake cycles in rodents.
Recommendation: Recording protocols for handling, preparation of the recording chambers, time of day, light/dark cycle, and duration of recordings should be reported and remain standardized across recordings included in an EEG study.
8. Factors influencing control EEG patterns
a. Source, strain, sex, and age
Genetic backgrounds and cross breeding protocols play a significant role in modulating rodent EEGs as related to both the circadian and ultradian sleep cycles.45–49 Significant differences both in sleep cycle durations and spectral power distribution during sleep indicated that genetic factors play a significant role in determining light vs. dark phase activity profiles as well as duration of slow-wave sleep and the associated delta wave densities and amplitude on EEG.
Task-driven gender differences have been reported in humans for EEGs in spectral power analysis especially in inter-hemispheric correlations.50–52 Studies to evaluate the effect of sex hormones on EEG53–55 have revealed different inter- and intra-hemispheric functional organization by sex.
Age-related EEG changes should be considered when recording long-term from rodents. The incidence of brain pathologies such as tumors (Figure 4) increases with age.56 Additionally, patterns that have been interpreted by the authors of these studies as spike-wave discharges (SWDs) have been reported in experimental controls in many strains (see Table 3).57–79
Figure 4.
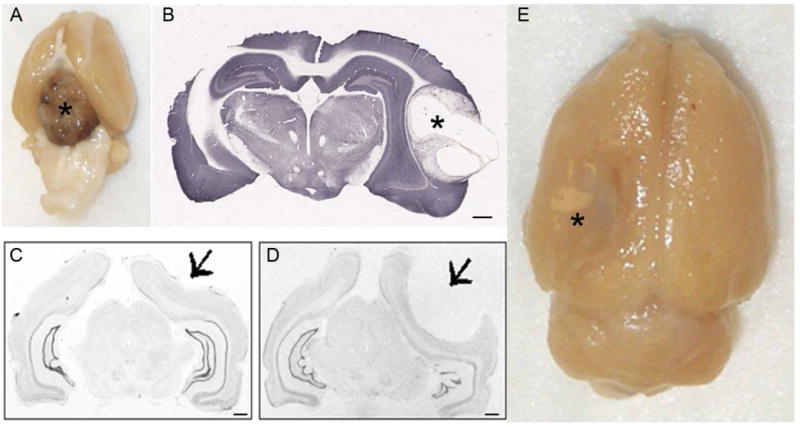
Brain malformations. A. A soft tissue mass was found between the ventral brain surface and skull base in a control 20 month old control F344 rat, consistent with pituitary adenoma. B. Brain section from a lesioned 20-month-old F344 rat showing a large tumor in the right neocortex. Courtesy: K. Kelly. C and D. Brain deformations observed in control Sprague Dawley rats. Cresyl violet staining of coronal sections is shown in black and white. Animals were received from Charles River. C. A deformation is evident in the right parietal cortex (arrow). D. A prominent arachnoid cyst resulted in gross deformation of the right hemisphere (arrow). Courtesy of R. D’Ambrosio. E. Brain of a control 20-month-old F344 rat showing a large area of infection in the left neocortex. Courtesy K. Kelly. Scale bar=1mm. The asterisks (*) indicate the location of the soft tissue mass in A, the large tumor in B and the arachnoid cyst in D.
Table 3.
Paroxysmal EEG patterns in experimental controls with different terminologies and interpretations across studies.
| Ref. | Breeder | Sex | Type of EEG | EEG event | Terminology/Morphology | Associated behavior | Events react to: | Age at EEG recording | Prevalence | Rate of occurrence | Duration of events | Interpretation by the authors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprague-Dawley rats | ||||||||||||
| Kleinlogel 198557 | C-R (Switzerland) | F | Chronic, Screws | 7–9 Hz SWD | High amplitude SW paroxysms | Chewing, vibrissae/eyelid twitching | ESM, DZP, NZP (reduced); PTZ (prolonged) | 220g at surgery; EEG after 6mo | 20% | NR | NR | SW paroxysms resemble “petit mal” |
| Aldinio 198558 | C-R (Italy) | M | 7–10 Hz spikes | SW discharges | Immobility | NR | 6mo | 16% | NR | 2–60 sec | Age dependent increase in asymptomatic SW bursts and impaired passive avoidance | |
| 10–>24 mo | 22–>93% | NR | ||||||||||
| Aporti 198659 | C-R (Italy) | M | Chronic, ESSS | 6.8 – 9.4 Hz spikes ; | Spontaneous EEG bursts of spikes, Bilateral monophasic parietal > frontal, | Immobility, freezing | Acoustic stimulation interrupts bursts | 4mo | 10% | 8/hr | Few sec | Epileptic-like seizures. Events increase with age (prevalence, rate, duration) |
| 24mo | 90% | 81/hr | ||||||||||
| Buzsáki 199060 | B-K | F | 30 min (awake only), ESSS | 6–9 Hz | High Voltage SW Spindle (HVS), frontal bilateral | Immobility; fine tremor of vibrissae / eyes or head/trunk | Acepromazine: increased HVS incidence, duration | 3mo | 0% | 0/hr | HVS: age and strain-dependent pattern | |
| 26–29mo | 71.4% | 54/hr | 10 sec | |||||||||
| Willoughby, Mackenzie 199261 | Australia | M | 7–9 Hz SW | SW discharges | >12mo | 0% | – | – | SW: Nonconvulsive electrographic paroxysms (absence epilepsy) | |||
| Kharlamov 200362 | Taconic (NY) | M | Chronic (screws) | 7–9 Hz SWD | Generalized SW discharges | Immobility | 2.5–8 mo | 100% | NR | 2 sec to > I min | Stereotypic generalized SWDs consistent with absence seizures | |
| D’Ambrosio 200563 | C-R (Hollister) | M | Chronic, ESSS | 6–8 Hz sharp – wave; | Idiopathic SW seizures; occipital maximal, bilateral | NR | 2–5mo | 0% | – | – | Nonconvulsive idiopathic generalized epileptic seizures | |
| 8mo | 33% | 0.24/hr | Few sec | |||||||||
| Pearce 201464 | C-R (Wilmington) | M | Chronic, 1 week long recordings (screws, depth) | 7–8 Hz SW | SW discharges, frontal > occipital | Racine 1–2, behavioral arrest | ESM, noise (reduced), Housing/lighting influences them | 2–3mo | 0% | 0/hr | Some predisposition to SWD. SWD were found in young adult females but only in older than 4.1+−0.9 months male rats. The challenges in differentiating SWD from hippocampal theta rhythm and stage 1–2 limbic seizures, as well as recommendations to circumvent the problem, were raised by the authors | |
| F | 2–3mo | 19% | 7.5/hr | 1–10 sec | ||||||||
| Rodgers 201565* | H-L | M | Chronic (screws) | 8 Hz SW; | SW discharges, frontal > parietal, synchronous > asynchronous | Inactivity, arrest, vibrissae tremor, bruxism, eye boggling | Sensory stimuli suppress | 1–3mo | 67–100% | 4–5/hr | 1–10 sec | SW may reflect absence seizures |
| 6–12 | 90–100% | 10–14/hr | 1–15 sec | |||||||||
| Reid 201666 | C-R (Hollister) | M | Chronic (screws, depth) | Age-dependent SWD; rHFOS and focal seizures (rhythmic spike-wave discharges 9 to 8Hz) reported in 1/7 sham controls | 2–12 months; | 0% | 2±1 sec | Age-dependent SWDs not seen in 7 male sham control rats; rHFOS and focal seizures recorded in one sham control | ||||
| Fischer 344 inbred | ||||||||||||
| Buzsáki 199060 | C-R (MA) | F | 30min recording, ESSS | 6–9 Hz | HVS, frontal > parietal; synchronous > asynchronous | Acepromazine (increased) | 3mo | 87.5% | 16/hr | 2.3 sec | HVS: age and strain-dependent pattern | |
| 12mo | 100% | 52/hr | 8.1 sec | |||||||||
| 26–28mo | 100% | 126/hr | 10.4 sec | |||||||||
| Willoughby, Mackenzie 199261 | Australia | M | 7–9 Hz | SW discharges | NR | 6–16mo | 100% | 24/hr | 10 sec | SW: Nonconvulsive electrographic paroxysms (absence epilepsy) | ||
| Jando 1995*67 | H-L (Indianapolis) | M, F | 12hr (night), ESSS | 7–12 Hz | HVS, bilateral synchronous (99%) | No or very low amplitude tremor/myoclonus | More SW in night hours | 7–8mo | 100%, no sex differences | 1.67/hr | 2.4 sec | HVS may reflect absence seizures |
| Nair 200868 | Taconic (NY) | M | Chronic (screws) | 7–12 Hz SWD | Generalized SW discharges; absence seizures | Immobility | 4 and 20 mo | NA | NR | NR | Age-related differences in the expression of absence seizures may be associated with the ability of the seizures to reset brain dynamics. | |
| Kelly 201169 | Taconic (NY) | M | Chronic (screws) | 7–12 Hz SWD | Generalized SW discharges | Immobility | 4 and 20 mo | NA | NR | NR | Dynamic resetting of both linear and nonlinear EEG characteristics occurred following SWDs with no significant interaction effect between age and infarction. | |
| Buffalo inbred | ||||||||||||
| Buzsaki 199060 | Simonsen Laboratories (CA) | F | 30min recording, ESSS | 6–9 Hz | HVS, frontal, bilaterally synchronous | Acepromazine (no effect); PTZ + acepromazine (induced) | 3mo | 0% | 0/hr | 0 sec | Neocortical HVS is genetically mediated | |
| 12mo | 58.3% | 7.6/hr | 2.4 sec | |||||||||
| Wistar rats | ||||||||||||
| Robinson Gilmore 198070 | C-R (Wistar-Lewis rats) | Chronic, ESSS | 6–10 Hz spikes or SW | Generalized SW discharges, bursts of polyspikes and SW generalized | Arrest, stare + vibrissae / mouth / neck twitching | 300–500g at surgery, up to 6 month follow up | 32% | 1–12 sec | Potential model of “absence with mild clonic components” | |||
| Vergnes 198271 | Strasbourg | M | Chronic, bipolar electrodes | 7–11 Hz | Paroxysmal electroclinical patterns; bursts of spike and SW discharges | Immobility, vibrissae / cervicofacial twitching | SW interrupted by sensory stimuli | 6–9mo | 34% | 78/hr | 2–15 sec | Model of generalized nonconvulsive epilepsy. The GAERS rat model of absence epilepsy was bred from this colony. |
| F | 80% | |||||||||||
| Willoughby, Mackenzie 199261 | Australia | M | 7–9 Hz | SW discharges | NR | 2–5mo | 0% | SW: Nonconvulsive electrographic paroxysms (absence epilepsy) | ||||
| 6–16mo | 100% | 64/hr | 7 sec | |||||||||
| Gralewicz 199572 | NR (Wistar imp: DAK rats) | M | 2 x 1hr recording, SSW bipolar | 7–11 Hz SW | SW discharges | 6mo | 60% | 25/hr | 2 sec | Age-related epileptic-like activity | ||
| Shaw 200973 | NR | M | 1hr x 2 days, epicortical SSS | 7–12 Hz SW | High voltage rhythmic spike discharges(HVRS), frontal-parietal, bilateral | Immobility, facial / whisker twitching | 6–8mo | 0% | – | – | HVRS: absence epileptic discharges | |
| 9–12mo | 0% | |||||||||||
| Long Evans rats | ||||||||||||
| Wiest Nicolelis 200374 | NR | F | Depth (microelectrode array) | 7–12Hz | Cortical (S1) oscillations | Immobility | Response to whisker stimulus unaffected | Adult | 100% | NR | 0.2–1.8 sec | Thalamocortical oscillations 7–12Hz are a functional correlate of the physiological μ rhythm in humans |
| Shaw 200475 | NR | F | Chronic, Epicortical SSS | 5–12 Hz SW | HVRS; frontal-parietal maximal | Immobility (wakefulness); whisker twitching (sleep) | ESM (reduced); HVRS: longer in wakefulness | 4–6mo | 90% | 48/hr | 4 sec | HVRS might not be associated with a voluntary μ rhythm behavior, instead it behaves as an absence-like seizure activity. |
| Kelly 200676 | Harlan (Indianapolis) | M | Chronic (screws) | 6.5–9 Hz SWD 7–8 Hz SWD |
Generalized SW discharges Focal or regionally bilateral SW discharges |
Immobility No change in normal motoric activities |
3.5–8.5 mo 3.5–8.5 mo |
100% 100% |
25.2/hr 6.6/hr |
1–130 sec 1.4 sec |
Brief focal or bilateral regional SWDs were considered interictal; Long generalized SWDs were considered ictal | |
| Shaw 200777 | NR | M | Chronic, Epicortical SSS | 7–12 Hz SW | HVRS; Frontal-parietal maximal | Whisker twitching | HVRS suppressed by ESM, DZP, VPA but not CBZP | 6–9mo | NR | 11 sec | HVRS may be an absence-like seizure activity rather than a μ rhythm. | |
| Shaw 200973 | NR | M | Chronic, Epicortical SSS | 7–12 Hz SW | SW discharges, absence seizures | Immobility, facial / whisker | 6–9mo | 100% | 40/hr | 3.3 sec | SWDs may increase depressive and anxious behaviors | |
| Huang 201278 | NR | M | Chronic, Epicortical SSS | 7–12 Hz SW | SW discharges | Immobility, facial / whisker twitching | LTG (reduced) | 5–8mo | 93% | 40–65/hr | Spontaneous absence epileptic discharges | |
| CD rats | ||||||||||||
| Robinson Gilmore 198070 | C-R | M | Chronic, ESSS | 6–10 Hz spikes or SW | Generalized SW discharges, Bursts of polyspikes and SW generalized | Arrest, stare + vibrissae / mouth / neck twitching | Not triggered by audio / photic stimulation | 300–500g at surgery, up to 6 month follow up | 10% | 1–12 sec | “The occurrence of these discharges is unrelated to surgical procedures used to create chronic recordings”. Similar to discharges/behavior induced by PTZ injection. |
|
| Brown Norway rats | ||||||||||||
| Jando 199567* | H-L (Indianapolis) | M | 12 night hours, ESSS | 7–12 Hz SW | HVS | Tremor / myoclonus | More SWD in night hours | 7–8mo | 47% | 1.3/hr | 8–9 sec | HVS may reflect absence seizures |
| F | 7% | 0.05/hr | ||||||||||
| Willoughby, Mackenzie 199261 | Australia | M | Chronic, ESSS | 7–9 Hz SW | SW discharges | 0–8.5mo | 0% | 2sec | SW: Nonconvulsive electrographic paroxysms (absence epilepsy) | |||
| 8.5–16mo | 100% | 11.3/hr | ||||||||||
The table compares the data from selected studies reporting paroxysmal rhythmic activities (bursts of SW or spikes or polyspikes, or oscillations) in experimental control rats. We list the various terminologies, attributes, and proposed interpretations, as presented by the original authors of such studies to demonstrate the challenges and heterogeneous opinions in interpreting their nature and significance. Similar to rats, studies in some strains of mice like DBA/2, A/J and some other inbred mouse strains have also reported 6–8 Hz SWDs79; however, other mouse strains, including Swiss, NMRI, FVB/N and C57BL/6, have not yet been reported to exhibit SWDs. The systematic review and unbiased study of the available data on these patterns will be the focus of a future study from our Task Force.
BIC: Bicuculline; B-K=Bantin and Kingman; CBZP: carbamazepine; C-R: Charles River; DZP: diazepam; ESSS: Epidural stainless steel screws; ESM: ethosuximide; F: female; GAERS: Generalized Absence Epilepsy Rats of Strasbourg; H-L = Harlan Laboratories; HVRS : High voltage rhythmic spike discharges ; HVS: High Voltage SW Spindle; LTG: lamotrigine; M: male; mo: months; NA: not applicable; NR: not reported; NZP: nitrazepam; PTZ: pentylenetetrazole; rHFOS: repeated high frequency oscillations with spikes, SSS: stainless steel screws; SSW: stainless steel wire; SW: spike waves; SWD: spike wave discharges, VPA: valproic acid.
Recommendation: Report strain, age, sex, and vendor of the animals because all of these may influence the onset and frequency of SWDs (Table 3).
b. Unexpected pathologies in experimental controls
Rats and mice delivered to the laboratory from a vendor are not necessarily healthy and free of pathology. In addition, modern specific pathogen-free facilities do not provide a guarantee against all acute or chronic infectious diseases, and animals may suffer from a range of different congenital or acquired disorders, just as it is for the human population. For example, upon receipt from the vendor, naive rats and mice can have congenital conditions such as tumor (Figure 4A and B), brain malformation or deformation (Figure 4C), arachnoid cyst (Figure 4D). The incidence of brain malformations in laboratory rodent strains is not well studied but such occurrences are relatively rare, based on the experience of the authors. It is evident that once detected, for example, in histology, those animals with malformations should not count in the final number of control animals.
The need for surgical implantation also presents a risk of mechanical or thermal damage to the underlying brain tissue (see section 3a above), infection (e.g., abscess; Figure 4E) and irritative/inflammatory lesions or reactions from the screw electrodes. Cautious aseptic and surgical techniques can minimize these risks, but sensitive assessment of brain pathology is important to determine whether unexpected or pathological EEG patterns may result from unintended brain damage.
While there is no description of the epidemiology of various types of epilepsies in naïve rodents, the possibility that epilepsies may occur spontaneously in naïve control rodents cannot be excluded. Several strains or inbred populations of rodents that reliably manifest certain types of epilepsies were bred specifically from naïve “control” rodents to create epilepsy-prone rodents modeling specific epilepsy syndromes.80–82, For instance, selective breeding of Wistar rats from a colony with a 30% incidence of SWDs, resulted, in just a few generations, in sub-strains in which either all animals or no animals developed such absence-like events.80 Classic Mendelian analyses have shown that this SWD trait in both the WAG/Rij and GAERS rat strains exhibits autosomal dominant transmission.81,82
Table 3 presents an overview of the current literature, summarizing the features, the variable terminologies utilized to describe some of these patterns, and interpretation provided by the authors of the original studies. The main reason for including this Table is to highlight that investigators find a high variability in these events, and therefore is an area that needs further evaluation and clarification to understand the nature, defining features, and significance of these patterns. Interpretations and comparisons of most EEG patterns are complicated further by the non-uniform manner in which vEEG studies in controls are performed and presented. Adoption of common ways of describing such patterns (morphology and localization in different montages, state, reactivity, associated behaviors (consensual glossaries or behavioral dictionaries are desirable), underlying pathology, etc.) would greatly facilitate comparisons. This topic is beyond the focus of this manuscript and will be one of our next goals, as it will require systematic review of the literature on this topic, as well as an unbiased review and comparison of raw vEEG data.
CONCLUSIONS
Whereas protocols for obtaining and reporting EEG data in human epilepsy patients have been standardized to facilitate diagnosis and inter-subject comparison, no such standardization has been established for rodent EEG. This hinders comparison of data across studies and between laboratories. While no particular methodology is likely to be ideal for all research purposes, we have provided a limited number of recommendations to minimize artifacts and facilitate robust and consistent comparison, analysis and interpretation of data from disparate sources. We have focused on the EEG recorded in control rodents that serves as the baseline against which the effects of a manipulation or feature of interest is determined. Although the literature cited here mainly focused on rat EEG data, the concepts discussed here also apply to mice.
In human studies, “normal” volunteers recruited for various clinical studies to provide control measurements often are screened to exclude a limited set of features and diagnoses that have been identified as likely to interfere with the investigation. Human control EEG features “physiological” patterns, sporadic “normal variant” patterns and (rarely) patterns associated with potentially pathological conditions that were not screened out by control patient exclusion criteria. Unlike humans, a premorbid medical history does not exist for laboratory animals, which often are not pre-screened for potentially confounding pathologies. It is important to understand that naïve “control” rodents obtained from laboratory animal vendors represent an undiagnosed population, and a major take-home message of this paper is that EEGs from naïve rodents cannot be assumed to be “normal” or free of pathological patterns.
Supplementary Material
KEY POINTS.
This work is part of a larger effort by the TASK1 group of the AES/ILAE Translational Task Force of the ILAE to harmonize vEEG interpretation in rodents.
We discuss advantages and pitfalls of common vEEG techniques in rodents and propose a set of recommended practices.
Common traces of EEG patterns and associated behaviors recorded from adult rats are discussed.
Acknowledgments
The authors are grateful to Dr. Lauren Harte-Hargrove for assistance at the preparation of this manuscript.
SDK acknowledges grant support by NICHD R21HD073105. RD acknowledges grant support by NINDS R21 NS085459. NGC acknowledges grant support by FAPESP-Brazil grant 2007/50261-4. ASG acknowledges grant support by NINDS RO1 NS091170, U54 NS100064, the US Department of Defense (W81XWH-13-1-0180), the CURE Infantile Spasms Initiative and research funding from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. ASG is co-Editor in Chief of Epilepsia Open. KMK acknowledges grant support by Department of Defense Contract 12097010 and NINDS 2R44 NS064647-05A1.
Footnotes
Conflict disclosures
This report was written by experts selected by the International League Against Epilepsy (ILAE) and the American Epilepsy Society (AES) and was approved for publication by the ILAE and the AES. Opinions expressed by the authors, however, do not necessarily represent the policy or position of the ILAE or the AES. We are also grateful to the AES and ILAE for partial sponsoring the activities of the AES/ILAE Translational Task Force of the ILAE.
Reference to websites, products or systems that are being used for EEG acquisition, storage or analysis was based on the resources known to the co-authors of this manuscript and is done only for informational purposes. The AES/ILAE Translational Research Task Force of the ILAE is a non-profit society that does not preferentially endorse certain of these resources, but it is the readers’ responsibility to determine the appropriateness of these resources for their specific intended experimental purposes.
SDK, NGC, MdC, ASG, KMK report no conflicts of interests. AI acknowledges departmental grant from GlaxoSmithKline K.K., Nihon Kohden Cooperation, Otsuka Pharmaceuticals Co., and UCB Japan Co., Ltd. RD is cofounder and equity holder in Therma Neurosciences. VD and CR are employees of SynapCell (http://www.synapcell.fr/). There are no financial relationships to the works of this manuscript. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Detari L, Rasmusson DD, Semba K. Phasic relationship between the activity of basal forebrain neurons and cortical EEG in urethane-anesthetized rat. Brain Res. 1997;759:112–121. doi: 10.1016/s0006-8993(97)00252-7. [DOI] [PubMed] [Google Scholar]
- 2.Hudetz AG. Effect of volatile anesthetics on interhemispheric EEG cross-approximate entropy in the rat. Brain Res. 2002;954:123–131. doi: 10.1016/s0006-8993(02)03358-9. [DOI] [PubMed] [Google Scholar]
- 3.Sleigh JW, Vizuete JA, Voss L, et al. The electrocortical effects of enflurane: experiment and theory. Anesth Analg. 2009;109:1253–1262. doi: 10.1213/ANE.0b013e3181add06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal D, Hambrecht-Wiedbusch VS, Silverstein BH, et al. Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br J Anaesth. 2015;114:979–989. doi: 10.1093/bja/aev095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modica PA, Tempelhoff R, White PF. Pro- and anticonvulsant effects of anesthetics (Part I) Anesth Analg. 1990;70:303–315. doi: 10.1213/00000539-199003000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Modica PA, Tempelhoff R, White PF. Pro- and anticonvulsant effects of anesthetics (Part II) Anesth Analg. 1990;70:433–444. doi: 10.1213/00000539-199004000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Myslobodsky Golovchinsky V, Ketamine Mintz M. Convulsant or anti-convulsant? Pharmacology Biochemistry and Behavior. 1981;14:27–33. doi: 10.1016/0091-3057(81)90099-x. [DOI] [PubMed] [Google Scholar]
- 8.Vaughn LK, Pruhs RJ. Strain-dependent variability in nitrous oxide withdrawal seizure frequency. Life Sciences. 1995;57:1125–1130. doi: 10.1016/0024-3205(95)02057-p. [DOI] [PubMed] [Google Scholar]
- 9.Sawaki K, Ohno K, Miyamoto K, et al. Effects of Anticonvulsants on Local Anaesthetic-Induced Neurotoxicity in Rats. Pharmacol Toxicology. 2000;86:59–62. doi: 10.1034/j.1600-0773.2000.d01-11.x. [DOI] [PubMed] [Google Scholar]
- 10.Zavisca FG, Kytta J, Heavner JE, et al. A rodent model for studying four well defined toxic endpoints during bupivacaine infusion. Reg Anesth. 1991;16:223–227. [PubMed] [Google Scholar]
- 11.Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis. 2005;24:649–653. doi: 10.1007/s10096-005-0021-y. [DOI] [PubMed] [Google Scholar]
- 12.Bellesi M, Vyazovskiy VV, Tononi G, Cirelli C, Conti F. Reduction of EEG Theta Power and Changes in Motor Activity in Rats Treated with Ceftriaxone. PLoS ONE. 2012;7(3):e34139. doi: 10.1371/journal.pone.0034139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yillar DO, Akkan AG, Akcasu A, et al. The effect of oral and subcutaneous meperidine on the maximal electroshock seizure (MES) in mice. J Basic Clin Physiol Pharmacol. 2009;20:159–168. doi: 10.1515/jbcpp.2009.20.2.159. [DOI] [PubMed] [Google Scholar]
- 14.Vezzani A. Anti-inflammatory drugs in epilepsy: does it impact epileptogenesis? Expert Opin Drug Saf. 2015;14:583–592. doi: 10.1517/14740338.2015.1010508. [DOI] [PubMed] [Google Scholar]
- 15.Pagliardini S, Greer JJ, Funk GD, et al. State-dependent modulation of breathing in urethane-anesthetized rats. J Neurosci. 2012;32:11259–1270. doi: 10.1523/JNEUROSCI.0948-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 17.Miller AL, Leach MC. The Mouse Grimace Scale: A Clinically Useful Tool? PLoS One. 2015;10(9):1–10. doi: 10.1371/journal.pone.0136000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viventi J, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14:1599–1605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernan AE, Schevon CA, Worrell GA, et al. Methodological standards and functional correlates of depth in vivo electrophysiological recordings in control rodents. A report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia Supplement. doi: 10.1111/epi.13905. (in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holsheimer J, Feenstra BW. Volume conduction and EEG measurements within the brain: a quantitative approach to the influence of electrical spread on the linear relationship of activity measured at different locations. Electroencephalogr Clin Neurophysiol. 1977 Jul;43(1):52–8. doi: 10.1016/0013-4694(77)90194-8. [DOI] [PubMed] [Google Scholar]
- 21.Johnston MV, Ammanuel S, O’Driscoll C, Wozniak A, Naidu S, Kadam SD. Twenty-four hour quantitative-EEG and in-vivo glutamate biosensor detects activity and circadian rhythm dependent biomarkers of pathogenesis in Mecp2 null mice. Front Syst Neurosci. 2014;8:118. doi: 10.3389/fnsys.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ambrosio R, Hakimian S, Stewart T, et al. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132(Pt 10):2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curia G, Eastman CL, Miller JW, et al. Modeling posttraumatic epilepsy for therapy development. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 10. [Google Scholar]
- 24.Eastman CL, Fender JS, Temkin NR, D’Ambrosio R. Optimized methods for epilepsy therapy development using an etiologically realistic model of focal epilepsy in the rat. Exp Neurol. 2015;264:150–162. doi: 10.1016/j.expneurol.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertram EH, Williamson JM, Cornett JF, Spradlin S, Chen ZF. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res Brain Res Protoc. 1997;2(1):85–97. doi: 10.1016/s1385-299x(97)00033-0. [DOI] [PubMed] [Google Scholar]
- 26.Dunne NJ, Orr JF. Curing characteristics of acrylic bone cement. J Mater Sci Mater Med. 2002;13(1):17–22. doi: 10.1023/a:1013670132001. [DOI] [PubMed] [Google Scholar]
- 27.Panagiotouni E, Karanika-Kouma A. Comparative study of heat release of various cement base materials during their setting. Bull Group Int Rech Sci Stomatol Odontol. 1995;38(1-2):45–50. [PubMed] [Google Scholar]
- 28.Gefen A, Gefen N, Zhu Q, Raghupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;20:1163–1177. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- 29.Downs J1, Bebbington A, Woodhead H, Jacoby P, Jian L, Jefferson A, Leonard H. Early determinants of fractures in Rett syndrome. Pediatrics. 2008 Mar;121(3):540–6. doi: 10.1542/peds.2007-1641. [DOI] [PubMed] [Google Scholar]
- 30.O’Callaghan JP, Jensen KF. Enhanced expression of glial fibrillary acidic protein and the cupric silver degeneration reaction can be used as sensitive and early indicators of neurotoxicity. Neurotoxicology. 1992;13(1):113–22. [PubMed] [Google Scholar]
- 31.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4(3):229–37. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox KS, Vezzani A. Does brain inflammation mediate pathological outcomes in epilepsy? Adv Exp Med Biol. 2014;813:169–183. doi: 10.1007/978-94-017-8914-1_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96(Pt A):70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Robel S, Buckingham SC, Boni JL, et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci. 2015;35:3330–3345. doi: 10.1523/JNEUROSCI.1574-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertram EH. In: Monitoring for seizures in rodents Chapter 46 in Models of seizures and epilepsy. Pitkanen A, Schwartzkroin PA, Moshé SL, editors. Elsevier Academic Press; Burlington MA: 2006. pp. 569–58. [Google Scholar]
- 36.Moyer JT, Gnatkovsky V, Ono T, Otáhal J, Wagenaar J, Stacey WC, Noebels J, Ikeda A, Staley KJ, de Curtis M, Litt B, Galanopoulou AS. Standards for data acquisition and software-based analysis of in vivo electroencephalography recordings from animals: report from the ILAE-AES joint translational task force of the. Epilepsia Supplement. doi: 10.1111/epi.13909. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paralikar K, Rao C, Clement RS. Automated reduction of non-neuronal signals from intra-cortical microwire array recordings by use of correlation technique. Conf Proc IEEE Eng Med Biol Soc. 2008:46–9. doi: 10.1109/IEMBS.2008.4649087. [DOI] [PubMed] [Google Scholar]
- 38.White AM, Williams PA, Ferraro DJ, et al. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods. 2006;152:255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Young CK, McNaughton N. Coupling of theta oscillations between anterior and posterior midline cortex and with the hippocampus in freely behaving rats. Cereb Cortex. 2009;19:24–40. doi: 10.1093/cercor/bhn055. [DOI] [PubMed] [Google Scholar]
- 40.Stern, Engel . Atlas of EEG patterns. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 41.Fisch BJ. Fisch and Spehlmann’s EEG Primer: Basic Principles of Digital and Analog EEG. 3rd. Elsevier; 1999. [Google Scholar]
- 42.Kitanishi T, Ujita S, Fallahnezhad M, et al. Novelty-Induced Phase-Locked Firing to Slow Gamma Oscillations in the Hippocampus: Requirement of Synaptic Plasticity. Neuron. 2015;86:1265–1276. doi: 10.1016/j.neuron.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Jeewajee A, Lever C, Burton S, et al. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus. 2008;18:340–348. doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyazovskiy VV, Delogu A. NREM and REM sleep: Complementary roles in recovery after wakefulness. Neuroscientist. 2014;20:203–219. doi: 10.1177/1073858413518152. [DOI] [PubMed] [Google Scholar]
- 45.Valatx JL. Genetics of EEG, of certain forms of epilepsy, sleep and learning. Rev Electroencephalogr Neurophysiol Clin. 1975;5:319–329. doi: 10.1016/s0370-4475(75)80042-6. [DOI] [PubMed] [Google Scholar]
- 46.Valatx JL. Possible embryonic origin of sleep interstrain differences in the mouse. Prog Brain Res. 1978;48:385–391. doi: 10.1016/S0079-6123(08)61036-5. [DOI] [PubMed] [Google Scholar]
- 47.Valatx JL, Cespuglio R, Paut L, et al. Genetic study of paradoxical sleep in mice. Connection with coloration genes. Waking Sleeping. 1980;4:175–183. [PubMed] [Google Scholar]
- 48.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 49.Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- 50.Arce C, Ramos J, Guevara MA, et al. Effect of spatial ability and sex on EEG power in high school students. Int J Psychophysiol. 1995;20:11–20. doi: 10.1016/0167-8760(95)00022-k. [DOI] [PubMed] [Google Scholar]
- 51.Davidson RJ, Schwartz GE, Pugash E, et al. Sex differences in patterns of EEG asymmetry. Biol Psychol. 1976;4:119–138. doi: 10.1016/0301-0511(76)90012-0. [DOI] [PubMed] [Google Scholar]
- 52.Corsi-Cabrera M, Ramos J, Guevara MA, et al. Gender differences in the EEG during cognitive activity. Int J Neurosci. 1993;72:257–264. doi: 10.3109/00207459309024114. [DOI] [PubMed] [Google Scholar]
- 53.del Rio-Portilla I, Ugalde E, Juarez J, et al. Sex differences in EEG in adult gonadectomized rats before and after hormonal treatment. Psychoneuroendocrinology. 1997;22:627–642. doi: 10.1016/s0306-4530(97)00056-5. [DOI] [PubMed] [Google Scholar]
- 54.Corsi-Cabrera M, Arce C, Ramos J, et al. Effect of spatial ability and sex on inter- and intrahemispheric correlation of EEG activity. Electroencephalogr Clin Neurophysiol. 1997;102:5–11. doi: 10.1016/s0013-4694(96)96091-5. [DOI] [PubMed] [Google Scholar]
- 55.Juarez J, Corsi-Cabrera M, del Rio-Portilla I. Effects of prenatal testosterone treatment on sex differences in the EEG activity of the rat. Brain Res. 1995;694:21–28. doi: 10.1016/0006-8993(95)00725-6. [DOI] [PubMed] [Google Scholar]
- 56.Prejean JD, Peckham JC, Casey AE, et al. Spontaneous tumors in Sprague-Dawley rats and Swiss mice. Cancer Res. 1973;33:2768–2773. [PubMed] [Google Scholar]
- 57.Kleinlogel H. Spontaneous EEG paroxysms in the rat: effects of psychotropic and alpha-adrenergic agents. Neuropsychobiology. 1985;13:206–213. doi: 10.1159/000118189. [DOI] [PubMed] [Google Scholar]
- 58.Aldinio C, Aporti F, Calderini G, et al. Experimental models of aging and quinolinic acid. Methods Find Exp Clin Pharmacol. 1985;7:563–568. [PubMed] [Google Scholar]
- 59.Aporti F, Borsato R, Calderini G, et al. Age-dependent spontaneous EEG bursts in rats: effects of brain phosphatidylserine. Neurobiol Aging. 1986;7:115–120. doi: 10.1016/0197-4580(86)90149-1. [DOI] [PubMed] [Google Scholar]
- 60.Buzsáki G, Laszlovszky I, Lajtha A, et al. Spike-and-wave neocortical patterns in rats: genetic and aminergic control. Neuroscience. 1990;38:323–333. doi: 10.1016/0306-4522(90)90031-x. [DOI] [PubMed] [Google Scholar]
- 61.Willoughby JO, Mackenzie L. Nonconvulsiveelectrocorticographic paroxysms (absence epilepsy) in rat strains. Lab Anim Sci. 1992;42:551–554. [PubMed] [Google Scholar]
- 62.Kharlamov EA, Jukkola PI, Schmitt KL, Kelly KM. Electrobehavioral characteristics of adult rats during epileptogenesis and the epileptic state following photothrombotic brain infarction. Epilepsy Res. 2003;6:185–203. doi: 10.1016/j.eplepsyres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 63.D’Ambrosio R, Fender JS, Fairbanks JP, et al. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearce PS, Friedman D, Lafrancois JJ, et al. Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 2014;32:121–131. doi: 10.1016/j.yebeh.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodgers KM, Dudek FE, Barth DS. Progressive, Seizure-Like, Spike-Wave Discharges Are Common in Both Injured and Uninjured Sprague-Dawley Rats: Implications for the Fluid Percussion Injury Model of Post-Traumatic Epilepsy. J Neurosci. 2015;35:9194–9204. doi: 10.1523/JNEUROSCI.0919-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reid AY, Bragin A, Giza CC, Staba RJ, Engel J. The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury. Epilepsia. 2016;57:1558–1567. doi: 10.1111/epi.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jandó G, Carpi D, Kandel A, et al. Spike-and-wave epilepsy in rats: sex differences and inheritance of physiological traits. Neuroscience. 1995;64:301–317. doi: 10.1016/0306-4522(94)00329-4. [DOI] [PubMed] [Google Scholar]
- 68.Nair SP, Jukkola PI, Quigley M, Wilberger A, Shiau DS, Sackellares JC, Pardalos PM, Kelly KM. Absence seizures as resetting mechanisms of brain dynamics. Cybern Syst Anal. 2008;44:664–672. doi: 10.1007/s10559-008-9051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly KM, Shiau DS, Jukkola PI, Miller ER, Mercadante AL, Quigley MM, Nair SP, Sackellares JC. Effects of age and cortical infarction on EEG dynamic changes associated with spike wave discharges in F344 rats. Exp Neurol. 2011;232:15–21. doi: 10.1016/j.expneurol.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson PF, Gilmore SA. Spontaneous generalized spike-wave discharges in the electrocorticograms of albino rats. Brain Res. 1980;201:452–458. doi: 10.1016/0006-8993(80)91052-5. [DOI] [PubMed] [Google Scholar]
- 71.Vergnes M, Marescaux C, Micheletti G, et al. Spontaneous paroxysmal electroclinical patterns in rat: a model of generalized non-convulsive epilepsy. Neurosci Lett. 1982;33:97–101. doi: 10.1016/0304-3940(82)90136-7. [DOI] [PubMed] [Google Scholar]
- 72.Gralewicz S, Luczak C, Wiaderna D, et al. Development of the age-related spontaneous spike-wave discharges in rat neocortex and exposure to a model neurotoxin. Acta Neurobiol Exp (Wars) 1995;55:65–71. doi: 10.55782/ane-1995-1062. [DOI] [PubMed] [Google Scholar]
- 73.Shaw FZ, Chuang SH, Shieh KR, et al. Depression- and anxiety-like behaviors of a rat model with absence epileptic discharges. Neuroscience. 2009;160:382–393. doi: 10.1016/j.neuroscience.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 74.Wiest MC, Nicolelis MA. Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat Neurosci. 2003;6:913–914. doi: 10.1038/nn1107. [DOI] [PubMed] [Google Scholar]
- 75.Shaw FZ. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J Neurophysiol. 2004;91:63–77. doi: 10.1152/jn.00487.2003. [DOI] [PubMed] [Google Scholar]
- 76.Kelly KM, Jukkola PI, Kharlamov EA, Downey KL, McBride JW, Strong R, Aronowski J. Long-term video-EEG recordings following transient unilateral middle cerebral and common carotid artery occlusion in Long-Evans rats. Exp Neurol. 2006;201:495–506. doi: 10.1016/j.expneurol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Shaw FZ. 7-12 Hz High-Voltage Rhythmic Spike Discharges in Rats Evaluated by Antiepileptic Drugs and Flicker Stimulation. J Neurophys. 2007;97:238–247. doi: 10.1152/jn.00340.2006. [DOI] [PubMed] [Google Scholar]
- 78.Huang HY, Lee HW, Chen SD, et al. Lamotrigine ameliorates seizures and psychiatric comorbidity in a rat model of spontaneous absence epilepsy. Epilepsia. 2012;53:2005–2014. doi: 10.1111/j.1528-1167.2012.03664.x. [DOI] [PubMed] [Google Scholar]
- 79.Loscher W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–184. doi: 10.1016/j.eplepsyres.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 80.Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg–a review. J Neural Transm Suppl. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- 81.Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33(6):635–55. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- 82.Serikawa T, Mashimo T, Kuramoro T, Voigt B, Ohno Y, Sasa M. Advances on genetic rat models of epilepsy. Exp Anim. 2015;64(1):1–7. doi: 10.1538/expanim.14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


