Abstract
Objective
Obesity, inflammation, and circulating 25-hydroxyvitamin D (25-OHD) have distinct roles in cancer prognosis. We investigated their interplay by evaluating the associations of body mass index (BMI) with circulating 25-OHD levels in cancer survivors, and determining whether associations were modified by inflammation, defined by C-reactive protein (CRP) levels.
Methods
Data on cancer survivors were aggregated from the U.S. National Health and Nutrition Examination Survey (2001–2010). Multivariable linear regression models were used to evaluate the associations of BMI with circulating 25-OHD. We stratified the analyses by CRP levels: low <1.0mg/L, moderate 1.0–3.0mg/L and high >3.0–9.9mg/L.
Results
Among 1,305 cancer survivors (mean age=60.8 years, mean BMI=28.0 kg/m2), circulating 25-OHD levels were 8.74 nmol/L lower (95%CI: 4.71 to 12.77) in cancer survivors with BMI ≥30kg/m2 compared to those with BMI <25kg/m2. This association was, however, limited to those with moderate CRP (−9.90 nmol/L, 95% CI: −16.45 to −3.36) and high CRP (−11.61 nmol/L, 95% CI: −18.71 to −5.05), but not among those with low CRP levels (−5.31 nmol/L, 95% CI: −12.66 to 2.04).
Conclusions
A greater understanding of the interplay between 25-OHD and inflammation in cancer survivors with obesity should allow for targeted secondary prevention and help improve prognosis in these patients.
Keywords: cancer survivors, obesity, vitamin D, Inflammation, NHANES
INTRODUCTION
The growing aging population contributes to an increasing cancer incidence globally (1), while advances in cancer prevention, diagnosis and treatment have contributed to an overall reduction in cancer mortality (2). Thus, the increasing prevalence and reduced mortality are expected to result in a growing number of survivors. There are currently more than 15.5 million cancer survivors in the U.S. and the number is expected to rise to 20 million by 2026 (3). Identifying modifiable factors that improve prognosis in this rapidly expanding demographic group, and how these factors interact, is of high priority.
Emerging evidence suggests that vitamin D status may be associated with improved cancer prognosis and survival, particularly colorectal and breast cancers (4, 5, 6, 7). On the other hand, obesity is associated with poor prognosis in many cancers (8) and low circulating 25-OHD levels in both cancer-free, and cancer survivors (9, 10, 11). There is emerging evidence that individuals with obesity who are metabolically normal may be protected from the adverse effects of obesity (12, 13). Thus, when evaluating the associations of obesity with health outcomes, it is important to consider the metabolic status so that interventions to reduce the adverse effects of obesity can be targeted towards individuals at the highest risk of detrimental health outcomes.
Although there are no unified criteria to define metabolically healthy obesity, previous studies have shown that metabolically healthy obese individuals have a favorable inflammation profile (12, 14). Further, it has been shown that an indivudual’s inflammatory status could mediate the effect of obesity on health outcomes including cancer (15), and could thus be used to identify individuals who may be at the highest risk of adverse health outcomes related with obesity. However, no studies have evaluated whether inflammation modifies the effect of obesity on circulating 25-OHD levels in cancer survivors.
To fill this knowledge gap, we evaluated the associations of body mass index (BMI) with circulating 25-OHD levels among cancer survivors using data from the U.S. National Health and Nutrition Examination Survey (NHANES). Further, we determined if this association is modified by inflammation, as determined C-reactive protein (CRP) levels.
METHODS
Study Population
The NHANES was designed to provide cross-sectional estimates on the prevalence of health, nutrition, and potential risk factors among the civilian non-institutionalized U.S. population up to 85 years old (16). In brief, NHANES surveys a nationally representative complex, stratified, multistage, probability clustered sample of about 5,000 participants each year in 15 counties across the country. The NHANES obtained approval from the National Center for Health Statistics Research Ethics Review Board and participants provided written consent.
We extracted demographic information, measures of adiposity, smoking history, time of blood draw, circulating CRP and 25-OHD levels, cancer diagnosis and vitamin D supplementation use and combined these into a single dataset for data collection from 2001–2002 to 2009–2010. Participants were considered as cancer survivors if they answered “yes” to the question “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” We excluded participants who had non-melanoma skin cancer. This interview question was given to males and females 20 years or older, subsequently restricted the analyzed sample to adult cancer survivors. We aggregated five waves’ data and excluded those who were never diagnosed with cancer, or were pregnant. (Figure 1)
Figure 1.
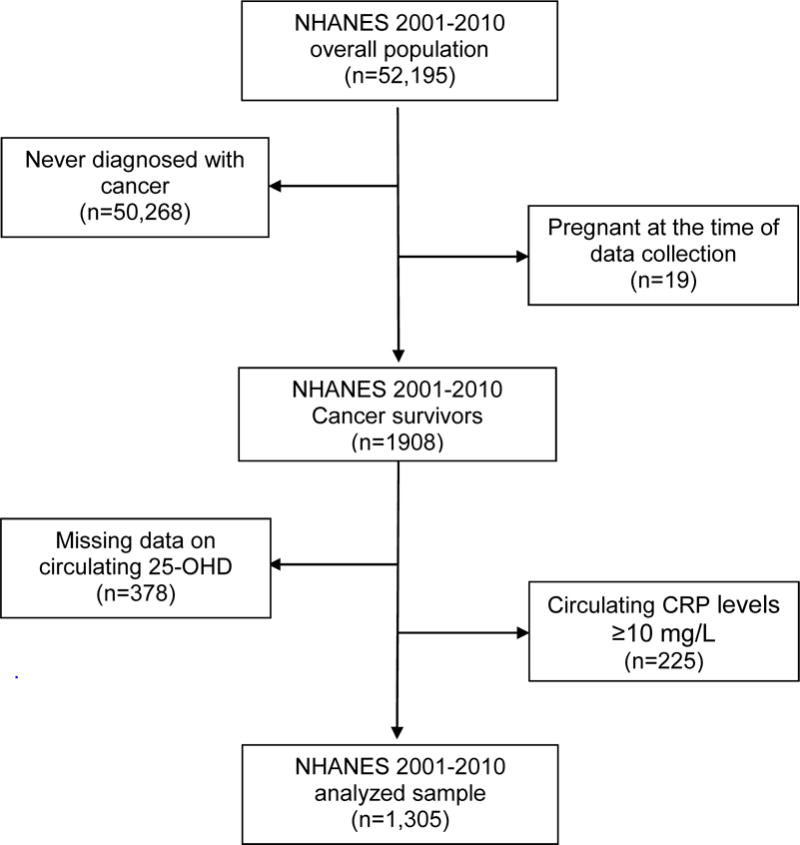
Flow diagram illustrating the U.S. National Health and Nutrition Examination Survey (2001 – 2010) study population and analyzed sample of cancer survivors aged 20 years or older.
Body mass index (BMI)
Weight and height were measured in a mobile examination centre (MEC) or in the participant’s home at the time of physical examination. The measurements followed standard procedures and were carried out by trained technicians using standardized equipment. BMI was calculated as weight in kg/(height in meters)2. We categorized study participants into BMI categories: underweight (<18.5kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0 – 29.9 kg/m2), and obesity (≥30.0 kg/m2). For analytic purposes, we combined underweight and normal weight into 1 category (≤25.0 kg/m2).
Circulating 25-OHD levels
The process of blood collection is detailed in the NHANES Laboratory/Medical Technologist Procedures Manual (17). The NHANES blood collection excluded participants who received chemotherapy within the preceding 4 weeks. Blood samples were collected, processed, stored and shipped to University of Washington, Seattle for testing. The lab methods for measuring 25-OHD for 2007–2010 changed from 2005–2006 and earlier in NHANES, and has been described previously (18). Briefly, circulating 25-OHD concentrations were measured at the National Center for Environmental health, CDC, Atlanta, GA using the DiaSorin RIA kit (Stillwater, MN) between 2001 and 2006. NHANES provided regression to convert the 2001–2006 measures to equivalent 25–OHD measurement from a standardized liquid chromatography-tandem mass spectrometry (LC-MS/MS) method, which was used in the analysis of 25-OHD in NHANES 2007–2010 data (19). This standardization procedure therefore ensures that 25-OHD data are comparable between 2001–2006 and 2007–2010.
Circulating C-reactive protein (CRP)
CRP levels were determined from blood samples. The process of blood collection is detailed in the NHANES Laboratory/Medical Technologist Procedures Manual (17). Circulating CRP was quantified by latex-enhanced nephelometry with a Behring Nephelometer Analyzer System. CRP levels were categorized to low (<1.0 mg/L), moderate (1.0–3.0 mg/L), and high (>3.0 mg/L) (20). Cancer survivors with CRP levels ≥10 mg/L were excluded since this may represent an acute infective episode.
Season of blood draw
Blood samples were collected at the same time as weight and height. Season of blood draw was determined from the month of physical examination. Months were documented in two groups: November 1st through April 30th, or May 1st through October 31st, and classified into winter or summer, respectively (21).
Dietary Vitamin D supplement use
Information on dietary vitamin D supplement was retrieved from the 30–day Dietary Supplement data. For 2001–2006 data, information on individual product for participants who reported taking vitamin supplement were obtained, and linked to the Dietary Supplements Ingredient Database (22). Products’ ingredients that contained Vitamin D were aggregated for each participant, and categorized into a binary variable (yes/no) for dietary vitamin D supplement use assessment. In 2007–2010 data, aggregated information on dietary supplement use (including vitamin D supplement use) was readily available, thus, was used to determine participants’ dietary vitamin D supplement use (yes/no).
Socio-demographic characteristics
Information on age, sex, race and ethnicity, and smoking status were extracted. Based on self-reported race and ethnicity, participants were classified into one of the three racial/ethnic groups: Non-Hispanic White, Non-Hispanic Black, and Hispanic and others. For smoking status, we classified participants into three groups: never smokers (did not smoke 100 cigarettes and not currently smoking), former smokers (smoked 100 cigarettes in life and not currently smoking), and current smokers (smoked 100 cigarettes in life and currently smoking).
Statistical analyses
Survey analysis procedures were used to account for the sample weights (MEC exam weight), stratification, and clustering of the complex sampling design to ensure nationally representative estimates. Information on socio-demographic characteristics, weight, height, season of blood draw, and CRP was complete among cancer survivors who had available data on circulating 25-OHD levels. We calculated the descriptive statistics for participants’ characteristics and CRP levels by quintiles of 25-OHD. We summarized weighted means and standard errors for continuous variables, and weighted proportions for categorical variables.
We estimated the linear associations of BMI categories with circulating 25-OHD, adjusted for socio-demographic characteristics (age, gender, race, and smoking status), season of blood draw, vitamin D supplement use, and CRP levels. A cross-product term with BMI and CRP categories was entered into the multivariable linear regression with the main effect terms. A statistical significant interaction (P-value<.001) was found using Wald statistic. We stratified the analyses by CRP categories, and estimated the linear associations of BMI categories and circulating 25-OHD in each CRP level strata. We examined the normality of residuals using kernel density estimate and standardized normal probability plots for all the linear regression models. Further sensitivity analyses were carried out using BMI as a continuous variable in all regression models. All statistical significance was set at p<0.05. All statistical analyses were performed using Stata version 14.0 (STATA Corp., College Station, Texas, USA).
RESULTS
There were 1,305 cancer survivors in the five NHANES waves who had data on circulating 25-OHD and CRP levels. The prevalent cancer sites were breast (19.6%), prostate (19.6%), cervix (10.0%), and colon (8.9%). Participants’ mean age was 60.8 years at the time of examination, and their mean BMI was 28.0 kg/m2. We observed statistically significant differences in circulating 25-OHD levels for most characteristics, except for age, and sex (Table 1). Cancer survivors with BMI ≥ 30.0 kg/m2, Non-Hispanic Black, smokers, reported no vitamin D supplement use had lower 25-OHD levels than those who with BMI < 25kg/m2, Non-Hispanic White/Hispanic, non-smokers and those who reported vitamin D supplement use, respectively.
Table 1.
Socio demographic Characteristics and Circulating C Reactive Protein Levels of Cancer Survivors Aged 20 years or Older from the NHANES (2001–2010), by Quintiles of Circulating 25-OHD Levels
| Circulating 25-OHD (nmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Quintile 9.1–47.1 |
Quintile 2 47.2–60.6 |
Quintile 3 60.8–72.5 |
Quintile 4 72.6–87.4 |
Quintile 5 87.6–206 |
P value | ||
|
| ||||||||
| N | 1305 | 326 | 263 | 257 | 241 | 218 | ||
| Age (years) | Mean (s.e.) | 60.94 (0.52) | 60.74 (1.27) | 61.45 (0.98) | 62.80 (1.15) | 61.90 (0.96) | 58.84 (1.28) | 0.60 |
| Body Mass Index (BMI, kg/m2) | ||||||||
| <18.5 | % | 2.1 | 2.1 | 0.1 | 2.5 | 1.9 | 4.1 | <0.001 |
| 18.5–24.9 | % | 32.1 | 30.6 | 23.4 | 29.6 | 34.5 | 42.6 | |
| 25.0–29.9 | % | 34.4 | 25.7 | 43.0 | 39.9 | 21.1 | 31.1 | |
| ≥ 30 | % | 31.4 | 41.6 | 33.5 | 28.0 | 31.5 | 22.2 | |
| Season of blood draw | 0.01 | |||||||
| Winter (Nov to Apr) | % | 32.6 | 41.3 | 34.8 | 32.7 | 21.9 | 32.2 | |
| Summer (May to Oct) | % | 67.4 | 58.7 | 65.2 | 67.3 | 78.1 | 67.8 | |
| C Reactive Protein (mg/l) | 0.009 | |||||||
| Low risk (<1.0) | % | 29.4 | 23.9 | 27.6 | 29.6 | 29.4 | 36.8 | |
| Average risk (1.1–3.0) | % | 36.9 | 32.4 | 40.3 | 37.7 | 33.2 | 41.0 | |
| High risk (3.1–9.9) | % | 33.7 | 43.7 | 32.1 | 32.7 | 37.4 | 22.2 | |
| Gender | 0.21 | |||||||
| Male | % | 36.3 | 30.0 | 38.3 | 40.1 | 40.1 | 32.9 | |
| Female | % | 63.7 | 70.0 | 61.7 | 59.9 | 59.9 | 67.1 | |
| Race | <.001 | |||||||
| Non-Hispanic White | % | 85.6 | 68.6 | 82.0 | 90.3 | 93.1 | 94.3 | |
| Non-Hispanic Black | % | 6.6 | 17.0 | 6.9 | 3.8 | 1.9 | 3.5 | |
| Hispanic and other | % | 7.8 | 14.4 | 11.1 | 5.9 | 5.0 | 2.2 | |
| Smoking | <.001 | |||||||
| Never smoked | % | 43.5 | 38.5 | 49.8 | 46.1 | 35.6 | 47.3 | |
| Former smoker | % | 37.2 | 29.8 | 34.8 | 40.6 | 47.6 | 33.4 | |
| Current smoker | % | 19.3 | 31.7 | 15.4 | 13.3 | 16.8 | 19.3 | |
| Vitamin D Supplement use | <.001 | |||||||
| Yes | % | 52.7 | 22.9 | 49.3 | 59.5 | 58.5 | 74.0 | |
| No | % | 47.3 | 77.1 | 50.7 | 40.5 | 41.5 | 26.0 | |
Associations between obesity and circulating 25-OHD levels
Table 2 summarizes both the non-adjusted and adjusted associations between BMI categories and circulating 25-OHD in linear regression models. Circulating 25-OHD levels were 8.74 nmol/L (95% CI: 4.71 to 12.77) lower among cancer survivors with BMI≥30kg/m2 compared to cancer survivors with BMI < 25kg/m2.
Table 2.
Associations between BMI and ciruclating 25-OHD from Unadjusted and Multivariable-adjusted Linear Regression models among Cancer Survivors Aged 20 years or Older from the NHANES 2001–2010 (n=1305)
| Circulating 25-OHD (nmol/L)
|
||
|---|---|---|
| Unadjusted beta coefficient (95% CI) | Adjusteda beta coefficient (95% CI) | |
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −3.36 (−7.38 to 0.68) | −3.10 (−7.05 to 0.85) |
| ≥ 30 | −8.80 (−13.61 to −4.00) | −8.74 (−12.77 to −4.71) |
| P-trend | <.001 | <.001 |
Adjusted for age, sex, race, smoking status, season of blood draw, dietary vitamin D supplement use and circulating creactive protein levels.
Table 3 summarizes analyses stratified by CRP categories. We observed an interaction (P-value <0.001) as the associations of BMI categories with circulating 25-OHD levels differ in each strata of CRP. In cancer survivors with low CRP level group, there were no statistically significant associations between BMI and circulating 25-OHD (−1.33 nmol/L, 95% CI: −9.33 to 6.67 in cancer survivors with 25.0 kg/m2 ≤ BMI<30.0 kg/m2; −5.31 nmol/L, 95% CI: −12.66 to 2.04 in cancer survivors with BMI ≥ 30kg/m2). Among those with moderate CRP levels, cancer survivors with BMI ≥ 30.0 kg/m2 (−9.90 nmol/L, 95% CI: −16.45 to −3.36), but not those with 25.0 kg/m2 ≤ BMI < 30.0 kg/m2 (−1.64 nmol/L, 95% CI: −7.86 to 4.58) had low 25-OHD levels. Among those with high CRP levels, cancer survivors with 25.0 kg/m2 ≤ BMI < 30.0 kg/m2 (−8.63 nmol/L, 95% CI: −14.33 to −2.92) and those with BMI ≥ 30.0 kg/m2 (−11.61 nmol/L, 95% CI: −18.17 to −5.05) both had low 25-OHD levels.
Table 3.
CRP Level Stratified Associations between BMI and ciruclating 25-OHD from Unadjusted and Multivariable-adjusted Linear Regression models among Cancer Survivors Aged 20 years or Older from the NHANES 2001–2010 (n=1305)
| Circulating 25-OHD (nmol/L) | ||
|---|---|---|
| Unadjusted beta coefficient (95% CI) | Adjusteda beta coefficient (95% CI) | |
|
Low risk CRP level (<1.0 mg/L), n=348 |
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −0.10 (−7.26 to 7.05) | −1.33 (−9.33 to 6.67) |
| ≥ 30 | −3.62 (−11.42 to 4.17) | −5.31 (−12.66 to 2.04) |
| P-trend | 0.46 | 0.20 |
|
| ||
| Moderate risk CRP level (1.1–3.0 mg/L), n=498
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −2.23 (−9.16 to 4.69) | −1.64 (−7.86 to 4.58) |
| ≥ 30 | −8.05 (−15.62 to −0.47) | −9.90 (−16.45 to −3.36) |
| P-trend | 0.04 | 0.004 |
|
| ||
| High risk CRP level (3.1–9.9 mg/L), n=459
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −6.87 (−13.34 to −0.40) | −8.63 (−14.33 to −2.92) |
| ≥ 30 | −9.46 (−16.96 to −1.95) | −11.61 (−18.17 to −5.05) |
| P-trend | 0.03 | 0.004 |
Adjusted for age, sex, race, smoking status, season of blood draw, and dietary vitamin D supplement use.
We conducted further analyses among breast (N=256) and prostate cancer survivors (N=255) because these were the two most prevalent cancer types among study participants (Tables 4 and 5). We observed similar trends in associations as we did in the overall analyses, especially for breast cancer, but the stratified analyses, though highly suggestive, were not statistically significant likely due to the small sample size. Breast cancer survivors with BMI ≥ 30.0 kg/m2 had low 25-OHD levels (−8.75, 95%CI −16.78 to −0.72). Among those with high CRP levels, although breast cancer survivors with BMI ≥ 30.0 kg/m2 had low 25-OHD levels (−14.87, 95% CI −29.77 to 0.03), the trend test was not statistically significant likely as a result of the reduced sample size (N=89).
Table 4.
Overall and CRP Level Stratified Associations between BMI and circulating 25-OHD from Unadjusted and Multivariable-adjusted Linear Regression models among breast Cancer Survivors Aged 20 years or Older from the NHANES 2001–2010 (n=256)
| Circulating 25-OHD (nmol/L) | ||
|---|---|---|
| Unadjusted beta coefficient (95% CI) | Adjusteda beta coefficient (95% CI) | |
|
All breast cancer survivors, n=256 |
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −6.66 (−14.50 to 1.19) | −5.21 (−12.09 to 1.68) |
| ≥ 30 | −8.52 (−16.15 to −0.89) | −8.75 (−16.78 to −0.72) |
| P-trend | 0.03 | 0.03 |
|
| ||
| Low risk CRP level (<1.0 mg/L), n=70
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −3.23 (−13.55 to 7.08) | −3.67 (−14.82 to 7.47) |
| ≥ 30 | −5.81 (−23.27 to 11.65) | −4.26 (−20.39 to 11.88) |
| P-trend | 0.46 | 0.52 |
|
| ||
| Moderate risk CRP level (1.1–3.0 mg/L), n=97
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −6.48 (−20.50 to 7.53) | −2.79 (−13.15 to 7.56) |
| ≥ 30 | −5.17 (−19.02 to 8.67) | −9.64 (−18.88 to −0.40) |
| P-trend | 0.41 | 0.06 |
|
| ||
| High risk CRP level (3.1–9.9 mg/L), n=89
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | −12.23 (−29.94 to 5.48) | −10.46 (−24.65 to 3.72) |
| ≥ 30 | −13.39 (−30.51 to 3.74) | −14.87 (−29.77 to 0.03) |
| P-trend | 0.16 | 0.08 |
Adjusted for age, race, smoking status, season of blood draw, dietary vitamin D supplement use, (and CRP in the overall analysis).
Table 5.
Overall and CRP Level Stratified Associations between BMI and ciruclating 25-OHD from Unadjusted and Multivariable-adjusted Linear Regression models among prostate Cancer Survivors Aged 20 years or Older from the NHANES 2001–2010 (n=255)
| Circulating 25-OHD (nmol/L) | ||
|---|---|---|
| Unadjusted beta coefficient (95% CI) | Adjusteda beta-coefficient (95% CI) | |
|
All prostate cancer survivors, n=255 |
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | 5.59 (−2.41 to 13.59) | −0.76 (−7.89 to 6.38) |
| ≥ 30 | 3.69 (−4.38 to 11.75) | −4.02 (−11.52 to 3.47) |
| P-trend | 0.41 | 0.27 |
|
| ||
| Low risk CRP level (<1.0 mg/L), n=74
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | 8.70 (−3.27 to 20.67) | 3.19 (−9.01 to 15.40) |
| ≥ 30 | 7.39 (−3.01 to 17.79) | 2.71 (−12.05 to 17.47) |
| P-trend | 0.15 | 0.72 |
|
| ||
| Moderate risk CRP level (1.1–3.0 mg/L), n=95
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | 4.87 (−9.38 to 19.12) | −1.74 (−15.05 to 11.57) |
| ≥ 30 | 2.51 (−12.30 to 17.32) | −7.30 (−20.91 to 6.32) |
| P-trend | 0.71 | 0.28 |
|
| ||
| High risk CRP level (3.1–9.9 mg/L), n=86
|
||
| BMI (kg/m2) | ||
| <25.0 | reference | reference |
| 25.0–29.9 | 2.66 (−11.49 to 16.82) | −3.12 (−15.37 to 9.14) |
| ≥ 30 | 1.87 (−9.79 to 13.54) | −7.73 (−18.75 to 3.29) |
| P-trend | 0.87 | 0.22 |
Adjusted for age, race, smoking status, season of blood draw, dietary vitamin D supplement use, (and CRP in the overall analysis).
Our findings were similar when using BMI as a continuous variable. One unit increase in BMI was associated with a reduction in 25-OHD levels (−0.57 nmol/L, 95% CI: −0.92 to −0.23) in the overall analyses (Data not shown). The obesity prevalence was higher in Non-Hispanic black cancer survivors than in Non-Hispanic white (46.1% Non-Hispanic Black with BMI ≥ 30.0 kg/m2 vs. 30.6% Non-Hispanic Whites with BMI ≥ 30.0 kg/m2, and 28.3% Hispanics with BMI ≥ 30.0 kg/m2). Similarly, race/ethnical differences were observed for CRP levels (mean CRP levels were 3.25 mg/L among Non-Hispanic Black vs. 2.63 mg/L among Non-Hispanic white, and 2.77 mg/L among Hispanics) (Data not shown).
DISCUSSION
In a U.S. nationally representative sample of 1,305 cancer survivors, higher BMI was associated with lower circulating 25-OHD levels. Nevertheless, this association was modified by the inflammation status. In analyses stratified by CRP categories, the association of higher BMI with lower circulating 25-OHD persisted among cancer survivors with elevated levels of CRP, yet attenuated to null among those with low CRP levels.
Our finding of an estimated 0.57 nmol/L (95% CI: 0.23 to 0.9) lower circulating 25-OHD with each unit increase of BMI in the overall analyses is consistent with previous studies. Using a hospital sample, Vashi and colleagues (10) found a similar trend of lower circulating 25-OHD levels with each increase in BMI among cancer survivors of mixed cancer sites. The magnitude of the decrease in that study was slightly larger (−0.42 ng/mL, 95% CI: −0.56 to −0.28, which translates to −1.05 nmol/L, 95% CI: −1.40 to −0.70) than what we found, probably due to the limited number of confounding factors (age and gender) adjusted in their multivariable linear regression model. Likewise, Friedman, et al (9) reported that the vitamin D deficiency rate was 3-fold higher (aOR=3.05, 95% CI: 1.72–5.41) in postmenopausal breast cancer survivors with obesity compared to those with normal weight. However, none of the aforementioned studies had included any inflammation assessment.
To the best of our knowledge, our study is the first to investigate the associations of obesity with circulating 25-OHD levels by strata of inflammation status. Our findings suggested that CRP modifies the association between obesity and circulating 25-OHD. Adipose tissue is a metabolically active endocrine organ that is involved in whole body tissue homeostasis. Obesity is characterized by a state of chronic inflammation and adipose tissue hypoxia resulting in dysregulation in adipokine production, and activation of pro-inflammatory pathways (23), which can promote tumor progression (24, 25, 26). Nevertheless, although the majority of individuals with obesity are metabolically unhealthy, with evidence of low-grade systemic inflammation, it has been shown that up to 28% of individuals with obesity are metabolically healthy (27), and have no evidence of systemic inflammation. Metabolically healthy individuals with or without obesity have lower circulating CRP concentrations compared to metabolically unhealthy individuals (28). After stratifying cancer survivors by CRP levels in our study, we observed that among cancer survivors whose BMI ≥ 30.0 kg/m2, only those with moderate and high CRP levels, but not those with low CRP, had low circulating 25-OHD levels. Similar associations have been reported among non-cancer individuals. In a large study among individuals with obesity, participants who were classified as metabolically healthy obesity (classified using CRP and other biomarkers), had higher circulating 25-OHD levels compared to those who were classified as metabolically unhealthy obesity (29). This is in line with a recent review of mechanistic studies (30) that pointed to the potential mediator role of inflammation to in vitamin D and cancer progression. It has been suggested that vitamin D status may modulate the favorable inflammatory profile among the metabolically healthy cancer survivors with obesity, because of vitamin D’s role in regulating chronic inflammation (29, 30). This needs to be investigated in a longitudinal setting with repeated measures of vitamin D and inflammation biomarkers in cancer survivors.
The main strength of this analysis is pooling a considerable size of adult cancer survivors from a U.S. nationally representative sample. In addition, we controlled for a range of factors that are known to affect the circulating 25-OHD levels. Further, we were able to compare associations of BMI with circulating 25-OHD by CRP categories, thereby providing further insight on the interplay between circulating 25-OHD and inflammation among cancer survivors with obesity.
There are a number of limitations to this study. First, interventional studies and mendelian randomization analyses supported a causal relationship between obesity and low circulating 25-OHD levels (31, 32, 33). The cross-sectional nature of this study makes it impossible to determine a causal association between circulating 25-OHD and inflammation. It is unclear if correcting vitamin D level could effectively lower inflammation among cancer survivors who are with obesity and evident systematic inflammation (34). Second, season, an important determinant of 25-OHD levels, was only available in two categories. Solar radiation is required for skin to synthesize vitamin D, yet it is weaker in winter compared to summer. Although the NHANES study collected blood samples in the Southern states during winter, and in the Northern states during summer. higher circulating 25-OHD levels were seen in the Northen States. Third, apart from breast and prostate cancers, we were not able to conduct analyses stratified by other cancer type and disease stage because of the limited number of individual cancers. Finally, participants who received chemotherapy within last 4 weeks were excluded from blood collection when they enrolled the NHANES study. Given the documented chemotherapy associated reduction of circulating 25-OHD level (35, 36, 37), our findings might not be generalizable to patients currently receiving chemotherapy.
To date, inflammation has been rarely considered in prospective studies investigating the impact of circulating 25-OHD on cancer prognosis (38). As evidence emerges of potential associations between low circulating 25-OHD levels and prognosis in cancer patients, secondary prevention efforts might be best served if interventions to correct low 25-OHD levels are directed towards cancer survivors with obesity and high CRP levels.
CONCLUSION
Obesity is associated with lower circulating 25-OHD levels in cancer survivors. This appears, however, to be limited to those with evidence of systemic inflammation. Further studies are needed to elucidate the casual relationship between circulating 25-OHD and inflammation in cancer prognosis, particularly among cancer survivors with obesity. Findings from such studies could open up opportunities to prioritize interventions to correct 25-OHD by stratifying cancer survivors with obesity based on their metabolic profile or inflammation markers.
What is already known about this subject?
Obesity is associated with poor prognosis, and survival in several cancers, possibility through metabolic dysregulation, and activation of pro-inflammatory pathways.
Emerging evidence suggest that vitamin D status be associated with cancer prognosis and survival, particularly colorectal and breast cancers.
What does your study add?
We evaluated the associations of body mass index (BMI) with circulating 25-OHD levels in a nationally representative sample of cancer survivors, taking into consideration systemic inflammation, defined by circulating C-reactive protein (CRP).
We found that cancer survivors with high BMI have low circulating 25-OHD levels. However, circulating CRP levels modified the association of BMI with 25-OHD levels such that the unfavourable association between BMI and circulating 25-OHD levels was limited to cancer survivors with elevated CRP levels, but not among those with low CRP levels.
A greater understanding of the interplay between 25-OHD and inflammation in cancer survivors with obesity should allow for targeted secondary prevention and help improve prognosis in these patients.
Acknowledgments
FUNDING: This work was supported by the Transdisplinary Research on Energetics and Cancer (TREC) Center at Washington University in St. Louis (LY) and the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital Foundation, and Washington University School of Medicine (ATT). The TREC Center is funded by the National Cancer Institute at NIH (U54 CA155496).
Footnotes
DISCLOSURE: The authors declared no conflict of interest
CONTRIBUTIONS: LY and ATT conceived and designed study, analyzed and interpreted data, drafted and reviewed manuscript.
References
- 1.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Toriola AT, Nguyen N, Scheitler-Ring K, Colditz GA. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:917–933. doi: 10.1158/1055-9965.EPI-14-0053. [DOI] [PubMed] [Google Scholar]
- 5.Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, Hong CC, et al. Association of Serum Level of Vitamin D at Diagnosis With Breast Cancer Survival: A Case-Cohort Analysis in the Pathways Study. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mondul AM, Weinstein SJ, Moy KA, Mannisto S, Albanes D. Circulating 25-Hydroxyvitamin D and Prostate Cancer Survival. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25:665–669. doi: 10.1158/1055-9965.EPI-15-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb PM, de Fazio A, Protani MM, Ibiebele TI, Nagle CM, Brand AH, et al. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. The American journal of clinical nutrition. 2015;102:109–114. doi: 10.3945/ajcn.114.102681. [DOI] [PubMed] [Google Scholar]
- 8.Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman CF, DeMichele A, Su HI, Feng R, Kapoor S, Desai K, et al. Vitamin d deficiency in postmenopausal breast cancer survivors. Journal of women’s health. 2012;21:456–462. doi: 10.1089/jwh.2011.3009. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vashi PG, Lammersfeld CA, Braun DP, Gupta D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutrition journal. 2011;10:51. doi: 10.1186/1475-2891-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring, Md) 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 12.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. The Journal of clinical endocrinology and metabolism. 2012;97:2482–2488. doi: 10.1210/jc.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, et al. Metabolically normal obese people are protected from adverse effects following weight gain. The Journal of clinical investigation. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring, Md) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disesae Control and Prevention. National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes.htm. Accessed June 21, 2016.
- 17.Centers for Disesae Control and Prevention. NHANES Laboratory/Medical Technologists Procedures Manua. 2009 https://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/lab.pdf, Accessed June 21, 2016.
- 18.Centers for Disesae Control and Prevention. Analytical Note for 25-Hydroxyvitamin D Data Analysis using NHANES III (1988–1994) NHANES 2001–2006, and NHANES 2007–2010. 2015 http://wwwn.cdc.gov/nchs/nhanes/VitaminD/AnalyticalNote.aspx, Accessed June 21, 2016.
- 19.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. The Journal of nutrition. 2010;140:2030s–2045s. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 21.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. American journal of epidemiology. 2008;168:577–586. doi: 10.1093/aje/kwn163. discussion 587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwyer JT, Picciano MF, Betz JM, Fisher KD, Saldanha LG, Yetley EA, et al. Progress in development of an integrated dietary supplement ingredient database at the NIH Office of Dietary Supplements. Journal of food composition and analysis : an official publication of the United Nations University, International Network of Food Data Systems. 2006;19:S108–s114. doi: 10.1016/j.jfca.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Molecular and cellular endocrinology. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC endocrine disorders. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? The Journal of clinical endocrinology and metabolism. 2013;98:E1610–1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 29.Esteghamati A, Aryan Z, Esteghamati A, Nakhjavani M. Differences in vitamin D concentration between metabolically healthy and unhealthy obese adults: associations with inflammatory and cardiometabolic markers in 4391 subjects. Diabetes & metabolism. 2014;40:347–355. doi: 10.1016/j.diabet.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 30.van Harten-Gerritsen AS, Balvers MG, Witkamp RF, Kampman E, van Duijnhoven FJ. Vitamin D, Inflammation, and Colorectal Cancer Progression: A Review of Mechanistic Studies and Future Directions for Epidemiological Studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:1820–1828. doi: 10.1158/1055-9965.EPI-15-0601. [DOI] [PubMed] [Google Scholar]
- 31.Himbert C, Ose J, Delphan M, Ulrich CM. A systematic review of the interrelation between diet- and surgery-induced weight loss and vitamin D status. Nutrition research (New York, NY) 2017;38:13–26. doi: 10.1016/j.nutres.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, et al. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring, Md) 2012;20:2296–2301. doi: 10.1038/oby.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS medicine. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermato-endocrinology. 2014;6:e983401. doi: 10.4161/19381980.2014.983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, Sunga AY. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. International journal of colorectal disease. 2009;24:219–224. doi: 10.1007/s00384-008-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fakih MG, Andrews C, McMahon J, Muindi JR. A prospective clinical trial of cholecalciferol 2000 IU/day in colorectal cancer patients: evidence of a chemotherapy-response interaction. Anticancer research. 2012;32:1333–1338. [PubMed] [Google Scholar]
- 37.Jacot W, Pouderoux S, Thezenas S, Chapelle A, Bleuse JP, Romieu G, et al. Increased prevalence of vitamin D insufficiency in patients with breast cancer after neoadjuvant chemotherapy. Breast cancer research and treatment. 2012;134:709–717. doi: 10.1007/s10549-012-2084-7. [DOI] [PubMed] [Google Scholar]
- 38.Conway FJ, McMillan DC. Plasma vitamin D concentration and survival in colorectal cancer: potential confounding by the systemic inflammatory response. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:224. doi: 10.1200/JCO.2014.59.2386. [DOI] [PubMed] [Google Scholar]


