Abstract
Aging is a global decline of physiological functions, leading to an increased susceptibility to diseases and ultimately death. Maximum lifespans differ up to 200-fold between mammalian species. Although considerable progress has been achieved in identifying conserved pathways that regulate individual lifespan within model organisms, whether the same pathways are responsible for the interspecies differences in longevity remains to be determined. Recent cross-species studies have begun to identify pathways responsible for interspecies differences in lifespan. Here, we review the evidence supporting the role of anti-cancer mechanisms, DNA repair machinery, insulin/IGF1 signaling, and proteostasis in defining species lifespans. Understanding the mechanisms responsible for the dramatic differences in lifespan between species will have a transformative effect on developing interventions to improve human health and longevity.
Keywords: Longevity, aging, mammals, comparative biology
From model organisms to multispecies comparisons
Genetic studies in model organisms identified multiple means to extend lifespan, including dietary restriction (see Glossary) [1], knockout of the insulin receptor or the insulin-like growth factor 1 (IGF-1) receptor [2, 3], knockout of the growth hormone (GH) receptor [4], deteriorated anterior pituitary gland [5], inhibition of the mechanistic target of rapamycin (mTOR) pathway (genetically [6] or by rapamycin administration [7]), activation of AMP kinase (AMPK) [8], augmentation of autophagy [9], overexpression of particular sirtuin proteins [10, 11], elimination of senescent cells [12], overexpression of fibroblast growth factor-21 (FGF-21) [13], knockout of the TRPV1 pain receptor [14], etc. Up to a 10-fold lifespan extension can be achieved in invertebrate models [15], where as in mammals lifespan extension typically ranges from 10–20%. The most striking lifespan extension (64%) from a single mutation was achieved in female Ames dwarf mice [5]. Overall, mammals have evolved lifespans that differ up to 200-fold between species [16]. However, the mechanisms responsible for the dramatic differences in natural lifespans remain mysterious. This review will focus on the following questions: Which of the mechanisms identified in model organisms are differentially regulated in short- and long-lived mammals? Are there unique mechanisms contributing to longevity of long-lived mammalian species?
Anti-cancer mechanisms
To achieve longevity species have to evolve mechanisms to protect themselves from cancer. Selection against cancer is very strong at the reproductive age, as developing cancer in a young organism would cut its chance of successfully reproduction. However, as the force of selection diminishes with age cancer emerges as an age-related disease. Interestingly, several lifespan modulating pathways identified by genetic studies in model organisms are implicated in cancer progression, such as GH, IGF-1, mTOR, and AMPK signaling. In fact, cancer incidence in long-lived dwarf mice that have perturbed GH signaling is greatly reduced [17, 18]. Interestingly, downregulation of these pro-growth pathways has both anti-cancer and anti-aging effect, suggesting longevity and cancer resistance mechanisms may be synergistic, at least in some cases.
Statistically, cancer risk increases in large and long-lived species, as they have more cells and more time to accumulate mutations that lead to malignant transformation. However, species with longer lifespan tend to have lower incidence of cancer than short-lived species. This apparent paradox, known as Peto’s paradox [19, 20], suggests that large and long-lived species evolved more potent anti-cancer mechanisms to overcome the increased cancer risk. Multiple lines of evidence support this notion. It has long been known that mouse and rat cells are more susceptible to oncogenic transformation than human cells [21]. Cancer-related mortality can reach up to 90% in mice, which have a maximum lifespan (MLS) of 4 years [22]. In contrast, the similar-sized naked mole rat (Heterocephalus glaber), which has an MLS of 32 years, rarely develops cancer [23, 25, 26], furthermore naked mole rat cells are resistant to experimental tumorigenesis [27, 28]. Another long-lived rodent, the blind mole rat (Spalax sp.), with an MLS of 21 years, is extremely resistant to spontaneous cancer and chemical-induced tumorigenesis [29, 30]. The African elephant (Loxodonta africana), which is more than 200,000 times larger than a mouse and lives up to 65 years [16], has a strikingly low (4.81%) cancer mortality rate [31]. Recent studies focusing on cancer resistant species have revealed multiple novel anti-cancer mechanisms.
Inhibition of somatic telomerase activity
Telomeres are structures composed of long stretches of TTAGGG repeats at the ends of chromosomes protecting them from degradation and from being recognized as double strand breaks. Telomeres are maintained by a special reverse transcriptase, telomerase. Telomerase expression is restricted in human somatic cells leading to telomere shortening and replicative senescence.
Telomeres shorten with age in old animals, including humans. Shorter telomeres in old people (>60 years) are a prognosis for higher mortality [36]. It has also been reported that telomeres shorten more slowly in longer-lived birds and mammals [37, 38].
However, mere telomere length does not positively correlate with species MLS. For instance, mouse telomeres are 10 times longer than human telomeres. Studies by our group and others [39, 40] suggest that telomere length and expression of telomerase activity are shaped primarily by selection for more efficient anti-cancer mechanisms rather than longer lifespan. Replicative senescence due to shortened telomeres prevents infinite cell division that occurs during cancer progression.
Expansion of the TP53 gene
The African elephant is the largest land mammal and is relatively long-lived (MLS is 65 years) [16]. As expected, elephant cells have limited telomerase activity and undergo replicative senescence [40]. However, replicative senescence may be insufficient to counteract the increased cancer risk due to the extremely large size of the elephant. Furthermore, with only 4.81% cancer mortality, elephants are considerably cancer resistant compared to humans, whose cancer mortality reaches up to 20% [31, 41]. Evolutionary genomic studies showed that the African elephant genome encodes 20 copies of tumor suppressor gene TP53, which accounts for enhanced sensitivity of elephant cells to DNA damage and augmented apoptosis in response to DNA damaging agents [31, 42]. The expansion of TP53 gene family may be a critical anti-cancer and longevity mechanism for this species.
High-molecular-mass hyaluronan
The naked mole rat and the blind mole rat are two subterranean rodent species showing extreme longevity and cancer resistance [23, 29, 30]. Remarkably, both of these species are small, have active telomerase in somatic tissues and lack replicative senescence [39, 43]. Therefore, cancer resistance in these species relies on alternative, telomere independent mechanisms. Cancer resistance in naked mole rats is mediated by the secretion of high-molecular-mass hyaluronan, which triggers hyper-sensitivity of their cells to contact inhibition and prevents hyperplasia [28, 44]. Degrading hyaluronan or inhibiting hyaluronan synthesis renders the naked mole-rat cells susceptible to malignant transformation [44].
Concerted cell death
Although blind mole rat cells also secret high-molecular-mass hyaluronan, they do not show hypersensitivity to contact inhibition. Instead, blind mole rat cells utilize an interferon-mediated “concerted cell death” mechanism to prevent tumorigenic transformation when they undergo hyper-proliferation [29]. This anti-cancer mechanism in blind mole rats potentially compensates for the weak TP53 in this species, which may have evolved to prevent apoptosis in the extremely hypoxic environment these animals are exposed to [45, 46].
In summary, in addition to conserved mechanisms, long-lived species have evolved novel mechanisms of tumor-suppression which are unique for each species. As these anti-cancer mechanisms are either lacking or underdeveloped in humans, once adapted to human cells, these mechanisms hold tremendous potential for developing interventions for prevention and treatment of cancer in humans.
DNA repair machinery
Genomic instability is a hallmark of aging [47]. Genomic DNA undergoes constant insults from intrinsic and extrinsic agents, such as reactive oxygen species (ROS), replication errors, and genotoxic chemicals. To counteract deleterious effects of DNA-damaging agents, cells rely on versatile DNA repair mechanisms, which include base excision repair (BER), nucleotide excision repair (NER), DNA mismatch repair (MMR), and DNA double-strand break repair (DSBR) [48]. However, DNA repair machinery is not perfect, resulting in accumulation of unrepaired DNA damage, mutations and genomic rearrangements, which in turn may contribute to aging and cancer. In addition, DNA repair efficiency declines with aging and replicative senescence [49–51], which may lead to more rapid accumulation of mutations in aged animals [52, 53].
Long-lived organisms had been proposed to possess more efficient genome maintenance mechanisms [54]. The first analysis of NER involving a small number of species, found a strong correlation between NER efficiency and MLS [54], while later studies with larger numbers of species failed to find such a relationship [55, 56]. DNA double-strand break recognition was found to correlate with species MLS, which may be attributable to higher abundance of Ku80 in longer-lived species [57]. For BER, a comparative study across 15 species identified a significant correlation between the activity of BER enzymes Polβ and AP-endonuclease and body mass, but not lifespan [58]. The picture that emerges is that not all DNA repair pathways contribute equally to interspecies differences in MLS, with DNA double-strand break repair being the most critical for longevity. In addition to different DNA repair efficiencies, species may also differ in their response to DNA damage. It was proposed that longer-lived species might have stricter threshold for activation of cell death or cell cycle arrest upon DNA damage [59].
Upregulation of DNA repair genes
To achieve more efficient DNA repair, species may evolve more abundant and/or more efficient DNA repair enzymes. Comparison of liver transcriptomes between mice, naked mole rats, and humans found that long-lived species have upregulated DNA repair genes and DNA damage signaling pathways compared to shorter-lived species [60]. A transcriptomics study of fibroblasts from 16 mammalian species, which aimed to identify longevity-associated gene expression signatures, found that expression of several DNA repair genes positively correlated with species MLS [61]. Several other DNA repair genes were found to be upregulated in long-lived species in a transcriptome study of liver, kidney, and brain from 33 mammalian species [62]. It is worth noting that different long-lived species upregulate different DNA repair-related genes, suggesting that each time a longer-lived species evolve evolution takes a different path to enhanced DNA repair (Table 1). Strikingly, DSBR genes were identified in multiple independent studies, further strengthening the link between DSBR repair and longevity.
Table 1.
Upregulation and sequence alterations of DNA repair genes in long-lived species
| Mechanisms | Genes | Alterations | Species | Control species |
Refs |
|---|---|---|---|---|---|
| DNA damage response | TP53 | upregulation | Human and naked mole rat | Mouse | [60] |
| Chek1, Rif1 | upregulation | Long-lived bats and rodents | Short-lived counterparts | [61] | |
| Base excision repair | MBD4, MUTYH, NEIL1, NEIL2, TDG, POLL | upregulation | Human and naked mole rat | Mouse | [60] |
| Nucleotide excision repair | Ercc1 | upregulation | Long-lived bats and rodents | Short-lived counterparts | [61] |
| Mutations | Bowhead whale | 9 mammals | [63] | ||
| Ercc3 | Positive selection | Bowhead whale | Minke whale, cow, and dolphin | [63] | |
| DNA Mismatch repair | MSH3 | upregulation | Human and naked mole rat | Mouse | [60] |
| Msh6, Pms2 | upregulation | Long-lived bats and rodents | Short-lived counterparts | [61] | |
| DNA double strand break repair | NHEJ1, XRCC6, POLL | upregulation | Human and naked mole rat | Mouse | [60] |
| Pnkp, Rad51b, Prpf19, Slx4 | upregulation | Long-lived bats and rodents | Short-lived counterparts | [61] | |
| ATM, PRKDC, RAD50, KU80 | Positive selection | Myotis davidii, Pteropus alecto | 8 non-bat mammals | [64] | |
| Fanconi anemia DNA repair | Faap100, Fancg | upregulation | Long-lived bats and rodents | Short-lived counterparts | [61] |
Sequence alteration of DNA repair genes
Long-lived species may also evolve more efficient DNA repair enzymes. One way to evaluate whether a particular gene is under selection pressure is to measure the ratio of nonsynonymous/ synonymous mutations in the protein coding sequence. The ratio above one indicates that a gene is under positive selection. The bowhead whale (Balaena mysticetus) is the longest-lived mammal on earth, whose MLS reaches 211 years [16]. A comparative genomic analysis identified unique sequence changes in DNA repair genes, such as ERCC1, ERCC3, and PCNA, in the bowhead whale genome compared to other shorter-lived cetaceans [63]. An analysis of two bat genomes found multiple DNA repair and DNA damage signaling genes are under positive selection including ATM, PRKDC, RAD50, XRCC5, TP53, etc. [64]. Thus DNA repair genes are highly enriched among the positively selected genes in long-lived species, suggesting the importance of genome maintenance mechanisms for longevity. Several other genome maintenance genes including CEBPG, GTF2H2C, RPA4 and TINF2 were found to have increased copy number in naked mole rats or humans compared to shorter-lived mammalian species [65].
The finding that DNA repair genes are frequently under positive selection does not provide information on if or how the resulting changes improve the function of DNA repair proteins. It would be of great interest to determine which of the identified sequence variants contribute to better genome maintenance, especially when transferred to human cells. Furthermore, the type and frequency of somatic mutations can now be analyzed using single cell sequencing [66, 67]. Thus it will soon be possible to compare mutation rates across species. Based on the types of mutations one could determine which DNA repair pathways begin to fail with age and specifically target these pathways to ameliorate age-related genomic instability.
Insulin/IGF-1 signaling
The insulin/IGF-1 signaling (IIS) pathway was the first pathway shown to modulate organismal lifespan in model organisms [68]. Worms and flies have a single receptor for IIS, daf-2 in C. elegans and InR in Drosophila. Mutation of daf-2 doubles a worm’s lifespan [69], while Drosophila with mutant InR exhibit a dwarf phenotype and 85% lifespan extension [70]. Unlike worms and flies, mammals have evolved separate receptors, INSR and IGF1R, for insulin and IGF-1 signaling, respectively. Mice with adipose -specific knockout of INSR live 18% longer than wild type mice [2]. In addition, mice heterozygous for IGF1R knockout live 26% longer than the wild type mice [3]. Genetic polymorphism studies have identified genetic variations that are associated with extreme human longevity in centenarians, including FOXO3A [71], IGF1R [72], and GHR [73], suggesting that the IIS pathway may influence health and longevity in humans.
Whether insulin/IGF-1 signaling is perturbed in long-lived animal breeds or long-lived species has just begun to be elucidated (Fig. 1). A lifespan study of 31 genetically diverse inbred mouse strains with strikingly different median lifespans ranging from 251 to 964 days found a significant inverse correlation between the plasma IGF-1 level and median lifespan [74]. The association of IGF levels with body mass or cancer rates was not addressed in this study.
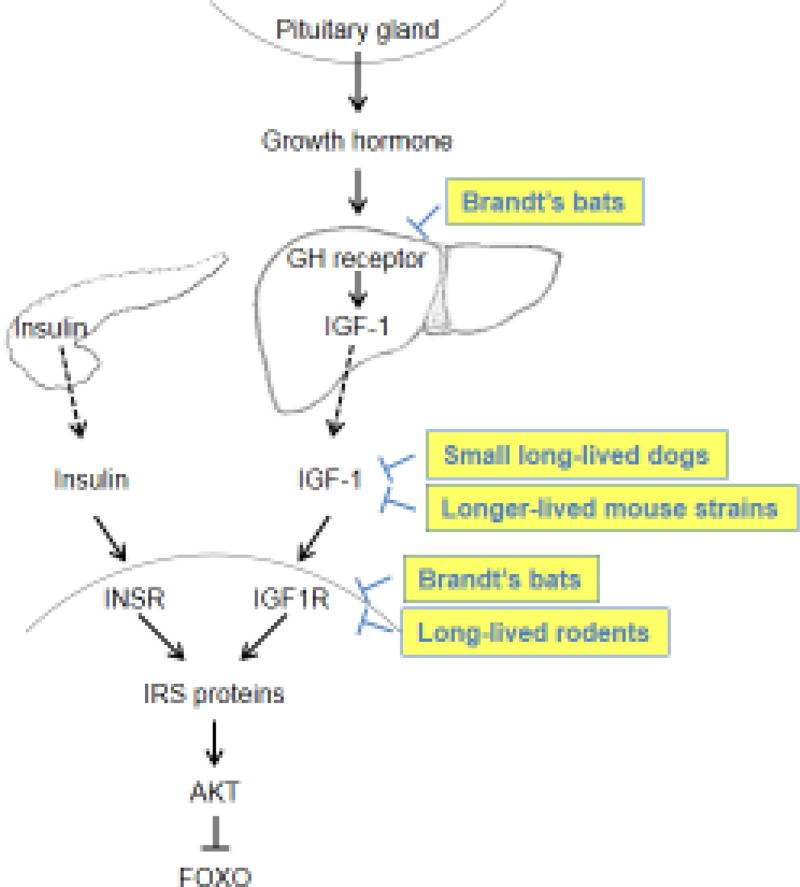
Fig. 1. Perturbation of insulin/IGF-1 signaling pathway in long-lived strains or species.
In mammals, IGF-1 is produced primarily in the liver in response to growth hormone secreted by the pituitary gland. IGF-1 mainly functions as an endocrine hormone by activating the IGF-1 receptor (IGF1R) in distant tissues. Insulin secreted by pancreas activates the insulin receptor (INSR). IGF1R and INSR activate similar downstream signaling pathways via insulin receptor substrate (IRS) proteins, which eventually inactivate FOXO transcription factors. Multiple mechanisms that perturb the insulin/IGF-1 signaling pathway have been identified in long-lived strains or species. For example, unique mutations in the growth hormone (GH) receptor and IGF1R are identified in Brandt’s bats, which downregulate insulin/IGF-1 signaling. Long-lived mouse strains and small dogs have reduced plasma IGF-1 levels compared to their counterparts. In addition, IGF1R expression levels in the brain negatively correlate with species maximum lifespan.
Domestic dogs vary greatly in body size between breeds, and larger body size is associated with a faster aging rate [75]. Several studies have linked the longer lifespans and smaller body size in long-lived dog breeds to reduced circulating IGF-1 levels [76–78]. Strikingly, a genomic study attributed the small size to a single IGF-1 single-nucleotide polymorphism (SNP) present in all small breeds of dogs [79].
The Brandt’s bat (Myotis brandtii), with an MLS of 41 years, is an exceptionally long-lived mammal relative to its small body mass (7g) [16, 80]. Whole-genome sequencing of Brandt’s bats and comparative genomic analysis of several other bat species have identified multiple mutations in the growth hormone receptor (GHR) and IGF1R genes in long-lived bats [80]. Importantly, several downstream genes in the IIS pathway in Brandt’s bats and GHR−/− mice have similarly altered expression compared to wild-type mice [80], which implies that the identified mutations in Brandt’s bats may perturb the signaling of the GH-IGF1 axis. However, considering the potential effect of GH-IGF1 signaling on both lifespan and body mass, the cause-and-effect of the downregulated insulin/IGF-1 signaling on bats’ longer lifespan, smaller body size, or both, needs further investigation. Furthermore, since it is unlikely that a single pathway fully explain the large lifespan differences between species, other longevity mechanisms such as alterations of DNA repair genes that were identified in long-lived bats (Table 1) may contribute to bats’ longevity.
Althougth downregulation of the IIS pathway is associated with longer lifespan within species, the exact role of this pathway in shaping interspecies differences in lifespan remains puzzling. Large body size is associated with longer lifespan across species [81], however downregulation of the IIS pathway results in smaller body size. A comparative study of 16 rodent species performed by our group provides some clues to the puzzle. We identified a significant negative correlation between the levels of IGF1R and MLS in the brain, but not in peripheral tissues [82]. Thus, lower IIS in the brain may be contributing to evolution of longer lifespan, while IIS in peripheral tissues does not, thus uncoupling the link between lower IIS and small body size.
Proteostasis network
Protein homeostasis (proteostasis) is maintained by the proteostasis network, which includes protein synthesis, chaperone-assisted protein folding, and proteolytic systems [83]. Proteostasis declines with age, which is largely attributable to the impaired chaperone response to stress [84, 85], and decreased proteasome and autophagy activities [86, 87]. Interventions that enhance proteostasis improve health or increase lifespan in model organisms including C. elegans [88], Drosophila [89, 90], and mice [9, 91].
Several lines of evidence indicate that long-lived species evolved more efficient proteostasis machinery. Naked mole-rat cells have up to 10-fold higher translational fidelity than mice [92]. This improved translational fidelity may result from a unique ribosomal structure where 28S ribosomal RNA is cleaved into two smaller fragments. Furthermore, translational fidelity correlates positively with MLS across multiple species of rodents [93]. More accurate protein synthesis may contribute to longevity of long-lived species by reducing the number of misfolded proteins that accumulate over time.
Naked mole rats express higher levels of chaperones than mice, such as HSP25 and HSP70 [94]. A genome analysis identified an expansion of HSP70 and HSP90 genes in the naked mole-rat genome [95]. Naked mole rats also have stronger proteasome activity than mice [96], which may be mediated by a cytosolic protein factor that prevents proteasome inhibition [97]. Autophagy activity in naked mole rats is also augmented [98, 99]. All these evidences suggest that naked mole rats evolved a more effective proteostasis network. Indeed, naked mole rats show no age-related increase of oxidative protein damage and maintain their high proteasome activity during aging [100]. In addition to naked mole rats, other long-lived mammals may also evolve more efficient proteostasis network. A comparative genomic analysis across 36 mammalian species identified accelerated evolution of proteasome-related genes specifically in lineages of longer-lived species [101]. Furthermore, proteostasis is likely to play an important role in long-lived bivalve mollusks including Arctica islandica (MLS>500 years) [102], suggesting a universal role of proteostasis in longevity across the animal kingdom.
Other lifespan-modulating mechanisms identified in model organisms
Manipulation of several conserved genes and pathways have been shown to extend lifespan in model organisms. These include Sirtuins, mTOR signaling pathway, AMP kinase (AMPK) pathway, cellular senescence, and pain receptor-mediated neuropeptide signaling. Further studies are needed to understand whether these pathways are differentially regulated in long-lived species. Not every perturbation that extends lifespan in a protected and pathogen free laboratory environment would be beneficial in the wild. Understanding which pathways extend lifespan without reducing fitness is important for development of anti-aging therapies.
Sirtuin protein family
Sirtuins are the mammalian homologs of yeast Sir2 (Silent Information Regulator 2). There are seven members (SIRT1–7) in this family, which are localized in different cellular compartments and are involved in multiple age-related processes [103]. Overexpression of SIRT6 extends lifespan in male mice [10]. Though whole-body SIRT1 overexpression in mice does not result in lifespan extension [104], overexpressing SIRT1 specifically in the brain extends lifespan in both male and female mice [11]. Considering the roles of sirtuins in regulating multiple age-related physiological functions [105, 106], stronger sirtuin activities could confer healthier metabolism, more efficient DNA repair, and more stable genome etc. It would be important to test whether Sirtuin levels or activity is enhanced in long-lived species of mammals.
mTOR signaling pathway
mTOR kinase is a nutrient and amino acid sensor that plays a key role in mediating the lifespan extending effect of dietary restriction. Inhibiting the mTOR pathway extends lifespan in all model organisms tested, including yeast [107], C. elegans [108, 109], Drosophila [110, 111], and mice [6, 7]. The exact mechanism by which downregulation of mTOR extends lifespan is not fully understood [112]. But inhibition of TOR activity is known to suppress protein translation, stimulate autophagy, and promote metabolic health [113]. Furthermore, mTOR integrates the upstream signals from important aging-related pathways including AMPK, IIS pathway, and PI3K signaling. Therefore, long-lived species could potentially modulate mTOR to promote healthy metabolism, proteostasis, stringent cell growth control, and lower cancer incidence. Considering the universal lifespan extending effect of mTOR inhibition in model organisms, its involvement in regulating mammalian lifespan between species deserves further investigation.
AMPK pathway
AMPK is an energy sensor that is activated during dietary restriction. AMPK maintains energy balance by inducing catabolic processes to produce energy and inhibiting anabolism such as protein synthesis via inhibiting mTOR. AMPK overexpression prolongs lifespan in C. elegans and Drosophila [114, 115]. Metformin, a biguanide drug that activates AMPK, when fed at a low dose (0.1% w/w in food), extends lifespan in male mice [8]. However, a higher dose (1% w/w in food) of metformin shortens lifespan, possibly due to off-target effects. AMPK is a master integrator of multiple signaling pathways known to promote longevity, including mTOR, sirtuins, FOXO transcription factors, and CREB-regulated transcriptional coactivators (CRTCs) etc. [116]. Therefore, fine-tuning AMPK activity could contribute to the evolution of longevity. However, whether AMPK signaling is modulated in long-lived species is unknown. Since long-lived species have unique metabolic signatures [117], it would be interesting to test the status of the AMPK signaling pathway.
Cellular senescence
Senescent cells accumulate with age [118, 119]. Cellular senescence can be induced by multiple intrinsic and extrinsic stressors, including oxidative stress, DNA damage, oncogene activation, epigenetic stress, mitotic spindle stress, etc. [120] Cellular senescence compromises tissue function, and impairs tissue regeneration potential when stem cells or progenitor cells are affected [121]. Moreover, senescent cells are characterized with a senescence-associated secretory phenotype (SASP), which leads to persistent inflammation [122]. Clearing senescent cells in wild-type adult mice improves their health and extends lifespan [12]. Antagonizing senescence could potentially contribute to longevity by improving the function of different organs, delaying the onset of tumors [12], and reducing chronic inflammation by attenuating SASP. Whether senescence response is attenuated in long-lived species is unknown. Long-lived species could potentially evolve mechanisms to reduce the deleterious effect of cellular senescence through 1) reducing the generation of senescent cells by augmenting maintenance mechanisms, such as DNA repair; 2) more efficient elimination of senescent or pre-senescent cells by immune-surveillance mechanisms; 3) counteracting the negative effects of cellular senescence by downstream protective mechanisms. As longevity evolved independently, different long-lived species may utilize distinct mechanisms to deal with the cellular senescence problem.
Pain and aging
Chronic pain is associated with increased mortality in humans [123]. A recent study showed that knockout of a pain receptor, TRPV1, extends lifespan in both male and female mice [14]. TRPV1 knockout decreases production of the neuropeptide CGRP, which de-represses insulin secretion and promotes metabolic health. Chronic pain is also associated with chronic inflammation, which is a hallmark and possibly a driver of aging. Interestingly, sensory neurons of the naked mole rat, naturally lack CGRP, which contributes to their pain insensitivity to high CO2-induced tissue acidosis [124, 125]. Naked mole rats live in deep borrows with low oxygen and high CO2 levels which was likely the reason they evolved this unique adaptation. However, the sensation of pain is critical for survival in the wild and it would be interesting to compare the pain thresholds and pain signaling pathways in a wider group of short- and long-lived species.
Concluding remarks
Mammalian radiation for the past 160 million years generated more than 5,000 species with an amazing diversity of lifespans. This is an amazing resource for understanding the mechanisms of longevity that is only beginning to be tapped into [126]. Molecular biology and genomics approaches have already identified several mechanisms that are differentially regulated in short- and long-lived species. These include enhanced tumor suppressor mechanisms, more efficient DNA repair, and perturbed insulin/IGF-1 signaling. As genomes and transcriptomes from a larger number of species become available, comparative genomics approaches will enable further insights into the mechanisms of longevity that evolved in nature.
Including long-lived species as research models in the future will be extremely important. Current model organisms have enabled researchers to identify conserved pathways of longevity. However, whether any of the mechanisms identified in model organisms, which are all very short-lived, promote longevity in humans is an open question. As human is a very long-lived species, these conserved pathways may be already optimized to the maximum leaving a limited window for improvement.
In each long-lived species MLS is determined by a set of conserved and species-specific longevity mechanisms (Figure 3). It is very important to understand which of the conserved mechanisms identified in model organisms also play a role in the evolution of longevity in the wild and do not reduce organismal fitness in a natural environment replete with pathogens and other environmental challenges.
Fig. 3. Evolution of longevity.
Mechanisms that determine lifespan in long-lived species are composed of species-specific and conserved mechanisms. Each long-lived species has evolved unique mechanisms of longevity, in addition the conserved pathways that control aging may be differentially regulated in long-lived species relative to the short-lived ones.
The species-specific longevity mechanisms evolved independently in each taxa to adapt to the species ecology and physiological requirements. For example, to counteract the increased cancer risk, different long-lived species evolved unique mechanisms, such as TP53 gene expansion in elephants, high-molecular-mass hyaluronan in naked mole rats, and concerted cell death in blind mole rats. As these mechanisms are missing in humans importing them from the long-lived species has a tremendous potential for improving human health and slowing down the rate of aging.
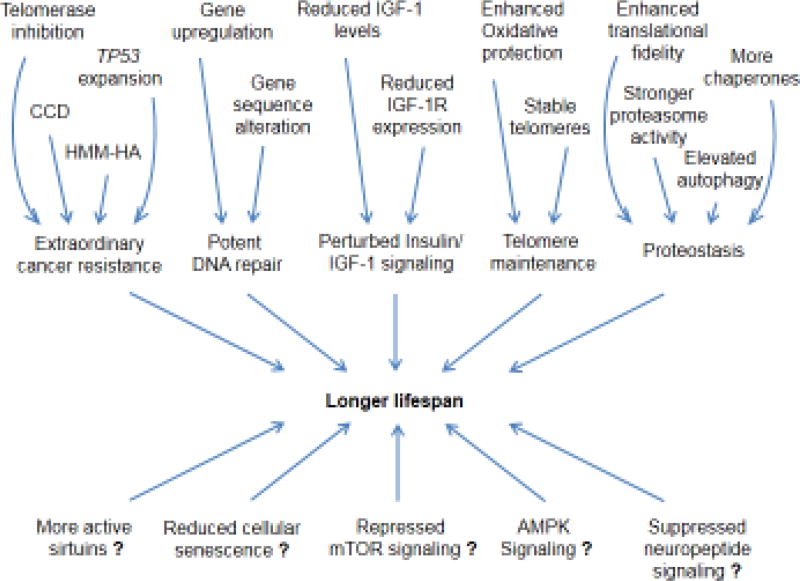
Fig. 2. Molecular mechanisms determining lifespan in mammalian species.
Multiple molecular mechanisms contributing to longer lifespans have been identified in long-lived mammalian species. Telomerase inhibition in large long-lived species (larger than 5–10 kg), TP53 expansion in elephants, concerted cell death (CCD) in blind mole rats, and high-molecular-mass hyaluronan (HMM-HA) in naked mole rats contribute to extraordinary cancer resistance. Strategies to enhance DNA repair capacity include upregulating the expression and altering the sequences of DNA repair genes in different species. Perturbed insulin/IGF-1 signaling due to reduced plasma IGF-1 and brain IGF1R expression or unique mutations in growth hormone receptor (GHR) and IGF1R were identified in some long-lived species. Long-lived species may protect telomeres by augmenting antioxidant response systems and upregulating shelterin proteins. Proteostasis in naked mole rats is enhanced by increasing translational fidelity, upregulating the expression of chaperones, and elevating autophagy and proteasome activity. Other longevity mechanisms identified in model organisms may contribute to longer lifespans of naturally long-lived species, which include more active sirtuins, reduced cellular senescence, repressed mTOR signaling, activated AMPK signaling and suppressed neuropeptide signaling.
Box 1. Evolution of telomere-maintenance mechanisms.
A prerequisite to replicative senescence is repression of telomerase activity in somatic tissues that causes telomere shortening. However, not all mammalian species possess replicative senescence mechanism. A comparative analysis of 15 rodent species revealed that telomerase activity is repressed in species with large body size [39]. As a result, cells isolated from large-bodied species undergo replicative senescence [43]. This anti-cancer mechanism appears to be utilized by almost all mammalian species with body mass larger than 5–10 kg [43, 126, 127]. Species with limited telomerase activity in the soma tend to have shorter telomeres, in the range of 8–12 kb, while small-bodies species express abundant telomerase in somatic cells and have longer telomeres. Negative correlation between telomerase activity and body mass was later confirmed in an analysis of more than 60 mammalian species [40]. Furthermore, it was found that telomere length is negatively correlated with MLS [40]. Thus selective pressures imposed by cancer and aging compete, with tumor suppression taking a more dominant role and favoring shorter telomere in long-live species.
Slower rate of telomere shortening observed in long-lived species [37, 38] may be explained by superior DNA repair machinery in these species (see below) that prevents oxidative damage to telomeres [128], rather than by more active telomerase. In addition, multiple genomic and transcriptomic studies identified positive selection or increased transcription or copy number of several telomere binding proteins in long-lived species (Table I), which may contribute to a more stable telomere structure and protect telomeres from damage.
Table I.
Alterations of shelterin proteins and telomere binding proteins in long-lived species
Trends.
It is important to understand molecular mechanisms responsible for longevity of naturally long-lived species to develop safe and effective anti-aging treatments and improve human health.
Novel anti-cancer mechanisms have been identified in several long-lived species including elephants, naked mole rats, and blind mole rats.
Long-lived species evolved more efficient DNA repair machinery by upregulating the expression or altering the sequences of DNA repair genes.
Long-lived species alter telomere binding proteins to protect telomeres.
Perturbation of the insulin/IGF-1 signaling pathway may contribute to longevity of long-lived mouse strains, small dog breeds, long-lived rodents and Brandt’s bats.
Naked mole rats improve translational fidelity, increase the expression of chaperones, and augment proteasome activity and autophagy to maintain proteostasis.
Outstanding Questions.
What are the anti-cancer mechanisms in other long-lived and/or large-sized species, such as the bowhead whale? Which of these species-specific anti-cancer mechanisms, when transferred to other species including humans, can decrease cancer incidence or promote longevity?
How to safely and efficiently strengthen the DNA repair machineries? Which of the identified DNA repair genes with either increased expression levels or altered sequences are responsible for increased genomic stability in long-lived species?
Multiple regulatory mechanisms are known to modulate lifespan in model organisms, such as the insulin/IGF-1 signaling, the mTOR pathway, and the AMPK pathway. To what extent do these mechanisms account for the huge variation of mammalian lifespans in natural species? How do long-lived species fine-tune these pathways? Can these mechanisms be further intervened in long-lived species without deleterious effects?
Considering that the large long-lived species have short telomeres and repressed telomerase activity, how they maintain telomere length is not fully understood. Do they eliminate senescent cells more efficiently? Do their stem cells renew aged cells more efficiently? How do their stem cells themselves maintain self-renew capacity to support longer lifespans?
How do other long-lived species maintain proteostasis? How can proteostasis be further improved in long-lived species?
How do humans regulate all the above-mentioned mechanisms? Given the fact that human is already a relatively long-lived species, is human lifespan or health span able to be extended? In other words, can we slow down the aging rate in humans?
Glossary
- Ataxia-telangiectasia mutated (ATM)
serine/threonine protein kinase that is activated by DNA double-strand breaks. ATM phosphorylates and activates multiple DNA repair genes.
- CEBPG
CCAAT/enhancer-binding protein gamma; transcription factor regulating CCAAT/enhancer-mediated transcription.
- Dietary restriction
reduction of food intake without causing malnutrition. Dietary restriction regimens used in experiments are typically reducing food intake by 20–50% of ad libitum consumption.
- ERCC1
excision repair cross-complementation group 1; nucleotide excision repair protein; forms a heterodimer with the XPF endonuclease.
- ERCC3
excision repair cross-complementation group 3; encodes a protein called XPB which functions in nucleotide excision repair.
- GTF2H2C
general transcription factor IIH, polypeptide 2C; transcription factor involved in nucleotide excision repair.
- Hyaluronan
also called hyaluronic acid, a nonsulfated glycosaminoglycan made of glucuronic acid and N-acetyl-glucosamine. It is an extracellular matrix component that regulates cell growth and cell migration.
- Interferon
a group of signaling proteins that are secreted by host cells to regulate the immune system. It is responsive to viruses, parasites, and cancer cells. There are three classes of interferons, i.e. alpha, beta, and gamma.
- Maximum lifespan
the maximum recorded lifespan of a given species that lives in a protected captive environment.
- PCNA
proliferating cell nuclear antigen; involved in DNA replication and DNA repair.
- PRKDC
protein kinase, DNA-activated, catalytic polypeptide; encodes a protein called DNA-PKcs; functions in DNA double-strand break repair and V(D)J recombination.
- RAD50
protein involved in DNA double-strand break repair.
- RPA4
replication protein A4; the 30-kDa subunit of RPA complex; involved in DNA double-strand break repair.
- Shelterin
protein complex that protects telomeres and regulates telomerase activity.
- Sirtuins
mammalian homologs of yeast Sir2 gene that is known to maintain silent heterochromatin. Sirtuins regulate multiple mechanisms related to aging and cancer.
- TINF2
TERF1 interacting nuclear factor 2; protein in the shelterin complex to protect telomere.
- XRCC5
X-Ray repair cross complementing 5; encodes a protein called Ku80; involved in DNA double-strand break repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontana L, et al. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluher M, et al. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 3.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 4.Coschigano KT, et al. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 5.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 6.Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Montalvo A, et al. Metformin improves healthspan and lifespan in mice. Nature communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyo JO, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nature communications. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanfi Y, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 11.Satoh A, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DJ, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riera CE, et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Ayyadevara S, et al. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 16.Tacutu R, et al. Human Ageing Genomic Resources: Integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res. 2013;41:D1027–D1033. doi: 10.1093/nar/gks1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeno Y, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeno Y, et al. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. The journals of gerontology. Series A, Biological sciences and medical sciences. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 19.Peto R, et al. Cancer and ageing in mice and men. British journal of cancer. 1975;32:411–426. doi: 10.1038/bjc.1975.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caulin AF, Maley CC. Peto's Paradox: evolution's prescription for cancer prevention. Trends in ecology & evolution. 2011;26:175–182. doi: 10.1016/j.tree.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn WC, et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipman R, et al. Genetic loci that influence cause of death in a heterogeneous mouse stock. J Gerontol a-Biol. 2004;59:977–983. doi: 10.1093/gerona/59.10.B977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 24.Delaney MA, et al. Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Veterinary pathology. 2013;50:607–621. doi: 10.1177/0300985812471543. [DOI] [PubMed] [Google Scholar]
- 25.Delaney MA, et al. Initial Case Reports of Cancer in Naked Mole-rats (Heterocephalus glaber) Vet Pathol. 2016;53:691–696. doi: 10.1177/0300985816630796. [DOI] [PubMed] [Google Scholar]
- 26.Taylor KR, et al. Four Cases of Spontaneous Neoplasia in the Naked Mole-Rat (Heterocephalus glaber), A Putative Cancer-Resistant Species. J Gerontol A Biol Sci Med Sci. 2017;72:38–43. doi: 10.1093/gerona/glw047. [DOI] [PubMed] [Google Scholar]
- 27.Liang S, et al. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9:626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seluanov A, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19352–19357. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorbunova V, et al. Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19392–19396. doi: 10.1073/pnas.1217211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manov I, et al. Pronounced cancer resistance in a subterranean rodent, the blind mole-rat, Spalax: in vivo and in vitro evidence. Bmc Biol. 2013;11:91. doi: 10.1186/1741-7007-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abegglen LM, et al. Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans. Jama. 2015;314:1850–1860. doi: 10.1001/jama.2015.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaziri H, et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrich U, et al. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 34.Hastie ND, et al. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 35.Coviello-McLaughlin GM, Prowse KR. Telomere length regulation during postnatal development and ageing in Mus spretus. Nucleic Acids Res. 1997;25:3051–3058. doi: 10.1093/nar/25.15.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cawthon RM, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 37.Haussmann MF, et al. Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp Gerontol. 2007;42:610–618. doi: 10.1016/j.exger.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Haussmann MF, et al. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proceedings. Biological sciences. 2003;270:1387–1392. doi: 10.1098/rspb.2003.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seluanov A, et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6:45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes NMV, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 42.Sulak M, et al. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife. 2016;5 doi: 10.7554/eLife.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seluanov A, et al. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7:813–823. doi: 10.1111/j.1474-9726.2008.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian X, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashur-Fabian O, et al. Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. Proc Natl Acad Sci U S A. 2004;101:12236–12241. doi: 10.1073/pnas.0404998101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avivi A, et al. p53--a key player in tumoral and evolutionary adaptation: a lesson from the Israeli blind subterranean mole rat. Cell Cycle. 2005;4:368–372. doi: 10.4161/cc.4.3.1534. [DOI] [PubMed] [Google Scholar]
- 47.Lepez-Otin C, et al. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 49.Seluanov A, et al. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7624–7629. doi: 10.1073/pnas.0400726101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao Z, et al. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11800–11805. doi: 10.1073/pnas.1200583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaidya A, et al. Knock-in reporter mice demonstrate that DNA repair by non-homologous end joining declines with age. PLoS genetics. 2014;10:e1004511. doi: 10.1371/journal.pgen.1004511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolle ME, et al. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997;17:431–434. doi: 10.1038/ng1297-431. [DOI] [PubMed] [Google Scholar]
- 53.Dolle ME, et al. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8403–8408. doi: 10.1073/pnas.97.15.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hart RW, Setlow RB. Correlation between Deoxyribonucleic-Acid Excision-Repair and Life-Span in a Number of Mammalian-Species. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato H, et al. Absence of Correlation between DNA-Repair in Ultraviolet Irradiated Mammalian-Cells and Life-Span of the Donor Species. Jpn J Genet. 1980;55:99–108. [Google Scholar]
- 56.Woodhead AD, et al. DNA repair and longevity in three species of cold-blooded vertebrates. Experimental gerontology. 1980;15:301–304. doi: 10.1016/0531-5565(80)90034-0. [DOI] [PubMed] [Google Scholar]
- 57.Lorenzini A, et al. Significant correlation of species longevity with DNA double strand break recognition but not with telomere length. Mech Ageing Dev. 2009;130:784–792. doi: 10.1016/j.mad.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Page MM, Stuart JA. Activities of DNA base excision repair enzymes in liver and brain correlate with body mass, but not lifespan. Age (Dordr) 2012;34:1195–1209. doi: 10.1007/s11357-011-9302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freitas AA, de Magalhaes JP. A review and appraisal of the DNA damage theory of ageing. Mutat Res. 2011;728:12–22. doi: 10.1016/j.mrrev.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 60.MacRae SL, et al. DNA repair in species with extreme lifespan differences. Aging. 2015;7:1171–1184. doi: 10.18632/aging.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma S, et al. Cell culture-based profiling across mammals reveals DNA repair and metabolism as determinants of species longevity. eLife. 2016;5 doi: 10.7554/eLife.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fushan AA, et al. Gene expression defines natural changes in mammalian lifespan. Aging Cell. 2015;14:352–365. doi: 10.1111/acel.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keane M, et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 2015;10:112–122. doi: 10.1016/j.celrep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang G, et al. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 2013;339:456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacRae SL, et al. Comparative analysis of genome maintenance genes in naked mole rat, mouse, and human. Aging Cell. 2015;14:288–291. doi: 10.1111/acel.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gundry M, et al. Direct, genome-wide assessment of DNA mutations in single cells. Nucleic Acids Res. 2012;40:2032–2040. doi: 10.1093/nar/gkr949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong X, et al. Accurate identification of single-nucleotide variants in whole-genome-amplified single cells. Nature methods. 2017 doi: 10.1038/nmeth.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Kenyon C, et al. A C-Elegans Mutant That Lives Twice as Long as Wild-Type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 70.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 71.Flachsbart F, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ben-Avraham D, et al. The GH receptor exon 3 deletion is a marker of male-specific exceptional longevity associated with increased GH sensitivity and taller stature. Science advances. 2017;3:e1602025. doi: 10.1126/sciadv.1602025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan R, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraus C, et al. The size-life span trade-off decomposed: why large dogs die young. The American naturalist. 2013;181:492–505. doi: 10.1086/669665. [DOI] [PubMed] [Google Scholar]
- 76.Eigenmann JE, et al. Body size parallels insulin-like growth factor I levels but not growth hormone secretory capacity. Acta endocrinologica. 1984;106:448–453. doi: 10.1530/acta.0.1060448. [DOI] [PubMed] [Google Scholar]
- 77.Eigenmann JE, et al. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta endocrinologica. 1988;118:105–108. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- 78.Greer KA, et al. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr) 2011;33:475–483. doi: 10.1007/s11357-010-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sutter NB, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seim I, et al. Genome analysis reveals insights into physiology and longevity of the Brandt's bat Myotis brandtii. Nature communications. 2013;4:2212. doi: 10.1038/ncomms3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J Gerontol. 1991;46:B47–53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- 82.Azpurua J, et al. IGF1R levels in the brain negatively correlate with longevity in 16 rodent species. Aging. 2013;5:304–314. doi: 10.18632/aging.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powers ET, et al. Biological and chemical approaches to diseases of proteostasis deficiency. Annual review of biochemistry. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 84.Calderwood SK, et al. The shock of aging: molecular chaperones and the heat shock response in longevity and aging--a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tower J. Hsps and aging. Trends in endocrinology and metabolism: TEM. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaushik S, Cuervo AM. Proteostasis and aging. Nature medicine. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 87.Rubinsztein DC, et al. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 88.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 89.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simonsen A, et al. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 91.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nature medicine. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azpurua J, et al. Naked mole-rat has increased translational fidelity compared with the mouse, as well as a unique 28S ribosomal RNA cleavage. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17350–17355. doi: 10.1073/pnas.1313473110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ke Z, et al. Translation fidelity co-evolves with longevity. Aging Cell. 2017 doi: 10.1111/acel.12628. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodriguez K, Buffenstein R. High levels of the small chaperone HSP25 in naked mole-rats may be a determinant of rodent longevity. Faseb Journal. 2014;28 [Google Scholar]
- 95.Yang Z, et al. Investigation of anti-cancer mechanisms by comparative analysis of naked mole rat and rat. Bmc Syst Biol. 2013;7(Suppl 2):S5. doi: 10.1186/1752-0509-7-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodriguez KA, et al. Altered composition of liver proteasome assemblies contributes to enhanced proteasome activity in the exceptionally long-lived naked mole-rat. PloS one. 2012;7:e35890. doi: 10.1371/journal.pone.0035890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodriguez KA, et al. A cytosolic protein factor from the naked mole-rat activates proteasomes of other species and protects these from inhibition. Bba-Mol Basis Dis. 2014;1842:2060–2072. doi: 10.1016/j.bbadis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao S, et al. High autophagy in the naked mole rat may play a significant role in maintaining good health. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;33:321–332. doi: 10.1159/000356672. [DOI] [PubMed] [Google Scholar]
- 99.Pride H, et al. Long-lived species have improved proteostasis compared to phylogenetically-related shorter-lived species. Biochemical and biophysical research communications. 2015;457:669–675. doi: 10.1016/j.bbrc.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 100.Perez VI, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, de Magalhaes JP. Accelerated protein evolution analysis reveals genes and pathways associated with the evolution of mammalian longevity. Age (Dordr) 2013;35:301–314. doi: 10.1007/s11357-011-9361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Treaster SB, et al. Superior proteome stability in the longest lived animal. Age (Dordr) 2014;36:9597. doi: 10.1007/s11357-013-9597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finkel T, et al. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herranz D, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nature communications. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends in endocrinology and metabolism: TEM. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends in biochemical sciences. 2014;39:72–81. doi: 10.1016/j.tibs.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 108.Jia K, et al. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 109.Vellai T, et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 110.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bjedov I, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell metabolism. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wullschleger S, et al. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 114.Apfeld J, et al. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes & development. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stenesen D, et al. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell metabolism. 2013;17:101–112. doi: 10.1016/j.cmet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burkewitz K, et al. AMPK at the nexus of energetics and aging. Cell metabolism. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma S, et al. Organization of the Mammalian Metabolome according to Organ Function, Lineage Specialization, and Longevity. Cell metabolism. 2015;22:332–343. doi: 10.1016/j.cmet.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herbig U, et al. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 119.Wang C, et al. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 120.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collado M, et al. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torrance N, et al. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain. 2010;14:380–386. doi: 10.1016/j.ejpain.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Park TJ, et al. Somatosensory organization and behavior in naked mole-rats: II. Peripheral structures, innervation, and selective lack of neuropeptides associated with thermoregulation and pain. The Journal of comparative neurology. 2003;465:104–120. doi: 10.1002/cne.10824. [DOI] [PubMed] [Google Scholar]
- 125.Park TJ, et al. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber) PLoS Biol. 2008;6:e13. doi: 10.1371/journal.pbio.0060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gorbunova V, et al. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat Rev Genet. 2014;15:531–540. doi: 10.1038/nrg3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gorbunova V, Seluanov A. Coevolution of telomerase activity and body mass in mammals: from mice to beavers. Mech Ageing Dev. 2009;130:3–9. doi: 10.1016/j.mad.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 129.Kim EB, et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479:223–227. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]