Abstract
Purpose of review
Distinction between discrete subvalvar aortic stenosis and other causes of left ventricular outflow obstruction has important implications for predicting natural history and guiding the timing and type of intervention. Imaging, primarily transthoracic echocardiography, plays a pivotal role in the diagnosis and management of adults with subvalvar stenosis.
Recent findings
The majority of systematic research on imaging of subvalvar aortic stenosis has focused on echocardiography. Transthoracic echocardiography, especially two-dimensional imaging with color and spectral Doppler, remains the main modality for delineation of the anatomic and hemodynamic features of subvalvar stenosis, associated anomalies, and involvement of accessory mitral valve attachments to the subaortic septum or abnormally placed papillary muscles. Transesophageal echocardiography may provide a more detailed definition of left ventricular outflow tract anatomy, including the presence and extension of the obstructive subaortic fibroelastic tissue onto the aortic or mitral valve, especially in patients with poor transthoracic windows. The clinical role for advanced imaging technologies, including 3-dimensional echocardiography, cardiac magnetic resonance, and computed tomography, is evolving but, largely because of the adequacy of established imaging with transthoracic echocardiography, remains relatively limited.
Summary
In the absence of other congenital heart defects or alternative indications (e.g., coronary angiography), transthoracic echocardiography is usually adequate for assessment of discrete subvalvar aortic stenosis in the adult. In specific clinical situations, supplemental imaging modalities can play an integral role in clinical decision making.
Keywords: subaortic stenosis, echocardiography, congenital heart disease, left ventricular outflow obstruction
Introduction
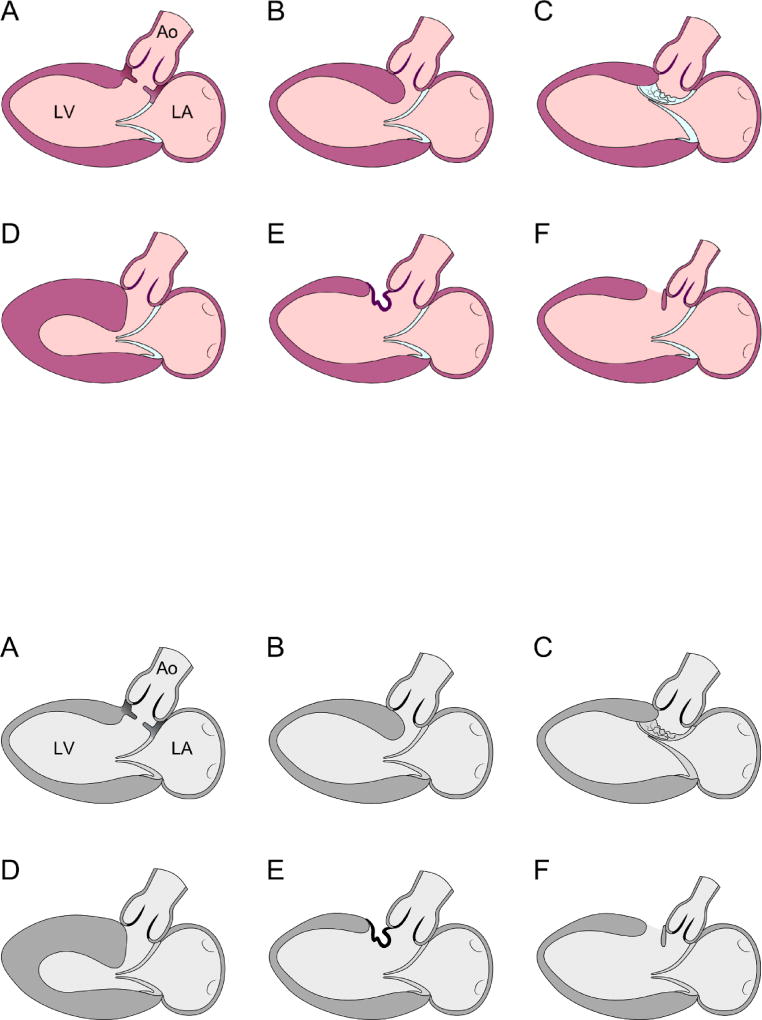
Left ventricular outflow tract (LVOT) obstruction can result from an array of causes and may be due to several distinct anatomic abnormalities (Figure 1).1 A common acquired cause of LVOT obstruction in adults is hypertrophic cardiomyopathy in the context of ventricular septal hypertrophy, often with systolic anterior motion of the mitral valve and abnormally positioned papillary muscles. A second form of LVOT obstruction referred to as “tunnel-type,” relates to long-segment narrowing due to the presence of abnormal muscle tissue in the outflow tract. A third form results from posterior deviation of the infundibular septum, often with either type B interrupted aortic arch, or a ventricular septal defect (VSD) and aortic coarctation. This paper will review imaging of discrete subaortic stenosis (DSS), a fourth distinct type of LVOT obstruction. The anatomy of DSS is importantly variable, as discussed below, but broadly involves a discrete ridge of fibroelastic tissue protruding from the ventricular septum into the outflow tract with variable involvement of the aortic and mitral valves.
FIGURE 1.
Diagrams representing parasternal long axis transthoracic echocardiogram images depicting various anatomic types of subaortic stenosis. The figure is modified from Freedom RM. The natural and modified history of congenital heart disease. Elmsford, N.Y.: Blackwell Pub./Futura; 2004. Page 175. Figure 14C-1,1 used with permission.
A: Discrete fibrous membrane
B: Fibromuscular tunnel
C: Mitral valve tissue or its tension apparatus attached to the septum
D: Hypertrophic obstructive cardiomyopathy
E. Tissue derived from the membranous septum or tricuspid valve herniating into the LVOT through a VSD
F. Posterior displacement of the infundibular septum.
Ao: aorta; LA: left atrium; LV: left ventricle.
Transthoracic echocardiography (TTE) is the dominant modality used for imaging of DSS. While other modalities have important niche roles, TTE is sufficient for the diagnosis and delineation of anatomy and physiology in a large majority of cases. Further, there is more systematic data on the use of TTE in DSS than for alternative imaging approaches. Therefore, we will focus mainly on TTE with a brief comment on the role of supplemental modalities in the management of DSS.
Epidemiology and Mechanisms
DSS is commonly considered under the rubric of congenital heart defects, though, akin to double chamber right ventricle, DSS is more accurately considered neither as clearly congenital nor independently acquired. Hemodynamically severe DSS is rarely documented in the fetus or soon after birth. However, identifiable structural abnormalities present at birth, namely elongated mitral-aortic intervalvular fibrosa and steep aortoseptal angle, are known to predispose to its development. While DSS can present as an isolated lesion, it is also frequently associated with other congenital heart defects.
Patients with DSS often have additional left-sided obstructive lesions. Shone complex, as initially described, involves the presence of either all or a subset of two or more of four components: subaortic stenosis, supravalvar mitral ring (also referred to as an annulo-leaflet mitral ring), parachute mitral valve, and aortic coarctation. The eponym is used inconsistently, but all eight of the patients in that initial report had both subaortic stenosis and a supravalvar mitral ring, while the other components were variably present.2
Membranous VSD is commonly associated with DSS (~32%). Double chamber right ventricle, frequently associated with a VSD, is also relatively common among patients with DSS (~9%); this may indicate that such patients are prone to excessive cardiac cellular or fibrotic proliferation in the setting of high shear stress (see below for further discussion). Atrioventricular canal defects are also associated with an increased risk for LVOT obstruction and DSS may be secondary to displacement of the aortic valve in relation to the atrioventricular valves and elongation of the outflow tract, as well as aberrant chordal attachments.
Anatomy
Isolated DSS rarely presents in infancy and often progresses over time. However, there are specific differences in LVOT morphometry and geometry between patients who do and do not develop DSS. Patients with DSS tend to have a greater degree of aortic override over the ventricular septum, a large distance between the hinge point of the non-coronary aortic valve leaflet to the hinge point of the anterior mitral valve leaflet, and a more acute aortoseptal angle.3–8 Experimental studies suggest these abnormalities, especially the relatively acute aortoseptal angle, are associated with abnormal ventricular septal shear stress.9 Presumably, the presence of increased shear stress acts as a stimulus to cellular proliferation, resulting in growth of subaortic tissue. However, not all patients with predisposing aortoseptal angle develop DSS, suggesting a multi-hit mechanism where LVOT geometry interacts with predisposing genetic, developmental, or anatomic and cellular features. The presence of a VSD, for example, further increases septal shear stress and is a known risk factor for development of DSS. Shorter distance between the VSD and aortic annulus is associated with greater increase in shear stress.9 There is also pathologic evidence that persistence and hypertrophy of an accessory anterolateral papillary muscle in the LVOT (sometimes referred to as the “muscle of Moulaert”) may play a role in development of subaortic stenosis in a subset of cases.10
Imaging
The goals of imaging in a patient with subaortic stenosis are to:
Identify the level(s) of LVOT obstruction and distinguish between anatomic types of subaortic stenosis
Define degree of obstruction
Understand relationship of the obstructive subaortic tissue to key cardiac structures
Identify associated lesions and adverse sequelae (e.g., aortic regurgitation)
Identify the level of LVOT obstruction and distinguish between types of subaortic stenosis
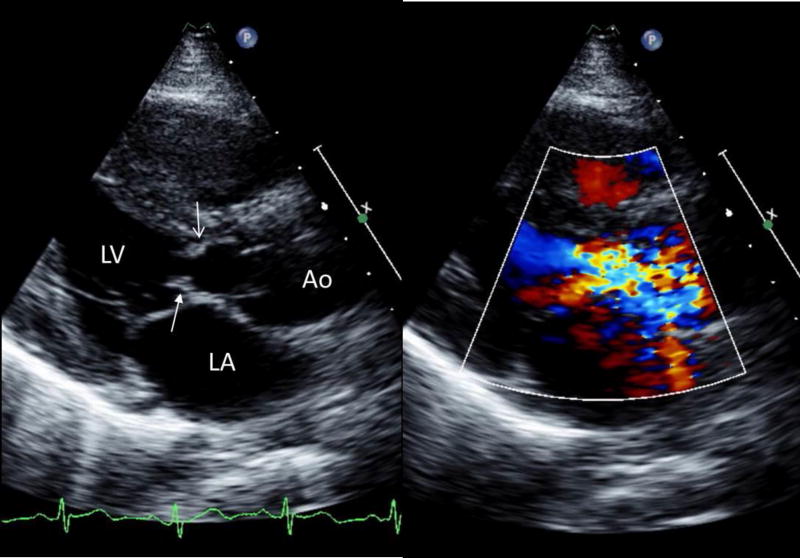
The presence of LVOT obstruction is largely based on evidence of hemodynamic impact (i.e., a pressure gradient); the severity of obstruction is likewise defined by the magnitude of the pressure gradient. As such, spectral Doppler, as described below, remains the most dependable noninvasive method to demonstrate and grade the severity of LVOT obstruction. The appearance of flow acceleration with turbulence below (proximal to) the level of the aortic valve provides further support for the presence of subaortic obstruction (Figure 2). The shape of the Doppler envelope, or response to physical maneuvers, can further hint at the underlying pathophysiology (e.g., fixed versus dynamic obstruction),11 but 2-dimensional echocardiography provides insight into the anatomy and structural basis for obstruction.
FIGURE 2.
Parasternal long-axis transthoracic echocardiographic images during systole, 2-dimensional (left) showing a discrete subaortic obstruction (top arrow, open head) approximately 1cm below the aortic valve. There is extension onto the anterior mitral leaflet (arrow with solid head). The addition of color Doppler (right) demonstrates flow acceleration with turbulence beginning at the level of the subaortic ridge.
Ao: aorta; LA: left atrium; LV: left ventricle.
It is key to determine whether obstruction is isolated to the subaortic region or involves multiple levels, as this has implications for surgical planning and the validity of spectral Doppler estimates. Bicommissural aortic valve and other congenital aortic valve abnormalities are often seen in conjunction with DSS, resulting in concomitant valvar stenosis. It is also important in these patients to identify more proximal obstruction due to septal hypertrophy or abnormal mitral valve attachments. The distinction between DSS due to a fibrous ridge and tunnel-type subaortic stenosis is not always easily delineated on preoperative imaging and may impact the extent of resection necessary to adequately relieve the obstruction. Fortunately, that pathologic distinction is less important for surgical planning and counseling than distinguishing these diagnoses from hypertrophic cardiomyopathy or aortic valve stenosis, and defining associated anatomic features (e.g., abnormal mitral valve attachments).
While careful 2-dimensional imaging is usually sufficient, 3-dimensional TTE may be useful in select cases to better understand the anatomy of subaortic stenosis.12 There has been little systematic investigation of this technology, however, and the current lower spatial and temporal resolution of 3D TTE imaging may limit its usefulness. One case report demonstrated the potential role of 3D transesophageal echocardiography (TEE) to use planimetry to better define the contributions of DSS and valvar aortic stenosis in the context of multiple lesions when Doppler methods are invalid.13, 14
Define degree of obstruction
Continuous wave Doppler measurement of maximal instantaneous and mean systolic gradients from the apical and suprasternal notch views, using the modified Bernoulli equation, provides an estimate of the severity of the LVOT obstruction. While evidence to support recommendations is sparse in adults, guideline documents have suggested a maximal instantaneous gradient of ≥50 mm Hg or mean gradient of ≥30 mm Hg constitutes an indication for surgical repair even in the absence of symptoms or adverse sequelae, such as aortic regurgitation.15 Therefore, understanding the limitations of echocardiographic estimation of pressure gradients is essential to prevent potentially unnecessary intervention. For patients with an isolated DSS, the assumptions of the modified simplified Bernoulli are reasonably met. However, there are several limitations to this approach that require note. First, the Bernoulli equation is not appropriate in the setting of sequential or long-segment stenoses. Second, as the equation assumes negligible proximal velocity, it would be more accurate to correct the peak instantaneous velocity for flow velocity proximal to the obstruction in settings of high output. Fourth, interrogation of the systolic flow jet through the LVOT may be contaminated by other flow jets, most commonly related to a VSD or mitral regurgitation. The mitral regurgitant jet velocity will always be higher than the LVOT velocity (unless the mitral jet velocity is underestimated because of an incomplete spectral envelope or suboptimal angle of interrogation).
Identify associated lesions and adverse sequelae
As noted above, DSS is often associated with additional congenital or acquired heart disease. Sequential LVOT obstruction is not infrequent, and it is important to determine whether stenosis is present only at the subaortic level or also at the aortic valve.16 Left ventricular hypertrophy, particularly basal septal hypertrophy, often coexist and can contribute to LVOT obstruction (Figure 3). VSD, aortic regurgitation, and double-chamber right ventricle are readily identified by transthoracic echocardiography as long as there is a high index of suspicion. Quantification of these defects and their hemodynamic burden, however, is sometimes better defined by other modalities, particularly cardiac magnetic resonance (MR). For example, some standard TTE criteria for grading aortic regurgitation severity may be misleading if the LVOT anatomy is such that there is obstruction to flow from the distal LVOT to LV body during diastole. While usually identified with post-operative TEE, TTE is also able to identify postoperative complications of DSS resection, including residual or de novo aortic regurgitation, mitral regurgitation, residual or recurrent subaortic obstruction, or iatrogenic VSD.
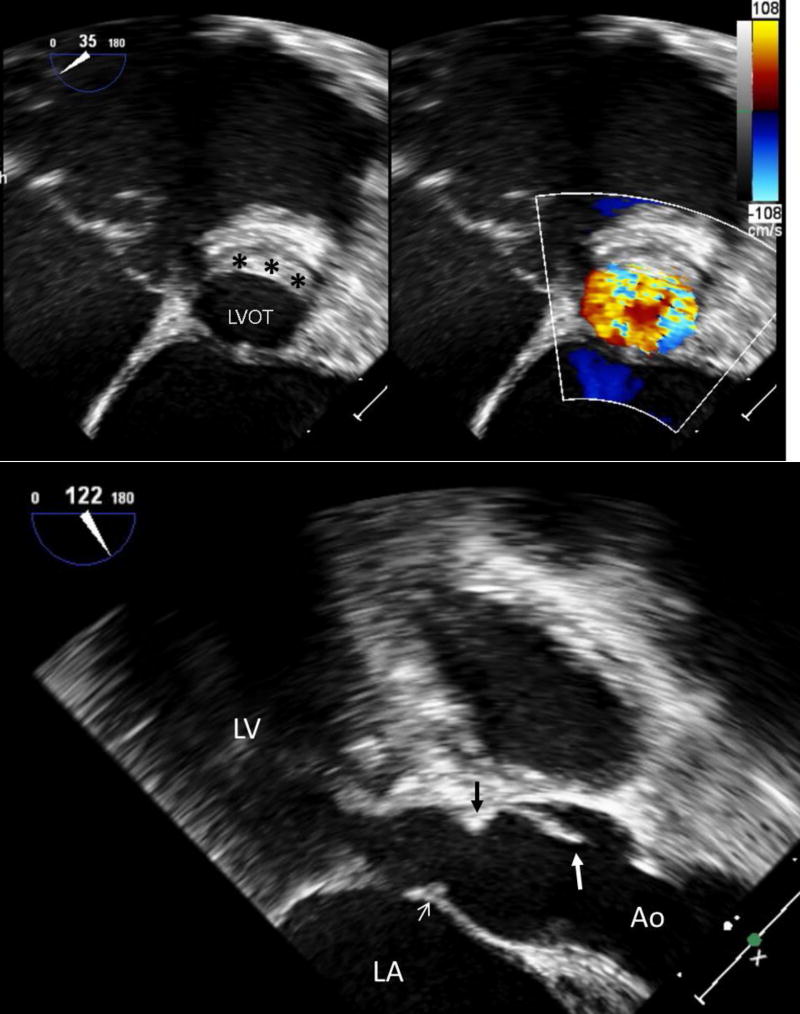
FIGURE 3.
Transesophageal echocardiography performed directly prior to surgical repair of discrete subaortic stenosis. In this example, the obstructive ridge inserts directly below the aortic valve and extends onto the anterior mitral valve.
A (Top): Long-axis view. The large white arrow identifies the aortic valve cusp, while the black arrow identifies the subaortic ridge directly below the insertion of the aortic valve. The small white arrow indicates extension of the obstructive tissue onto the anterior mitral valve leaflet. There is also hypertrophy of the basal ventricular septum proximal to the discrete subaortic membrane.
B (Bottom): Short-axis view. The black asterisks designate the subaortic ridge in cross-section.
Ao: aorta; LA: left atrium; LV: left ventricle; LVOT: left ventricular outflow tract
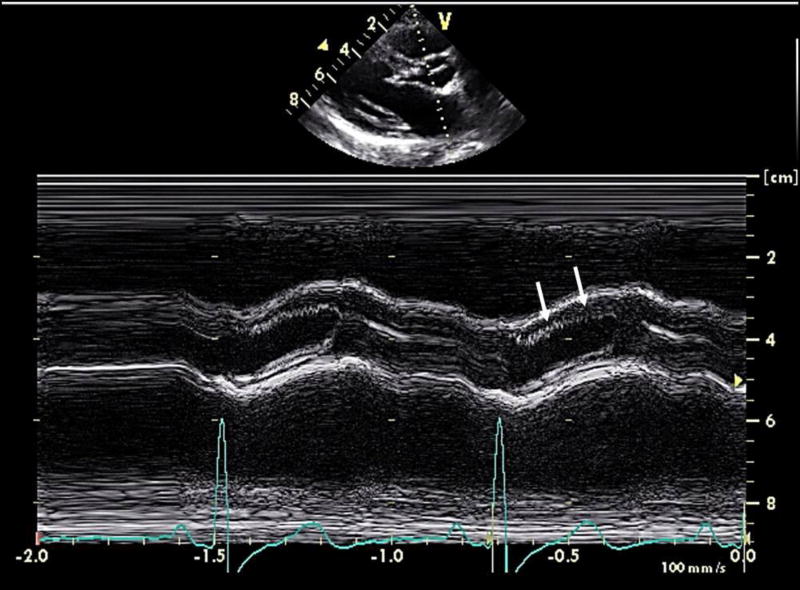
High-frequency oscillation (“fluttering”) of the aortic valve leaflet on M-mode echocardiography is a classic finding with DSS (Figure 4), even in mild cases without hemodynamically significant LVOT obstruction.17 While not of actionable clinical relevance, this observation illustrates one hemodynamic impact of DSS, increased turbulence of flow across the aortic valve. This abnormal flow has potentially adverse consequences in part independent of the degree of stenosis, including development of aortic regurgitation and an increased risk for subacute bacterial endocarditis.18,19 Aortic regurgitation in DSS also develops as a consequence of growth of the obstructive fibroelastic tissue onto the ventricular surface of the aortic valve leaflets, causing restriction of leaflet motion and valve incompetence.
FIGURE 4.
M-Mode echocardiography just above the aortic annulus in the parasternal long axis view in a patient with subaortic stenosis. There are high-frequency oscillations of the right coronary aortic cusp (white arrows, second beat; first beat not annotated).
The presence of progressive aortic regurgitation and either left ventricular dilation or dysfunction (left ventricular end-systolic dimension ≥50 mm or ejection fraction <55%) is also considered indications for intervention. There has been ongoing debate if proactive intervention for DSS should be considered in the presence of mild aortic regurgitation to prevent progressive aortic valve degeneration (and avoid the need for valve replacement); at present, the consensus is that this would not constitute a reason to intervene.15
Given the frequent co-occurrence of DSS and membranous VSDs, attention should be paid to ruling out the latter, which may be subtle if flow is restricted by tricuspid valve attachments.20 A membranous VSD may also result in aortic regurgitation unrelated to the DSS as a result of prolapse of the aortic valve leaflet into the defect. Finally, the creation of an iatrogenic VSD is a rare complication of membrane resection and myomectomy, a complication that can be detected by intraoperative TEE allowing for prompt repair. 21
Understand relationship of stenosis to key cardiac structures
Determination of distance of the subaortic ridge from the aortic valve, typically measured on TTE from the parasternal long-axis view in end-diastole from the septal attachment of the ridge to the hinge point of the right coronary leaflet, is an important element of the anatomic assessment due to its prognostic implications for rate of progression of obstruction and risk of reoperation (further discussed below).3 The membrane may also extend circumferentially to adhere to the anterior mitral leaflet and accessory attachments of the mitral valve to the ventricular septum are a potential additional source of obstruction. 3-D TTE may aid in defining the morphologic details of the subaortic obstruction and in evaluating the mitral valve apparatus.12 Subaortic stenosis may also develop following repair of complex congenital heart disease in which patch material is used to baffle the left ventricle to the aorta via a VSD, such as in a Rastelli-type repair, with resultant development of subaortic obstruction related to turbulent flow across a tortuous outflow tract and fibrous growth on the patch material. In such cases, supplemental modalities, such as cardiac MR, may aid in anatomic assessment and operative planning.
The risk of progression, recurrence, and survival
DSS is a variably progressive disease. Bezold and colleagues derived a model to predict the progression of DSS stenosis in children, with progression defined as those who underwent intervention or for whom there was an intention to intervene with a peak instantaneous gradient of >40 mm Hg. The 3 independent predictors of progression were: higher initial gradient, involvement of the anterior mitral leaflet, and distance between the DSS and aortic valve indexed to body surface area. Interestingly, in univariate modeling the presence of associated congenital lesions was associated with a lower probability of progression. These findings were robust on validation in an independent sample at a separate institution. Of note, neither the aortoseptal angle nor the mitral-to-aortic valve separation distance nor the severity of aortic override were reported to be associated with progressive disease.22 This suggests that factors predisposing to development of DSS may differ from those that favor hemodynamic progression.
Despite adequate surgical relief of obstruction, recurrence of DSS requiring reoperation occurs relatively frequently with contemporary series estimating 19–25%.23–25 In a multicenter study of adults with prior operation for DSS, van der Linde and colleagues report a reoperation rate of 1.8% per patient-year.23 Female sex and progression of LVOT gradient over time were the strongest independent predictors of reoperation. In a pediatric long-term follow-up study, predictors of reoperation included younger age of initial resection, preoperative gradient ≥60 mm Hg, peeling of the membrane off the aortic or mitral valve, distance of the membrane to the aortic valve <7 mm, and concomitant valvar aortic stenosis.5 While the risk of reoperation appears to plateau after 10 years in the pediatric series, van der Linde et al. demonstrate a steady decline in intervention-free survival over 20 years of postoperative follow-up. Overall survival of adults with DSS without associated congenital lesions or valvar aortic stenosis is excellent and equivalent to that of the age-matched population. 24
Imaging Modalities Beyond Echocardiography
Echocardiography is usually sufficient to define the anatomy and hemodynamic pathophysiology of DSS. However, adjunct imaging approaches are useful in specific situations, namely cardiac magnetic resonance (CMR), computed tomography (CT), and cine-angiography. CMR is useful when echocardiographic imaging is of inadequate quality or when more precise quantification of flow or chamber volumes or mass is necessary for clinical decision-making. Defining the severity of aortic regurgitation is probably the most common indication for CMR in the context of isolated DSS. As above, TTE estimation of regurgitation severity is challenging, and standard criteria may be misleading in the context of a subaortic chamber partly separated from the main left ventricular cavity. The more precise measurements of left ventricular volumes and mass provided by CMR presumably provide some degree of incremental information, but clinical decisions rarely rest upon demonstration of subtle dilation or hypertrophy. CMR can provide better definition of LVOT geometry and DSS morphology; this may have some benefit in surgical planning and patient counseling, though the ultimate surgical approach will depend on direct inspection and intraoperative TEE. While flow velocities can also be measured by phase contrast MR imaging, this is rarely necessary. Except in patients with extraordinarily poor acoustic windows, TTE continuous wave Doppler can identify the peak velocity across the LVOT and is preferable to CMR for this purpose. Likewise, cardiac MR is often critically important in the setting of more complex congenital heart disease, but that utility is usually not related directly to the DSS. Cardiac CT is rarely indicated for evaluation of DSS unless CMR is contraindicated. Cardiac CT provides precise estimates of ventricular volumes and mass, as well as LVOT geometry. However, neither the severity of aortic regurgitation nor the flow acceleration across the DSS can be measured. Likewise, catheter-based ventriculography plays a supporting role in the contemporary evaluation of DSS. Specifically, invasive hemodynamic evaluation and ventriculography are generally the approach of last resort when echocardiography (and other imaging) is either of inadequate quality or unable to delineate the level of obstruction. Either cardiac CT or catheterization is indicated in adults with risk factors for coronary artery disease as part of preoperative planning, though this evaluation is distinct from the assessment of DSS itself.
Conclusion
Imaging plays a central role in the evaluation of discrete subvalvar aortic stenosis, to distinguish from other or coexisting causes of LVOT obstruction, and to direct the timing and type of intervention. Transthoracic and transesophageal echocardiography remains the main tool for imaging these patients, while recent advances in 3D echocardiography, CT, and MR imaging have valuable roles in specific situations.
Acknowledgments
We are deeply grateful to Emily Harris for her expert medical illustration work in adapting Figure 1.
Financial support and sponsorship: ARO is supported by the Dunlevie Family Fund and receives research grant funding from Actelion and Roche Diagnostics. SSP is supported by NIH/NHLBI T32 HL007572-32. TG is supported by the Farb Family Fund.
Abbreviations
- CT
computed tomography
- DSS
discrete subaortic stenosis
- LVOT
left ventricular outflow tract
- CMR
cardiac magnetic resonance
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
- VSD
ventricular septal defect
Footnotes
Conflicts of interest: The authors have no other conflicts of interest pertinent to the topic of this manuscript.
References
- 1.Freedom RM. The natural and modified history of congenital heart disease. Elmsford, N.Y.: Blackwell Pub./Futura; 2004. [Google Scholar]
- 2.Shone JD, Sellers RD, Anderson RC, Adams P, Jr, Lillehei CW, Edwards JE. The developmental complex of "parachute mitral valve," supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol. 1963;11:714–25. doi: 10.1016/0002-9149(63)90098-5. [DOI] [PubMed] [Google Scholar]
- 3••.Kleinert S, Geva T. Echocardiographic morphometry and geometry of the left ventricular outflow tract in fixed subaortic stenosis. Journal of the American College of Cardiology. 1993;22:1501–8. doi: 10.1016/0735-1097(93)90563-g. Describes the association between acute aortoseptal angle, wider separation between the mitral valve and aortic valve and greater aortic override and the development of DSS. [DOI] [PubMed] [Google Scholar]
- 4.Gewillig M, Daenen W, Dumoulin M, Van der Hauwaert L. Rheologic genesis of discrete subvalvular aortic stenosis: a Doppler echocardiographic study. Journal of the American College of Cardiology. 1992;19:818–24. doi: 10.1016/0735-1097(92)90524-q. [DOI] [PubMed] [Google Scholar]
- 5.Sigfússon G, Tacy TA, Vanauker MD, Cape EG. Abnormalities of the left ventricular outflow tract associated with discrete subaortic stenosis in children: an echocardiographic study. Journal of the American College of Cardiology. 1997;30:255–9. doi: 10.1016/s0735-1097(97)00151-4. [DOI] [PubMed] [Google Scholar]
- 6.Barboza LA, Garcia FeM, Barnoya J, Leon-Wyss JR, Castañeda AR. Subaortic membrane and aorto-septal angle: an echocardiographic assessment and surgical outcome. World J Pediatr Congenit Heart Surg. 2013;4:253–61. doi: 10.1177/2150135113485760. [DOI] [PubMed] [Google Scholar]
- 7.Barkhordarian R, Wen-Hong D, Li W, Josen M, Henein M, Ho SY. Geometry of the left ventricular outflow tract in fixed subaortic stenosis and intact ventricular septum: an echocardiographic study in children and adults. The Journal of thoracic and cardiovascular surgery. 2007;133:196–203. doi: 10.1016/j.jtcvs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Tutar HE, Atalay S, Türkay S, Gümüş H, Imamoglu A. Echocardiographic, morphologic, and geometric variations of the left ventricular outflow tract: possible role in the pathogenesis of discrete subaortic stenosis. Angiology. 2000;51:213–21. doi: 10.1177/000331970005100305. [DOI] [PubMed] [Google Scholar]
- 9••.Cape EG, Vanauker MD, Sigfússon G, Tacy TA, del Nido PJ. Potential role of mechanical stress in the etiology of pediatric heart disease: septal shear stress in subaortic stenosis. Journal of the American College of Cardiology. 1997;30:247–54. doi: 10.1016/s0735-1097(97)00048-x. Makes a case, using fluid dynamic modeling, connecting the anatomic and geometric abnormalities described in other papers with abnormal shear stress of the ventricular septum in the pathophysiology of DSS. [DOI] [PubMed] [Google Scholar]
- 10.Moulaert AJ, Oppenheimer-Dekker A. Anterolateral muscle bundle of the left ventricle, bulboventricular flange and subaortic stenosis. Am J Cardiol. 1976;37:78–81. doi: 10.1016/0002-9149(76)90503-8. [DOI] [PubMed] [Google Scholar]
- 11•.Hong JH, Schaff HV, Nishimura RA. Fixed versus dynamic subaortic stenosis: Hemodynamics and resulting differences in Doppler echocardiography and aortic pressure contour. The Journal of thoracic and cardiovascular surgery. 2016;151:883–4. doi: 10.1016/j.jtcvs.2015.10.082. A recent report describing the differences between typical continuous wave Doppler contours in fixed subaortic stenosis versus dynamic LVOT obstruction. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha T, Ho SY, Vettukattil JJ. Multiplanar review analysis of three-dimensional echocardiographic datasets gives new insights into the morphology of subaortic stenosis. Eur J Echocardiogr. 2008;9:614–20. doi: 10.1093/ejechocard/jen008. [DOI] [PubMed] [Google Scholar]
- 13.de Agustin JA, Gomez de Diego JJ, Marcos-Alberca P, Macaya C, Perez de Isla L. Combined subaortic membrane and aortic valve stenosis: additive value of three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2014;15:388. doi: 10.1093/ehjci/jet193. [DOI] [PubMed] [Google Scholar]
- 14.Misra A, McCulloch M, Gangopadhyay S, Lawrie G, Dokainish H. Images in cardiology: remarkable correlation of subaortic membrane visualization by three-dimensional echocardiography and at surgery. Clinical cardiology. 2005;28:356. doi: 10.1002/clc.4960280711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss A, Motro M, Shem-Tov A, Blieden LC, Neufeld HN. Discrete subaortic stenosis associated with congenital valvular aortic stenosis--a diagnostic challenge. American heart journal. 1983;106:55–9. doi: 10.1016/0002-8703(83)90439-8. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox WD, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Discrete subaortic stenosis: two-dimensional echocardiographic features with angiographic and surgical correlation. Mayo Clin Proc. 1980;55:425–33. [PubMed] [Google Scholar]
- 18.Shem-Tov A, Schneeweiss A, Motro M, Neufeld HN. Clinical presentation and natural history of mild discrete subaortic stenosis. Follow-up of 1–17 years. Circulation. 1982;66:509–12. doi: 10.1161/01.cir.66.3.509. [DOI] [PubMed] [Google Scholar]
- 19.Frommelt MA, Snider AR, Bove EL, Lupinetti FM. Echocardiographic assessment of subvalvular aortic stenosis before and after operation. Journal of the American College of Cardiology. 1992;19:1018–23. doi: 10.1016/0735-1097(92)90287-w. [DOI] [PubMed] [Google Scholar]
- 20.Vogel M, Smallhorn JF, Freedom RM, Coles J, Williams WG, Trusler GA. An echocardiographic study of the association of ventricular septal defect and right ventricular muscle bundles with a fixed subaortic abnormality. Am J Cardiol. 1988;61:857–60. doi: 10.1016/0002-9149(88)91079-x. [DOI] [PubMed] [Google Scholar]
- 21.Kuralay E, Ozal E, Bingöl H, Cingöz F, Tatar H. Discrete subaortic stenosis: assessing adequacy of myectomy by transesophageal echocardiography. J Card Surg. 1999;14:348–53. doi: 10.1111/j.1540-8191.1999.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 22.Bezold LI, Smith EO, Kelly K, Colan SD, Gauvreau K, Geva T. Development and validation of an echocardiographic model for predicting progression of discrete subaortic stenosis in children. Am J Cardiol. 1998;81:314–20. doi: 10.1016/s0002-9149(97)00911-9. [DOI] [PubMed] [Google Scholar]
- 23.van der Linde D, Roos-Hesselink JW, Rizopoulos D, Heuvelman HJ, Budts W, van Dijk AP, Witsenburg M, Yap SC, Oxenius A, Silversides CK, Oechslin EN, Bogers AJ, Takkenberg JJ. Surgical outcome of discrete subaortic stenosis in adults: a multicenter study. Circulation. 2013;127:1184–91. e1–4. doi: 10.1161/CIRCULATIONAHA.112.000883. [DOI] [PubMed] [Google Scholar]
- 24•.Pickard SS, Geva A, Gauvreau K, del Nido PJ, Geva T. Long-term outcomes and risk factors for aortic regurgitation after discrete subvalvular aortic stenosis resection in children. Heart. 2015;101:1547–53. doi: 10.1136/heartjnl-2015-307460. A recent report analyzing outcome after DSS resection including risk factors for recurrent DSS requiring surgical intervention. [DOI] [PubMed] [Google Scholar]
- 25.Donald JS, Naimo PS, d'Udekem Y, Richardson M, Bullock A, Weintraub RG, Brizard CP, Konstantinov IE. Outcomes of Subaortic Obstruction Resection in Children. Heart, lung & circulation. 2017;26:179–186. doi: 10.1016/j.hlc.2016.05.120. [DOI] [PubMed] [Google Scholar]