Abstract
Background
Profiling of plasma metabolites to predict the course of heart failure (HF) appears promising but validation and incremental value are less established.
Methods
Patients meeting Framingham HF criteria with history of reduced ejection fraction were (n=1032) were randomly divided into derivation and validation cohorts (n=516 each). Amino acids, organic acids, and acylcarnitines were quantified using mass spectrometry in fasting plasma samples. We derived a prognostic metabolite profile (PMP) in the derivation cohort using Lasso-penalized Cox regression. Validity was assessed by 10-fold cross-validation in the derivation cohort, and by standard testing in the validation cohort. The PMP was analyzed as both a continuous variable (PMPscore) and dichotomized at the median (PMPcat), in univariate and multivariate models adjusted for clinical risk score and NTproBNP.
Results
Overall 48% of patients were African American, 35% were female, and average age was 69 years. After median follow-up of 34 months, there were 256 deaths (127 and 129 in derivation and validation cohorts, respectively). Optimized modeling defined the 13 metabolite PMP, which cross-validated as both PMPscore (hazard ratio [HR] 3.27, p<2×10−16) and PMPcat (HR=3.04, p=2.93×10−8). The validation cohort showed similar results; PMPscore HR=3.9 (p<2×10−16) and PMPcat HR=3.99 (p=3.47×10−9). In adjusted models PMP remained associated with mortality in the cross-validated derivation cohort (PMPscore HR=1.63, p=0.0029; PMPcat HR=1.47, p=0.081) and validation cohort (PMPscore HR=1.54, p=0.037; PMPcat HR=1.69, p=0.043).
Conclusion
Plasma metabolite profile varies across HF subgroups and is associated with survival incremental to conventional predictors. Additional investigation is warranted to define mechanisms and clinical applications.
Keywords: Metabolomic Profiling, Congestive Heart Failure, Prognosis, Risk Stratification
INTRODUCTION
Despite the cumulative success of neurohormonal interventions, heart failure (HF) remains an enormous health problem with a substantial residual disease burden and exhibiting a wide range of disease course and response to treatment.(1,2) Powerful risk prediction models of survival have been produced using clinical risk scores and natriuretic peptides,(3,4) but crucial knowledge gaps still exist regarding variability in the course of disease, additional biologic axes at play, and our limited ability to recognize pathophysiologic subgroups that comprise the overall HF population.
Our ability to biologically characterize patients has exploded due to powerful technology platforms such as genomics and proteomics, offering new hope of answering this challenge. A more recently maturing platform to arrive on the scene is metabolomics, which measures the levels of many small molecules of intermediary metabolism (i.e., metabolites) in tissues or fluids. Metabolomics is increasingly utilized to probe organ function and dysfunction, as well as to identify novel disease pathways. An early application of metabolomics profiling has been cardiovascular disease (5–7) with a focus on coronary syndromes (8–10). Similarly, there is growing data derived from the setting of HF, in which specific plasma metabolite levels were reported to be associated with incident disease or risk of death (11–15). More recently, a few systematic evaluations of the metabolites in HF were published which further support the overall hypothesis that circulating metabolites may be deranged in the setting of HF and indeed may reflect the underlying disease state (16–18). Larger scale validation of the plasma metabolome regarding prognostic value, or as way to stratify HF phenotypes, is still urgently needed.
Herein, we profiled a pool of targeted metabolites in plasma obtained from an adequately sized group of patients with HF and reduced ejection fraction (HFrEF) in order to describe phenotypic associations with the circulating metabolome, unveil plasma metabolites associated with mortality, and assess their risk association incremental to established predictors.
METHODS
Patients
This study was conducted at Henry Ford Hospital and was approved by the study site Institutional Review Board. All patients provided written informed consent at time of enrollment. The Henry Ford Heart Failure Pharmacogenomic registry has enrolled more than 1700 HF patients of any type and collected blood/plasma samples and detailed phenotypic information. Patients enrolled in the registry are required to meet Framingham Criteria for diagnosis of HF, including 2 major criteria (presence of paroxysmal nocturnal dyspnea, neck vein distention, rales, radiographic cardiomegaly, acute pulmonary edema, an S3 gallop, elevated central venous pressure, positive hepatojugular reflux, or sufficient weight loss with diuresis) or 1 major with 2 minor criteria (bilateral lower extremity edema, nocturnal cough, dyspnea with usual activity, hepatomegaly, pleural effusions, tachycardia, or decreased vital capacity). Registry participants are also required to have had an assessment of ejection fraction (EF) prior to enrollment, and patients on chronic dialysis are excluded.
For the current study, all registry patients with EF <50% at the time of HF diagnosis were included (n=1070) and underwent metabolomic profiling of stored plasma (detailed below). Among these patients, we restricted analysis to those with NTproBNP levels as well as complete data for calculation of the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) score. Thus, an analytic cohort of 1032 patients ultimately comprised the study. This entire group was utilized for testing the association of metabolites with demographic and clinical characteristics. For the survival analyses, the total study group was divided randomly in a 1:1 ratio (n=516 each) into a derivation cohort and a validation cohort.
MAGGIC scores were calculated as described in the original work, utilizing 13 routinely available clinical factors including age, sex, comorbid diabetes or COPD, creatinine, duration of heart failure, tobacco abuse, current medications (ACEI/ARB or beta blockers), systolic blood pressure, ejection fraction, BMI and NYHA class.(3) Data used to populate the MAGGIC score was derived from direct abstraction of the medical record or from administrative data, including claims data. The duration of HF diagnosis was the time elapsed between first diagnostic code for HF in the system and date of study enrollment; this was categorized as greater or less than 18 months for MAGGIC score calculation. The NTproBNP levels were measured on stored plasma samples, which were obtained at the time of study enrollment and were frozen at −70C until processing. These were quantified using an immunoelectrochemiluminescence assay on the Modular Analytics E 170 system. This assay has less than 0.001% cross-reactivity with bioactive BNP.
Metabolomic Studies
Quantitative targeted metabolite profiling of individual amino acids (AA), organic acids (OA), and acylcarnitines (AC) was performed at the Sanford Burnham Prebys Medical Discovery Institute using HPLC/mass spectrometry or GC/mass spectrometry. To demonstrate feasibility as well as direct subsequent efforts, we completed metabolomic profiling of all 1032 patients in the Henry Ford registry with HFrEF. Blood samples were drawn at the time of registry enrollment after an overnight fast. Plasma was prepared, aliquoted, and stored at −70°C. ACs (up to n=57) were assayed using an Agilent 1290 HPLC/Agilent 6490 triple quadrupole mass spectrometer with electrospray ionization. Plasma samples were spiked with stable isotope-labeled internal standards followed by extraction with methanol. ACs were derivatized using benzylhydroxylamine. Multiple reaction monitoring was used to quantitate fragment ions derived from the parent ion ACs. A similar process was performed to measure 23 AAs including Gly, Ala, Pro, Val, Arg, Thr, Lys, Gln, Ser, Leu, Ile, Met, His, Phe, Tyr, Asn, Asp, Glu, Trp, Orn, Cit, 1-MeHis, and 3-MeHis, using a quinolone functional group for derivatization. Quantitation of OAs (lactate, pyruvate, succinate, fumarate, malate, α-ketoglutarate, citrate, and 3-hydroxybutyrate) was performed using a Thermo Scientific GC single quadrupole mass spectrometer with electron ionization. These samples were derivatized with ethylhydroxylamine and extracted with ethylacetate. The ethyl acetate layer was dried down and OAs were derivatized to their corresponding silyl ethers with N,O-Bis(trimethylsilyl)trifluoroacetamide prior to GC/mass spectrometry.
Statistical Analysis
Of 87 total analytes, 38 metabolites with coefficient of variation <0.05 were considered non-variable and not analyzed further. Data was log transformed and scaled (mean-centered and divided by the standard deviation) to maintain a symmetric and comparable distribution. Metabolites were individually tested for association with each phenotype of interest (gender, race, diabetes status, NYHA class, 6 min walk distance (6MWD), EF change) using the t test, ANOVA, or Pearson’s correlation as appropriate. To account for multiple comparisons, the False Discovery Rate using Benjamini-Hochberg procedure is also presented with False Discovery Rate <0.05 considered statistically significant (19).
Individual metabolites were additionally evaluated for association with survival and used to identify a risk-associated plasma metabolite profile. To identify a robust combination of metabolites, data were randomly and equally divided into a derivation cohort and a validation cohort as noted above. The derivation cohort was used to build a multi-variable Cox regression model. Lasso penalized Cox regression with was used for variable selection (20). The selected metabolites were fitted using the model output to generate a numeric prognostic metabolic profile (PMP). Based on the selected metabolites and coefficients, we calculated the minimal and maximal raw PMP that are possible in our cohort, which ranged from −9.00 to 10.84. To make the score more easily understandable and intuitive, we rescaled the raw PMP to PMPscore with possible scores ranging from 0 to 10 using the formula:
Higher PMPscore indicates a greater likelihood of death. We then evaluated PMPscore, and its categorical separation at the median (PMPcat) to assess its value alone and in addition to established risk predictors, using Cox regression modeling and Kaplan-Meier survival curves. The proportionality assumption was assessed using Schoenfeld Residuals Test, which did not indicate significant departure.
To test PMP validity we took two separate approaches. First, the PMP was retested in the derivation cohort using 10-fold cross-validation. To briefly summarize, the derivation cohort was divided randomly into 10 subsamples. A Cox regression model is developed using 9 subsamples for metabolite selection and regression coefficient estimation. The PMP was then calculated using the remaining (held-out) subsample. This process was repeated for each of 10 loops of cross-validation so that in the end each patient had a PMP, with which overall (cross-validated) association and inference was tested. Second, we also performed standard testing using the distinct validation cohort. The score coefficients and cutoffs used in validation testing were as defined in the derivation cohort (i.e. strict validation of the parameters as initially defined in a distinct cohort of subjects). To test whether PMP could add incrementally to current best practice risk stratification, we then repeated the above analyses including the MAGGIC score and NTproBNP levels as covariates in the Cox models.(3,21). All analyses were implemented in R (version 3.2.1).
RESULTS
The total study analytic cohort included 1032 patients with a history of HFrEF. These subjects were randomly divided 1:1 into a derivation cohort and a validation cohort (each n= 516). The baseline characteristics of the study participants are shown in Table 1. Overall, 48% of the patient participants were African American, 35% were female and 43% had documented ischemic cardiomyopathy. The derivation and validation cohorts did not differ across a broad range of characteristics. There were 127 (24.6%) deaths over a median follow up of 34.2 months in the derivation cohort and 129 deaths over 33.1 months in the validation cohort.
Table 1.
Baseline characteristics of the entire study population (n=1032) and divided into derivation and validation cohorts (n=516 each).
| Overall (N=1032) |
Derivation (N=516) |
Validation (N=516) |
p | |
|---|---|---|---|---|
| Age | 67.9 (12.0) | 68.4 (11.5) | 67.5 (12.4) | 0.21 |
| Male | 671 (65.0%) | 353 (68.4%) | 318 (61.6%) | 0.03 |
| African American | 500 (48.4%) | 254 (49.2%) | 246 (47.7) | 0.64 |
| COPD | 227 (22.0%) | 116 (22.5%) | 111 (21.5%) | 0.76 |
| CKD | 229 (22.2%) | 112 (21.7%) | 117 (22.7%) | 0.76 |
| Diabetes | 428 (41.5%) | 206 (39.9%) | 222 (43.0%) | 0.34 |
| Ischemic Etiology | 583 (56.5%) | 294 (57.0%) | 289 (56.0%) | 0.80 |
| Stroke/TIA | 125 (12.1%) | 52 (10.1%) | 73 (14.1%) | 0.06 |
| ACE/ARB | 667 (64.6%) | 335 (64.9%) | 332 (64.3%) | 0.90 |
| Beta Blocker | 617 (59.8%) | 309 (59.9%) | 308 (59.7%) | 1.00 |
| Heart Rate, bpm | 71.1 (13.0) | 71.2 (13.6) | 71.0 (12.4) | 0.81 |
| Systolic BP, mmHg | 128.8 (23.1) | 128.5 (23.61) | 129.2 (22.7) | 0.65 |
| Creatinine, mg/dL | 1.33 (1.03) | 1.35 (1.14) | 1.30 (0.91) | 0.51 |
| NTproBNP, pmol/L | 355.2 (380.2) | 361.7 (379.2) | 348.7 (381.4) | 0.58 |
| Ejection Fraction | 36.8 (12.5) | 36.9 (12.8) | 36.8 (12.3) | 0.97 |
| MAGGIC Score | 19.0 (7.3) | 19.3 (7.3) | 18.7 (7.4) | 0.19 |
| 6MWD, m | 318 (105) | 313 (107) | 323 (103) | 0.18 |
| NYHA = 1 | 590 (57.2%) | 293 (56.8%) | 297 (57.6%) | 0.76 |
| 2 | 180 (17.4%) | 95 (18.4%) | 85 (16.5%) | |
| 3 | 119 (11.5%) | 60 (11.6%) | 59 (11.4%) | |
| 4 | 121 (11.7%) | 59 (11.4%) | 62 (12.0%) | |
| Survival (days) | 1055.7 (617.3) | 1047.6 (595.8) | 1063.8 (638.6) | 0.67 |
| Death | 256 (24.8%) | 127 (24.6%) | 129 (25.0%) | 0.94 |
Plasma Metabolite Association with Demographics, Comorbidities, and Symptoms
We analyzed metabolite associations with various phenotypes of interest including sex, race (African American vs. white), etiology of HF (ischemic vs. nonischemic), presence of diabetes, change in EF over time, 6-minute walk distance (6MWD) and NYHA classification. There were numerous significant associations of individual metabolites with patient characteristics and clinical phenotype (Table 2). The levels of certain amino acids (Leu, Phe, and Val) were lower in women (compared to men). Fumarate and α-ketoglutarate were higher in women and were also positively correlated with age. We found a relatively greater abundance of short branch chained ACs among diabetics, though the corresponding AAs were not clearly elevated.
Table 2.
(A–G). Metabolomic Associations across Patient Characteristics and Clinical Phenotypes
| A. Sex (Male vs. Female) | ||
|---|---|---|
| Metabolite | Fold Change | Adj. p value |
| Glycine | 1.154 | 1.15E-08 |
| Leucine | 0.874 | 1.15E-08 |
| Isoleucine | 0.876 | 2.08E-08 |
| Valine | 0.9 | 5.88E-08 |
| C5.Isovaleryl | 0.829 | 9.31E-08 |
| Tryptophan | 0.914 | 2.24E-05 |
| Glutamate | 0.867 | 6.86E-05 |
| C8.OH | 0.818 | 9.00E-05 |
| C5.DC | 0.781 | 9.07E-05 |
| C5.2.Methylbutyryl | 0.894 | 0.00024 |
| C3 | 0.892 | 0.000405818 |
| Phenylalanine | 0.942 | 0.002297143 |
| Citrate | 1.095 | 0.002297143 |
| Succinate | 1.081 | 0.002297143 |
| a.KG | 1.105 | 0.002592 |
| C12.1 | 0.965 | 0.0225 |
| Serine | 1.052 | 0.026666667 |
| Fumarate | 1.112 | 0.026666667 |
| B. Diabetes (Present vs. Absent) | ||
|---|---|---|
| Metabolite | Fold Change | Adj. p value |
| Lactate | 1.158 | 1.25E-07 |
| Glutamate | 1.193 | 1.58E-06 |
| Citrulline | 0.917 | 4.32E-06 |
| Pyruvate | 1.12 | 2.28E-05 |
| C5.2.Methyl-butyryl | 1.185 | 4.61E-05 |
| C4.Isobutyryl | 1.251 | 0.0003 |
| Malate | 1.135 | 0.0005 |
| Glycine | 0.936 | 0.0014 |
| Valine | 1.076 | 0.0016 |
| C3 | 1.119 | 0.0016 |
| Isoleucine | 1.099 | 0.0021 |
| Tryptophan | 0.943 | 0.0027 |
| C4.Butyryl | 1.15 | 0.0048 |
| Histidine | 0.953 | 0.0051 |
| Alanine | 1.079 | 0.0054 |
| C6 | 1.106 | 0.0108 |
| C2 | 1.103 | 0.0141 |
| Glutamine | 0.961 | 0.0141 |
| Fumarate | 1.123 | 0.0149 |
| C8 | 1.051 | 0.0264 |
| Leucine | 1.071 | 0.0389 |
| C. Race (African American vs. Not) | ||
|---|---|---|
| Metabolite | Fold Change | Adj. p value |
| Proline | 0.856 | 3.20E-10 |
| Phenylalanine | 0.889 | 2.66E-07 |
| Ornithine | 0.908 | 2.66E-07 |
| Tryptophan | 0.905 | 1.41E-06 |
| C14.1 | 0.705 | 1.79E-06 |
| Lysine | 0.914 | 6.97E-06 |
| C16.1 | 0.757 | 3.29E-05 |
| Alanine | 0.905 | 3.41E-05 |
| C12 | 0.791 | 4.70E-05 |
| Tyrosine | 0.923 | 0.0001175 |
| C18.1 | 0.911 | 0.00030942 |
| C5.2.Methyl-butyryl | 0.88 | 0.000309 |
| C12.1 | 0.791 | 0.000318 |
| Citrulline | 0.911 | 0.000877 |
| C16 | 0.914 | 0.000877 |
| C14.2 | 0.815 | 0.00194 |
| Threonine | 1.075 | 0.00331765 |
| Glutamate | 0.888 | 0.00339 |
| C2 | 0.925 | 0.00396 |
| Asparagine | 0.957 | 0.006345 |
| Valine | 0.951 | 0.01499 |
| C6 | 0.877 | 0.0278 |
| X3.HBA | 0.864 | 0.0409 |
| C18.2 | 0.919 | 0.0431 |
| Histidine | 0.979 | 0.0432 |
| D. Heart Failure Etiology (Ischemic vs. Non-ischemic) | ||
|---|---|---|
| Metabolite | Fold Change | Adj. p value |
| C14.1 | 1.452 | 2.11E-06 |
| C14.2 | 1.401 | 2.11E-06 |
| C18.2 | 1.189 | 3.04E-05 |
| C4.Isobutyryl | 1.252 | 4.56E-05 |
| C18.1 | 1.137 | 5.09E-05 |
| C10 | 1.198 | 4.32E-04 |
| C8 | 1.194 | 4.32E-04 |
| C12 | 1.196 | 5.86E-04 |
| C6 | 1.229 | 5.86E-04 |
| C4.Butyryl | 1.142 | 0.00154 |
| Glutamine | 0.947 | 0.00196 |
| Proline | 1.075 | 0.0022 |
| C2 | 1.111 | 0.00222 |
| C8.OH | 1.181 | 0.00264 |
| Fumarate | 1.154 | 0.00269 |
| Serine | 0.944 | 0.00291 |
| Citrate | 1.068 | 0.00339 |
| C5.2.Methyl-butyryl | 1.085 | 0.00507 |
| Ornithine | 1.066 | 0.0104 |
| Malate | 1.104 | 0.0142 |
| C5.Isovaleryl | 1.077 | 0.0165 |
| Lactate | 1.063 | 0.0185 |
| C3 | 1.051 | 0.0355 |
| Succinate | 1.089 | 0.04 |
| Arginine | 0.945 | 0.0403 |
| E. Age | ||
|---|---|---|
| Metabolite | Corr. Coef. | Adj. p value |
| C6 | 0.125 | 0.000127 |
| Phenylalanine | 0.124 | 0.000151 |
| C16.1 | 0.123 | 0.000157 |
| C4.Butyryl | 0.122 | 0.000164 |
| Leucine | −0.119 | 0.00023 |
| Glycine | 0.118 | 0.000276 |
| C18.2 | 0.115 | 0.000372 |
| Asparagine | 0.106 | 0.001073 |
| Valine | −0.099 | 0.002464 |
| Isoleucine | −0.09 | 0.006186 |
| C5.DC | 0.087 | 0.008127 |
| C18 | 0.079 | 0.016323 |
| Citrulline | 0.252 | 1.01E-14 |
| C14.2 | 0.174 | 1.15E-07 |
| C5.2.Methylbutyryl | 0.144 | 1.27E-05 |
| C10 | 0.172 | 1.28E-07 |
| C12.1 | 0.142 | 1.48E-05 |
| C8.OH | 0.221 | 1.50E-11 |
| C18.1 | 0.141 | 1.69E-05 |
| C12 | 0.197 | 1.75E-09 |
| Succinate | 0.14 | 1.84E-05 |
| Malate | 0.169 | 1.98E-07 |
| C8 | 0.169 | 2.05E-07 |
| Aspartate | −0.139 | 2.08E-05 |
| Ornithine | 0.182 | 2.76E-08 |
| C2 | 0.206 | 3.53E-10 |
| Fumarate | 0.134 | 4.09E-05 |
| C14.1 | 0.18 | 4.14E-08 |
| a.KG | 0.132 | 4.84E-05 |
| C3 | 0.162 | 6.90E-07 |
| Glutamate | −0.202 | 7.02E-10 |
| F. NYHA Class | |
|---|---|
| Metabolite | Adj. p value |
| Arginine | 2.20E-12 |
| C18.1 | 2.20E-12 |
| C18.2 | 1.31E-09 |
| C2 | 6.16E-09 |
| Lactate | 3.17E-07 |
| Histidine | 9.53E-07 |
| C6 | 1.26E-06 |
| Tryptophan | 1.76E-06 |
| X3.HBA | 2.30E-06 |
| Valine | 5.72E-06 |
| Serine | 9.60E-06 |
| Citrate | 9.90E-06 |
| C10 | 2.74E-05 |
| C4.Isobutyryl | 4.40E-05 |
| C14.2 | 4.99E-05 |
| C16 | 0.000129 |
| C4.Butyryl | 0.000194 |
| C8 | 0.000293 |
| Leucine | 0.000602 |
| Pyruvate | 0.001012 |
| C14.1 | 0.001488 |
| C3 | 0.0058 |
| Isoleucine | 0.008609 |
| C12 | 0.0088 |
| Alanine | 0.015312 |
| C5.2.Methylbutyryl | 0.019556 |
| C18 | 0.019556 |
| Tyrosine | 0.022 |
| C16.1 | 0.034897 |
| C8.OH | 0.042533 |
| G. Six Minute Walk Distance | ||
|---|---|---|
| Metabolite | Corr. Coef. | Adj. p value |
| C2 | −0.202 | 2.40E-08 |
| a.KG | −0.204 | 2.40E-08 |
| Citrate | −0.18 | 9.76E-07 |
| C10 | −0.163 | 1.14E-05 |
| C4.Isobutyryl | −0.155 | 1.86E-05 |
| C6 | −0.157 | 1.86E-05 |
| C8 | −0.155 | 1.86E-05 |
| Malate | −0.157 | 1.86E-05 |
| C12 | −0.135 | 0.00024 |
| Succinate | −0.135 | 0.00024 |
| Leucine | 0.126 | 6.00E-04 |
| Tryptophan | 0.127 | 6.00E-04 |
| C4.Butyryl | −0.126 | 0.000628 |
| C12.1 | −0.122 | 0.000857 |
| Valine | 0.117 | 0.00144 |
| Fumarate | −0.113 | 0.00216 |
| C8.OH | −0.112 | 0.002231 |
| C16.1 | −0.107 | 0.003467 |
| Arginine | 0.103 | 0.005053 |
| C5.2.Methylbutyryl | −0.101 | 0.006 |
| Serine | 0.095 | 0.010286 |
| C14.1 | −0.093 | 0.011345 |
| C18.1 | −0.092 | 0.012104 |
| C16 | −0.091 | 0.013 |
| Lactate | −0.09 | 0.013824 |
| Histidine | 0.087 | 0.0168 |
| C14.2 | −0.085 | 0.019556 |
| C3 | −0.084 | 0.020571 |
| C5.Isovaleryl | 0.079 | 0.031448 |
| Asparagine | 0.072 | 0.048 |
| Phenylalanine | −0.072 | 0.048 |
| Aspartate | 0.071 | 0.0495 |
Additionally, a broad array of metabolites were shown to be associated with NYHA class and 6MWD. Substantial overlap existed between metabolites associated with NYHA class and those associated with 6MWD. Among the subjects with a repeat echocardiogram (n=688), we examined whether baseline metabolite levels were associated with change in EF. Over a median of 29.6 months the mean change in EF was 3.22% (±13.84). In crude analyses several metabolites were associated with EF change, however none withstood adjustment for multiple comparisons (FDR>0.05).
Plasma Metabolites and Survival
Plasma metabolites associated with survival were identified using a Lasso-penalized Cox regression model of survival (without covariates). The optimized model identified 13 metabolites which together comprised a prognostic metabolite profile (Table 3). The normalized metabolite values and coefficients from the Cox model were used to calculate a PMP score for each patient and rescale from 0 to 10. As expected, the PMP score was strongly associated with survival (p=2.0×10−16) in the derivation cohort. Two stages of validation were performed, first using 10-fold cross-validation in the derivation cohort, and then using standard statistical approaches in the distinct validation cohort. When testing the validity of the PMP score (a continuous variable), the 10-fold CV within the derivation cohort revealed a HR of 3.27 per point (p=2.0 × 10−16). This result was confirmed in the independent validation cohort (n=516, 129 deaths), which produced a very similar risk association (HR = 3.92 per point, p<2.0 × 10−16). In parallel fashion, PMPcat (dichotomized at median of derivation cohort) validated similarly in both the cross-validation (in the derivation cohort) and the standard validation cohort. Cross-validation showed a HR for death of 3.04 (p=2.93 × 10−8). The validation cohort high-risk PMP category exhibited a HR of 3.99 (p=3.47 × 10−9). Survival curves for each subcohort (cross-validated for the derivation cohort and validation cohort) are shown in Figure 1.
Table 3.
Selected Metabolites and Corresponding Coefficients from Multiple Variable Cox Regression
| Metabolite | HR | Pr(>|z|) |
|---|---|---|
| Arginine | 0.841449 | 0.093212 |
| Leucine | 0.769837264 | 0.159228 |
| Phenylalanine | 1.510985775 | 0.0001 |
| Valine | 0.979394201 | 0.91114 |
| C2 | 1.387656169 | 0.007185 |
| C4.Isobutyryl | 1.577933326 | 0.000177 |
| C5.Isovaleryl | 0.654209879 | 0.000385 |
| C5.DC | 1.193786537 | 0.08468 |
| C18.1 | 1.186870605 | 0.196161 |
| X3.HBA | 0.818738983 | 0.010068 |
| Succinate | 1.153386974 | 0.177973 |
| Fumarate | 1.029689807 | 0.85332 |
| α-KG | 0.955228362 | 0.733616 |
Figure 1.
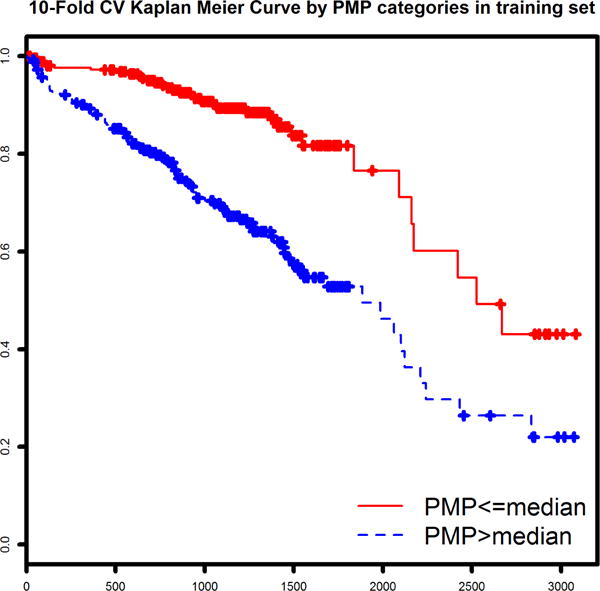
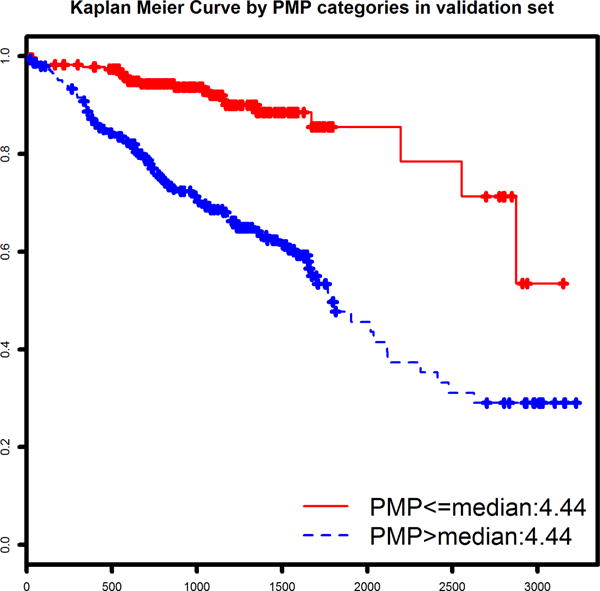
We also wanted to test whether the PMP added incremental information to established prognostication tools. To represent current recommended risk stratification with clinical factors and biomarkers we chose to utilize the MAGGIC score and NTproBNP. We tested Cox regression models of survival time using PMPs both as a continuous and categorical variable as described above, but this time including MAGGIC score and NTproBNP levels as covariates. The results of these models are shown in Tables 4a and 4b. The two validation methods yielded generally consistent results. While the strength of association with survival was, as expected, mitigated by adjustment for these powerful predictors, overall PMP remained statistically significant with HRs ranging from 1.4 to 1.7.
Table 4a.
Cox models of survival with PMP as Continuous Variable and including MAGGIC score and NTproBNP as covariates
| 10-fold Cross-validated Derivation cohort (N=513, 127 deaths) |
Validation Cohort (n=516, 129 deaths) |
|||
|---|---|---|---|---|
| HR | Pr(>|z|) | HR | Pr(>|z|) | |
| MAGGIC | 1.10 | 6.9 ×10–11 | 1.07 | 8.95 ×10-6 |
| NTproBNP | 1.0005 | 0.0074 | 1.0011 | 3.7 ×10-8 |
| PMP | 1.63 | 0.0029 | 1.54 | 0.0373 |
Table 4b.
Cox models of survival with dichotomized PMP (at the median) and including MAGGIC and NTproBNP as covariates
| 10-fold Cross-validated Derivation cohort (N=513, 127 deaths) |
Validation Cohort (n=516, 129 deaths) |
|||
|---|---|---|---|---|
| HR | Pr(>|z|) | HR | Pr(>|z|) | |
| MAGGIC | 1.10 | 2.1 ×10–12 | 1.08 | 2.6 ×10-6 |
| NTproBNP | 1.0007 | 0.0002 | 1.0012 | 4.3 ×10-10 |
| PMP | 1.47 | 0.0808 | 1.69 | 0.0426 |
DISCUSSION
Our investigation clearly confirms the vast potential of the circulating metabolome in the setting of HF, establishing strong phenotypic associations, unveiling a blood borne metabolite profile that is predictive of survival in HF patients, and even augmenting optimal conventional risk prediction (i.e. MAGGIC score and NTproBNP levels). The importance of these observations is not that our PMP be considered as the next biomarker of interest, but more broadly that the plasma metabolome will, we predict, be a quantitative tool for recognizing important variations in this disease that are not clinically obvious.
Our data is derived from one of the largest HF patient cohorts in which a broadly representative sweep of key plasma metabolites has been examined. We demonstrated a variety of associations of known metabolites with comorbidities, demographics, HF severity, and reverse remodeling. While not all of our findings align perfectly, they generally appear to be internally consistent. For example, there is substantial overlap of metabolites and congruency in direction of effect regarding symptom severity measures (6MWD and NYHA class) and those associated with survival. Viewing these data in the context of the literature-based evidence reveals good consistency as well. For example, with regards to metabolite associates of race and disease severity in HF, our findings agree with those from earlier reports.(13,22) In the setting of diabetes, our data showed significant differences in several key intermediates of fatty acid metabolism including pyruvate and malate, consistent with other studies.(23) Interestingly, we found branched chained ACs to be associated with diabetes but not the corresponding AAs as has been described elsewhere. The reasons for this are unclear but could suggest increased utilization in the setting of HF that partially offsets the diabetes effect.
Regarding survival prediction, the PMP devised herein is comprised of 13 metabolites. Some of these were previously associated with survival in HF while others appear to be novel. Specifically, the associations of αKG, Citric Acid Cycle intermediates, Arg pathway, and others, are consistent with earlier publications.(24,25) Notably, our data confirmed certain findings by Ahmad et al. who recently reported that long-chain ACs were associated with survival in HF patients and respond to treatment (in the form of an LVAD). Despite taking a very different statistical approach, two factors of our PMP (Arg, C18.1 AC) were also contributing factors in the most predictive principle component identified from the Ahmad study.(18) This consistency of our findings with those of previous investigators strongly supports external validity.(7,18,26– 29) Moreover, although the primary aim here was to validate the importance of circulating metabolites in HF and not define a biomarker, the PMP did statistically add to current optimal prognosticators; a high hurdle that few markers (such as troponin and neutral endopeptidase) have achieved (30,31)
The pathophysiologic link between circulating metabolites and HF phenotypes remains uncertain, and our data does not address this directly. The numerous significant associations involving metabolites linked to the Citric Acid Cycle (e.g. succinate, α-ketoglutarate, and fumarate) may reveal perturbed oxidative metabolism. The energetic state and substrate utilization of the heart is known to be altered in the setting of HF (32–37). It has been hypothesized that this mediates specific differences in circulating metabolite levels (25,38) such as long-chain ACs (18). However, it could be that the circulating metabolite profile reflects a systemic metabolic response (perhaps sourced largely by skeletal muscle) to the HF state, reflecting an enhanced catabolism that is correlated with severity and risk. This key question requires additional research to evaluate the correspondence of the circulating metabolite profile with the state of energy metabolism in the heart and skeletal muscle. Moreover, the answer to this question will likely require experimental studies using tissue and model systems in order to establish mechanistic links.
Limitations
Our study has limitations to be considered when interpreting the findings. We chose to define the PMP without MAGGIC or NTproBNP in the initial models. The goal was to identify circulating metabolites intrinsically associated with survival, and not specifically to identify metabolite biomarkers. Hence, while evaluating the PMP incremental to these established predictors lends additional support to the notion that they are independent predictor of risk in HF, this would not be the ideal approach if the goal were simply to define an additional biomarker. Additional analyses can be done to optimize metabolomic biomarkers, but that is a different objective from the current study. Another potential limitation is the use of targeted metabolomic profiling of 3 specific metabolite classes (OAs, AAs, ACs) as opposed to unbiased global metabolomics. Importantly, although a relatively small group of total metabolites was surveyed, OAs, AAs and ACs as a group have been shown to provide a comprehensive snapshot of intermediary metabolism.(24,39) While it is possible that impactful associations were missed due to our use of targeted metabolite profiling, our chosen approach was optimal for the study size and practical goals of interrogating promising groups of metabolites in a quest for novel biomarkers. Moreover, the highly quantitative nature of our targeted metabolomics approach is crucial to reveal distinctions between plasma samples from the different patient groups. Finally, though our study population was large enough to divide and create a distinct validation cohort, additional investigation with similar patients from other studies or geographic regions might be worthwhile.
Conclusions
The plasma metabolite profile encompassing OAs, AAs and ACs varies across subgroups of HF patients and is associated with HF symptom severity. We defined and then validated a profile of 13 circulating metabolites (PMP) that is a strong predictor of survival in HF patients, which remains statistically significant after accounting established clinical risk factors and NTproBNP levels. These findings suggest that the plasma metabolite profile may be a useful tool to better understand phenotypic subgroups of HF and potentially further illuminate the pathophysiology of HF. Additional investigation is warranted to define underlying mechanisms and potential clinical applications.
Clinical Perspectives.
Circulating plasma metabolites differ across heart failure phenotypes and associate with severity. A 13-metabolite profile was validated as a predictor of mortality, and remained statistically significant in survival models adjusted for clinical factors and natriuretic peptides.
Translational Outlook.
Additional research is warranted to investigate the biologic underpinnings of differences in the circulating metabolome in heart failure, and the mechanistic relationship of metabolites to heart failure progression and/or response to treatment. This could lead to greater understanding of phenotypic variation across heart failure patients and illuminate novel mechanisms at work during HF progression.
Acknowledgments
Financial Support: This research was supported by the National Heart, Lung, and Blood Institute (Lanfear R01HL103871, R01HL132154). Dr. Williams is supported by NHLBI (R01HL118267), the National Institute of Allergy and Infectious Diseases (R01AI079139) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK064695). Dr. Sabbah’s work is supported by the National Heart, Lung, and Blood Institute (PO1HL074237, R01HL132154). Drs. Gardell and Petucci are supported by the NIH Common Fund (Southeast Center for Integrated Metabolomics U24 DK097209).
Abbreviations
- HF
Heart Failure
- HFrEF
Heart Failure with Reduced Ejection Fraction
- EF
Ejection Fraction
- MAGGIC
Meta-Analysis Global Group in Chronic Heart Failure
- AA
Amino Acids
- OA
Organic Acids
- AC
Acylcarnitines
- NYHA
New York Heart Association
- 6MWD
6 Minute Walk Distance
- PMP
Prognostic Metabolite Profile
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CITATIONS
- 1.Roger VLGA, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. Heart disease and stroke statistics – 2012 update: a report from the American heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talameh JA, Lanfear DE. Pharmacogenetics in chronic heart failure: new developments and current challenges. Curr Heart Fail Rep. 2012;9:23–32. doi: 10.1007/s11897-011-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–13. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 4.van Kimmenade RR, Januzzi JL., Jr Emerging biomarkers in heart failure. Clin Chem. 2012;58:127–38. doi: 10.1373/clinchem.2011.165720. [DOI] [PubMed] [Google Scholar]
- 5.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126:1110–20. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163:844–850. doi: 10.1016/j.ahj.2012.02.005. e1. [DOI] [PubMed] [Google Scholar]
- 7.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–7. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Chen B, Chen T, et al. Comprehensive metabolomics identified lipid peroxidation as a prominent feature in human plasma of patients with coronary heart diseases. Redox Biol. 2017;12:899–907. doi: 10.1016/j.redox.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFilippis AP, Trainor PJ, Hill BG, et al. Identification of a plasma metabolomic signature of thrombotic myocardial infarction that is distinct from non-thrombotic myocardial infarction and stable coronary artery disease. PLoS One. 2017;12:e0175591. doi: 10.1371/journal.pone.0175591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trainor PJ, Hill BG, Carlisle SM, et al. Systems characterization of differential plasma metabolome perturbations following thrombotic and non-thrombotic myocardial infarction. J Proteomics. 2017;160:38–46. doi: 10.1016/j.jprot.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsushita Kunihiro WE, Mongraw-Chaffin Morgana, et al. The association of plasma lactate with incident cardiovascular outcomes: the ARIC study. American Journal of Epidemiology. 2013;178:401–409. doi: 10.1093/aje/kwt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turer Aslan LG, O’Sullivan John, et al. Increases in myocardial workload induced by rapid atrial pacing trigger alterations in global metabolism. PLOS ONE. 2014;9:e99058. doi: 10.1371/journal.pone.0099058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Yu B, Alexander D, et al. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC Study. Am J Epidemiol. 2013;178:534–42. doi: 10.1093/aje/kwt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn WB, Broadhurst DI, Deepak SM, et al. Serum metabolomics reveals many novel metabolic markers of heart failure, including pseudouridine and 2-oxoglutarate. Metabolomics. 2007;3:413–426. [Google Scholar]
- 15.Tang WH, Shrestha K, Wang Z, Troughton RW, Klein AL, Hazen SL. Diminished global arginine bioavailability as a metabolic defect in chronic systolic heart failure. J Card Fail. 2013;19:87–93. doi: 10.1016/j.cardfail.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aubert GMO, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SH, Kruger M, Hoppel CL, Lewandowskin ED, Crawford Pa, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemesle GMF, Beseme O, Ovart L, Amouyel P, Lamblin N, de Groote P, Bauters C, Pinet F. Multimarker proteomic profiling for prediction of cardiovascular mortality in patients with chronic heart failure. PLOS ONE. 2015;10:e0119265. doi: 10.1371/journal.pone.0119265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad T, Kelly JP, McGarrah RW, et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility With Mechanical Circulatory Support. J Am Coll Cardiol. 2016;67:291–9. doi: 10.1016/j.jacc.2015.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini YDD, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioral Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 20.Simon RMSJ, Li M, Menezes S. Using cross-validation to evaluate predictive accuracy of survival risk classifiers based on high-dimensional data. Briefings in Bioinformatics. 2011;12:201–214. doi: 10.1093/bib/bbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alba AC, Agoritsas T, Jankowski M, et al. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail. 2013;6:881–9. doi: 10.1161/CIRCHEARTFAILURE.112.000043. [DOI] [PubMed] [Google Scholar]
- 22.Sharma AC-AN, Yancy CW. Heart failure in African Ameericans: disparities can be overcome. Cleveland Clinic Journal of Medicine. 2014;81:301–311. doi: 10.3949/ccjm.81a.13045. [DOI] [PubMed] [Google Scholar]
- 23.Devarshi PP, McNabney SM, Henagan TM. Skeletal Muscle Nucleo-Mitochondrial Crosstalk in Obesity and Type 2 Diabetes. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah SHKW, Newgard CB. Metabolic profiling for identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126:1110–1120. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turer AT, SR, Bain JR, Muehlbauer MJ, et al. Metabolomic profiling reveals distinct patterns fo myocardial substrate utilization in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia-reperfusion. Circulation. 2009;110:1736–1746. doi: 10.1161/CIRCULATIONAHA.108.816116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng ML, Wang CH, Shiao MS, et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65:1509–20. doi: 10.1016/j.jacc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya S, Granger CB, Craig D, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232:191–6. doi: 10.1016/j.atherosclerosis.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PA, Xu ZH, Huang YL, et al. Increased serum 2-oxoglutarate associated with high myocardial energy expenditure and poor prognosis in chronic heart failure patients. Biochim Biophys Acta. 2014;1842:2120–5. doi: 10.1016/j.bbadis.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Dunn WBD, Deepak S, et al. Serum metabolomics reveals many novel metabolic markers of heart failure, including pseudouridine and 2-oxoglutarate. Metabolomics. 2007;3:413–26. [Google Scholar]
- 30.Bayes-Genis ABJ, Galan A, de Antonio M, Domingo M, Zamora E, Urrutia A, Lupton J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. Journal of the American College of Cardiology. 2015;65:657–665. doi: 10.1016/j.jacc.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 31.Pascual-Figal DADM, Casas T, Gich I, Ordonez-Llanos J, Martinez P, Cinca J, Valdes M, Januzzi JL, Bayes-Genis A. Usefulness of clinical and NT-proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. European Heart Journal. 2008;29:1011–1018. doi: 10.1093/eurheartj/ehn023. [DOI] [PubMed] [Google Scholar]
- 32.Rosca MG, Vazquez EJ, Kerner J, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovascular research. 2008;80:30–9. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanley WC, Sabbah HN. Metabolic therapy for ischemic heart disease: the rationale for inhibition of fatty acid oxidation. Heart Fail Rev. 2005;10:275–9. doi: 10.1007/s10741-005-7542-4. [DOI] [PubMed] [Google Scholar]
- 34.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–7. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 35.Molina AJ, Bharadwaj MS, Van Horn C, et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart failure. 2016;4:636–45. doi: 10.1016/j.jchf.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell SP, Adkisson DW, Ooi H, Sawyer DB, Lawson MA, Kronenberg MW. Impairment of subendocardial perfusion reserve and oxidative metabolism in nonischemic dilated cardiomyopathy. J Card Fail. 2013;19:802–10. doi: 10.1016/j.cardfail.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohte N, Narita H, Iida A, et al. Impaired myocardial oxidative metabolism in the remote normal region in patients in the chronic phase of myocardial infarction and left ventricular remodeling. Journal of Nuclear Cardiology. 2009;16:73–81. doi: 10.1007/s12350-008-9006-4. [DOI] [PubMed] [Google Scholar]
- 38.Turer AT. Using metabolomics to assess myocardial metabolism and energetics in heart failure. J Mol Cell Cardiol. 2013;55:12–8. doi: 10.1016/j.yjmcc.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Bain JR, SR, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from inofrmation to knowledge. Diabetes. 2009;58:2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]


