Abstract
The plentiful assortment of natural and synthetic materials can be leveraged to accommodate diverse wound types, as well as different stages of the healing process. An ideal material is envisioned to promote tissue repair with minimal inconvenience for patients. Traditional materials employed in the clinical setting often invoke secondary complications, such as infection, pain, foreign body reaction, and chronic inflammation. This review surveys the repertoire of surgical sutures, wound dressings, surgical glues, orthopedic fixation devices and bone fillers with drug eluting capabilities. It highlights the various techniques developed to effectively incorporate drugs into the selected material or blend of materials for both soft and hard tissue repair. The mechanical and chemical attributes of the resultant materials are also discussed, along with their biological outcomes in vitro and/or in vivo. Perspectives and challenges regarding future research endeavors are also delineated for next-generation wound repair materials.
Keywords: Material, Medical device, Wound repair, Drug delivery
1. Introduction
A wound constitutes any physical injury to the body arising from injuries, diseases, or surgical interventions, characterized by superficial lacerations or penetration to underlying tissues, such as muscles, ligaments or bones [1, 2]. Minor wounds often heal through the body’s intrinsic repair process that entails four consecutive phases: coagulation and hemostasis, inflammation, proliferation, and remodeling orchestrated by multiple cell populations (neutrophils, macrophages and fibroblasts), as well as through extracellular matrix formation and action of soluble mediators including growth factors and cytokines [3, 4]. Restoration is mostly impaired, however, in injuries of greater severity and may lead to wound exposure or tissue abnormalities [5, 6].
Consequently, materials frequently employed in the clinic are designed to stabilize the site of injury and aid in the healing process [7–9]. In order to be effective, wound repair devices should ideally possess similar mechanical properties to the tissue undergoing reconstruction [10–12]. Soft tissues (skin, tendon, ligaments, muscles) require more elastic and pliant materials such as polymers, as well as glues, sutures or dressings for wound closure [13–17]. On the other hand, stiff and strong materials, such as ceramics, metals and their alloys, are preferable for repairing hard tissues (bone, cartilage) [18–21].
The need for wound repair devices continues to steadily increase with greater than 114 million patients worldwide endure wounds from surgical procedures annually [22]. In the United States alone, 36 million patients experienced surgery-related wounds in 2012, and 31 million injured persons visited the emergency room in 2011 [23, 24]. The global wound care market totaled $15.6 billion in 2014 and is anticipated to grow to $18.3 billion by 2019 [25].
While many wound repair materials in current clinical use are reported to be effective, devastating wounds – mostly large defects – are highly susceptible to infection, pain, and abnormal inflammation [26, 27]. Cumbersome devices often employed for treatment may invoke secondary complications, such as foreign body reactions and chronic inflammation [28–33]. Multiple administrations of oral or injectable drugs may therefore be prescribed to combat these issues [34–36]. Such strategies rely primarily on systemic drug exposure, which may not optimally address local wound complications [37, 38].
As a result, developing wound repair devices coupled with localized drug delivery represents an avenue of tremendous interest. This review begins with a general discussion on materials for wound repair and related complications that may arise. Subsequent sections focus on soft and hard tissues – each surveying the landscape for drug-eluting materials and their influence on different aspects of wound healing. Finally, perspectives on future directions in this field are offered.
2. Wound repair devices
To begin, wound repair devices can be categorized according to the mechanical properties of the damaged tissue, namely soft and hard (Fig. 1 and Fig. 2). Soft tissues include skin, muscle, tendon, and ligaments, which exhibit relatively high flexibility and elasticity [39], whereby in contrast, hard tissues consisting of bone or cartilage tend to have higher stiffness [40, 41].
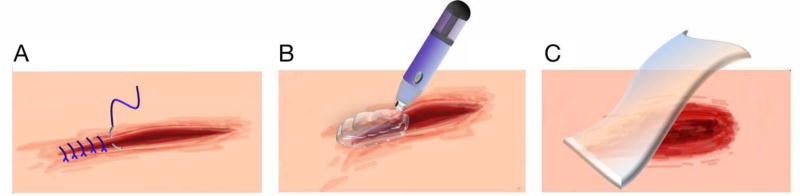
Figure 1.
Representative schematic images of medical devices for soft tissue repair: (A) surgical suture, (B) surgical glue, and (C) wound dressing.
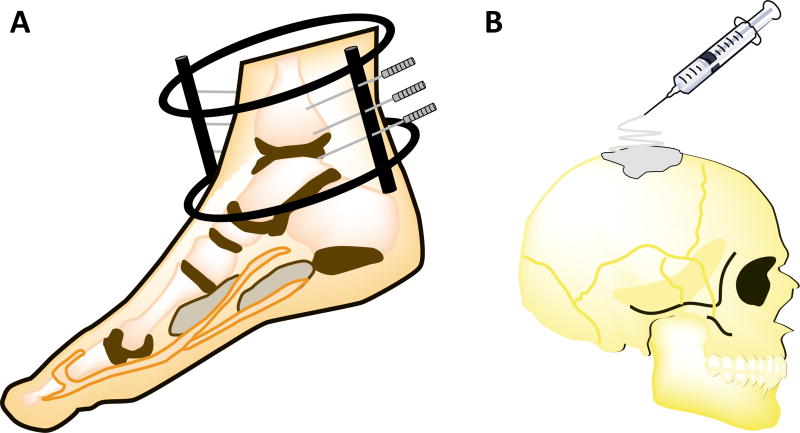
Figure 2.
Representative schematic images of medical devices for hard tissue repair: (A) orthopedic fixation device with pins affixed to bone and (B) bone filler. Illustrations were adapted from ChemBioDraw (version 14.0, PerkinElmer, Waltham, MA)
Soft tissue wounds are inflicted via abrasion, laceration, avulsion, amputation, and penetration, as well as arising from burns, diseases, infections, or tumors [41]. Wound closure at these sites commonly involves sutures, staples or glues [42–44]. In the event of significant tissue loss, the defect can be covered or filled with dressings to prevent dryness or infection, as well as to potentially encourage tissue regeneration [45–47].
Filamentous surgical sutures (Fig. 1A) derived from either absorbable or non-absorbable materials are most prevalently used to seal dermal wounds and internal organs or to ligate blood vessels [42, 44, 48]. Absorbable sutures consist of biodegradable materials, such as catgut, collagen, poly(glycolic acid) (PGA), poly(dioxanone) (PDX), poly(glyconate) (PGC), poly(glactin) (PGL), poly(lactic-co-glycolic acid) (PLGA) and poly(trimethylene carbonate) (PTMC) [44, 48–53]. Since they do not require subsequent removal, they are preferable for treating internal organ wounds, such as in the stomach, colon and bladder [44, 48–50]. Non-absorbable sutures are composed of non-biodegradable materials, including silk, cotton, nylon, polyester and metal [49, 50]. Despite needing to be extracted upon conclusion of wound healing, non-absorbable sutures possess relatively higher tensile strength and are often applied to tissues warranting greater mechanical support, such as the skin, fascia, and tendons [44, 48].
Under circumstances demanding urgent care, surgical staples can be applied more readily than conventional sutures [54, 55]. They can be evenly spaced along large open wounds in the gastrointestinal organs, thereby better preventing leakage [54, 56, 57]. To ensure proper mechanical strength, surgical staples are primarily metal-based (stainless steel and titanium). Alternatively, absorbable staples consisting of synthetic polymers such as PGA and poly(lactic acid) (PLA) have been developed to minimize patient discomfort [53, 58–60].
Aside from sutures and staples, liquid surgical glue (Fig. 1B) provides a third option. It creates insoluble networks almost instantaneously to function as an adhesive, sealant and hemostat when applied to a wound [42, 43, 61–64]. Surgical glues have been prepared from an assortment of natural materials, such as fibrin, collagen and gelatin [43, 61, 65–67], as well as from synthetic polymers based on cyanoacrylate, dendrimers, urethane and ethylene glycol [43, 63, 68].
For large gaps in soft tissues, wound dressings (Fig. 1C) provide a physical barrier to maintain moist and airy environments that may stave off infection or further damage [37, 45, 69–71]. Wound dressings are largely fabricated from synthetic biopolymers, including poly(L-lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA), poly(ethylene glycol) (PEG) and polyurethane (PU), due to reproducibility and lower susceptibility to biological contamination [69, 72]. They are amenable to the majority of wound types [73] and help facilitate exudate removal from wounds [37, 74]. Furthermore, dressings serve as scaffolds for tissue regeneration, especially in severe burn wounds [75–78], and in these instances, they may consist of highly biocompatible, natural hydrogels, such as alginate, collagen or chitosan [69, 73].
Because wound conditions differ for hard tissues, the aforementioned items for soft tissues would therefore not provide adequate repair. Severe insults to bone lead to breakage as opposed to tears or lacerations. Bone fixation devices (Fig. 2A) are consequently employed to align and support fractured bones in desired positions [79, 80]. They come in a range of configurations depending on the application. Pins are used to temporarily fix fracture fragments or to serve as a guide for other fixation systems, and are commonly applied onto the wrist and arthrodesis. Additionally, screws are widely used for bone fixation alone or in combination with plates, wires or nails. Plates hold fractured bones together (generally in the spine, wrist, and long bones) to prevent their displacement and are typically attached with screws. Wires provide tension, position long bone fractures, or hold bone and soft tissue together. Intramedullary nails and rods introduced into the center of fractured bones to shield them from torsion or bending are generally applied to long bone fractures, such as the tibia and femur [81].
Indeed, bone fixation devices must be sufficiently rigid and able to withstand mechanical loads sustained by the body during the course of fracture repair. Metals and metal alloys address this criteria [18, 82] and are consequently integrated at high load bearing sites, such as the femur, hip, and tibia [82–84]. Synthetic polymer-based bone fixation devices have also been gaining considerable attention, since usage of biodegradable materials could negate subsequent removal. During the healing process, these synthetic materials also notably lose their strength to minimize stress-shielding and gradually confer mechanical loads back to native bone [79, 85, 86]. Polymeric fixation devices are more suitable for regions enduring relatively lower load, such as the ankles, hands and craniofacial bones. Commonly used bioabsorbable formulations include PGA, PLA, PDX, PLLA and poly(L,DL-lactic acid) (PLDLLA) [18, 79, 87]. To strike a better balance between elasticity and load-bearing strength, polymer-ceramic composites have been extensively investigated [18, 72, 83, 85].
In other instances, bone fillers (Fig. 2B) of various biological or synthetic substances can infiltrate large defects [18, 88]. Autologous bone derived from a patient’s own body represents the gold standard material. However, its low availability and invasiveness of the extraction surgery prompt a critical need for other alternatives. Allogeneic bone and its demineralized form have been suggested as substitutes for autografts to improve tissue reconstruction in craniofacial defects and fractured gaps [88–90], but supply limitations remain. Consequently, synthetic alternatives, such as calcium phosphates [89, 91] and calcium sulfates have been widely incorporated into bone fillers [18]. Bone cements formulated from poly(methylmethacrylate) (PMMA), poly(ethylmethacrylate) (PEMA), and polyethylene (PE) have additionally been explored [72, 89, 90, 92]. As with bone fixation devices, composite bone fillers (often ceramic-polymer) ensure structural reinforcement and bioactivity in dental and load-bearing applications.
3. Device-related complications
Even after receiving appropriate treatment, large exposed wounds still face an array of complications, including infection [35, 93, 94], abnormal inflammation [95] and poor regeneration [96]. These issues lead to low patient compliance and prolonged hospitalization, which create a substantial socioeconomic burden [35, 93, 97, 98]. For instance, the overall cost of healthcare-associated infections in the United States ranges from $35.7 to $45.0 billion annually and involves roughly 1.8 million patients [99]. It is therefore imperative to devise strategies that better address the challenges at hand.
Among the aforementioned issues, infection represents the most immediate complication. Its occurrence stems from microorganism infiltration into the body upon material implantation at the wound site [35, 100]. Severe wounds remain highly susceptible to infection primarily from the following two factors: (1) surgical/nosocomial transmission or (2) residual microbial existence on the surface of an implanted medical device [101], which becomes rapidly coated with extracellular matrix proteins, such as fibrinogen, fibronectin and collagen. These proteins promote multilayered microbial attachment and proliferation that manifest in the formation of a biofilm, becoming more resistant to antibacterial treatment [32, 98, 102]. The lingering presence of these biofilms causes abnormal chronic inflammation at the wound site and jeopardizes the healing process. Moreover, biofilms can contribute to orthopedic device failure, eventually culminating in the need for implant removal [103]. Surgical sutures, especially of the multi-filament variety, have been reported to be more susceptible to harboring microorganisms in their micro-gaps [104–106].
Aside from infection, inflammation can also result from injury [107, 108]. Following tissue damage, coagulation components such as a platelet and fibrin work to prevent blood loss [1, 109]. The cascade then recruits inflammatory cells such as neutrophils, macrophages and lymphocytes, whose functions include disposing of cellular debris and foreign matter [6], regenerating native cells, or forming scar tissue under circumstances requiring rapid wound closure [110]. While inflammation remains essential for wound healing, its associated symptoms, such as redness, heat, swelling, and pain, often intensify with large wounds and may contribute to patient discomfort. The foreign body reaction can additionally be triggered around the wound site. This complication extends the period of inflammation and retards the wound healing process, which can lead to device failure via rapid degradation, excessive fibrosis, restenosis, or calcification [111–114].
Furthermore, large wounds typically impede the normal reparative process [115]. Severe, prolonged acute inflammation results in chronic inflammation, subsequently generating excessive scar formation [108, 116] or unhealed wounds [108, 117]. Delayed wound closure comes with a higher risk for infection [118, 119] and may also cause severe inflammation. A handful of diseases have also been demonstrated to disrupt wound repair. For example, complications of late-stage diabetes may include damaged nerves or limited blood supply, leading to ulcers particularly at the body’s lower extremities [120]. Patients with keloids are subjected to excessive scar formation from abnormal fibroblast proliferation and collagen deposition [121]. Moreover, osteoporosis hampers bone regeneration due to imbalanced activation between osteoclasts and osteoblasts (ex. higher bone resorption than bone formation) [122, 123].
To alleviate the aforementioned issues, drug therapy has been employed in clinical practice, oftentimes entailing systemic delivery through oral administration or intravenous injection [124, 125]. Antibiotics are widely used to prevent infection at the wound site; penicillin, cephalosporins and tetracyclines are commonly available in the clinic [126–128]. However, these drugs may potentially cause diarrhea, vomiting, hearing loss, vertigo, kidney damage or liver failure [129–131]. Anti-inflammatory medications categorized as either steroid or non-steroid are commonly employed to modulate intense inflammatory symptoms. The most widely used drugs include dexamethasone, prednisone, acetaminophen, ibuprofen and naproxen by oral administration, or cortisone and ketorolac by local injection.[132–137]. Anti-inflammatories, however, also cause problems due to stimulation of vagus nerve activity, leading to increases in gastric acidity and ulcer formation [138, 139]. To minimize these undesirable side effects, wound treatment may rely on the local drug delivery strategies of bolus solution, emulsion, suspension, ointment, gel or dry powder [37, 140, 141], yet these approaches still require tedious procedures. The development of materials with localized drug delivery capabilities are thus envisioned to concurrently mitigate complications associated with large wounds and to promote effective repair.
4. Drug delivery medical devices for soft tissue repair
4.1. Surgical sutures
Selecting the appropriate suture type for a patient remains essential but challenging. The nature of the soft tissue wound and any possible allergic reactions the individual may have to a given material must be taken into consideration. As mentioned in an earlier section, suture materials can be classified as absorbable or non-absorbable. Absorbable sutures are biodegradable and conveniently do not require subsequent removal. On occasion, they may potentially elicit inflammatory responses. Non-absorbable sutures generally need to be recovered, but exhibit more robust mechanical strength than their absorbable counterparts. Presently, sutures coated with antimicrobial drugs have already received approval for select clinical applications [142–147]. Meanwhile in the laboratory, drug-eluting sutures that modulate inflammation or promote wound healing have also been developed (Table 1).
Table 1.
Various examples of drug-loaded surgical sutures. The sutures were commonly dip-coated into a drug solution or into a blend of drug and encapsulation materials. Drug-loaded sutures prepared using other methods are annotated.
| Functionality | Drug | Drug Loading Strategy | Drug Encapsulating Materials | Drug Release Timeframe | Reference |
|---|---|---|---|---|---|
| Anti-infection | Triclosan | Adsorption | N/A | N/A | [98, 142–146, 148, 149] |
| Ciprofloxacin | Immobilization | N/A | 95–120 h | [153, 241] | |
| Amoxicillin | Impregnation | N/A | 336 h | [154] | |
| Chlorhexidine, Octenidine | Fatty acid | Lauric acid/palmitic acid | 96 h, 168 h | [150, 151] | |
| Tetracycline, Rifampin, Chloramphenicol | Polymer | Chitosan, alginate | 96 h | [152] | |
| Tetracycline, Cefotaxime | Polymer nanofibers | PLLA | 144 h, 240 h | [157, 158]a | |
| Anti-inflammation | Ibuprofen | Polymer | Copolymer of PLA, PCL and PTMC | 120 h | [160] |
| Poly(allylamine hydrochloride) and dextran | 240 h | [161] | |||
| Polymer strands | PLGA | 144 h | [155]b | ||
| Dexamethasone | Polymer microparticles | PLGA | 672 h | [156]c | |
| Aceclofenac or insulin | Polymer nanofibers | PLGA | 240 h or 168 h | [162] |
Drug was encapsulated in the polymer nanofibers, which were then braided to prepare drug-loaded surgical sutures.
Drug was encapsulated in the polymer strands, which were then wound around a surgical suture.
Sutures were dip coated in suspension of drug-loaded polymer microparticles.
Surgical sutures for anti-infection
With the prevalence of wound infections, surgical suture materials with capacity for localized drug release have been actively investigated for soft tissue wound treatment over the past decade. The most prevalent strategy involves dip-coating the suture in a given drug solution [98, 142–146, 148–151]. The drug simply adsorbs onto the suture surface, resulting in low drug loading efficiency and nearly instantaneous drug release.
Because drug retention and prolong anti-infection effects are beneficial for wound healing, suture surface modifications can be made prior to drug loading. For example, sutures may be coated with biocompatible hydrogel materials, such as chitosan, sodium alginate and calcium alginate, and then immersed into an antimicrobial drug solution, such as tetracycline, rifampin or chloramphenicol [152]. This approach improves drug retention in the hydrogel layer, as well as better sustained release. In another study, vinylimidazole grafted onto suture material provided imidazole units that served as interaction sites for ciproflaxin immobilization [153]. As a result, the antibiotic was released slowly over the course of four to five days and demonstrated an evident zone of inhibition against E. coli. To further sustain drug release, Choudhury el al. impregnated amoxicillin into the suture core using oxygen plasma treatment to greatly enhance the hydrophilicity of muga (Antheraea assama) silk fibroin (AASF)-based sutures [154]. The drug-loaded AASF suture exhibited a clear zone of inhibition against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli), whereas its drug-adsorbed counterpart exhibited no apparent antibacterial activity. In vivo studies corroborated this finding, revealing that drug-loaded AASF sutures facilitated better wound healing due to reduced infection risk.
In addition to the aforementioned strategies, drug-loaded strands [155], microparticles [156] or nanofibers [157, 158] have been used to coat the suture surface or to comprise the body of the suture. For example, nanofibers were fabricated by electrospinning a solution consisting of PLLA and either tetracycline or cefotaxime, which were then braided to produce a multifilament surgical suture. The drug release from these sutures was reported to exceed five days. Drug release kinetics could be tailored by modulating the mass ratio between PLLA and drug. With coaxial fibers, the drug and PLLA were localized within the core and on the surface respectively, thereby permitting more sustained drug release [158].
Regarding commercialization of drug-eluting sutures, Ethicon developed Coated Vicryl® Plus Antibacterial (polyglactin 910), an absorbable surgical suture containing the drug triclosan, in 2004. In vitro studies with S. aureus and E. coli showed that Coated Vicryl® Plus exhibited a zone of inhibition correlating to its apparent antimicrobial activity and significantly reduced the adherence of methicillin-resistant S. aureus (MRSA) on the surface [98]. Biocompatibility tests performed with Coated Vicryl® Plus revealed that the suture was neither toxic nor irritating [143]. Furthermore, an in vivo study in which Coated Vicryl® Plus and non-drug-containing sutures were separately implanted into guinea pigs for 48 hours and subsequently explanted demonstrated that Coated Vicryl® Plus with triclosan reduced the bacterial population by 96.7% compared to conventional sutures [144]. Ethicon later launched MONOCRYL®_Plus Antibacterial (poliglecaprone 25) and PDS® Plus Antibacterial (polydioxanone) in 2008. These drug-loaded sutures implanted into mouse and guinea pig infection models also showed better inhibition of bacterial colonization compared to their uncoated counterparts [145, 146].
The efficacy of triclosan-coated sutures was further demonstrated in clinical studies. Non-drug-containing and triclosan-coated sutures were employed for wound closure in cerebrospinal fluid shunt surgery [148]. Among the 84 patients involved, triclosan-coated sutures significantly decreased the rate of shunt infection from 21% to 4.3%. Another study consisting of 2088 individuals triclosan-coated sutures applied to abdominal wound closure decreased the rate of infection from 10.8% to 4.9% [149]. In addition to triclosan, chlorhexidine has also been employed in commercialized antimicrobial surgical sutures [159], but reports on the efficacy of this suture type prove scarce.
Surgical sutures for inflammation modulation
In contrast to infections that can be treated with early, extensive drug exposure, inflammation lingers locally at injured sites for a relatively long period until completion of wound healing and requires prolonged, sustained delivery of anti-inflammatory drugs. To achieve this goal, a variety of drug-loading strategies have been proposed for long-term drug release.
In one scenario, sutures were dip-coated with a nonsteroidal anti-inflammatory drug (NSAID), ibuprofen, prepared in the organic solvent dichloromethane [160]. This way, the suture could swell during immersion in the solution, allowing more drug to diffuse deeply into the suture core. Drug loading concentration and release pattern could therefore be controlled by varying the duration of suture immersion. Consequently, drug was released for 7 days with different loading amounts depending on the immersion time. Ibuprofen was also incorporated into surgical sutures using a layer-by-layer deposition method [161]. In this instance, the surface of the silk surgical suture was first coated with multiple layers of chemically cross-linked poly(allylamine hydrochloride) and dextran microgels. Due to the electrostatic interaction between the microgel layers and drug molecules, ibuprofen was released in a sustained manner for 10 days. Applying a third method, Lee et al. prepared a separate drug-loaded strand by electrospinning a PLGA solution with ibuprofen, which was then physically braided with the surgical suture in clinical use [155]. Outcomes from pain-induced animal models revealed that the drug was released for six days, and that pain could be mitigated until completion of wound healing.
In another electrospinning approach, Padmakumar et al. [162] attempted a core-sheath electrospinning configuration to fabricate mechanically strong sutures with a PLLA center encased by PLGA containing either aceclofenac or insulin. The authors observed that the aforementioned drugs could be released in a sustained manner over several days in vitro. Moreover, aceclofenac-loaded sutures contributed to inflammation reduction in vivo, whereas sutures with insulin promoted cell migration when assayed in vitro.
Finally, another strategy for extending the drug release timeframe entailed synthesizing polyethyleneimine (PEI)-coated PLGA microparticles loaded with dexamethasone [156]. This coating conferred a positive charge to the microparticles, thereby enabling them to be immobilized via electrostatic interactions onto the negatively charged suture surface. The resulting material was reported to elute drug for up to 28 days in vitro.
4.2. Wound dressings
Larger soft tissue injuries may require augmentation with wound dressings as opposed to sutures, which consist of a sterile patch or pad to promote healing and prevent further infection. Their chief roles include absorbing exudate, encouraging healing, easing pain, and protecting from infection. Traditionally, wet or dry gauze was employed to conceal a wound. These basic dressings have gradually evolved into materials specifically designed to control environmental moisture and to deliver drugs or active ingredients (specific chemicals, cells, growth hormones) to the site of interest.
Particularly in recent years, much effort has been devoted to designing wound dressing materials that administer a diverse range of drugs for anti-infection [76, 163–166], anti-inflammation [119] and tissue regeneration [167, 168]. Drugs are often first blended with polymers, which are subsequently used as constituents to prepare the wound dressings [167, 169–171]. Akin to methods for deriving drug-loaded sutures, a drug can be absorbed (or adsorbed) directly onto the material [172, 173] or encapsulated in carriers, such as polymer-based microparticles, prior to incorporation into the wound dressings [166, 168]. The following section discusses commercialized materials and relevant technologies (Table 2).
Table 2.
Various examples of drug-loaded wound dressings. Drugs were largely absorbed into wound dressings or blended with the encapsulation materials. Drug-loaded dressings prepared through other strategies are annotated.
| Functionality | Drug | Drug Loading Strategy | Drug Encapsulation Materials | Drug Release Timeframe | Reference |
|---|---|---|---|---|---|
| Anti-infection | Chlorhexidine | Polymer | Chitosan, 5-methylpyrrolidinone chitosan | 48 h | [179] |
| Ciprofloxacin | Poly(2-hydroxyethyl methacrylate) | 144 h | [169] | ||
| Tetracycline | PLA, PCL | 48 h | [171] | ||
| Ciprofloxacin, levofloxacin, moxifloxacin | PLDLLA, PEG | 6.7 h | [181] | ||
| Levofloxacin | Chitosan, 2-hydroxyethylacrylate | 132 h | [184] | ||
| Cefazolin | PLGA | N/A | [182] | ||
| Benzalkonium chloride | Styrene-isoprene-styrene copolymer, Sodium alginate | N/A | [183] | ||
| Nitrofurazone | Hydrogel | PVA, Alginate | N/A | [170] | |
| Sulfamethoxazole | Microparticles | PNIPAAm | 75 h | [166]a | |
| Anti-infection and pain relief | Mupirocin, Lidocaine | Polymer | PLLA | 72 h | [185] |
| Anti-infection and anti-inflammation | Streptomycin, Diclofenac | Polymer | Poly(ethylene oxide) | 72 h | [186] |
| Tissue regeneration | Human growth hormone | Hydrogel | Collagen | 168 h | [167] |
| Epidermal growth factor | Microparticles | Gelatin | N/A | [168]a |
Drug-loaded polymer microparticles were separately prepared and incorporated in wound dressings.
Wound dressings for anti-infection
For decades, wound dressings have been commercially developed with infection prevention in mind. Products that have been marketed for various applications include Iodosorb®, Bactigras®, and Xeroform®. Iodosorb® was formulated with iodine and proved effective in treating both acute and chronic human wounds [174–177]. Bactigras® consisted of tulle wound dressings medicated with chlorhexidine, which were designed to prevent infection in partial and full-thickness wounds in the short-term. Xeroform®, a petrolatum impregnated with antiseptic agent bismuth tribromophenate, was developed specifically with surgical incisions, lacerations and burns in mind. In addition to these aforementioned materials, wound dressings containing povidone, polymyxin, neomycin, and bacitracin were also made available in the clinic [172, 173, 178].
However, many commercialized drug-eluting wound dressings did not consider the timeframe for local drug exposure. Various techniques have therefore been executed to achieve sustained drug release. In an earlier study, Lin et al. [166] prepared wound dressings consisting of sulfamethoxazole-loaded poly(N-isopropylacrylamide) (PNIPAAm) microparticles with Eudragit E film. The resulting material effectively absorbed wound fluid, and sulfamethoxazole was released from the PNIPAAm microparticles for 75 hours. In another approach, Rossi et al. fabricated wound dressings by freeze-drying a solution of chitosan hydrochloride, 5-methyl-pyrrolidinone chitosan and chlorhexidine [179], which exhibited sustained release of chlorhexidine for over 48 hours. According to in vitro studies with bacterial (S. aureus, Staphylococcus epidermidis (S. epidermidis), Pseudomonas aeruginosa (P. aeruginosa)) and fungal (Candida albicans) strains, the material loaded with chlorhexidine was observed to have effective antimicrobial activity.
Applying a UV-radiation polymerization strategy, Tsou et al. [169] fabricated ciprofloxacin-loaded 2-hydroxymethacrylate wound dressings. The resulting product demonstrated in vitro drug release for 6 days and retained antibacterial activity for up to 12 days. In another study, Kim et al. [170] pursued a sol-gel process to fabricate poly(vinyl alcohol) (PVA)/alginate hydrogel matrices containing nitrofurazone. Alginate was shown to enhance protein adsorption on these wound dressings. In vivo results demonstrated that nitrofurazone-loaded hydrogels improved wound healing in rats as a consequence of enhanced antibacterial activity.
In addition to sustained antibiotic release, another important endeavor has been to mimic the nanofibrous structure of the extracellular matrix in soft tissues. To that end, electrospinning has been leveraged to generate wound dressings since drugs can be readily introduced into a polymeric solution and their release properties controlled by changing the material and altering fiber morphology [180]. Antibiotics such as tetracycline, ciprofloxacin, levofloxacin, moxifloxacin and cefazolin have been successfully incorporated into solutions of biocompatible polymers, such as PLA, poly(ε-caprolactone) (PCL), PLDLLA, PEG, and PLGA to produce drug-loaded nanofibrous mats [171, 181, 182]. These dressings also exhibited prolonged drug release and enhanced antimicrobial activity.
Recently, hydrocolloid wound dressings loaded with benzalkonium chloride (BC) were prepared using a hot melting method [81, 183]. The product displayed better mechanical strength and flexibility compared to that of commercial wound dressings. BC-containing wound dressings contributed to a decrease in antimicrobial activity (S. aureus, E. coli, P. aeruginosa) in vitro, whereas this outcome was not observed in the untreated and commercial wound dressing groups. Furthermore, a rat-based excision wound model revealed that using BC-loaded wound dressings led to a higher percentage of healing (~84%) compared to the controls (untreated ~36%, commercial wound dressings ~57%). Using a modified thermally activated phase separation method, Siafaka et al. designed porous dressings for levofloxacin delivery [184]. Levofloxacin was loaded into the dressing (5–30%) by free-radical polymerization using chitosan with 2-hydroxyethylacrylate materials and released for up to 132 hours. Antibacterial performance was evaluated using three different bacterial strains (S. aureus, M. staphylococcusaureus and P. aeruginosa) and results showed a significant effect on the zone of inhibition.
Wound dressings for inflammation modulation
Additionally, the delivery of anti-inflammatory drugs, together with an anti-infection drug, has been utilized to improve wound healing. Thakur et al. reported on a wound dressing containing both the anesthetic lidocaine and the antibiotic mupirocin [185]. A dual spinneret electrospinning apparatus was used to collect two distinct PLLA fibers – each loaded with lidocaine or mupirocin, respectively. The resulting dressing exhibited antibacterial action for over 72 hours, with a comparably large initial release of lidocaine for potential treatment of early pain in large wounds. In another study, polyethylene oxide films were produced by solvent casting to contain the antibiotic streptomycin and the anti-inflammatory diclofenac [186], and demonstrated that both drugs could be released in a sustained manner for 72 hours. Using drugs with dual functions offers a good strategy for more comprehensive wound care.
Wound dressings for tissue regeneration
To more actively aid tissue regeneration at local wound sites, several groups have investigated dressings with growth factor delivery capabilities. Maeda et al. developed a collagen-based dressing loaded with human growth hormone (hGH), whose release profiles varied based on material composition and preparation conditions [167]. Wound healing was significantly improved due to enhanced tissue regeneration in the presence of hGH. In a similar vein, gelatin-based wound dressings containing epidermal growth factor (EGF) were prepared by Ulubayram et al. [168]. When applied to wounds on the dorsal region of rabbits, a substantial effect on wound healing was observed compared to that of control groups sans EGF.
4.3. Surgical glues
Surgical glues prove advantageous for wound closure because they enable quick application, minimal pain and no subsequent removal. Materials used in surgical glues, such as fibrin glue, albumin and urethane, can rapidly form a rigid, hardened structure and also demonstrate good biocompatibility [187–189]. Among the available options, fibrin glue has been widely investigated as a carrier for drugs, biological factors, or genes [190–194]. Due to its high water uptake and pore-forming abilities, fibrin glue has additionally been studied in the form of tissue engineering scaffolds [195–197]. However, few reports discuss the application of medicated surgical glues for enhancing wound healing processes.
In recent years, drug-eluting surgical glues have been leveraged to mitigate infection and pain. Osada et al. [198] evaluated the safety and pharmacokinetics of fibrin glue loaded with the antibiotic sisomicin. In live animals, the local drug concentration at the glued site was observed to be higher, when compared to that of the group receiving intravenously injections of sisomicin. When tested in ten human subjects, the drug-loaded glue did not cause any infection. For pain relief, Zhibo et al. [199] and Kitajiri et al. [200] prepared a fibrin glue containing lidocaine and tested its efficacy in sub-pectoral breast augmentation and tonsillectomy, respectively. In both cases, individuals receiving the combined entity of surgical glue and lidocaine reportedly experienced less pain compared to the control groups that had either lidocaine only or fibrin glue only. While these clinical studies were small in scale, preliminary results show promise and should be further elaborated.
5. Drug-eluting medical devices for hard tissue repair
5.1. Orthopedic fixation devices
Fixation devices in the form of plates, rods, screws or pins afford stability during bone fracture repair. The two modes of fixation are external and internal. External fixation involves positioning wires or pins (connected to rods) that penetrate the skin around the fracture [81]. Internal fixation aims to expedite the return of mobility and function at the site of injury, where the various implants may be directly attached to the bone fragments in need of repair [81]. Metals have been traditionally employed as the material of choice because of their sturdy mechanical properties exceeding that of native bone. Stainless steel is most commonly used, though titanium has also been applied due to its more desirable bio logical characteristics. Polymers have more recently been explored as an alternative; however, balancing mechanical strength and degradation properties remains a significant challenge for researchers.
Orthopedic fixation devices for anti-infection
In an effort to reduce the incidence of infections, current research endeavors have largely focused on designing fixation devices with localized drug delivery capabilities. Gulati et al. [201] designed titanium nanotube arrays on titanium wires as a release platform for gentamicin, which exhibited a two-phase release profile (burst release, followed by zero-order kinetics). In vitro studies showed that covalently binding vancomycin to titanium rods could prevent growth of S. aureus, even after exposure to serum proteins, prolonged incubation in physiological buffer, and serial exposures to the bacterial strain [202]. Forster et al. [203] developed a gentamicin-coated polyurethane sleeve, allowing for localized administration to combat pin tract infections. In vitro release studies revealed that the eluted antibiotic concentrations exceeded performance standards outlined by the National Committee for Clinical Laboratory Standards. Tobramycin impregnated into hydroxyapatite (HA)-coated external fixation pins was released above the S. aureus mean inhibitory concentration over eight days [204]. While these preliminary results seem promising, the implant’s in vivo performance should next be studied. In another study, Lucke et al. [205] used a rat model of osteomyelitis to test the efficacy of PDLLA-coated titanium Kirschner wires containing gentamicin. The group found that incorporating antibiotic significantly minimized S. aureus colonization, whereas coating-only and uncoated controls failed to stop bacterial growth in vivo. Sanchez and colleagues studied gentamicin release profiles both in vitro and in vivo from implants consisting of a calcium phosphate-synthetic polymer blend [206]. The addition of PDLLA or PLGA coatings was shown to prolong the release rate. Notably, implants with an outer layer of PDLLA sustained gentamicin concentrations over four weeks in vivo exceeding the minimum bactericidal concentration. Histology and radiographs demonstrated that PDLLA-coated implants promoted bone formation in rat femurs over 20 weeks. Mäkinen et al. [207] incorporated ciprofloxacin into PLGA screws, which were contaminated with S. aureus prior to implantation into rabbits. Upon retrieval six weeks later, these antibiotic-impregnated devices did not cause infections; conversely, stainless steel screws were unable to stave off infection. In this case, no fracture had been induced in the animals.
While antibiotic-laden implants show promise, the path to clinical translation demands strategies that will ensure sufficient antibiotic concentrations to swiftly eradicate any bacteria around the wound site [208]. Concerns regarding potential development of antibiotic resistance have also prompted the use of alternative antimicrobial compounds, namely chlorhexidine, poly(hexamethylenebiguanide) and chloroxylenol [208]. More insight is nevertheless warranted to better understand the effects of antimicrobial-eluting implants on neighboring tissues. Exploring other candidate agents, Holt et al. [209] showed that titanium external fixation pins layered with xerogel films releasing nitric oxide could substantially diminish the presence of bacteria, as well as the potential for infection following 28 days in rat vertebrae.
Orthopedic fixation devices for tissue regeneration
Aside from reducing infection, fixation devices have also been developed to promote tissue regeneration at the injury site. In an early study, Schmidmeier et al. [210] incorporated transforming growth factor β1 (TGF-β1) and insulin-like growth factor-1 (IGF-1) into PDLLA coatings for steel and titanium Kirschner wires. 42 days post-implantation in a rat fracture model, the growth factors retained their bioactivity and improved the biomechanics of animals in comparison to uncoated counterparts. A later work using the aforementioned coating and therapeutic molecules on titanium plates reported significant mineralization in vivo, while providing stability to the fracture site in parallel [211]. Titanium alloy discs coated with calcium phosphate and BMP-2 were able to induce bone formation in rats [212]. Likewise, Bae et al. [213] functionalized titanium discs with BMP-2, and osteoblast cells seeded onto these materials were shown to proliferate faster. Moreover, upregulation of osteogenic markers was observed. Lee et al. [214] designed dual-function heparin-dopamine titanium implants that administered substrate, as well as significant stimulation of osteoblasts. Likewise, Baas et al. [215] incorporated recombinant human BMP-2 and pamidronate into coatings for titanium screws. Dual administration prevented fibrous tissue formation, but unfortunately did not have the intended effect of lowering resorption accompanying BMP usage. Additionally, these implants exhibited the worst fixation. Alternatively, cholesterol-lowering drugs such as statins have been employed since they are cost-effective. For example, titanium Kirschner wires with high dose simvastatin-loaded PLGA coating loaded with a high dose of simvastatin were implanted into rat tibial fractures and performed similarly to BMP-2 in terms of bone formation [216].
5.2. Bone fillers
A wide array of bone fillers has been developed in the laboratory to augment voids in large defects or to serve as a bonding agent in joint replacement procedures, such as total hip arthroplasty. Injectable variants are particularly attractive, since they can minimize the invasiveness often associated with surgical intervention. As with any foreign material, bone fillers introduced into the human body create the potential for infection, and consequently, tremendous efforts have been undertaken to develop materials that can thwart microbes. Furthermore, bone fillers may be used to stimulate tissue repair via the incorporation of various compounds or therapeutic molecules.
Bone fillers for anti-infection
To combat infection, antibiotics have been loaded into a variety of materials, and have largely yielded favorable results against specific strains of bacteria in laboratory studies. Polymeric materials have been utilized as antibiotic carriers due to their compositional tunability and their degradation properties. Brooks et al. [217] evaluated tobramycin release kinetics from PCL coatings on bone allograft and coralline ceramic fillers over six weeks in vitro. Encapsulating the antibiotic in oil and incorporating PEG into the PCL coating was demonstrated to minimize the initial burst release typically characteristic of drug-loaded materials. In a subsequent study, several members from the aforementioned group employed molten-casting to produce PCL-calcium carbonate/phosphate composites that could release tobramycin exceeding 10 weeks [218]. A short-term study demonstrated that these bone fillers could effectively inhibit S. aureus growth. Koort et al. [219] administered ciproflaxin via incorporation into PDLLA 50:50 pellets for 300 days, though noting a substantial delay period of 60 days. This observed lag period needs to be shortened for optimal antibiotic release. Henslee et al. [220] developed polypropylene fumarate/carboxymethylcellulose (CMC) space maintainers loaded with antibiotic-containing PLGA microparticles. Colistin and clindamycin were successfully released for prolonged periods exceeding relevant bacterial minimal inhibitory concentrations (MICs). Finally, Shah et al. [221] loaded clindamycin either encapsulated in PLGA microparticles or directly in the CMC porogen of PMMA space maintainers and evaluated these constructs in a rabbit model of critical-size mandibular defects infected with Prevotella melaninogenica. The PLGA microparticles were intended to extend the antibiotic release timeframe and attenuate the typical profile of a burst release seen with direct incorporation of the drug. At 12 weeks post-implantation, bony bridging of the defect was seen in one-third of the rabbits, but this outcome was not distinct to a specific experimental group.
Another prevalent strategy involves calcium-based ceramics and bioactive glass, which are commonly used due to their osteoconductive properties. Domingues et al. [222] loaded tetracycline or tetracycline/β-cyclodextrin into sol-gel solutions of bioactive glass and discovered that the latter delayed antibiotic release. Both experimental conditions significantly impeded Aggregatibacter actinomycetemcomitans colonization in vitro compared to pure bioglass surfaces. Xia and Chang [223] adsorbed gentamicin onto mesoporous bioactive glass for controlled release. The resulting system retained more antibiotic, had a slower rate of release that hinged on pH or ionic concentration, and stimulated HA formation in vitro. Tobramycin elution from calcium carbonate HA ceramic composites implanted in rabbit radial defects resulted in minimized infection susceptibility to S. aureus [224]. Control animals necessitated premature euthanasia, whereas no infection but some foreign body response was noted at eight weeks when the rabbits receiving antibiotic were sacrificed. Xie et al. [225] compared vancomycin delivery from calcium sulfate and borate-based bioglass in a rabbit model, finding that both were highly MRSA-negative compared to non-antibiotic containing controls. Interestingly, histological results identified good bone formation through antibiotic-eluting borate glass, but very little in the antibiotic-eluting calcium sulfate counterparts. Tuning the porosity of brushite-forming bone cement could influence the release of vancomycin and ciproflaxin, with low porosity being optimal [226]. However, results were contingent on drug solubility as well. The authors observed in this case a largely negated burst release and a practically linear elution trend.
Composite bone void fillers have also been developed to leverage the attractive properties of different materials. Arcos et al. [227] designed glass/PMMA composites, with the underlying rationale being that the glass would provide bioactivity conducive for bone regeneration, while PMMA’s hydrophobic nature would prevent a burst release of gentamicin. In another study, Mäkinen et al. [228] evaluated the therapeutic efficacy of ciproflaxin, which was incorporated into pellets consisting of biodegradable PLGA and osteoconductive bioglass microparticles, in a rabbit model. Over three months, the antibiotic was release locally, and new bone was observed around the implant site. A noted drawback of using bioceramics is that they tend to require high processing temperatures, which may compromise the incorporated drug’s bioactivity. With this in mind, Lee et al. chose silica xerogels because they conveniently undergo a sol-gel transition at room temperature and modified them with the natural polymer chitosan with the aim of making their mechanical properties and drug release profiles more favorable [229]. Based on the preliminary in vitro data, chitosan improved the implant’s mechanical properties closer to that of cortical bone and enabled extended, lower concentration vacomycin release still exceeding the MIC for S. aureus.
Potential synergism stemming from dual antibiotic release has additionally been investigated, namely by incorporating both β-lactam- and aminoglycoside-based drugs. For example, vancomycin and gentamicin loaded in PMMA spacers exhibited a high initial release and sustained profiles when implanted into clinical subjects undergoing total hip replacement surgery [230]. The majority of patients (17 out of 20) displayed lower markers for infection. More recently, Arias et al. [231] conducted a study using vancomycin, daptomycin, or gentamicin, as well as PEG600, a compound that helps prevent S. epidermis biofilm formation. The group observed that vancomycin yielded superior results to daptomycin and gentamicin, but further noted that combining daptomycin with gentamicin or PEG600 was able to entirely prevent biofilm formation by S. epidermis.
Bone fillers for tissue regeneration
Infection prevention aside, bone fillers have also served as carriers to stimulate bone repair. Thormann et al. [232] prepared calcium phosphate cements containing strontium, which was identified in a number of previous studies to possess osteonconductive properties. The resulting constructs were implanted into rat osteotomy defects and led to bone formation at the site of injury that was significantly greater than with calcium phosphate cements alone. Tahara and Ishii [233] loaded the anti-cancer drug cis-diamminedichloroplatinum (CDDP) into calcium phosphate cements, aiming to locally restore tissue at sites of resected bone tumors. They evaluated the bone fillers containing different weight percentages of CDDP using a rabbit model and found that exceeding 10% CDDP was unconducive for bone formation. In another work, Maus et al. [234] investigated the effects of adding recombinant human BMP-2 to β-tricalcium phosphate (β-TCP) in situ hardening cements implanted in an ovine trepanation defect model. The resulting bone was comparable in amount to that observed when using autografts, though it was still less than that achieved with pure β-TCP. The authors surmised that BMP-2 did not provide additional osteoinductive effects because the dense cement composition may have impeded tissue ingrowth during the early timeframe at which BMP-2 is mostly released. Likewise with a BMP-2 delivery system in mind, Saito et al. [235] discussed a body of work focused on poly(D,L-lactic acid)-poly(p-dioxanone)-poly(ethylene glycol) block copolymer (PLA-DX-PEG), noted for its biodegradability and thermosensitivity. Recombinant human BMP-2/PLA-DX-PEG was implanted into critical-size iliac bone defects in rats, and four weeks later, bone formation was observed. Moreover, to demonstrate minimal invasiveness, a heated solution of BMP-2 and PLA-DX-PEG was injected percutaneously into the femurs of mice, and new bone tissue was noted at that area three weeks later. The effectiveness of BMP-2/PLA-DX-PEG was also evaluated in concert with other materials, such as porous HA blocks and titanium fiber-mesh cylinders. Signs of bone formation were evident when these composites were implanted in their respective animal models. Finally, Maehara et al. [236] observed that a lower concentration (10µg/ml) of fibroblast growth factor-2 (FGF-2) administered from HA/collagen composites resulted in superior repair of rabbit osteochondral defects compared to both higher FGF-2 dosage-containing (100µg/ml) constructs or material alone.
6. Perspectives on next-generation wound repair devices
The design and fabrication of efficient drug delivery materials remains of vital importance to the field of healthcare. As shown in previous work, medical devices have been used to administer a wide range of drugs and proteins to combat infection, modulate inflammation and enhance tissue regeneration. A pivotal role of drug-incorporated medical devices during the wound repair process is to provide mechanical strength at the site of injury, as well as to prevent undesirable biological complications.
In the quest to design suitable carriers for bioactive agents, researchers have taken into consideration the attributes of natural and synthetic materials. Natural polymers are generally endowed with the properties of biocompatibility and biodegradability; moreover, they can potentially influence cell behavior. These materials, however, are not as amenable to customization and tend to have less robust mechanical strength. Conversely, synthetic polymers can be relatively easily tailored, but do not have bioactive properties. Composite materials have thus been developed to leverage the advantages of both types. Material configuration will furthermore be informed by the nature of the injury. Soft tissue wounds can be repaired using sutures, glues or dressings, whereas hard tissue injuries require fixation devices or bone fillers.
Ongoing work should focus on improving the release kinetics of drugs and bioactive factors. The characteristic initial burst release often observed from medical devices may cause some toxicity in vivo and could be further minimized by designing “smart” materials that are more controllable, perhaps by incorporating stimulus-responsive mechanisms [237,238]. Approaches that better harness polymer degradation to be aligned with the rate of tissue regeneration will also be invaluable. Additionally, studies that delve into furthering our understanding of how various material properties affect tissue repair will be tremendously beneficial, creating new insights for rational tuning of these materials to enhance their interactions with host tissue. Addressing all of these points would help increase the safety and efficacy of drug-eluting medical devices and bring them one step closer to clinical translation.
In aspects of manufacturing, improved technologies for materials processing are imperative. Simplified methods that could circumvent the use of harsh organic solvents [239] or high processing temperatures [240] would be particularly attractive, since these conditions may potentially render therapeutic drugs biologically inactive. The feasibility of such techniques would also depend on how well they can be scaled-up for commercial production with high quality and at low cost.
Since wound healing encompasses a complex, coordinated series of events, next-generation drug-eluting medical devices should place more emphasis on multiple drug combinations with varying release profiles. Only a few studies to-date have employed multiple drugs in one system, so this is a direction with exciting potential for growth in the coming years.
Summarily, wound repair through drug-incorporated materials has made large strides in the research setting. The hurdle from bench-to-bedside is not insignificant, but with promising results from in vitro and in vivo studies, the next task at hand will be clinical validation of these technologies. Continued innovations in material development, material processing strategies, and drug loading are highly anticipated in the coming years, and will lead to safer and more effective wound treatments.
7. Conclusions
An array of medical devices has been developed to support the wound healing process. However, several complications still remain: infection, inflammation and limited tissue regeneration. In the past 15 years, many studies have focused on developing materials with local drug delivery capabilities to enhance soft and hard tissue wound repair. Various drug loading techniques such as dip coating, absorption and simple blending method have been successfully coupled with diverse materials to vary drug release profiles and improve efficacy of wound healing.
Indeed, many challenges and further improvement still remain. The wound healing process entails complex stages involving various factors and cytokines, proving difficult to control different aspects of device-related complications simply through the use of one drug. Even if multiple drug-eluting medical devices are successfully developed in the laboratory, clinical trials and commercialization may still present significant hurdles. Nevertheless, we hope that this review provides insights that could direct further developments in drug-loaded materials for wound repair.
Table 3.
Various examples of drug-loaded orthopedic fixation devices.
| Functionality | Drug | Drug Loading Strategy | Drug Encapsulation Materials | Drug Release Timeframe | Reference |
|---|---|---|---|---|---|
| Anti-infection | Gentamicin | Polymer | PDLLA | 48 hours | [205] |
| Phosphate, PDLLA | 28 days | [206] | |||
| Ciprofloxacin | PLGA | 84 days | [207] | ||
| Gentamicin | Adsorption | TNT | 11 day | [201] | |
| Chlorhexidine, poly(hexamethylenebiguanide), Chloroxylenol | Titanium dioxide layer | N/A | [208] | ||
| Vancomycin | Covalent bonding | Titanium rods | N/A | [202] | |
| Gentamicin | Impregnation | N/A | 182 days | [203] | |
| Tobramycin | Immersion | HA | 8 days | [204] | |
| Nitrogen oxide | Dip coating | Xerogel | 3 days | [209] | |
| Tissue regeneration | TGF-β1/IGF-1 | Polymer | PDLLA | 42 days | [210, 211] |
| BMP-2 | Immersion | N/A | 35 days | [212, 213] | |
| Gentamicin/BMP-2 | Immobilization | Heparin-Dopamine | 28 days | [214] | |
| Simvastatin | PDLLA | N/A | [216] |
Table 4.
Various examples of drug-loaded bone fillers.
| Functionality | Drug | Drug Loading Strategy | Drug Encapsulation Materials | Drug Release Timeframe | Reference |
|---|---|---|---|---|---|
| Anti-infection | Tobramycin | Polymer | PCL, PEG | 42 days | [217] |
| [218] | |||||
| HA, PCL, PEG | 70 days | [224] | |||
| Ciproflaxin | PDLLA | 300 days | [219] | ||
| Clindamycin | PLGA | 28 days | [221] | ||
| Gentamicin | Mixture | Bioactive glass and PMMA | 14 days | [227] | |
| Ciprofloxacin | PLGA | 150 days | [228] | ||
| Vancomycin | Borate glass | 28 days | [225] | ||
| Xerogel-chitosan | 30 days | [229] | |||
| Gentamicin/vancomycin | PMMA | 10 days | [230] | ||
| Gentamycin/vancomycin/daptomycin | PMMA | N/A | [231] | ||
| Clindamycin Colistin | Microparticles | Poly(propylene fumarate) PLGA | 77 days | [220] | |
| Tetracycline | Sol-gel | Bioactive glass | 80 days | [222] | |
| Gentamicin | Adsorption | Mesoporous bioactive glass | 20 days | [223] | |
| Vancomycin/ciprofloxacin | Cement | Calcium phosphate | 4 days (vancomycin), 14 days (ciprofloxacin) | [226] | |
| Tissue regeneration | Strontium | Cement | Calcium phosphate | N/A | [232] |
| CDDP | Calcium phosphate | 28 days | [233] | ||
| BMP-2 | Calcium phosphate | N/A | [234] | ||
| BMP-2 | Mixture | PLA-DX-PEG | N/A | [235] | |
| FGF-2 | Impregnation | HA/collagen | N/A | [236] |
Acknowledgments
Funding: This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Ministry of Health & Welfare, Republic of Korea (HI15C1744 and HI14C2194) (YBC); the National Institutes of Health (R01 AR068073 and R21 AR067527) (AGM); the Army, Navy, National Institutes of Health, Air Force, Veterans Affairs, and Health Affairs to support the AFIRM II effort under Award No. W81XWH-14-2-0004 (AGM); and a National Science Foundation Graduate Research Fellowship (EJL).
Abbreviations
- AASF
Antheraea assama silk fibroin
- BC
benzalkonium chloride
- BMP-2
bone morphogenetic protein-2
- β-TCP
β-tricalcium phosphate
- CDDP
cis-diamminedichloroplatinum
- CMC
carboxymethylcellulose
- E. coli
Escherichia coli
- EGF
epidermal growth factor
- FGF-2
fibroblast growth factor-2
- hGH
human growth hormone
- HA
hydroxyapatite
- IGF-1
insulin-like growth factor-1
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- NSAID
nonsteroidal anti-inflammatory drug
- P. aeruginosa
Pseudomonas aeruginosa
- PCL
poly(ε-caprolactone)
- PDLLA
poly(D,L-lactic acid)
- PDX
poly(dioxanone)
- PE
poly(ethylene)
- PEG
poly(ethylene glycol)
- PEI
poly(ethyleneimine)
- PEMA
poly(ethylmethacrylate)
- PGA
poly(glycolic acid)
- PGC
poly(glyconate)
- PGL
poly(glactin)
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PLA-DX-PEG
poly(D,L-lactic acid)-poly(p-dioxanone)-poly(ethylene glycol)
- PLDLLA
poly(L,DL-lactic acid)
- PLLA
poly(L-lactic acid)
- PMMA
poly(methylmethacrylate)
- PNIPAAm
poly(N-isopropylacrylamide)
- PTMC
poly(trimethylene carbonate)
- PU
polyurethane
- PVA
poly(vinyl alcohol)
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- TGF-β1
transforming growth factor-β1
- TNT
titania nanotube
Biographies

Esther J. Lee is a bioengineering Ph.D. student in the research group of Dr. Antonios G. Mikos at Rice University. She received her B.S.E. and M.S. degrees in biomedical engineering from Duke University in 2011 and 2012, respectively. Her current research focuses on leveraging optogenetic tools from synthetic biology for bone tissue engineering applications. Lee was the recipient of a National Science Foundation Graduate Research Fellowship (2012). To date, she has co-authored 13 peer-reviewed journal papers and 1 book chapter.

Beom Kang Huh is a bioengineering Ph.D. student in the research group of Dr. Young Bin Choy at Seoul National University. He received his B.S. (2011) in biomedical engineering from University of Wisconsin - Madison and his M.S. (2013) in bioengineering from the University of Pennsylvania. His current research focuses on the development of drug-loaded medical devices for various applications. Recently, he has co-authored 8 peer-reviewed journal papers.

Se Na Kim is a bioengineering Ph.D. student in the research group of Dr. Young Bin Choy at Seoul National University. She received her B.S.E. and M.S. degrees in chemical engineering from Inha University in 2007 and 2012, respectively. She is currently working on the fabrication of sustained drug delivery formulations using biocompatible polymer based nanoparticles and microparticles and metal-organic frameworks for glaucoma, pain and cancer therapy. To-date, she has co-authored 11 peer-reviewed journal papers.

Jae Yeon Lee is a bioengineering M.S. student in the research group of Dr. Young Bin Choy at Seoul National University. She received her B.S.E. in biomedical engineering from Chung-Ang University in 2015. She is currently working on the delivery of biopolymer nanoparticles for ocular application. She has co-authored 2 peer-reviewed journal papers.

Chun Gwon Park received his B.S. (2009) from Hanyang University before earning his Ph.D. (2014) from Seoul National University under the supervision of Dr. Young Bin Choy in the department of biomedical engineering. Dr. Park’s graduate research dealt primarily with the design, fabrication, and evaluation of novel drug delivery devices. Specifically, he used nanofiber-structured biopolymers in order to achieve effective and sustained delivery of drugs at desired sites in the body. Dr. Park is currently a research fellow in the research group of Dr. Michael Goldberg at Harvard Medical School and Dana-Farber Cancer Institute, where he focuses mainly on biomaterial-based cancer immunotherapy. To date, Dr. Park has published 18 papers.

Dr. Antonios G. Mikos is the Louis Calder Professor of Bioengineering and Chemical and Biomolecular Engineering at Rice University. He obtained his Dipl.Eng. (1983) from the Aristotle University of Thessaloniki, Greece, followed by a Ph.D. (1988) in chemical engineering from Purdue University. Dr. Mikos conducted postdoctoral research at the Massachusetts Institute of Technology and Harvard Medical School until starting a faculty position at Rice University in 1992. His research encompasses biomaterials development, drug delivery, and gene therapy, with present emphasis on bone and cartilage tissue engineering. Dr. Mikos’ work has thus far garnered over 550 publications and 28 patents. Moreover, he has served as editor of 15 books and authored one textbook. Dr. Mikos has received numerous accolades, including the Lifetime Achievement Award from the Tissue Engineering and Regenerative Medicine International Society – Americas, the Founders Award of the Society for Biomaterials, and the Robert A. Pritzker Distinguished Lecturer Award of the Biomedical Engineering Society. He is a Member of the National Academy of Engineering, the National Academy of Medicine, the Academy of Medicine, Engineering, and Science of Texas, and the Academy of Athens, as well as a Fellow of the National Academy of Inventors.

Dr. Young Bin Choy is an Associate Professor in the Department of Biomedical Engineering at Seoul National University College of Medicine, Korea. He received his B.S. (1999) from Seoul National University, his M.S. (2000) in electrical engineering from University of Wisconsin - Madison and his Ph.D. (2006) in electrical engineering from University of Illinois at Urbana, Champaign. Dr. Choy worked as a postdoctoral fellow at the Georgia Institute of Technology until he became a faculty member at Seoul National University in 2009. His research is focused on developing biomaterial-based devices for various applications in medicine, such as drug delivery, tissue engineering and biomedical implants. Dr. Choy has published over 55 papers, and applied and issued over 40 patents. Dr. Choy has received the Young Biomedical Engineer Award and the Special Contribution Award from the Korean Society of Medical and Biological Engineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Leaper DJ, Harding KG. Wounds: biology and management. Oxford University Press; USA: 1998. [Google Scholar]
- 3.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Enoch S, Leaper DJ. Basic science of wound healing. Surgery (Oxford) 2008;26:31–7. [Google Scholar]
- 5.Bowler P, Duerden B, Armstrong D. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 7.Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19:66–75. doi: 10.1016/j.jse.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Ovington LG. Advances in wound dressings. Clin Dermatol. 2007;25:33–8. doi: 10.1016/j.clindermatol.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res. 1997;38:155–82. doi: 10.1002/(sici)1097-4636(199722)38:2<155::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 11.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad CS, Gardner TR, Groh M, Arnouk J, Levine WN. Mechanical properties of soft tissue femoral fixation devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:635–40. doi: 10.1177/0363546503261714. [DOI] [PubMed] [Google Scholar]
- 13.Khan TA, Peh KK, Ch’ng HS. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharmaceut Sci. 2000;3:303–11. [PubMed] [Google Scholar]
- 14.Chu C. Mechanical properties of suture materials: An important characterization. Annals of surgery. 1981;193:365. doi: 10.1097/00000658-198103000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne D, Hardy J, Wood R, McIntosh R, Cuschieri A. Effect of fibrin glues on the mechanical properties of healing wounds. Brit J Surg. 1991;78:841–3. doi: 10.1002/bjs.1800780723. [DOI] [PubMed] [Google Scholar]
- 16.Kull S, Martinelli I, Briganti E, Losi P, Spiller D, Tonlorenzi S, et al. Glubran2 surgical glue: in vitro evaluation of adhesive and mechanical properties. J Surg Res. 2009;157:e15–e21. doi: 10.1016/j.jss.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Rho KS, Jeong L, Lee G, Seo B-M, Park YJ, Hong S-D, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–61. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–224. [Google Scholar]
- 19.Niinomi M. Mechanical properties of biomedical titanium alloys. Mater Sci Eng, A. 1998;243:231–6. [Google Scholar]
- 20.Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354–61. [PubMed] [Google Scholar]
- 21.Bauer TW, Muschler GF. Bone Graft Materials: An Overview of the Basic Science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 22.Kazemzadeh-Narbat M, Annabi N, Khademhosseini A. Surgical sealants and high strength adhesives. Mater Today. 2015;18:176–7. [Google Scholar]
- 23.Control CfD, Prevention. National hospital ambulatory medical care survey. 2011 [Google Scholar]
- 24.MedMarket Diligence, LLC, Report #S190, “Worldwide Surgical Sealants, Glues, Wound Closure and Anti-Adhesion Markets, 2010–2017. 2012 [Google Scholar]
- 25.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 27.Franz MG, Steed DL, Robson MC. Optimizing healing of the acute wound by minimizing complications. Curr Probl Surg. 2007;44:691–763. doi: 10.1067/j.cpsurg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Ruff ME, Lubbers LM. Treatment of subtrochanteric fractures with a sliding screw-plate device. J Trauma Acute Care Surg. 1986;26:75–80. doi: 10.1097/00005373-198601000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Nadarajah R, Mahaluxmivala J, Amin A, Goodier D. Clavicular hook–plate: complications of retaining the implant. Injury. 2005;36:681–3. doi: 10.1016/j.injury.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Flores LA, Harrington I, Heller M. The stability of intertrochanteric fractures treated with a sliding screw-plate. J Bone Joint Surg Brit Vol. 1990;72:37–40. doi: 10.1302/0301-620X.72B1.2298792. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Staffenberg D, Petronio J, Wood R. Bioabsorbable plates and screws in pediatric craniofacial surgery: a review of 22 cases. J Craniofac Surg. 1997;8:97–9. doi: 10.1097/00001665-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergsma EJ, Rozema FR, Bos RR, De Bruijn WC. Foreign body reactions to resorbable poly (L-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J Oral Maxillofac Surg. 1993;51:666–70. doi: 10.1016/s0278-2391(10)80267-8. [DOI] [PubMed] [Google Scholar]
- 34.Falotico R, Kopia GA, Llanos GH, Siekierka J, Carter AJ. Antiinflammatory drug and delivery device. Google Patents. 2004 [Google Scholar]
- 35.Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–67. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 36.Heal CF, Buettner PG, Cruickshank R, Graham D, Browning S, Pendergast J, et al. Does single application of topical chloramphenicol to high risk sutured wounds reduce incidence of wound infection after minor surgery? Prospective randomised placebo controlled double blind trial. BMJ. 2009;338 doi: 10.1136/bmj.a2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 38.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma Acute Care Surg. 1984;24:742–6. doi: 10.1097/00005373-198408000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Holzapfel GA. Biomechanics of soft tissue. The handbook of materials behavior models. 2001;3:1049–63. [Google Scholar]
- 40.Currey JD. The design of mineralised hard tissues for their mechanical functions. J Exp Biol. 1999;202:3285–94. doi: 10.1242/jeb.202.23.3285. [DOI] [PubMed] [Google Scholar]
- 41.Crecelius C. Soft Tissue Trauma. Atlas of the Oral and Maxillofacial Surgery Clinics. 2013;21:49–60. doi: 10.1016/j.cxom.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Chu C-C, Von Fraunhofer JA, Greisler HP. Wound closure biomaterials and devices. CRC Press; 1996. [Google Scholar]
- 43.Duarte A, Coelho J, Bordado J, Cidade M, Gil M. Surgical adhesives: systematic review of the main types and development forecast. Prog Polym Sci. 2012;37:1031–50. [Google Scholar]
- 44.Pillai CKS, Sharma CP. Review paper: absorbable polymeric surgical sutures: chemistry, production, properties, biodegradability, and performance. J Biomater Appl. 2010;25:291–366. doi: 10.1177/0885328210384890. [DOI] [PubMed] [Google Scholar]
- 45.Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Tech. 2010;21:77–95. [Google Scholar]
- 46.Abdelrahman T, Newton H. Wound dressings: principles and practice. Surgery (Oxford) 2011;29:491–5. [Google Scholar]
- 47.Park SU, Lee BK, Kim MS, Park KK, Sung WJ, Kim HY, et al. The possibility of microbial cellulose for dressing and scaffold materials. Int Wound J. 2014;11:35–43. doi: 10.1111/j.1742-481X.2012.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chellamani K, Veerasubramanian D, Balaji RV. Surgical Sutures: An overview [Google Scholar]
- 49.Capperauld I. Suture materials: A review. Clin Mater. 1989;4:3–12. [Google Scholar]
- 50.Bennett RG. Selection of wound closure materials. J Am Acad Dermatol. 1988;18:619–37. doi: 10.1016/s0190-9622(88)70083-3. [DOI] [PubMed] [Google Scholar]
- 51.Lim TY, Poh CK, Wang W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J Mater Sci Mater Med. 2009;20:1669–75. doi: 10.1007/s10856-009-3727-z. [DOI] [PubMed] [Google Scholar]
- 52.Luis AL, Rodrigues JM, Amado S, Veloso AP, Armada-Dasilva PA, Raimondo S, et al. PLGA 90/10 and caprolactone biodegradable nerve guides for the reconstruction of the rat sciatic nerve. Microsurgery. 2007;27:125–37. doi: 10.1002/micr.20317. [DOI] [PubMed] [Google Scholar]
- 53.Tajirian AL, Goldberg DJ. A review of sutures and other skin closure materials. J Cosmet Laser Ther. 2010;12:296–302. doi: 10.3109/14764172.2010.538413. [DOI] [PubMed] [Google Scholar]
- 54.Johnson A, Rodeheaver GT, Durand LS, Edgerton MT, Edlich RF. Automatic disposable stapling devices for wound closure. Ann Emerg Med. 1981;10:631–5. doi: 10.1016/s0196-0644(81)80086-8. [DOI] [PubMed] [Google Scholar]
- 55.Stegmaier OC. Use of skin stapler in dermatologic surgery. J Am Acad Dermatol. 1982;6:305–9. doi: 10.1016/s0190-9622(82)70020-9. [DOI] [PubMed] [Google Scholar]
- 56.Mery CM, Shafi BM, Binyamin G, Morton JM, Gertner M. Profiling surgical staplers: effect of staple height, buttress, and overlap on staple line failure. Surg Obes Relat Dis. 2008;4:416–22. doi: 10.1016/j.soard.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Gatt D, Quick C, Owen-Smith M. Staples for wound closure: a controlled trial. Ann R Coll Surg Engl. 1985;67:318. [PMC free article] [PubMed] [Google Scholar]
- 58.Hirashima T, Eto T, DenBesten L. Lactomer copolymer absorbable staples in gastrointestinal surgery. Am J Surg. 1985;150:381–5. doi: 10.1016/0002-9610(85)90084-4. [DOI] [PubMed] [Google Scholar]
- 59.Bonney WW, Robinson RA. Absorbable staples in continentileal urinary pouch. Urology. 1990;35:57–62. doi: 10.1016/0090-4295(90)80014-e. [DOI] [PubMed] [Google Scholar]
- 60.Bond SJ, Harrison MR, Slotnick RN, Anderson J, Flake AW, Adzick NS. Cesarean delivery and hysterotomy using an absorbable stapling device. Obstet Gynecol. 1989;74:25–8. [PubMed] [Google Scholar]
- 61.Laurens N, Koolwijk P, De Maat M. Fibrin structure and wound healing. J Thromb Haemost. 2006;4:932–9. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 62.Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J Biomater Appl. 1993;7:309–52. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 63.Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. The American journal of emergency medicine. 2008;26:490–6. doi: 10.1016/j.ajem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 64.Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World J Surg. 2010;34:632–4. doi: 10.1007/s00268-009-0252-7. [DOI] [PubMed] [Google Scholar]
- 65.Chen C-F, Shen H-H, Lin T-Y, Yu Y-J, Chen W-C, Chu I-M. Studies on the preparation and characterization of mPEG-polyester biodegradable bioglue for bone defect repair. J Med Biol Eng. 2011;31:13–7. [Google Scholar]
- 66.Coulthard P, Worthington H, Esposito M, van der Elst M, van Waes OJ. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst Rev. 2002;3 doi: 10.1002/14651858.CD004287.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Passage J, Jalali H, Tam RK, Harrocks S, O’Brien MF. BioGlue Surgical Adhesive—an appraisal of its indications in cardiac surgery. Ann Thorac Surg. 2002;74:432–7. doi: 10.1016/s0003-4975(02)03689-5. [DOI] [PubMed] [Google Scholar]
- 68.Blondeel PN, Murphy JW, Debrosse D, Nix JC, Puls LE, Theodore N, et al. Closure of long surgical incisions with a new formulation of 2-octylcyanoacrylate tissue adhesive versus commercially available methods. Am J Surg. 2004;188:307–13. doi: 10.1016/j.amjsurg.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–36. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Paul W, Sharma CP. Chitosan and alginate wound dressings: a short review. Trends Biomater Artif Organs. 2004;18:18–23. [Google Scholar]
- 71.Queen D, Evans J, Gaylor J, Courtney J, Reid W. Burn wound dressings—a review. Burns. 1987;13:218–28. doi: 10.1016/0305-4179(87)90170-7. [DOI] [PubMed] [Google Scholar]
- 72.Razak SIA, Sharif N, Rahman W. Biodegradable polymers and their bone applications: a review. Int J Basic Appl Sci. 2012;12:31–49. [Google Scholar]
- 73.Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013;9:7093–114. doi: 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 74.Hanna JR, Giacopelli JA. A review of wound healing and wound dressing products. J Foot Ankle Surg. 1997;36:2–14. doi: 10.1016/s1067-2516(97)80003-8. [DOI] [PubMed] [Google Scholar]
- 75.Sawada Y, Ara M, Yotsuyanagi T, Sone K. Treatment of dermal depth burn wounds with an antimicrobial agent-releasing silicone gel sheet. Burns. 1990;16:347–52. doi: 10.1016/0305-4179(90)90007-j. [DOI] [PubMed] [Google Scholar]
- 76.Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330:138–45. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 77.Still J, Glat P, Silverstein P, Griswold J, Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003;29:837–41. doi: 10.1016/s0305-4179(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 78.Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003;55:1595–611. doi: 10.1016/j.addr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Daniels A, Chang MK, Andriano KP, Heller J. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J Appl Biomater. 1990;1:57–78. doi: 10.1002/jab.770010109. [DOI] [PubMed] [Google Scholar]
- 80.Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34:3174–83. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taljanovic MS, Jones MD, Ruth JT, Benjamin JB, Sheppard JE, Hunter TB. Fracture Fixation 1. Radiographics. 2003;23:1569–90. doi: 10.1148/rg.236035159. [DOI] [PubMed] [Google Scholar]
- 82.Long PH. Medical devices in orthopedic applications. Toxicol Pathol. 2008;36:85–91. doi: 10.1177/0192623307310951. [DOI] [PubMed] [Google Scholar]
- 83.Rodríguez-González FÁ. Biomaterials in Orthopaedic Surgery. ASM International; 2009. [Google Scholar]
- 84.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials science: an introduction to materials in medicine. Academic Press; 2004. [Google Scholar]
- 85.Vaccaro AR, Singh K, Haid R, Kitchel S, Wuisman P, Taylor W, et al. The use of bioabsorbable implants in the spine. Spine J. 2003;3:227–37. doi: 10.1016/s1529-9430(02)00412-6. [DOI] [PubMed] [Google Scholar]
- 86.Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615–21. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 87.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 88.Pryor LS, Gage E, Langevin C-J, Herrera F, Breithaupt AD, Gordon CR, et al. Review of bone substitutes. Craniomaxillofac Trauma Reconstr. 2009;2:151. doi: 10.1055/s-0029-1224777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nandi S, Roy S, Mukherjee P, Kundu B, De D, Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res. 2010;132:15–30. [PubMed] [Google Scholar]
- 90.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 91.Ginebra M-P, Traykova T, Planell J. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113:102–10. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Kenny S, Buggy M. Bone cements and fillers: a review. J Mater Sci Mater Med. 2003;14:923–38. doi: 10.1023/a:1026394530192. [DOI] [PubMed] [Google Scholar]
- 93.Campton-Johnston SM, Wilson JA. Infected Wound Management: Advanced Technologies, Moisture-Retentive Dressings, and Die-Hard Methods. Crit Care Nurs Q. 2001;24:64–77. doi: 10.1097/00002727-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 94.Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–15. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 95.Phillips SJ. Physiology of wound healing and surgical wound care. ASAIO J. 2000;46:S2–S5. doi: 10.1097/00002480-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 96.Kunt TK. Disorders of wound healing. World J Surg. 1980;4:271–7. doi: 10.1007/BF02393382. [DOI] [PubMed] [Google Scholar]
- 97.Fleck T, Moidl R, Blacky A, Fleck M, Wolner E, Grabenwoger M, et al. Triclosan-coated sutures for the reduction of sternal wound infections: economic considerations. Ann Thorac Surg. 2007;84:232–6. doi: 10.1016/j.athoracsur.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 98.Edmiston CE, Seabrook GR, Goheen MP, Krepel CJ, Johnson CP, Lewis BD, et al. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg. 2006;203:481–9. doi: 10.1016/j.jamcollsurg.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 99.Scott RD. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. 2009 [Google Scholar]
- 100.Wilson BA, Salyers AA, Whitt DD, Winkler ME. Bacterial pathogenesis: a molecular approach. American Society for Microbiology (ASM); 2011. [Google Scholar]
- 101.Weinstein RA, Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis. 2001;33:1567–72. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- 102.Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2:176–94. doi: 10.4161/biom.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ercan B, Kummer KM, Tarquinio KM, Webster TJ. Decreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation. Acta Biomater. 2011;7:3003–12. doi: 10.1016/j.actbio.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 104.Edlich RF, Panek PH, Rodeheaver GT, Kurtz LD, Edgerton MT. Surgical sutures and infection: a biomaterial evaluation. J Biomed Mater Res. 1974;8:115–26. doi: 10.1002/jbm.820080312. [DOI] [PubMed] [Google Scholar]
- 105.Choi H-J, Chae H-D. Comparison of E. coli infiltration between new synthetic absorbable sutures. J Korean Surg Soc. 2009;77:1–6. [Google Scholar]
- 106.Stashak TS, Yturraspe DJ. Considerations for selection of suture materials. Vet Surg. 1978;7:48–55. [Google Scholar]
- 107.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 108.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 109.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 110.Anderson JM. Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993;2:33–41. [Google Scholar]
- 111.Deodhar AK, Rana R. Surgical physiology of wound healing: a review. J Postgrad Med. 1997;43:52. [PubMed] [Google Scholar]
- 112.Anderson JM, Rodriguez A, Chang DT. Semin Immunol. Elsevier; 2008. Foreign body reaction to biomaterials; pp. 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luttikhuizen DT, Harmsen MC, Luyn MJV. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12:1955–70. doi: 10.1089/ten.2006.12.1955. [DOI] [PubMed] [Google Scholar]
- 114.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2:1003–15. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stroncek JD, Reichert WM. Overview of wound healing in different tissue types. In: Reichert WM, editor. Indwelling neural implants: strategies for contending with the in vivo environment. Boca Raton, FL: CRC Press; 2008. [Google Scholar]
- 116.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 117.Harris I, Yee K, Walters C, Cunliffe WJ, Kearney J, Wood E, et al. Cytokine and protease levels in healing and non-healing chronic venous leg ulcers. Exp Dermatol. 1995;4:342–9. doi: 10.1111/j.1600-0625.1995.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 118.Dorai AA. Wound care with traditional, complementary and alternative medicine. Indian J Plast Surg. 2012;45:418. doi: 10.4103/0970-0358.101331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS. Curcumin-loaded poly (ε-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin Exp Pharmacol Physiol. 2009;36:1149–56. doi: 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laing P. Diabetic foot ulcers. Am J Surg. 1994;167:S31–S6. doi: 10.1016/0002-9610(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 121.Sawada Y, Sone K. Hydration and occlusion treatment for hypertrophic scars and keloids. Br J Plast Surg. 1992;45:599–603. doi: 10.1016/0007-1226(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 122.Augat P, Simon U, Liedert A, Claes L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005;16:S36–S43. doi: 10.1007/s00198-004-1728-9. [DOI] [PubMed] [Google Scholar]
- 123.Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9:699–712. doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seppala MD, Rose ME. Prescription painkillers: history, pharmacology, and treatment. Hazelden Publishing; 2013. [Google Scholar]
- 125.Barajas-Nava L, López-Alcalde J, Solà I. Antibiotics to prevent burn wounds becoming infected. Health (NY) 2013 [Google Scholar]
- 126.Kumar S, Singh BR. An overview of mechanisms and emergence of antimicrobials drug resistance. Emergence. 2013;2013:10–24. [Google Scholar]
- 127.Van Krimpen P, Van Bennekom W, Bult A. Penicillins and cephalosporins. Pharm Weekbl. 1987;9:1–23. doi: 10.1007/BF01956487. [DOI] [PubMed] [Google Scholar]
- 128.Geddes AM, Gould IM. Ampicillin, amoxicillin and other ampicillin-like penicillins. In: Grayson ML, Crowe SM, McCarthy JS, Mills J, Mouton JW, Norrby SR, editors. Kucers’ the use of antibiotics. London: Hodder Arnold; 2010. pp. 65–92. [Google Scholar]
- 129.Aronson JK. Meyler's Side Effects of Antimicrobial Drugs. Oxford: Elsevier; 2009. [Google Scholar]
- 130.Kahlmeter G, Dahlager JI. Aminoglycoside toxicity–a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984;13:9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 131.Avent M, Rogers B, Cheng A, Paterson D. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 2011;41:441–9. doi: 10.1111/j.1445-5994.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- 132.Penniston SG, Hargreaves KM. Evaluation of periapical injection of Ketorolac for management of endodontic pain. J Endod. 1996;22:55–9. doi: 10.1016/S0099-2399(96)80272-X. [DOI] [PubMed] [Google Scholar]
- 133.Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacother. 2005;25:1566–91. doi: 10.1592/phco.2005.25.11.1566. [DOI] [PubMed] [Google Scholar]
- 134.Urbanska AM, Zhang X, Prakash S. Bioengineered Colorectal Cancer Drugs: Orally Delivered Anti-Inflammatory Agents. Cell Biochem Biophys. 2015:1–13. doi: 10.1007/s12013-015-0528-5. [DOI] [PubMed] [Google Scholar]
- 135.Troullos ES, Hargreaves KM, Butler DP, Dionne RA. Comparison of nonsteroidal anti-inflammatory drugs, ibuprofen and flurbiprofen, with methylprednisolone and placebo for acute pain, swelling, and trismus. J Oral Maxillofac Surg. 1990;48:945–52. doi: 10.1016/0278-2391(90)90007-o. [DOI] [PubMed] [Google Scholar]
- 136.Serhan CN. Controlling the resolution of acute inflammation: a new genus of dual anti-inflammatory and proresolving mediators. J Periodontol. 2008;79:1520–6. doi: 10.1902/jop.2008.080231. [DOI] [PubMed] [Google Scholar]
- 137.Kokki H. Nonsteroidal anti-inflammatory drugs for postoperative pain. Pediatr Drugs. 2003;5:103–23. doi: 10.2165/00128072-200305020-00004. [DOI] [PubMed] [Google Scholar]
- 138.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 139.Bjarnason I, Hayllar J, Macpherson AJ, Russell A. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterol. 1993;104:1832–47. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- 140.Daniels R, Knie U. Galenics of dermal products–vehicles, properties and drug release. J Dtsch Dermatol Ges. 2007;5:367–83. doi: 10.1111/j.1610-0387.2007.06321.x. [DOI] [PubMed] [Google Scholar]
- 141.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect (Larchmt) 2002;3:s79–s87. doi: 10.1089/sur.2002.3.s1-79. [DOI] [PubMed] [Google Scholar]
- 143.Barbolt TA. Chemistry and safety of triclosan, and its use as an antimicrobial coating on Coated VICRYL* Plus Antibacterial Suture (coated polyglactin 910 suture with triclosan) Surg Infect (Larchmt) 2002;3:s45–s53. doi: 10.1089/sur.2002.3.s1-45. [DOI] [PubMed] [Google Scholar]
- 144.Storch ML, Rothenburger SJ, Jacinto G. Experimental efficacy study of coated VICRYL plus antibacterial suture in guinea pigs challenged with Staphylococcus aureus. Surg Infect (Larchmt) 2004;5:281–8. doi: 10.1089/sur.2004.5.281. [DOI] [PubMed] [Google Scholar]
- 145.Ming X, Rothenburger S, Nichols MM. In vivo and in vitro antibacterial efficacy of PDS plus (polidioxanone with triclosan) suture. Surg Infect (Larchmt) 2008;9:451–7. doi: 10.1089/sur.2007.061. [DOI] [PubMed] [Google Scholar]
- 146.Ming X, Nichols M, Rothenburger S. In vivo antibacterial efficacy of MONOCRYL plus antibacterial suture (Poliglecaprone 25 with triclosan) Surg Infect (Larchmt) 2007;8:209–14. doi: 10.1089/sur.2006.004. [DOI] [PubMed] [Google Scholar]
- 147.Samyang_biopharm. Antimicrobial Suture Materials (Neosorb®Plus) 2010 https:// http://www.samyangbiopharm.com/eng.
- 148.Rozzelle CJ, Leonardo J, Li V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J Neurosurg Pediatr. 2008;2:111–7. doi: 10.3171/PED/2008/2/8/111. [DOI] [PubMed] [Google Scholar]
- 149.Justinger C, Moussavian MR, Schlueter C, Kopp B, Kollmar O, Schilling MK. Antibiotic coating of abdominal closure sutures and wound infection. Surgery. 2009;145:330–4. doi: 10.1016/j.surg.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Obermeier A, Schneider J, Wehner S, Matl FD, Schieker M, von Eisenhart-Rothe R, et al. Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS One. 2014;9:e101426. doi: 10.1371/journal.pone.0101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Obermeier A, Schneider J, Föhr P, Wehner S, Kühn K-D, Stemberger A, et al. In vitro evaluation of novel antimicrobial coatings for surgical sutures using octenidine. BMC Microbiol. 2015;15:1. doi: 10.1186/s12866-015-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shanmugasundaram O, Gowda RM, Saravanan D. Drug release and antimicrobial studies on polylactic acid suture. Int J Biotechnol Mol Biol Res. 2011;2:80–9. [Google Scholar]
- 153.Gupta B, Anjum N, Gulrez S, Singh H. Development of antimicrobial polypropylene sutures by graft copolymerization. II. Evaluation of physical properties, drug release, and antimicrobial activity. J Appl Polym Sci. 2007;103:3534–8. [Google Scholar]
- 154.Choudhury AJ, Gogoi D, Chutia J, Kandimalla R, Kalita S, Kotoky J, et al. Controlled antibiotic-releasing Antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery. 2015 doi: 10.1016/j.surg.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 155.Lee JE, Park S, Park M, Kim MH, Park CG, Lee SH, et al. Surgical suture assembled with polymeric drug-delivery sheet for sustained, local pain relief. Acta Biomater. 2013;9:8318–27. doi: 10.1016/j.actbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 156.Lee D-H, Kwon T-Y, Kim K-H, Kwon S-T, Cho D-H, Jang SH, et al. Anti-inflammatory drug releasing absorbable surgical sutures using poly (lactic-co-glycolic acid) particle carriers. Polym Bull. 2014;71:1933–46. [Google Scholar]
- 157.He CL, Huang ZM, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2009;89:80–95. doi: 10.1002/jbm.a.32004. [DOI] [PubMed] [Google Scholar]
- 158.Hu W, Huang Z-M, Liu X-Y. Development of braided drug-loaded nanofiber sutures. Nanotechnology. 2010;21:315104. doi: 10.1088/0957-4484/21/31/315104. [DOI] [PubMed] [Google Scholar]
- 159.Watret L, White R. Surgical wound management: the role of dressings. Nurs Stand. 2001;15:59–69. doi: 10.7748/ns2001.07.15.44.59.c3060. [DOI] [PubMed] [Google Scholar]
- 160.Zurita R, Puiggalí J, Rodríguez-Galán A. Loading and Release of Ibuprofen in Multi- and Monofilament Surgical Sutures. Macromol Biosci. 2006;6:767–75. doi: 10.1002/mabi.200600084. [DOI] [PubMed] [Google Scholar]
- 161.Wang L, Chen D, Sun J. Layer-by-layer deposition of polymeric microgel films on surgical sutures for loading and release of ibuprofen. Langmuir. 2009;25:7990–4. doi: 10.1021/la9004664. [DOI] [PubMed] [Google Scholar]
- 162.Padmakumar S, Joseph J, Neppalli MH, Mathew SE, Nair SV, Shankarappa SA, et al. Electrospun Polymeric Core–sheath Yarns as Drug Eluting Surgical Sutures. ACS Appl Mater Interfaces. 2016;8:6925–34. doi: 10.1021/acsami.6b00874. [DOI] [PubMed] [Google Scholar]
- 163.Chellamani K, Balaji R, Veerasubramanian D. Development of Wound Dressing Made of Electro Spun Tetracycline Hydrochloride Drug Incorporated PCL (Poly (Ε-Caprolactone)) Nanomembrane. Int J Emerg Technol Adv Eng. 2010;4:1–6. [Google Scholar]
- 164.Sung JH, Hwang M-R, Kim JO, Lee JH, Kim YI, Kim JH, et al. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392:232–40. doi: 10.1016/j.ijpharm.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 165.Maneerung T, Tokura S, Rujiravanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym. 2008;72:43–51. [Google Scholar]
- 166.Lin S-Y, Chen K-S, Run-Chu L. Design and evaluation of drug-loaded wound dressing having thermoresponsive, adhesive, absorptive and easy peeling properties. Biomaterials. 2001;22:2999–3004. doi: 10.1016/s0142-9612(01)00046-1. [DOI] [PubMed] [Google Scholar]
- 167.Maeda M, Kadota K, Kajihara M, Sano A, Fujioka K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. Journal of controlled release. 2001;77:261–72. doi: 10.1016/s0168-3659(01)00512-0. [DOI] [PubMed] [Google Scholar]
- 168.Ulubayram K, Cakar AN, Korkusuz P, Ertan C, Hasirci N. EGF containing gelatinbased wound dressings. Biomaterials. 2001;22:1345–56. doi: 10.1016/s0142-9612(00)00287-8. [DOI] [PubMed] [Google Scholar]
- 169.Tsou T-L, Tang S-T, Huang Y-C, Wu J-R, Young J-J, Wang H-J. Poly (2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release studies. J Mater Sci Mater Med. 2005;16:95–100. doi: 10.1007/s10856-005-5954-2. [DOI] [PubMed] [Google Scholar]
- 170.Kim JO, Park JK, Kim JH, Jin SG, Yong CS, Li DX, et al. Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int J Pharm. 2008;359:79–86. doi: 10.1016/j.ijpharm.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 171.Zahedi P, Karami Z, Rezaeian I, Jafari SH, Mahdaviani P, Abdolghaffari AH, et al. Preparation and performance evaluation of tetracycline hydrochloride loaded wound dressing mats based on electrospun nanofibrous poly (lactic acid)/poly (ε-caprolactone) blends. J Appl Polym Sci. 2012;124:4174–83. [Google Scholar]
- 172.Campbell N, Campbell D. Evaluation of a non-adherent, povidone-iodine dressing in a case series of chronic wounds. J Wound Care. 2013;22 doi: 10.12968/jowc.2013.22.8.401. [DOI] [PubMed] [Google Scholar]
- 173.Vermeulen H, Ubbink D, Goossens A, De Vos R, Legemate D, Westerbos S. Dressings and topical agents for surgical wounds healing by secondary intention. Cochrane Database Syst Rev. 2004;2:CD003554. doi: 10.1002/14651858.CD003554.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moberg S, Hoffman L, Grennert ML, Holst A. A randomized trial of cadexomer iodine in decubitus ulcers. J Am Geriatr Soc. 1983;31:462–5. doi: 10.1111/j.1532-5415.1983.tb05117.x. [DOI] [PubMed] [Google Scholar]
- 175.Skog E, Arnesjö B, Troeng T, Gjöres J, Bergljung L, Gundersen J, et al. A randomized trial comparing cadexomer iodine and standard treatment in the out-patient management of chronic venous ulcers. Br J Dermatol. 1983;109:77–83. doi: 10.1111/j.1365-2133.1983.tb03995.x. [DOI] [PubMed] [Google Scholar]
- 176.Mertz PM, Oliveira-Gandia MF, Davis SC. The evaluation of a cadexomer iodine wound dressing on methicillin resistant Staphylococcus aureus (MRSA) in acute wounds. Dermatol Surg. 1999;25:89–93. doi: 10.1046/j.1524-4725.1999.08055.x. [DOI] [PubMed] [Google Scholar]
- 177.Zhou L, Nahm W, Badiavas E, Yufit T, Falanga V. Slow release iodine preparation and wound healing: in vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Br J Dermatol. 2002;146:365–74. doi: 10.1046/j.1365-2133.2002.04605.x. [DOI] [PubMed] [Google Scholar]
- 178.Sagrillo DP, Kunz S. Effective Scar Management: The Old and the New. Plast Surg Nurs. 2002;22:74–6. [Google Scholar]
- 179.Rossi S, Marciello M, Sandri G, Ferrari F, Bonferoni MC, Papetti A, et al. Wound dressings based on chitosans and hyaluronic acid for the release of chlorhexidine diacetate in skin ulcer therapy. Pharm Dev Technol. 2007;12:415–22. doi: 10.1080/10837450701366903. [DOI] [PubMed] [Google Scholar]
- 180.Abrigo M, McArthur SL, Kingshott P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci. 2014;14:772–92. doi: 10.1002/mabi.201300561. [DOI] [PubMed] [Google Scholar]
- 181.Toncheva A, Paneva D, Maximova V, Manolova N, Rashkov I. Antibacterial fluoroquinolone antibiotic-containing fibrous materials from poly (L-lactide-co-D, L-lactide) prepared by electrospinning. Eur J Pharm Sci. 2012;47:642–51. doi: 10.1016/j.ejps.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 182.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. J Biomed Mater Res B. 2004;70:286–96. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 183.Jin SG, Yousaf AM, Jang SW, Son MW, Kim KS, Kim DW, et al. In Vivo Wound-Healing Effects of Novel Benzalkonium Chloride-Loaded Hydrocolloid Wound Dressing. Drug Dev Res. 2015;76:157–65. doi: 10.1002/ddr.21253. [DOI] [PubMed] [Google Scholar]
- 184.Siafaka PI, Zisi AP, Exindari MK, Karantas ID, Bikiaris DN. Porous dressings of modified chitosan with poly (2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr Polym. 2016;143:90–9. doi: 10.1016/j.carbpol.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 185.Thakur R, Florek C, Kohn J, Michniak B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int J Pharm. 2008;364:87–93. doi: 10.1016/j.ijpharm.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 186.Boateng JS, Pawar HV, Tetteh J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int J Pharm. 2013;441:181–91. doi: 10.1016/j.ijpharm.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 187.Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J Control Release. 2010;148:49–55. doi: 10.1016/j.jconrel.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hidas G, Kastin A, Mullerad M, Shental J, Moskovitz B, Nativ O. Sutureless nephron-sparing surgery: use of albumin glutaraldehyde tissue adhesive (BioGlue) Urology. 2006;67:697–700. doi: 10.1016/j.urology.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 189.Walgenbach KJ, Bannasch H, Kalthoff S, Rubin JP. Randomized, prospective study of TissuGlu® surgical adhesive in the management of wound drainage following abdominoplasty. Aesthetic Plast Surg. 2012;36:491–6. doi: 10.1007/s00266-011-9844-3. [DOI] [PubMed] [Google Scholar]
- 190.Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573–82. [PubMed] [Google Scholar]
- 191.Catelas I, Dwyer JF, Helgerson S. Controlled release of bioactive transforming growth factor beta-1 from fibrin gels in vitro. Tissue Eng Part C Methods. 2008;14:119–28. doi: 10.1089/ten.tec.2007.0262. [DOI] [PubMed] [Google Scholar]
- 192.Breen A, Dockery P, O'Brien T, Pandit A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J Biomed Mater Res A. 2009;89:876–84. doi: 10.1002/jbm.a.32039. [DOI] [PubMed] [Google Scholar]
- 193.Wan L, Li D, Wu Q. Perivenous application of fibrin glue as external support enhanced adventitial adenovirus transfection in rabbit model. J Surg Res. 2006;135:312–6. doi: 10.1016/j.jss.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 194.Yau L, Molnar P, Moon MC, Buhay S, Werner JP, Molnar K, et al. Metaiodobenzylguanidine, an inhibitor of arginine-dependent mono (ADP-ribosyl) ation, prevents neointimal hyperplasia. J Pharmacol Exp Ther. 2008;326:717–24. doi: 10.1124/jpet.108.137513. [DOI] [PubMed] [Google Scholar]
- 195.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–14. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 196.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587–95. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 197.Le Nihouannen D, Le Guehennec L, Rouillon T, Pilet P, Bilban M, Layrolle P, et al. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006;27:2716–22. doi: 10.1016/j.biomaterials.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 198.Osada T, Yamamura K, Yano K, Fujimoto K, Mizuno K, Sakurai T, et al. Distribution and serum concentration of sisomicin released from fibrin glue-sealed Dacron graft in the rat and human. J Biomed Mater Res. 2000;52:53–7. doi: 10.1002/1097-4636(200010)52:1<53::aid-jbm7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 199.Zhibo X, Miaobo Z. Effect of sustained-release lidocaine on reduction of pain after subpectoral breast augmentation. Aesthet Surg J. 2009;29:32–4. doi: 10.1016/j.asj.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 200.Kitajiri Si, Tabuchi K, Hiraumi H, Kaetsu H. Relief of Post-Tonsillectomy Pain by Release of Lidocaine From Fibrin Glue. Laryngoscope. 2001;111:642–4. doi: 10.1097/00005537-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 201.Gulati K, Aw MS, Losic D. Drug-eluting Ti wires with titania nanotube arrays for bone fixation and reduced bone infection. Nanoscale Res Lett. 2011;6:1. doi: 10.1186/1556-276X-6-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Antoci V, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858–66. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 203.Forster H, Marotta JS, Heseltine K, Milner R, Jani S. Bactericidal activity of antimicrobial coated polyurethane sleeves for external fixation pins. J Orthop Res. 2004;22:671–7. doi: 10.1016/j.orthres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 204.Sörensen JH, Lilja M, Sörensen TC, Åstrand M, Procter P, Fuchs S, et al. Biomechanical and antibacterial properties of Tobramycin loaded hydroxyapatite coated fixation pins. J Biomed Mater Res B. 2014;102:1381–92. doi: 10.1002/jbm.b.33117. [DOI] [PubMed] [Google Scholar]
- 205.Lucke M, Schmidmaier G, Sadoni S, Wildemann B, Schiller R, Haas NP, et al. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32:521–31. doi: 10.1016/s8756-3282(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 206.Sanchez E, Baro M, Soriano I, Perera A, Evora C. In vivo–in vitro study of biodegradable and osteointegrable gentamicin bone implants. Eur J Pharm Biopharm. 2001;52:151–8. doi: 10.1016/s0939-6411(01)00169-2. [DOI] [PubMed] [Google Scholar]
- 207.Mäkinen TJ, Veiranto M, Knuuti J, Jalava J, Törmälä P, Aro HT. Efficacy of bioabsorbable antibiotic containing bone screw in the prevention of biomaterial-related infection due to Staphylococcus aureus. Bone. 2005;36:292–9. doi: 10.1016/j.bone.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 208.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B. 2009;91:470–80. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 209.Holt J, Hertzberg B, Weinhold P, Storm W, Schoenfisch M, Dahners L. Decreasing bacterial colonization of external fixation pins via nitric oxide release coatings. J Orthop Trauma. 2011;25:432. doi: 10.1097/BOT.0b013e3181f9ac8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schmidmaier G, Wildemann B, Stemberger A, Haas NP, Raschke M. Biodegradable poly (D, L-lactide) coating of implants for continuous release of growth factors. J Biomed Mater Res. 2001;58:449–55. doi: 10.1002/jbm.1040. [DOI] [PubMed] [Google Scholar]
- 211.Wildemann B, Bamdad P, Holmer C, Haas NP, Raschke M, Schmidmaier G. Local delivery of growth factors from coated titanium plates increases osteotomy healing in rats. Bone. 2004;34:862–8. doi: 10.1016/j.bone.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 212.Liu Y, De Groot K, Hunziker EB. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005;36:745–57. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 213.Bae SE, Choi J, Joung YK, Park K, Han DK. Controlled release of bone morphogenetic protein (BMP)-2 from nanocomplex incorporated on hydroxyapatite-formed titanium surface. J Control Release. 2012;160:676–84. doi: 10.1016/j.jconrel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 214.Lee D-W, Yun Y-P, Park K, Kim SE. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone. 2012;50:974–82. doi: 10.1016/j.bone.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 215.Baas J, Elmengaard B, Jensen TB, Jakobsen T, Andersen NT, Soballe K. The effect of pretreating morselized allograft bone with rhBMP-2 and/or pamidronate on the fixation of porous Ti and HA-coated implants. Biomaterials. 2008;29:2915–22. doi: 10.1016/j.biomaterials.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 216.Pauly S, Luttosch F, Morawski M, Haas NP, Schmidmaier G, Wildemann B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone. 2009;45:505–11. doi: 10.1016/j.bone.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 217.Brooks AE, Brooks BD, Davidoff SN, Hogrebe PC, Fisher MA, Grainger DW. Polymer-controlled release of tobramycin from bone graft void filler. Drug Del Transl Res. 2013;3:518–30. doi: 10.1007/s13346-013-0155-x. [DOI] [PubMed] [Google Scholar]
- 218.Brooks BD, Sinclair KD, Davidoff SN, Lawson S, Williams AG, Coats B, et al. Molded polymer-coated composite bone void filler improves tobramycin controlled release kinetics. J Biomed Mater B. 2014;102:1074–83. doi: 10.1002/jbm.b.33089. [DOI] [PubMed] [Google Scholar]
- 219.Koort JK, Suokas E, Veiranto M, Mäkinen TJ, Jalava J, Törmälä P, et al. In vitro and in vivo testing of bioabsorbable antibiotic containing bone filler for osteomyelitis treatment. J Biomed Mater Res A. 2006;78:532–40. doi: 10.1002/jbm.a.30766. [DOI] [PubMed] [Google Scholar]
- 220.Henslee AM, Shah SR, Wong ME, Mikos AG, Kasper FK. Degradable, antibiotic releasing poly (propylene fumarate)-based constructs for craniofacial space maintenance applications. J Biomed Mater Res A. 2015;103:1485–97. doi: 10.1002/jbm.a.35288. [DOI] [PubMed] [Google Scholar]
- 221.Shah SR, Tatara AM, Lam J, Lu S, Scott DW, Bennett GN, et al. Polymer-Based Local Antibiotic Delivery for Prevention of Polymicrobial Infection in Contaminated Mandibular Implants. ACS Biomateri Sci Eng. 2016;2:558–66. doi: 10.1021/acsbiomaterials.5b00545. [DOI] [PubMed] [Google Scholar]
- 222.Domingues ZR, Cortés ME, Gomes TA, Diniz HF, Freitas CS, Gomes JB, et al. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials. 2004;25:327–33. doi: 10.1016/s0142-9612(03)00524-6. [DOI] [PubMed] [Google Scholar]
- 223.Xia W, Chang J. Well-ordered mesoporous bioactive glasses (MBG): a promising bioactive drug delivery system. J Control Release. 2006;110:522–30. doi: 10.1016/j.jconrel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 224.Brooks BD, Sinclair KD, Grainger DW, Brooks AE. A resorbable antibiotic-eluting polymer composite bone void filler for perioperative infection prevention in a rabbit radial defect model. PloS One. 2015;10:e0118696. doi: 10.1371/journal.pone.0118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xie Z, Liu X, Jia W, Zhang C, Huang W, Wang J. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J Control Release. 2009;139:118–26. doi: 10.1016/j.jconrel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 226.Hofmann MP, Mohammed AR, Perrie Y, Gbureck U, Barralet JE. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009;5:43–9. doi: 10.1016/j.actbio.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 227.Arcos D, Ragel CV, Vallet-Regı M. Bioactivity in glass/PMMA composites used as drug delivery system. Biomaterials. 2001;22:701–8. doi: 10.1016/s0142-9612(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 228.Mäkinen TJ, Veiranto M, Lankinen P, Moritz N, Jalava J, Törmälä P, et al. In vitro and in vivo release of ciprofloxacin from osteoconductive bone defect filler. J Antimicrob Chemoth. 2005;56:1063–8. doi: 10.1093/jac/dki366. [DOI] [PubMed] [Google Scholar]
- 229.Lee E-J, Jun S-H, Kim H-E, Kim H-W, Koh Y-H, Jang J-H. Silica xerogel-chitosan nano-hybrids for use as drug eluting bone replacement. J Mater Sci Mater Med. 2010;21:207–14. doi: 10.1007/s10856-009-3835-9. [DOI] [PubMed] [Google Scholar]
- 230.Minelli EB, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two-stage revision of infected arthroplasty. J Antimicrob Chemoth. 2004;53:329–34. doi: 10.1093/jac/dkh032. [DOI] [PubMed] [Google Scholar]
- 231.Arias PP, Tafin UF, Bétrisey B, Vogt S, Trampuz A, Borens O. Activity of bone cement loaded with daptomycin alone or in combination with gentamicin or PEG600 against Staphylococcus epidermidis biofilms. Injury. 2015;46:249–53. doi: 10.1016/j.injury.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 232.Thormann U, Ray S, Sommer U, ElKhassawna T, Rehling T, Hundgeburth M, et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials. 2013;34:8589–98. doi: 10.1016/j.biomaterials.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 233.Tahara Y, Ishii Y. Apatite cement containing cis-diamminedichloroplatinum implanted in rabbit femur for sustained release of the anticancer drug and bone formation. J Orthop Sci. 2001;6:556–65. doi: 10.1007/s007760100012. [DOI] [PubMed] [Google Scholar]
- 234.Maus U, Andereya S, Gravius S, Ohnsorge JAK, Siebert CH, Niedhart C. BMP-2 incorporated in a tricalcium phosphate bone substitute enhances bone remodeling in sheep. J Biomater Appl. 2008 doi: 10.1177/0885328207083311. [DOI] [PubMed] [Google Scholar]
- 235.Saito N, Murakami N, Takahashi J, Horiuchi H, Ota H, Kato H, et al. Synthetic biodegradable polymers as drug delivery systems for bone morphogenetic proteins. Adv Drug Deliv Rev. 2005;57:1037–48. doi: 10.1016/j.addr.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 236.Maehara H, Sotome S, Yoshii T, Torigoe I, Kawasaki Y, Sugata Y, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2) J Orthop Res. 2010;28:677–86. doi: 10.1002/jor.21032. [DOI] [PubMed] [Google Scholar]
- 237.James HP, John R, Alex A, Anoop KR. Smart polymers for the controlled delivery of drugs–a concise overview. Acta Pharm Sin B. 2014;4:120–7. doi: 10.1016/j.apsb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hardy JG, Palma M, Wind SJ, Biggs MJ. Responsive Biomaterials: Advances in Materials Based on Shape-Memory Polymers. Adv Mater. 2016 doi: 10.1002/adma.201505417. [DOI] [PubMed] [Google Scholar]
- 239.Kim S-S, Park MS, Jeon O, Choi CY, Kim B-S. Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 240.Kumar A, Mandal S, Barui S, Vasireddi R, Gbureck U, Gelinsky M, et al. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater Sci Eng R Rep. 2016;103:1–39. [Google Scholar]
- 241.Anjum N, Gulrez S, Singh H, Gupta B. Development of antimicrobial polypropylene sutures by graft polymerization. I. Influence of grafting conditions and characterization. J Appl Polym Sci. 2006;101:3895–901. [Google Scholar]