Abstract
Objective
The study examined the effects of a group-phone based weight management intervention on change in physical activity as measured via accelerometer and self-report in rural breast cancer survivors. The study also evaluated the role of physical activity on clinically meaningful cut-points for weight loss (baseline to 6 months) and weight loss maintenance (6 to 18 months).
Methods
Participants were breast cancer survivors in a weight management intervention who provided valid weight and accelerometer data (N=142). We categorized participants into four groups based on weight loss ≥10% and weight regain ≥5% at 18 months.
Results
Accelerometer-measured moderate-to-vigorous physical activity (MVPA) significantly increased from baseline to 6 months (+46.9 minutes). MVPA declined during maintenance; however remained significantly greater than baseline. Self-reported MVPA followed a similar pattern as accelerometer MVPA, but estimates were significantly higher. Participants in the high loss, low regain group had significantly higher MVPA at all points.
Conclusions
A distance-based weight management intervention for survivors improved physical activity outcomes over 18 months. Self-reported physical activity was substantially higher than accelerometer-measured. Findings highlight the importance of device-based measurement for characterizing the magnitude of physical activity change, as well as the role of physical activity in weight management outcomes.
Keywords: Physical activity, obesity, breast cancer, weight loss, weight maintenance
Introduction
Obesity and physical activity are modifiable risk factors for breast cancer and cancer recurrence (1, 2). Physical activity may reduce breast cancer risk directly through its effects on chronic inflammation and sex hormones, as well as indirectly through its effects on obesity-related factors such as adiposity, insulin resistance, and adipokines (3, 4). During weight loss, moderate to vigorous physical activity (MVPA) helps induce caloric expenditure to reach a prescribed caloric deficit needed for weight loss. A systematic review of weight loss studies targeting both diet and physical activity changes over 3–12 months reported a small, positive additive effect of physical activity during weight loss (pooled estimate = −1.65 kg) (5). Whereas physical activity may have a smaller role in weight loss, physical activity is crucial for successful long-term weight loss maintenance (6, 7). The American College of Sports Medicine recommends 200–300 MVPA min/week for sustained weight loss maintenance (8). In this regard, Jakicic (2014) examined weight loss maintenance patterns among participants from the general population and found that those who sustained ≥10% weight loss at 6 and 18 months were more likely to complete 200+ minutes of MVPA per week (7).
Comprehensive clinical weight management trials tailored to breast cancer survivors that targeted reduced caloric intake, increased energy expenditure, and behavioral strategies have yielded initial evidence of physical activity improvements during initial (up to 6 months) and more long-term (up to 18–24 months) intervention periods (9). The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial included a home-based (non-supervised) physical activity component (10). During weight loss (0 to 6 months), self-reported MVPA min/wk increased 150% in the intervention group (94 to 238 min/wk), and increased by 50% in the non-intervention control group (103 to 163 min/wk) (10). Similar improvements were found in 18-month outcomes of the LISA weight loss trial (11). Participants randomized to the phone-based intervention had self-reported improvements from 0 to 18 months (110% increase: 120 to 250 min/wk), as did participants in the mail-based intervention (122% increase: 95 to 210 min/wk) (11).
Measuring physical activity using self-report is common in lifestyle interventions for breast cancer survivors; it affords low participant burden and can be highly scalable large trials. However, measuring physical activity using self-report questionnaires can overestimate physical activity as compared to device-based measurement with accelerometers (12, 13), possibly because these measures assess different aspects of physical activity or different constructs (13). For example, self-reported physical activity participation may also reflect an individual’s perceived ease or difficulty in physical activity participation (13). To our knowledge, only two pilot weight loss trials breast cancer survivors reported findings on physical activity change using device-based measurement. One single-arm pilot study designed to provide extended care for weight loss maintenance via text messages indicated that accelerometer-measured MVPA significantly increased from baseline to 18 months; however the magnitude of the increase was lower (38% increase) than the aforementioned self-reported physical activity increases in other breast cancer trials (14). In the Lifestyle, Exercise, and Nutrition (LEAN) randomized pilot study, pedometer steps increased by 37% in the in-person treatment group and 16% in the telephone treatment group from baseline to 6 months (15). Importantly, participants in both LEAN treatment groups self-reported a higher increase in MVPA min/wk (86–115%). Thus, self-report of physical activity may overestimate treatment effects on physical activity among breast cancer survivors. This has important implications for addressing unanswered questions about the relative importance of weight loss versus physical activity change in prognostic factors for recurrence and survival. However, given that pedometer-measured physical activity can yield substantial measurement error (16) and no self-report measures were reported for comparison in the accelerometer study, it is difficult to determine the degree to which self-report may overestimate physical activity in treatment samples of breast cancer survivors.
The goals of the current study were threefold. The first aim was to examine the effects of an 18-month group phone-based weight loss intervention for rural breast cancer survivors on change in physical activity from 0 to 6 months (weight loss phase) and from 6 to 18 months (weight loss maintenance phase). Our second aim was to compare estimates of physical activity change across accelerometer versus self-report measures. The third aim was to compare physical activity changes across weight loss and weight regain groups as defined by clinically meaningful cut-points.
Methods
Study design
This study was designed to compare continued group phone-based counseling versus mailed newsletters on weight loss maintenance following weight loss. Study details and primary outcomes were presented previously (17, 18), and briefly described herein. Participants (N=210) were postmenopausal female breast cancer survivors residing in rural areas of the Midwestern United States, with a BMI of 27 to 45 kg/m, age <75 years, diagnosis of Stage 0-IIIc disease, and completion of breast cancer treatment.
The intervention consisted of 1) a 6-month weight loss phase where all participants received weekly group phone sessions, followed by 2) a 12-month weight loss maintenance phase (6 to 18 months) during which participants were randomized to continued phone sessions or a newsletter condition. Participants in the phone group received biweekly phone sessions, whereas newsletter condition participants received mailed newsletters at the same frequency as the phone sessions that covered the same content as the group calls.
The primary endpoint for the main trial was weight was regain from 6–18 months among participants who lost ≥5% by 6 months. However, all participants continued in the intervention and were followed throughout the study and are reported here. The study was approved by the University’s Institution Review Board and informed consent was obtained from all participants.
Study Sample
The sample for the current analyses consisted of participants who provided valid weight and accelerometer data at baseline, 6, and 18 months. Figure 1 depicts how we obtained our final sample of N=142.
Figure 1.
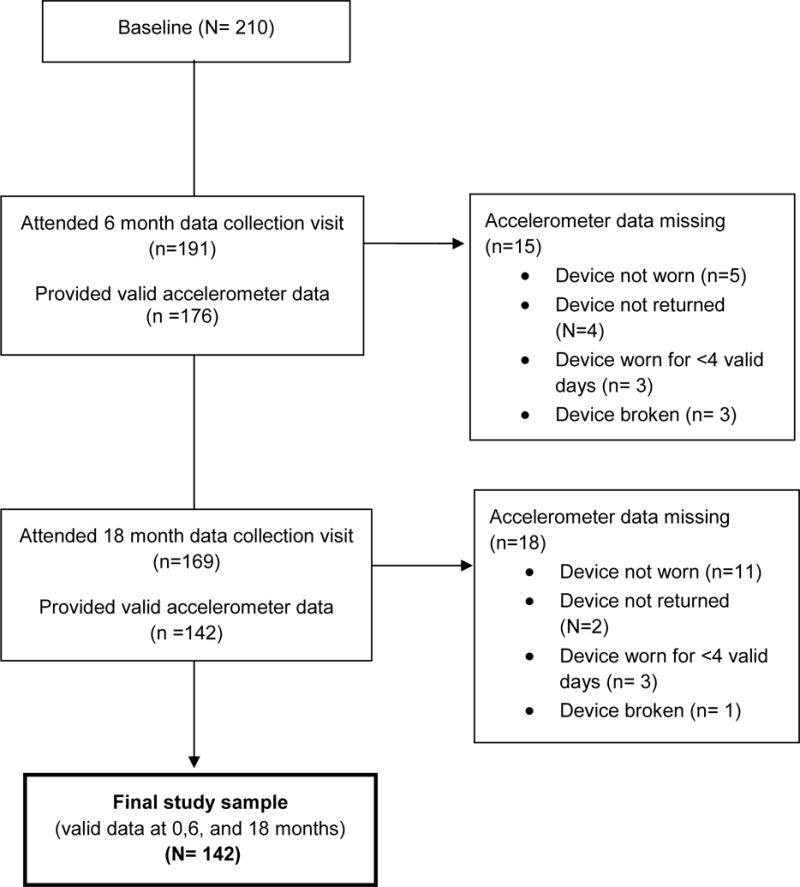
Flow Diagram for Study Sample
Intervention
During the 6-month weight loss phase, participants met via conference call for one hour weekly. Participants were instructed to follow a structured meal plan and to gradually increase their physical activity, with the goal of 225 min/wk of MVPA by week 12, consistent with recommendations (8). Participants received a Physical Activity Tool Kit with two DVDs, a pedometer, and self-monitoring charts. Brisk walking was the activity of choice for most participants. Physical activity sessions addressed both education to ensure safety, monitor and regulate intensity, and increase exercise variety, as well as problem-solving activities to address common barriers, including issues regarding the built environment in rural settings (lack of sidewalks, extreme temperatures, etc), sustaining motivation, and social support. Participants submitted a weekly self-monitoring report form detailing their dietary intake, physical activity minutes at ≥10 minute bouts, and steps/day (goal of 10,000/day). During each session, participants reported whether they met nutrition and physical activity goals from the previous session.
Assessments
Participants attended assessment visits at baseline, 6, 12, and 18 months. At each visit, participants were weighed in light clothing (shorts, t-shirt) in a fasting state using a calibrated digital scale accurate to 0.1 kg (Befour PS5700). Height was measured with a stadiometer.
Both self-report and accelerometer measures of physical activity were collected at all time points. Participants were instructed to wear a GT3X+ Actigraph Accelerometer (Fort Walton Beach, FL) for seven consecutive days at each point. The ActiGraph has been shown to provide valid assessments of activity intensity during both walking/running (19) and daily living activities (20). The device does not have a display screen to minimize reactivity. Participants received verbal and written instructions accompanied by a wear time log to help encourage adherence. Devices were returned in a pre-stamped envelope. Participants’ data were included if data was available for ≥10 hours/day for ≥ 4 days, an algorithm that has been demonstrated to validly estimate physical activity patterns (21). The accelerometer outcome variable was total ≥10-minute MVPA bouted minutes per week, consistent with the intervention guidelines for counting only ≥10 minute bouts toward total weekly minutes. Prior studies found this measure to be more predictive of weight loss outcomes than non-bouted minutes (7). Data in counts per minute summed across 10-second epochs were downloaded and number of minutes per day at various activity levels were calculated using the cut-points suggested by Matthews et al. (22). Moderate to vigorous activity (counts ≥1952 per min) bouts wherein at least 8 minutes were at/above the 1952 threshold were used to identify 10-minute MVPA bouts. Bouted MVPA minutes per valid day (≥10 hours worn) were calculated and multiplied by 7 to obtain weekly estimates.
The Paffenbarger Physical Activity Questionnaire (23) includes questions on stairs climbed, blocks walked, and other sports, leisure, and recreational activities on a typical week during the past month. All activities were assigned MET values (24) and MVPA minutes (MET values >3) were summed to obtain weekly MVPA min/week estimates. Total MVPA min/wk was used as the self-report outcome variable to allow for direct comparison to the accelerometer measure. Self-report physical activity measures were available for 139 of the 142 participants in the sample (98%). Two participants at 6 months and one at 18 months had self-report measures that were statistical outliers and were physically implausible.
Weight-change groupings
We classified participants into low/high weight loss (< 10% loss versus ≥ 10% loss from baseline to 6 months) and further into low/high weight regain (<5% regain versus ≥ 5% regain from 6 to 18 months). This resulted four weight-change groups (see Table 2 for sample sizes).
Table 2.
Accelerometer and Self-Reported Physical Activity by Time
| Change over time | Difference: accelerometer vs self-report | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | 18 months | Baseline 6 months | 6 – 18 months | 6 months | 18 months | |
| Median (IQR) |
Median (IQR) |
Median (IQR) |
Median (IQR) |
P valuea | P valueb | P valuec | P valuec | |
| Accelerometer bouted MVPA min/week | ||||||||
| Full sample (N=142) |
18.4(62.1) | 65.3(166.3) | 40.3(131.5) | 38.1(119.3) | .001 | .01 | .001 | .001 |
| Low loss, low regain (N= 25) |
9.3(37.0) | 20.2(79.2) | 17.5(75.9) | 0(61.3) | .488 | .560 | .001 | .001 |
| Low loss, high regain (N=18) |
18.8(58.0) | 16.1(36.5) | 7.4(30.3) | 22.7(85.2) | .843 | .376 | .001 | .034 |
| High loss, low regain (N=53) |
29.8(80.1) | 134.0(204.7) | 88.1(124.2) | 109.4(167.6) | .001 | .027 | .001 | .001 |
| High loss, high regain (N=46) |
18.2(55.3) | 89.7(178.5) | 26.3(147.0) | 10.5(72.2) | .001 | .001 | .001 | .001 |
| Paffenbarger self – reported MVPA min/week | ||||||||
| Full sample | 0(120.0) | 227.5(183.7) | 142.5(220.0) | 150.0(222.7) | .001 | .001 | – | – |
| Low loss, low regain (N=23) |
30.0(120.0) | 150.0(141.2) | 60.0(200.0) | 60.0(170.0) | .001 | .002 | – | – |
| Low loss, high regain (N=18) |
0(90.0) | 180.0(218.5) | 100.0(162.8) | 130.0(210.0) | .001 | .059 | – | – |
| High loss, low regain (N=52) |
30.0(147.5) | 272.5(168.8) | 200.0(208.8) | 235.0(191.3) | .001 | .001 | – | – |
| High loss, high regain (N=47) |
0(140.0) | 240.0(230.0) | 125.0(200.0) | 125.0(220.0) | .001 | .004 | – | – |
P value corresponding to within subjects effects in repeated measures ANOVA
P value corresponding to within subjects change in linear mixed model
P value corresponding to paired t-test
Note:
Low loss, low regain: <10% loss at 6 months, <5% regain at 18 months
Low loss, high regain: <10% loss at 6 months, ≥ 5% regain at 18 months
High loss, low regain: ≥ 10% loss at 6 months, <5% regain at 18 months
High loss, high regain: ≥ 10% loss at 6 months, ≥ 5% regain at 18 months
Analyses
We first calculated descriptive statistics for the outcome measures, as well as the percentage of participants who met physical activity guidelines of ≥ 150 min/wk. Both outcomes were square-root transformed to correct for skewedness. We examined the effects of randomization condition at 6 months on MVPA change during maintenance (6–18 months) using a linear mixed model. There were no significant differences in MVPA change between the conditions; therefore the groups were combined for the main analyses.
Separate repeated measures ANOVAs were run to examine changes in accelerometer and self-reported physical activity during the weight loss phase (baseline to 6 months) across the full sample, as well as the four weight-change groups. A Bonferroni adjustment was used for multiple comparisons between groups. A series of paired t tests were used to test for significant differences between accelerometer-measured and self-reported MVPA at each time point. Separate linear mixed models using a compound symmetry correlation structure were constructed to examine changes in accelerometer and self-reported MVPA outcomes across the three time points during weight loss maintenance phase (6,12, and 18 months) for the full sample, as well as the weight-change groups. Finally, an interaction between time and weight-change group was included to examine differences between groups by time point, using a Bonferroni adjustment. We included the following variables as covariates in the models: randomization assignment, age, education, rurality (large rural vs. small/isolated rural (25)), and time since end of cancer treatment. We used Restricted Maximum Likelihood Estimation (REML) to cope with missing data.
Results
Table 1 presents participant characteristics. Participants were a mean of 58.6 (SD =8.0) years old, 76% were married and 22% had a 4-year college degree. Participants were a mean of 3.6 (SD =2.5) years beyond cancer treatment excluding anti-hormone therapy. Mean baseline BMI was 33.7 (4.0). Weight loss at 6 months was 13.9% (SD= 5.74) and participants regained a mean of 4.6% (SD = 5.9) by 18 months.
Table 1.
Demographic Characteristics (N=142)
| Demographic Variable | M (SD) or n (%) |
|---|---|
| Age | 58.6 (8.0) |
| Marital Status | |
| Married/Cohabitating | 108 (76%) |
| Race/Ethnicity (% Caucasian) | 138 (97%) |
| Education | |
| High School/GED | 33 (23%) |
| Some college/Associate’s | 54 (38%) |
| Bachelor’s | 31 (22%) |
| Graduate (Masters, PhD) | 24 (17%) |
| Rurality (% small/isolated rural) | 80 (56%) |
| Age at diagnosis | 54.6 (8.3) |
| Stage at diagnosisa | |
| 0 | 13 (9%) |
| I | 58 (41%) |
| II | 53 (37%) |
| III | 17 (12%) |
| Time since treatment (years) | 3.6 (2.5) |
| Treatment Received | |
| Lumpectomy | 90 (63%) |
| Mastectomy | 64 (45%) |
| Radiation | 103 (73%) |
| Chemotherapy | 92 (65%) |
| Anti-hormone Therapy | 104 (73%) |
| BMI | 33.7 (4.0) |
| % weight loss at 6 months | 13.9 (5.74) |
| % regain at 18 months | 4.6 (5.9) |
One participant’s stage at diagnosis was unknown
Participants in the current sample did not significantly differ on age, baseline BMI, or cancer treatment-related variables (all p values > .05) from those who attended the 6-month visit but were excluded from the current analyses based on missing or invalid accelerometer data. Additionally, participants in the current sample did have significantly higher percent weight loss at 6 months (13.9% vs 10.1%, p = .001) than those who attended the 6-month visit but were excluded from the current analyses based on missing or invalid accelerometer data. However, these participants did not significantly differ in percent weight regain from 6 to 18 months (4.6% vs 6.4%, p = .083).
Change in physical activity
Table 2 shows within-subject changes in MVPA over 18 months. Accelerometer-measured median MVPA bouted minutes significantly increased from baseline to 6 months (18.4 vs 65.3, p = .001), an increase of 350% from baseline, and significantly decreased from 6 to 18 months (65.3 vs 38.1, p = .01; 42% decrease from 6 months). MVPA at 18 months remained significantly higher than baseline (18.4 vs 38.1, p = .001; 210% increase).
Within the weight-change groups, accelerometer MVPA significantly increased from baseline to 6 months among both high weight loss groups, but not the low loss groups. In addition, accelerometer MVPA significantly decreased from 6 to 18 months in the high loss groups, but not in the low loss groups.
Self-reported MVPA min/week significantly increased in the full sample (0 vs 227.5, p = .001; 227% increase), as well as in all weight-change groups (p values =.001) from baseline to 6 months. From 6 to 18 months, self-reported MVPA min/week significantly decreased in the full sample (227.5 vs 150.0; a 34% decrease), as well as in all groups except the low loss, high regain group (Table 2). Self-reported MVPA min/week at 18 months were 150% higher than baseline.
Table 3 shows the percentage of participants in each weight-change group that met MVPA guidelines. Accelerometer data indicated <15% of participants in both low loss groups met MVPA guidelines of ≥ 150 min/wk at any time point. In contrast, 30–43% of participants in the high loss groups met ≥ 150 min/wk guidelines at 6 months. In the high loss, low regain group, 25% met guidelines at 12 months, which increased to 32% at 18 months.
Table 3.
Percentage of participants meeting physical activity recommendations of ≥150 minutes per week over time
| Baseline | 6 months | 12 months | 18 months | |
|---|---|---|---|---|
| Accelerometer | %(N) | %(N) | %(N) | %(N) |
| Low loss, low regain | 4%(1) | 8% (2) | 14%(3) | 8%(2) |
| Low loss, high regain | 0%(0) | 6%(1) | 0%(0) | 11%(2) |
| High loss, low regain | 2%(1) | 43%(23) | 25%(13) | 32%(17) |
| High loss, high regain | 9%(4) | 30%(14) | 21%(9) | 11%(5) |
| Paffenbarger self – report | ||||
|---|---|---|---|---|
| Low loss, low regain | 17%(4) | 57%(13) | 36%(8) | 26%(6) |
| Low loss, high regain | 22%(4) | 58%(10) | 35%(6) | 39%(7) |
| High loss, low regain | 25%(13) | 90%(47) | 65%(34) | 75%(39) |
| High loss, high regain | 23%(11) | 76%(35) | 47%(21) | 48%(22) |
Note:
Low loss, low regain: <10% loss at 6 months, <5% regain at 18 months
Low loss, high regain: <10% loss at 6 months, ≥ 5% regain at 18 months
High loss, low regain: ≥ 10% loss at 6 months, <5% regain at 18 months
High loss, high regain: ≥ 10% loss at 6 months, ≥ 5% regain at 18 months
By self-report, over 60% of participants in all weight-change groups reported meeting ≥150 min/wk at 6 months. At 12 and 18 months, 26–39% of participants in the low loss groups reported meeting guidelines compared to 47–75% in the high loss groups.
Accelerometer vs self-reported MVPA
Self-reported MVPA was significantly higher than accelerometer-measured MVPA at 6 months (p= .001) and 18 months (p=.001) in the full sample. Findings were similar among all four weight-change groups (Table 2).
Physical activity across weight-change groups
Group comparisons for accelerometer-measured MVPA over time are depicted in Figure 2. There were no baseline group differences in MVPA. There was a significant interaction between group and time (p= .031). At 6 months, median MVPA in both of the high loss groups was significantly higher compared to the low loss groups (p values <.05). At 12 and 18 months, the high loss, low regain group had significantly higher MVPA than all other groups. Notably, the high loss, low regain group’s MVPA decreased at 12 months but stabilized by 18 months, while the high loss, high regain group’s MVPA decreased at 12 months and 18 months.
Figure 2.
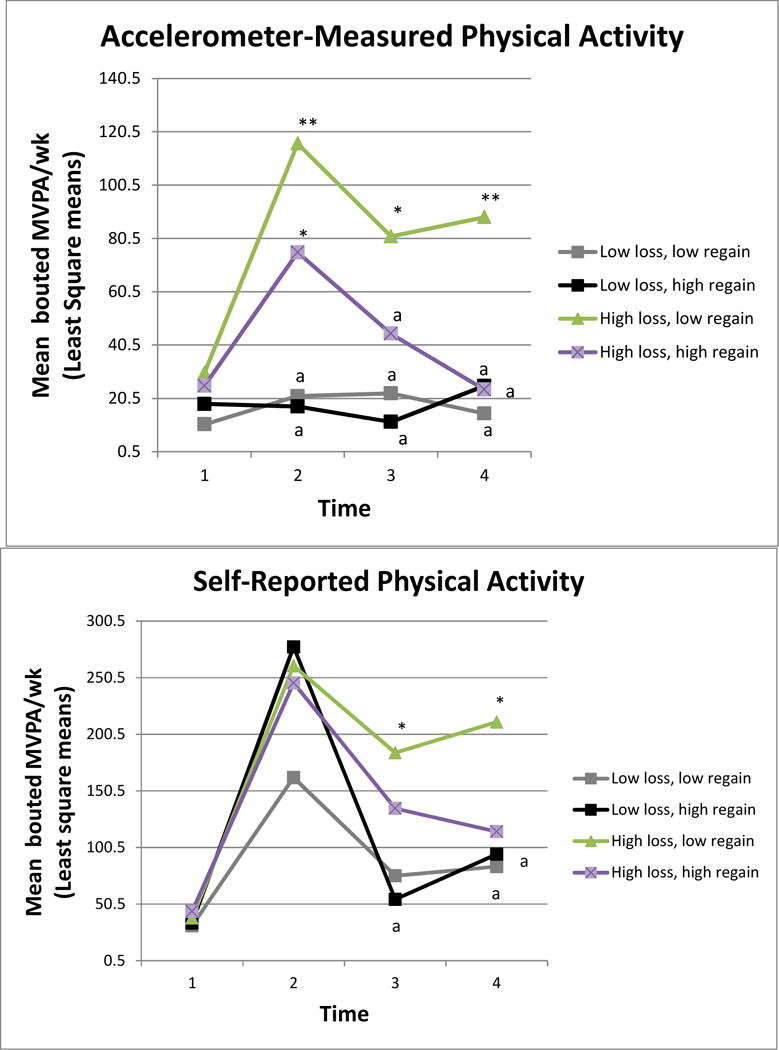
Accelerometer measured and self-reported physical activity
*Significantly different at p<.05 level
**Significantly different at p<.01 level
acomparator group
Discussion
The present study demonstrated that a distance-based weight management intervention for breast cancer survivors that targeted home-based physical activity improved physical activity outcomes over 18 months. Specifically, MVPA assessed via accelerometer significantly increased (+46.9 minutes) from baseline to 6 months in the full sample. MVPA bouted minutes did decline during weight loss maintenance (6–18 months); however MVPA at 18 months was still significantly greater than baseline, suggesting some maintenance of effects. Our observed changes in MVPA (Median=46.9 min) are consistent with the increases in MVPA min/wk observed in The Weight Loss Maintenance Randomized Controlled Trial in the general population (Mean= 48 min at 6 mo) (26). Compared to previous weight loss intervention trials in breast cancer survivors, self-reported physical activity changes from baseline to 18 months among our participants (150%) were similar in magnitude to the LISA phone-intervention condition (110%: 120 to 250 min/wk) (11) and higher than those of the ENERGY trial (79%, 94 to 168 min/wk). Participants in our study had lower physical activity at baseline compared to these other trials, a finding that may reflect environmental, cultural, and access-related barriers to physical activity in rural settings (27, 28).
Only a minority of our participants achieved a level of physical activity consistent with guidelines, and there were no substantial differences between the phone and mail-based maintenance interventions during the maintenance phase. Novel strategies are needed to improve adherence to physical activity recommendations in non-supervised settings, where the broadest population impact is likely to occur (29). Our previous qualitative findings indicated that participants appeared to underestimate the impact of environmental barriers on their physical activity, which may be a point of focus for future interventions (30). In a recent pilot study examining tailored text messages for promoting weight loss maintenance, Spark et al found a drop in physical activity from 6 to 12 months, but a return to 6 month physical activity levels by 18 months (16). This finding suggests maintenance of effects is possible in this population and warrants further attention.
Our findings add to the preliminary evidence indicating self-reported physical activity can be substantially inflated as compared to device-based measurement among breast cancer survivors (12, 14, 15). The discrepancy between self-report and accelerometer measures in the current study was striking; median self-reported MVPA at 6 and 18 months was more than triple accelerometer-measured MVPA. Self-report estimates were particularly inflated in the low loss groups at 6 months, and in all groups except the high loss, low regain group at 18 months, indicating bias in self-reported physical activity may be greater among participants experiencing poorer weight loss and maintenance outcomes. Thus, these findings underscore the need for objective measurement of physical activity, particularly during weight loss trials during which social desirability to report physical activity adherence may be high and may influence study conclusions regarding the role of physical activity in weight management. Our findings further highlight the importance of using objective measurement of physical activity in studies that aim to disentangle the relative effects of diet, physical activity, and weight loss on biomarker modulation and disease outcomes among breast cancer survivors.
Our findings are consistent with those of Jakicic et al (2014) who reported a similar pattern of MVPA stabilization at 12 and 18 month among participants from the general population who successfully maintained 10% weight loss at 18 months. Taken together, these findings highlight the importance of preventing or minimizing the decline in physical activity during maintenance. A stepped care approach focused on sustaining or further increasing MVPA during maintenance may have benefits for preventing weight regain long-term. Given that motivation and engagement tend to decline during maintenance, a shift in intervention focus from primarily maintaining compliance with weight management skills learned during weight loss to expanding participants’ physical activity goals and participation (PA type, duration, and frequency) may provide novelty and variety that could better sustain physical activity and may thus improve weight management outcomes.
The study had several limitations. First, our analyses were limited to participants who provided valid accelerometer wear time, and this group had higher weight loss compared to those without valid accelerometer data. If we had obtained data from the excluded participants, our estimates of physical activity during the program may have been lower overall. However, the percentage of participants who provided valid accelerometer data was high (89–92%) and in line with national cohort research that used accelerometers to measure physical activity (31), as well as with some other intervention studies in the literature (32, 33), although it is difficult to compare with most weight management trials because valid wear time statistics are often not reported (7, 14, 26), a problem identified previously (34). Additionally, our sample was comprised of primarily White, older survivors living in the rural Midwest and may not generalize to other groups. Also, our MVPA outcome variables were skewed. While we coped with this statistically using transformed variables as is commonly done (7), this issue results from a minority of participants meeting recommended physical activity levels, a problem which requires further attention. Finally, participants reported on their physical activity during a typical week in the past month, whereas accelerometers were worn the week directly after each visit. Thus, it is possible that participants’ reports of their typical physical activity over the past month might not directly align with their actual physical activity the following week. Future research should examine agreement among accelerometer and self-report measures for the same week (12).
The major strength of the study is the use of both accelerometer and self-reported measures of physical activity in addition to the broad range of high and low weight loss and regain groups, which allowed for comparisons across the four corresponding subgroups.
Conclusions
A phone-based weight management intervention for breast cancer survivors that targeted non-supervised physical activity improved MVPA min/wk at 6 months, with partial maintenance of effects at 18 months. However, the majority of participants did not meet guideline thresholds, pointing to the continued need for enhancing intervention effects on physical activity. Self-reported physical activity changes were more than triple those of the accelerometer-measured changes, suggesting that measurement is crucial for accurately specifying the magnitude of physical activity changes during a weight management intervention. The highest levels of sustained physical activity were important for clinically significant weight loss, as well as successful weight loss maintenance by 18 months.
Study importance.
Clinical weight loss trials for breast cancer survivors have yielded initial evidence of physical activity improvements; however most have assessed physical activity using only self-report. Additionally, little is known about the role of physical activity during weight maintenance among breast cancer survivors.
The present study used both accelerometer and self-reported measures of physical activity in what, to our knowledge, is the largest weight loss trial to date among breast cancer survivors reporting physical activity changes across these two measurement methods.
The study evaluated the role of physical activity on clinical meaningful cut-points for weight loss (baseline to 6 months) and weight loss maintenance (6 to 18 months) among survivors.
Acknowledgments
Funding: This study was supported by NIH/NCI R01CA155014.
Footnotes
Clinical trial registration: ClinicalTrials.gov identifier NCT01441011
Disclosure: The authors have no conflicts of interest to declare.
References
- 1.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 2.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 3.Dieli-Conwright CM, Lee K, Kiwata JL. Reducing the Risk of Breast Cancer Recurrence: an Evaluation of the Effects and Mechanisms of Diet and Exercise. Curr Breast Cancer Rep. 2016;8:139–150. doi: 10.1007/s12609-016-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res Fortschritte Krebsforsch Progres Dans Rech Sur Cancer. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 5.Goode AP, Hall KS, Batch BC, et al. The Impact of Interventions that Integrate Accelerometers on Physical Activity and Weight Loss: A Systematic Review. Ann Behav Med Publ Soc Behav Med. 2017;51:79–93. doi: 10.1007/s12160-016-9829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly JE, Smith B, Jacobsen DJ, et al. The role of exercise for weight loss and maintenance. Best Pract Res Clin Gastroenterol. 2004;18:1009–1029. doi: 10.1016/j.bpg.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Jakicic JM, Tate DF, Lang W, et al. Objective physical activity and weight loss in adults: the step-up randomized clinical trial. Obes Silver Spring Md. 2014;22:2284–2292. doi: 10.1002/oby.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine Position Stand Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 9.Chlebowski R, Reeves M. Weight Loss Randomized Intervention Trials in Female Cancer Survivors: Journal of Clinical Oncology: Vol 34, No 35. J Clin Oncol. 2016;34:4238–4248. doi: 10.1200/JCO.2016.69.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock CL, Byers TE, Colditz GA, et al. Reducing breast cancer recurrence with weight loss, a vanguard trial: The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial. Contemp Clin Trials. 2013;34:282–295. doi: 10.1016/j.cct.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoni A-S, Nordin K, Berntsen S, Demmelmaier I, Igelström H. Comparison between logbook-reported and objectively-assessed physical activity and sedentary time in breast cancer patients: an agreement study. BMC Sports Sci Med Rehabil. 2017;9:8. doi: 10.1186/s13102-017-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spark LC, Fjeldsoe BS, Eakin EG, Reeves MM. Efficacy of a Text Message-Delivered Extended Contact Intervention on Maintenance of Weight Loss, Physical Activity, and Dietary Behavior Change. JMIR MHealth UHealth. 2015;3 doi: 10.2196/mhealth.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrigan M, Cartmel B, Loftfield E, et al. Randomized Trial Comparing Telephone Versus In-Person Weight Loss Counseling on Body Composition and Circulating Biomarkers in Women Treated for Breast Cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J Clin Oncol. 2016;34:669–676. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang M, Bassett DR, Barreira TV, Tudor-Locke C, Ainsworth BE. Measurement Effects of Seasonal and Monthly Variability on Pedometer-Determined Data. J Phys Act Health. 2012;9:336–343. doi: 10.1123/jpah.9.3.336. [DOI] [PubMed] [Google Scholar]
- 17.Befort CA, Klemp JR, Sullivan DK, et al. Weight loss maintenance strategies among rural breast cancer survivors: The rural women connecting for better health trial. Obesity. 2016;24:2070–2077. doi: 10.1002/oby.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Befort CA, Bennett L, Christifano D, Klemp JR, Krebill H. Effective recruitment of rural breast cancer survivors into a lifestyle intervention. Psychooncology. 2014;24:487–490. doi: 10.1002/pon.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brage S, Wedderkopp N, Franks PW, Andersen LB, Froberg K. Reexamination of validity and reliability of the CSA monitor in walking and running. Med Sci Sports Exerc. 2003;35:1447–1454. doi: 10.1249/01.MSS.0000079078.62035.EC. [DOI] [PubMed] [Google Scholar]
- 20.Bassett DR, Ainsworth BE, Swartz AM, Strath SJ, O’Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 22.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34:1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Paffenbarger RS, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 25.United States Department of Agriculture. USDA Economic Research Service – Rural-Urban Commuting Area Codes. 2015 [WWW document]. URL http://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx.
- 26.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 27.Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health Off J Am Rural Health Assoc Natl Rural Health Care Assoc. 2004;20:151–159. doi: 10.1111/j.1748-0361.2004.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox S, Castro C, King AC, Housemann R, Brownson RC. Determinants of leisure time physical activity in rural compared with urban older and ethnically diverse women in the United States. J Epidemiol Community Health. 2000;54:667–672. doi: 10.1136/jech.54.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity. 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazzino TL, Sporn NJ, Befort CA. A qualitative evaluation of a group phone-based weight loss intervention for rural breast cancer survivors: Themes and mechanisms of success. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2016;24:3165–3173. doi: 10.1007/s00520-016-3149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard VJ, Rhodes JD, Mosher A, et al. Obtaining Accelerometer Data in a National Cohort of Black and White Adults. Med Sci Sports Exerc. 2015;47:1531–1537. doi: 10.1249/MSS.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. Effect of physical activity on weight loss, energy expenditure, and energy intake during diet induced weight loss. Obesity. 2014;22:363–370. doi: 10.1002/oby.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuger SL, Barry VW, Sui X, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy SL. Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Prev Med. 2009;48:108–114. doi: 10.1016/j.ypmed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


