Abstract
Myeloid-derived suppressor cells (MDSCs) are a major obstacle to promising forms of cancer immunotherapy, but tools to broadly limit their immunoregulatory effects remain lacking. In this study, we assessed the therapeutic effect of the humanized anti-Jagged1/2 blocking antibody CTX014 on MDSC-mediated T cell suppression in tumor-bearing mice. CTX014 decreased tumor growth, impacted the accumulation and tolerogenic activity of MDSCs in tumors, and inhibited the expression of immunosuppressive factors arginase I and iNOS. Consequently, anti-Jagged therapy overcame tumor-induced T cell tolerance, increased the infiltration of reactive CD8+ T-cells into tumors, and enhanced the efficacy of T cell-based immunotherapy. Depletion of MDSC-like cells restored tumor growth in mice treated with anti-Jagged, whereas co-injection of MDSC-like cells from anti-Jagged-treated mice with cancer cells delayed tumor growth. Jagged1/2 was induced in MDSCs by tumor-derived factors via NFkB-p65 signaling, and conditional deletion of NFkB-p65 blocked MDSC function. Collectively, our results offer a preclinical proof of concept for the use of anti-Jagged1/2 to reprogram MDSC-mediated T cell suppression in tumors, with implications to broadly improve the efficacy of cancer therapy.
Keywords: MDSC, Jagged, Notch, Tumor immunity
Introduction
The coordinated activity of the innate and adaptive arms of the immune system is essential to protect individuals against cancer. However, established tumors create a highly tolerogenic microenvironment that blocks the development of protective immunity (1). Myeloid-derived suppressor cells (MDSCs) are primary components of the immunosuppressive tumor milieu, and are emerging as a major obstacle in the successful development of promising cancer treatments (2). Therapeutic inhibition of the immunosuppressive effects induced by MDSCs in cancer patients treated with chemotherapy, radiotherapy, or immunotherapy regimens is thought to be a highly promising strategy with potentially significant clinical impact. However, current approaches to clinically inhibit MDSCs are limited to multi-tyrosine kinase inhibitors or myelosuppressive agents that are only partially effective and cause rebounds in MDSC numbers as the bone marrow recovers (3,4). Therefore, new therapeutic strategies to block MDSCs in cancer patients are needed. Numerous monoclonal antibody (mAb)-based therapies have shown promising efficacy as cancer therapeutics through the direct targeting of tumor antigens or tolerogenic molecules on immune cells (5,6). Although several mAb-based approaches have been developed to directly target exhausted or immunosuppressed T-cells in cancer patients, there are limited mAb-based therapies that could effectively inhibit MDSCs (7).
The Notch family of receptors regulate a highly conserved pathway that controls the development, differentiation, survival, and function of many cell types, including immune cells (8). Mammals have four Notch receptors (Notch1 through 4) that are bound by five ligands of the Jagged (Jagged1 and Jagged2) and the Delta-like (DLL1, DLL3, and DLL4) families (9). Binding of Notch receptors to the Jagged or DLL membrane ligands induces a two-step proteolytic activation leading to the intracellular release and nuclear translocation of the Notch intracellular active domain (NICD). Once there, NICD binds to the recombination signal-binding protein-Jκ (RBP-Jκ) and recruits a mastermind-like (MAML1–3) co-activator, promoting the transcription of multiple genes (10). In addition, NICD triggers a number of non-canonical responses (11,12). Binding of the Notch receptors on CD8+ T-cells to DLL ligands on antigen-presenting cells induces anti-tumor cytotoxic responses (13–15), whereas binding of Notch to Jagged members resulted in suppressive signals (16). The mechanism for this opposite effect remains unclear with possible explanations, including different kinetics of Notch activation or selectivity of DLL and Jagged ligands for Notch receptors. Although the effects of Notch signaling in innate and adaptive immune responses are the focus of active research, the mechanisms leading to the expression of Jagged molecules in tumors and the potential effect of their blockade as a cancer therapy remain largely unknown.
In this study, we aimed to test the anti-tumor effects of CTX014, a humanized IgG1 blocking antibody that recognizes human and mouse Jagged1 and 2. Our results show that anti-Jagged therapy triggered anti-tumor T-cell responses through the induction of potentially immunogenic MDSC-like cells (MDSC-LC) (17). Additional findings demonstrate the role of NFκB-p65 in the upregulation of Jagged1–2 ligands in tumor-associated MDSCs. Thus, results identify a promising therapeutic tool to potentially block MDSCs in tumors, thereby addressing a major obstacle in the development of successful therapies against cancer.
Material and Methods
Cell lines and treatment of mice
3LL Lewis lung carcinoma, MCA-38 colon carcinoma, EL-4 thymoma, B16–F10 melanoma, and ovalbumin-expressing EL-4 (EG-7) cells (American Type Culture Collection, Manassas, VA) were injected s.c. into mice, as described (18). Tumor cell lines were authenticated on May 2016 and validated to be mycoplasma-free using an ATCC detection kit in June 2016. All experiments were conducted with cells within 6 passages. C57BL/6 mice (6 to 8-wk-old female) were purchased from Harlan-Envigo (Indianapolis, IN). OT-1 and recombination activating gene 1 null (Rag) mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Previously reported NFκB-p65flox/flox mice (19) were crossed with Lysozyme Cre+ (LysM-Cre) mice. Tumor-bearing mice were treated i.p. with non-toxic concentrations of the anti-Jagged antibody (CTX-014, Cytomx, 5 mg/kg, every 3 days) or isotype IgG control (BioXcell, 5 mg/kg) starting on day 6 post-tumor injection and throughout the experiment. To deplete CD8+ T-cells or MDSC-LC, 3LL-bearing mice were pre-treated 1 day before the anti-Jagged injection with 400 µg anti-CD8 (clone 53.6.72, BioXcell) or 250 µg anti-Gr-1 (clone RB6-8C5, BioXcell), respectively. Maintenance i.p. doses of depleting antibodies were given every 3th day until tumor endpoint. In MDSCs co-injection studies, 1×106 tumor-MDSCs from 3LL-bearing mice treated with anti-Jagged or isotype control were co-injected s.c. with 1×106 3LL cells. Tumor volume was measured using calipers and calculated using the formula [(small diameter)2 × (large diameter) × 0.5]. Experiments using mice were approved by the Augusta University-IACUC, following the recommended guidelines.
Antibodies
Purified antibodies against arginase I (clone19), iNOS (54/iNOS), gp91phox (53/gp91), and fluorochrome-conjugated antibodies against CD8 (53-6.7), CD11b (M1/70), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD49f (GoH3), CD69 (H1.2F3), Gr-1 (RB6-8C5), XCR1 (ZET), CD103 (2E7), IFNγ (XMG1.2) and Ki-67 (16A8) were obtained from Becton Dickinson (San Jose, CA) or Biolegend (San Diego, CA). Antibodies against β-actin (AC-74) and vinculin (V284) were from Sigma-Aldrich (St. Louis, MO) and anti-p84 (5E10) from Abcam (Cambridge, MA). Polyclonal antibodies against NFκB-p65 (D14E1) and Jagged1 (28H8) were obtained from Cell Signaling Technologies (Beverly, MA), while anti-Jagged2 (H-143) was from Santa Cruz Biotechnologies.
Western Blot
Cell lysates were electrophoresed in 8% Tris-Glycine gels, transferred to PVDF membranes, and immunoblotted with the corresponding primary antibodies. Membrane-bound immune complexes were detected using ECL in a Chemi-Doc imaging system (Bio-Rad). Densitometry of NFκB-p65 normalized to nuclear p84 was calculated using the Bio-Rad Image-Lab software.
Cell isolation and suppression assays
Tumors digested with DNAse and Liberase (Roche, Branchburg, NJ) were used to isolate different cellular populations by flow cytometry. 3LL cancer cells were recovered by sorting the CD45neg CD49f+ cells, whereas tumor-infiltrating myeloid cells were isolated based on the expression of CD45+ CD11b+. For functional assays, MDSCs from tumors or spleens of tumor-bearing mice or immature myeloid cells (iMCs) from spleens of mice without tumors were harvested using magnetic beads, as described (18,20). Purity for each population ranged from 90–99%, as detected by flow cytometry. Isolated MDSCs were co-cultured for 72 hours with anti-CD3/CD28-activated T-cells labeled with CFSE and T-cell proliferation or IFNγ expression monitored by flow cytometry (14). Splenic-MDSCs were cultured for 48 hours with GM-CSF (20 ng/mL) and 30% 3LL-tumor explants (TES) (21) in the presence of anti-Jagged antibody (2 µg/ml).
Adoptive Cellular Therapy
For adoptive T-cell transfer (ACT) therapy, CD45.2+ mice were injected s.c. with EG-7 cells and started receiving the anti-Jagged or control treatments 6 days post-tumor injection. One day later, mice received ACT with 1×106 negatively sorted CD45.1+ CD8+ OT-1 cells that were pre-activated for 48 hours with SIINFEKL (14). Ten days later, spleens and tumors were tested for the presence of the transferred CD45.2neg CD45.1+ CD8+ OT-1 cells and for the expression of IFNγ. For Elispot assays, spleens were collected 10 days after OT-1 transfer and activated with 2 µg/ml SIINFEKL for 24 hours before measuring IFNγ production.
H&E staining and Immunofluorescence
Formalin-fixed-paraffin-embedded tissue sections were stained with hematoxylin & eosin (H&E) for histology. For immunofluorescence, de-paraffinization and antigen retrieval were completed and sections blocked in 2% donkey serum and incubated overnight with rat anti-mouse CD8 (53-6.7, Novus Biologicals) or double labeled with mouse anti-pan-cytokeratin (C-11, Thermo) and rabbit anti-mouse cleaved caspase 3 (5A1E, Cell Signaling Technologies), followed by washing in PBS and incubation in donkey anti-rat or anti-mouse/rabbit IgG Alexa Fluor® 488/647 (Thermo Fisher Scientific). Next, sections were washed in PBS, mounted in aqueous mounting media with DAPI (Thermo-Fisher), and visualized in a Zeiss-LSM-780 Upright-Confocal microscope.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were done using SimpleChip kit (Cell Signaling Technologies), following the vendor’s recommendations. Briefly, digested and cross-linked chromatin was prepared from 4×106 tumor-MDSCs or splenic-MDSCs treated or not with TES for 48 hours, followed by immunoprecipitation with antibodies against NFκB-p65, Histone H3, or rabbit IgG (Cell Signaling Technologies). Eluted and purified DNA was analyzed by qPCR with pre-validated ChIP primers targeting the Jagged1 or Jagged2 promoters, purchased from Qiagen. Primers against RPL30 promoter were used as housekeeping gene control.
Quantitative RT-PCR
Total RNA was isolated from cells using TRIzol (Life Technologies). Reverse transcription was performed using the Bio-Rad iScript-cDNA synthesis kit. Quantitative-PCR was achieved on an Applied Biosystems thermocycler using Bio-Rad SYBR green supermix with pre-validated primers targeting mouse Jagged1, Jagged2, Notch1, Notch2, or Hes1 (QuantiTect, Qiagen). Fold change expression was calculated comparing the RNA values from experimental samples relative to the endogenous Actin control (forward, TGTGATGGTGGGAATGGGTCAGAA; reverse TGTGGTGCCAGATCTTCTCCATGT), compared to the results obtained from a pooled sample. Thus, fold change =2−Δ(ΔCT), where ΔCT=CT (target)−CT (actin); and Δ(ΔCT)=ΔCT (target)−ΔCT(control, pool of all samples).
Statistical Analysis
Statistical analyses were performed in GraphPad Prism. Significance tests were conducted at a 5% significance level. Experimental differences between endpoints were assessed by ANOVA, whereas means comparisons were carried out with the Tukey procedure or the Dunnet procedure for comparisons with controls.
Results
Anti-tumor effects of anti-Jagged therapy
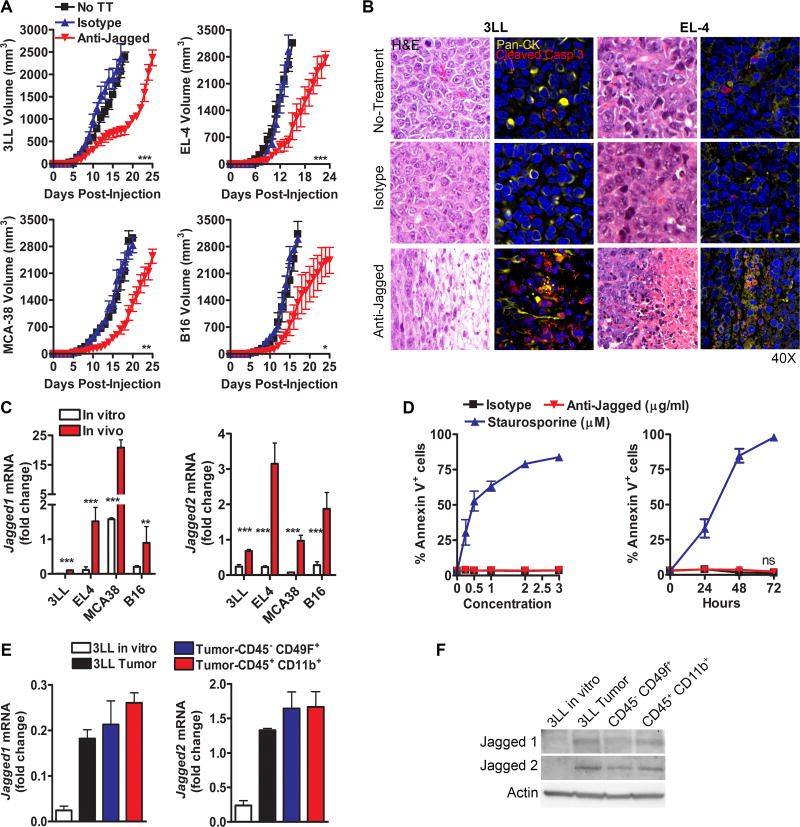
We aimed to determine the therapeutic effects of the anti-Jagged antibody in several s.c. tumor models, including 3LL, EL-4, MCA-38, and B16. Mice were treated starting on day 6 post-tumor injection and continued to be injected every 3 days throughout the experiment. A significant delay in tumor growth was found in all the tested cancer models after treatment with the anti-Jagged antibody, compared to untreated mice or animals injected with the same dose of an isotype control (Fig. 1A). Also, evaluation of tumor morphology by H&E indicated that tumors from anti-Jagged-treated mice show coagulative necrosis and moderate edema, surrounded by a mild inflammatory infiltrate (Fig. 1B, left), which correlated with the co-expression of tumor cell marker, cytokeratin, and cell death marker, cleaved caspase 3 (Fig. 1B, right); and a similar distribution of vascular CD31+ cells (Suppl. Fig. 1). Thus, anti-Jagged therapy induced anti-tumor effects and cancer cell death in vivo independently of changes in tumor vascularization.
Figure 1. Anti-Jagged therapy induces anti-tumor effects.
(A) Tumor volume in mice bearing s.c. 3LL, EL-4, MCA-38, or B16 tumors and treated with anti-Jagged or isotype antibodies, as described in the methods. Results are means ± SEM of 15 mice per group. (B) Tumor morphology by H&E (left) and immunofluorescence against Pan-cytokeratin (Pan-CK) and cleaved caspase 3 (right) in 3LL or EL-4-bearing mice treated with anti-Jagged or isotype. Representative images from 3 repeats. (C) Jagged1–2 mRNA by quantitative-PCR in 3LL, EL-4, MCA-38, and B16 cells cultured in vitro and s.c. tumor suspensions. Data are means ± SD from 3 repeats. (D) Annexin V+ in 3LL cells cultured in the presence of anti-Jagged, isotype (µg/ml) or staurosporine (µM) (left) for 24 hours, or with anti-Jagged, isotype (2 µg/ml) or staurosporine (1 µM) for 0–72 hours (right). (E–F) Expression of Jagged1–2 mRNA and protein by quantitative-PCR (E) or representative western blot (F) in control 3LL cell line and sorted cancer cells (CD49f+ CD45neg) and myeloid cells (CD45+ CD11b+) from s.c. 3LL tumors. RNA levels are means ± SD from 6 mice and tested in triplicates. ***, P< 0.001
In order to identify potential populations targeted by the anti-Jagged therapy, we first measured the expression of Jagged1 and 2 in tumors cultured in vitro and those collected from mice. A higher expression of Jagged1 and 2 was detected in the s.c. tumors obtained from mice, compared to the cancer cells cultured in vitro (Fig. 1C). Consistent with these data, the anti-Jagged antibody failed to induce cytotoxicity against cultured 3LL cells (Fig. 1D). Next, we measured the expression of Jagged1 and 2 in sorted cells representing the two most abundant populations in 3LL tumors, the 3LL cancer cells (CD45neg CD49f+) and myeloid cells (CD45+ CD11b+) (Suppl. Fig. 2). The elevated levels of Jagged1 and 2 in tumors were distributed among the cancer cells and myeloid cells (Fig. 1E–F), suggesting the potential recognition of both cellular populations by anti-Jagged.
Anti-Jagged therapy impacted the accumulation and function of tumor-MDSCs
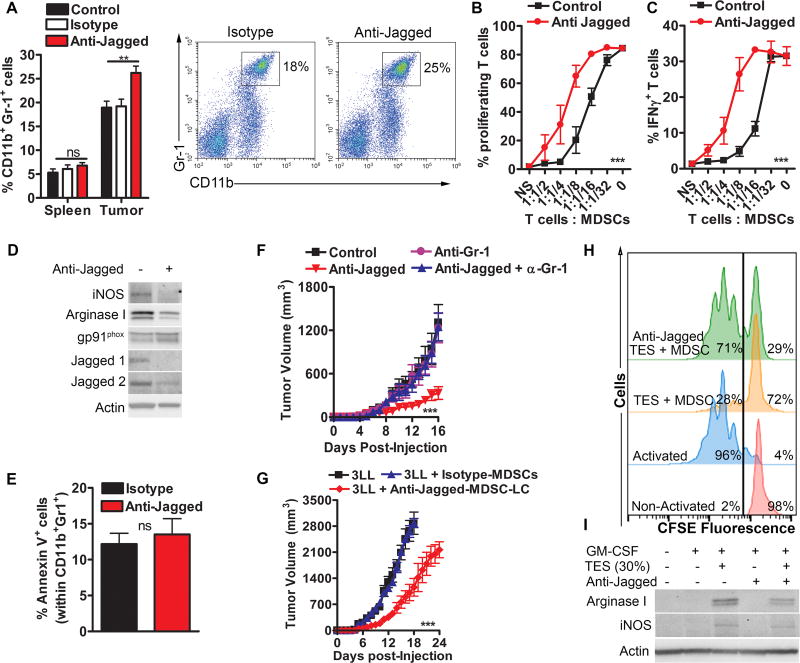
Because MDSCs (CD11b+ Gr-1+) were one of the major cellular subsets expressing Jagged ligands in tumors (14), we tested the effect of the anti-Jagged therapy on the accumulation and function of MDSCs. Surprisingly, we found an elevated frequency of CD11b+ Gr-1+ cells in tumors from mice treated with anti-Jagged, compared to control mice, while similar percentages of these cells were noted in the spleen (Fig. 2A). Moreover, tumor-linked CD11b+ Gr-1+ cells from anti-Jagged-treated 3LL-bearing mice had a lower ability to block CFSE-based T-cell proliferation or IFNγ production, compared to MDSCs from control mice (Fig. 2B–C), suggesting an increase in MDSC-LC populations (17). The low tolerogenic activity of MDSC-LC from anti-Jagged-treated mice correlated with a lower expression of MDSC-suppressive mediators, iNOS and arginase I and decreased levels of the antibody targets, Jagged1 and 2 (Fig. 2D). Interestingly, MDSC-LC from anti-Jagged-treated mice showed slight higher levels of MDSC-regulator, gp91phox, and similar proportions of Annexin V+ (Fig. 2D–E), suggesting that anti-Jagged differentially modulates MDSC-regulatory pathways, without inducing apoptosis. Next, we aimed to determine the role of MDSC-LC in the anti-tumor effects induced by anti-Jagged. As such, we depleted MDSC-LC in tumor-bearing mice treated with anti-Jagged using an anti-Gr-1 antibody (Suppl. Fig. 3), or co-injected MDSC-LC from anti-Jagged-treated mice with 3LL tumors. Results show that elimination of MDSC-LC restored tumor growth in mice treated with anti-Jagged (Fig. 2F), whereas co-injection of 3LL cells with MDSC-LC from anti-Jagged-treated mice, but not isotype-treated, resulted in delayed tumor growth (Fig. 2G). Taken together, data suggest that treatment of tumor-bearing mice with anti-Jagged induces the accumulation of potentially anti-tumor MDSC-LC.
Figure 2. Anti-Jagged impacts the suppressive activity of tumor-MDSC.
(A) Percentages of CD11b+ Gr-1+ cells by flow cytometry in tumor and spleen of 3LL-bearing mice treated with anti-Jagged or isotype. Cumulative data from 10 mice per group (left) and representative results (right). (B–C) Proliferation and IFNγ expression in anti-CD3/CD28-activated CFSE-labeled T-cells co-cultured for 72 hours with tumor-MDSCs from anti-Jagged or control-treated mice bearing 3LL tumors. Means ± SD from 3 experiments. (D) Western blots from 3 similar repeats using protein lysates of tumor-MDSCs obtained from mice treated with anti-Jagged or isotype. (E) Annexin V in MDSCs from (D). (F) Tumor volume in 3LL-bearing mice treated or not with anti-Jagged and receiving anti-Gr-1, as described in methods. n=5. (H) Tumor growth in mice injected with 3LL cells alone or co-injected at a 1:1 ratio with tumor-MDSCs from 3LL-bearing mice treated with anti-Jagged or isotype. Means ± SEM from 5 mice per group. (H–I) Ability to block proliferation of CFSE-labelled T-cells primed with anti-CD3/CD28 and expression of arginase I and iNOS were measured in splenic MDSCs from 3LL-bearing mice cultured for 48 hours in GM-CSF plus 30% TES with and without anti-Jagged. Representative data from 3 similar experiments. ** P< 0.01, *** P, < 0.001
Impaired MDSC activity in anti-Jagged-treated mice could be a secondary result of direct anti-tumor effects since smaller tumors may have less MDSC-stimulatory factors. To rule out this, splenic-MDSCs from 3LL-bearing mice were cultured with TES for 48 hours in the presence of anti-Jagged antibody, after which they were co-cultured with CFSE-labeled activated T-cells. A decreased ability to block T-cell proliferation was noted in the anti-Jagged-treated MDSCs, compared to control MDSCs, which correlated with lower levels of arginase I and iNOS (Fig. 2H–I), suggesting a direct inhibitory effect of the anti-Jagged antibody on tumor-exposed MDSCs.
Anti-Jagged therapy induces anti-tumor effects through CD8+ T-cell responses
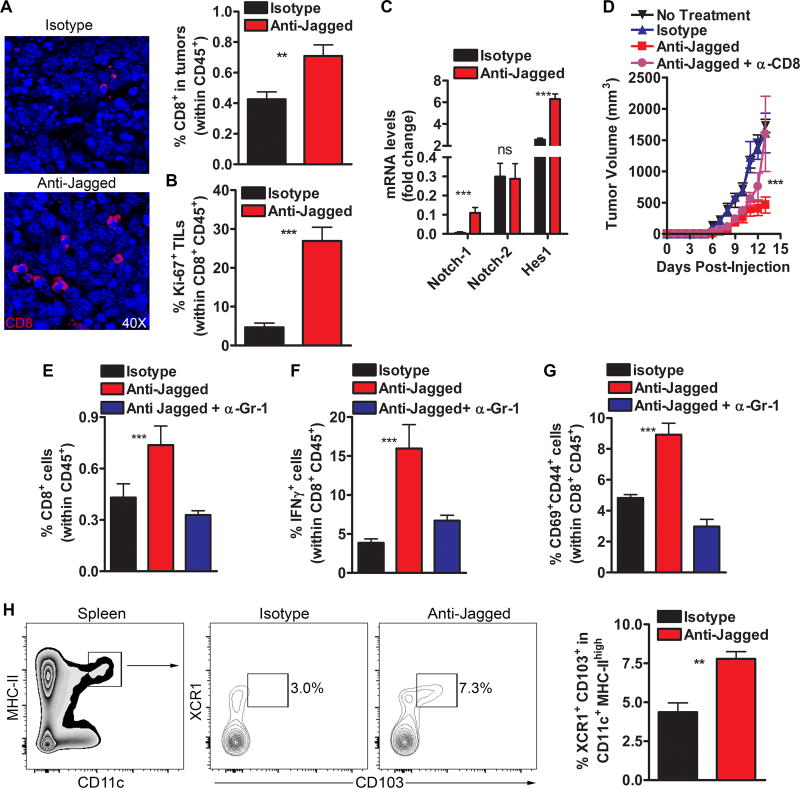
The expansion of MDSCs in tumors is a key driver in the inhibition of protective anti-tumor T-cell immunity. Thus, we tested the role of CD8+ T-cells in the anti-tumor effects induced by anti-Jagged. Although CD8+ T-cells in tumors did not express Jagged1 or 2 (data not shown), we found that treatment of 3LL-bearing mice with anti-Jagged increased the accumulation of CD8+ T-cells in tumors (Fig. 3A). Interestingly, anti-Jagged also elevated the proliferation and paradoxically enhanced Notch1 signaling of tumor-CD8+ T-cells, as suggested by the increased expression of replication marker, Ki-67 (Fig. 3B), and the higher levels of Notch1 and Notch target gene, Hes1, but not Notch2 (Fig. 3C), respectively. Moreover, elimination of CD8+ T-cells partially restored tumor growth in 3LL-bearing mice treated with anti-Jagged (Fig. 3D), confirming the key role of CD8+ T-cells in the anti-tumor effects induced by anti-Jagged. Next, we determined the functional interaction between the anti-Jagged-induced MDSC-LC and CD8+ T-cells. Depletion of MDSC-LC prevented the anti-Jagged-induced expansion of IFNγ-expressing and antigen-experienced and activated (CD44+ CD69+) CD8+ T-cells in tumors (Fig. 3E–G), suggesting that the anti-Jagged-induced MDSC-LC promoted anti-tumor CD8+ T-cell reactivity.
Figure 3. Anti-tumor effects induced by anti-Jagged antibody are mediated by CD8+ T-cells.
(A) Tumors from 3LL-bearing mice treated with anti-Jagged or control were tested for CD8+ T-cell infiltration by immunofluorescence (left) and flow cytometry (right). Results from 5 tumors per group. (B). Percentage of CD45+ CD8+ Ki-67+ T-cells was tested by flow cytometry in tumor suspensions from (A). (C) Tumor-associated CD45+ CD8+ T-cells were sorted by flow cytometry from 3LL-bearing mice treated with anti-Jagged or control and tested for specific mRNAs using quantitative-PCR. (D) Tumor growth in 3LL-bearing mice treated with anti-Jagged therapy receiving or not anti-CD8. n=5. (E–G) Percentage of CD45+ gated CD8+ T-cells, IFNγ-producing CD8+ T-cells (upon activation with PMA/ionomycin), and CD44+ CD69+ CD8+ T-cells in tumors from 3LL-bearing mice treated with anti-Jagged therapy with or without anti-Gr-1. Means from 5 mice ± SEM. (H) Spleens from (A) were tested for CD11C+ MHC-II+ CD103+ XCR1+ cells by flow cytometry. Representative finding (right) and means of 5 mice ± SEM (left). **, P<0.01 ***, P < 0.001.
The expansion of immunogenic CD11c+ MHC-II+ CD103+ XCR1+ cells has been correlated with development of protective anti-tumor T-cell immunity (22). An elevated abundance of this cellular subset was noted in 3LL-bearing mice treated with the anti-Jagged therapy (Fig. 3H). However, CD11c+ MHC-II+ CD103+ XCR1+ cells failed to develop from MDSCs cultured with anti-Jagged, and MDSC-LC were not found in gated immunogenic cells from anti-Jagged-treated mice (Suppl. Fig. 4A–B), indicating the independent nature of these populations in mice receiving anti-Jagged.
Anti-Jagged therapy overcomes tumor-induced tolerance and increases the effect of T-cell-based immunotherapy
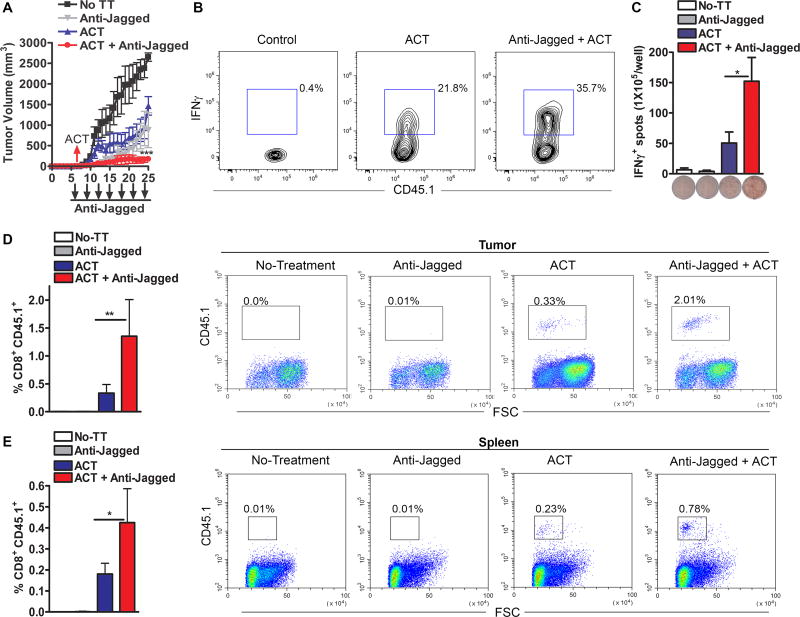
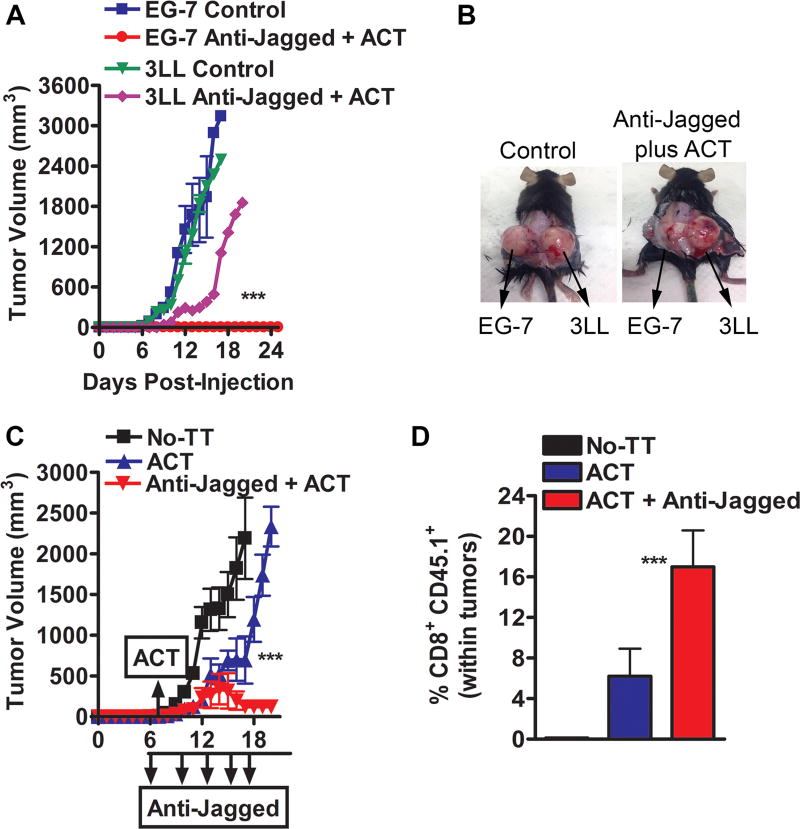
To address the effect of the anti-Jagged therapy in tumor-induced CD8+ T-cell tolerance, we used an ACT model against the experimental tumor-antigen ovalbumin (OVA), in which activated anti-OVA257–264 (SIINFEKL) transgenic CD45.1+ OT-1 cells were transferred into mice bearing OVA-expressing EG-7 tumors. Anti-Jagged therapy started 6 days after the EG-7 injection, and one day later, the mice were transferred with SIINFEKL-pre-activated CD45.1+ CD8+ OT-1 cells. A higher anti-tumor effect was observed in mice treated with anti-Jagged plus ACT, compared to those receiving anti-Jagged or ACT single treatments or untreated controls (Fig. 4A). Accordingly, EG-7-bearing mice treated with anti-Jagged plus ACT had a higher frequency of IFNγ-expressing CD45.1+ CD8+ OT-1 cells in the tumor (Fig. 4B), elevated frequency of clones producing IFNγ upon ex vivo activation of splenocytes with SIINFEKL (Fig. 4C), and increased yield of the transferred CD45.1+ OT-1 cells in the tumor and spleen (Fig. 4D–E). A similar effect was found after ACT with non-activated OT-1 cells (Suppl. Fig. 5A–C). Thus, results show the potential benefit of the anti-Jagged therapy as a strategy to overcome tumor-induced T-cell tolerance and as an approach to increase the efficacy of T-cell-based immunotherapy.
Figure 4. Jagged blockade overcomes tumor-induced T-cell tolerance and enhances T-cell immunotherapy.
(A) Splenocytes from CD45.1+ OT-1 mice were activated with SIINFEKL (2 µg/ml) for 48 hours, after which CD8+ T-cells were negatively sorted and transferred into CD45.2+ mice bearing established EG-7 tumors. Mice received anti-Jagged or transferred T-cells alone, or combination of ACT plus anti-Jagged. n=10. (B–C) Ten days after ACT, tumors were activated for 6 hours with PMA/ionomycin and percentages of IFNγ-producing OT-1 cells tested by flow cytometry. (B). Spleens were challenged with SIINFEKL and IFNγ production measured by Elispot (C). Results represent means ± SD from 5 mice. (D–E) Representative image showing accumulation of CD45.1+ CD8+ T-cells in tumor and spleen of mice from (A). **, P < 0.01; ***, P < 0.001.
During our experiments, we noticed that some of the mice treated with the combination of anti-Jagged and ACT completely rejected the EG-7 tumors. Thus, we studied the potential development of protective memory against the initial tumor. EG-7 cells failed to re-grow in mice previously treated with anti-Jagged plus ACT that had rejected the initial tumor, while they grew at the usual rate in naïve mice (Fig. 5A–B). Moreover, a partial protection against 3LL tumors was also noted in mice initially treated with anti-Jagged and ACT therapy that had rejected the EG-7 cells (Fig. 5A–B). Thus, combination of anti-Jagged and ACT induced the development of protective immune memory responses against tumors.
Figure 5. Anti-Jagged and adoptive T-cell therapy combination induces permanent responses against tumors.
(A–B) Mice that were initially treated with anti-Jagged plus ACT therapy and that had completely rejected their tumors, were injected with EG-7 (left flank) and 3LL (right flank) and followed for tumor growth. n=3 mice per group. (C) Tumor volume in immunodeficient Rag-mice bearing established EG-7 tumors that received combination of ACT plus anti-Jagged. n=5 mice per group. (D) Ten days after ACT, tumors were evaluated for infiltration of CD45.1+ CD8+ OT-1 cells by flow cytometry. n= 4 mice per group. ***, P < 0.001.
Next, we aimed to determine whether the anti-tumor effect induced after anti-Jagged plus ACT therapy was specifically mediated through the transferred T-cells. To test this, ACT alone or anti-Jagged plus ACT therapy were administered to T-cells-deficient Rag mice. Similar to the findings noted in wild type mice, a significant anti-tumor effect was found after combination of anti-Jagged and ACT, compared to ACT alone or no treatment controls (Fig. 5C), which correlated with a higher frequency of the transferred CD45.1+ CD8+ OT-1 cells in the tumor (Fig. 5D). Thus, the transferred T-cells mediated the anti-tumor effects induced by anti-Jagged plus ACT.
Tumors induce Jagged ligands in MDSCs through NFκB-p65
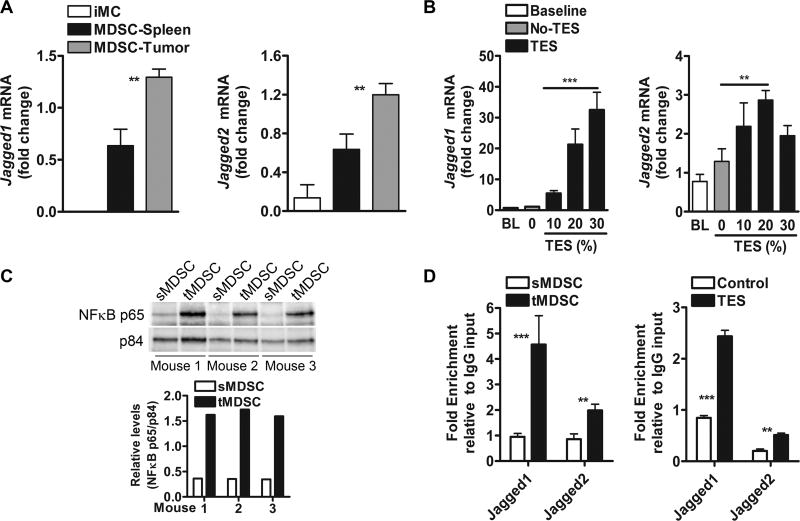
We aimed to investigate the role of the tumors in the upregulation of Jagged1 and 2 in MDSCs. Increased levels of Jagged1 and 2 were noted in tumor-infiltrating MDSCs, compared to splenic-MDSCs from tumor-bearing mice or iMCs from animals without tumors (Fig. 6A, Suppl. Fig. 6A). To determine the direct influence of tumor factors in the expression of Jagged in MDSCs, splenic-MDSCs were cultured in medium containing increasing concentrations of TES. A dose-dependent induction of Jagged ligands was found in TES-treated MDSCs, compared to untreated controls (Fig. 6B, Suppl. Fig. 6B). Next, we aimed to identify the intracellular mediators leading to the expression of Jagged1 and 2 in tumor-MDSCs. Activation of NFκB-p65 has been proposed as a common transcription factor regulating MDSC function and Jagged expression (23,24). Accordingly, we observed elevated nuclear expression of NFκB-p65 in tumor-MDSCs, compared to splenic-MDSCs (Fig. 6C). Also, enhanced endogenous binding of NFκB-p65 to Jagged1 and 2 promoters was detected in tumor-MDSCs and TES-treated MDSCs (Fig. 6D), compared to control MDSCs, suggesting the direct role of NFκB-p65 in the expression of Jagged1 and 2 in tumor-exposed MDSCs.
Figure 6. Tumor-induced NFκB-p65 drives the expression of Jagged ligands in tumor MDSC.
(A) Jagged1 and 2 mRNA in tumor and splenic-MDSCs from mice bearing 3LL tumors and in iMC from mice without tumors. Data represent means ± SD of 5 samples from different mice. (B) Jagged1 and 2 mRNA in splenic-MDSCs from 3LL-bearing mice cultured for 48 hours in GM-CSF-containing medium supplemented with increasing levels of TES. Results are from 3 independent experiments. (C) NFκB-p65 in nuclear extracts from tumor and splenic-MDSCs sorted from 3LL-bearing mice. A representative result from 3 repeats. (D) ChIP assays to detect the endogenous binding of NFκB-p65 to Jagged1 and 2 promoters were assessed in cells from (C). Results represent means ± SD from 2 experiments. **, P < 0.01; ***, P < 0.001.
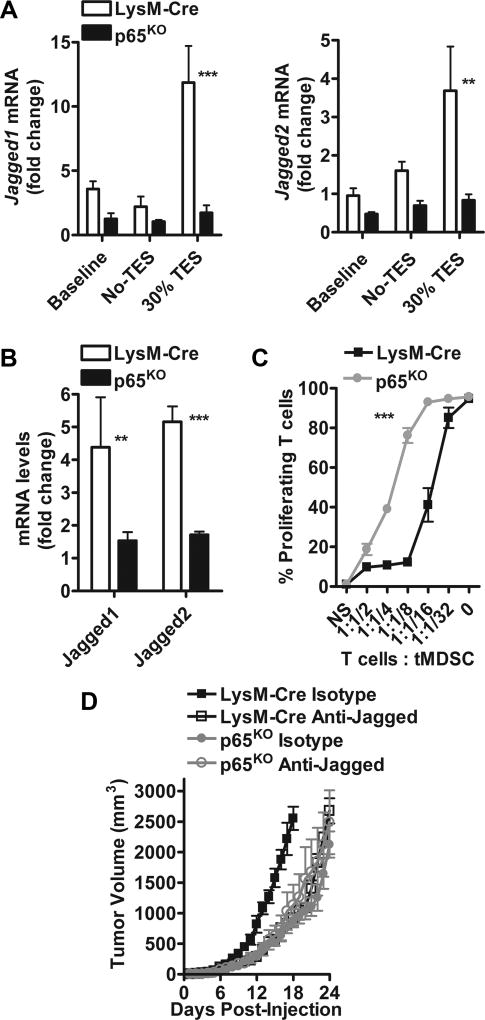
To confirm the role of the NFκB signaling in the induction of Jagged in MDSCs, we used myeloid cell-conditional NFκB-p65 knockout mice (p65KO), which were obtained after crossing floxed p65 mice with LysM-Cre mice. Lower induction of Jagged ligands was noted in TES-treated MDSCs and tumor-MDSCs from p65KO mice, compared to LysM-Cre controls (Fig. 7A–B), which also correlated with a significant inability of the p65KO MDSCs to block T-cell proliferation (Fig. 7C). These results show the role of NFκB-p65 in the induction of Jagged1 and 2 in tumor-MDSCs. Next, we tested the anti-tumor effects of anti-Jagged therapy in p65KO mice. Although anti-Jagged induced significant anti-tumor responses in LysM-Cre controls, we found similar kinetics of tumor growth in p65KO mice treated with anti-Jagged or isotype (Fig. 7D), indicating the potential role of the NFκB-p65-dependent upregulation of Jagged in MDSCs as a target for anti-Jagged therapy.
Figure 7. Deletion of NFκB-p65 decreases Jagged1 and 2 expression and blocks MDSC suppression in tumors.
(A) Splenic-MDSCs harvested from LysM-Cre controls and myeloid cell-conditional NFκB-p65KO mice bearing similar sized 3LL tumors were cultured in the presence of 30% TES for 48 hours, after which the levels of Jagged1 and 2 mRNA were tested by quantitative-PCR. Data are means ± SD from 3 repeats. (B) Jagged1 and 2 mRNA levels in tumor-MDSCs collected from LysM-Cre controls and myeloid cell-conditional NFκB-p65KO mice bearing 3LL tumors. Means ± SD from 5 different tumors. (C) Immunosuppressive activity of MDSCs from (B) against CFSE-labelled T-cells stimulated with anti-CD3/CD28. Percentages of proliferating T-cells were measured at 72 hours by flow cytometry. Results are means ± SD from 3 repeats. (D) Tumor volume in NFκB-p65KO and LysM-Cre mice bearing 3LL-tumors and treated or not with anti-Jagged. n=5. **, P < 0.01; ***, P < 0.001.
Discussion
In this study, we aimed to test the efficacy of the Jagged1–2 blocking antibody, CTX014, in tumor-bearing mice. The results characterize a promising therapeutic tool to reprogram MDSC-mediated T-cell suppression in tumors, thereby addressing a major obstacle in the development of successful therapies against cancer. Findings also advance in the understanding of the role of Notch ligands as major modulators of tumor-associated immune responses.
Accumulating evidence indicates the opposite effects of the activation of Notch in T-cells through DLL or Jagged ligands. DLL1 and 4 induced development of Th1 cells and effector memory CD8+ T-cells (15,25–29), whereas Jagged1 and 2 skewed T-cells into Th2, Treg, and anergic CD8+ T-populations (16,30–33). In agreement with the proposed immune inhibitory role of Jagged, we found that anti-Jagged overcame tumor-T-cell suppression through the induction of potentially immunogenic MDSC-LC. Our data also showed the expression of Jagged ligands in cancer cells and tumor-myeloid cells. Although anti-Jagged directly modulated MDSC function in vitro, it remains unknown whether the induced anti-tumor effects are a combination of Jagged targeting on cancer cells and MDSCs. In fact, it is possible that direct effects of anti-Jagged on tumor cells could result in immunogenic cell death-related responses promoting MDSC-LC. Thus, differential modulation of Jagged forms in cancer cells vs. myeloid cells will allow us to test the contribution of Jagged on these subsets in the inhibition of anti-tumor immunity. Also, understanding of the mechanisms of action of the anti-Jagged antibody, including the role of complement activation, antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP) will better characterize the mediators for this therapy in cancer.
Inhibition of Notch signaling in T-cells is emerging as a major mechanism of immune evasion in tumors. However, the pathways blunting Notch activity in T-cells remain unclear. MDSCs blocked the induction of full-length and cleaved Notch in T-cells through nitric oxide-dependent pathways (14). Also, the impaired Notch activity found in T-cells from tumor-bearing hosts was rescued by the proteasome inhibitor bortezomib, suggesting the post-translational regulation of T-cell-Notch in tumors (34). Additional data showed that methylation of the Notch regulator, Numb, triggered Notch activity upon T-cell stimulation, indicating the epigenetic regulation of Notch signaling in T-cells (35). Because impaired Notch activity in T-cells blocks their anti-tumor potential, it is conceivable that restoring T-cell-Notch signaling could promote anti-tumor immunity. Indeed, ectopic expression of NICD rendered CD8+ T-cells less susceptible to the regulatory effects of tumors and increased their efficacy upon ACT (14). Moreover, activation of T-cell-Notch through a DLL1-Fc fusion complex induced central memory CD8+ T-cells that had heightened anti-tumor effects (15,36). Accordingly, systemic DLL1 delivery promoted T-cell infiltration into tumors and enhanced efficacy of EGFR-targeted therapy (36). Collectively, these studies show that activation of DLL-Notch signaling could represent an opportunity to restore CD8+ T-cell responses in tumors. Interestingly, we found a paradoxical elevation of Notch1 signaling in tumor-linked CD8+ T-cells from anti-Jagged-treated mice. This effect could be explained by the induction of MDSC-LC with impaired regulatory function that fail to block Notch1 in T-cells. An additional possibility is that reprogramming of MDSCs by anti-Jagged could lead to the induction of DLL1 and 4, which induce Notch activation in T-cells. The specific role of these possibilities is currently under investigation.
Similar to T-cells, DLL and Jagged ligands induced opposite effects in myeloid cells. Myeloid precursors cultured with fibroblasts expressing DLL1 differentiated into functional DCs, while activation of Notch through Jagged promoted immature myeloid cells (37) and induced IL-10 (38). A potential explanation for the opposite roles of DLL and Jagged in myeloid cells is their differential effects on Wnt pathway (39,40). Our data show that tumor-derived factors trigger the expression of Jagged1–2 in tumor-associated MDSCs through NFκB-p65. However, the tumor factors increasing NFκB signaling remain unknown. Potential mediators include TNFα and IL-1β, both highly elevated in most tumor microenvironments and key regulators of MDSC function (41,42). Additional candidates include the exposure of MDSCs to hypoxia, tumor exosomes, or S100A8/A9 (43–45). In addition to inducing Jagged, NFκB controls the production of several cytokines, including IL-6, IL-10, TNFα and IL-1β, which occurs by direct NFκB transcriptional activity, but also through partnering with other factors, including Notch, hypoxia inducible factor alpha (HIF-1α), and the activator of transcription 3 (Stat3) (46–50).
In summary, our study describes a new therapeutic strategy to overcome MDSC-mediated T-cell suppression in tumors, which could increase the efficacy of several cancer treatments, such as T-cell-based immunotherapies that remain highly limited by the regulatory action of MDSCs.
Supplementary Material
Acknowledgments
We would like to thank CytomX (San Francisco, CA) for providing the anti-Jagged antibody.
Financial support: This work was partially supported by NIH-CA18485 to P.C. Rodriguez and by a pilot program from CytomX to P.C. Rodriguez and L. Miele.
Abbreviations
- ACT
Adoptive T-cell transfer
- DLL
Delta-like ligand
- MAML1–3
mastermind-like co-activator
- MDSCs
Myeloid-derived suppressor cells
- NICD
Notch intracellular active domain
- RBP-Jκ
Recombination signal-binding protein-Jκ
References
- 1.Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16(10):599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 4.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 5.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148(6):1081–4. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, et al. Selective Targeting of Myeloid-Derived Suppressor Cells in Cancer Patients Using DS-8273a, an Agonistic TRAIL-R2 Antibody. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13(6):427–37. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 9.Guruharsha KG, Kankel MW, rtavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13(9):654–66. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radtke F, Fasnacht N, MacDonald HR. Notch signaling in the immune system. Immunity. 2010;32(1):14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7(1):64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 12.Minter LM, Osborne BA. Canonical and non-canonical Notch signaling in CD4(+) T cells. Curr Top Microbiol Immunol. 2012;360:99–114. doi: 10.1007/82_2012_233. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto K, Maekawa Y, Kitamura A, Nishida J, Koyanagi A, Yagita H, et al. Notch2 signaling is required for potent antitumor immunity in vivo. J Immunol. 2010;184(9):4673–78. doi: 10.4049/jimmunol.0903661. [DOI] [PubMed] [Google Scholar]
- 14.Sierra RA, Thevenot P, Raber PL, Cui Y, Parsons C, Ochoa AC, et al. Rescue of notch-1 signaling in antigen-specific CD8+ T cells overcomes tumor-induced T-cell suppression and enhances immunotherapy in cancer. Cancer Immunol Res. 2014;2(8):800–11. doi: 10.1158/2326-6066.CIR-14-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Lin L, Shanker A, Malhotra A, Yang L, Dikov MM, et al. Resuscitating cancer immunosurveillance: selective stimulation of DLL1-Notch signaling in T cells rescues T-cell function and inhibits tumor growth. Cancer Res. 2011;71(19):6122–31. doi: 10.1158/0008-5472.CAN-10-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kijima M, Iwata A, Maekawa Y, Uehara H, Izumi K, Kitamura A, et al. Jagged1 suppresses collagen-induced arthritis by indirectly providing a negative signal in CD8+ T cells. J Immunol. 2009;182(6):3566–72. doi: 10.4049/jimmunol.0803765. [DOI] [PubMed] [Google Scholar]
- 17.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, et al. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41(3):389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180(4):2588–99. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 20.Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME, et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2014;134(12):2853–64. doi: 10.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3(11):1236–47. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162(6):1257–70. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston DA, Dong B, Hughes CC. TNF induction of jagged-1 in endothelial cells is NFkappaB-dependent. Gene. 2009;435(1–2):36–44. doi: 10.1016/j.gene.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Li B, Li X, Zhao X, Wan L, Lin G, et al. Transmembrane TNF-alpha promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol. 2014;192(3):1320–31. doi: 10.4049/jimmunol.1203195. [DOI] [PubMed] [Google Scholar]
- 25.Meng L, Bai Z, He S, Mochizuki K, Liu Y, Purushe J, et al. The Notch Ligand DLL4 Defines a Capability of Human Dendritic Cells in Regulating Th1 and Th17 Differentiation. J Immunol. 2016;196(3):1070–80. doi: 10.4049/jimmunol.1501310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backer RA, Helbig C, Gentek R, Kent A, Laidlaw BJ, Dominguez CX, et al. A central role for Notch in effector CD8(+) T cell differentiation. Nat Immunol. 2014;15(12):1143–51. doi: 10.1038/ni.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skokos D, Nussenzweig MC. CD8− DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204(7):1525–31. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backer RA, Helbig C, Gentek R, Kent A, Laidlaw BJ, Dominguez CX, et al. A central role for Notch in effector CD8(+) T cell differentiation. Nat Immunol. 2014;15(12):1143–51. doi: 10.1038/ni.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathieu M, Duval F, Daudelin JF, Labrecque N. The Notch signaling pathway controls short-lived effector CD8+ T cell differentiation but is dispensable for memory generation. J Immunol. 2015;194(12):5654–62. doi: 10.4049/jimmunol.1402837. [DOI] [PubMed] [Google Scholar]
- 30.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):116–24. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 31.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27(1):89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202(8):1037–42. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auderset F, Schuster S, Coutaz M, Koch U, Desgranges F, Merck E, et al. Redundant Notch1 and Notch2 signaling is necessary for IFNgamma secretion by T helper 1 cells during infection with Leishmania major. PLoS Pathog. 2012;8(3):e1002560. doi: 10.1371/journal.ppat.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thounaojam MC, Dudimah DF, Pellom ST, Jr, Uzhachenko RV, Carbone DP, Dikov MM, et al. Bortezomib enhances expression of effector molecules in anti-tumor CD8+ T lymphocytes by promoting Notch-nuclear factor-kappaB crosstalk. Oncotarget. 2015;6(32):32439–55. doi: 10.18632/oncotarget.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17(1):95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biktasova AK, Dudimah DF, Uzhachenko RV, Park K, Akhter A, Arasada RR, et al. Multivalent Forms of the Notch Ligand DLL-1 Enhance Antitumor T-cell Immunity in Lung Cancer and Improve Efficacy of EGFR-Targeted Therapy. Cancer Res. 2015;75(22):4728–41. doi: 10.1158/0008-5472.CAN-14-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng P, Nefedova Y, Corzo CA, Gabrilovich DI. Regulation of dendritic-cell differentiation by bone marrow stroma via different Notch ligands. Blood. 2007;109(2):507–15. doi: 10.1182/blood-2006-05-025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bugeon L, Gardner LM, Rose A, Gentle M, Dallman MJ. Cutting edge: Notch signaling induces a distinct cytokine profile in dendritic cells that supports T cell-mediated regulation and IL-2-dependent IL-17 production. J Immunol. 2008;181(12):8189–93. doi: 10.4049/jimmunol.181.12.8189. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Zhou J, Cheng P, Ramachandran I, Nefedova Y, Gabrilovich DI. Regulation of dendritic cell differentiation in bone marrow during emergency myelopoiesis. J Immunol. 2013;191(4):1916–26. doi: 10.4049/jimmunol.1300714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity. 2009;30(6):845–59. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14(5):408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122(11):4094–104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–9. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osipo C, Golde TE, Osborne BA, Miele LA. Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest. 2008;88(1):11–7. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- 47.Aguilera C, Hoya-Arias R, Haegeman G, Espinosa L, Bigas A. Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2004;101(47):16537–42. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nefedova Y, Cheng P, Gilkes D, Blaskovich M, Beg AA, Sebti SM, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175(7):4338–46. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172(2):989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 50.Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res. 2010;16(3):784–9. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.