Abstract
Descriptions of the changeable, striking colors associated with secreted natural products date back well over a century. These molecules can serve as extracellular electron shuttles (EESs) that permit microbes to access substrates at a distance. In this review, we argue that the colorful world of EESs has been too long neglected. Rather than simply serving as a diagnostic attribute of a particular microbial strain, redox-active natural products likely play fundamental, underappreciated roles in the biology of their producers, particularly those that inhabit biofilms. Here, we describe the chemical diversity and potential distribution of EES producers and users, discuss the costs associated with their biosynthesis, and critically evaluate strategies for their economical usage. We hope this review will inspire efforts to identify and explore the importance of EES cycling by a wide range of microorganisms so that their contributions to shaping microbial communities can be better assessed and exploited.
Keywords: extracellular electron shuttle, metabolism, microbial diversity, public goods, redox chemistry
1. INTRODUCTION
The microbial world is nothing if not colorful. Microbes are colorful figuratively in that they accomplish stunning metabolic feats, which are increasingly being recognized for their important roles in promoting human health (57, 117) and shaping the composition of the atmosphere, lithosphere, and hydrosphere (59) and for their biotechnological potential (72), to list only a few examples. Microbes are colorful literally in that they produce a spectacular range of pigments, representing every hue of the rainbow. This latter aspect of microbial identity is often the first thing one notices about a strain when it is streaked upon a plate, whether its pigments are tightly associated with its colonies or rapidly diffuse away (Figure 1). Flipping through Bergey’s Manual of Systematic Bacteriology (42), one can find numerous examples of organisms that have been named after the colors they produce, for example, Pseudomonas aeruginosa (“rusted copper”; i.e., greenish-blue) (129), Pseudoalteromonas luteoviolacea (“yellow-violet”) (43), and Streptomyces coelicolor (“heavenly color”; Figure 2b) (19); yet the biological functions of these defining pigments are seldom discussed. Both old (39) and emerging (48, 55, 93) evidence, however, suggests that literal colorfulness may underpin figurative colorfulness, particularly when the pigment in question has the property of changing color.
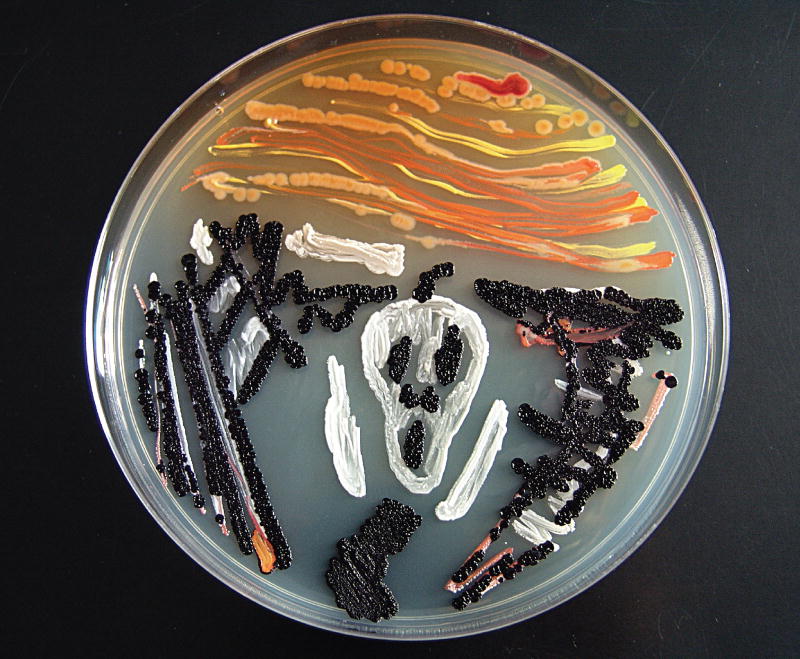
Figure 1.
The colorful microbial world: The Scream rendered in bacteria. Black bacteria are Chromobacterium violaceum, and white bacteria are Staphylococcus epidermidis. Others are a mix of Micrococcus luteus (yellow) and unidentified pink and orange strains from different environmental samples. Reproduced courtesy of Tomislav Ivankovic, University of Zagreb, Croatia.
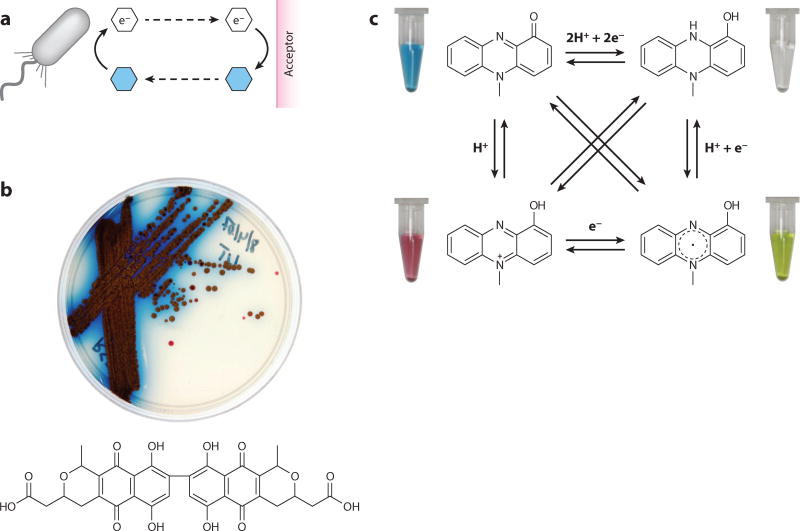
Figure 2.
General concept and structures of representative extracellular electron shuttles (EESs). (a) Action at a distance. Cells can transfer electrons to small molecules, which then transfer these electrons to distant extracellular acceptors. (b) Redox-active pigment production by Streptomyces coelicolor, including actinorhodin, whose structure is shown below. (c) One molecule, four colors. The color of pyocyanin depends on both pH and reduction potential. The tubes pictured each contain approximately 200-µM pyocyanin in water. The radical and fully reduced forms can be prepared by titrating pyocyanin with sodium dithionite, producing an immediate and stunning color change. Note that “e−” represents a single- or multiple-electron transfer reaction, depending on the particular EES.
Color changes are often indicative of redox reactions, and the redox properties of many colorful excreted metabolites confer upon them rich potential (bio)chemical reactivity. Such reactivity, when combined with the metabolites’ ability to cycle in and out of the cells that produce them, permits these molecules to serve as extracellular electron shuttles (EESs). Although we use the word extracellular to draw attention to the fact that these metabolites can leave the cell in their reduced state to transfer electrons to a distant extracellular oxidant, it is equally important to the definition of microbial electron shuttles that they can return to the cell in the oxidized state, whereupon they are re-reduced (Figure 2a). It is the cycling of EESs and their facilitation of electron transfer both within and without the cell that underpins their important physiological functions. Several years ago, we made a distinction between two types of EESs, classifying them according to whether microorganisms produce them (endogenous) or whether they are already present in an environment (exogenous) (55). Endogenous EESs, for example, include microbial metabolites that are often considered to be redox-active antibiotics (93); exogenous EESs include humic substances, a chemically heterogeneous fraction of organic compounds that derive from the degradation of microbial and plant matter (15, 61, 98). Though pigmented proteins, such as cytochromes, can also be involved in extracellular electron transfer, for the purpose of this review, we restrict our focus to nonproteinaceous natural products.
EESs are particularly relevant to situations where microbes have limited access to a critical substrate. For example, an electron acceptor for catabolism might be poorly soluble, as is the case for minerals in many groundwater and sedimentary systems or electrodes in biofuel applications. Alternatively, the substrate might be locally depleted due to rapid consumption by other cells, outpacing its diffusion, as is the case for oxygen in biofilms, be they on the surface of a corroding steel pipeline or in the mucus-filled lungs of an individual living with cystic fibrosis (22, 68, 111, 125). Finally, the substrate might be utilized by another organism in an intimate syntrophic partnership, requiring the passage of electrons between different cell types to catalyze an important biogeochemical reaction, such as the anaerobic oxidation of methane achieved by mixed archaeal-bacterial aggregates (76, 101). In each of these cases, extracellular electron transfer permits the microbes at a distance from the terminal electron acceptor to remain metabolically active.
Cycling of EESs represents a strategy whereby microbes can facilitate extracellular electron transfer but is by no means the only one. Over the past three decades, investigations into how organisms transfer electrons to or from minerals have revealed that many possess outer-membrane cytochromes that are critically important for these processes (105). Whether and/or how electrons traverse through nanowires, cables, or some type of extracellular matrix is still being debated (74, 86, 114, 127). We simply note that in some organisms, such as the mineral-reducing bacteria Shewanella and Geobacter species, the proteinaceous machinery that is required for extracellular electron transfer can interact with and reduce EESs of different types (16, 66, 71, 109, 116). Thus, it is important to be mindful of the potential involvement of EESs in any context where extracellular electron transfer matters, be it the soil of the rhizosphere or the inflamed tissues of chronic infections. Moreover, due to their versatile redox activity, EESs can play other important biological roles, such as serving as signaling molecules and promoting iron acquisition (31, 54, 93, 121).
In this review, we focus our discussion primarily on colorful, endogenous EESs, drawing upon our experience studying phenazines produced by Pseudomonas aeruginosa (20, 21, 30–33, 45, 46, 54, 55, 58, 93–95, 115, 119–121) (Figure 2c). We use phenazines for illustrative purposes only to pique the reader’s curiosity about what other molecules may have similar physiological functions. We critically discuss costs of EES biosynthesis, as well as bioenergetic concerns related to the cell biology of EES reduction and potential loss to the environment. Underpinning this review is the conviction that recent advances in mass spectrometry, imaging, and sequencing have the potential to enable dramatic progress in understanding the importance of endogenous EESs across diverse microbial systems.
2. DIVERSITY OF ENDOGENOUS EESs AND ORGANISMS
How many endogenous EESs exist in nature and which organisms produce them? We are only at the threshold of being able to answer these questions. The two best-characterized EES-producing organisms are P. aeruginosa and Shewanella oneidensis, known for their use of phenazines and flavins, respectively (13, 45, 66, 75, 119). Other putative EES-producing organisms, for example, Lactococcus lactis, Sphingomonas xenophaga, and Klebsiella pneumonia, are reported to utilize quinone shuttles (27, 38, 63, 83, 85) (Table 1). A few other observations in the literature hint at the production of EESs by diverse organisms, including Geothrix and Geobacter species (11, 116), yet in most cases, the molecular nature of the putative EESs is unknown. Although many secreted redox-active natural products, such as indigoidine, have been well known for decades, only recently have their physiological functions begun to be explored (24). Because these types of natural products are genetically encoded in biosynthetic clusters, bioinformatic analysis predicts that EES production by microorganisms may be extensive (Figure 3). In this section, we discuss the chemical diversity and potential distribution of EES producers and users as predicted by current bioinformatics platforms. We emphasize that these are predictions that must be validated experimentally (see the sidebar titled How to Characterize a Putative EES).
Table 1.
Putative extracellular electron shuttles
| Molecule | Structure | Aqueous midpoint potential (mV versus SHE) |
LogP or Kowa |
Organism | Reference(s) |
|---|---|---|---|---|---|
| 2-Amino-3-dicarboxy-1,4-naphthoquinone |
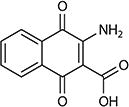
|
−71 | 0.15 | Lactococcus lactis | 38 |
| 4-Amino-1,2-naphthoquinone |
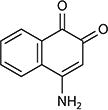
|
−96 | 0.81 | Sphingomonas xenophaga BN6 | 63 |
| 2,6-Di-tert-butyl-1,4-benzoquinone |
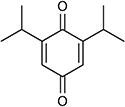
|
−253 | 3.9 | Klebsiella pneumonia | 27 |
| Flavin mononucleotide |
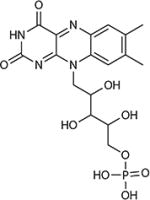
|
−220 | −2.18 | Alkaliphilic community, Shewanella spp. | 40, 75 |
| Phenazine-1-carboxylic acid |
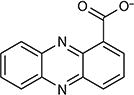
|
−177 | 2.17 (Kow)/1.75 (logP) | Pseudomonas spp., Streptomyces spp. | 120 |
| Pyocyanin |
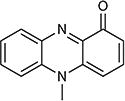
|
−34 | 1.6 (Kow) | Pseudomonas aeruginosa | 93 |
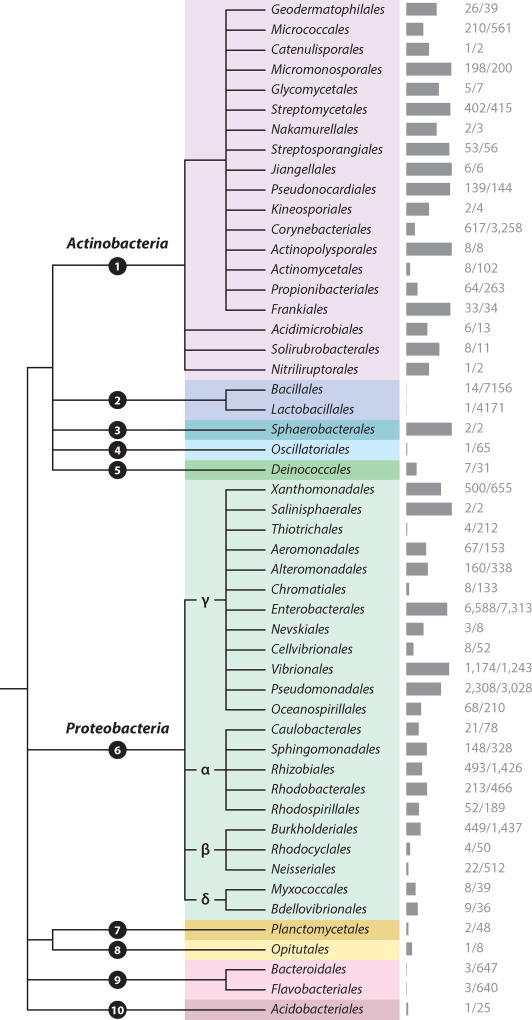
Figure 3.
Phylogenetic distribution of SoxR, a transcription factor that senses redox-active metabolites. The phylogenetic tree shows all bacterial orders containing at least one genome with a SoxR homolog in the Integrated Microbial Genomes database [SoxR TIGRfam 01950 (14)]. The gray bars represent the percentage of genomes from each order that contain SoxR, with the number of SoxR-containing genomes over the total listed on the right. The tree was generated using the National Center for Biotechnology Information taxonomic classification with the Phylogenetic Tree Generator and Interactive Tree of Life (37, 69). Phyla: ❶ Actinobacteria, ❷ Firmicutes, ❸ Chloroflexi, ❹ Cyanobacteria, ❺ Deinococcus, ❻ Proteobacteria, ❼ Planctomycetes, ❽ Verrucomicrobia, ❾ Bacteroidetes, ❿ Acidobacteria.
2.1. Chemical Diversity of EESs
Much like their intracellular cousins [e.g., NAD(P)H, FAD, and quinones], EESs are distinguished by the presence of conjugated bonds, often in the form of heterocyclic aromatic rings. It is this system of conjugated bonds that confers color upon EESs. Generally, conjugated bonds are also the molecular origin of an electron shuttle’s redox activity, as double bonds can be chemically reduced and rearranged at biologically accessible reduction potentials. Rearrangement of the conjugated bond system necessarily entails a change in the molecule’s absorption spectrum, and so a shuttle’s redox activity is inextricably linked to its vibrant and interconvertible colors (Figure 2c).
Unlike intracellular redox-active metabolites, the endogenous EESs described in the literature to date generally lack modifications such as adenylation or lipidation. Instead, they can be decorated with functional groups (e.g., amines, carboxylic acids, amides) that tune physical and chemical properties such as the reduction potential, charge state, or solubility. Advances in mass spectrometry and natural product discovery are only beginning to unearth the extensive combinatorial diversity of these modifications: As of February 2017, the Dictionary of Natural Products database contains over 190 unique phenazine derivatives, 1,200 quinones, 1,600 naphthoquinones, and 2,200 anthraquinones (1). Although the growth of natural products databases has far outpaced our ability to empirically study the functions of these molecules, computational chemistry has shown progress in predicting their properties, such as reduction potential (4). We anticipate that a convergence of metabolomics and computational chemistry, together with the ongoing detailed study of select molecules such as phenazines, will implicate many of these redox-active natural products as ubiquitous and diverse EESs.
2.2. Phylogenetic Diversity
To predict which organisms may produce or utilize EESs, we may consider the phylogenetic distribution of genes used to produce or interact with known EESs. We might speculate that most organisms could shuttle electrons, because S. oneidensis and other species use common flavin cofactors (13, 40). However, it is unclear how many organisms may use flavins in a similar manner, so we will instead focus on more specialized natural products. As an example, we will see that biosynthetic clusters for phenazines are widespread, as is the redox transcription factor SoxR, which can sense phenazines.
2.2.1. Biosynthetic clusters
Bioinformatic tools (e.g., antiSMASH) are rapidly improving our ability to identify specific natural products (122). The authors of the Integrated Microbial Genomics–Atlas of Biosynthetic Gene Clusters (IMG-ABC) database showcased their toolkit with phenazines by identifying approximately 1,000 genomes that contained at least six of seven genes required for phenazine biosynthesis. These species included the well-known phenazine producers Streptomyces species and pseudomonads but also Alphaproteobacteria, Betaproteobacteria, and other Actinobacteria. It was suggested that a much wider set of species probably produce phenazines than previously thought and these new putative producers likely synthesize phenazines with novel chemical structures and biosynthetic pathways (51). As the accuracy of these types of bioinformatics tools advances, we may be able to predict an organism’s ability to make EESs from its genome with reasonable confidence.
2.2.2. SoxR redox sensors
One way to identify potential EES users is to search genomes for machinery that can sense them. Presently, the best-known example is the SoxR transcription factor. SoxR was originally studied in Escherichia coli for its role in upregulating the oxidative stress response upon exposure to superoxide-generating molecules. It was discovered that SoxR sensed redox-active molecules through an Fe-S cluster bound by a unique cysteine motif, and its activation prompted sequence-specific binding and transcriptional activation (92). Studies outside of the Enterobacteriaceae then showed that SoxR did not upregulate the oxidative stress response in P. aeruginosa (82) and Streptomyces coelicolor (23) but that it responded to endogenous redox-active molecules (33, 108) and upregulated factors likely to be involved in their cycling. Comparative genomic analysis found that SoxR is widely distributed through the Bacteria, and it predicted that most organisms do not conform to the original E. coli oxidative stress paradigm (33).
Today, the IMG database contains approximately 15,000 bacterial genomes with high-confidence SoxR homologs out of approximately 47,000 total bacterial genomes (14), approximately the same proportion as those genomes with the stress sigma factor RpoS. Figure 3 shows that SoxR homologs are present in 10 bacterial phyla and the most representatives occur in the orders of Proteobacteria and Actinobacteria (37, 69). Homologs can be found in the majority of Pseudomonadales (2,308 genomes) and Streptomycetales (402 genomes), which are well known for their production of natural (often redox-active) products. Strikingly, SoxR homologs have also been identified in the emerging and established pathogens of the genus Mycobacterium (approximately 400 genomes within the order Corynebacteriales), the agriculturally important Rhizobium (493 genomes) and Frankia (33 genomes), and recently discovered phyla (6 genomes). Whether these organisms use SoxR to sense endogenous EESs remains to be determined, but it suggests that they might sense exogenous EESs at a minimum. It is even possible that organisms could upregulate their own EES-processing machinery to “cheat,” analogous to organisms that steal exogenous siderophores (50).
Although the diversity of redox-active metabolites, biosynthetic clusters, and redox-sensing homologs satisfy important preconditions for potential widespread EES usage in the microbial world, the numbers we have presented come with two important caveats. First, not all secreted redox-active molecules can serve as EESs due to redox-potential constraints (see the sidebar titled How Does Redox Potential Define an EES?). Second, although we have good reason to think that SoxR is a robust predictor of an organism’s ability to sense EESs, it is just one of many possible transcription factors used to sense, modify, and transport small redox-active molecules (33, 100). Our bioinformatic analysis only suggests the potential for widespread production and usage of EESs in the microbial world, which we hope will stimulate experimental follow-up (see the sidebar titled How to Characterize a Putative EES).
3. COSTS OF EES BIOSYNTHESIS
At first glance, electron shuttles might appear expensive to produce, especially for a molecule that can be lost to diffusion or used by competitors in a microbial community. We discuss strategies for overcoming diffusive losses below, but here we wish to critically ask whether shuttles are “expensive.” Although a cell certainly loses carbon material by secreting a shuttle, the cost should not be compared to the energy that might be gained from fully oxidizing the shuttle to CO2. It is, after all, precisely the condition of oxidant limitation where EESs are important. In a microenvironment where organic carbon is abundant but oxidants are limited or absent, there is essentially no opportunity cost to discarding carbon. Based on biosynthetic pathways, what is the cost of producing EESs?
All the known phenazine-producing bacteria use the same biosynthetic pathway, reviewed in detail elsewhere (9), in which two molecules of chorismate are modified and condensed to produce the core phenazine ring. Chorismate synthesis begins with two ATP equivalents and requires two additional ATP equivalents for completion (28), and so the core phenazine molecule requires a total of eight ATP. Some quinones require only a single ATP in their conversion from chorismate (28). Riboflavin synthesis requires only one ATP in its conversion from guanosine triphosphate (5), and including the precursors, its cost has been estimated at up to 25 ATP per molecule (75). For these representative examples, the energy required to synthesize a shuttle is on the order of 5–25 ATP per molecule. How does this compare to other cellular processes? In protein synthesis, the addition of a single amino acid to a nascent peptide chain requires an estimated 4.5–7.9 ATP per amino acid (3), whereas RNA transcription further requires one ATP equivalent per nucleotide. Thus, the biosynthetic proteins themselves may represent a significant portion of a shuttle’s cost. A quantitative analysis in Shewanella spp. illustrates that their endogenous shuttles, flavin derivatives, cost less than 0.1% of a cell’s energy budget to secrete at the concentration required to promote extracellular electron transfer (as low as 250 nM) (75).
The striking conclusion is that for a redox-limited microbial community, shuttle synthesis may pose only a minor metabolic cost. Furthermore, bacteria are known to carefully regulate production of secreted molecules. Xavier et al. (124) coined the term metabolic prudence to describe how P. aeruginosa specifically secretes carbon-rich rhamnolipids (used for swarming motility) when excess carbon is available and the cost of production is low. This regulation ensures that secreting cells are more fit than cheaters in competition experiments, because cost is minimized and the benefit of swarming remains high. Examples from P. aeruginosa and Salmonella typhimurium suggest that metabolic prudence is a broadly used strategy for regulating secretions under diverse nutritional conditions, such as iron, phosphate, or nitrogen limitation (44, 47). We expect that metabolically prudent regulation could further reduce the effective cost of EESs under oxidant limitation.
4. CELL BIOLOGY OF ELECTRON SHUTTLING
The biochemical reduction of EESs poses a unique topological challenge. Perhaps the best-elucidated mechanism of extracellular electron transport (EET) occurs in S. oneidensis MR-1, which has been reviewed in detail elsewhere (13, 106). This organism uses the Mtr pathway, which is distinguished by a set of proteins that directly transfer electrons through the inner membrane, across the periplasm, and to the outside of the outer membrane where they directly reduce extracellular shuttles (Figure 4a). The reduction of quinone by NADH dehydrogenase can be accompanied by the translocation of up to four protons across the inner membrane (41), thus directly contributing to the proton motive force. In contrast, many other membrane-bound quinone reductases, such as succinate dehydrogenase or lactate dehydrogenase, are not known to translocate protons. Thus, the direct benefit of EET depends on the particular metabolism of a cell.
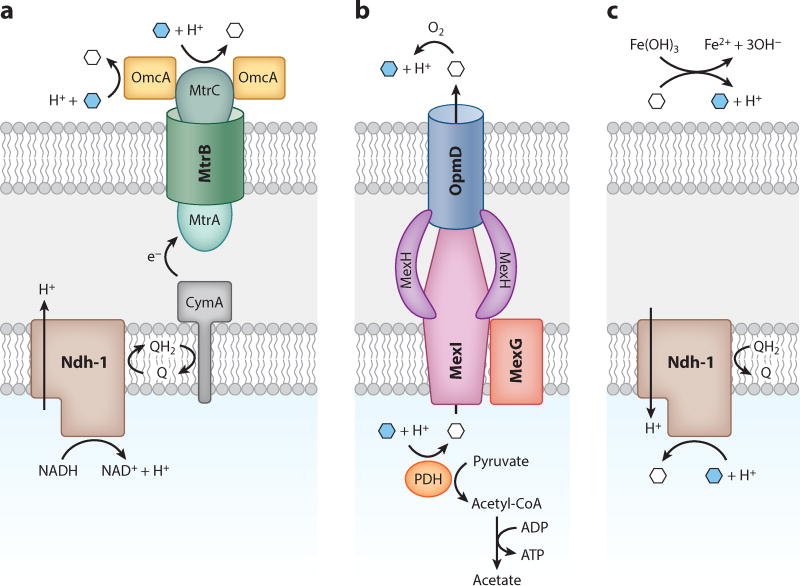
Figure 4.
The topology of electron shuttle reduction (white hexagons) and oxidation (blue hexagons). For simplicity, we show only single electrons and protons without considering the stoichiometry of individual reactions. (a) The Mtr system in Shewanella oneidensis MR-1. Shuttles are reduced extracellularly and do not need to reenter the cell. (b) A model of phenazine reduction in Pseudomonas aeruginosa that allows for energy conservation by electron shuttling. By substituting for NAD+ in the pyruvate dehydrogenase complex (PDH), phenazines enable the synthesis of acetyl-CoA, which can drive ATP synthesis through the enzymes phosphate transacetylase and acetate kinase (45, 46). Efflux of reduced phenazines by MexGHI-OpmD (100) may be coupled to proton translocation. (c) A condition where electron shuttling is costly. Operating the NADH dehydrogenase in reverse can consume the proton motive force to drive paraquat reduction (18). Extracellular reduction of some minerals, such as Fe(OH)3, can alkalize the medium, possibly depleting the proton motive force further and causing pH stress for the cell.
In contrast to Shewanella systems, specific pathways of extracellular electron transfer in other systems, such as phenazine shuttling in P. aeruginosa, remain ambiguous. Recent work from our laboratory shows that cytoplasmic (flavo)proteins can catalyze phenazine reduction in P. aeruginosa (46). Specifically, the enzyme dihydrolipoamide dehydrogenase enables phenazines to substitute for NAD+ in the pyruvate and α-ketoglutarate dehydrogenase complexes. This activity may allow phenazines to promote ATP synthesis during glucose oxidation by increasing the flux through pyruvate dehydrogenase and acetate kinase (46) (Figure 4b). Similar findings have been observed in fermenting organisms, where the presence of humic substances or analogs increases the ratio of oxidized-to-reduced products (7), presumably increasing the ATP yield of fermentation.
The invocation of cytoplasmic EES reduction has interesting implications with respect to the proton motive force. Upon reduction, phenazines, quinones, and flavins take on two protons at circumneutral pH (Figure 2c). The subsequent secretion and oxidation of the reduced shuttle would therefore release those two protons outside the cell, essentially translocating two protons across the inner membrane (Figure 4b). If this shuttle does not require active transport into or out of the cell, redox cycling of a shuttle could drive the generation of a proton motive force. Conversely, shuttles that accept electrons from the cytoplasmic face of NADH dehydrogenase, such as the artificial compound paraquat, might consume the proton motive force (18) (Figure 4c). Although progress is being made on phenazine transport systems (100), where different phenazines are reduced, how their intracellular reactivity is controlled and how they enter the appropriate efflux pump machinery remain open questions. The take-away message is that the subcellular localization of shuttle reduction greatly influences the energy that can be conserved, and we expect that future work will elucidate new and interesting mechanisms to accompany the Mtr system.
Finally, although we have focused here on the mechanisms of EES reduction, extracellular electron transfer entails a more subtle point with respect to the terminal electron acceptor. The reduction potential of minerals, and the kinetics of their reduction by shuttles (120), depends strongly on pH, and so the free energy available to a cell necessarily depends on its microenvironment. Moreover, mineral reduction is accompanied by alkalization (Figure 4c), which further inhibits the available free energy and may even negatively affect the proton motive force if it happens close to the cell. From the perspective of the cell, the energy that can be conserved via electron shuttling may depend on the pH-buffering capacity of its environment and the rate of proton diffusion.
5. EESs IN THE EXTRACELLULAR ENVIRONMENT
In addition to defining the constraints that shape intracellular EES biosynthesis and processing, what is central to any predictive understanding of EES usage is knowledge of what happens to EESs once they leave the cell. Because most bacteria live in biofilms (81, 112), we will consider the extracellular environment to be a self-produced matrix of extracellular polymeric substances (EPSs) and water. Here, we explore under what conditions EESs can be efficiently recycled.
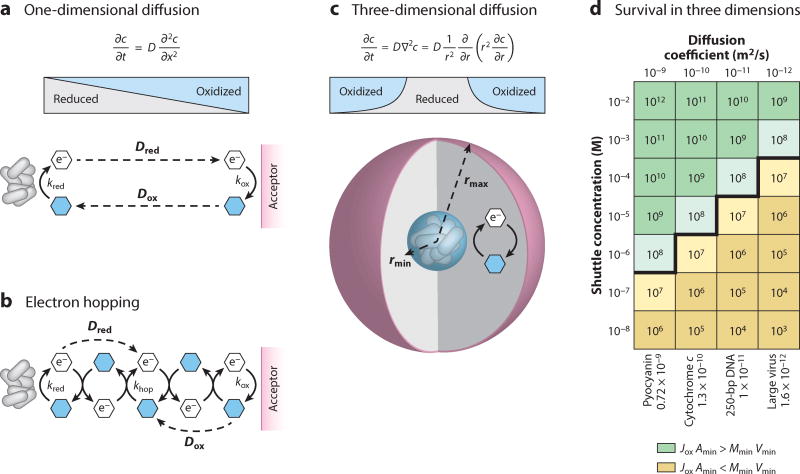
5.1. Shuttle Diffusion
Secreted metabolites diffuse down their concentration gradients away from the cells that produce them. In the environment, losses to diffusion could limit the effectiveness of EESs as a metabolic strategy. Moreover, it is unclear whether diffusion alone provides sufficient electron transfer rates to support metabolism. However, two studies have shown that diffusion is theoretically fast enough to explain EES-supported metabolism in a closed system (64, 89). In a diffusion model, the flux of reduced EESs away from cells (Jred) and towards the electron acceptor is driven by the concentration gradient that forms as cells reduce EESs. Simultaneously, the concentration gradient of oxidized EESs drives flux (Jox) back to the cells that use EESs to run their metabolism (Figure 5a). To model an oxygen-limited P. aeruginosa biofilm (73, 77, 79, 87), we adapted a simple model of three-dimensional diffusion comprising an inner sphere of anoxic cells that shuttles electrons to an outer shell of electron acceptors (Figure 5c). Using realistic values of phenazine diffusion coefficients (approximately 10−9–10−10 m2/s) and concentrations (1–100 µM), the model produces redox gradients that can theoretically drive sufficient flux of oxidized EESs to support survival (Figure 5d), assuming a conservative estimate of the minimum energy needed to survive (32, 45, 64). Importantly, this model predicts that EESs are a viable strategy over a wide range of concentrations and/or diffusion coefficients (Figure 5d). Therefore, in an ideal scenario where EESs are not lost to the environment, we expect that diffusion alone is sufficient to support EES-mediated survival.
Figure 5.
Mechanisms of electron transfer by extracellular electron shuttles (EESs). (a) Diffusion only. The reduced EES can diffuse down its concentration gradient to an electron acceptor, whereas the oxidized form simultaneously returns along its own concentration gradient. At steady state (∂c/∂t = 0), the concentration gradients are linear in this closed, one-dimensional system. (b) The self-exchange rate (khop) allows electrons to hop between oxidized and reduced EESs. If khop is significantly faster than the rate of diffusion, electron hopping will accelerate the apparent diffusion of electrons. EES diffusion can also co-occur. (c) In three dimensions, with an inner sphere of cells and an outer sphere of electron acceptor, the concentration gradient at steady state is nonlinear. The gradient is steeper near the inner sphere, allowing for higher flux than in the one-dimensional case. (d) Results of a closed, steady-state model of EES diffusion in three dimensions, with different EES concentrations and diffusion coefficients. The results were obtained by solving for the concentration profile at steady state (∂c/∂t = 0) and applying Fick’s first law [J = −D (∂c/∂r)] to obtain . The number in each box corresponds to the modeled flux of oxidized EES (J evaluated at r = rmin) through the surface area (Amin) of the inner sphere, in units of molecules per second. Green indicates regions where diffusion can account for the minimum flux needed for cells to survive, and tan indicates regions where diffusion is too slow. The number of molecules per second needed to support the inner sphere with volume Vmin was calculated to be 1.5 × 107 based on published parameters for Mmin, the minimum energy required for survival in Pseudomonas aeruginosa (64). The inner sphere radius was rmin = 10 µm, and the outer sphere radius was rmax = 100 µm. For simplicity, we have taken the diffusion constants of the oxidized and reduced EES (Dred and Dox) to be equivalent, and the rates of EES reduction and oxidation (kred and kox) to be instant. Representative aqueous diffusion coefficients spanning a range of biomolecular structures, pyocyanin, cytochrome c, 250-bp DNA, and a large virus are listed below the plot for comparison (29, 73, 77, 79).
5.2. Electron Hopping as an Alternative to Shuttle Diffusion
In a more realistic open system, where EESs can be lost or degraded, what strategies might facilitate efficient EES cycling? An attractive mechanism that might prevent EES loss and facilitate fast electron transfer is electron hopping between EESs bound in the biofilm matrix (Figure 5b) (12, 89). The flux of hopping electrons from the cells to the acceptor is driven by the concentration gradient of electrons (i.e., redox gradient) (110). In this scenario, EESs are bound within the extracellular matrix, and electrons propagate from cells to the terminal metabolic electron acceptor (e.g., O2) by sequential electron transfer reactions among the EESs, allowing EES retention and reuse (Figure 5b). Hopping steps (khop) are expected to happen rapidly compared to shuttle diffusion (D) (99, 118).
Significant theoretical and experimental work has already been done to describe electron hopping in electrode-grown biofilms of Geobacter sulfurreducens. Such biofilms, which can exceed tens of microns in thickness, utilize the underlying electrode, poised at a sufficient oxidizing potential, as the terminal metabolic acceptor. Cells not in direct contact with the electrode can transport their respired electrons to the electrode via hopping through a proposed network of c-type cytochromes as opposed to EESs. This model is supported experimentally by measurements of biofilm electrical conductivity (12, 88, 97, 110, 113, 126–128). Much of the theory comes from models of synthetic redox polymers and proteins that contain bound redox cofactors (10, 12, 49, 91, 114). This work provides a proof of principle that motivates experiments to test for electron hopping in other biofilms where EESs may dominate extracellular electron transfer.
5.3. Evolutionary Strategies that Maintain EESs in Open Environments
Regardless of the electron transfer mechanism, sufficient concentrations of EESs must be maintained for viability. What strategies do microbes take in producing and maintaining public goods in open environments with competing organisms? We view EESs as public goods because they are secreted molecules that benefit nearby individuals as well as the producer cell (123). Kin selection theory posits that public goods production can evolve when individuals’ actions increase not only their own reproductive success but also the success of genetically related individuals (123). Microbes are therefore more likely to produce public goods when they are highly related to their neighbors, the direct and indirect benefits are high, and the cost of production is low (78). Moreover, the molecular properties of a public good may minimize cheating by other community members.
In this context, it makes sense that EESs would be regulated by quorum-sensing (QS) molecules, signals that direct population behaviors when cell numbers are high. QS molecules can provide cells with information on nearby relatives and diffusion conditions (53). By dividing quickly and producing an extracellular matrix, many bacteria can establish highly related microcolonies that favor the evolution of public goods (78). Laboratory studies show that producers of the siderophore pyoverdin outcompete nonproducer mutants when populations are highly related but not when strains are mixed (34, 50). Because QS molecules are secreted into the environment and subject to diffusion, they enable cells to distinguish between closed and open systems (96). A good example of QS regulation of EES production can be found in how Pseudomonas species regulate phenazine production (31).
Other strategies that can facilitate the viability of EES utilization relate to mechanisms of privatization, such as physical retention or a requirement for special machinery to utilize the secreted molecule (123). Physical retention can be achieved via noncovalent interactions between small molecule and biofilm components, which in the case of EESs could conceivably also provide a scaffold for electron hopping. Marine microbes that retain siderophores by embedding them in the outer membrane with lipophilic side chains (67) provide an example of selective retention of a secreted metabolite near producing cells. However, other chemical mechanisms, such as electrostatic attraction between EESs and specific components of a biofilm matrix, may facilitate retention. This may help rationalize coregulation of EESs and EPSs, as in the case of phenazines, eDNA, and particular types of EPSs (25, 26, 32). Discrimination can be achieved with highly specific receptors for siderophores and QS molecules that enable the uptake of their secreted products only by producers (80, 103, 123). Another strategy is to make the public goods themselves toxic, which requires specialized detoxification systems (102). Interestingly, many EESs were first identified as antibiotics (24, 93), and special proteins are required to deal with their toxic byproducts (52).
6. OUTLOOK
This is an exciting time to care about microbes that change color. The explosion of computational platforms that can predict structures and properties of natural products, combined with the exponential growth of genomic databases, is ushering in a golden age for EES discovery. Based on these databases, and lessons learned from experimental systems where redox-active pigment production and utilization have been well documented, the available evidence suggests that EESs are likely to be widespread and play underappreciated roles in the physiology of diverse microbes.
Going forward, it will be important to prioritize systematic screens to test for EES production and usage. Above, the first sidebar (How to Characterize a Putative EES) provides an example of how one can approach this, but innovations that would enable high-throughput screens are needed to stimulate the search process. Although such technologies could be developed by individual laboratories at universities, an opportunity for efficient scale-up exists if national laboratories that are interested in metabolomics, such as the Joint Genome Institute (6, 14, 107), were to take on this challenge. The creation of pipelines to interface metabolomics with complementary bioinformatics and physiological screens would greatly accelerate EES discovery. In our view, the identification of genetically tractable, EES-producing microbes should be a priority for future research. Genetically tractable systems are now more achievable than ever before (17, 84); thus, the isolation of EES-producing or -utilizing microbes has the potential to quickly reach a mechanistic level of understanding.
Identification of machinery that senses and processes EESs across diverse microorganisms will permit the type of comparative analysis needed to enable general principles to be resolved. For example, the better we understand the conservation of EES transporters or sensors, the more confidently we will be able to scan genomes to predict EES production or usage by new isolates. In this way, we will be able to learn whether the strategies that govern EES cycling by the handful of organisms that have been studied in detail to date represent special cases or broader paradigms. Metabolic strategies generally fall into a limited number of categories that are viable for cells; thus, it will be exciting to discover the range of strategies that can be used by organisms that employ EES for energy generation and other purposes.
We close by noting that much of what we understand about microbial metabolism springs from research that has been done when nutrients are replete. Yet, microbial existence in the natural world is usually limited by a variety of substrates (8), and it is in this context that we believe EESs will prove to be broadly important. Not only can EESs enable energy generation in oxidant-limited biofilms, which are ubiquitous (81, 112), but the broader reactivity of EESs can impact the fate and transport of pollutants in soils and sediments (60–62). From both fundamental and applied viewpoints, a comprehensive understanding of EES production, sensing, and utilization thus stands to improve our ability to rationalize and control the composition and activities of microbial communities across a variety of natural, engineered, and clinical environments.
HOW TO CHARACTERIZE A PUTATIVE EES.
What criteria establish that a molecule functions as an EES? Based on our definition, the molecule must facilitate extracellular electron transfer, undergo multiple redox cycles, and provide a physiological benefit to its producer via cycling. The following steps can be used to determine whether a particular secreted metabolite is an EES.
-
■
Find a tractable extracellular electron transfer phenotype and implicate a soluble EES. Quantifiable extracellular electron transfer phenotypes can be detected in assays with minerals (40, 54), electrodes (35, 45, 119), or soluble electron acceptors (65, 93). Secreted EESs can be implicated by demonstrating electron acceptor reduction in the absence of direct cell contact using different strategies, such as separation by agar, permeable membranes, glass frits, or compartments in microfluidic devices.
-
■
Isolate and characterize the EES molecule. Once an EES phenotype is established, the responsible molecule can be purified using chromatography and other standard separation methods from organic chemistry. Its spectral properties and redox potential can then be measured using different types of spectroscopy (ultraviolet-visible, electron paramagnetic resonance) and cyclic voltammetry, respectively (e.g., 39, 104, 120).
-
■
Genetically inhibit EES biosynthesis. Because EESs are not essential under all conditions, it is possible to isolate mutants defective in their production. Such mutants can offer insights into EES biosynthesis, regulation, and even cycling mechanisms (e.g., 30, 90, 119). Control over EES production, which is enabled by mutant strains, is necessary to rigorously determine the physiological benefits of an EES.
-
■
Measure EES turnover. EES uptake and reduction can be quantified using biosynthetic mutants in the presence of a defined EES concentration and oxidant (e.g., 119). The molecule can be defined as an EES if it enables a superstoichiometric reaction with the distal electron acceptor.
HOW DOES REDOX POTENTIAL DEFINE AN EES?
The essence of a shuttle is summarized by its midpoint reduction potential, a broad generalization of a compound’s ability to act as electron acceptor or donor. To avoid misusing this parameter, one must remain cognizant that it is descriptive of standard conditions for reactions heading toward thermodynamic equilibrium, conditions that rarely describe biological systems. Importantly, many redox reactions involve proton exchange, and so the true reduction potential depends on the environmental pH. A distinction must also be drawn between the one- and two-electron potentials of a shuttle, which can vary by hundreds of millivolts; this difference has even been exploited by some microorganisms to drive the reduction of low-potential acceptors in a process known as electron bifurcation (56, 70). Moreover, the reduction potential says nothing about the kinetics or chemical sensibility of the reaction. A striking example of this distinction is the thermodynamic favorability of biological nitrogen fixation (2), a process that nonetheless requires catalysis and energy input to proceed at rates that support life. Conversely, the artificial redox cycling drug paraquat has a midpoint potential below that of most intracellular donors, rendering paraquat reduction thermodynamically unfavorable under standard conditions; even so, the paraquat radical reacts with oxygen at a rate that is nearly diffusion limited (36), allowing paraquat to drive redox cycling under biological conditions. And so, although a shuttle’s reduction potential is a core physical parameter that captures the essence of redox cycling, it must be carefully considered with respect to environmental conditions, reaction kinetics, and other chemical properties. A rigorous quantitative prediction of these effects remains an ongoing challenge, but further advancements will undoubtedly enrich the already informative natural products databases.
Acknowledgments
We thank Leonard Tender, Matthew Yates, and members of the Newman lab for helpful comments on the manuscript; Muir Morrison, Tal Einav, and Manuel Razo for guidance in deriving the three-dimensional diffusion model; and Tomislav Ivankovic for allowing us to use his memorable microbial artwork in Figure 1. Financial support from the Donna and Benjamin M. Rosen Bioengineering Center, NIH (1R01AI127850-01A1), and ARO (W911NF-17-1-0024) enabled this work.
Glossary
- Extracellular electron shuttle (EES)
self-produced small molecule that accepts metabolic electrons and donates them to extracellular electron acceptors
- Biosynthetic cluster
an organized set of genes that encodes enzymes that produce specific metabolites
- Redox gradient
a concentration gradient of electrons across a field of redox-active molecules
- Public goods
secreted molecules that benefit nearby individuals that did not originally produce the molecules
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Chemnetbase. Dictionary of Natural Products 25.2. Taylor and Francis; Oxford, UK: 2017. [accessed February, 2017]. http://dnp.chemnetbase.com/ [Google Scholar]
- 2.Alberty RA. Thermodynamics of the nitrogenase reactions. J. Biol. Chem. 1994;269:7099–102. [PubMed] [Google Scholar]
- 3.Amthor JS. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Ann. Bot. 2000;86:1–20. [Google Scholar]
- 4.Assary RS, Brushett FR, Curtiss LA. Reduction potential predictions of some aromatic nitrogen-containing molecules. RSC Adv. 2014;4:57442–51. [Google Scholar]
- 5.Bacher A, Eberhardt S, Fischer M, Kis K, Richter G. Biosynthesis of vitamin B2 (riboflavin) Annu. Rev. Nutr. 2000;20:153–67. doi: 10.1146/annurev.nutr.20.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Baran R, Ivanova NN, Jose N, Garcia-Pichel F, Kyrpides NC, et al. Functional genomics of novel secondary metabolites from diverse cyanobacteria using untargeted metabolomics. Mar. Drugs. 2013;11:3617–31. doi: 10.3390/md11103617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benz M, Schink B, Brune A. Humic acid reduction by Propionibacterium freudenreichii and other fermenting bacteria. Appl. Environ. Microbiol. 1998;64:4507–12. doi: 10.1128/aem.64.11.4507-4512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergkessel M, Basta DW, Newman DK. The physiology of growth arrest: uniting molecular and environmental microbiology. Nat. Rev. Microbiol. 2016;14:549–62. doi: 10.1038/nrmicro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankenfeldt W, Parsons JF. The structural biology of phenazine biosynthesis. Curr. Opin. Struct. Biol. 2014;29:26–33. doi: 10.1016/j.sbi.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blauch DN, Saveant JM. Dynamics of electron hopping in assemblies of redox centers. Percolation and diffusion. J. Am. Chem. Soc. 1992;114:3323–32. [Google Scholar]
- 11.Bond DR, Lovley DR. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 2005;71:2186–89. doi: 10.1128/AEM.71.4.2186-2189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd DA, Erickson JS, Roy JN, Snider RM, Strycharz-Glaven SM, Tender LM. Theory of redox conduction and the measurement of electron transport rates through electrochemically active biofilmsn. In: Beyenal H, Babauta JT, editors. Biofilms in Bioelectrochemical Systems: From Laboratory Practice to Data Interpretation. Hoboken, NJ: Wiley; 2015. pp. 177–210. [Google Scholar]
- 13.Brutinel ED, Gralnick JA. Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl. Microbiol. Biotechnol. 2012;93:41–48. doi: 10.1007/s00253-011-3653-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen I-MA, Markowitz VM, Chu K, Palaniappan K, Szeto E, et al. IMG/M: integrated genome and metagenome comparative data analysis system. Nucleic Acids Res. 2016;45:D507–16. doi: 10.1093/nar/gkw929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates JD, Cole KA, Chakraborty R, O’Connor SM, Achenbach LA. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl. Environ. Microbiol. 2002;68:2445–52. doi: 10.1128/AEM.68.5.2445-2452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coates JD, Ellis DJ, Blunt-Harris EL, Gaw CV, Roden EE, Lovley DR. Recovery of humic-reducing bacteria from a diversity of environments. Appl. Environ. Microbiol. 1998;64:1504–9. doi: 10.1128/aem.64.4.1504-1509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cobb RE, Wang Y, Zhao H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth. Biol. 2014;4:723–28. doi: 10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochemé HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J. Biol. Chem. 2008;283:1786–98. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 19.Conn JE. The pigment production of Actinomyces coelicolor and A. violaceus-ruber. J. Bacteriol. 1943;46:133. doi: 10.1128/jb.46.2.133-149.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa KC, Bergkessel M, Saunders S, Korlach J, Newman DK. Enzymatic degradation of phenazines can generate energy and protect sensitive organisms from toxicity. mBio. 2015;6:e01520–15. doi: 10.1128/mBio.01520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa KC, Glasser NR, Conway SJ, Newman DK. Pyocyanin degradation by a tautomerizing demethylase inhibits Pseudomonas aeruginosa biofilms. Science. 2017;355:170–73. doi: 10.1126/science.aag3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowley ES, Kopf SH, LaRiviere A, Ziebis W, Newman DK. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. mBio. 2015;6:e00767–15. doi: 10.1128/mBio.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz RD, Gao Y, Penumetcha S, Sheplock R, Weng K, Chander M. Expression of the Streptomyces coelicolor SoxR regulon is intimately linked with actinorhodin production. J. Bacteriol. 2010;192:6428–38. doi: 10.1128/JB.00916-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cude WN, Mooney J, Tavanaei AA, Hadden MK, Frank AM, et al. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl. Environ. Microbiol. 2012;78:4771–80. doi: 10.1128/AEM.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das T, Kutty SK, Tavallaie R, Ibugo AI, Panchompoo J, et al. Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation. Sci. Rep. 2015;5:8398. doi: 10.1038/srep08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das T, Manefield M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLOS ONE. 2012;7:e46718. doi: 10.1371/journal.pone.0046718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng L, Li F, Zhou S, Huang D, Ni J. A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin. Sci. Bull. 2010;55:99–104. [Google Scholar]
- 28.Dewick PM. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 1994;11:173–203. doi: 10.1039/np9941100173. [DOI] [PubMed] [Google Scholar]
- 29.Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J. Diabetes Res. 2014;2014:562625. doi: 10.1155/2014/562625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006;61:1308–21. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006;61:1308–21. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich LEP, Okegbe C, Price-Whelan A, Sakhtah H, Hunter RC, Newman DK. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. 2013;195:1371–80. doi: 10.1128/JB.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietrich LEP, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–6. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–14. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 35.Emde R, Swain A, Schink B. Anaerobic oxidation of glycerol by Escherichia coli in an amperometric poised-potential culture system. Appl. Microbiol. Biotechnol. 1989;32:170–75. [Google Scholar]
- 36.Farrington JA, Ebert M, Land EJ, Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim. Biophys. Acta Bioenerg. 1973;314:372–81. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- 37.Federhen S. The NCBI taxonomy database. Nucleic Acids Res. 2012;40:D136–43. doi: 10.1093/nar/gkr1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freguia S, Masuda M, Tsujimura S, Kano K. Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry. 2009;76:14–18. doi: 10.1016/j.bioelechem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Friedheim E, Michaelis L. Potentiometric study of pyocyanine. J. Biol. Chem. 1931;91:355–68. [Google Scholar]
- 40.Fuller SJ, McMillan DG, Renz MB, Schmidt M, Burke IT, Stewart DI. Extracellular electron transport-mediated Fe(III) reduction by a community of alkaliphilic bacteria that use flavins as electron shuttles. Appl. Environ. Microbiol. 2014;80:128–37. doi: 10.1128/AEM.02282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galkin AS, Grivennikova VG, Vinogradov AD. →H+/2e stoichiometry in NADH-quinone reductase reactions catalyzed by bovine heart submitochondrial particles. FEBS Lett. 1999;451:157–61. doi: 10.1016/s0014-5793(99)00575-x. [DOI] [PubMed] [Google Scholar]
- 42.Garrity GM, Bell JA, Lilburn TG. Taxonomic Outline of the Prokaryotes. Bergey’s Manual of Systematic Bacteriology. New York: Springer; 2004. [Google Scholar]
- 43.Gauthier M, Flatau G. Antibacterial activity of marine violet-pigmented Alteromonas with special reference to the production of brominated compounds. Can. J. Microbiol. 1976;22:1612–19. doi: 10.1139/m76-237. [DOI] [PubMed] [Google Scholar]
- 44.Gerstel U, Römling U. Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella typhimurium. Environ. Microbiol. 2001;3:638–48. doi: 10.1046/j.1462-2920.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 45.Glasser NR, Kern SE, Newman DK. Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol. Microbiol. 2014;92:399–412. doi: 10.1111/mmi.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glasser NR, Wang BX, Hoy JA, Newman DK. The pyruvate and α-ketoglutarate dehydrogenase complexes of Pseudomonas aeruginosa catalyze pyocyanin and phenazine-1-carboxylic acid reduction via the subunit dihydrolipoamide dehydrogenase. J. Biol. Chem. 2017;292:5593–607. doi: 10.1074/jbc.M116.772848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glick R, Gilmour C, Tremblay J, Satanower S, Avidan O, et al. Increase in rhamnolipid synthesis under iron-limiting conditions influences surface motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 2010;192:2973–80. doi: 10.1128/JB.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grahl N, Kern SE, Newman DK, Hogan DA. The yin and the yang of phenazine physiology. In: Chincholkar S, Thomashow L, editors. Microbial Phenazines: Biosynthesis, Agriculture and Health. Heidelberg, Ger.: Springer; 2013. pp. 43–70. [Google Scholar]
- 49.Gray HB, Winkler JR. Electron transfer in proteins. Annu. Rev. Biochem. 1996;65:537–61. doi: 10.1146/annurev.bi.65.070196.002541. [DOI] [PubMed] [Google Scholar]
- 50.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–27. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 51.Hadjithomas M, Chen I-MA, Chu K, Ratner A, Palaniappan K, et al. IMG-ABC: a knowledge base to fuel discovery of biosynthetic gene clusters and novel secondary metabolites. mBio. 2015;6:e00932–15. doi: 10.1128/mBio.00932-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassan HM, Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 1979;196:385–95. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 53.Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft J-U. Does efficiency sensing unify diffusion and quorum sensing? Nat. Rev. Microbiol. 2007;5:230–39. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez ME, Kappler A, Newman DK. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 2004;70:921–28. doi: 10.1128/AEM.70.2.921-928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez ME, Newman DK. Extracellular electron transfer. Cell. Mol. Life Sci. 2001;58:1562–71. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herrmann G, Jayamani E, Mai G, Buckel W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 2008;199:784–91. doi: 10.1128/JB.01422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hum. Microbiome Proj. Consort. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 2012;47:738–45. doi: 10.1165/rcmb.2012-0088OC. [DOI] [PubMed] [Google Scholar]
- 59.Jelen BI, Giovannelli D, Falkowski PG. The role of microbial electron transfer in the coevolution of the biosphere and geosphere. Annu. Rev. Microbiol. 2016;70:45–62. doi: 10.1146/annurev-micro-102215-095521. [DOI] [PubMed] [Google Scholar]
- 60.Jiang J, Kappler A. Kinetics of microbial and chemical reduction of humic substances: implications for electron shuttling. Environ. Sci. Technol. 2008;42:3563–69. doi: 10.1021/es7023803. [DOI] [PubMed] [Google Scholar]
- 61.Kappler A, Benz M, Schink B, Brune A. Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 2004;47:85–92. doi: 10.1016/S0168-6496(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 62.Kappler A, Haderlein SB. Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ. Sci. Technol. 2003;37:2714–19. doi: 10.1021/es0201808. [DOI] [PubMed] [Google Scholar]
- 63.Keck A, Rau J, Reemtsma T, Mattes R, Stolz A, Klein J. Identification of quinoide redox mediators that are formed during the degradation of naphthalene-2-sulfonate by Sphingomonas xenophaga BN6. Appl. Environ. Microbiol. 2002;68:4341–49. doi: 10.1128/AEM.68.9.4341-4349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kempes CP, Okegbe C, Mears-Clarke Z, Follows MJ, Dietrich LE. Morphological optimization for access to dual oxidants in biofilms. PNAS. 2014;111:208–13. doi: 10.1073/pnas.1315521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan MT, Duncan SH, Stams AJ, van Dijl JM, Flint HJ, Harmsen HJ. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic–anoxic interphases. ISME J. 2012;6:1578–85. doi: 10.1038/ismej.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kotloski NJ, Gralnick JA. Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. mBio. 2013;4:e00553–12. doi: 10.1128/mBio.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kömmerli R, Schiessl KT, Waldvogel T, McNeill K, Ackermann M. Habitat structure and the evolution of diffusible siderophores in bacteria. Ecol. Lett. 2014;17:1536–44. doi: 10.1111/ele.12371. [DOI] [PubMed] [Google Scholar]
- 68.Lee A, Newman D. Microbial iron respiration: impacts on corrosion processes. Appl. Microbiol. Biotechnol. 2003;62:134–39. doi: 10.1007/s00253-003-1314-7. [DOI] [PubMed] [Google Scholar]
- 69.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–45. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Hinderberger J, Seedorf H, Zhang J, Buckel W, Thauer RK. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J. Bacteriol. 2008;190:843–50. doi: 10.1128/JB.01417-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl. Environ. Microbiol. 2005;71:4414–26. doi: 10.1128/AEM.71.8.4414-4426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lovley DR. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006;4:497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- 73.Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman A. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 2000;275:1625–29. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 74.Malvankar NS, Rotello VM, Tuominen MT, Lovley DR. Reply to ‘Measuring conductivity of living Geobacter sulfurreducens biofilms’. Nat. Nanotechnol. 2016;11:913–14. doi: 10.1038/nnano.2016.191. [DOI] [PubMed] [Google Scholar]
- 75.Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. PNAS. 2008;105:3968–73. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGlynn SE, Chadwick GL, Kempes CP, Orphan VJ. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature. 2015;526:531–35. doi: 10.1038/nature15512. [DOI] [PubMed] [Google Scholar]
- 77.Murray AG, Jackson GA. Viral dynamics: a model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. Oldendorf. 1992;89:103–16. [Google Scholar]
- 78.Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol. Rev. 2009;33:206–24. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 79.Nauman JV, Campbell PG, Lanni F, Anderson JL. Diffusion of insulin-like growth factor-I and ribonuclease through fibrin gels. Biophys. J. 2007;92:4444–50. doi: 10.1529/biophysj.106.102699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neilands J. Iron absorption and transport in microorganisms. Annu. Rev. Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- 81.O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 82.Palma M, Zurita J, Ferreras JA, Worgall S, Larone DH, et al. Pseudomonas aeruginosa SoxR does not conform to the archetypal paradigm for SoxR-dependent regulation of the bacterial oxidative stress adaptive response. Infect. Immun. 2005;73:2958–66. doi: 10.1128/IAI.73.5.2958-2966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pence HE, Williams A. ChemSpider: an online chemical information resource. J. Chem. Educ. 2010;87:1123–24. [Google Scholar]
- 84.Peters JM, Silvis MR, Zhao D, Hawkins JS, Gross CA, Qi LS. Bacterial CRISPR: accomplishments and prospects. Curr. Opin. Microbiol. 2015;27:121–26. doi: 10.1016/j.mib.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petrauskas AA, Kolovanov EA. ACD/Log P method description. Perspect. Drug Discov. Des. 2000;19:99–116. [Google Scholar]
- 86.Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, et al. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218–21. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- 87.Phalak P, Chen J, Carlson RP, Henson MA. Metabolic modeling of a chronic wound biofilm consortium predicts spatial partitioning of bacterial species. BMC Syst. Biol. 2016;10:90. doi: 10.1186/s12918-016-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phan H, Yates MD, Kirchhofer ND, Bazan GC, Tender LM, Nguyen T-Q. Biofilm as a redox conductor: a systematic study of the moisture and temperature dependence of its electrical properties. Phys. Chem. Chem. Phys. 2016;18:17815–21. doi: 10.1039/c6cp03583c. [DOI] [PubMed] [Google Scholar]
- 89.Picioreanu C, Head IM, Katuri KP, van Loosdrecht MC, Scott K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007;41:2921–40. doi: 10.1016/j.watres.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Pierson LS, III, Thomashow LS. Cloning and heterologous expression of the phenazine biosynthetic. Mol. Plant-Microbe Interact. 1992;5:330–39. doi: 10.1094/mpmi-5-330. [DOI] [PubMed] [Google Scholar]
- 91.Pirbadian S, El-Naggar MY. Multistep hopping and extracellular charge transfer in microbial redox chains. Phys. Chem. Chem. Phys. 2012;14:13802–8. doi: 10.1039/c2cp41185g. [DOI] [PubMed] [Google Scholar]
- 92.Pomposiello PJ, Demple B. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 2001;19:109–14. doi: 10.1016/s0167-7799(00)01542-0. [DOI] [PubMed] [Google Scholar]
- 93.Price-Whelan A, Dietrich LE, Newman DK. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2006;2:71–78. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- 94.Price-Whelan A, Dietrich LE, Newman DK. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J. Bacteriol. 2007;189:6372–81. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramos I, Dietrich LE, Price-Whelan A, Newman DK. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 2010;161:187–91. doi: 10.1016/j.resmic.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002;10:365–70. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 97.Robuschi L, Tomba JP, Schrott GD, Bonanni PS, Desimone PM, Busalmen JP. Spectroscopic slicing to reveal internal redox gradients in electricity-producing biofilms. Angew. Chem. Int. Ed. 2013;52:925–28. doi: 10.1002/anie.201205440. [DOI] [PubMed] [Google Scholar]
- 98.Roden EE, Kappler A, Bauer I, Jiang J, Paul A, et al. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat. Geosci. 2010;3:417–21. [Google Scholar]
- 99.Rosso KM, Smith DM, Wang Z, Ainsworth CC, Fredrickson JK. Self-exchange electron transfer kinetics and reduction potentials for anthraquinone disulfonate. J. Phys. Chem. A. 2004;108:3292–303. [Google Scholar]
- 100.Sakhtah H, Koyama L, Zhang Y, Morales DK, Fields BL, et al. The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. PNAS. 2016;113:E3538–47. doi: 10.1073/pnas.1600424113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheller S, Yu H, Chadwick GL, McGlynn SE, Orphan VJ. Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science. 2016;351:703–7. doi: 10.1126/science.aad7154. [DOI] [PubMed] [Google Scholar]
- 102.Schertzer JW, Boulette ML, Whiteley M. More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol. 2009;17:189–95. doi: 10.1016/j.tim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 103.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu. Rev. Microbiol. 2013;67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 104.Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 1998;32:2984–89. [Google Scholar]
- 105.Shi L, Dong H, Reguera G, Beyenal H, Lu A, et al. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016;14:651–62. doi: 10.1038/nrmicro.2016.93. [DOI] [PubMed] [Google Scholar]
- 106.Shi L, Rosso KM, Clarke TA, Richardson DJ, Zachara JM, Fredrickson JK. Molecular underpinnings of Fe(III) oxide reduction by Shewanella oneidensis MR-1. Front. Microbiol. 2012;3:46–55. doi: 10.3389/fmicb.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Silva LP, Northen TR. Exometabolomics and MSI: deconstructing how cells interact to transform their small molecule environment. Curr. Opin. Biotechnol. 2015;34:209–16. doi: 10.1016/j.copbio.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 108.Singh AK, Shin JH, Lee KL, Imlay JA, Roe JH. Comparative study of SoxR activation by redox-active compounds. Mol. Microbiol. 2013;90:983–96. doi: 10.1111/mmi.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith JA, Tremblay P-L, Shrestha PM, Snoeyenbos-West OL, Franks AE, et al. Going wireless: Fe(III) oxide reduction without pili by Geobacter sulfurreducens strain JS-1. Appl. Environ. Microbiol. 2014;80:4331–40. doi: 10.1128/AEM.01122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Snider RM, Strycharz-Glaven SM, Tsoi SD, Erickson JS, Tender LM. Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. PNAS. 2012;109:15467–72. doi: 10.1073/pnas.1209829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stewart PS. Diffusion in biofilms. J. Bacteriol. 2003;185:1485–91. doi: 10.1128/JB.185.5.1485-1491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 113.Strycharz-Glaven SM, Snider RM, Guiseppi-Elie A, Tender LM. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci. 2011;4:4366–79. [Google Scholar]
- 114.Subramanian P, Pirbadian S, El-Naggar MY, Jensen GJ. The ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryo-tomography. bioRxiv. 2017;103242 doi: 10.1073/pnas.1718810115. https://doi.org/10.1101/103242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sullivan NL, Tzeranis DS, Wang Y, So PT, Newman D. Quantifying the dynamics of bacterial secondary metabolites by spectral multiphoton microscopy. ACS Chem. Biol. 2011;6:893–99. doi: 10.1021/cb200094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tan Y, Adhikari RY, Malvankar NS, Ward JE, Nevin KP, et al. The low conductivity of Geobacter uraniireducens pili suggests a diversity of extracellular electron transfer mechanisms in the genus Geobacter. Front. Microbiol. 2016;7:980. doi: 10.3389/fmicb.2016.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature. 2007;449:804. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uchimiya M, Stone AT. Reversible redox chemistry of quinones: impact on biogeochemical cycles. Chemosphere. 2009;77:451–58. doi: 10.1016/j.chemosphere.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Kern SE, Newman DK. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 2010;192:365–69. doi: 10.1128/JB.01188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, Newman DK. Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol. 2008;42:2380–86. doi: 10.1021/es702290a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J. Bacteriol. 2011;193:3606–17. doi: 10.1128/JB.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weber T, Blin K, Duddela S, Krug D, Kim HU, et al. antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–43. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 124.Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol. Microbiol. 2011;79:166–79. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 1998;64:4035–39. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yates MD, Golden JP, Roy J, Strycharz-Glaven SM, Tsoi S, et al. Thermally activated long range electron transport in living biofilms. Phys. Chem. Chem. Phys. 2015;17:32564–70. doi: 10.1039/c5cp05152e. [DOI] [PubMed] [Google Scholar]
- 127.Yates MD, Strycharz-Glaven SM, Golden JP, Roy J, Tsoi S, et al. Measuring conductivity of living Geobacter sulfurreducens biofilms. Nat. Nanotechnol. 2016;11:910–13. doi: 10.1038/nnano.2016.186. [DOI] [PubMed] [Google Scholar]
- 128.Yates MD, Strycharz-Glaven SM, Tender LM. Toward understanding long-distance extracellular electron transport in an electroautotrophic microbial community. Energy Environ. Sci. 2016;9:3544–58. [Google Scholar]
- 129.Young G. Pigment production and antibiotic activity in cultures of Pseudomonas aeruginosa. J. Bacteriol. 1947;54:109. doi: 10.1128/jb.54.2.109-117.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]