Abstract
Background
Although cardiovascular disease (CVD) is the primary killer of women in the U.S., women and female animals have traditionally been omitted from research studies. In reports that do include both sexes, significant sexual dimorphisms have been demonstrated in development, presentation and outcome of CVD. However, there is little understanding of the mechanisms underlying these observations. A more thorough understanding of sex-specific cardiovascular differences both at baseline and in disease is required to effectively consider and treat all CVD patients.
Methods & Results
We analyzed contractility in the whole rat heart, adult rat ventricular myocytes (ARVMs) and myofibrils from both sexes of rats and observed functional sex differences at all levels. Hearts and ARVMs from female rats displayed greater fractional shortening than males, and female ARVMs and myofibrils took longer to relax. To define factors underlying these functional differences, we performed an RNA-sequencing experiment on ARVMs from male and female rats and identified ~600 genes were expressed in a sexually dimorphic manner. Further analysis revealed sex-specific enrichment of signaling pathways and key regulators. At the protein level, female ARVMs exhibited higher PKA activity, consistent with pathway enrichment identified through RNA-seq. Additionally, activating the PKA pathway diminished the contractile sexual dimorphisms previously observed.
Conclusions
These data support the notion that sex-specific gene expression differences at baseline influence cardiac function, particularly through the PKA pathway, and could potentially be responsible for differences in CVD presentation and outcomes.
Keywords: Sex-specific, cardiac myocytes, cardiovascular disease, gene expression
Introduction
Sex differences in baseline cardiac function & disease
Cardiovascular disease (CVD) is the leading cause of death of American men and women.1 Even though CVD causes 1/3 of women’s deaths each year, women have traditionally been excluded from clinical trials and female animals have been utilized less or sex was not reported in basic research studies.1, 2 Until recently, consideration of both sexes was not required in clinical and preclinical studies focusing on CVD.3, 4 This research bias has led to the development of CVD therapeutics that are either less effective or have different side effects in women when compared to males.5 Sex-specific differences in baseline cardiac function are observed in healthy adults, with women displaying better diastolic function compared to men as well as preserved systolic function compared to men over the course of aging.6 Similar to humans, baseline differences in cardiac function have been reported in rodent studies, with females generally having better function compared to their male counterparts.7–9 These sex differences are also observed at the level of the cardiac myocyte with male rodent cardiac myocytes generally contracting more strongly and rapidly than female cells, but this difference diminishes with age.10–12 However, the mechanisms responsible for these functional differences are not well understood.
Additionally, differences between men and women are apparent in a variety of CVDs.Reviewed in 13, 14 With respect to myocardial infarction (MI) and heart failure, women are protected in that they develop the disease later in life than men.1, 15 Sexually dimorphic responses to cardiac disease stimuli are also observed in a variety of animal models. For example, females are less likely to progress to heart failure in response to a variety of different pathological stressors and male hypertensive rats typically develop greater increases in blood pressure.Reviewed in 14
Estrogen signaling has been credited for the cardioprotection observed in women since this protection is generally lost after the onset of menopause.Reviewed in 13 However, contradictory results from many studies reveals that this issue is still not completely understood and highlights that pathways other than estrogen signaling are undoubtedly playing a role. To completely understand these sex specific differences the mechanisms underlying these differences, particularly at baseline need to be defined.
Baseline sexual dimorphisms in cardiac gene expression
Left ventricular gene expression is sexually dimorphic in humans and rodents. In reports analyzing the left ventricle of humans, mice and rats, cardiac genes are differentially expressed between males and females, many of which are expressed on sex chromosomes.16–18 Expression of autosomal cardiac genes also differ between the sexes, and in mice, these differences are not affected by the estrous cycle, suggesting that these differences are not due to varying circulating estrogen levels.16, 18 Upon further investigation, enrichment of GeneOntology categories such chemotaxis, mitochondrial function, cell cycle, inflammation and particularly metabolism are sexually dimorphic, suggesting these baseline gene expression differences have functional consequences.16, 19–21 While these sexual dimorphisms are intriguing, these studies analyzed whole ventricles, which are a complex mixture of multiple cell types including fibroblasts, myocytes, smooth muscle and endothelial cells. Additionally, most of these previous studies utilized microarrays, which are limited compared to next generation sequencing methods, which provide a more unbiased and in-depth approach. To address how biological sex impacts the genetic profile of the contractile cells of the heart, we conducted an RNA-sequencing experiment to analyze baseline gene expression differences between male and female rat cardiac myocytes. This study defines the basic gene expression profiles in cardiac myocytes from each of the sexes and describes the functional consequences of sex-specific gene expression. We believe these findings could be useful in the development of cardiovascular therapeutics for both men and women.
Materials and Methods
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado at Boulder. Rats were fed ad libitum standard rodent chow and housed in a facility with a 12 hour light, 12 hour dark cycle. Male and female (300–400g, 3–4 months old) Sprague-Dawley, CD, rats were purchased from Charles River Laboratory and acclimated to the facility prior to the described experiments. The females were not staged by estrous cycle for the following experiments. For the ovariectomy experiment, 3-month-old female Sprague Dawley, CD, rats that underwent either a sham or ovariectomy (OVX) procedure were purchased from Charles River Laboratory. Cardiac myocytes were isolated from sham and OVX animals three weeks after surgery.
Echocardiography
One week prior to cardiac myocyte isolation, all rats were subjected to transthoracic echocardiography. Non-invasive echocardiographic images and measurements were made using the Philips Sonos 5500 system as previously described.22 Heart rate measurements were not different between groups (Supplemental Table 1).
Adult rat ventricular myocyte (ARVM) isolation and culture
ARVMs were isolated from the left ventricle of adult rats using a Langendorff apparatus with modification of previously published protocols.23 Cells were plated on 60-mm plates coated with 10ug/ml laminin (Invitrogen, Carlsbad, CA) in Springhorn media23 and allowed to adhere for 45 minutes. For cells used for RNA analysis, the media was removed after 45 minutes of culture, and the cells were flash frozen in liquid nitrogen for downstream experiments.
Cardiac myocyte contractility assay and analysis
ARVMs were isolated as described above with 30mmol/liter blebbistatin (Cayman Chemical, Ann Arbor, MI) added to the Type II collagenase (Worthington Biochemical, Lakewood, NJ) solution, plated on laminin-coated coverslips and allowed to settle for two hours in culture prior to the beginning of each contractility experiment. The coverslips were then transferred to the microscope (Nikon Diaphot) and superfused in Tyrode’s solution [137mmol/liter NaCl, 2.7mmol/liter KCl, 1 mmol/liter MgCl2, 1.8mmol/liter CaCl2, 0.2mmol/liter Na2HPO4, 12mmol/liter NaHCO3 & 5.5mmol/liter D-Glucose, pH 7.4]. Myocytes were electrically paced via field stimulation at room temperature by using the IonOptix MyoPacer with a stimulus duration of 4ms, voltage of 1.2× stimulation threshold and frequency of 1Hz. Transients from at most five randomly selected myocytes per coverslip were recorded for at least 30 seconds per cell. Cell length measurements and shortening dynamics were determined by edge detection
IonWizard software (IonOptix, Westwood, MA) in which at least ten transients per cell were averaged and analyzed. To analyze differences in contractile function in response to alterations in the PKA pathway, ARVMs from both sexes were treated with 1µmol/liter bucladesine (Cayman Chemical, Ann Arbor, MI) or DMSO two hours after isolation for 30 minutes. Contractility experiments were then performed as described above with the Tyrode’s solution supplemented with either 1µmol/liter bucladesine or DMSO for the respective treatments. Cells were not in culture for more than six hours post isolation for the duration of the contractility experiments.
Left ventricular myofibrillar isolation and analysis
We used previously published techniques to measure the force and kinetics of isolated myofibrils activated and relaxed by fast solution switching.24–26 Myofibrils were stretched 5–10% above slack myofibril length to set the sarcomeres to optimally loaded length of 2.1–2.3 µm. Average sarcomere length and myofibril diameter were measured using ImageJ (NIH). Mounted myofibrils were activated and relaxed by rapidly translating the interface between two flowing streams of solutions of different pCa.24, 27 Data was collected and analyzed using a customized LabView software. Measured mechanical and kinetic parameters were defined as follows: rate constant of early slow force decline (Linear kREL) - the slope of the linear regression normalized to the amplitude of relaxation transient and duration of early slow force decline - measured from onset of solution change to the beginning of the exponential force decay.
RNA extraction, Library Preparation and Sequencing
Total RNA was isolated using the miRNeasy mini kit (Qiagen, Valencia, CA) on-column DNase digestion. RNA concentration was determined using the Qubit RNA BR Assay (Invitrogen, Carlsbad, CA) and RNA integrity was analyzed with the Agilent Bioanalyzer (Agilent, Santa Clara, CA) with all of the RNA samples used for sequencing having a RNA integrity number (RIN) of at least 9. Ribo-zero gold depleted paired-end sequencing libraries were then constructed from 650ng total RNA using the TruSeq Stranded Total RNA Sample Kit (Illumina, San Diego, CA). All libraries were sequenced using Illumina’s HiSEQ2500 with 2×125bp v4 chemistry.
RNA-sequencing mapping, differential gene expression analysis & pathway analysis
All paired-end reads were demultiplexed with the Casava pipeline (v1.8.2) and adapter trimmed with Trimmomatic (v0.32). Reads were aligned to the rat rn6 genome using Tophat (v2.0.12, Supplemental Table 2). Both the rn6 fasta reference file as well as the gene annotation (gtf) file was downloaded from the UCSC genome brower database (http://genome.ucsc.edu). To be able to distinguish between multiple isoforms, the UCSC rn6 gtf file was converted with UCSC genome browser’s genePredToGtf Utility and then used for all mapping and differential expression analysis. Aligned reads were counted with HTseq count (v0.6.1) and differentially expressed genes were identified using DESeq (v1.10.1). Genes identified to be differentially expressed between sexes by at least 1.5 fold with a padj value of <0.01 by DESeq were then analyzed using Qiagen’s Ingenuity Pathway Analysis (IPA) software (Content version: 24718999) to identify enriched networks (For complete list see: Supplemental Tables 3 and 4). We noticed inconsistencies with the gene annotation of Hsp90aa1 between NCBI, Ensemble and UCSC, so this gene was excluded from our DESeq and IPA analysis.
Estrogen and androgen response element analysis
We utilized the Genome-wide position weight matrix (PWM) scanner available on the Computational Cancer Genomics website (http://ccg.vital it.ch/pwmtools/pwmscan.php) to detect estrogen (EREs) and androgen response elements (AREs) in genes that were enriched in the either sex using the JASPAR core vertebrate motif library searching for the Esr1 MA0112.3 and Ar MA0007.3 motifs with a p-value cutoff of 0.00001. We detected the presence of the identified response elements within 10kb of a transcription start site of genes utilizing the Bedtools (v2.22.0) window option. Gene Ontology enrichments for distinct sets of genes, including the presence or absence of ERE or ARE in genes up-regulated in male or female cardiac myocytes, was analyzed using David Bioinformatics Resource 6.8.28
Quantitative PCR
cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) and random hexamer primers. Gene expression was determined by qRT-PCR using SYBR Green dye (Invitrogen, Carlsbad, CA) and gene specific primer sets. All genes were normalized to 18S expression. All primer sequences used are listed in Supplemental Table 5. Data were collected and analyzed using a Bio-Rad CFX-96 Real-Time PCR system.
Protein Kinase A Activity Assay
Sexual dimorphisms in Protein Kinase A activity in ARVMs was determined using the Protein Kinase A activity kit (Abcam, Cambridge, United Kingdom). After isolation, ARVMs from both sexes were cultured for 45 minutes then washed with phenol-red free media (ThermoFisher, Rochester, NY) and flash frozen. ARVMs were collected in lysis buffer [20 mmol/L MOPS, 50 mmol/L β-glycerolphosphate, 50 mmol/L sodium fluoride, 1mmol/M sodium vanadate, 5 mmol/L EGTA, 2 mmol/L EDTA, 1% NP40, 1 mmol/L dithiothreitol (DTT), benzamidine, phenylmethane- sulphonylfluoride (PMSF) and 10 µg/mL leupeptin and aprotinin] and this crude enzyme lysate was used for the remainder of the assay according the manufacturer’s protocol.
Statistical analysis
The number of animals and cells used for each experiment are denoted in the figure legends. Statistical analysis of differentially expressed genes (17,257 genes in total) was performed with DESeq (v1.10.1) DESeq uses a negative binomial model to test for differential expression. Raw p values were then adjusted (padj values) for FDR control with the Benjamini-Hochberg procedure.29 We only considered genes for downstream analysis that had a DESeq reported padj value of at least 0.01 and also exhibited at least a 1.5 fold difference in expression relative to the opposite sex. This adjusted p-value of 0.01 assumes a FDR of 1%.29 Differences between two groups were evaluated for statistical significance using Student’s two-tailed t test using GraphPad PRISM 6 software with data presented as mean ± SEM. P <0.05 was considered significant. For the Bucladesine experiment, significance was determined using a two-way ANOVA, followed by a Tukey’s multiple comparisons post hoc test. For DAVID gene ontology analysis, the top 10 enriched biological keywords with at least p<0.05 were sorted based on fold-enrichment scores. Enrichment of EREs or AREs was analyzed for significance by using the hypergeometric probability calculator (https://www.geneprof.org/GeneProf/tools/hypergeometric.jsp).
Results
Sex differences exist in baseline cardiac function from the level of the whole heart to cardiac myofibrils
At the level of the whole heart, in vivo echocardiography demonstrated that cardiac function was enhanced in the female animals with fractional shortening being higher in the female hearts compared to their male counterparts Figure 1A). Similar functional differences between the sexes were also observed in isolated ARVMs with female cells displaying greater percent shortening than the male myocytes (Figure 1B). Interestingly, female ARVMs also took more time than male cells to reach peak contraction as well as to relax. This sex-specific difference during the relaxation phase was also observed at the level of the myofibril. Female myofibrils exhibited slower relaxation duration and rate constant of the linear relaxation phase (Figure 1C).
Figure 1. Cardiac function is sexually dimorphic from the level of the whole heart down to the myofibril.
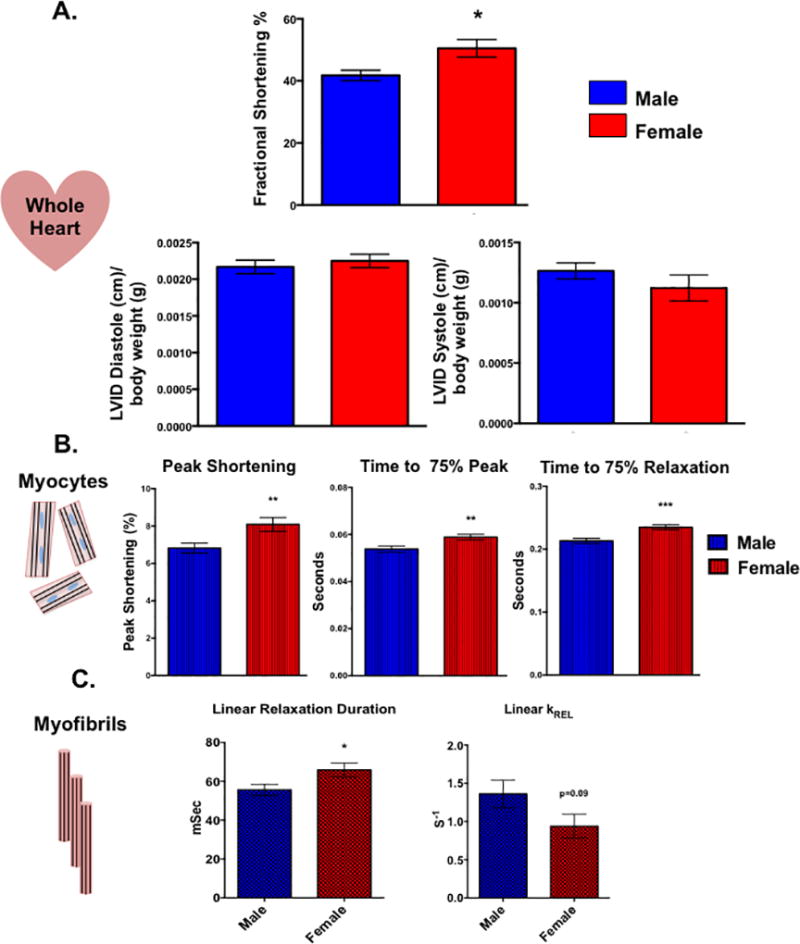
(A) In vivo whole heart function as measured by echocardiography in male and female rats. *p<0.05 relative to male, N=5 female and 6 males. (B) Contractile function of electrically paced ventricular myocytes from rats of both sex. **p<0.01, ***p<0.001 relative to male, N=97 female cells and 110 male cells pooled from 6 animals of each sex. (C) Kinetic properties of left ventricular myofibrils isolated from male and female rats. Linear relaxation duration: *p<0.05 relative to female, N=21 female and 25 male myofibrils from 3 separate animals. Linear KREL: N= 15 female and 14 myofibrils from 3 separate animals. All data reported as mean ± SEM.
Cardiac myocyte gene expression is sexually dimorphic
We next asked what molecular mechanisms could be responsible for the functional differences we observed in the cardiac myocytes by performing an RNA-sequencing experiment with ARVMs from both sexes. All paired-end sequencing reads were mapped to the rat rn6 genome using the Tophat pipeline leading to around 100 million reads mapping per sample with an overall mapping rate of at least 80% (Supplemental Table 2). Differential expression analysis carried out using DESeq identified ~600 genes that are differentially expressed between male and female cardiac myocytes at baseline by at least 1.5 fold (Figure 2; Supplemental Tables 3 and 4). These differences in gene expression appear to be sex-specific and not merely differences among biological replicates, as there are almost no statistical differences in gene expression when the samples were analyzed with DESeq randomly (Supplemental Figure 1). Furthermore, the two genes with the largest difference in expression between sexes, Ddx3 and Eif2s3y, are located on the Y chromosome (Figure 2A). Additionally, there were ~300 genes that were differentially expressed by at least 1.5 fold in one sex compared to the other (Figure 2B; Supplemental Tables 3 and 4). To validate our results, we performed qPCR on a subset of mRNAs that were differentially expressed between the sexes and observed the same expression profile that was shown in the RNA-sequencing results (Figure 3). For example, genes such as the transferrin receptor (Tfrc) and cytochrome C oxidase (Cox6c) were more highly expressed in the male cardiac myocytes by both qPCR and RNA-sequencing methods (Figure 3A). Similarly, expression of the uncoupling protein 2 (Ucp2) and P450 oxidoreductase (Por) genes was higher in the female myocytes in both the RNA-sequencing and qPCR datasets (Figure 3B).
Figure 2. Male and female cardiac myocytes exhibit different transcriptome profiles.
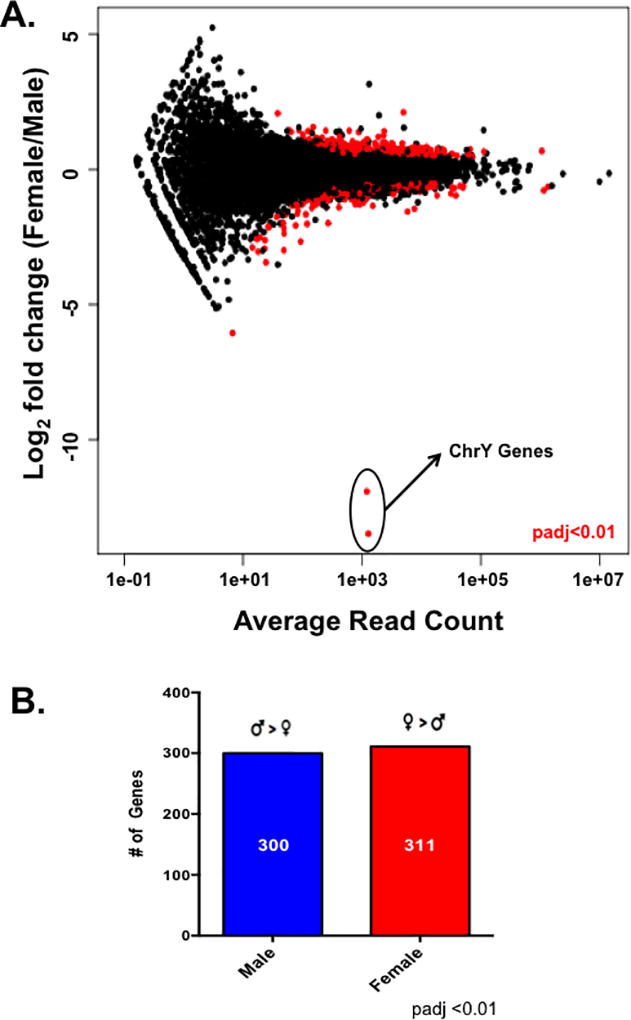
(A) Differential gene expression analysis between male and female cardiac myocytes as measured with DESeq. Differentially expressed genes between the sexes are indicated by the red circles; padj <0.01 female relative to male, N=5 female and 6 male isolated cardiac myocyte preparations. (B) The number of differentially expressed genes (padj<0.01) genes at least 1.5× higher in one sex relative to the other as measured by DESeq; this list of genes was used for downstream analysis with IPA.
Figure 3. Validation of differentially expressed genes with qPCR also demonstrates that cardiac myocyte gene expression is sexually dimorphic.
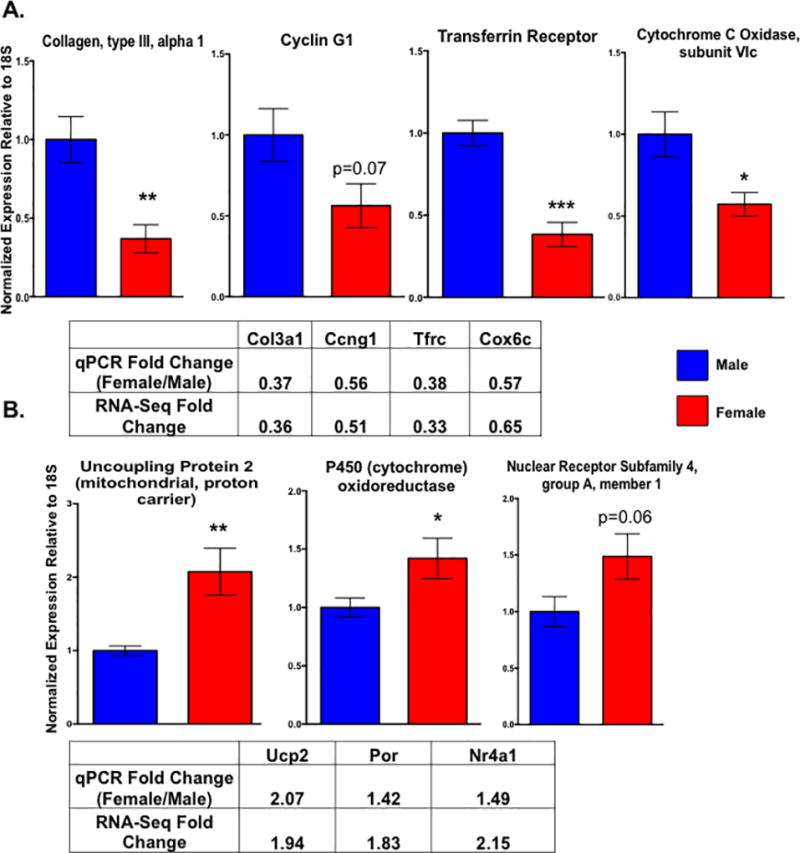
Expression of Col3a1, Ccng1, Tfrc, Cox6c (A), Ucp2, Por, and Nr4a1 (B) as measured by qPCR in male and female cardiac myocytes. The mean fold changes (female relative to male) for each gene as found by qPCR and the RNA-sequencing experiment are indicated in the supplied tables. All genes were normalized to levels of 18S; *p<0.05, **p<0.01, ***p<0.001 female relative to male; N=5 female and 6 male isolated cardiac myocyte preparations. All data reported as mean ± SEM.
Enriched pathways and regulators are distinct between the sexes
We next wanted to investigate the potential functional relevance of the sex-specific gene expression differences observed in cardiac myocytes by performing pathway analysis on genes that were up-regulated in either sex (Supplemental Tables 3 and 4). Ingenuity pathway analysis (IPA), which integrates data across previously published studies, was used to identify different pathways, functions and upstream regulators enriched in genes that were at least 1.5 fold higher in expression between male or female cardiac myocytes. We observed that canonical pathways predicted to be activated by IPA were distinct between sexes (Figure 4A; Supplemental Tables 6 and 7). For example, signaling pathways such as Protein Kinase A (PKA), Wnt/Ca2+ and inducible nitric oxide synthase (iNOS) were activated in the female dataset, whereas integrin, Rac, and insulin receptor pathways were enriched in genes up-regulated in male ARVMs. Furthermore, genes that were more highly expressed in female cardiac myocytes are involved processes such as gene expression, lipid and energy metabolism, and small molecular biochemistry (Figure 4B). However, the male enriched genes are more likely to function in regulating cell growth and movement, the cell cycle and cellular death processes. To understand more about the effectors responsible for the observed gene expression and pathway differences observed between male and female ARVMs, we used the upstream regulator tools provided by IPA. We discovered that the types of upstream regulators enriched in either sex were largely distinct, similar to what we observed with the activated pathways and cellular functions (Figure 4C). In total, it appears that the sexually dimorphic gene expression differences in cardiac myocytes are pathway-specific, which could have functional contractile consequences.
Figure 4. Specific pathways and functions are enriched in cardiac myocytes from either sex.
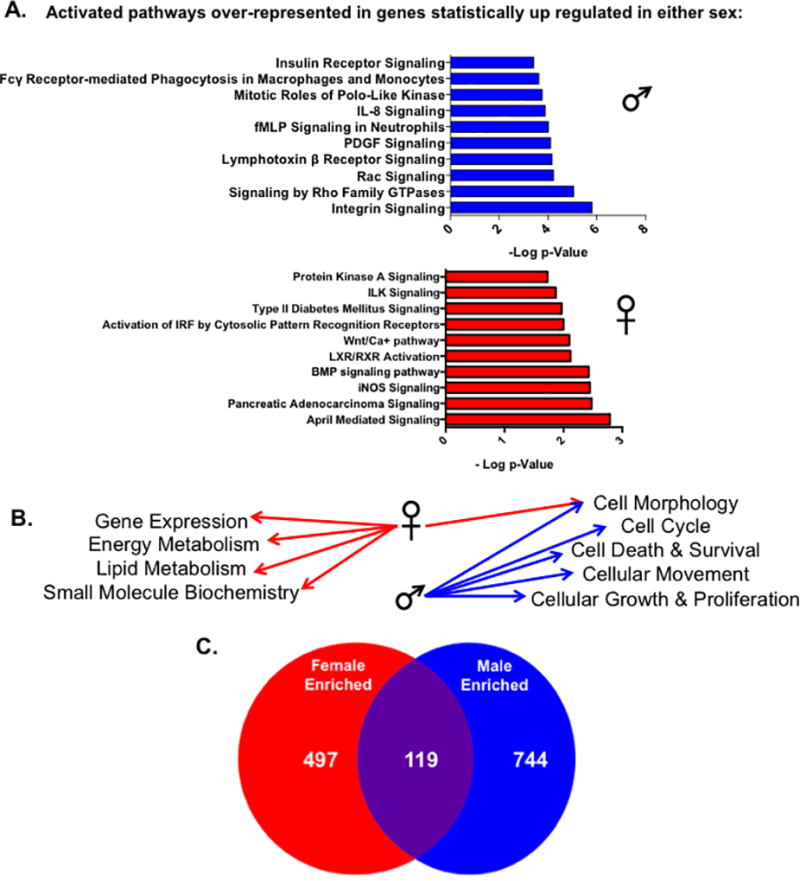
IPA analysis of significantly expressed genes by at least 1.5 fold in either sex. (A) The top ten statistically significant canonical pathways predicted to be activated by IPA in genes unregulated in either male or female cardac myocytes. Pathways were deemed statistically significant by IPA if p<0.05 using the Fischer exact test. Activated pathways were indicated by positive z-scores calculated by IPA; for pathways that contained the same genes, the pathway with the lower p-value was listed. (B) The top five statistically significant (lower end of p-value overlap at least 0.05) molecular and cellular functions enriched in genes found to be upregulated in either male or female myocytes by IPA. (C) The number of statistically significant (p<0.05) upstream regulators enriched in up-regulated genes from either sex as measured by IPA analysis.
To understand the role of sex hormones in mediating these differences, we identified EREs or AREs within the regulatory regions of genes that were differentially expressed between male and female ARVMs. Almost half of the genes that were more highly expressed in female cardiac myocytes also harbored an ERE within 10kb of their start site (Supplemental Figures 2 and 3, Supplemental Table 8). This was also true for genes enriched in female myocytes that contributed to the activated pathways in our IPA analysis (Supplemental Figure 2). We observed similar, but not significant, results for genes harboring an ARE that were enriched in male myocytes (Supplemental Figures 2 and 4; Supplemental Table 9). Furthermore, we performed gene ontology analysis on genes upregulated in either sex with or without an ERE or ARE and discovered enrichment of distinct biological keywords (Supplemental Figures 3 and 4; Supplemental Tables 10–13). However, it is important to note that not all genes that were differentially expressed between the sexes contained these response elements (Supplemental Figures 2–4; Supplemental Tables 8 and 9). Additionally, genes not differentially expressed between the sexes also harbored EREs or AREs (Supplemental Figure 5). We also analyzed the expression of sex specific differentially expressed genes we validated by qPCR in Figure 3 in sham control and ovariectomized (OVX) female animals but did not observe differences in expression of these genes despite estrogen depletion observed as decreased uterine weights in the OVX animals (Supplemental Figure 6).
Protein kinase A activity exhibits sex-specific profiles
Because PKA signaling is an important mediator in cardiac physiology12 and our IPA analysis indicated this pathway was activated in female myocytes (Figure 4A), we measured PKA activity in male and female cells. Female ARVMs displayed increased PKA activity compared to their male counterparts (Figure 5A). We next perturbed the PKA pathway and performed contractility assays in ARVMs from both sexes to determine the functional relevance of the increased PKA activity in the female cells. We treated male and female ARVMs with the cyclic adenosine monophosphate (cAMP) mimic, bucladesine, for 30 minutes to investigate how activating the PKA pathway in cells of both sexes affects contractile function. Similar to what we observe in non-treated cells (Figure 1B), the female DMSO treated cells took longer to reach peak shortening as well as to fully relax relative to their male counterparts (Figure 5B). However, the time it took the male and female bucladesine treated myocytes to reach peak shortening and relaxation was not statistically different from each other (Figure 5B). Additionally, bucladesine treatment appears to affect myocytes of either sex differently as the two-way ANOVA performed in this experiment resulted in statistically significant p-values for an interaction effect between bucladesine treatment and biological sex (p<0.05) for the time it took the cells to relax after treatment. This suggests that activating the PKA pathway in both sexes is mitigating the functional differences observed in the DMSO control cells.
Figure 5. PKA activity is sexually dimorphic in cardiac myocytes.
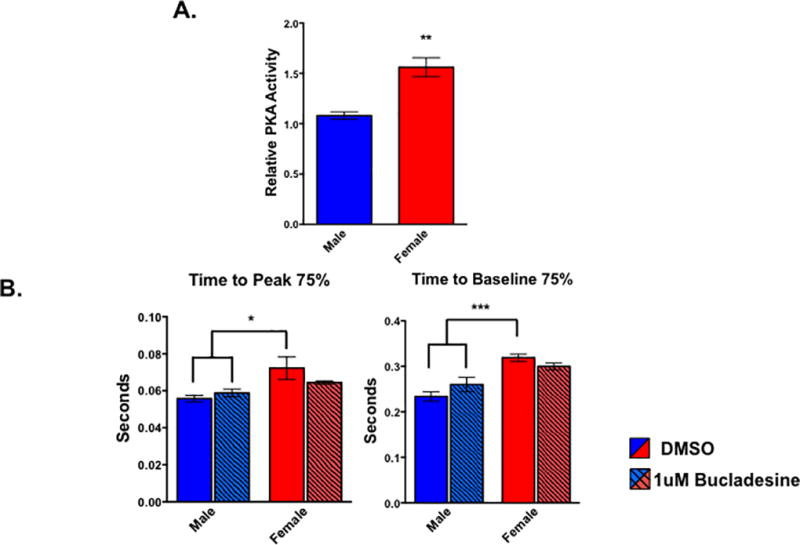
(A) Levels of PKA activity in male and female cardiac myocytes as measured by PKA activity assay. **p<0.01 female relative to male; n=4 animals of each sex. All data reported as mean ± SEM. (B) Contractile function of electrically paced ventricular myocytes treated with bucladesine or DMSO for 30 minutes from rats of both sex. *p<0.05 relative to female DMSO; n= at least 19 cells per group pooled from 4 animals of each sex.
Discussion
Baseline cardiac function is sexually dimorphic at multiple tissue levels
While there are multiple reports analyzing the functional differences between male and female cardiac myocytes at baseline, to our knowledge, this is the first report to demonstrate that functional sex differences exist from the level of the whole heart down to isolated myofibrils. Sex differences in whole heart function have been debated within the literature. However, in our study we observed higher fractional shortening in the female rats relative to the males, which is consistent with previously published work in humans, mice and rats.6, 8, 9 Our data also demonstrate this functional difference is apparent at the level of the cardiac myocyte with female cells displaying greater percent shortening but taking longer to contract and relax. While other studies have also observed that female myocytes reach peak shortening and relaxation more slowly that male cells, previous reports have commonly observed male myocytes to exhibit greater contractile function, which contrasts with our findings.30 However, a variety of differences in procedures during the contractility studies, such as load conditions, temperature or pacing frequency,12 could account for these differences and it is promising that our in vivo echocardiograph data support the functional differences we observed in the isolated myocytes.
This is also the first report comparing isolated myofibrillar function between sexes, further validating that the duration of the relaxation phase is consistently longer in females. Additionally, it is encouraging that we observed similar functional relaxation sex differences in both our myocyte and myofibril data, as these analyses utilized distinct experimental loading conditions. These results are particularly interesting considering that women are more likely to develop heart failure with preserved ejection fraction (HFpEF), which is characterized by left ventricular diastolic dysfunction and stiffness.31 The prolonged relaxation times we observed in female cardiac myocytes and myofibrils at baseline, in combination with aging, may provide the underlying mechanism that predisposes women to developing HFpEF, but more focused researched on this observation is required.
Sexually dimorphic gene expression mediates the functional differences observed at baseline
Many past studies have focused on how estrogen signaling mediates the sexual dimorphisms in cardiac disease development and while we acknowledge the importance of estrogen in the heart, we also sought to understand what other mechanisms are involved. Additionally, we previously described that ER transcript expression is quite low, restricted to ERα and not sexually dimorphic in cardiac myocytes,32 prompting our interest in understanding what other pathways are involved in mediating the functional sex differences we observed at baseline. However, differences in hormone signaling between the sexes could be a source of variation leading to the genetic and functional differences in the myocytes. The ERE and ARE motif analysis we report further implicates a role for sex hormones in mediating gene expression differences we observed (Supplemental Figures 2–5). Even so, it is important to keep in mind that sexual dimorphisms are much more complex than just hormone signaling. Estrogen and androgen receptors (ERs/ARs) signal in a variety of genomic and rapid non-genomic mechanisms in a variety of different cell types, including cardiac cells.Reviewed in:13, 33, 34 It is well understood that ERs can interact with and tether to other transcription factors such as Fos, Jun and SP1, promoting expression of genes that do not contain EREs.Reviewed in:33 While we acknowledge the presence of an ERE/ARE within a promoter region of a particular gene suggests that gene could be regulated by that hormone, it does not necessary mean it actually is affected by estrogen/androgen signaling. Additionally, delineating which pathways are not regulated by sex hormones is complex because estrogen and androgen signaling to activate a plethora critical cellular pathways, such as MAPK, PI3K, JNK, calcium and Akt.13, 34 Furthermore, we did not observe any difference in expression of the sexually dimorphic genes we validated with qPCR (Figure 3) between the sham and OVX cardiac myocytes, suggesting that estrogen is not mediating these expression differences and other factors are undoubtedly playing a role. By exploring broad gene expression differences between sexes in myocytes from healthy animals, we aimed to gain further insight into how biological sex, not just sex hormones, influences cardiac function and eventually disease development within the cardiac myocyte.
Sexually dimorphic gene expression differences are apparent in multiple somatic tissues including, liver, adipose, brain and muscle.18, 35, 36 Similar to what we observed in cardiac myocytes, there are many genes that are differentially expressed between the sexes with modest fold-changes, however, the gene changes are highly tissue specific and are enriched for distinct signaling pathways.35 Several previous reports have sought to describe sex differences in cardiac gene expression. Studies analyzing healthy human as well as mouse hearts reported only a modest number of genes (~30–125) that were expressed in a sexually dimorphic manner.16, 37 However, a previous microarray study in our lab analyzing mouse left ventricles reported sex-specific expression of ~2000 genes with most of the genes being enriched in the male samples23. While we detected around 600 genes that were differentially expressed in ARVMs from either sex, we did not observe a difference in the overall number of genes that were more highly expressed in one sex relative to the other (Figure 2B). These previous studies analyzed whole heart and ventricle samples, which due to the many different cardiac cell types could account for the contrast between our findings. Additionally, the previous studies utilized microarray methods to detect differential expression, which would have missed genes not present on the array, whereas our RNA-sequencing data provides an unbiased and large-scale approach to detect differences. Interestingly, a large number of genes (~1800) were differentially expressed in left ventricle samples from male and female patients with dilated cardiomyopathy.17 Similar to our findings, the majority of the fold changes in gene expression reported in the study were modest (~1.5–2.0), suggesting that biological sex does not mediate large individual changes, but perhaps the enriched pathways are the most important for mediating functional differences.
Irrespective of tissue type, all studies reporting sex specific differences in gene expression also observed enrichment for distinct pathways. Sexually dimorphic enrichment of pathways such as the immune response and lipid metabolism appear to be strongly conserved among a variety of tissues such as liver, adipose, skeletal muscle, heart as well as in our cardiac myocyte samples (Figure 4).16, 18, 20, 35–37 Previous reports in the heart have observed GeneOntology categories such as metabolismReviewed in:21, signaling transduction, regulation of cell growth, size and cell death to be enriched in a sex-specific manner.16, 20, 23, 37 Our results agree with these reports as Rho and Rac signaling and genes involved in regulating cell growth and death were enriched in male ARVMs, whereas female myocytes were enriched for processes such as energy metabolism and gene expression (Figure 4). Our findings suggest these enriched pathways are myocyte specific, but a more thorough gene expression analysis in the whole heart would need to be performed to definitively understand this observation. Additionally, we observed the PKA and inducible nitric oxide synthase (iNOS) pathways to be enriched in genes that were more highly expressed in female cardiac myocytes, but this was not observed in the whole heart studies16, 23. Many of the pathways we observed to be sex-specific are important in maintaining cardiac function. Integrin signaling, which was enriched in male cardiac myocytes relative to the females, is an integral pathway within the cardiac myocyte as it is involved in maintaining adhesions with the extracellular matrix, but also in mechanotransduction and responding to hypertrophic stimuli.38 Additionally, estrogen supplementation of cardiac fibroblasts attenuated the angiotensin-II increases in β1-integrin expression39, suggesting a potential mechanism by which integrin signaling is lower in female cardiac myocytes.
PKA signaling is extremely important in maintaining proper cardiac function as well as in responding to increases in metabolic demands that was enriched in a sexually dimorphic manner in cardiac myocytes. We also observed that the female cardiac myocytes exhibited higher PKA activity at baseline relative to their male counterparts, further validating our RNA-sequencing results (Figure 5A). Additionally, treating ARVMs with the cAMP mimic bucladesine abrogated the sex specific difference in the time it took the cells to reach peak shortening or relax (Figure 5B). This suggests altering PKA signaling is affecting the cells from either sex differently since the males experienced a slight, but not statistically significant, increase in the time it took them to reach peak shortening and baseline, but the opposite was observed in the female cells.
Because PKA signaling generally results in decreased calcium sensitivity in addition to increased relaxation rates, the increased PKA activity in the female cells was surprising. However, there are conflicting reports regarding the effect PKA activation has on myofibril relaxation rates, and it appears, based on our data, that sex mediates differences in this pathway.40, 41 Additionally, our results could be due to the bucladesine treatment protocol. A common dose of bucladesine or its equivalent dibutyryl cAMP in the literature is 1mmol/liter42, which is much higher than the dose we used for our experiments. However, when we initially performed our contractility experiments with the 1mmol/liter dose, the cells could not maintain proper contraction upon electrical pacing, prompting us to use a lower concentration. Our chosen bucladesine concentration led to both an increase in p-troponin I levels as well as consistent, reliable contractions in male ARVMs (data not shown), suggesting this dose appropriately activated the pathway without negatively impacting the cells.
To our knowledge, this study is the first to observe sex differences at multiple levels of the cardiac structure in a single model as well as report sexually dimorphic gene expression within cardiac myocytes. Additionally, our RNA-sequencing analysis was able to identify an important cardiac signaling pathway, the PKA pathway, which appears to be differentially regulated between the sexes. While understanding the influence biological sex exerts on the cardiovascular system is complex, more studies, particularly during healthy baseline conditions, are needed. By obtaining a more thorough picture of how the cardiovascular system differs between men and women, we will be better prepared to effectively treat all CVD patients.
Supplementary Material
Clinical Perspective.
Although cardiovascular disease (CVD) is the primary killer of women in the U.S., women and female animals have traditionally not been included in research studies. In reports that do include both sexes, significant sexual dimorphisms have been demonstrated in the development and presentation and outcome of CVD. However, there is little understanding of the mechanisms underlying these observations. A more thorough understanding of sex-specific cardiovascular differences both at baseline and in disease is required to effectively consider and treat all CVD patients. To investigate these differences at multiple levels more thoroughly, we analyzed contractility in the whole rat heart, adult rat ventricular myocytes (ARVMs) and myofibrils from both sexes of rats and observed functional sex differences in all preparations. Additionally, we performed an RNA-sequencing experiment with isolated ARVMs of each sex and demonstrated that sex specific gene expression differences are apparent at the level of the cardiac myocyte under physiological conditions. We were able to utilize this large-scale gene expression approach to identify the PKA pathway as a potential mediator of the functional differences we observed at the level of the cardiac myocyte.
Acknowledgments
We would like to thank the BioFrontiers Computing Core at the University of Colorado at Boulder. We also thank Drs. Angela Peter, Pamela Harvey and Mary Allen for helpful assistance, discussions and preparation of this manuscript.
Sources of Funding: This work was supported in part by the American Heart Association 14PRE20380468 (CLT), the National Science Foundation NSF DBI 1262410 (RDD), the NIH CCTSI KL2, 5KL2TR001080-02 (MYJ) and The Tom Marsico Chair of Excellence (LAL).
Footnotes
All RNA-sequencing data is publicly available through GEO accession: GSE95231.
Disclosures: None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Miller VM, Best PJ. Implications for reproductive medicine: Sex differences in cardiovascular disease. Sex Reprod Menopause. 2011;9:21–28. [PMC free article] [PubMed] [Google Scholar]
- 3.Consideration of sex as a biological variable in nih-funded research. 2015 [Google Scholar]
- 4.Clayton JA, Collins FS. Policy: Nih to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5:425–438. doi: 10.1038/nrd2032. [DOI] [PubMed] [Google Scholar]
- 6.Prabhavathi K, Selvi KT, Poornima KN, Sarvanan A. Role of biological sex in normal cardiac function and in its disease outcome - a review. J Clin Diagn Res. 2014;8:BE01–04. doi: 10.7860/JCDR/2014/9635.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: The dallas heart study. Circulation. 2006;113:1597–1604. doi: 10.1161/CIRCULATIONAHA.105.574400. [DOI] [PubMed] [Google Scholar]
- 8.Haines CD, Harvey PA, Leinwand LA. Estrogens mediate cardiac hypertrophy in a stimulus-dependent manner. Endocrinology. 2012;153:4480–4490. doi: 10.1210/en.2012-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litwin SE, Katz SE, Litwin CM, Morgan JP, Douglas PS. Gender differences in postinfarction left ventricular remodeling. Cardiology. 1999;91:173–183. doi: 10.1159/000006906. [DOI] [PubMed] [Google Scholar]
- 10.Grandy SA, Howlett SE. Cardiac excitation-contraction coupling is altered in myocytes from aged male mice but not in cells from aged female mice. American journal of physiology. Heart and circulatory physiology. 2006;291:H2362–2370. doi: 10.1152/ajpheart.00070.2006. [DOI] [PubMed] [Google Scholar]
- 11.Howlett SE. Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. American journal of physiology. Heart and circulatory physiology. 2010;298:H659–670. doi: 10.1152/ajpheart.00214.2009. [DOI] [PubMed] [Google Scholar]
- 12.Parks RJ, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Archiv : European journal of physiology. 2013;465:747–763. doi: 10.1007/s00424-013-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiological reviews. 2017;97:1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 14.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circulation research. 2016;118:1294–1312. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenzen MJ, Rosengren A, Scholte op Reimer WJ, Follath F, Boersma E, Simoons ML, et al. Management of patients with heart failure in clinical practice: Differences between men and women. Heart. 2008;94:e10. doi: 10.1136/hrt.2006.099523. [DOI] [PubMed] [Google Scholar]
- 16.Isensee J, Witt H, Pregla R, Hetzer R, Regitz-Zagrosek V, Noppinger PR. Sexually dimorphic gene expression in the heart of mice and men. Journal of molecular medicine. 2008;86:61–74. doi: 10.1007/s00109-007-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fermin DR, Barac A, Lee S, Polster SP, Hannenhalli S, Bergemann TL, et al. Sex and age dimorphism of myocardial gene expression in nonischemic human heart failure. Circ Cardiovasc Genet. 2008;1:117–125. doi: 10.1161/CIRCGENETICS.108.802652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Fuscoe JC, Zhao C, Guo C, Jia M, Qing T, et al. A rat rna-seq transcriptomic bodymap across 11 organs and 4 developmental stages. Nat Commun. 2014;5:3230. doi: 10.1038/ncomms4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haines CD, Harvey PA, Luczak ED, Barthel KK, Konhilas JP, Watson PA, et al. Estrogenic compounds are not always cardioprotective and can be lethal in males with genetic heart disease. Endocrinology. 2012;153:4470–4479. doi: 10.1210/en.2012-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijay V, Han T, Moland CL, Kwekel JC, Fuscoe JC, Desai VG. Sexual dimorphism in the expression of mitochondria-related genes in rat heart at different ages. PloS one. 2015;10:e0117047. doi: 10.1371/journal.pone.0117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy E, Amanakis G, Fillmore N, Parks RJ, Sun J. Sex differences in metabolic cardiomyopathy. Cardiovascular research. 2017 doi: 10.1093/cvr/cvx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugach EK, Richmond PA, Azofeifa JG, Dowell RD, Leinwand LA. Prolonged cre expression driven by the alpha-myosin heavy chain promoter can be cardiotoxic. Journal of molecular and cellular cardiology. 2015;86:54–61. doi: 10.1016/j.yjmcc.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haines CD, Harvey PA, Luczak ED, Barthel KK, Konhilas JP, Watson PA, et al. Estrogenic compounds are not always cardioprotective and can be lethal in males with genetic heart disease. Endocrinology. 2012 doi: 10.1210/en.2012-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C. The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophysical journal. 2000;78:3081–3092. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colomo F, Nencini S, Piroddi N, Poggesi C, Tesi C. Calcium dependence of the apparent rate of force generation in single striated muscle myofibrils activated by rapid solution changes. Adv Exp Med Biol. 1998;453:373–381. doi: 10.1007/978-1-4684-6039-1_42. discussion 381-372. [DOI] [PubMed] [Google Scholar]
- 26.Demos-Davies KM, Ferguson BS, Cavasin MA, Mahaffey JH, Williams SM, Spiltoir JI, et al. Hdac6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-ii signaling. American journal of physiology. Heart and circulatory physiology. 2014;307:H252–258. doi: 10.1152/ajpheart.00149.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C. Modulation by substrate concentration of maximal shortening velocity and isometric force in single myofibrils from frog and rabbit fast skeletal muscle. The Journal of physiology. 1999;516(Pt 3):847–853. doi: 10.1111/j.1469-7793.1999.0847u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curl CL, Wendt IR, Kotsanas G. Effects of gender on intracellular. Pflugers Archiv : European journal of physiology. 2001;441:709–716. doi: 10.1007/s004240000473. [DOI] [PubMed] [Google Scholar]
- 31.den Ruijter H, Pasterkamp G, Rutten FH, Lam CS, Chi C, Tan KH, et al. Heart failure with preserved ejection fraction in women: The dutch queen of hearts program. Neth Heart J. 2015;23:89–93. doi: 10.1007/s12471-014-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugach EK, Blenck CL, Dragavon JM, Langer SJ, Leinwand LA. Estrogen receptor profiling and activity in cardiac myocytes. Mol Cell Endocrinol. 2016;431:62–70. doi: 10.1016/j.mce.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: How do they signal and what are their targets. Physiological reviews. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 34.Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindholm ME, Huss M, Solnestam BW, Kjellqvist S, Lundeberg J, Sundberg CJ. The human skeletal muscle transcriptome: Sex differences, alternative splicing, and tissue homogeneity assessed with rna sequencing. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:4571–4581. doi: 10.1096/fj.14-255000. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji M, Kawasaki T, Matsuda T, Arai T, Gojo S, Takeuchi JK. Sexual dimorphisms of mrna and mirna in human/murine heart disease. PloS one. 2017;12:e0177988. doi: 10.1371/journal.pone.0177988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Israeli-Rosenberg S, Manso AM, Okada H, Ross RS. Integrins and integrin-associated proteins in the cardiac myocyte. Circulation research. 2014;114:572–586. doi: 10.1161/CIRCRESAHA.114.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart JA, Jr, Cashatt DO, Borck AC, Brown JE, Carver WE. 17beta-estradiol modulation of angiotensin ii-stimulated response in cardiac fibroblasts. Journal of molecular and cellular cardiology. 2006;41:97–107. doi: 10.1016/j.yjmcc.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Walker JS, Walker LA, Margulies K, Buttrick P, de Tombe P. Protein kinase a changes calcium sensitivity but not crossbridge kinetics in human cardiac myofibrils. American journal of physiology. Heart and circulatory physiology. 2011;301:H138–146. doi: 10.1152/ajpheart.00838.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao V, Cheng Y, Lindert S, Wang D, Oxenford L, McCulloch AD, et al. Pka phosphorylation of cardiac troponin i modulates activation and relaxation kinetics of ventricular myofibrils. Biophys J. 2014;107:1196–1204. doi: 10.1016/j.bpj.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase a-dependent and epac-dependent signals downstream of camp in cardiac myocytes. Circulation research. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


