Abstract
Objective:
To assess in a longitudinal study whether subjective cognitive decline (SCD) and brain β-amyloid (Aβ) contribute unique information to cognitive decline.
Methods:
One hundred thirty-six healthy elderly from the Berkeley Aging Cohort Study were followed up for a mean of 4 years. SCD and affective measures were generated from the Geriatric Depression Scale (GDS) with factor analysis on data from a larger set of 347 healthy, nondepressed (GDS <11) elderly individuals. Cognition was summarized with previously validated factor scores. Pittsburgh compound B (PiB)-PET scans were acquired to determine the presence (PiB+) or absence (PiB−) of Aβ pathology. Mixed models were used to assess the independent and interactive effects of SCD, affective features, PiB status, and time on cognition, with adjustment for demographic variables.
Results:
SCD score demonstrated good construct validity compared to an existing measure of subjective memory and was partially explained by several lower-order measurements. Mixed models revealed that SCD interacted with PiB status to predict change in episodic memory and global cognition over time, with adjustment for affective features. PiB+ individuals with more severe SCD demonstrated the steepest cognitive decline. Worse SCD predicted faster decline in working memory independently of PiB status. No such effects were seen for affective scores when adjusted for SCD.
Conclusions:
PiB+ individuals with SCD are at greatest risk of cognitive decline. Evidence for amyloid alone is not sufficient to indicate risk of rapid cognitive decline in healthy elderly. Effects of GDS on cognitive decline in nondepressed cohorts may be driven by SCD rather than subsyndromal depression.
Alzheimer disease (AD) is preceded by a long preclinical period characterized by neuropathology without accompanying symptoms1,2 and the accumulation of β-amyloid (Aβ) plaques in the cerebral cortex.1,3 Because clinical trials of Aβ-lowering therapies have consistently failed in symptomatic people, several large clinical trials are recruiting cognitively intact people with evidence of brain Aβ to treat a preclinical phase of AD.4 Because this preclinical phase can last decades and clinical trials cannot, there is a critical need to identify markers that indicate a high risk of converting to a symptomatic phase of AD.
Both Aβ plaques, which can be measured in vivo with PET,5 and subjective cognitive decline (SCD), the subjective report of one's own cognition decline over time,6 are highly relevant to longitudinal cognitive change. The presence and extent of both brain Aβ1,3,7 and SCD8–11 at baseline are associated with cognitive decline over time, although associations with the latter are sometimes confounded by disparities in definition6 and the presence of neuropsychiatric deficits,12 which themselves may be independently associated with cognitive decline.13 While brain Aβ and SCD are frequently concurrent phenomena,14–16 little is known about how these factors are related to one another and to longitudinal cognitive change. For example, it is unclear whether SCD and Aβ exert independent additive effects on cognitive outcomes or if 1 factor is a principal driver of predicting cognitive change. In addition, assessing both Aβ and SCD may be more informative than measuring either alone; individuals with SCD but without Aβ may be experiencing cognitive or affective changes that are not due to preclinical AD, while individuals with Aβ but without SCD may be at lesser risk of imminent decline. Measuring both factors may increase power to detect cognitively intact individuals at greatest risk for incipient dementia.
In this longitudinal study, we measured brain Aβ and SCD in 136 healthy older people who were followed up for a mean of 4 years and were undergoing regular cognitive assessment. We used mixed models to ascertain the independent and interactive effects of SCD and Aβ on cognition over time. Given the potential role of depressive symptoms in AD and cognitive decline,13 we also tested models examining the relationship between these 2 variables. We hypothesized that SCD and Aβ would exert additive effects on cognition such that individuals exhibiting evidence for both markers would demonstrate the steepest cognitive decline.
METHODS
Participant characteristics.
Participants made up a sample of 346 cognitively intact elderly individuals from the Berkeley Aging Cohort Study (BACS). Information about recruitment and inclusion criteria is reported elsewhere.17 All participants demonstrated intact cognition at baseline, evidenced by a score ≥25 on the Mini-Mental State Examination18 and >1.5 SDs below the age-, sex-, education-adjusted population mean for 2 delayed recall memory tests (California Verbal Learning Test Long Delay Free Recall and Wechsler III Visual Recall Long Delay Free Recall). Finally, all participants were required to score ≤10 on the Geriatric Depression Scale (GDS)19 and were thus considered to be nondepressed. Each participant underwent at least 1 standardized interview and neuropsychological testing procedure assessing health and demographic information, lifestyle factors, family history of AD, and multiple domains of cognition.
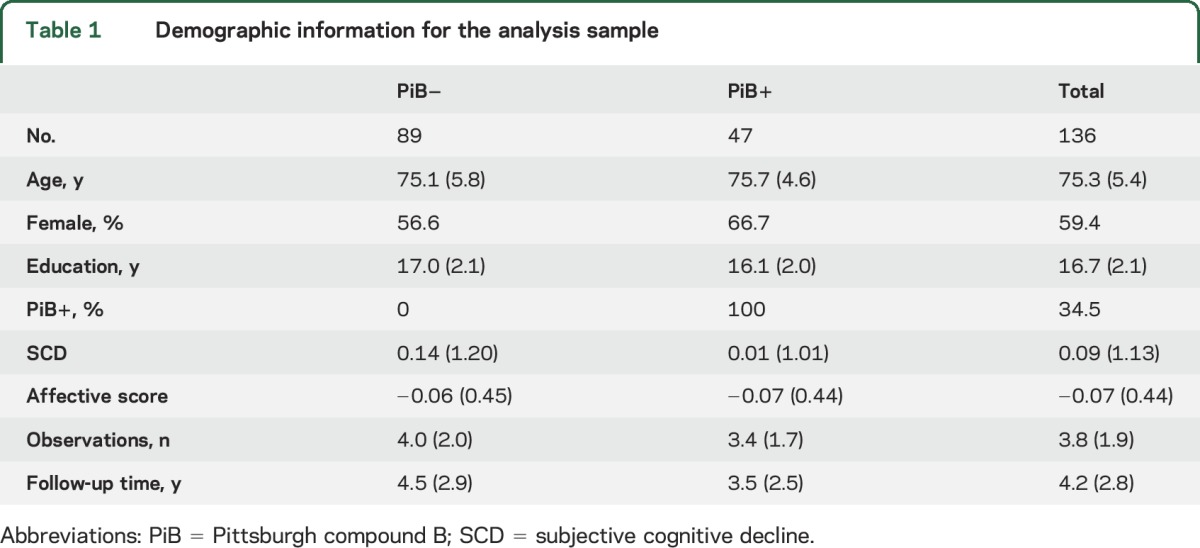
The entire cohort was used to develop variables that measured SCD and other measures of cognitive function. A subsample of 136 participants with all data on or before November 13, 2015, was used for all subsequent analyses. Demographics from this sample can be found in table 1. Participants were followed up from 1 to 10 years (table 1), amounting to a total of 519 observations across all participants.
Table 1.
Demographic information for the analysis sample
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained at each assessment and before all scanning protocols, and all procedures were approved by the Institutional Review boards of the University of California, Berkeley and the Lawrence Berkeley National Laboratory.
Measurement of SCD and affective variables.
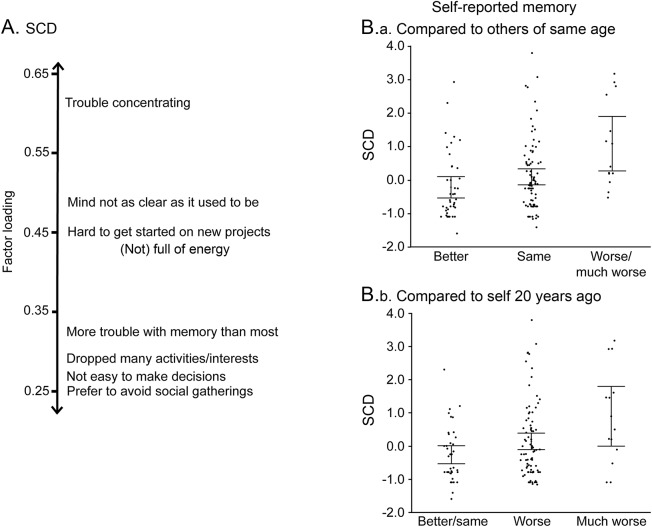
Previous literature has variably demonstrated that cognitive complaint is a principal component of the GDS.20,21 We extracted a latent measure of SCD by performing a principal axis factor analysis with direct oblimin rotation on a tetrachoric correlation matrix22 generated from 29 yes/no questions from the GDS at baseline using the Psych package from R (http://cran.r-project.org/package=psych). One question, “Do you feel that your situation is hopeless?,” was excluded from the analysis because 0% of the sample endorsed this statement. Each yes/no question was dummy coded so that the “depressed” answer was equal to 1. Parallel analysis23 suggested a 4-factor solution, which included a factor that grouped questions assessing cognitive complaints and was called SCD (figure 1A). Factor loadings and weights for each question can be found in tables e-1 and e-2 at Neurology.org, respectively. The resulting SCD factor scores were generated for each participant with regression. Factor scores from the remaining 3 factors (interpreted as dysphoric mood, anxiety, and apathy/hopelessness) were averaged to create 1 variable, affective score, representing negative affect. Scores were computed at the baseline visit. A simple command-line executable function to convert GDS scores into these factor scores can be found at https://github.com/illdopejake/Neurology_2017_SCD_score.
Figure 1. SCD factor score.
(A) GDS questions with the highest loadings into the SCD factor are shown. Higher factor loadings indicate greater contribution of the GDS question to the SCD factor. Questions contributing to the SCD factor are related largely to subjective reports of cognitive state or changes in behavior that may come about in relation to changes in cognitive state. (B) The SCD factor shows a strong linear relationship to an independent set of 2 questions assessing subjective report of memory: (B.a) self-reported memory compared to others of same age and (B.b) self-reported memory compared to self 20 years ago (other: F1,136 = 12.96, p < 0.001; self: F1,137 = 15.22, p < 0.001). GDS = Geriatric Depression Scale; SCD = subjective cognitive decline.
Measurement of cognition.
Cognitive factor scores were derived from the baseline sample as previously described24 and validated.25 Cognitive factors included episodic memory, executive function, and processing speed (table e-3). Factor scores were calculated for each participant at each time point by applying factor weights derived from the whole sample to z-scored cognitive test scores. Global cognition scores were generated by averaging all 3 cognitive factor scores.
Imaging.
PET scans were acquired an average of 1.02 years (SD = 1.61 years) after cognitive baseline. Structural MRI scans were acquired with a Siemens 1.5T Magnetom Avanto system (Siemens Medical Systems, Iselin, NJ) using a 12 channel head-coil run in triple mode. Details of the acquisition procedures have been documented elsewhere.17 All [11C]-Pittsburgh compound B (PiB)-PET scans were acquired at Lawrence Berkeley National Laboratory with either a Siemens ECAT EXACT HR PET scanner or Siemens Biograph Truepoint 6 scanner (Siemens Medical Systems, Malvern, PA) in 3-dimensional acquisition mode and a 90-minute dynamic scan as previously reported.17 Data were analyzed as mean distribution volume ratios as previously reported, with a threshold for PIB positivity of 1.08.17,26
Statistical analyses.
To validate our factor analysis–derived SCD score, we compared the score to 2 independent subjective memory measurements.16 The questions were as follows: “How would you rate your memory compared to others your same age?” and “How would you rate your memory compared to yourself 20 years ago?” Participants could rate themselves as better, the same, worse, or much worse. Categories with <4 respondents were merged with adjacent categories (e.g., worse/much worse). One-way analyses of variance (ANOVAs) were used to assess the relationship between SCD and each subjective memory measurement.
Relationships between SCD scores, PiB status, affective score, and demographic variables (age, sex, education, PET scanner, and duration of follow-up) were assessed with correlations, t tests, and Fisher exact tests. Linear models adjusted for age, sex, education, and affective score were used to assess cross-sectional relationships between SCD score and cognition. To better understand some of the underlying constructs contributing to the SCD score, a single linear model was fit with SCD as the dependent variable and age, sex, education, PiB status, family history of AD, global cognition, and affective score as independent variables. Cross-sectional associations between PiB status and cognition were established with linear models adjusted for age, sex, education, and PET scanner.
Mixed models were performed to evaluate the main effects of and interactions between PiB status, SCD, and time on each cognitive variable. These models are robust to missing cognitive data and differing follow-up times. All models used the restricted maximum likelihood method and were fit with an AR(1) covariance structure and type III sum of squares. Denominator degrees of freedom were calculated with the Satterthwaite approximation. Confidence intervals were calculated with Wald statistics. For each model, 1 cognitive domain was entered as the dependent variable. Time since baseline (time), SCD, PiB status, and every interaction between them were used as independent variables. All models included age, sex, education, PET scanner, and affective score as covariates of no interest. Additional models including interactions between these variables and time did not affect results. One model was run for each cognitive domain for a total of 4 models. Later, models were further adjusted for family history of AD and baseline cognitive scores specific to the dependent variable. All models first were fit with a random effect of participant and then were fit with random slopes (time|participant) if ANOVAs comparing the likelihood ratio suggested a significant improvement in model fit (all models except those with processing speed as a dependent variable and those adjusted for baseline cognition). Effects are reported only for covariates of interest. The 4 primary models were repeated, substituting affective score for SCD and adding SCD as a covariate of no interest. To interpret significant interactions, effects were plotted with JMP version 11.2 (SAS Institute Inc, Cary, NC, 2013). All statistical analyses were performed with R version 3.1.3.
RESULTS
Examination of SCD measure and affective score.
With 1-way ANOVAs, SCD was significantly related to each of the 2 subjective memory measurements in a stepwise fashion (figure 1B) in which the mean SCD score significantly increased at each step of progressively worsening subjective memory rating (other: F2,136 = 8.39, p < 0.0001; self: F2,136 = 9.56, p < 0.0001). Participants with higher SCD scores (more severe SCD) demonstrated worse episodic memory (t136 = −2.80, p < 0.01), working memory (t136 = −3.03, p < 0.005), and global cognition (t136 = −3.35, p = 0.001) and slightly worse processing speed (t136= −1.66, p = 0.099) at baseline.
The multivariate model assessing the contribution of several independent variables to the SCD score revealed that individuals with more severe SCD also demonstrated more severe affective features (t136 = 5.78, p < 0.0001), worse global cognition (t136 = −3.48, p < 0.001), and a greater likelihood of a family history of AD (t136 = 2.92, p < 0.005), which together explained 31% of variance in SCD. There were no relationships between SCD and age, sex, or education.
Individuals with higher affective scores were older on average (t136 = 2.02, p < 0.05). PiB+ individuals tended to be less educated (t136 = −2.65, p < 0.01) and had on average 1 year less follow-up time (t136= −2.23, p < 0.05). Neither SCD scores nor affective scores differed between PiB groups. There were no cross-sectional associations between amyloid and any of the cognitive scores.
Contribution of SCD, affective features, and brain Aβ to cognitive decline.
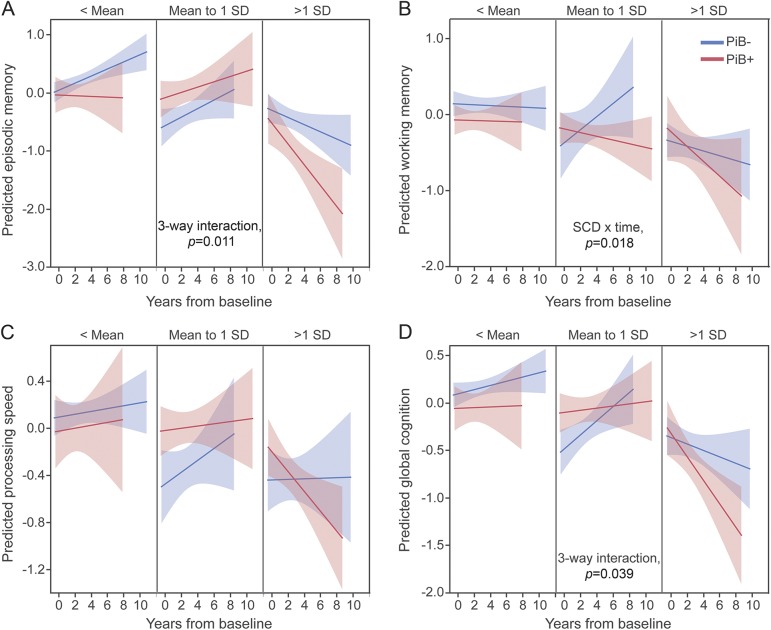
Mixed models were used to assess the effect of SCD, brain Aβ, and their interaction on cognition over time, with adjustment for demographic and affective variables. Results for the effects of interest can be found in table 2. A significant 3-way time × PiB status × SCD interaction was found for episodic memory and global cognition. Plotting these effects revealed that PiB+ individuals exhibited steeper cognitive decline as they demonstrated more severe SCD (figure 2). No 3-way interactions were observed for working memory or processing speed; however, a 2-way SCD × time interaction was observed for working memory such that individuals with worse SCD declined more in working memory over time, independently of PiB status (figure 2). Finally, processing speed declined over time independently of other factors. Adjusting models for family history of AD and baseline cognition dropped the 3-way interaction with global cognition to trend levels, although a significant SCD × time interaction on global cognition emerged (table e-4). Models including only participants with ≥3 cognitive testing sessions did not change results (data not shown).
Table 2.
Interactive and main effects of SCD and affective scores, PiB status, and time on cognition
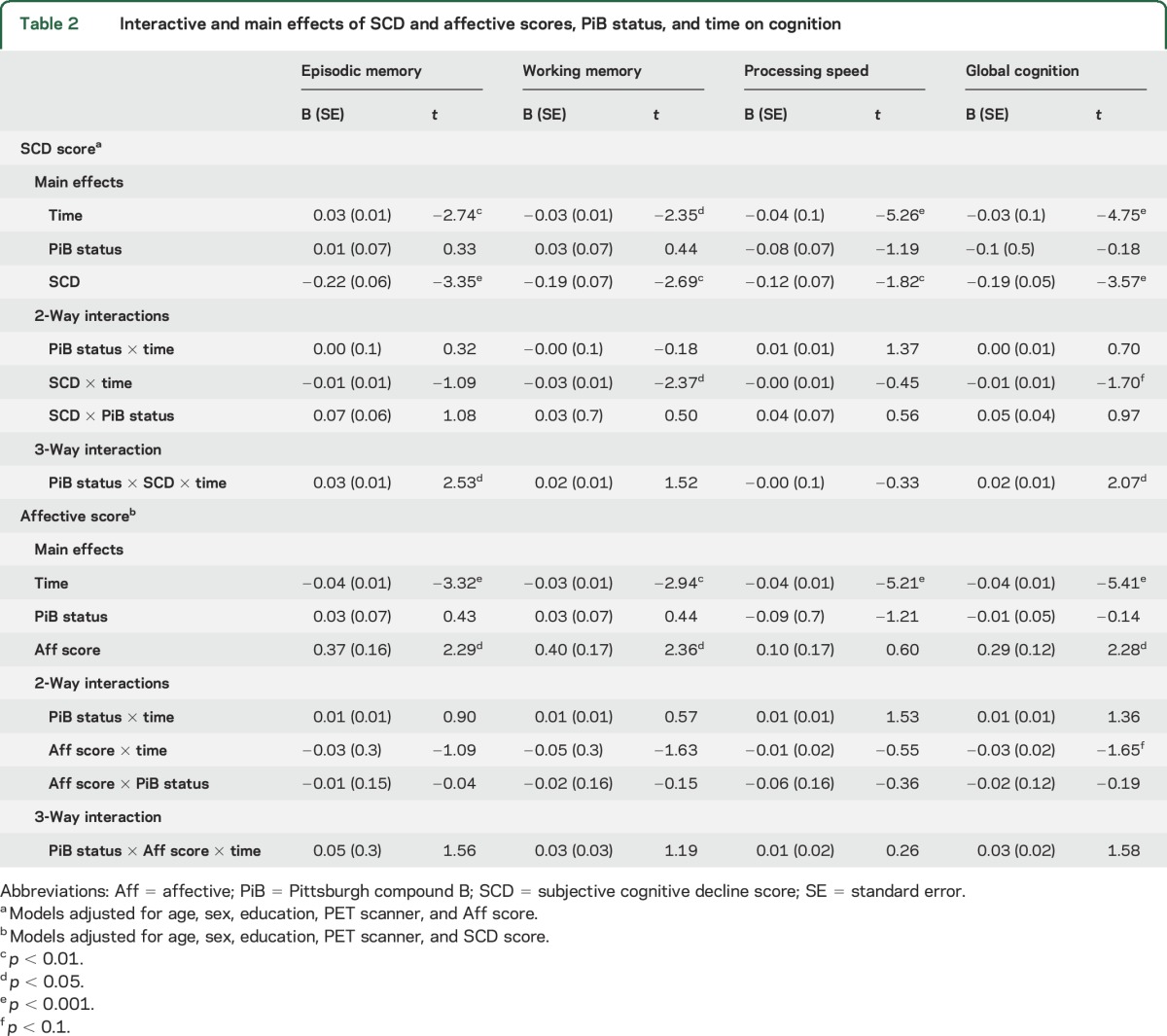
Figure 2. Interactions between PiB status and SCD severity on rate of cognitive change over time.
(A) Episodic memory, (B) working memory, (C) processing speed, and (D) global cognition. The y-axis of each plot represents the cognitive factor values predicted by the models; the x-axis represents time from baseline. To visualize 3-way interactions, cognition over time is presented separately for PiB− (blue) and PiB+ (red) groups. These relationships are further split at differing levels of SCD score (panels). Because SCD is a population-normalized variable, panels represent splits in reference to the mean and SD. This is meant only to aid in interpretation of the results. In the actual models, SCD was entered as a scalar variable. Main effects and interactions of interest are given in table 2. GDS = Geriatric Depression Scale; PiB = Pittsburgh compound B; SCD = subjective cognitive decline.
Finally, the same linear mixed models were run, substituting affective score for SCD and instead adjusting for SCD score. No significant 2- or 3-way interactions were observed, although there was a trend-level affective score × time interaction for global cognition (table 2). All cognitive variables declined significantly over time. Across all observations, individuals with worse (higher) affective scores tended to demonstrate better cognition.
DISCUSSION
Our results add to previous studies that found that both SCD and Aβ are related to cognitive decline1,7,8,11 and suggest that brain Aβ and SCD contribute complementary value to the prediction of cognitive decline when both measures are present. Results from our sample suggest that cognitively intact elderly with evidence for brain Aβ and SCD undergo objective cognitive decline at a higher rate than individuals with either Aβ or SCD alone or neither. This is true despite the fact that individuals with more severe SCD showed evidence of more cognitive impairment at baseline, suggesting that subjective assessments add valuable information over objective cognitive measures. The results were also domain specific: individuals with more severe SCD demonstrated significant cognitive decline in working memory and global cognition independently of Aβ, but decline in episodic memory was observed only when both SCD and Aβ were present.
Episodic memory is typically the first and most profoundly impaired cognitive domain during the course of AD,1 and individuals with SCD who later develop dementia show selective impairment in episodic memory over time.27 While longer follow-up is needed to assess conversion to dementia in the BACS cohort, the presence of brain Aβ, SCD, and decline in episodic memory in these individuals is consistent with a profile for preclinical AD. Indeed, our models predicted that PiB+ individuals in the top 80% of SCD scores demonstrate an average rate of decline of 0.18 SD a year in episodic memory and 0.12 SD in global cognition, suggesting a decline of a full SD in only 5 to 8 years. In contrast, PiB− individuals in the top 80% of SCD score demonstrated little change in cognitive scores over time (0.1 SD/y). These results are convergent with studies finding SCD to be associated with worse cognition in APOE4 carriers compared to noncarriers9 and studies examining SCD specifically in amyloid-positive participants.28 The presence of brain Aβ alone was not associated with cognitive decline on average. In some cases, PiB+ individuals even showed increases in cognitive test scores over time. These results imply that assessment of both brain Aβ and SCD may enable enrichment of clinical trial samples for faster decline over a shorter time period.
Individuals reporting more severe SCD showed declining cognition globally, most markedly in working memory. Working memory deficits in aging are well described,29 and in cases when Aβ is not present, the biology underlying this cognitive decline is unlikely to be related to AD.30 Instead, white matter abnormalities31 and frontal lobe integrity32 have consistently been reported to drive age-related variation in working memory, leading to speculation that vascular dysfunction32 may contribute to its etiology. Another factor showing a prominent association with working memory in aging is late-life depression.33 This is an important and relevant consideration because, as with our study, many previous studies have reported a link between SCD and the presence of subtle psychiatric symptoms such as anxiety and subsyndromal depression.12,34 This is unlikely to explain our findings, however, because models looking specifically at the contribution of affective scores to cognitive decline failed to find an effect when adjusted for SCD. Our results suggest that associations between GDS scores and cognitive decline in healthy, nondepressed elderly participants may actually be driven by those GDS questions assessing cognitive symptoms rather than depressive symptoms.
SCD is a difficult construct to measure because of its very subjectivity. Our study used an unsupervised data-driven method that identified a series of collinear GDS questions relating to subjective cognitive state and related functional complaints, summarized into a weighted SCD score. Our analyses suggest that these complaints may be driven by several independent factors, including symptoms of subsyndromal depression or anxiety, current objective cognitive state, and knowledge of a family history of AD. The last feature may be related to anxiety concerning the knowledge that family history increases risk of AD35 or could even be a manifestation of preclinical AD symptoms in these high-risk individuals. However, these features together explained only 31% of the variance in SCD and had little bearing on our longitudinal findings when models were adjusted for them. This indicates that individuals may be able to communicate a contextual awareness of cognitive state that goes beyond what a cross-sectional cognitive measurement might reveal. In other words, while SCD and baseline cognition are somewhat collinear, they are not redundant with respect to predicting future cognitive decline.
There are several limitations to this study. SCD is currently a field of major interest in aging and AD research, but only recently have there been efforts to standardize its measurement.6 Therefore, previous studies have used a wide range of instruments to characterize SCD, and our study is no different. The GDS is not specific for SCD, and we recognize that the questions in our SCD factor are not specific to memory loss. However, previous studies have shown that SCD questionnaire items that best distinguish clinical groups or amyloid-positive and -negative groups are not solely memory specific.22 In addition, the association between our SCD score and independent measures of subjective memory increases confidence in the construct that we are measuring. It is also important to note that our participants were nondepressed, healthy individuals who did not report to a memory clinic for evaluation.36 Finally, PET scans were recorded on average a year after cognitive baseline. Brain Aβ accumulates slowly and is not expected to change much over only a few years,1 although it is possible that some individuals categorized as PiB− in this study experienced enough amyloid accumulation over the course of follow-up to transition to PiB+.
Our results provide evidence that cognitively intact elderly with both brain Aβ and appreciable SCD are at the greatest risk of decline and clinical impairment. This provides a potential way of enriching the participant sample in clinical trials of Aβ-lowering therapies. The complementary nature and domain specificity with which SCD and Aβ predict cognitive decline suggest that they are 2 independent entities. While studies are underway to better understand the relationship of Aβ to incipient AD, future studies are required to elucidate the neurobiology underlying the manifestation of SCD and how it may vary according to amyloid status.
Supplementary Material
ACKNOWLEDGMENT
Rachel Bell, Taylor Mellinger, and Kaitlin Swinnerton (University of California, Berkeley) assisted in data collection and participant recruitment. Kris Norton (University of California, Berkeley) helped perform PET scans. Suzanne Baker (Lawrence Berkeley National Laboratory) processed PET data.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ANOVA
analysis of variance
- BACS
Berkeley Aging Cohort Study
- GDS
Geriatric Depression Scale
- PiB
Pittsburgh compound B
- SCD
subjective cognitive decline
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Jacob W. Vogel: study concept and design, analysis and interpretation of data, statistical analysis, manuscript preparation, data collection. Monika Varga Doležalová: study concept and design, analysis and interpretation of data, critical revision of manuscript. Renaud La Joie: interpretation of data, critical revision of manuscript. Shawn M. Marks: data collection, analysis and interpretation of data, critical revision of manuscript. Henry D. Schwimmer: data collection and preprocessing, critical revision of manuscript. Susan M. Landau: analysis and interpretation of data, critical revision of manuscript. William J. Jagust: study concept and design, interpretation of data, manuscript preparation, study supervision.
STUDY FUNDING
This study was funded by NIH grant AG034570.
DISCLOSURE
J. Vogel, M. Varga Doležalová, R. La Joie, S. Marks, and H. Schwimmer report no disclosures relevant to the manuscript. S. Landau is consulting for Cortexyme, Inc. W. Jagust is consulting for Bioclinica, Novartis, Genentech, and Biogen. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–367. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement 2011;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagust W. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain 2016;139:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron 2014;84:608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 6.Jessen F, Amariglio RE, Van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doraiswamy PM, Sperling RA, Johnson K, et al. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry 2014;19:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessen F. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67:414–422. [DOI] [PubMed] [Google Scholar]
- 9.Samieri C, Proust-Lima CM, Glymour M, et al. Subjective cognitive concerns, episodic memory, and the APOEε4 allele. Alzheimers Dement 2014;10:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 2010;6:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 2014;83:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid LM, MacLullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 2006;22:471–485. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Capuano AW, Boyle PA, et al. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology 2014;83:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amariglio RE, Mormino EC, Pietras AC, et al. Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology 2015;85:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 2006;67:1581–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging. Arch Neurol 2012;62:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, et al. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain 2015;138:2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. J Psychiatr Res 2017;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 20.Adams KB, Matto HC, Sanders S. Confirmatory factor analysis of the Geriatric Depression Scale Gerontologist 2004;44:818–826. [DOI] [PubMed] [Google Scholar]
- 21.Kim G, DeCoster J, Huang C-H, Bryant AN. A meta-analysis of the factor structure of the Geriatric Depression Scale (GDS): the effects of language. Int Psychogeriatrics 2013;25:71–81. [DOI] [PubMed] [Google Scholar]
- 22.La Joie R, Perrotin A, Egret S, et al. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: association with medical help seeking, cognitive deficits, and β-amyloid imaging. Alzheimers Dement (Amst) 2016;5:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika 1965;30:179–185. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber S, Vogel J, Schwimmer HD, Marks SM, Schreiber F, Jagust W. Impact of lifestyle dimensions on brain pathology and cognition. Neurobiol Aging 2016;40:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016;89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mormino EC, Brandel MG, Madison CM, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage 2012;59:1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kryscio RJ, Abner EL, Jicha GA, et al. Self-reported memory complaints: a comparison of demented and unimpaired outcomes. J Prev Alzheimers Dis 2016;3:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley RF, Maruff P, Ames D, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement 2017;12:796–804. [DOI] [PubMed] [Google Scholar]
- 29.Salthouse TA. The aging of working memory. Neuropsychology 1994;8:535–543. [Google Scholar]
- 30.Oh H, Madison C, Villeneuve S, Markley C, Jagust WJ. Association of gray matter atrophy with age, Aβ-amyloid, and cognition in aging. Cereb Cortex 2014;24:1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage 2009;44:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia 2003;41:1929–1941. [DOI] [PubMed] [Google Scholar]
- 33.Nebes RD, Butters MA, Mulsant BH, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 2000;30:679–691. [DOI] [PubMed] [Google Scholar]
- 34.Buckley R, Saling MM, Ames D, et al. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int Psychogeriatr 2013;25:1307–1315. [DOI] [PubMed] [Google Scholar]
- 35.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 2006;63:168–174. [DOI] [PubMed] [Google Scholar]
- 36.Perrotin A, La Joie R, de La Sayette V, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement 2017;13:550–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.