SECTION 1
A 4-year-old girl presented to an outside hospital after waking up with inability to stand or walk. She had a viral prodrome with fever for several days. Brain and spine MRIs revealed lesions in the left caudate, bilateral insular cortex, right thalamus, and right temporal cortex as well as longitudinally extensive cervical and thoracic cord lesions. Spinal fluid showed pleocytosis with 200 white blood cells (mixed neutrophils and lymphocytes), elevated CSF protein (103 mg/dL), and negative bacterial and viral cultures and PCR. Oligoclonal bands were negative. During the hospitalization, she developed encephalopathy and was diagnosed with acute disseminated encephalomyelitis (ADEM). She received a course of IV steroids followed by an oral steroid taper, and her neurologic symptoms resolved fully. However, within 2 weeks, she experienced painless bilateral vision loss. A repeat brain and orbit MRI revealed swelling in the bilateral optic nerves and decrease in size or resolution of prior brain lesions. Optic neuritis (ON) was diagnosed based on clinical and radiographic presentation. She was given a course of IV immunoglobulin, followed by IV steroids, and a longer steroid taper.
Questions:
What are the diagnostic criteria for ADEM?
What is the differential diagnosis for a first demyelinating episode in a child?
SECTION 2
The International Pediatric Multiple Sclerosis Study Group defined ADEM as a first polyfocal clinical CNS event with encephalopathy that cannot be explained by fever, presumably of inflammatory demyelinating etiology.1 Brain MRI is abnormal during the acute phase with diffuse, poorly demarcated, large T2-hyperintense lesions involving predominantly cerebral white matter. ADEM requires that there be no new clinical or MRI findings 3 months or more after onset.1 Our patient's paraparesis and encephalopathy with multifocal brain and spinal cord lesions would fit with these diagnostic criteria. An episode of ON within 3 months of symptom onset falls within the allowed time period for ADEM. Other clinical considerations include first presentation of multiple sclerosis (MS) or neuromyelitis optica spectrum disorder (NMOSD). ADEM-like attack is the presenting symptom of MS in 5%–15% of children.1 ADEM followed by ON (ADEM-ON) can be associated with antibodies to myelin oligodendrocyte glycoprotein (MOG).2 It can sometimes be difficult at initial presentation to stratify the risk of future demyelinating events in these patients and ongoing follow-up and repeat imaging is necessary.
Two years later (age 6), the patient had acute onset of total loss of vision in the right eye. Vision improved again to baseline following IV steroids. For the next 4 years, she had 3 more recurrent episodes of ON in either eye, each time improving back to near baseline after IV steroids. From age 10 to 15 years she was relapse-free, but after age 15 she experienced further episodes of ON, mostly involving the right eye. Recurrences generally occurred at the time of steroid withdrawal and recovery was more protracted and less complete. At age 16, she had an episode of headache and nystagmus and was found to have nonenhancing lesions in the left cerebellum and left thalamus. Symptoms improved after steroids and MRI lesions fully resolved. During steroid taper, she had a single focal seizure with secondary generalization and was started on antiepileptic therapy. Subsequent brain MRIs were normal except for involvement of the optic nerves. Aquaporin-4 antibody testing was repeatedly negative.
Questions:
How does this change your differential diagnosis?
What additional testing would you perform?
What treatment would you consider to prevent further relapses?
SECTION 3
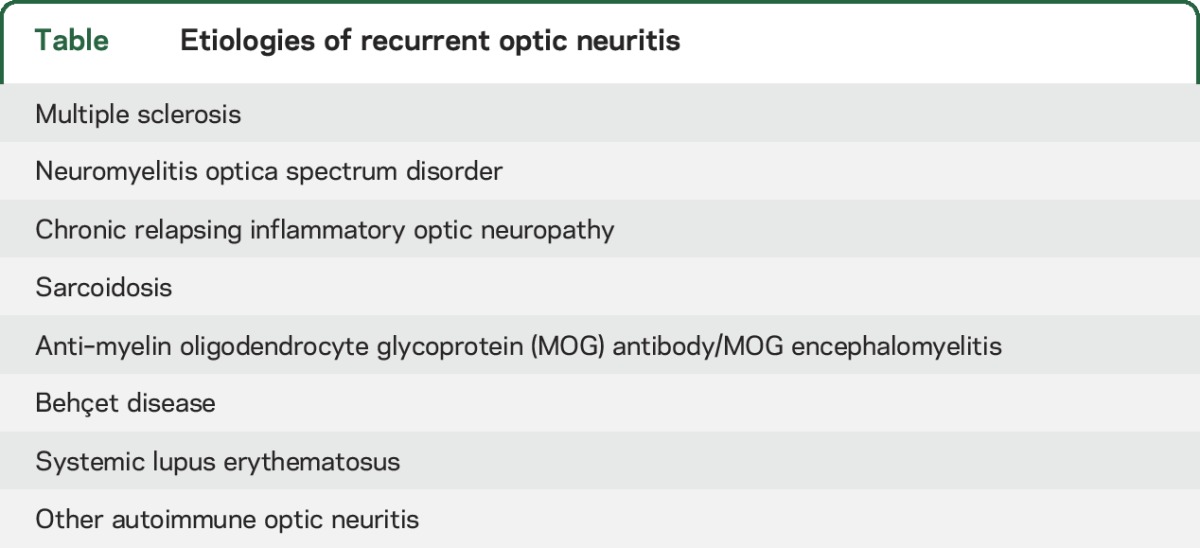
The patient has a relapsing-remitting, presumably inflammatory CNS disorder with predilection for the optic nerves, following an initial event of longitudinally extensive myelitis with brain lesions. The most common relapsing inflammatory disorder of CNS is MS; however, absence of lesions on brain MRI after more than a decade of disease would be highly unusual. The current 2015 International Panel of Neuromyelitis Optica Diagnostic (IPND) criteria stratify patients with NMOSD according to aquaporin-4 (AQP4) antibody status.3 Recognized presentations of NMO include ON (sometimes affecting both optic nerves or the chiasm), longitudinally extensive transverse myelitis (LETM), area postrema syndrome (intractable hiccups or vomiting), and diencephalic, brainstem, and cerebral syndromes. Our patient experienced recurrent ON and had LETM at onset, so, in the absence of an alternative explanation, she would meet the IPND criteria for seronegative NMOSD. The table lists additional causes of recurrent ON, including chronic relapsing inflammatory optic neuropathy, characterized by recrudescence of inflammation when steroids are tapered,4 neurosarcoidosis, lupus, and others. Testing for rheumatologic diseases can also be performed when relevant systemic symptoms are present. Recent studies have consistently documented that some AQP4-seronegative patients with NMOSD have antibodies to MOG.5,6
Table.
Etiologies of recurrent optic neuritis
Because of frequent recurrences, at age 16 the patient was started on immunosuppressive therapy (azathioprine) for presumed seronegative NMOSD. She continued to have relapses of ON while on azathioprine and 2 years later she was switched to rituximab infusions every 6 months. She had multiple episodes of otitis and sinusitis while on rituximab, which prompted the addition of monthly IV immunoglobulin. Less frequent episodes of ON continued during the first 2 years on rituximab, but the patient then stabilized without further episodes of ON during the last 2 years. To date (age 23), the patient has had 15 documented episodes of ON. Her last examination is notable for poor vision (20/200) in the right eye with profound pallor of the fundi. Optical coherence tomography (OCT) showed striking bilateral thinning of the retinal nerve fiber layer (RNFL) (22 μm on the right and 24 μm on the left); normal RNFL thickness on OCT is around 90–100 μm.
A reanalysis of the patient's serum samples at Mayo Clinic laboratories revealed anti-MOG antibodies at high titer in multiple samples since 2010.
DISCUSSION
Even with optimal testing for AQP4 antibodies, 10%–20% of patients with NMOSD are seronegative.5 Increasingly sensitive and specific testing via cell-based assays have revealed anti-MOG antibodies in up to 27% of seronegative patients with NMOSD. MOG is expressed exclusively on the surface of oligodendrocytes in the CNS and is thought to be a late component in the development of myelin.6 MOG was found to be an autoantigen in the experimental autoimmune encephalomyelitis model of MS in mice.6 There is some controversy regarding phenotypic correlations of anti-MOG antibodies in humans. An earlier study by Sato et al.7 found that patients with MOG antibodies tended to have a more limited phenotype of mostly ON or caudal myelitis with generally better recovery from attacks and less accrual of disability than AQP4 antibody–positive patients. Another study of pediatric patients presenting with an ADEM phenotype found 19 of 39 patients with positive anti-MOG antibodies (age range 1–17 years at disease onset), 15 of whom had monophasic disease.8 However, in recent retrospective series from Germany4 with 50 MOG antibody–positive patients and from India9 with 25 MOG antibody–positive patients, most patients had a relapsing-remitting course, and a minority have accumulated considerable disability. Among pediatric patients, an association was found between MOG antibodies and a presentation of ADEM followed by monophasic or recurrent ON, as in our case.2 Conflicting results concerning disease phenotypes and outcomes may be, in part, due to the fact that studies carried out before 2015 may have suffered from immunoglobulin M contamination; only studies with anti–immunoglobulin G1 (IgG1) isotype as the secondary antibody are reliable for anti-MOG disease testing.
Some anti-MOG–positive patients appear to respond to rituximab, though several patients in the German study were noted to have relapses within the first year of starting rituximab, as was also the case with our patient, and at end-dose intervals with reconstitution of B cells.5 Importantly, several patients with anti-MOG antibodies who were initially diagnosed with MS and treated with interferons experienced increased relapse rates,5,9 as is sometimes seen in AQP4 antibody–positive NMOSD.
Our case emphasizes the importance of re-examining cases of unexplained neuroinflammatory disease as new knowledge and testing become available. MOG antibody testing should be considered for patients who have recurrent transverse myelitis (especially LETM), recurrent ON, or both; atypical ADEM syndromes such as multiphasic ADEM or ADEM-ON; atypical imaging for MS; and in seronegative NMOSD. Interestingly, seizures associated with ON, as noted in our patient, have been associated with anti-MOG antibodies in a few patients in Japan.10 While testing is currently only available in specialized research settings, it is expected that rigorous testing for anti-IgG1 isotype of anti-MOG antibodies will become commercially available in the near future.
Our patient, with very young onset (4 years) and 18 years of close clinical follow-up during which she experienced ADEM-like attack, brainstem relapse, and 15 episodes of ON, adds to our knowledge of clinical phenotypes associated with anti-MOG. Immunotherapy may be warranted in cases of recurrent neuroinflammatory conditions even with atypical manifestations. The discovery of MOG antibodies highlights the evolving spectrum of autoantibodies in CNS demyelination. Evidence-based therapies for anti-MOG and seronegative NMOSD are lacking, but improved understanding of the molecular mechanisms of these autoimmune diseases will allow for more targeted therapies in the future.
ACKNOWLEDGMENT
The authors thank the patient for cooperation and review of the manuscript.
AUTHOR CONTRIBUTIONS
Josef Maxwell Gutman: study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content. Michael Levy: drafting of the manuscript, critical revision of the manuscript for important intellectual content. Steven Galetta: drafting of the manuscript, critical revision of the manuscript for important intellectual content. Ilya Kister: drafting of the manuscript, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J. Gutman reports no disclosures relevant to the manuscript. M. Levy receives research support from NIH, Guthy-Jackson Charitable Foundation, Shire, Acorda, Sanofi/Genzyme, Alnylam, Neural Stem, and Genentech and serves as a consultant for Chugai Pharmaceuticals, Shire, GlaxoSmithKline, and Alexion Pharmaceuticals. S. Galetta has been a consultant for Biogen Idec. I. Kister served on the scientific advisory board for Biogen Idec and Genentech and received research support from Guthy-Jackson Charitable Foundation, National Multiple Sclerosis Society, Biogen Idec, Serono, Genzyme, and Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Tardieu M, Banwell B, Wolinsky JS, Pohl D, Krupp LB. Consensus definitions for pediatric MS and other demyelinating disorders in childhood. Neurology 2016;87(suppl 2):S8–S11. [DOI] [PubMed] [Google Scholar]
- 2.Pohl D, Alper G, Haren KV, et al. Acute disseminated encephalomyelitis updates on an inflammatory CNS syndrome. Neurology 2016;87:S38–S45. [DOI] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain 2003;126:276–284. [DOI] [PubMed] [Google Scholar]
- 5.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients: part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016;13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 2016;15:307–324. [DOI] [PubMed] [Google Scholar]
- 7.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry 2015;86:265–272. [DOI] [PubMed] [Google Scholar]
- 9.Pandit L, Sato DK, Mustafa S, et al. Relapsing optic neuritis and isolated transverse myelitis are the predominant clinical phenotypes for patients with antibodies to myelin oligodendrocyte glycoprotein in India. Mult Scler J Exp Transl Clin 2016;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody–positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm 2017;4:e322. [DOI] [PMC free article] [PubMed] [Google Scholar]


