Abstract
Background
Data from broad populations has established associations between incidental carotid plaque and vascular events. Among persons living with HIV (PLWHIV), the risk of vascular events is increased; however, whether incidental carotid plaque is increased and there is an association between incidental carotid plaque, plaque characteristics and vascular events among PLWHIV is unclear.
Methods and Results
Data from the multi-institutional Research Patient Data Registry was used. Presence and characteristics (high-risk plaque (HRP) including spotty calcification and low attenuation) of carotid plaque by CT among PLWHIV without known vascular disease were described. Data were compared to uninfected controls similar in age, sex and cardiovascular risk factors including DM, HLD and cigarette smoking to cases. Primary outcome was an ASCVD event and secondary outcome was ischemic stroke. Cohort consisted of 209 PLWHIV (45±10 years, 72% male) and 168 controls. Using CT, PLWHIV without vascular disease had a higher rates of any carotid plaque (34 vs. 25%, p=0.04), non-calcified (18 vs. 5%, p<0.001) and HRP (25 vs. 16%, p=0.03). Over a follow-up of 3 years, 19 ASCVD events (9 strokes) occurred. Carotid plaque was independently associated with a 3-fold increase in ASCVD events among PLWHIV (HR: 2.91, CI: 1.10–7.7, p=0.03) and a 4-fold increased risk of stroke (HR: 4.43, CI: 1.17–16.70, p=0.02); HRP was associated with a 3-fold increased risk of ASCVD events and a 4-fold increased risk of stroke.
Conclusion
There is an increase in incidental carotid plaque, non-calcified plaque and HRP among PLWHIV and the presence and characteristics of carotid plaque are associated with subsequent vascular events.
Keywords: HIV, carotid plaque, atherosclerotic cardiovascular disease events
Introduction
Among persons living with HIV (PLWHIV), studies have shown a disproportionate vulnerability to select cardiovascular (CV) diseases including myocardial infarction and stroke1, 2. Specifically, PLWHIV face a more than two-fold greater risk of vascular events including both myocardial infarction and ischemic stroke as compared to uninfected individuals3, 4. The mechanisms involved in the increased risk of vascular events in HIV are incompletely understood5 and more effective means of identifying PLWHIV at highest risk of incurring atherosclerotic cardiovascular disease (ASCVD) events may be of value.
Computerized tomography (CT) allows for the detailed anatomical delineation of large and medium sized vessels and the reproducible early detection and characterization of atherosclerosis and atherosclerotic plaque6. Among PLWHIV, studies using CT focused on coronary imaging have established that PLWHIV have an increased prevalence of coronary atherosclerosis, non-calcified and high-risk coronary atherosclerotic plaque (HRP) and studies have shown an association between coronary atherosclerosis and plaque features with subsequent ASCVD events7, 8. Among PLWHIV, there are no data testing whether HIV status is associated with an increase in carotid plaque using CT and whether the presence or characteristics of carotid plaque using CT in HIV is associated with an increase in subsequent ASCVD events. Therefore, we compared the presence and characteristics of incidental carotid plaque among a population of PLWHIV without known vascular disease to an uninfected control group similar with regards to age, sex and cardiovascular risk factors including DM, HLD and cigarette smoking to cases and tested the association between the presence of plaque and plaque features with adverse outcomes.
Methods
Study Design and Patient population
The study was retrospective in design and our data set was developed from an established and validated research registry of PLWHIV and non-HIV controls1, 9–12. In brief, PLWHIV were identified via a broad initial search which included the ICD 9 codes for HIV (ICD-9-CM codes 042, 043 or V08) used in the research registry plus HIV-related diagnosis related codes (DRG’s: DRG 488, 489, 702, 705, 707, 708, 710, 711, 714, 790) and specific search terms (e.g. HIV, ART, AIDS) generating a broad initial sample size of (n): 9375 (Figure A). From this dataset, we confirmed a diagnosis of HIV on chart review and identified all PWLHIV who had a neck CT with contrast. Subjects with a prior history of ASCVD including myocardial infarction (MI), coronary artery disease (CAD), cerebrovascular disease (stroke and transient ischemic attack (TIA), prior carotid or peripheral vascular disease and prior atrial fibrillation were excluded. Subjects with non-diagnostic/unavailable neck CT scans and subjects in whom the indication for the CT scan was stroke were also excluded. Control subjects were derived from the same database. In brief, 441 subjects were noted to be erroneously coded under the extended search term “HIV” and served as the control group. A through chart review demonstrated that these subjects had the word “HIV” in the medical record typically due to HIV testing and did not have HIV. Non-HIV control subjects with a prior history of ASCVD, CAD, and cerebrovascular disease were excluded (stroke and transient ischemic attack). Data collection, image analysis and event adjudication were performed by three independent and blinded groups. One team performed electronic medical record review to ascertain data on baseline CV risk factors and HIV-specific parameters, another adjudicated clinical outcomes and a third performed image analysis of the neck CT’s. Our study was approved by the Institutional Review Board.
Data Collection
Traditional CV risk factors
Baseline covariates were collected within one year of the neck CT scan by electronic medical record review. Data were collected on traditional CV risk factors including hypertension, dyslipidemia, diabetes mellitus, and family history of coronary heart disease13. The use of CV medications, body mass index (BMI), sleep apnea, creatinine levels, smoking, alcohol use, and illicit drug use were also collected.
HIV-specific parameters
Data were collected on duration since HIV diagnosis, duration of antiretroviral therapy, category of antiretroviral therapies, viral load, CD4 count, and nadir CD4 count as previously described14. All efforts were made to collect the HIV-specific parameters from the electronic medical record at a time point closest to the CT scan.
CT neck image analysis
The types of CT scans included CT neck with contrast, CT angiography and PET-CT with contrast, performed for a variety of indications. Indications for neck CT included assessment for trauma (27%), neck mass (17%), neck cancer (13%), lymphadenopathy (8%), abscess (7%), neck pain (7%), headache (2%), dysphagia (1%) and varied (12%). We assessed the presence of plaque, plaque composition (calcified, mixed, and non-calcified), degree of stenosis based on NASCET evaluation (minimal 1–30%, mild 31–50%, moderate: 51–69%, severe: 70–99%), presence of high risk plaque (HRP) features including spotty calcification (size of calcification <3 mm in all directions) and low attenuation (if circular ROI with diameter of 1mm shows area with mean attenuation <40 HU)15. A plaque score was computed by summing the maximum thickness of plaque in each segment (mm) on both sides according to Gupta et al16. The carotid images were analyzed by 3 experienced readers (S.J., P.S., R.T.), blinded to the clinical data, on a cardiac workstation (Tera Recon, Foster City, California). All readers completed standardized CT training to assess inter-observer variability and had excellent inter-observer agreement for presence of plaque (kappa: 0.92), grade of stenosis (kappa: 0.77), type of calcification (kappa: 0.74), low attenuation (kappa: 0.95) and spotty calcification (kappa: 0.69).
Outcomes
The primary outcome was an ASCVD event, defined as myocardial infarction (MI), coronary heart disease (CHD) death or ischemic stroke, in accordance with ASCVD endpoints defined in the 2013 ACC/AHA Risk Assessment Guidelines17. The secondary outcome was ischemic stroke as defined by the AHA/ASA consensus document18. All events were adjudicated by blinded board certified physicians using standardized definitions blinded to other variables19.
Statistical Analysis
Continuous variables were presented as mean±SD or median and interquartile range, whereas categorical variables were presented as percentages. Parameters were compared between the two groups using student t test or Mann Whitney test for continuous variables and chi square or Fisher’s exact test for categorical variables, as appropriate. Univariate and multivariate Cox proportional hazard regression analyses were performed to determine the association between the presence of any carotid plaque or HRP and outcomes among the entire cohort and then just limited to PLWHIV. The multivariate models were adjusted for the number of traditional CV risk factors including hypertension (HTN), diabetes mellitus (DM), hyperlipidemia (HLD), current smoking and family history of CAD. Hazard ratios (HR) are presented with 95% confidence interval (CI). Kaplan-Meier curves were generated to compare event-free survival among PLWHIV and un-infected individuals with and without plaque. Between group differences were assessed using the log-rank test. A P value < 0.05 was considered significant. Statistical calculations were performed using SPSS software (SPSS version 22; IBM Corp., Armonk, New York) and STATA software (Version 13, StataCorp LP., College Station, TX).
Results
Study flow diagrams for the derivation of both the PLWHIV and uninfected control individuals are presented in Figures 1A and 1B. After exclusion of subjects in each cohort for prior vascular disease, there were 209 PLWHIV and 168 controls. The distribution of type of CT scans included CT neck with contrast (65%), CT angiography (CTA) (44%) and PET-CT with contrast (4%). PLWHIV had a higher frequency of CT neck with contrast while controls had a higher number of CT angiography. The indication categories in the two groups were similar for abscess, cancer, dysphagia, pain, mental status change (p<0.05 for all), whereas significant differences were found for the numbers in each group where the indication was lymphadenopathy, mass and trauma (p>0.05 for all). Tables 1 and 2 describe the baseline characteristics of PLWHIV and uninfected controls. There were no significant differences in age (45±10 vs. 43±17 years, HIV+ vs. HIV-, p=0.07), gender (72 vs. 65% male, p=0.18), prevalence of diabetes (10 vs. 11%, p=0.61), hyperlipidemia (15 vs.17%, p=0.67) and cigarette smoking (33 vs. 30%, p=0.57). Cardiovascular medication use was similar between the two groups.
Figure 1.
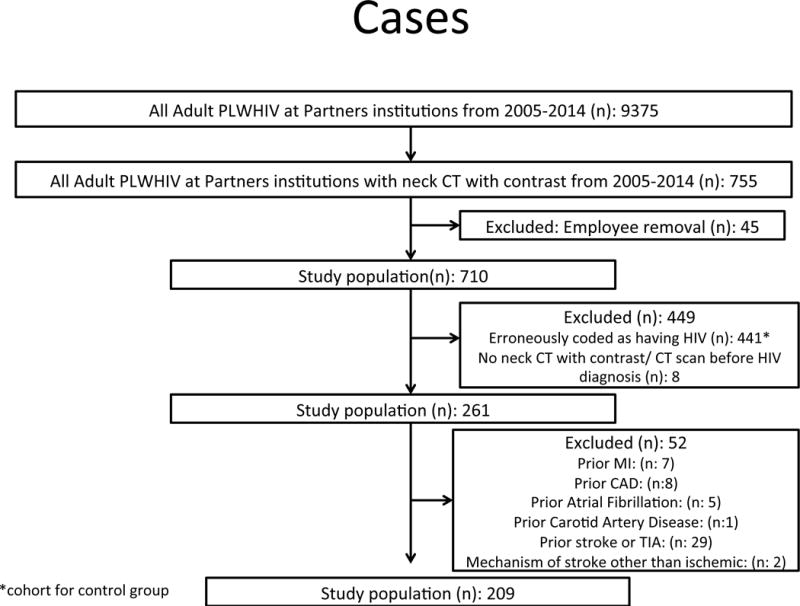
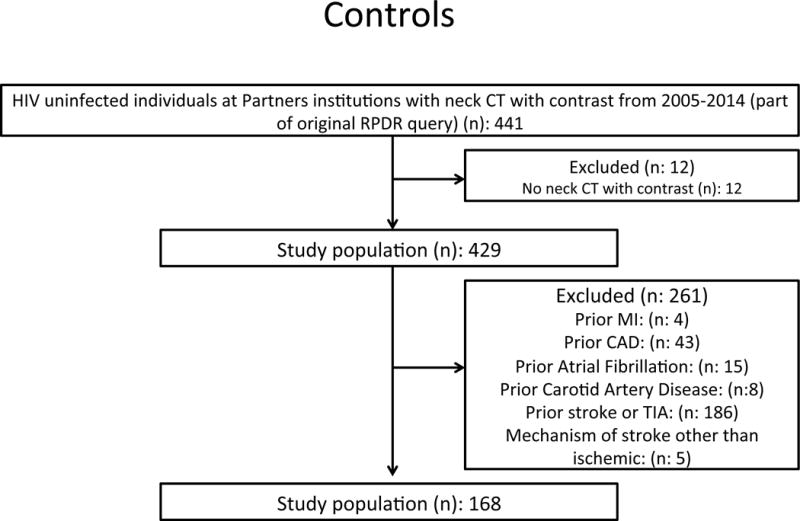
A: Consort diagram describing inclusion and exclusion criteria for the PLWHIV cohort. After excluding 52 subjects with prior cerebrovascular and cardiovascular disease, our eligible study population was 209.
B: Consort diagram describing the inclusion and exclusion criteria for the uninfected cohort. After excluding 261 subjects with prior cerebrovascular and cardiovascular disease, our eligible study population was 168.
Table 1.
Baseline characteristics of PLWHIV vs. uninfected individuals
| PLWHIV (n=209) |
uninfected (n=168) |
P value | |
|---|---|---|---|
| Age (mean±SD) | 45.6±10 | 43.1±17 | 0.07 |
| Male sex | 150(72) | 109(65) | 0.18 |
| Race | 0.02 | ||
| White | 106(51) | 30(65) | |
| Black | 73(35) | 5(11) | |
| Asian | 4(2) | 2(1) | |
| Other | 19(9) | 6(13) | |
| Hispanic/Latino | 27(13) | 3(6) | 0.35 |
| Diabetes mellitus | 20(10) | 19(11) | 0.61 |
| Hypertension | 46(22) | 53(31) | 0.04 |
| Hyperlipidemia | 31(15) | 28(17) | 0.67 |
| Current Smoker | 71(33) | 51(30) | 0.57 |
| Family history of CAD | 10(6) | 2(1) | 0.07 |
| Family history of stroke/TIA | 11(5) | 7(5) | 1.00 |
| Number of risk factors | |||
| 0 | 82(21) | 107(28) | <0.001 |
| 1 | 93(25) | 30(8) | |
| 2 | 20(5) | 21(6) | |
| 3 | 12(3) | 10(3) | |
| 4 | 2(1) | 0(0) | |
| IVDU | 0.01 | ||
| Current | 17(8) | 0(0) | |
| Past | 19(9) | 0(0) | |
| Cocaine use | <0.01 | ||
| Current | 17(8) | 0(0) | |
| Past | 35(17) | 2(4) | |
| CV Medications | |||
| Aspirin | 19(9) | 15(9) | 1.00 |
| ACE inhibitor | 19(9) | 15(9) | 1.00 |
| ARB | 5(1) | 2(1) | 0.46 |
| Betablocker | 30(14) | 18(11) | 0.35 |
| Statin | 27(12) | 18(11) | 0.74 |
Values are mean±SD or n (%). CAD: coronary artery disease, TIA: transient ischemic attack, IVDU: intravenous drug use, ACE: angiotensin converting enzyme, ARB: angiotensin receptor blocker, HDL: high density lipoprotein, LDL: low density lipoprotein, BMI: body mass index
Table 2.
Baseline characteristics of PLWHIV vs. uninfected individuals
| PLWHIV (n=209) |
HIV uninfected (n=168) |
P value | |
|---|---|---|---|
| Total cholesterol, mg/dL | 170±48 | 182±42 | 0.09 |
| Trigylcerides, mg/dL | 172±148 | 148±104 | 0.09 |
| HDL, mg/dL | 44±22 | 52±19 | 0.01 |
| LDL, mg/dL | 92±38 | 101±37 | 0.14 |
| BMI, kg/m2 | 26±5.3 | 28±7.4 | 0.01 |
| Systolic Blood pressure, mmHg | 123±19 | 126±18 | 0.31 |
| Diastolic Blood pressure, mmHg | 75±11 | 74±11 | 0.42 |
| Heart Rate, beats/min | 83±17 | 83±16 | 0.99 |
| Creatinine, mg/dL | 1.1±1.2 | 1.1±1.2 | 0.85 |
| HCV co-infection | 38 (18) | ||
| Duration since HIV diagnosis (years) | 14±7 | ||
| Endocarditis | 6(3) | ||
| CD4 count (cells/mm3) | 378±358 | ||
| CD4 nadir (cells/mm3) | 184±194 | ||
| CD8 count (cells/mm3) | 825±550 | ||
| ART at time of CT | 194 (93) | ||
| NNRTI | 67 (32) | ||
| NRTI | 159 (76) | ||
| PI | 87 (42) | ||
| Integrase inhibitors | 23 (11) | ||
| Duration of ART (years) | 9.8 (4.9, 15.0) | ||
| Undetectable viral load (copies/ml) | 104 (49) |
Values are mean±SD, n (%) or median (IQR). HDL: high density lipoprotein, LDL: low density lipoprotein, BMI: body mass index, ART: antiretroviral therapy; NNRTI: non nucleotide reverse transcriptase inhibitors; NRTI: nucleotide reverse transcriptase inhibitors; PI: protease inhibitors
PLWHIV varied significantly with regards to race (more African- American) and had increased illicit drug use, lower BMI and lower HDL as compared to uninfected controls. The mean duration of HIV was 14±7 years, 49% had an undetectable viral load, the mean CD4 count was 378±358 cells/mm3 and 93% (n=194) of the PLWHIV were on ART. Variables including duration of ART, CD4 nadir and CD4 count were present in 93% of the cases (missing 7%), absolute CD8 count present for 69% of the cases (missing 31%) and undetectable viral load for 88% of the cases (missing 12%).
Carotid plaque characteristics
PLWHIV free of known vascular disease had an increased rate of any carotid plaque (34 vs. 25%, p= 0.04), non-calcified carotid plaque (18 vs. 5%, p<0.001) and higher plaque score (1.8±3.4 vs. 1.2±2.7, p=0.03). PLWHIV also had a significantly higher number of any HRP (25 vs. 16%, p=0.03), higher number of HRP features per patient (p=0.03) and a higher number of low attenuation plaque (16 vs. 7%, p=0.007, Table 3, Figure 2).
Table 3.
Carotid Plaque characteristics in PLWHIV vs. uninfected individuals
| PLWHIV (n=209) |
uninfected (n=168) |
P value | |
|---|---|---|---|
| Any plaque | 72(34) | 42(25) | 0.04 |
| Plaque score | 1.8±3 | 1.21±3 | 0.03 |
| Composition | |||
| Calcified plaque | 6(3) | 8(5) | 0.33 |
| Non-calcified plaque | 38(18) | 8(5) | <0.001 |
| Partially calcified plaque | 46(22) | 34(20) | 0.67 |
| Stenosis | |||
| Moderate (>50%) | 10(5) | 2(1) | 0.07 |
| Severe (>70%) | 3(1) | 0(0) | 0.25 |
| Any high risk plaque | 53(25) | 27(16) | 0.03 |
| Spotty calcification | 25(12) | 13(8) | 0.22 |
| Low attenuation | 34(16) | 12(7) | 0.007 |
| Number of high risk plaque features | 0.03 | ||
| 0 | 156(75) | 141(82) | |
| 1 | 42(20) | 22(13) | |
| 2 | 10(5) | 5(3) | |
| 3 | 1(0.5) | 0(0) |
Values are mean±SD or n (%).
Figure 2.
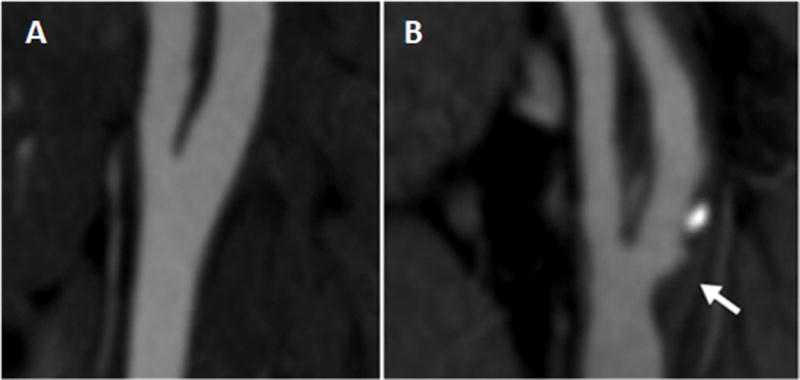
A CT neck image of the right carotid artery in a uninfected individual free of carotid plaque (A) as compared to a neck CT image from a PLWHIV with carotid plaque with a non-calcified component (B arrow)
Outcomes - ASCVD/Stroke
The primary outcome was an ASCVD event. Over a median follow up of 3 years, there were 17 ASCVD (ischemic stroke: 9, MI: 6, CHD death: 2) events among 209 PLWHIV and 2 ASCVD events (CHD death: 2) among uninfected controls (p=0.002). The secondary outcome of interest was ischemic stroke. There were 9 ischemic strokes among PLWHIV as compared to 0 strokes among controls (p=0.005). Among PLWHIV there were 10 ASCVD events among the 72 PLWHIV with carotid plaque (14%) as compared to 7 events among the 137 PLWHIV without plaque (5%, p=0.027). Similarly, among PLWHIV, there were 6 ischemic stroke events among the 72 PLWHIV (8%) with any carotid plaque as compared to 3 events among 137 PLWHIV (2%) without any plaque (p=0.046). Among PLWHIV, there were 8 ASCVD events among the 53 PLWHIV with any HRP (15%) as compared to 9 events among the 156 PLWHIV without HRP (6%, p=0.032) and there were 5 ischemic stroke events among the 53 PLWHIV (9%) with any HRP as compared to 4 events among 156 PLWHIV (2%) without any HRP (p=0.048).
Univariate and Multivariate Modeling for Outcomes
Entire cohort
Among the whole cohort, there was an unadjusted association between HIV status and ASCVD events (HR: 5.81, CI 1.34–25.2, p=0.02) and ischemic stroke and presence of any carotid plaque with ASCVD events (HR 3.32, CI 1.34–8.29, p=0.01) and ischemic stroke (HR: 4.89, CI 1.22–19.60, p=0.03). After adjustment for the number of CV risk factors, HIV status (HR: 5.22, CI 1.20–22.67, p=0.027) and carotid plaque (HR: 2.77, CI 1.08–7.12, p=0.03) were independent predictors of ASCVD events (Table 4).
Table 4.
Multivariate regression model for ASCVD events in PLWHIV vs. uninfected individuals
| Univariate analysis | p value | Multivariate analysis | p value | |
|---|---|---|---|---|
| HIV | 5.80 (1.34–25.15) | 0.01 | 5.22 (1.20–22.6) | 0.03 |
| Any carotid plaque | 3.32 (1.33–8.28) | 0.01 | 2.77 (1.08–7.12) | 0.03 |
| Number of CV factors* | 1.12 (0.70–1.77) | 0.62 |
number of CV risk factors include DM, HTN, HLD, current smoker and family history of CAD
PLWHIV
In analysis confined to PLWHIV, the presence of any carotid plaque was associated with an almost 3-fold increased risk of ASCVD events (HR: 2.91, CI 1.10–7.7, p=0.03) and a 4-fold increased risk of ischemic stroke (HR: 4.14, CI 1.03–16.6, p-0.04). In multivariate analysis confined to PLWHIV, after adjustment for CV risk factors, the presence of any carotid plaque was associated with an increased risk for ASCVD events (HR: 2.84, CI 1.05–7.63, p=0.03) and with a trend toward an increased risk for ischemic stroke (HR: 3.57, CI 0.86–14.7, p=0.07) (Table 5, Figure 3A and 3B). Also among PLWHIV, the presence of any HRP was associated with an unadjusted 3-fold increased risk for ASCVD events (HR: 3.02, CI 1.15–7.88, p=0.02) and a 4-fold increased risk of stroke (HR: 4.43, CI 1.17–16.70, p=0.02). In multivariate analysis, after adjustment for number of CV risk factors, the presence of any HRP feature remained associated with an increased risk of ASCVD events (HR: 2.93, CI 1.11–7.77, p=0.03) and with an increased risk of stroke (HR: 3.89, CI 1.01–14.89, p=0.04) (Table 6, Figure 4A and 4B). We also evaluated the association of non-calcified plaque and events: the presence of non-calcified plaque was associated with an unadjusted 3-fold increased risk for ASCVD events (HR: 3.21, CI 1.22–8.45, p=0.02) and stroke (HR: 3.71, CI 0.992–13.85, p=0.051). In multivariate analysis the presence of any non-calcified plaque remained associated with an increased risk of ASCVD events (HR: 3.36, CI 1.26–8.91, p=0.02) and an independent association with stroke (HR: 4.65, CI 1.17–18.53, p=0.03).
Table 5.
Multivariate regression model for carotid plaque and outcomes in PLWHIV
| ASCVD | ||||
|---|---|---|---|---|
|
| ||||
| Univariate analysis | p value | Multivariate analysis | p value | |
| Any carotid plaque | 2.91(1.10–7.67) | 0.03 | 2.84 (1.05–7.63) | 0.03 |
| Number of CV factors* | 1.06 (0.64–1.75) | 0.79 | ||
|
| ||||
| Stroke/TIA | ||||
|
| ||||
| Any carotid plaque | 4.14 (1.03–16.6) | 0.04 | 3.57 (0.86–14.7) | 0.07 |
| Number of CV factors* | 1.42 (0.79–2.54) | 0.23 | ||
number of CV risk factors include DM, HTN, HLD, current smoker and family history of CAD
Figure 3.
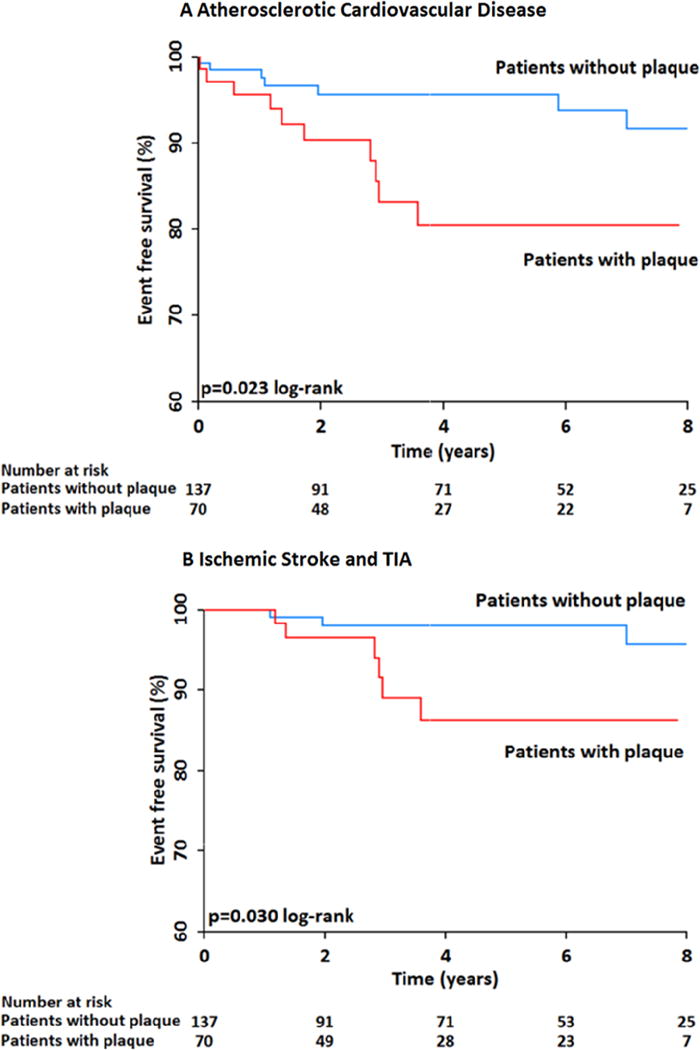
A: Kaplan-Meier event free survival curve for ASCVD events in PLWHIV with and without any carotid plaque showing that PLWHIV with any carotid plaque have a significantly increased risk for ASCVD events as opposed to those without plaque.
B: Kaplan-Meier event free survival curve for ischemic stroke in PLWHIV with and without any carotid plaque showing that PLWHIV with carotid plaque have an increased risk for stroke as opposed to those without plaque
Table 6.
Multivariate regression model for high risk plaque and outcomes in PLWHIV
| ASCVD | ||||
|---|---|---|---|---|
|
| ||||
| Univariate analysis | p value | Multivariate analysis | p value | |
| Any high risk plaque | 3.02 (1.15–7.88) | 0.024 | 2.93 (1.11–7.77) | 0.03 |
| Number of CV factors* | 1.08(0.65–1.79) | 0.74 | ||
|
| ||||
| Stroke/TIA | ||||
|
| ||||
| Any high risk plaque | 4.43 (1.17–16.70) | 0.02 | 3.89 (1.01–14.89) | 0.04 |
| Number of CV factors* | 1.47 (0.81–2.68) | 0.204 | ||
number of CV risk factors include DM, HTN, HLD, current smoker and family history of CAD
Figure 4.
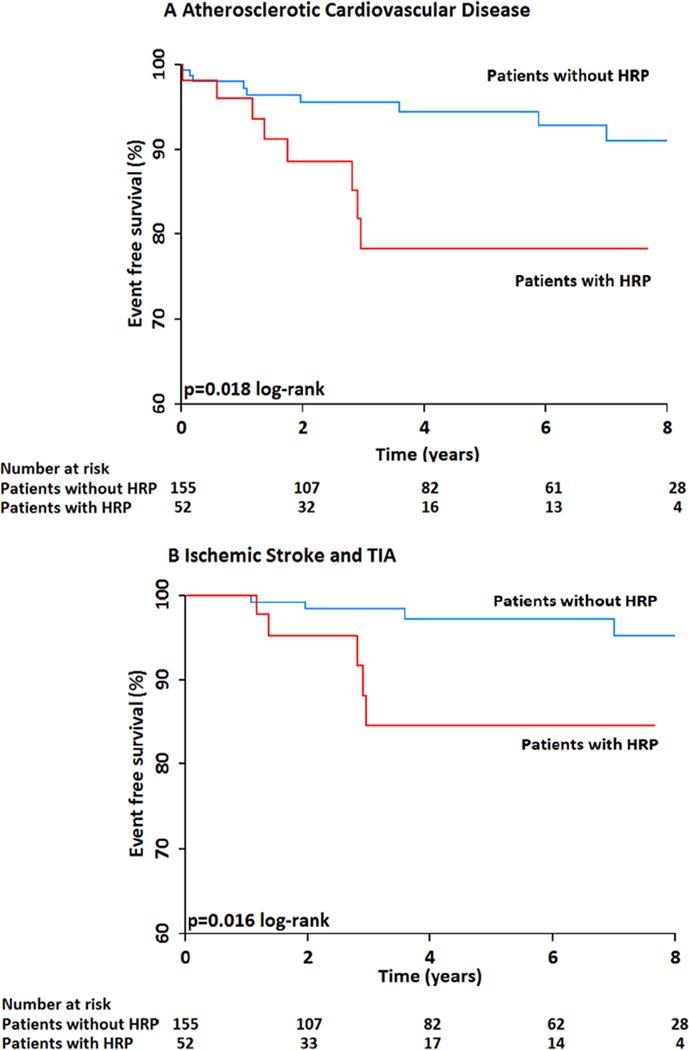
A: Kaplan-Meier event free survival curve for ASCVD events in PLWHIV with and without high-risk morphology carotid plaque showing that PLWHIV with any high risk plaque are at an increased risk for ASCVD events as opposed to those without any high risk plaque.
B: Kaplan-Meier event free survival curve for ischemic stroke in PLWHIV with and without high-risk morphology carotid plaque showing that HIV infected individuals with any high risk plaque have an increased risk for ischemic stroke as opposed to those without any high risk plaque.
Discussion
Leveraging data from a large US health care system, we tested, using CT, whether HIV status was associated with an increase in incidental carotid plaque, whether HIV status was associated with high risk and non-calcified plaque and whether incidental carotid plaque and plaque characteristics were associated with subsequent vascular events among PLWHIV. In a cohort free of known vascular disease, we found that HIV status was associated with an increased rates of incidental carotid plaque, non-calcified plaque and HRP as compared to uninfected controls. Additionally, in follow-up, among PLWHIV the presence of carotid plaque, non-calcified plaque and HRP was associated with an increased risk of both any atherosclerotic event and stroke after adjustment for CV risk factors.
Our hypothesis that there would be an increase in sub-clinical carotid plaque in HIV was based on prior data from several groups showing an increase in sub-clinical coronary plaque in HIV20, 21. For example, Lo et al showed in a study of 78 men living with HIV without a history of vascular disease, that incidental coronary atherosclerosis by CT was almost two fold higher in men living with HIV as compared to demographic and risk-factor uninfected men (59 vs. 34%)20. Based on these findings, we hypothesized that there would be an increase in sub-clinical carotid plaque by CT among PLWHIV free of known vascular disease. The hypothesis was also supported by robust data using ultrasound for detection of carotid plaque in HIV and data from other inflammatory diseases associated with an increased risk of vascular events22, 23. For example, in a study of 1,822 PLWHIV using serial ultrasound, Hanna et al showed that PLWHIV had a 61% greater risk of new focal carotid artery plaque formation over seven years compared with uninfected controls, regardless of baseline vascular phenotype and after controlling for cardiometabolic risk factors22. There are also significant data in other diseases that share pathophysiological overlap with HIV. Specifically, the mechanisms of increased vascular risk in HIV are likely related to immune activation and an increase in inflammation24, 25 and in other diseases where an immune activation and inflammation play a key pathophysiological role, an increase in incidental carotid plaque has been reported26, 27. For example, in rheumatoid arthritis and diabetes, data have described an increase in incidental carotid plaque28, 29. We extend these findings to HIV and provide, to our knowledge, the first study characterizing carotid plaque by CT in HIV and demonstrate an increased number of incidental carotid artery plaque in PLWHIV as compared to a uninfected cohort.
Given its spatial resolution, CT is an excellent technique for characterizing atherosclerotic plaque composition and data using CT in broad populations, has shown that the association between atherosclerotic plaque in the coronaries and adverse outcomes is markedly influenced by plaque composition30, 31. Among PLWHIV, data using CT have shown that there is a marked increase in the prevalence of both non-calcified plaque32 and HRP in coronary arteries of PLWHIV8, 33. For example, Post et al, showed in a study of 618 PLWHIV that the prevalence of any non-calcified coronary plaque was 63% as compared to 53% in uninfected controls21. Similar, D’Ascenzo et al, in a meta-analysis of 9 studies (1229 PLWHIV and 1029 controls), showed that the rates of non-calcified plaque were >3-fold higher in PLWHIV (58% vs. 17%)32. Prior to this current work, there were no data testing the prognostic significance of non-calcified or high-risk plaque in the carotid arteries among PLWHIV. However, there are data testing the prevalence and prognostic significance of non-calcified and high-risk plaque in the carotid in non-HIV populations34. For example, Homburg et al evaluated the associations between carotid plaque ulceration and plaque characteristics in ischemic stroke patients and found that a lipid-rich core (non- calcified plaque) was associated with plaque ulceration, whereas calcification (plaque stability) was inversely associated with plaque ulceration. We extend these prior observations to PLWHIV and find that PLWHIV have an increase number of non-calcified carotid plaque and HRP and that these plaque characteristics are associated with an increase risk of subsequent clinical vascular events.
Data have established that the risk of vascular events is increased in HIV5. For example, Chow et al, showed that the incidence rate of ischemic stroke was 5.27 per 1000 person years in PLWHIV compared with 3.75 in un-infected patients (adjusted HR of 1.40) and that HIV remained an independent predictor of stroke after controlling for demographics and stroke risk factor1. Additionally, studies in broad cohorts have demonstrated that the presence of carotid plaque is associated with an increased incidence of vascular events. For example, Gepner et al reported that in 6779 subjects without prior CV disease from the Multi- Ethnic Study of Atherosclerosis (MESA) cohort, carotid plaque presence by carotid ultrasound (c=0.782 to c=0.787; P=0.045) marginally improved prediction of stroke/TIA, above the traditional CV risk factors35. However, no prior study has connected carotid plaque to vascular events and stroke specifically in HIV. In this study, consistent with prior data, the baseline risk of a vascular event was higher in PLWHIV as compared to a uninfected cohort and, additively, we show that the adjusted risk for a vascular event in PLWHIV with carotid plaque or high-risk plaque was about 3-fold higher than those without plaque.
Strengths of our study include the first ever CT-based assessment of the presence of incidental carotid plaque in HIV, as well as first reporting on quantitative and qualitative plaque carotid analysis in relation to events in this population. However, some limitations merit discussion. This was a retrospective study and HIV-specific parameters were not recorded at the time of the CT but were gathered from the electronic medical record at a time close to the study. All efforts were made to collect the viral load assay data closest to the date well. We suspect that the majority of patients in whom detectable viral load was noted were experiencing “blips” – temporary low level, detectable viremia observed in the context of transient ART non-adherence. We consider it likely that transient non-adherence with ART is relatively common in this cohort, as it is in other contemporary cohorts. Indeed, a recent investigation published by the Swiss HIV Cohort team suggested that blips can be observed in up to 41% of HIV-infected patients to whom ART is prescribed. Since uninfected controls were generated from the original query, there may be an element of referral/selection bias. Additionally, cocaine use was only available for 65% of the subjects and its interpretation as significantly different between the cases and controls should be done with caution and the observational nature precludes definitive determinations of causal drivers of outcomes. We also acknowledge that the type of CT was not pre-specified and CT scans included arterial and venous phase CT studies, which differ in spatial resolution. However, CTA scans, with their higher resolution for plaque analysis, were more prevalent in the uninfected cohort.
Conclusions
PLWHIV without prior vascular disease have an increased number of carotid plaque, non-calcified plaque and HRP as compared to uninfected controls and in follow-up the presence of carotid plaque, non-calcified plaque and HRP is associated with an increase risk of adverse vascular outcomes. Further research is needed to identify the underlying mechanisms, both general or HIV-specific, contributing to this increased risk and whether interventions, such as statins can reduce risk in this vulnerable population.
Clinical Perspective.
Our study aimed to provide insight into the prevalence, characteristics and prognostic associations of CT-assessed carotid plaque among persons living with HIV (PLWHIV) free of known vascular disease as compared to uninfected individuals. Using a large established and validated registry, we show that, as compared to uninfected controls, PLWHIV have an increased prevalence of carotid plaque, non-calcified plaque and high-risk plaque. In the overall cohort, HIV status and any carotid plaque were independent predictors of cardiovascular and cerebrovascular events. Within PLWHIV, the presence and composition of carotid plaque was associated with a 3–4 fold increase in adverse vascular outcomes. This is the first study, to our knowledge, to report CT-based assessment of presence of carotid plaque in HIV, as well as reporting on quantitative and qualitative carotid plaque analysis with relation to events in this population. With this data we provide evidence of sub-clinical high risk-plaque in PLWHIV that is associated with worse cardiovascular outcomes as compared to uninfected controls. Although this is an observational data set and randomized control trials are needed to delineate causality, this data can help clinicians in identifying high risk PLWHIV and provide aggressive risk factor modification.
Acknowledgments
Sources of Funding
Dr. Janjua was supported by NIH/National Heart, Lung, and Blood Institute 5T32HL076136. Dr. Neilan has the following support: The Kohlberg Foundation, an American Heart Association Fellow to Faculty Award (12FTF12060588), NIH/National Heart, Lung, and Blood Institute (1R01HL130539-01A1; 1R01HL137562 - 01A1) and NIH/Harvard Center for Acquired Immune Deficiency Syndrome Research (P30 AI060354).
Footnotes
Disclosures
Dr Grinspoon is a consultant for Gilead and Bristol-Myers Squibb (modest). All other co-authors have no disclosures of relevance.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, McGinnis K, Kuller LH, Kraemer KL, Rimland D, Bidwell Goetz M, Butt AA, Rodriguez-Barradas MC, Gibert C, Leaf D, Brown ST, Samet J, Kazis L, Bryant K, Freiberg MS, Veterans Aging Cohort S HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933–40. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus JL, Leyden WA, Chao CR, Chow FC, Horberg MA, Hurley LB, Klein DB, Quesenberry CP, Jr, Towner WJ, Silverberg MJ. HIV infection and incidence of ischemic stroke. AIDS. 2014;28:1911–9. doi: 10.1097/QAD.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 4.D’Ascenzo F, Cerrato E, Biondi-Zoccai G, Moretti C, Omede P, Sciuto F, Bollati M, Modena MG, Gaita F, Sheiban I. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J. 2012;33:875–80. doi: 10.1093/eurheartj/ehr456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow FC. HIV infection, vascular disease, and stroke. Semin Neurol. 2014;34:35–46. doi: 10.1055/s-0034-1372341. [DOI] [PubMed] [Google Scholar]
- 6.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–7. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 7.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, Oh J, Zimmerman CO, Hwang J, Abbara S, Plutzky J, Robbins G, Tawakol A, Hoffmann U, Grinspoon SK. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263–72. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suchindran S, Regan S, Meigs JB, Grinspoon SK, Triant VA. Aspirin Use for Primary and Secondary Prevention in Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Patients. Open Forum Infect Dis. 2014;1:ofu076. doi: 10.1093/ofid/ofu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow FC, He W, Bacchetti P, Regan S, Feske SK, Meigs JB, Grinspoon SK, Triant VA. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83:1705–11. doi: 10.1212/WNL.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. Journal of acquired immune deficiency syndromes. 2012;60:351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LJ, Ismail A, McMeekin W, Lambert D, Mendelow AD, Birchall D. Computed tomography angiography for the evaluation of carotid atherosclerotic plaque: correlation with histopathology of endarterectomy specimens. Stroke. 2002;33:977–81. doi: 10.1161/01.str.0000013562.73522.82. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Baradaran H, Kamel H, Pandya A, Mangla A, Dunning A, Marshall RS, Sanelli PC. Evaluation of computed tomography angiography plaque thickness measurements in high-grade carotid artery stenosis. Stroke. 2014;45:740–5. doi: 10.1161/STROKEAHA.113.003882. [DOI] [PubMed] [Google Scholar]
- 17.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice G. 2013;14 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council CoCS, Anesthesia, Council on Cardiovascular R, Intervention, Council on C, Stroke N, Council on E, Prevention, Council on Peripheral Vascular D, Council on Nutrition PA and Metabolism An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirwan BA, Lubsen J, de Brouwer S, Danchin N, Battler A, Bayes de Luna A, Dunselman PH, Glasser S, Koudstaal PJ, Sutton G, van Dalen FJ, Poole-Wilson PA, investigators A Diagnostic criteria and adjudication process both determine published event- rates: the ACTION trial experience. Contemp Clin Trials. 2007;28:720–9. doi: 10.1016/j.cct.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, Nasir K, Grinspoon SK. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Post WS, George RT, Budoff M. HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;161:923–4. doi: 10.7326/L14-5033-2. [DOI] [PubMed] [Google Scholar]
- 22.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, Anastos K, Gange SJ, Landay AL, Lazar JM, Palella FJ, Tien PC, Witt MD, Xue X, Young MA, Kaplan RC, Kingsley LA. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin Infect Dis. 2015;61:640–50. doi: 10.1093/cid/civ325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 24.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Kalapus SC, Deeks S, Sereti I, Hsue PY. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. AIDS. 2016;30:2065–74. doi: 10.1097/QAD.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbour JD, Jalbert EC, Chow DC, Gangcuangco LM, Norris PJ, Keating SM, Heitman J, Nagamine L, Seto T, Ndhlovu LC, Nakamoto BK, Hodis HN, Parikh NI, Shikuma CM. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis. 2014;232:52–8. doi: 10.1016/j.atherosclerosis.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao AH, Lertratanakul A, Elliott JR, Sattar A, Santelices L, Shaw P, Birru M, Avram Z, Thompson T, Sutton-Tyrrell K, Ramsey-Goldman R, Manzi S. Relation of carotid intima-media thickness and plaque with incident cardiovascular events in women with systemic lupus erythematosus. Am J Cardiol. 2013;112:1025–32. doi: 10.1016/j.amjcard.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlandi M, Suvan J, Petrie A, Donos N, Masi S, Hingorani A, Deanfield J, D’Aiuto F. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236:39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, Pincus T, Raggi P, Gebretsadik T, Shintani A, Stein CM. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–5. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostling G, Hedblad B, Berglund G, Goncalves I. Increased echolucency of carotid plaques in patients with type 2 diabetes. Stroke. 2007;38:2074–8. doi: 10.1161/STROKEAHA.106.480830. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez-Figueroa JG, Rinehart S, Qian Z, Joshi PH, Sharma A, Lee J, Anderson H, Murrieta L, Wilmer C, Carlson H, Taylor K, Ballard W, Karmpaliotis D, Kalynych A, Brown C, 3rd, Voros S. Prospective validation that vulnerable plaque associated with major adverse outcomes have larger plaque volume, less dense calcium, and more non-calcified plaque by quantitative, three-dimensional measurements using intravascular ultrasound with radiofrequency backscatter analysis : results from the ATLANTA I Study. J Cardiovasc Transl Res. 2013;6:762–71. doi: 10.1007/s12265-013-9473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–26. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 32.D’Ascenzo F, Cerrato E, Calcagno A, Grossomarra W, Ballocca F, Omede P, Montefusco A, Veglia S, Barbero U, Gili S, Cannillo M, Pianelli M, Mistretta E, Raviola A, Salera D, Garabello D, Mancone M, Estrada V, Escaned J, De Marie D, Abbate A, Bonora S, Zoccai GB, Moretti C, Gaita F. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis. 2015;240:197–204. doi: 10.1016/j.atherosclerosis.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, Wai B, Hoffmann U, Abbara S, Grinspoon S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164–71. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homburg PJ, Rozie S, van Gils MJ, van den Bouwhuijsen QJ, Niessen WJ, Dippel DW, van der Lugt A. Association between carotid artery plaque ulceration and plaque composition evaluated with multidetector CT angiography. Stroke. 2011;42:367–72. doi: 10.1161/STROKEAHA.110.597369. [DOI] [PubMed] [Google Scholar]
- 35.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]


