Abstract
Hypertension is considered a powerful cardiovascular risk factor and is present in up to two-thirds of patients who suffer from diabetes. In the background of an established epidemiological association between lower blood pressure (BP) and improvement in long-term clinical outcomes, several large landmark trials and analyses have attempted to examine the possible benefit of tighter BP control in patients with Type 2 diabetes mellitus. Although aggressive BP targets in patients with diabetes have been advocated for a long time, currently accepted evidence from these studies has led to a general recommendation of systolic BP <140 mmHg and diastolic BP <90 mmHg. Therapy consists of lifestyle management, including weight loss if overweight or obese, a Dietary Approaches to Stop Hypertension (DASH)-style based nutrition counselling, and reduced sodium intake. Timely initiation and subsequent titration of antihypertensive medications to achieve individualised BP goals is recommended. A therapeutic agent that acts on the renin-angiotensin-aldosterone pathway, such as an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, should generally be included in the pharmacologic therapy for hypertension in patients with Type 2 diabetes mellitus. A multi-drug combination, particularly including a thiazide diuretic, is very often necessary and should be started early in the course of management. Finally, an accurate and standardised method of BP measurement in the outpatient setting is essential to ensure proper monitoring and gauge the effectiveness of treatment.
Keywords: Type 2 diabetes mellitus (T2DM), hypertension, blood pressure (BP), cardiovascular risk
INTRODUCTION
Patients with diabetes mellitus (DM) are at risk of adverse cardiovascular (CV) outcomes, including microvascular and atherosclerotic complications. In Type 2 diabetes mellitus (T2DM), a clustering of CV risk factors, very often with underlying insulin resistance, leads to a propensity for increased morbidity and mortality. The American Diabetes Association (ADA) has revised its general blood pressure (BP) goals in persons with DM to <140/90 mmHg, additionally advising that “lower systolic targets, such as <130 mmHg, may be appropriate for certain individuals with DM, such as younger patients, those with albuminuria, and/or those with hypertension and one or more additional atherosclerotic CV disease risk factors, if they can be achieved without undue treatment burden.”1 The optimal therapeutic approach involves both lifestyle measures, consisting mainly of dietary modification, weight loss, and restricting salt ingestion, and the evidence-based use of an individualised regimen of antihypertensive drug treatment. Close follow-up, timely modification of therapy, and active BP management in patients with DM has been shown to be beneficial; however, treatment can be challenging in the long run. In the clinical setting, healthcare professionals are frequently faced with the key question of what approach would be best to reduce the possibility of future CV events, morbidity, and mortality. Herein is a review of the significance and management of hypertension in individuals with T2DM.
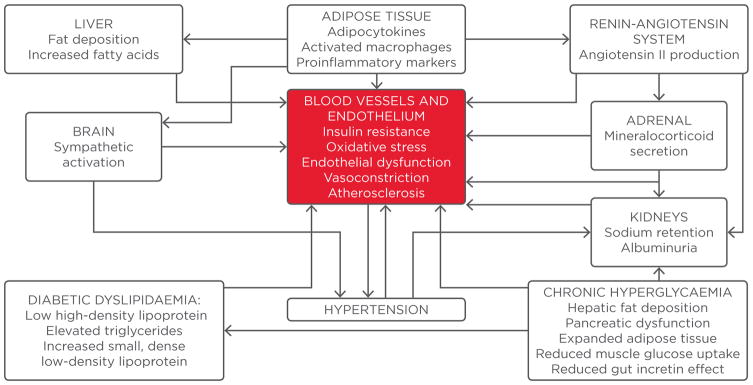
PATHOGENESIS OF INCREASED CARDIOVASCULAR RISK IN TYPE 2 DIABETES MELLITUS AND HYPERTENSION: ROLE OF THE KIDNEYS AND ENDOTHELIUM
The presence of hypertension in individuals with DM is a strong determinant of atherosclerotic disease, endothelial inflammation, and vascular damage. Statistics show that almost 40% of individuals with T2DM are already hypertensive at diagnosis, a situation that is very often accompanied by obesity and a higher risk of developing CV disease.2 In contrast, most patients with Type 1 diabetes mellitus (T1DM) do not have hypertension when diagnosed with DM.3 The development of essential hypertension and complications from target organ damage, in particular nephropathy, is thought to be responsible for the increase in prevalence with longer duration of DM.
The kidneys and the cardiovascular system are inextricably intertwined as determinants of ambient BP levels in both normal and diseased conditions. The earliest detectable pathologic increase in urinary albumin excretion, termed ‘moderately increased albuminuria’ (urinary albumin loss of 30–300 mg/24 hours),4 results from DM as well as hypertension, and the presence of both conditions is multiplicative in its emergence. In a bidirectional manner, the incidence and severity of hypertension increases with the emergence and progression of nephropathy. The complex interplay of hypertension and renal disease appears to be especially evident in persons with DM, who are inherently at high risk for progressive glomerular damage (Figure 1). Eighty-five percent of patients with overt diabetic nephropathy have hypertension.5 Additionally, increased extracellular volume results from sodium retention secondary to hyperfiltration of glucose; the reabsorption of both is increased because of upregulation of the sodium-glucose cotransporter enzyme in the proximal tubule.3,6 The resultant elevation in BP due to volume expansion tends to be exacerbated by salt intake and is responsive to sodium restriction. Advanced glycosylated end-products have a direct correlation with chronic hyperglycaemia and, together with atherosclerotic manifestations, contribute to reduced arterial pliability.7 The ensuing changes in blood vessels increase vascular stiffness, particularly resulting in a rise in the systolic BP. Endothelial dysfunction and increased oxidative stress are believed to play a pathologic role in both hypertension and diabetes early in their natural history, increasing the risk of atherothrombosis.8 Central sympathoadrenal activation is especially evident in hypertensive states. The roles of adipose tissue as a proinflammatory organ and the characteristic ‘diabetic dyslipidaemia’ further contribute to endovascular toxicity in a vicious cycle. In summary, the coexistence of both DM and hypertension combine to multiply the risk of the development, as well as progression, of nephropathy, while concurrently instigating endothelial damage and elevating the risk for adverse CV outcomes through multiple mechanisms.
Figure 1.
Multiple, interlinked pathophysiologic mechanisms that increase the risk of cardiovascular complications in hypertension and diabetes.
BLOOD PRESSURE CONTROL IN INDIVIDUALS WITH DIABETES MELLITUS: A REVIEW OF THE EVIDENCE
Insights into the significance of BP management in subjects with DM have been derived from trials designed primarily to examine glycaemic control, and from analysis of CV outcomes in subsets of DM subjects in hypertension studies. The data concerning BP management in patients with DM are notable for their heterogeneity and lack of uniformity. In general, the preponderance of evidence demonstrates that patients treated to lower BP targets have a reduced propensity to vascular events and lower rates of development and progression of microvascular complications, such as diabetic nephropathy. The benefits, although clinically important, were achieved with combination therapy with multiple medications, and were accompanied with increased risk of drug-induced side effects. Major trials pertaining to the significance of BP in patients with DM are summarised in Table 1, and what follows is a brief description of the prominent clinical studies that have contributed to our current understanding of the subject.
Table 1.
A summary of the clinical trial data on blood pressure and diabetes mellitus.
| RESULTS OF CLINICAL STUDIES | |
|---|---|
| Clinical Trial | Results |
| HOT9 | Significant reduction in CV events in DM subjects with systolic goal <80 versus <90 mmHg |
| UKPDS10 | Lower BP resulted in lower DM-related mortality, stroke, and microvascular complications |
| ABCD13 | A significant reduction in stroke, but no difference in composite CV events with more aggressive antihypertensive therapy |
| ADVANCE14 | Decreased microvascular and CV events and all-cause mortality in the lower BP group |
| SANDS15 | No difference in clinical CV events between the standard and intensive treatment groups |
| ACCORD BP16 | Reduction in stroke and more side effects in the intensive arm versus the standard arm |
| HOPE-318 | Statin use, but not BP lowering, was associated with CV risk reduction |
| ACCORDION17 | Observational 9-year follow-up showed that the difference in BP and stroke risk was no longer sustained |
| CONCLUSIONS FROM LARGE META-ANALYSES | |
| Study | Results |
| McBrien et al.19 | BP lowering in patients with diabetes significantly lowered the incidence of stroke |
| Emdin et al.20 | Antihypertensive therapy significantly reduced the rates of mortality, total CV disease, myocardial infarction, and stroke compared with placebo |
| Xie et al.21 | Significant reduction in major CV events with more intensive as compared with less intensive BP lowering |
BP: blood pressure; CV: cardiovascular; DM: diabetes mellitus.
In the HOT trial,9 the diastolic pressures in almost 19,000 participants were targeted to ≤90, ≤85, or ≤80 mmHg; the achieved average values were 144/85, 141/83, and 140/81 mmHg, respectively. In the subset of 3,000 patients with DM, but not in other patients, the relative risk (RR) of a CV event was significantly reduced in the ≤80 mmHg group compared to the ≤90 mmHg group (RR: 0.49; 95% confidence interval [CI]: 0.29–0.81).
The landmark UKPDS10 studied 1,148 patients with T2DM with a mean baseline BP of 160/94 mmHg. Compared to the standard arm (<180/105, achieved BP 154/87 mmHg), patients assigned to a lower BP target (<150/85, achieved BP 144/82 mmHg) had a 32% reduction in DM-related mortality (24% versus 35%), a 44% reduction in stroke, and a 24% reduction in microvascular disease after >8 years. There was no difference in outcomes between captopril and atenolol as the primary therapy. The benefits were not sustained and were lost within 2 years of post-trial observational monitoring.11 Follow-up a decade later indicated that each 10 mmHg reduction in systolic pressure was associated with a 12% risk reduction; the lowest risk occurred at a systolic pressure <120 mmHg.12 However, since the UKPDS was not designed to assess the usefulness of systolic BP <140 mmHg, cause-and-effect conclusions could not be made.
The normotensive ABCD trial13 enrolled close to 500 patients with T2DM into a moderate control (placebo) arm or an intensive arm with a target diastolic BP 10 mmHg below the initial baseline level, using either enalapril or nisoldipine. At 5 years, mean attained BP for the moderate and intensive control groups were 137/81 and 128/75 mmHg, respectively. Glomerular filtration rates showed no difference, whereas intensive BP control slowed progression of retinopathy and albuminuria. Apart from a significant reduction in stroke, there was no difference in composite CV events with more aggressive antihypertensive therapy.
An important study was the ADVANCE trial,14 comparing the use of a perindopril/indapamide fixed combination as antihypertensive treatment in patients with T2DM of long duration who were at high risk of vascular complications. The baseline BP was 145/81 mmHg and no BP goal was aimed for. Over 11,000 patients were studied and a placebo arm was included. The mean BP values were 134.5/74 versus 140/76 mmHg after 4 years. The intensively treated group had fewer macro and microvascular events and decreased CV mortality (3.8% versus 4.6%), as well as all-cause mortality (7.3% versus 8.5%). Taking into account a post-trial observational phase of 6 years, all-cause mortality was significantly lower among those in the lower BP group.
Aggressive BP lowering seems to have a beneficial effect on surrogate markers of morbidity and end-organ changes. In this respect, the SANDS trial15 was conducted on 499 Native American men and women with T2DM without prior history of CV disease, who were randomised to either a standard arm or an intensively-treated arm with regard to BP and low-density lipoprotein (LDL) cholesterol. At 3 years, the mean attained systolic BP was 117 and 129 mmHg in the aggressive and standard groups, respectively, with more adverse events related to antihypertensive drugs in the former. Although there was no difference in clinical events (1.6 versus 1.5 per 100 person-years), intensive therapy was associated with slower progression of atherosclerosis and a greater reduction in left ventricular mass.
The unresolved issue of safety and benefits of lowering the systolic BP to <120 mmHg were specifically addressed in the ACCORD BP trial.16 The results of this trial were destined to impact the formulation of BP guidelines in DM subjects. Patients with T2DM (n=4,733) who had established CV disease or at least two additional CV risk factors were randomly assigned to either a goal systolic BP <120 mmHg or <140 mmHg. The mean attained BPs in the two groups after 4.7 years of follow-up were 119.3 and 133.5 mmHg, respectively, compared to 139/76 mmHg at baseline. The primary composite outcome of non-fatal myocardial infarction, non-fatal stroke, or death from CV causes was comparable between the intensive versus standard therapy groups (1.87% versus 2.09%; hazard ratio [HR]: 0.88; 95% CI: 0.73–1.06), as was the annual all-cause mortality rate between intensive and standard therapy groups (1.28% versus 1.19%) and the rate of death from CV causes (0.52% versus 0.49%). Interestingly, intensive therapy was associated with significant reductions in the annual rates of total stroke and nonfatal stroke (0.32% versus 0.53%; HR: 0.59; 95% CI: 0.39–0.89, for total stroke; and 0.3% versus 0.47%; HR: 0.63; 95% CI: 0.41–0.96, for nonfatal stroke). The intensive group experienced more serious adverse events, including hypotension, syncope, bradycardia or arrhythmia, hyperkalaemia, angioedema, and renal failure, and an increase in serum creatinine >1.5 mg/dL. In summary, ACCORD studied BP management in patients with T2DM at high CV risk, essentially revealing a reduction in cerebrovascular events and more drug side effects in the intensive versus the standard treatment group. A subsequent 9-year follow-up of the ACCORD BP subjects, termed ACCORDION,17 demonstrated a significant interaction between glucose and BP control. The intensive treatment group had a significant 21% reduction in the primary endpoints compared to the standard group. Interestingly, the initial favourable reduction in stroke rate in the intensive-treatment group in ACCORD was absent in ACCORDION; it is worth noting that the original BP difference between the two groups no longer remained.
The HOPE-3 study18 was a primary CV prevention trial in 12,705 intermediate-risk individuals. While not specifically a trial of DM, a major finding was that lowering LDL cholesterol by approximately 35 mg/dL with rosuvastatin significantly decreased the primary outcome (CV related death, non-fatal stroke, or non-fatal myocardial infarction) in comparison to placebo. The combination of candesartan/hydrochlorothiazide, which lowered BP by 6 mmHg systolic and 3 mmHg diastolic compared to placebo, did not significantly lower the primary outcome measure. The largest benefit (4.8% versus 6.5%) was observed in the subgroup with the highest systolic BP measuring 143 mmHg at baseline.
A combined analysis of three of the previously mentioned trials (ACCORD BP, ABCD, and HOT) suggested that intensive BP lowering in patients with DM significantly lowered the incidence of stroke (2.0% versus 3.1%), but not mortality (5.5% versus 6.3%) or myocardial infarction (7.9% versus 8.5%).19 A recent meta-analysis of 40 trials examined the effects of antihypertensive therapy in studies that ranged in duration from 6 months to 8 years. In >100,000 subjects with DM, antihypertensive therapy significantly reduced the rates of mortality, total CV disease, myocardial infarction, and stroke compared with placebo.20 However, the benefit was seen only in those with initial systolic pressures >140 mmHg; in these subjects, a 10 mmHg reduction was associated with a HR for death of 0.87 (95% CI: 0.78–0.96) and for total CV disease of 0.89 (95% CI: 0.83–0.95). Among those with lower initial systolic pressures, therapy reduced the risk of stroke only. Whereas beta-blocker use was associated with an increased the risk of stroke compared with other agents (RR: 1.25; 95% CI: 1.05–1.50), calcium channel blockers decreased it in comparison with other agents (RR: 0.86; 95% CI: 0.97–0.77). It is noteworthy that, in general, no single class of drugs showed clear advantages over others for most clinical outcomes.
Finally, a meta-analysis of 19 BP trials, including 5 that included patients with DM, with a combined 44,989 individuals, found a significant reduction in major CV events with more intensive as compared to less intensive BP lowering (RR: 0.86; 95% CI: 0.78–0.96).21 The effect of intensive BP lowering in the five trials of DM patients was similar (RR: 0.83; 95% CI: 0.71–0.96) to other trials. All-cause mortality was also lower with intensive treatment, although it was not statistically significant (RR: 0.91; 95% CI: 0.81–1.03).
WHAT SHOULD BE THE BLOOD PRESSURE GOALS IN PATIENTS WITH DIABETES?
For many years, the recommended BP goal in persons with diabetes was <130/80 mmHg,22,23 based on the assumption that lower goals may slow the rate of progression of diabetic nephropathy and proteinuria.24 The treatment of hypertension in DM patients was associated with significant clinical benefits in the HOT,9 UKPDS,10 and ADVANCE trials,14 as detailed previously. However, although these observations support a goal BP for DM patients of <140/90 mmHg, as recommended in the majority of patients with hypertension in general, lower targets of <130/80 mmHg were not clearly justified by available data. In fact, the results from ACCORD BP argue against the presumption that ‘lower is better’.16,17 The SPRINT25 findings suggest that, in non-DM patients, early use of lower goals may result in benefits, despite the increased risk of side effects and adverse events. Individualisation of therapy, taking into account the risks and benefits and using clinical judgement, is sensible.26
BLOOD PRESSURE MANAGEMENT IN INDIVIDUALS WITH DIABETES MELLITUS: A RATIONAL THERAPEUTIC APPROACH
Lifestyle-based interventions as the pillar of early, as well as ongoing, treatment of hypertension is particularly important in patients with DM, both to prevent CV disease and to minimise progression of nephropathy and retinopathy.27,28 Non-pharmacologic methods include weight reduction; dietary modifications that increase consumption of fresh fruits, vegetables, and low-fat dairy products; physical activity; avoidance of processed foods that are high in sodium content; and avoidance of tobacco and excess alcohol intake. The ADA guidelines advise that among patients with a systolic BP of 120–139 mmHg, or a diastolic pressure of 80–89 mmHg, lifestyle changes and primarily non-drug modalities should be introduced to reduce BP (Table 2).1
Table 2.
Recommendations for management of hypertension in patients with diabetes, based on the recommendations from the American Diabetes Association (ADA) Standards of Medical Care.1
| BP Goals | Patients with DM and hypertension should be treated to systolic and diastolic BP goals of 140 mmHg and <90 mmHg, respectively. Lower targets, such as <130/<80 mmHg, may be appropriate in younger patients, those with albuminuria, and/or those with hypertension and one or more additional atherosclerotic CV disease risk factors, provided this can be achieved without undue treatment burden and side-effects. |
| Caution in older adults | Pharmacologic therapy to achieve treatment goals of <130/70 mmHg is not recommended; treating to systolic BP <130 mmHg has not been shown to improve CV outcomes and treating to diastolic BP <70 mmHg has been associated with higher mortality. |
| Non-pharmacologic Interventions | Lifestyle therapy for elevated BP consists of weight loss, if overweight or obese; a DASH-style dietary pattern, including reducing sodium and increasing potassium intake; moderation of alcohol intake; and increased physical activity. |
| Drug therapy | Patients with confirmed office-based BP >140/90 mmHg should, in addition to lifestyle therapy, have early initiation and timely subsequent titration of pharmacologic therapy to achieve BP goals. |
| Choice of antihypertensive agents | Pharmacologic therapy for patients with DM and hypertension should comprise a regimen that includes either an ACE-I or an ARB, but not both. If one class is not tolerated, the other should be substituted. These two classes of drugs should be especially considered in patients with evidence of nephropathy and/or heart failure. |
| Multi-drug therapy | A combination of a thiazide diuretic and ACE-I/ARB at maximal doses, a calcium-channel blocker, or a beta-blocker is generally required to achieve BP targets. |
| Individualisation of treatment | The choice of initial agent as well as subsequent combinations should be based on individual patient characteristics, preferences, potential side-effects, and cost. |
ACE-I: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BP: blood pressure; CV: cardiovascular; DASH: Dietary Approaches to Stop Hypertension; DM: diabetes mellitus.
Based on available evidence, patients with DM and persistent BP readings >140/90 mmHg should be started on antihypertensive drug therapy.29,30 These data are clear that drug therapy in hypertensive DM patients is effective in reducing mortality; preventing adverse CV events, such as myocardial infarction, stroke, and heart failure; and slowing the progression of existing kidney disease.31,32 It is important to keep in mind that the degree of BP reduction is the major determinant of reduction in CV risk, superseding the choice of antihypertensive drug; a dictum that is valid in patients with DM.10
The choice of initial agent is based on the individual clinical situation. Monotherapy can attain goal BP in some patients with DM and hypertension, especially when the BP is only modestly elevated. However, combination therapy is eventually required in most patients.
In patients with DM nephropathy, angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) may slow kidney disease progression more effectively than other antihypertensive drugs. These medications may have CV benefits in high-risk patients that exceed those of other agents.33,34 In randomised trials comparing ACE-I or ARB with placebo in patients at increased CV risk who had a baseline systolic BP >130 mmHg, the outcomes were similar in patients with or without DM.35–37 Results of a meta-analysis comprising 48 trials found that ACE-I significantly reduced mortality compared with placebo (9.3% versus 10.5%), whereas ARB did not (5% versus 5%), the caveat being that many trials included low-risk patients.38 However, both ACE-I and ARB were superior to other antihypertensive drugs (10.2% versus 11.9% and 8.5% versus 10.5%, respectively), and had significant benefits on heart failure; ACE-I significantly reduced the risk of myocardial infarction and ARB significantly reduced the risk of stroke. Other meta-analyses found that both classes had comparable beneficial effects on mortality and end-stage renal disease,39 and, in patients with and without DM, on mortality and CV events.40 Combining agents from both classes, however, does not yield additional benefit and is, in fact, contraindicated.
In patients without increased albuminuria, initial monotherapy can consist of an ACE-I, ARB, thiazide diuretic, or calcium channel blocker. Thiazide diuretics and beta-blockers have the disadvantage of worsening glucose metabolism and potentially aggravating hyperglycaemia. In the ALLHAT trial,41 chlorthalidone was associated with a mild rise in the plasma glucose; in non-DM patients, an elevation in fasting glucose into the DM range (≥126 mg/dL) occurred significantly more often with chlorthalidone (11.6% versus 9.8% and 8.1% with amlodipine and lisinopril, respectively). Although the IDNT42 and RENAAL trial43 found that patients treated with an ARB had achieved renal protection and had significant reductions in hospitalisations for heart failure, neither trial showed a significant CV mortality reduction. A loop diuretic is likely to be required in patients with renal disease or heart failure who have a propensity to fluid retention.
Beta-blockers have a reputation as unsuitable agents for patients with DM, a notion that is grounded in earlier findings that suggested aggravation of insulin resistance and masking of the warning symptoms of hypoglycaemia. The LIFE reduction in hypertension DM parallel study44 showed that the ARB agent losartan provided significantly more protection from adverse CV outcomes than atenolol (18% versus 23% at a mean follow-up of 4.7 years), CV mortality (6% versus 10%), and total mortality (11% versus 17%). Regression in left ventricular hypertrophy induced by losartan administration may have conferred a beneficial action. Interestingly, however, in the UKPDS, atenolol was found to be as effective as captopril in terms of both BP lowering and protection against microvascular disease in patients with T2DM.45 The beta-blocker carvedilol has combined non-selective beta and alpha-1 adrenergic antagonist actions; it improves survival in patients with heart failure and may not be as deleterious for glucose control.46,47
In patients who require more than one drug for BP control, a combination of an ACE-I or ARB and a dihydropyridine calcium channel blocker (e.g., amlodipine) is appropriate.48 Amlodipine may provide better protection against CV events than hydrochlorothiazide in this setting. Better efficacy and lack of adverse effects on lipid or carbohydrate metabolism also apply to the non-dihydropyridine calcium channel blockers (diltiazem and verapamil).49 Low-dose thiazides in combination with other agents work at least in part by countering volume expansion.50 ACE-I may minimise or prevent some of the metabolic complications associated with diuretic therapy, such as hypokalaemia and hyperuricaemia.51 The ACCOMPLISH trial52 evaluated combination therapy in 11,506 hypertensive patients with hypertension, 60% of whom had DM, showing that an ACE-I/calcium channel blocker regimen was superior to an ACE-I/diuretic combination. Of note, the evidence for a role of aldosterone blockade by selective and non-selective mineralocorticoid receptor antagonists, such as spironolactone or eplerenone, in patients with T2DM is limited; however, they may be considered in resistant cases as long as careful monitoring of renal function and serum potassium is maintained.53
To summarise, the weight of currently available evidence suggests that lifestyle and pharmacologic therapies in hypertensive persons with DM that reduces BP levels to <140/90 mmHg are associated with clinically significant lowering of vascular complications.20 The higher the BP level, the greater the benefit accrued from decreasing it. Although a combination of multiple medications, most frequently either an ACE-I or an ARB with one or more agents is necessary, such an approach is successful in attaining BP goals in the majority of patients with DM. It would be expected, therefore, that the patient with T2DM would optimally benefit from a combination of antihypertensive agents, one of which would act on the renin-angiotensin-aldosterone pathway (i.e. ACE-I or ARB).
CONCLUSIONS
Patients with coexisting DM and hypertension appear to be especially prone to CV disease and DM hypertensive microvascular complications. Recent data from several randomised clinical trials have led to the recommendation of target systolic BP of <140 mmHg and diastolic of <90 mmHg. In clinical practice, these goals necessitate the use of two or more antihypertensive medications, most often combining an ACE-1 or ARB with one or more agents from other drug classes. Individualisation of targets, choice of pharmacotherapeutic agents, and attention to cost and side effect profile are important considerations in management. Lifestyle modifications to reduce weight, improve diet, restrict salt intake, and incorporate physical activity have beneficial actions not only on BP, but also on glycaemic control, lipid profile, and overall CV risk. Efforts are needed to translate the knowledge already gained into population-based implementation while conducting further research into understanding pathogenetic mechanisms and expanding the therapeutic armamentarium.
Footnotes
Disclosure: The author has declared no conflicts of interest.
References
- 1.American Diabetes Association. Cardiovascular Disease and Risk Management. Diabetes Care. 2017;40(Suppl 1):S75–87. doi: 10.2337/dc17-S012. [DOI] [PubMed] [Google Scholar]
- 2.Hypertension in Diabetes Study (HDS) Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11(3):309–17. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Epstein M, Sowers JR. Diabetes mellitus and hypertension. Hypertension. 1992;19:403–18. doi: 10.1161/01.hyp.19.5.403. [DOI] [PubMed] [Google Scholar]
- 4.Kidney International Supplements. [Last accessed: 18 August 2017];KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.
- 5.Mogensen CE, et al. Renal factors influencing blood pressure threshold and choice of treatment for hypertension in IDDM. Diabetes Care. 1991;14(Suppl 4):13–26. doi: 10.2337/diacare.14.4.13. [DOI] [PubMed] [Google Scholar]
- 6.Nosadini R, et al. Role of hyperglycemia and insulin resistance in determining sodium retention in non-insulin-dependent diabetes. Kidney Int. 1993;44(1):139–46. doi: 10.1038/ki.1993.224. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank K, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation. 2002;106(16):2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 8.Wong WT, et al. Endothelial dysfunction in diabetes and hypertension: cross talk in RAS, BMP4, and ROS-dependent COX-2-derived prostanoids. J Cardiovasc Pharmacol. 2013;61(3):204–14. doi: 10.1097/FJC.0b013e31827fe46e. [DOI] [PubMed] [Google Scholar]
- 9.Hansson L, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351(9118):1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 10.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RR, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–76. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 12.Adler AI, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrier RW, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61(3):1086–97. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, et al. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–40. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 15.Howard BV, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the stop atherosclerosis in native diabetics study (SANDS) JAMA. 2008;299(14):1678–89. doi: 10.1001/jama.299.14.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ACCORD study group. nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39(5):701–8. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusuf S, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374(21):2032–43. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 19.McBrien K, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus. systematic review and meta-analysis. Arch Intern Med. 2012;172(17):1296–303. doi: 10.1001/archinternmed.2012.3147. [DOI] [PubMed] [Google Scholar]
- 20.Emdin CA, et al. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA. 2015;313(6):603–15. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 21.Xie X, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435–43. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.Bakris GL, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36(3):646–61. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(Suppl 1):11–3. [PubMed] [Google Scholar]
- 25.Perkovic V, Rodgers A. Redefining Blood-Pressure Targets--SPRINT Starts the Marathon. N Engl J Med. 2015;373(22):2175–8. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 26.Magnan EM, et al. The relationship of individual comorbid chronic conditions to diabetes care quality. BMJ Open Diabetes Res Care. 2015;3(1):e000080. doi: 10.1136/bmjdrc-2015-000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowlin SY, et al. Diet, inflammation, and glycemic control in type 2 diabetes: An integrative review of the literature. J Nutr Metab. 2012;2012:542698. doi: 10.1155/2012/542698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buse JB, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115(1):114–26. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 29.James PA, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 30.Mancia G, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 31.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: Blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138(7):593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 32.Snow V, et al. Clinical Efficacy Assessment Subcommittee of the American College of Physicians. The evidence base for tight blood pressure control in the management of type 2 diabetes mellitus. Ann Intern Med. 2003;138(7):587–92. doi: 10.7326/0003-4819-138-7-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 33.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253–9. [PubMed] [Google Scholar]
- 34.Daly CA, et al. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: Results from the PERSUADE substudy. Eur Heart J. 2005;26(14):1369–78. doi: 10.1093/eurheartj/ehi225. [DOI] [PubMed] [Google Scholar]
- 35.Fox KM EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362(9386):782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 36.Braunwald E, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058–68. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yusuf S, et al. The Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174–83. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 38.Cheng J, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174(5):773–85. doi: 10.1001/jamainternmed.2014.348. [DOI] [PubMed] [Google Scholar]
- 39.Wu HY, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: Systematic review and bayesian network meta-analysis. BMJ. 2013;347:f6008. doi: 10.1136/bmj.f6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savarese G, et al. A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013;61(2):131–42. doi: 10.1016/j.jacc.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 41.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 42.Lewis EJ, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 43.Brenner BM, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 44.Lindholm LH, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359(9311):1004–10. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 45.UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317(7160):713–20. [PMC free article] [PubMed] [Google Scholar]
- 46.Giugliano D, et al. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med. 1997;126(12):955–9. doi: 10.7326/0003-4819-126-12-199706150-00004. [DOI] [PubMed] [Google Scholar]
- 47.Bakris GL, et al. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292(18):2227–36. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- 48.Gradman AH, et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4(2):90–8. doi: 10.1016/j.jash.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Birkenhäger WH, Staessen JA. Treatment of diabetic patients with hypertension. Curr Hypertens Rep. 1999;1(3):225–31. doi: 10.1007/s11906-999-0025-6. [DOI] [PubMed] [Google Scholar]
- 50.National High Blood Pressure Education Program Working Group report on hypertension in diabetes. Hypertension. 1994;23(2):145–58. [PubMed] [Google Scholar]
- 51.Weinberger MH. Influence of an angiotensin converting-enzyme inhibitor on diuretic-induced metabolic effects in hypertension. Hypertension. 1983;5(5 Pt 2):III132–8. doi: 10.1161/01.hyp.5.5_pt_2.iii132. [DOI] [PubMed] [Google Scholar]
- 52.Jamerson K, et al. ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–28. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi S, et al. Effects of mineralocorticoid receptor antagonists in patients with hypertension and diabetes mellitus: a systematic review and meta-analysis. J Hum Hypertens. 2016;30(9):534–42. doi: 10.1038/jhh.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]