Abstract
The goal of this review is to show how transcranial magnetic stimulation (TMS) techniques can make a contribution to the study of brain networks. Brain networks are fundamental in understanding how the brain operates. Effects on remote areas can be directly observed or identified after a period of stimulation, and each section of this review will discuss one method. EEG analyzed following TMS is called TMS-evoked potentials (TEPs). A conditioning TMS can influence the effect of a test TMS given over the motor cortex. A disynaptic connection can be tested also by assessing the effect of a pre-conditioning stimulus on the conditioning-test pair. Basal ganglia-cortical relationships can be assessed using electrodes placed in the process of deep brain stimulation therapy. Cerebellar-cortical relationships can be determined using TMS over the cerebellum. Remote effects of TMS on the brain can be found as well using neuroimaging, including both positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). The methods complement each other since they give different views of brain networks, and it is often valuable to use more than one technique to achieve converging evidence. The final product of this type of work is to show how information is processed and transmitted in the brain.
Keywords: Transcranial magnetic stimulation, EEG, basal ganglia, cerebellum, positron emission tomography, functional magnetic resonance imaging, connectome, brain network
Introduction
The brain operates in networks to carry out its function. At one level, information passes between neurons via synapses, and, at another level, information passes from region to region. Visual information flows from occipital areas to the parietal areas and then to the motor areas to properly move the arm and shape the hand to pick up an object. Olfactory information from the smell of coffee integrates with pleasant limbic memories to drive a decision to have another cup. If we want to understand how the brain works, we need to know its anatomical connectivity and what pathways are utilized in different functional tasks.
In recent years, several neuroimaging techniques have been exploited with the objective of defining a comprehensive map of the neural connections in the human brain, the so-called human “connectome”, both in terms of anatomical and functional connectivity (Van Essen et al., 2013). This goal of modern neuroscience has recently received additional impetus from the integration of innovative mathematical approaches, such as the graph theory, which allows large scale evaluations of networks (Sporns et al., 2004). Brain stimulation methods, particularly with transcranial magnetic stimulation (TMS) have multiple applications in advancing this line of investigation, and that is the focus of this review.
TMS can be delivered to the brain as a single-pulse, repetitive TMS (rTMS), or in a patterned fashion, such as theta-burst TMS or quadripulse TMS. At the moment of delivery, called the online approach, TMS will generally interfere with brain function, creating a “virtual lesion”. However, in some circumstances it could also augment brain activity. Effects can be local, at the site of stimulation, or in distant brain regions connected with the site of stimulation. rTMS and patterned TMS can also have influences on brain activity that last for some time after the stimulation. These effects are induced by short or longer lasting neural plasticity, and when studied for connectivity effects, this is called the off-line approach.
While techniques such as EEG, fMRI and PET are able to evaluate functional connectivity (Friston et al., 1993), defined as the temporal correlations between spatially remote neurophysiological events, the integration of TMS with the above mentioned neuroimaging methodologies could be a potential tool to reveal the effective connectivity (Friston et al., 1997), defined as the influence one neural system exerts over another through causal or non-causal effects (Seghier et al., 2013). Specifically, TMS–PET and TMS–fMRI co-registration can reveal the spatial effects of TMS with high spatial resolution, but because they are based on changes in blood flow and oxygenation, these techniques have a reduced temporal resolution of a few seconds (fMRI) or minutes (PET). Instead, the co-registration of the EEG activity – which has a temporal resolution of a few milliseconds and can be simultaneously sampled from a large number of scalp sites – during TMS provides the opportunity of tracking temporal dynamics of brain activity and therefore of evaluating effective time-resolved connectivity (Ferreri et al., 2013).
TMS-EEG technique
In the evaluation of brain connectivity, TMS-EEG has several advantages, mainly due to its high temporal resolution. First, starting from the assumption that if area A is active prior to area B, then area A might cause increased (or reduced) activity in area B through excitatory (or inhibitory) connections between the two areas (Sporns et al., 2004), TMS-EEG conveys precise information about the temporal order of activations of connected cortical areas (either adjacent or remote), defining at the same time the causal interactions (excitatory or inhibitory) between two areas within functional brain networks. Second, TMS-EEG high temporal resolution allows the identification of critical periods during which the stimulated area and its connections to other brain regions make a critical contribution to the experimental task. Thereby it enables differentiating the connectivity pattern of different cognitive processes related to specific tasks or to different brain states and whether or how they are modified by learning and training (or by a lesion, such as a stroke). Third, the co-registration of TMS-EEG, given the precision of TMS in activating cortical regions and even specific neurons, has higher spatial resolution compared to EEG alone (that notoriously can capture neural activity of the cortical neurons, but not from deep-brain structures). A more accurate spatial resolution can be often obtained combining TMS-EEG with structural neuroimaging, although the poor temporal resolution of fMRI would still not permit localizing with accuracy the TMS-induced potentials and the eventual network hierarchy. Taking in account these points, TMS-EEG co-registration allows the evaluation of the spatiotemporal pattern of neural activity that determines the connections across brain areas, hence providing measures of effective connectivity mainly in the time domain.
From the first attempt to measure TMS-evoked brain responses made in 1989 by Cracco et al. (Cracco et al., 1989), several efforts have been made to overtake the severe technical limitations related to the coupling of a stimulation artefact (thousands times higher than the signal of interest) to the recording system. Using a sample-and-hold circuit able to block the acquisition of EEG signal for several milliseconds immediately adjacent to the TMS pulse, TMS-evoked brain EEG responses were successfully measured in 1997 (Ilmoniemi et al., 1997), succeeding to track TMS-evoked brain activity with a temporal window of a few milliseconds after the stimulus. Subsequent studies have begun to describe the scalp topography and study the possible generator sources of the TMS-evoked EEG potentials (TEPs). Probably, most of the EEG signals record a linear projection of the postsynaptic currents indirectly induced by TMS: then, EEG signals can be used to locate and quantify these synaptic current distributions and evaluate local excitability and effective connectivity in the nervous system.
Within the so-called “inductive approach”, applying a single TMS pulse on the brain cortex, a network of neuronal connections is triggered and the TMS-induced activation - a summation of post-synaptic potentials - spreads from the stimulation site to other interconnected parts of the brain producing deflections in scalp EEG signals, starting a few milliseconds after the stimulus and lasting about 300 ms, first in the form of rapid oscillations and then as lower-frequency waves (Miniussi et al., 2010). Increased EEG activity following the magnetic stimulus can be observed in a number of neighboring electrodes, suggesting the spread of TMS-evoked activity to anatomically interconnected cortical areas; particularly, TMS-evoked activity spreads from the stimulation site ipsilaterally via association fibers, contralaterally via transcallosal fibers, and to subcortical structures and spinal cord via projection fibers. Therefore, TMS-EEG gives the possibility to study cortico-cortical interactions and how the activity in one area affects the ongoing activity in other areas. It has been suggested that the first part of the TMS-evoked EEG signals reflects the excitability, i.e. the functional state, of the stimulated cortex, whereas the following spatiotemporal distribution over the scalp corresponds to the spread of activation to other cortical areas, i.e., the effective connectivity of the stimulated area (Komssi et al., 2006). The amplitude, latency, and scalp topography of single-pulse TMS-evoked EEG responses have been clearly described (Komssi et al., 2002); nevertheless, the characteristics of these responses and their diffusion over the scalp strongly depend on the stimulation intensity, the local activation of the stimulated cortex at the time of stimulus delivery as well as the functional state of the overall brain. Beyond this variability, TMS-evoked EEG averaged responses are generally highly reproducible, provided that the delivery and targeting of TMS is well controlled and stable from pulse to pulse and between experiments.
Several components of the EEG response to single-pulse TMS applied on the motor cortex have been identified and - benefiting from knowledge of the anatomical connectivity of the brain as seen by diffusion tensor imaging studies - their spatiotemporal spread has been followed (Nikulin et al., 2003): particularly, single-pulse TMS is able to evoke EEG activity composed at vertex by a sequence of deflections of negative polarity peaking at approximately 7, 18, 44, 100, and 280 ms alternating with positive polarity peaks at approximately 13, 30, 60, and 190 ms post-TMS (Bonato et al., 2006, Daskalakis et al., 2012, Casula et al., 2014, Premoli et al., 2014) (Figure 1). However, the previously described pattern of TEPs is not invariable because, in addition to inter-individual differences, it depends on the exact coil location and orientation, the state of the cortex, and the vigilance of the subject.
Figure 1.
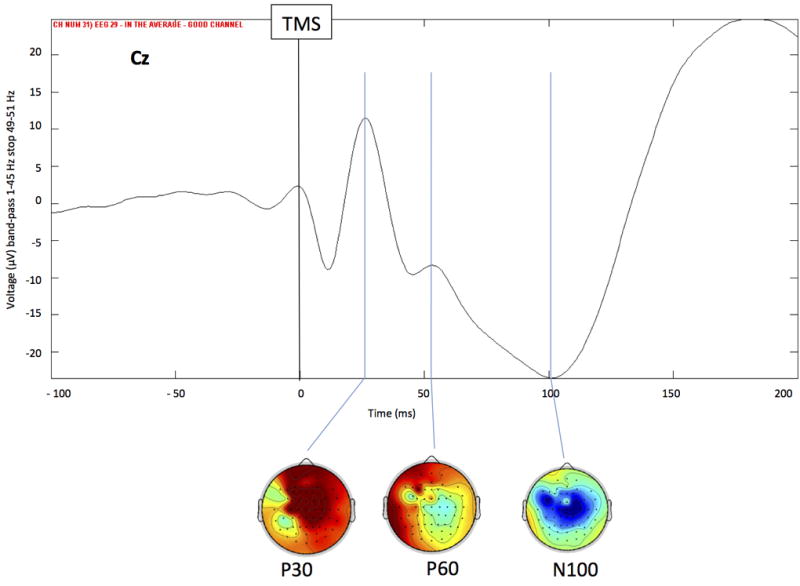
Grand average of the EEG responses recorded at vertex (Cz) and related scalp distribution maps of General Estimating Equation model EEG pattern at 30, 60 and 100 ms after single pulse stimulation over the left M1in a healthy subject.
The TMS-EEG technique presents major challenges and disadvantages. For example, the strong TMS magnetic field could induce an unwanted electric field in nearby conductors including the EEG electrodes, skin, nerves, muscles, skull, and CSF, could evoke muscle and nerve activations, and eye blinks or movements, and could lead, with a loud click and a tapping sensation, to the generation of auditory-evoked potentials (AEP) and sensory-evoked potentials (SEP), respectively. All these factors could generate large-amplitude artifacts in the EEG signal. Although some of these artifacts can be minimized through dedicated TMS-compatible EEG equipment and noise removal techniques, a number of control conditions, data recording considerations, and offline noise removal techniques are recommended in TMS-EEG studies (for technical aspects of TMS-EEG co-registration, see (Farzan et al., 2016).
Despite these technical limitations, TMS-EEG appears very suitable to test and evaluate the effective connections between different brain areas, both at rest and during motor and cognitive processes (Bortoletto et al., 2015). Several TMS-EEG studies on the motor system at rest have demonstrated that the TMS-induced activity spreads from the stimulated neural assembly to other ones of the same motor network: the TMS of the primary motor cortex causes the successive activation of ipsilateral supplementary/premotor areas and contralateral motor region with a short conduction time (Litvak et al., 2007). The nature of these connections seems to be inhibitory rather than excitatory and depends on the level of cortical activation (Giambattistelli et al., 2014). Other studies on the motor cortex at rest have revealed that the stimulation of M1 generates also the late activation of areas outside the ‘motor’ network, including the cingulate gyrus and temporo-parietal junction (Litvak et al., 2007). The spread of the later components of TEPs to other areas over the motor network suggests the involvement of further cerebral areas implicated in the transmission of information across the brain. Additional evidence about this bottom-up signal propagation has been provided by studies on the visual system (Garcia et al., 2011).
In the cognitive domain, TMS-EEG has been used to examine and confirm the causal relationship between the brain spatiotemporal dynamics and behavior by means of both offline and online studies. In the former approach, TMS-EEG protocols can be employed during a cognitive task performance before and after the introduction of interferences (e.g., rTMS) to quantify the neurophysiological correlates of the brain state (for a review of this modality see (Thut et al., 2010)). In the latter approach, TMS can be applied during EEG recording to one or more brain regions or network at specific time intervals during a cognitive performance to induce or suppress or facilitate the activity of functionally interacting neural assemblies (measured by EEG signal features), thus examining the effect of this interference on brain (areas or network)-behavior relationship. For example, Vernet and colleagues (Vernet et al., 2015), applying a paired-pulse TMS on two different cortical areas (i.e. “dual-site” TMS-EEG), demonstrated a mutual interaction of the parietal and prefrontal cortices and their influence on neurophysiological events (e.g., TEPs) and perception mechanisms. Other online (r)TMS-EEG studies have applied rTMS or single-pulse TMS concurrent with EEG recording during several cognitive tasks such as spatial attention control, inhibition control, perceptual decision making, goal-directed action, face-processing, temporal encoding, sensorimotor integration, and multisensory processing (Farzan et al., 2016). In this set of works, the target region of stimulation is represented by multimodal associative areas responsible for cognitive processes during task performance. In some cases it has been demonstrated that the spread of TMS-induced activity from these areas across the brain could be divergent depending on the task context, preferring the engagement of one network rather than another, therefore suggesting the existence of top-down modulation. For example, the results obtained by Akaishi et al. (Akaishi et al., 2013) by using concurrent TMS and EEG recording suggested the presence of dissociation between the frontal eye field (FEF) and ventral prefrontal cortex (VPFC) networks for their computational roles in processes of perceptual decision-making. These findings, taken together, highlight the potential role of TMS-EEG to test the dynamic changes of effective cortico-cortical connectivity, identifying the segregation or the integration of specialized brain areas or network rather than others both during the resting state and during cognitive demands.
In addition to standard TEPs, single-pulse TMS or frequency-tuned train of pulses can also trigger or enhance brain oscillatory activity or perturb ongoing rhythms of the target cortical, eliciting event-related phenomena, such as EEG rhythm synchronization or desynchronization (Rosanova et al., 2009, Thut et al., 2011). Brain oscillations may represent a mechanism through which the communication of neuronal assemblies - by synchronization in specific frequency bands - is rendered more effective, precise, and selectively tuned on the transmission of the relevant information (Fries, 2015). It has already been demonstrated that different cortical areas when stimulated respond at a characteristic frequency, i.e., their natural frequency, and that functionally segregated networks could oscillate at different frequencies at rest (Rosanova et al., 2009). Given these assumptions, some authors speculated that TMS could interact with such oscillatory patterns in the directly stimulated cortical area and in distant areas belonging to the same neural network thus inducing a resonant frequency activity in all “synchronized” areas of the same network by mechanisms of longer-range synchronization (interregional coherence) (Thut et al., 2012). This frequency-specific “resonant effect” should ensure a better information transfer across brain structures, even able to determine changes in the behavioral performance. Therefore, the “rhythmic TMS-EEG approach” appears as a promising tool in mapping the natural frequency of different cortical areas and identifying the role of a specific frequency oscillatory activity in distinct brain functions. As example, a relevant study was performed by Hanslmayr et al. (Hanslmayr et al., 2014), who integrated rhythmic stimulation with simultaneous EEG recording and behavior measurement, providing evidence for the causal role of prefrontal cortex beta frequency desynchronization in memory formation. The authors showed that beta (~19 Hz) rTMS, compared to ~7 or 11 Hz rTMS or sham stimulation, to the inferior frontal gyrus impaired memory formation, illustrating that only beta rTMS led to lasting beta entrainment post TMS.
With all these premises, it is easy to realize how TMS–EEG can be used to examine normal and altered effective brain connectivity under both physiological and pathological specific conditions, indicating the strengthening or weakening of existing cortico-cortical connections or the recruitment of compensatory networks. Indeed, TMS-EEG can be used to track the interactions of brain areas during sensory processing, cognition or motor control and, moreover, to evaluate such neurological disorders as Alzheimer disease (AD) characterized by altered connectivity (Ferreri et al., 2016). The few studies regarding this field already showed that stimulation of the motor cortex in AD patients was associated with a significant decrease in TMS-induced activity over several brain areas compared with healthy controls, suggesting a potential role of TMS-EEG as a neurophysiological marker for diagnosis and early identification of mild cognitive impairment and AD.
Another application of TMS-EEG concerns the measures of state consciousness (Tononi et al., 2016). In a series of initial experiments, Massimini and co-workers (Massimini et al., 2012) recorded the EEG response to a direct cortical stimulation in humans during wakefulness, NREM sleep, REM sleep and anesthesia. While during wakefulness and REM sleep the brain is able to sustain long-range specific patterns of activation, during NREM sleep and anesthesia, when consciousness fades, this ability is lost. The authors hypothesized that the ability of thalamocortical circuits to sustain long-range, differentiated patterns of activation, is a theoretical requisite for consciousness. Subsequently, TMS-EEG has been successfully employed for the differential diagnosis of vigilance disorders (e.g., vegetative state and minimally conscious state) (Ragazzoni et al., 2013, Napolitani et al., 2014). In addition, TMS-EEG has also been used for studying the mechanisms underlying human epilepsies (Shafi et al., 2015) and psychiatric disorders (Frantseva et al., 2014).
Recently, new multimodal approaches have been developed coupling the recording of TMS-evoked potentials before, during and after non-invasive brain stimulation (rTMS or transcranial electrical stimulation, tES), in order to better understand the mechanisms through which these techniques can induce changes in cortical functions both in physiological and pathological conditions (Chung et al., 2015, Bailey et al., 2016, Hill et al., 2016). For example, Romero Lauro and co-workers (Romero Lauro et al., 2014) used TMS-EEG to explore local and global cortical excitability modulation during and after active and sham transcranial direct current stimulation (tDCS). These authors applied TMS over the left posterior parietal cortex before, during, and after 15 min of anodal tDCS over the right posterior parietal cortex, while EEG was recorded. There was a diffuse rise of cortical excitability highlighting the spreading of the effects of anodal tDCS over remote cortical regions of stimulated and contralateral hemispheres. In another study, Cipollari and colleagues (Cipollari et al., 2015) studied the TMS-EEG in six chronic aphasics who underwent 3 weeks of language training coupled with tDCS over the right inferior frontal gyrus. The patients improved, and the components of the TMS-EEG from stimulation over the right inferior frontal gyrus were increased in the region of stimulation.
Bortoletto et al. (Bortoletto et al., 2015) showed how TMS-EEG could support recent models of functional brain architecture inferred by graph theory, i.e., the organization and configuration of brain networks. Graph theory is a mathematical tool to quantify the properties of complex networks and describe the interrelationships between network elements (nodes) by means of connections (edges), which in the case of human brain represent brain regions and their connections, respectively. According to graph theory analyses of structural and functional neuroimaging, the human brain “connectome” architecture resembles a “small world” network, characterized by modules (or sub-networks) of nodes that perform segregated and highly specialized processing and that are linked through a few long-range connections that ensure integrated processing (Achard et al., 2006, Bassett et al., 2006). Modules of nodes correspond to functional networks of areas, e.g., the motor network, visual network, dorsal attention network, default mode network, medial lobe memory network and fronto- parietal control network (Smith et al., 2009, Power et al., 2011). The communication among networks is via brain hubs, nodes with connections with multiple networks. By examining the responses within a network when one of its nodes is stimulated, TMS–EEG co-registration could provide measures of effective connectivity that may test the predictions of graph theory models in terms of brain interactions more directly and along a causal dimension. For example, during different specific cognitive tasks, connections via hubs to the different modules could switch.
TMS-EEG is clearly a valuable technique for study of physiology and pathophysiology and may soon have clinical applications. There are technical challenges in its application, however, and there will need be some improvements before it will have widespread use.
TMS-TMS
Effective connectivity studies can be conducted using TMS at two sites, often called the “twin coil” approach. Many of these studies look at the influences on the primary motor cortex (M1) coming from various brain regions at rest, before and during movement. These paradigms involve stimulating other brain regions prior to M1. Motor-evoked potential (MEP) amplitudes are compared between baseline single-pulse MEP amplitudes and conditioned MEP amplitudes. Based on these findings, effective connectivity is considered to be facilitatory or inhibitory, or to have no effect.
Influences on M1 come from other brain regions such as bilateral posterior parietal cortices (PPC), ventral (PMv) and dorsal (PMd) premotor cortices, supplementary motor area (SMA), pre-SMA and cerebellum, as well as contralateral M1. Ferbert et al. first looked at effective connectivity between bilateral M1 using a twin-coil approach, and found that a conditioning stimulus of one M1 5–6 ms or more prior to a test stimulus of the other M1 resulted in inhibition of the MEP amplitude (Ferbert et al., 1992). This was termed interhemispheric inhibition, and other groups have confirmed this effect (Di Lazzaro et al., 1999). Interhemispheric inhibition consists of two phases with maximum inhibition at about 10 ms and 40–50 ms (Ni et al., 2009). Others have found interhemispheric facilitatory influences on the motor cortex at ISIs of 1–5 ms, and late (ISI≥11 ms) inhibitory influences following this early facilitation (Hanajima et al., 2001). These influences, mediated by callosal fibers between the homotopic areas of the motor cortices, have been found to depend on current direction as well as conditioning intensity.
Both facilitation and inhibition can also be seen depending on the exact position and latency. An example is the different intra- and inter-hemispheric parietal-motor interactions seen from the inferior parietal lobule (IPL). A study showed that a facilitatory effect on right M1 is produced with stimulation of the right, ipsilateral caudal IPL (cIPL) at 4 to 15 ms interstimulus intervals (ISI) and stimulation of the left, contralateral cIPL at 6 to 12 ms ISIs (Koch et al., 2007, Koch et al., 2009). Additionally, right, ipsilateral anterior IPL stimulation (aIPL) resulted in an inhibitory effect at 4 ms ISI and left, contralateral aIPL stimulation had an inhibitory effect at 10 to 12 ms ISIs. These effects were studied in more detail during reach toward contralateral or ipsilateral space, and PPC-M1 facilitatory connections were found to be specific to contralateral (leftward) reach with stimulation of the right PPC-right M1 at two time intervals (50 and 125 ms), and contralateral (rightward) reach with stimulation of the left PPC-left M1 at two time intervals (50 and 100 ms) (Koch et al., 2008a). Another study confirmed the inhibitory effects of aIPL stimulation on the ipsilateral M1 in both hemispheres of subjects at rest, and found facilitatory effects with the left, ipsilateral central and posterior IPL stimulation on M1, but not a similar influence on the right side (Karabanov et al., 2013) (Figure 2) Stimulation of area 5 had an influence on M1 during sensorimotor tasks, but not at rest (Ziluk et al., 2010).
Figure 2.

The TMS response curves for the left hemisphere. Each data point represents the mean ± SEM for either the test stimulus alone or for one of the four paired-pulse interstimulus intervals (2, 4, 6, 8 ms). MEPs are normalized to the test stimulus. Data for the anterior inferior parietal lobule (IPL) is plotted in black, for the central IPL in dotted black and for the posterior IPL in grey. Modified from (Karabanov et al., 2013) with permission.
Studies have also shown different networks to be modulated by motor tasks, such as grasping. A precision grip modulated the left aIPL-left M1 interaction, whereas a whole hand grasp modulated the left cIPL-left M1 interaction (Koch et al., 2010). Another example of such modulation is the inhibitory influence of the left PMv on ipsilateral M1 at rest seen at 4–8 ms ISIs, which becomes facilitation during grasping (Houdayer et al., 2012). This resting premotor-to-motor inhibition disappears with power grip and turns into a net facilitation during precision grip, suggesting that the PMv-M1 connection is activated in conveying information used to assume a specific hand posture (Davare et al., 2008). This grasp-specific facilitation was also noted in the movement preparation period, in the specific muscle used in the upcoming grasp (Davare et al., 2009). Further work showed that stimulation over the parietal operculum affected grasp of an object in the dark when the object was known visually, but that stimulation over PMv affected grasp when the object was known haptically (Maule et al., 2015). A facilitatory net effect was found in a surround muscle not involved in the movement, occurring 100 ms before movement onset, which turned back to inhibition 50 ms later (Houdayer et al., 2012). These data collectively suggest that PMv has a dynamic influence on M1 excitability in preparing and executing the specific grasp for each required task.
Whereas PMv-M1 connections seem to be modulated according to the particular type of upcoming grasp, PMd-M1 connections appear to be involved in preparation for movements made in response to sensory cues. For example, low-intensity conditioning pulses of the left PMd resulted in a facilitatory influence on contralateral M1 both at rest (ISI 8 ms) and after cues (ISI 75 ms) instructing the subject to make movements with the left hand (O'Shea et al., 2007), whereas inhibitory connections manifest with cues (ISI 100ms) instructing the subject to make movements with the right hand (Koch et al., 2006). These data suggest that certain connections are modulated only for muscles involved in the upcoming movement. PMd also has strong effects on the M1 representation of the facial muscles (Parmigiani et al., 2015). There is also a very fast connection between PMd and M1, less than 5 ms, demonstrated by facilitation of an MEP even when the PMd stimulus follows the M1 stimulus (presumably mediated by later i-waves) (Groppa et al., 2012a, Groppa et al., 2012b). Others have demonstrated that TMS over the right PMd has inhibitory effects on contralateral M1 at ISIs of 8–10 ms (Mochizuki et al., 2004). These data, along with those of functional brain imaging studies confirm the presence of interhemispheric commissural fibers, which may play a role in bimanual coordination. Whether such influences involve “premotor to contralateral premotor to contralateral motor” or “premotor to ipsilateral motor to contralateral motor” is not clear, and possibly both pathways are relevant.
The supplementary motor area, an area important for preparation and execution of voluntary movements, has also been studied using a twin-coil TMS method. Facilitatory connections were found with 140% active motor threshold (AMT) conditioning stimuli of the contralateral SMA prior to M1 stimulation (Arai et al., 2012). This facilitation was only found with current directed towards the SMA and antero-medially directed current in M1. Oliveri et. al found paired-pulse of SMA-M1 to have this facilitatory effect only in the visual-emotional triggered movement condition, compared with paired TMS during presentation of neutral visual cues or single-pulse M1 stimulation, suggesting that the SMA could be an interface of the limbic and motor systems (Oliveri et al., 2003). Another group looked at the SMA-M1 connection at both rest and in movement of the lower extremities (Byblow et al., 2007). At rest, extensor carpi radialis (ECR) MEPs were inhibited by conditioning SMA at 90%AMT, while facilitatory effects of SMA stimulation on M1 were seen in movement conditions involving dorsiflexion and plantarflexion of the lower extremities. This could be interpreted as modulation of resting inhibition that may be relevant in the production of hand-foot coordination.
There are also reports on the differential effects of pre-SMA stimulation on M1, depending on the direction of the conditioning current or the subject’s cognitive state. Inhibitory effects were found with anteroposterior (AP) current direction, while posteroanterior (PA) current induced facilitatory effects (Civardi et al., 2001). Another study found stimulation of the pre-SMA to induce a facilitatory influence at a short latency of 6 ms, 125 ms after the cue to make a movement, suggesting that the functional connectivity of pre-SMA is dependent on the cognitive state of subjects (Mars et al., 2009).
There is a method now developed to study the disynaptic relationship among three (intrahemispheric) regions using a three single-pulse TMS paradigm. A study using a “triple-coil” paradigm composed of sequential single pulses over PPC and PMv followed by a third pulse over M1 found reversal of PMv-M1 inhibition to occur (Shields et al., 2016). These results demonstrate that the parietal connection to the PMv, known from anatomical studies including diffusion tensor imaging, plays a role in the modulation of M1.
Another way of looking at cortico-cortical relationships with TMS is cortico-cortical paired associative stimulation (ccPAS). Stimulation is delivered over a target site and a conditioning stimulus is delivered over another site that has input to the target site. The two sites are stimulated repetitively at intervals where the timing of the arrival of the two stimuli at the target site might give rise to a long-term potentiation (LTP)-like effect or a long-term depression (LTD)-like effect. These effects have been successfully demonstrated, for example, between the M1s in the two hemispheres (Koganemaru et al., 2009, Rizzo et al., 2009), from SMA to M1 (Arai et al., 2011), and from parietal areas to M1 (Koch et al., 2013, Chao et al., 2015). Production of these effects not only shows the effective connectivity, but might be useful for producing plastic changes that could have functional benefits.
One technical challenge of this method is being able to properly position two (or three) coils on the head. Generally small diameter coils are needed for these studies.
In summary, there are various facilitatory and inhibitory influences that are time, task and condition-dependent. These effects have also been found to be variable among subjects, and the exact location of the stimulated brain regions have found to vary as well, suggesting that a hotspot localization may be necessary similar to that used for M1. The observed effects might arise from differently weighted simultaneous facilitation and inhibition, and be mediated polysynaptically via other regions. For example, the parietal areas connect to M1 mainly through premotor regions. Changes of these effects are seen in neurological disorders, enhancing our understanding of the areas involved in pathophysiology. For example, studies of cortical networks with TMS-TMS have shown a number of connections with loss of inhibition in patients with focal hand dystonia (Hallett, 2011). Precise knowledge of the functional significance and clinical relevance of these networks is still lacking, and further studies employing stimulation paradigms that look at polysynaptic relationships may be helpful. As a final note, it should be recognized that changes in amplitude of an MEP is a gross measure of brain function. Even concepts of facilitation and inhibition are simple concepts, and as we learn more, we might find better ways to describe brain function. Nevertheless, the current methods have been informative.
Cerebellum-Cerebral Cortex as example of TMS-TMS
Using the paired-pulse TMS technique it is possible to assess connectivity between the cerebellum (CB) and motor cortex (CB-M1 connectivity). This approach has gained traction in recent years given that it permits determining in humans whether the normal inhibitory tone the CB exerts over M1 increases or diminishes in the context of different behaviors or when other non-invasive brain stimulation protocols are applied over the CB to modulate its excitability. Importantly, this method becomes particularly relevant if the changes in CB-M1 connectivity are above and beyond, or independent, of M1 changes; which would imply that the observed modifications are due to CB plasticity.
To assess CB-M1 connectivity using non-invasive brain stimulation a conditioning stimulus is applied over the CB near the inion in the back of the head, and a subsequent test stimulus is applied over M1. Changes in the motor evoked potential (MEP) show the effect of CB stimulation. Although earlier studies used electrical stimulation (Ugawa et al., 1991), subsequent investigations have employed TMS since it is less painful (Ugawa et al., 1995, Pinto et al., 2001). With this approach, it has been shown that delivering a conditioning pulse over the CB using a cone coil 5 to 7 msec prior to a test pulse over M1 results in a reduction of MEPs relative to single test pulses over M1. This inhibitory effect has been interpreted as the result of Purkinje cells activation in the cerebellar cortex, which inhibits dentate nucleus neurons that are in turn excitatory to M1 via thalamic connections. This paired-pulse measurement can be used as a marker of the amount of inhibition the CB exerts over M1, and has been termed cerebellar inhibition or CBI.
One important consideration for the technique and interpretation of CBI studies is that the conditioning stimulus should activate the cerebellum without engaging other structures that can block or modified the responses elicited by the M1 stimulation. For instance, if the conditioning pulse intensity is too high it could activate the corticospinal track antidromically with the consequent collision of descendent volleys coming from the TMS pulse over M1 (Fisher et al., 2009). To avoid this potential confound, it is important to first determine the TMS intensity required to elicit MEPs from the posterior fossa (brainstem threshold) and then use a conditioning pulse intensity of at least 5% below the brainstem threshold to assesses CBI. Similarly, using a flat coil over the back of the head could potentially activate the brachial plexus modifying the responses elicited from the paired pulse technique (Werhahn et al., 1996). To avoid this problem, most investigators use the cone coil over the CB, which wraps around the back of the head and thus reduces the likelihood of activating the brachial plexus.
Using CBI, numerous investigations have explored the role the CB has on motor control. For instance, tonic muscle contraction reduces the amount of CBI (Pinto et al., 2001) only for the activated muscle, but not for the surrounding ones (Panyakaew et al., 2016). This suggests that the CBI measurement and the CB activation during movement are somatotopic specific. Other studies have shown that the dominant hand expresses larger magnitudes of CBI relative to the non-dominant side, a finding that is proportional to the ability to execute precise movements (Schlerf et al., 2015).
A different line of research has explored the role of the CB during human motor learning and shown that learning tasks with the upper (Schlerf et al., 2012) or lower extremity (Jayaram et al., 2011) modulate CBI. Specifically, the magnitude of CBI is reduced when learning motor tasks, with a return towards baseline levels with continued practice (Schlerf et al., 2012). This has been interpreted as learning reducing excitability of Purkinje cells, as shown in animal models (Medina et al., 2008, D'Angelo, 2014), which ultimately leads to reduced activation of these cells by the conditioning TMS pulse (Celnik, 2015).
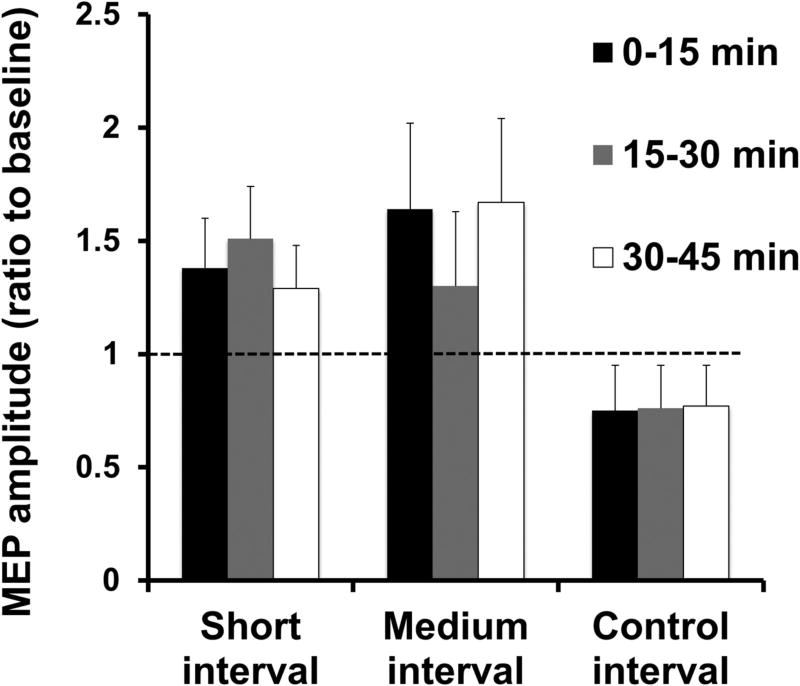
Given that CBI reflects the magnitude of inhibition the CB exerts over M1, numerous studies have explored the effects different non-invasive brain stimulation protocols applied over the CB (i.e., rTMS, TBS, tDCS, tACS) have on its connectivity with M1. For instance, it has been shown that anodal tDCS augments, while cathodal tDCS and cTBS reduce CBI (Koch et al., 2008b, Galea et al., 2009, Popa et al., 2010). tACS at 50Hz reduced CBI with an associated increase in M1 excitability, while the same protocol at 300Hz increased the magnitude of CBI (Naro et al., 2016). However, other studies reported opposite effects of non-invasive brain stimulation over the CB, such as reduction of CBI following iTBS in stroke patients (Bonni et al., 2014) or after anodal tDCS (Bradnam et al., 2015) (Figure 3). These differences might be due to testing in patient populations (as opposed to testing healthy subjects) and/or the specific TMS methodology used to assess CBI (e.g., using a flat coil to deliver conditioning stimuli over the cerebellum rather than a cone coil which is known to reach deeper structures). Further investigations are needed to clearly understand the optimal way to modulate CB excitability to obtain consistent CBI effects. However, it is critical that this assessment is performed using a consistent standardized TMS technique as described previously.
Figure 3.
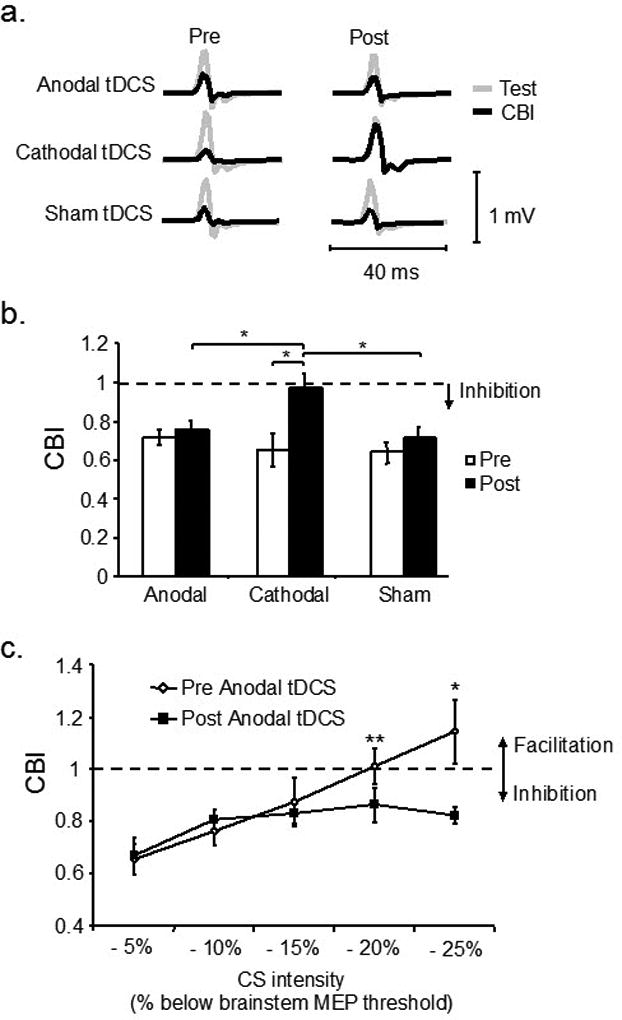
Modulation of cerebellar excitability by tDCS as assessed by CBI. (a) Single EMG traces representing MEP from test (single M1 TMS) and CBI (paired TMS over the CB+M1). Cathodal tDCS reduced CBI. (b) Group data showing magnitude of CBI (ratio of conditioning over test MEP) before and after tDCS. Cathodal tDCS reduces CBI, or the ability of CB TMS to engage the dentate-thalamic-cortical pathway. (c) A recruitment curve of the conditioning TMS intensities over the CB shows that after application of anodal tDCS it is possible to elicit CBI even at very low intensities. In other words, anodal tDCS increased the excitability of the CB. Modified from (Galea et al., 2009) with permission.
CBI has been assessed in numerous neurological conditions to better understand their pathophysiology. For instance, patients with focal hand dystonia (Brighina et al., 2009), progressive supranuclear palsy and Parkinson’s disease (Ni et al., 2010, Carrillo et al., 2013, Brusa et al., 2014) all expressed reduced CBI relative to healthy controls. These investigations are very important because they can help us design the most appropriate interventions to modulate CB excitability (e.g., cerebellar TMS or tDCS paired with specific training exercises or medications) with the ultimate goal of developing novel therapeutic approaches.
TMS-DBS and DBS-TMS
TMS has been used to investigate the interactions between basal ganglia and the cortex in conjunction with implanted deep brain stimulation (DBS) electrodes. Despite the widespread use of DBS to treat Parkinson’s disease and dystonia, its mechanisms of action remains unclear (Udupa et al., 2015). TMS can be used to examine the effects of DBS on cortical excitability and plasticity to shed light on the mechanisms of DBS. The main technical challenge is induction of current in the DBS system that could potentially lead to tissue injury or damage to the implanted pulse generator. Ex-vivo studies have shown that TMS is safe in patients with DBS (Kuhn et al., 2011) or subdural electrodes (Phielipp et al., 2017), and no severe adverse effect has been reported during TMS studies in DBS patients. However, there is potential to induce significant current if TMS is delivered over loops of leads (Deng et al., 2010) and in some studies this was sufficient to activate the internal capsule near the DBS electrodes (Hidding et al., 2006). Therefore, investigators should exercise caution in these studies and monitor adverse effects.
DBS can influence cortical excitability. Thalamic DBS in essential tremor increased MEP amplitude from motor cortical stimulation, consistent with activation of excitatory thalamocortical projections (Molnar et al., 2005). While an early study reported that internal globus pallidus (GPi) DBS increased cortical excitability as measured by motor threshold and MEP amplitude in dystonia patients, other studies did not confirm these changes (Ruge et al., 2011b). In Parkinson’s disease (PD) patients, GPi or subthalamic nucleus (STN) DBS did not change motor threshold or MEP amplitude. However, when TMS is timed to the delivery of a DBS pulse, motor cortical excitability was increased at about 3 ms and 23 ms after an STN stimulus (Kuriakose et al., 2010). These times coincided with the latencies of cortical evoked potentials from STN DBS and could represent antidromic stimulation of the hyperdirect pathway (~ 3 ms) and transmission along the STN-GPi thalamocortical pathway (~23 ms).
DBS also influences cortical inhibitory measures tested by TMS. GPi DBS in PD decreased the silent period (Chen et al., 2001), a measure of cortical inhibition measured by single pulse TMS and likely mediated by GABAB receptors. This is opposite to the effects of levodopa and may be related to the antidyskinetic effect of GPi DBS. The silent period was not changed by GPi DBS for dystonia (Kuhn et al., 2003). For STN DBS in PD, one study found increased silent period, but another study found no change.
Short-interval intracortical inhibition (SICI) is measured by paired pulse TMS and is likely mediated by GABAA receptors. Patients with generalized dystonia have decreased SICI. A longitudinal study showed that SICI increased with GPi DBS over time, and the changes correlated with the degree of improvement in dystonia (Ruge et al., 2011b). PD patients also have decreased SICI, which was normalized by STN DBS (Cunic et al., 2002, Pierantozzi et al., 2002) but not by GPi DBS (Chen et al., 2001).
Another set of cortical inhibitory circuits are short (SAI) and long (LAI) latency afferent inhibition that examine the inhibitory effects of sensory input (typically from median nerve stimulation) on the motor cortex. SAI was found to be normal in PD patients off medication, reduced in PD patients on medication (Sailer et al., 2003), and then restored by STN DBS (Sailer et al., 2007). LAI was decreased in PD patients and was not influenced by dopaminergic medications (Sailer et al., 2003), and was also normalized by STN DBS (Sailer et al., 2007). A longitudinal study showed that the effects of STN DBS on SAI and LAI required long-term stimulation (> 1 month), suggesting that it is related to the long-term plasticity effects of STN DBS (Wagle Shukla et al., 2013). These studies showed that some physiological abnormalities in dystonia and PD are reversed by DBS and correlate with clinical measures, but also showed that DBS in PD may have synergistic effects with medications, may improve non-dopaminergic features of PD or alleviate side effects of dopaminergic medications.
DBS can also affect cortical plasticity. Cortical plasticity can be assessed with a method known as paired associative stimulation (PAS), which involves long-term potentiation (LTP)-like, spike-timing dependent plasticity. PAS plasticity was found to be increased in dystonia. Treatment with GPi DBS led to decrease in PAS plasticity (Tisch et al., 2007) with maximum change occurring before maximum improvement in dystonia (Ruge et al., 2011b), leading to the suggestion that reduction of excessive plasticity may drive the improvement in dystonia. Moreover, higher degree of plasticity in dystonia patients on GPi DBS may be a marker for resistance to the re-emergence of dystonia when the stimulator is turned off (Ruge et al., 2011a). In advanced PD patients with dyskinesia treated with chronic dopaminergic medications, deficient PAS was not corrected with dopaminergic medications (Morgante et al., 2006) but was restored with STN DBS together with medications (Kim et al., 2015), suggesting that there may be synergistic effects between DBS and medications. Repeated applications of STN DBS and TMS timed to coincide with periods of increased motor cortical excitability from STN DBS (at ~3 and 23 ms) also led to long-term potentiation-like plasticity in the motor cortex (Udupa et al., 2016) (Figure 4). These studies suggest that modulation of cortical plasticity is a potential mechanism of action of DBS in the treatment of PD and dystonia.
Figure 4.
Induction of plasticity by pairing subthalamic nucleus deep brain stimulation (STN DBS) and motor cortical transcranial magnetic stimulation (TMS) at specific intervals in patients with Parkinson’s disease. The short (~ 3 ms) and medium (~23 ms) intervals from STN DBS to motor cortical TMS that produced the greatest cortical facilitation was first determined for each Parkinson’s disease patient. Three plasticity protocols with 180 pairs of STN DBS and motor cortical TMS delivered at the individually determined short and medium intervals and at a control (167 ms, DBS set at 3 Hz) interval were tested. The figure shows the motor-evoked potential (MEP) amplitude from a hand muscle (first dorsal interosseous) at different times after the plasticity intervention as a ratio to the MEP amplitude at baseline before the plasticity intervention. Ratios > 1 indicate facilitation and ratios <1 indicate inhibition. The plasticity protocol increased MEP amplitudes at the short and medium intervals, but not at the control interval. From (Udupa et al., 2016) with permission.
Cortical stimulation can influence the basal ganglia. Beta (13 – 30 Hz) oscillations are increased in the basal ganglia in PD patients and is considered a marker of the parkinsonian state. TMS of the primary motor cortex and the supplementary motor area transiently reduced beta oscillations in the STN as measured with DBS electrodes (Gaynor et al., 2008).
The combination of TMS and DBS in various ways can be useful to study pathophysiology and also improve understanding of the mechanism of action of therapeutic DBS. The studies reviewed here show that DBS can change cortical excitability and plasticity with important differences between acute and chronic stimulation. In PD, the effects of DBS may be different from dopaminergic medications. Modulation of cortical plasticity could be involved in mediating the clinical effects of DBS. While these studies are clearly useful, their invasive nature means that this technique will not have clinical application.
TMS-PET
Repetitive transcranial magnetic stimulation (rTMS) is a powerful technique to alter human brain behavior and activity. The advance of neuroimaging techniques, such as positron emission tomography (PET) has allowed researchers to see what structures are affected outside of M1, and thereby learn more about the underlying neural mechanisms of rTMS (Siebner et al., 2009). Another advantage of PET imaging is the ability to measure changes in neurotransmitters in cortical and sub-cortical regions. The majority of rTMS-PET studies have focused on changes in regional cerebral blood flow (rCBF), glucose metabolism and the dopaminergic system. Among the first studies to combine rTMS and PET, stimulation of the frontal eye field and M1 caused focal increases in rCBF change at the site of stimulation and in functionally connected areas (Fox et al., 1997, Paus et al., 1997). Further, Siebner et al. (Siebner et al., 2001) reported a positive relationship between rTMS frequency and cortical activation. When applied over M1 or left DLPFC (Knoch et al., 2006), both 1 Hz and 5 Hz rTMS increased rCBF in the stimulated region while remote areas were differentially modulated, suggesting that rTMS frequency may affect differently anatomically and functionally connected areas. The observation of rTMS-induced modulation in regions outside the stimulated area (Cho et al., 2009) is indeed a phenomenon commonly observed with rTMS-PET, thus providing a direct measure of synaptic activity and neurotransmitter release.
Of particular interest is the dopamine (DA) pathways due to their involvement in many brain functions (e.g., executive function, reward, motor learning) as well as in disease states such as Parkinson’s disease (PD), addiction, schizophrenia and depression. PET radiotracers for DA receptors have been used extensively in neuroscience research, and the development of high-affinity ligands such as [11C]FLB457 and [18F]fallypride may allow for quantification of DA receptors outside of the striatal region (Ko et al., 2012). These radiotracers while offering the ability to study DA release may also allow investigating cortical-subcortical neural networks in the healthy and brain diseases. Thus, the benefit of PET imaging in this regard is the ability to map rTMS-induced changes in neurotransmitters to advance our understanding of brain connectivity. Animal studies have demonstrated the influence of the frontal cortex on the release of striatal DA. Using microdialysis techniques, rTMS of the frontal cortex modulates DA release in subcortical regions such as nucleus accumbens and striatal regions (Keck et al., 2000). Using rTMS-PET, Strafella et al. (Strafella et al., 2001) demonstrated this phenomenon also in the human brain. Specifically, rTMS of the PFC and M1 induced DA release in the ipsilateral caudate nucleus and putamen, respectively (Strafella et al., 2001, Strafella et al., 2003). These subcortical regions receive most of their projections from the stimulated area, thus underlying the importance of the prefrontal-striatal circuitry in modulating dopamine. In a later study, Cho and Strafella (Cho et al., 2009) used the PET ligand [11C]FLB 457 to investigate extrastriatal DA changes following rTMS of the dorsolateral prefrontal cortex (DLPFC). rTMS induced DA release in the ipsilateral subgenual anterior cingulate (ACC), and medial orbitofrontal cortex following stimulation of only the left DLPFC. The dense anatomical connections between DLPFC and ACC suggest the possibility that these effects might be mediated by local activation of the nerve terminals. Overall these observations have implications for testing dopaminergic-related neural networks impacting on brain connectivity and potentially the therapeutic role of rTMS in disease states, such as PD, depression, addition and schizophrenia.
In cognitive neuroscience, rTMS is often used to investigate functional associations between cognitive tasks and brain circuits. Inhibitory continuous theta-burst stimulation (cTBS) applied to the left DLPFC of healthy adults can disrupt the performance of an executive task (e.g., Montreal card sorting task [MCST]) and interfere with the underlying neural network (Ko et al., 2008). Impulsive decision making involves the fronto-striatal dopaminergic pathways and can be probed using a delayed-discounting (DD) or go/no-go tasks combined with rTMS (Obeso et al., 2013). Using the DD task, rTMS of the medial prefrontal cortex (MePFC) increased subject preference towards larger-delayed rewards and influenced subcortical neural pathways with striatal DA release in the bilateral dorsal striatum (Cho et al., 2015) (Figure 5). Instead, in a glucose metabolism PET study, cTBS over the right pre-supplementary motor area (pre-SMA) increased rCBF in the underlying region and influenced the inhibitory control circuits during the go/no-go task (Obeso et al., 2013).
Figure 5.
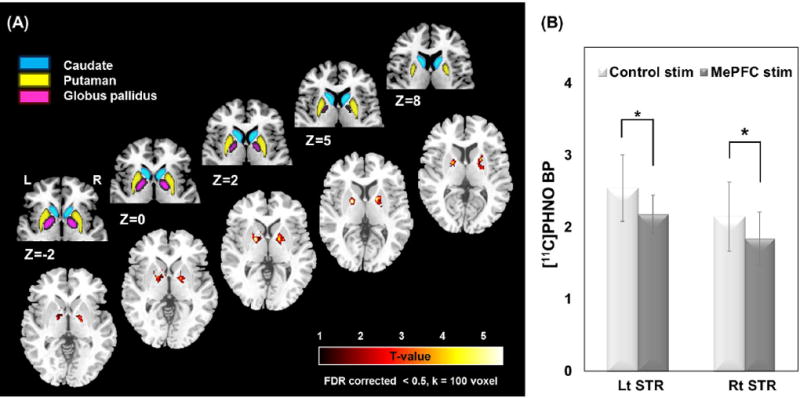
Release of dopamine is assessed using PET following rTMS of the medial prefrontal cortex. Release is demonstrated by a reduction in [11C]-(+)-PHNO binding. Part a shows an anatomical representation of the basal ganglia (upper row) and the t-value of the release highest in the bilateral dorsal putamen (DPu) and bilateral dorsal/ventral globus pallidus (GP) (lower row). Quantification of the reduction in the striatum is illustrated in part b. This observation shows that the prefrontal-striatal fibers can modulate release of dopamine. Image from (Cho et al., 2015) with permission.
The cortical-subcortical effects of brain stimulation provide the rationale for rTMS to be used as a disease-modifying therapy in neurological conditions. Kim et al. (Kim et al., 2008) delivered 5 Hz rTMS over M1 hand area and reported increased DA in the putamen and caudate nucleus bilaterally. In subsequent studies motor performance improved following repeated rTMS treatments and was paralleled by an increased serum DA levels (Khedr et al., 2007). These findings suggest that rTMS-induced modulation of dopaminergic networks may influence motor performance. The use of rTMS has highlighted the dysfunctional circuitry in depression, and rTMS may correct this abnormality and may alleviate depressive symptoms (Dell'Osso et al., 2015). Not all reports, however, have found patients with depression to be responsive to rTMS, In fact, in a double-blind controlled trial, rTMS was not significantly superior to sham rTMS for treatment-resistant depressed patients (Boutros et al., 2002). Similar observations were also reported in other two studies (Loo et al., 2003, Hansen et al., 2004), where rTMS did not show evidence of better outcome compared to sham stimulation in treating resistant depression. These reports highlight the importance of placebo controlled experiments when testing rTMS efficacy on symptom improvements.
PET imaging has certainly contributed to new exciting information on rTMS-induced effects on brain activity and to investigate the connectivity of cortical-subcortical neural networks. New developments in radioligand specificity will provide further understanding of neural circuitries in the healthy and brain diseases.
TMS-fMRI
There are two methods for recording fMRI, event-related fMRI and steady-state fMRI. Event-related fMRI detects BOLD changes while the subject performs a task or there is a perturbation such as brain stimulation. The aim of this kind of study is to identify the cortical areas activated by the event, revealing functional anatomy. Steady-state fMRI can detect low frequency fluctuations in BOLD signal (<0.1 Hz), and when done during relaxation can be called resting-state fMRI. The aim of this method is to identify synchronization between different brain regions, revealing functional connectivity and networks. Consequently, fMRI has been successful to assess functional anatomy, functional connectivity and networks.
For TMS-fMRI studies, both the on-line approach (fMRI during TMS) and off-line approach (fMRI before and after TMS) have been used. In the online approach, TMS is delivered while fMRI data are acquired. Online TMS-fMRI experiments are technically demanding because fMRI has an adverse influence on TMS setup due to the high magnetic field strength and TMS has an adverse influence on fMRI data acquisition due to the electromagnetic artifacts. In the offline approach, TMS is performed before and after fMRI. Off-line TMS-fMRI experiments are technically easier to accomplish because rTMS and fMRI can be separated in time and space.
Based on the above arguments, we have several kinds of TMS-fMRI method for connectivity analysis: Event-related or steady-state analysis, single-pulse TMS or rTMS intervention, and online or offline recording. We summarize some results in the following sections.
In the online approach, Bohning et al. (Bohning et al., 1999, Bohning et al., 2000) demonstrated that suprathreshold rTMS on primary motor cortex (M1) increased the BOLD signal at the stimulated site and remote areas of the motor network, comprising supplementary motor area (SMA), premotor cortex, cingulate, putamen, and thalamus. However, when subthreshold rTMS was applied, no activity changes were observed in M1 and only the remote motor network activities were affected (Bestmann et al., 2004). Subsequent experiments showed that the activity in M1 after suprathreshold rTMS is induced by processing of hand muscle afferents (Shitara et al., 2011). Thus, online TMS-fMRI holds great promise to supplement our understanding about the immediate and rapid changes generated by TMS in cortical networks (Ko et al., 2013).
Additionally, in the online approach, we can assess higher cortical function using “virtual lesion” induced by TMS. In one illustrative example, Sack et al. (Sack et al., 2007) performed TMS over left or right parietal cortex in normal subjects during a cognition task to produce visuospatial neglect. Right parietal TMS disrupted spatial performance. Online fMRI revealed effects of right parietal TMS on BOLD signals in right frontal and parietal cortexes that correlated with the behavioral effects. These findings were observed in right parietal TMS but not left. This study suggested that visuospatial neglect following parietal damage is mediated by specific fronto-parietal networks rather than the lesioned parietal cortex alone.
In one type of offline approach, i.e., fMRI before TMS, task-based fMRI is employed to guide the target cortical areas such as language networks. Here, “virtual lesion” is often applied to the fMRI-navigated regions. For example, Murakami et al. (Murakami et al., 2015) investigated left dorsal and ventral speech stream components and their contribution to phonological processing, which revealed the connection between those speech stream areas and the M1 for lip muscles. This study demonstrated brain connectivity and networks for phonological processing, especially the importance of left dorsal speech stream.
In another type of offline approach, the after effect induced by rTMS needs to be sustained during a resting-state fMRI study. For example, the bidirectional effect of quadripulse stimulation (QPS) has been studied by using resting-state fMRI (Watanabe et al., 2014). Excitatory QPS decreased interhemispheric functional connectivity between bilateral M1, whereas inhibitory QPS increased it. The bidirectional effects of QPS were also observed in the stimulation over prefrontal and parietal association areas. These findings provided evidence for the robust bidirectional effects of excitatory and inhibitory QPS on functional connectivity (Figure 6). Also, using this stimulation method, Watanabe et al. (Watanabe et al., 2015) studied the effects of QPS over pre-SMA on stop-signal task performance. Based on the correlation between the performance changes and fMRI changes induced by QPS, the authors speculated that the indirect pathway in the basal ganglia may play an important role in the task of stopping a preprogrammed action. These studies demonstrated the impact of QPS over M1 or pre-SMA on brain connectivity and networks in TMS-fMRI research.
Figure 6.
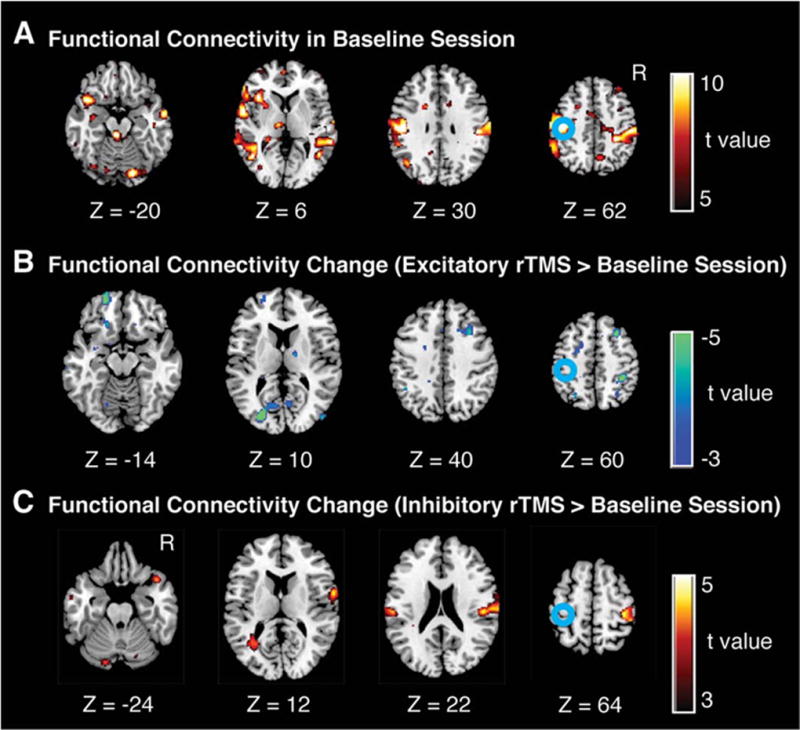
Changes in resting-state fMRI after QPS over the left M1. A. Statistical maps of functional connectivity with the left M1 in pre-QPS sessions (i.e., baseline sessions). B and C. Changes in resting-state fMRI with the left M1 induced by excitatory QPS (panel B) and inhibitory QPS (panel C). The color scale shown on the right indicates statistical significance level. The blue circles indicate the approximate location of the left M1, which was determined by the functional localizer scan. These panels indicate the subtraction between the functional connectivity maps of the pre-QPS and post-QPS sessions; as a consequence, the local effects were eliminated. (Cited from (Watanabe et al., 2014), with permission)
TMS-fMRI research has been applied not only to normal subjects, but also to patients. Li et al. (Li et al., 2004) used online TMS-fMRI approach in patients with depression to investigate the brain regions affected by rTMS of left dorsolateral prefrontal cortex (DLPFC) which is targeted by rTMS therapy for major depression. Increased activity was found at the site of stimulation as well as in connected limbic regions. This study demonstrated that TMS to DLPFC can indeed affect entire brain networks associated with depression.
The combination of TMS and fMRI has revealed normal brain connectivity and networks such as motor (Moisa et al., 2012), sensory (Blankenburg et al., 2008), visual (Ruff et al., 2008), auditory (Andoh et al., 2015), verbal (Murakami et al., 2015), and oculomotor networks (Neggers et al., 2007). This method has also been applied to analyze the networks responsible for higher cerebral functions such as attention (Leitao et al., 2015), working memory (Lorenc et al., 2015), perception (Pitcher et al., 2014), learning (Steel et al., 2016), and decision making (Rahnev et al., 2016). Furthermore, recently, the application of these methods has been expanded to some patients with neurological and psychological disorders such as Parkinson’s disease (Cerasa et al., 2015), stroke (Cunningham et al., 2015), epilepsy (Makela et al., 2013), pain (Martin et al., 2013), addiction (Hanlon et al., 2015), brain injury (Guller et al., 2014), peripheral nerve injury (Li et al., 2015), depression (Li et al., 2004), and schizophrenia (Gromann et al., 2012, Guller et al., 2012).
One feature of TMS-fMRI is that the changes in BOLD signal are often more evident in regions distant from the stimulated one (Arfeller et al., 2013). This result reflects functional connectivity effects, either direct or compensatory. One possible explanation is that direct activation by electricity utilizes no synaptic activation, but distant areas need synaptic activation. Synaptic activation needs more than 10 times the energy of axonal activation (Attwell et al., 2001). Thus the distant area would be associated with more BOLD changes. Another feature is that the changes in BOLD may take different directions (increase or decrease) unpredictably.
In assessing the functional or effective connectivity, mathematical models have played important roles in TMS-fMRI studies, just like TMS-EEG and TMS-PET studies. Among the various mathematical models, we need to select the appropriate one for the question to be addressed, e.g., graph-theoretical analysis, dynamic causal modeling, and computational modeling (Hartwigsen et al., 2013, Park et al., 2014, Cocchi et al., 2016).
While fMRI has certainly contributed to new information about rTMS-induced effects on brain activity and on the connectivity of various neural networks in healthy subjects and in neurological and psychological disorders, there are several limitations. For on-line TMS-fMRI experiments, the electromagnetic fields generated by both techniques have an effect on the data acquisition of the other. Therefore, on-line approaches are technically demanding. Off-line TMS-fMRI approaches are technically easier. However, to obtain robust data in off-line experiments, a stable long lasting rTMS effect, such as those by QPS, is required.
While TMS-fMRI is a fruitful method, there are clearly technical challenges. Hence, it will be used more for research studies than in the clinical area.
Conclusion
TMS, and other non-invasive stimulation techniques not reviewed here, integrate well with a number of assessment methods to explore brain circuitry. Stimulation can either probe a network or alter a network for later analysis. EEG detects electrophysiological activity, PET detects blood supply changes or metabolic changes, TMS detects changes in excitability, and fMRI usually detects the complex BOLD signal. When looking for the same TMS effect, if, for example, some inhibitory interneurons are active, metabolism of neurons, and blood supply for that action may increase (PET and fMRI increase), and TMS excitability may decrease, and EEG might show some inhibition. Each technique has advantages and disadvantages. The different techniques complement each other, and the use of several of these combinations will likely give a clearer picture of the physiology than by using only one of them.
The methods described here are certainly useful for research into brain physiology and, as they mature, at least some of them will likely also be useful for clinical assessment and diagnosis. Advances in recent years have been possible due to improvements in technology, and further improvements are to be expected both for stimulation and recording. We can expect to learn more about how the brain works, how it malfunctions in disease, and how we might treat patients better – also perhaps with non-invasive stimulation.
Highlights.
Non-invasive brain stimulation can reveal brain connections
Results can be assessed with tools such as EEG or the effects on another brain stimulus
Neuroimaging can be used to map TMS-induced changes in activity and connectivity.
Acknowledgments
Dr. Hallett is supported by the NINDS Intramural Program.
FULL DISCLOSURES
Mark Hallett: Dr. Hallett serves as Chair of the Medical Advisory Board for and may receive honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Editorial Board of approximately 20 journals, and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, and Elsevier. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by UniQure for a clinical trial of AAV2-GDNF for Parkinson Disease, Merz for treatment studies of focal hand dystonia, and Allergan for studies of methods to inject botulinum toxins.
Riccardo Di Iorio: None
Paolo Maria Rossini: None
Jung E Park: Dr. Park received grants from the Dystonia Medical Research Foundation and SK chemicals
Robert Chen; Dr. Chen received research support from the Canadian Institutes of Health Research, Catherine Manson Chair in Movement Disorders, Medtronic Inc and Merz. He was a consultant for Merz, Allergan and UCB.
Pablo Celnik: Dr Celnik receives financial support from the National Institutes of Health, National Institute of Child Health and Human Development, Grants R01HD053793 and R01HD073147
Antonio P. Strafella: Dr. Strafella received research funding from Canadian Institute of Health Research, Canada Research Chair Program, National Parkinson Foundation and Parkinson Society Canada.
Hideyuki Matsumoto: None
Yoshikazu Ugawa; Dr. Ugawa was supported by a Research Project Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by grants from the Research Committee on degenerative ataxia from the Ministry of Health and Welfare of Japan; by the Research Committee on Insomnia in Parkinson’s Disease from the Ministry of Health and Welfare of Japan; by a grant from the Committee of the Study of Human Exposure to EMF from the Ministry of Public Management, Home Affairs, Post and Telecommunications; and by a grant from the Uehara Memorial Foundation. He has also received speaker’s honorariums from the Taiwan Movement Disorders Society, Astellas Pharma Inc., Eisai Co., Ltd., FP Pharmaceutical Corporation, Otsuka Pharmaceutical Co., Ltd., Elsevier Japan K. K., Kissei Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., GlaxoSmithKline K. K., Sanofi-Aventis K.K., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Teijin Pharmaceutical Ltd., Nippon Chemiphar Co., Ltd., Nihon Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin, Ltd., and Mochida Pharmaceutical Co., Ltd. He has received royalties from Chugai-Igakusha, Igaku-Shoin Ltd., Medical View Co. Ltd., and Blackwell Publishing KK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None of the authors have potential conflicts of interest to be disclosed.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaishi R, Ueda N, Sakai K. Task-related modulation of effective connectivity during perceptual decision making: dissociation between dorsal and ventral prefrontal cortex. Front Human Neurosci. 2013;7:365. doi: 10.3389/fnhum.2013.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh J, Matsushita R, Zatorre RJ. Asymmetric Interhemispheric Transfer in the Auditory Network: Evidence from TMS, Resting-State fMRI, and Diffusion Imaging. J Neurosci. 2015;35:14602–11. doi: 10.1523/JNEUROSCI.2333-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N, Lu MK, Ugawa Y, Ziemann U. Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp Brain Res. 2012;220:79–87. doi: 10.1007/s00221-012-3117-5. [DOI] [PubMed] [Google Scholar]
- Arai N, Muller-Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, et al. State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network. J Neurosci. 2011;31:15376–83. doi: 10.1523/JNEUROSCI.2271-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfeller C, Schwarzbach J, Ubaldi S, Ferrari P, Barchiesi G, Cattaneo L. Whole-brain haemodynamic after-effects of 1-Hz magnetic stimulation of the posterior superior temporal cortex during action observation. Brain Topogr. 2013;26:278–91. doi: 10.1007/s10548-012-0239-9. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bailey NW, Thomson RH, Hoy KE, Hernandez-Pavon JC, Fitzgerald PB. TDCS increases cortical excitability: Direct evidence from TMS-EEG. Cortex. 2016;74:320–2. doi: 10.1016/j.cortex.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–23. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–62. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–8. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, et al. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45:385–94. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Wassermann EM, Ziemann U, Lorberbaum JP, Nahas Z, et al. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS) J Magn Reson Imaging. 2000;11:569–74. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bonato C, Miniussi C, Rossini PM. Transcranial magnetic stimulation and cortical evoked potentials: a TMS/EEG co-registration study. Clin Neurophysiol. 2006;117:1699–707. doi: 10.1016/j.clinph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Bonni S, Ponzo V, Caltagirone C, Koch G. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct Neurol. 2014;29:41–5. [PMC free article] [PubMed] [Google Scholar]
- Bortoletto M, Veniero D, Thut G, Miniussi C. The contribution of TMS-EEG coregistration in the exploration of the human cortical connectome. Neurosci Biobehav Rev. 2015;49:114–24. doi: 10.1016/j.neubiorev.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Gueorguieva R, Hoffman RE, Oren DA, Feingold A, Berman RM. Lack of a therapeutic effect of a 2-week sub-threshold transcranial magnetic stimulation course for treatment-resistant depression. Psychiatry Res. 2002;113:245–54. doi: 10.1016/s0165-1781(02)00267-6. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Human Neurosci. 2015;9:286. doi: 10.3389/fnhum.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Romano M, Giglia G, Saia V, Puma A, Giglia F, et al. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res. 2009;192:651–6. doi: 10.1007/s00221-008-1572-9. [DOI] [PubMed] [Google Scholar]
- Brusa L, Ponzo V, Mastropasqua C, Picazio S, Bonni S, Di Lorenzo F, et al. Theta burst stimulation modulates cerebellar-cortical connectivity in patients with progressive supranuclear palsy. Brain Stimul. 2014;7:29–35. doi: 10.1016/j.brs.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Muller JF, et al. Functional connectivity between secondary and primary motor areas underlying hand-foot coordination. J Neurophysiol. 2007;98:414–22. doi: 10.1152/jn.00325.2007. [DOI] [PubMed] [Google Scholar]
- Carrillo F, Palomar FJ, Conde V, Diaz-Corrales FJ, Porcacchia P, Fernandez-Del-Olmo M, et al. Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson's disease. Brain Stimul. 2013;6:582–9. doi: 10.1016/j.brs.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Casula EP, Tarantino V, Basso D, Arcara G, Marino G, Toffolo GM, et al. Low-frequency rTMS inhibitory effects in the primary motor cortex: Insights from TMS-evoked potentials. Neuroimage. 2014;98:225–32. doi: 10.1016/j.neuroimage.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Celnik P. Understanding and modulating motor learning with cerebellar stimulation. Cerebellum. 2015;14:171–4. doi: 10.1007/s12311-014-0607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Koch G, Donzuso G, Mangone G, Morelli M, Brusa L, et al. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain. 2015;138:414–27. doi: 10.1093/brain/awu329. [DOI] [PubMed] [Google Scholar]
- Chao CC, Karabanov AN, Paine R, Carolina de Campos A, Kukke SN, Wu T, et al. Induction of motor associative plasticity in the posterior parietal cortex-primary motor network. Cereb Cortex. 2015;25:365–73. doi: 10.1093/cercor/bht230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Garg RR, Lozano AM, Lang AE. Effects of internal globus pallidus stimulation on motor cortex excitability. Neurology. 2001;56:716–23. doi: 10.1212/wnl.56.6.716. [DOI] [PubMed] [Google Scholar]
- Cho SS, Koshimori Y, Aminian K, Obeso I, Rusjan P, Lang AE, et al. Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40:546–53. doi: 10.1038/npp.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB. Measuring Brain Stimulation Induced Changes in Cortical Properties Using TMS-EEG. Brain Stimul. 2015;8:1010–20. doi: 10.1016/j.brs.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Cipollari S, Veniero D, Razzano C, Caltagirone C, Koch G, Marangolo P. Combining TMS-EEG with transcranial direct current stimulation language treatment in aphasia. Expert Rev Neurother. 2015;15:833–45. doi: 10.1586/14737175.2015.1049998. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–53. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Sale MV, L LG, Bell PT, Nguyen VT, Zalesky A, et al. A hierarchy of timescales explains distinct effects of local inhibition of primary visual cortex and frontal eye fields. eLife. 2016;5 doi: 10.7554/eLife.15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417–24. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson's disease. Neurology. 2002;58:1665–72. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- Cunningham DA, Machado A, Janini D, Varnerin N, Bonnett C, Yue G, et al. Assessment of inter-hemispheric imbalance using imaging and noninvasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil. 2015;96:S94–103. doi: 10.1016/j.apmr.2014.07.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E. The organization of plasticity in the cerebellar cortex: from synapses to control. Prog Brain Res. 2014;210:31–58. doi: 10.1016/B978-0-444-63356-9.00002-9. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Radhu N, Fitzgerald PB. Combined transcranial magnetic stimulation and electroencephalography: its past, present and future. Brain Res. 2012;1463:93–107. doi: 10.1016/j.brainres.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586:2735–42. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Montague K, Olivier E, Rothwell JC, Lemon RN. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–7. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Osso B, Oldani L, Camuri G, Dobrea C, Cremaschi L, Benatti B, et al. Augmentative repetitive Transcranial Magnetic Stimulation (rTMS) in the acute treatment of poor responder depressed patients: a comparison study between high and low frequency stimulation. Eur Psychiatry. 2015;30:271–6. doi: 10.1016/j.eurpsy.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. Transcranial magnetic stimulation in the presence of deep brain stimulation implants: Induced electrode currents. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:6821–4. doi: 10.1109/IEMBS.2010.5625958. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, et al. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–4. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Farzan F, Vernet M, Shafi MM, Rotenberg A, Daskalakis ZJ, Pascual-Leone A. Characterizing and Modulating Brain Circuitry through Transcranial Magnetic Stimulation Combined with Electroencephalography. Front Neural Circuits. 2016;10:73. doi: 10.3389/fncir.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol (London) 1992;453:525–46. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Rossini PM. TMS and TMS-EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev Neurosci. 2013;24:431–42. doi: 10.1515/revneuro-2013-0019. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Vecchio F, Vollero L, Guerra A, Petrichella S, Ponzo D, et al. Sensorimotor cortex excitability and connectivity in Alzheimer's disease: A TMS-EEG Co-registration study. Hum Brain Mapp. 2016;37:2083–96. doi: 10.1002/hbm.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KM, Lai HM, Baker MR, Baker SN. Corticospinal activation confounds cerebellar effects of posterior fossa stimuli. Clin Neurophysiol. 2009;120:2109–13. doi: 10.1016/j.clinph.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, et al. Imaging human intracerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–91. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Frantseva M, Cui J, Farzan F, Chinta LV, Perez Velazquez JL, Daskalakis ZJ. Disrupted cortical conductivity in schizophrenia: TMS-EEG study. Cereb Cortex. 2014;24:211–21. doi: 10.1093/cercor/bhs304. [DOI] [PubMed] [Google Scholar]
- Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88:220–35. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principalcomponent analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–22. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JO, Grossman ED, Srinivasan R. Evoked potentials in large-scale cortical networks elicited by TMS of the visual cortex. J Neurophysiol. 2011;106:1734–46. doi: 10.1152/jn.00739.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor LM, Kuhn AA, Dileone M, Litvak V, Eusebio A, Pogosyan A, et al. Suppression of beta oscillations in the subthalamic nucleus following cortical stimulation in humans. Eur J Neurosci. 2008;28:1686–95. doi: 10.1111/j.1460-9568.2008.06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambattistelli F, Tomasevic L, Pellegrino G, Porcaro C, Melgari JM, Rossini PM, et al. The spontaneous fluctuation of the excitability of a single node modulates the internodes connectivity: a TMS-EEG study. Hum Brain Mapp. 2014;35:1740–9. doi: 10.1002/hbm.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromann PM, Tracy DK, Giampietro V, Brammer MJ, Krabbendam L, Shergill SS. Examining frontotemporal connectivity and rTMS in healthy controls: implications for auditory hallucinations in schizophrenia. Neuropsychology. 2012;26:127–32. doi: 10.1037/a0026603. [DOI] [PubMed] [Google Scholar]
- Groppa S, Schlaak BH, Munchau A, Werner-Petroll N, Dunnweber J, Baumer T, et al. The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum Brain Mapp. 2012a;33:419–30. doi: 10.1002/hbm.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Werner-Petroll N, Munchau A, Deuschl G, Ruschworth MF, Siebner HR. A novel dual-site transcranial magnetic stimulation paradigm to probe fast facilitatory inputs from ipsilateral dorsal premotor cortex to primary motor cortex. Neuroimage. 2012b;62:500–9. doi: 10.1016/j.neuroimage.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Guller Y, Ferrarelli F, Shackman AJ, Sarasso S, Peterson MJ, Langheim FJ, et al. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry. 2012;69:662–71. doi: 10.1001/archgenpsychiatry.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller Y, Giacino J. Potential applications of concurrent transcranial magnetic stimulation and functional magnetic resonance imaging in acquired brain injury and disorders of consciousness. Brain Inj. 2014;28:1190–6. doi: 10.3109/02699052.2014.920527. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42:177–84. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, et al. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–59. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, DeVries W, Dowdle LT, West JA, Siekman B, Li X, et al. A comprehensive study of sensorimotor cortex excitability in chronic cocaine users: Integrating TMS and functional MRI data. Drug Alcohol Depend. 2015;157:28–35. doi: 10.1016/j.drugalcdep.2015.07.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PE, Videbech P, Clemmensen K, Sturlason R, Jensen HM, Vestergaard P. Repetitive transcranial magnetic stimulation as add-on antidepressant treatment. The applicability of the method in a clinical setting. Nord J Psychiatry. 2004;58:455–7. doi: 10.1080/08039480410011678. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Matuschek J, Fellner MC. Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr Biol. 2014;24:904–9. doi: 10.1016/j.cub.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, Saur D, Price CJ, Ulmer S, Baumgaertner A, Siebner HR. Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proc Natl Acad Sci U S A. 2013;110:16402–7. doi: 10.1073/pnas.1310190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidding U, Baumer T, Siebner HR, Demiralay C, Buhmann C, Weyh T, et al. MEP latency shift after implantation of deep brain stimulation systems in the subthalamic nucleus in patients with advanced Parkinson's disease. Mov Disord. 2006;21:1471–6. doi: 10.1002/mds.20951. [DOI] [PubMed] [Google Scholar]
- Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci Biobehav Rev. 2016;64:175–84. doi: 10.1016/j.neubiorev.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia. Eur J Neurosci. 2012;35:478–85. doi: 10.1111/j.1460-9568.2011.07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–40. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21:1901–9. doi: 10.1093/cercor/bhq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov AN, Chao CC, Paine R, Hallett M. Mapping different intra-hemispheric parietal-motor networks using twin Coil TMS. Brain Stimul. 2013;6:384–9. doi: 10.1016/j.brs.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Sillaber I, Ebner K, Welt T, Toschi N, Kaehler ST, et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur J Neurosci. 2000;12:3713–20. doi: 10.1046/j.1460-9568.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Foly N, Hamdy A. Dopamine levels after repetitive transcranial magnetic stimulation of motor cortex in patients with Parkinson's disease: preliminary results. Mov Disord. 2007;22:1046–50. doi: 10.1002/mds.21460. [DOI] [PubMed] [Google Scholar]
- Kim JY, Chung EJ, Lee WY, Shin HY, Lee GH, Choe YS, et al. Therapeutic effect of repetitive transcranial magnetic stimulation in Parkinson's disease: analysis of [11C] raclopride PET study. Mov Disord. 2008;23:207–11. doi: 10.1002/mds.21787. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Udupa K, Ni Z, Moro E, Gunraj C, Mazzella F, et al. Effects of subthalamic nucleus stimulation on motor cortex plasticity in Parkinson disease. Neurology. 2015;85:425–32. doi: 10.1212/WNL.0000000000001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Treyer V, Regard M, Muri RM, Buck A, Weber B. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage. 2006;31:641–8. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[(11)C]raclopride PET study. Eur J Neurosci. 2008;28:2147–55. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Strafella AP. Dopaminergic neurotransmission in the human brain: new lessons from perturbation and imaging. Neuroscientist. 2012;18:149–68. doi: 10.1177/1073858411401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Tang CC, Eidelberg D. Brain stimulation and functional imaging with fMRI and PET. Handb Clin Neurol. 2013;116:77–95. doi: 10.1016/B978-0-444-53497-2.00008-5. [DOI] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, et al. In vivo definition of parieto-motor connections involved in planning of grasping movements. Neuroimage. 2010;51:300–12. doi: 10.1016/j.neuroimage.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–22. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, et al. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci. 2008a;28:5944–53. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, et al. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 2006;26:7452–9. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Mori F, Marconi B, Codeca C, Pecchioli C, Salerno S, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008b;119:2559–69. doi: 10.1016/j.clinph.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Koch G, Ponzo V, Di Lorenzo F, Caltagirone C, Veniero D. Hebbian and anti-Hebbian spike-timing-dependent plasticity of human cortico-cortical connections. J Neurosci. 2013;33:9725–33. doi: 10.1523/JNEUROSCI.4988-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, et al. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009;587:4281–92. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Nakatsuka M, Ueki Y, Fukuyama H, Domen K. Human motor associative plasticity induced by paired bihemispheric stimulation. J Physiol. 2009;587:4629–44. doi: 10.1113/jphysiol.2009.174342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S, Aronen HJ, Huttunen J, Kesaniemi M, Soinne L, Nikouline VV, et al. Ipsi- and contralateral EEG reactions to transcranial magnetic stimulation. Clin Neurophysiol. 2002;113:175–84. doi: 10.1016/s1388-2457(01)00721-0. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kahkonen S. The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev. 2006;52:183–92. doi: 10.1016/j.brainresrev.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Huebl J. Safety of transcranial magnetic stimulation for the newer generation of deep brain stimulators. Parkinsonism Relat Disord. 2011;17:647–8. doi: 10.1016/j.parkreldis.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Meyer BU, Trottenberg T, Brandt SA, Schneider GH, Kupsch A. Modulation of motor cortex excitability by pallidal stimulation in patients with severe dystonia. Neurology. 2003;60:768–74. doi: 10.1212/01.wnl.0000044396.64752.4c. [DOI] [PubMed] [Google Scholar]
- Kuriakose R, Saha U, Castillo G, Udupa K, Ni Z, Gunraj C, et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson's disease. Cereb Cortex. 2010;20:1926–36. doi: 10.1093/cercor/bhp269. [DOI] [PubMed] [Google Scholar]
- Leitao J, Thielscher A, Tunnerhoff J, Noppeney U. Concurrent TMS-fMRI Reveals Interactions between Dorsal and Ventral Attentional Systems. J Neurosci. 2015;35:11445–57. doi: 10.1523/JNEUROSCI.0939-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Hua XY, Zheng MX, Wang WW, Xu JG, Gu YD, et al. Different cerebral plasticity of intrinsic and extrinsic hand muscles after peripheral neurotization in a patient with brachial plexus injury: A TMS and fMRI study. Neurosci Lett. 2015;604:140–4. doi: 10.1016/j.neulet.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Li X, Nahas Z, Kozel FA, Anderson B, Bohning DE, George MS. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol Psychiatry. 2004;55:882–90. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Litvak V, Komssi S, Scherg M, Hoechstetter K, Classen J, Zaaroor M, et al. Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage. 2007;37:56–70. doi: 10.1016/j.neuroimage.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Loo CK, Mitchell PB, Croker VM, Malhi GS, Wen W, Gandevia SC, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med. 2003;33:33–40. doi: 10.1017/s0033291702006839. [DOI] [PubMed] [Google Scholar]
- Lorenc ES, Lee TG, Chen AJ, D'Esposito M. The Effect of Disruption of Prefrontal Cortical Function with Transcranial Magnetic Stimulation on Visual Working Memory. Front Syst Neurosci. 2015;9:169. doi: 10.3389/fnsys.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela JP, Vitikainen AM, Lioumis P, Paetau R, Ahtola E, Kuusela L, et al. Functional plasticity of the motor cortical structures demonstrated by navigated TMS in two patients with epilepsy. Brain Stimul. 2013;6:286–91. doi: 10.1016/j.brs.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, et al. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci. 2009;29:6926–31. doi: 10.1523/JNEUROSCI.1396-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Borckardt JJ, Reeves ST, Frohman H, Beam W, Nahas Z, et al. A pilot functional MRI study of the effects of prefrontal rTMS on pain perception. Pain Med. 2013;14:999–1009. doi: 10.1111/pme.12129. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Sarasso S, Tononi G. Cortical mechanisms of loss of consciousness: insight from TMS/EEG studies. Arch Ital Biol. 2012;150:44–55. doi: 10.4449/aib.v150i2.1361. [DOI] [PubMed] [Google Scholar]
- Maule F, Barchiesi G, Brochier T, Cattaneo L. Haptic working memory for grasping: the role of the parietal operculum. Cereb Cortex. 2015;25:528–37. doi: 10.1093/cercor/bht252. [DOI] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–92. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Thut G. Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr. 2010;22:249–56. doi: 10.1007/s10548-009-0083-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561:331–8. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisa M, Siebner HR, Pohmann R, Thielscher A. Uncovering a context-specific connectional fingerprint of human dorsal premotor cortex. J Neurosci. 2012;32:7244–52. doi: 10.1523/JNEUROSCI.2757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar GF, Sailer A, Gunraj CA, Cunic DI, Lang AE, Lozano AM, et al. Changes in cortical excitability with thalamic deep brain stimulation. Neurology. 2005;64:1913–9. doi: 10.1212/01.WNL.0000163985.89444.DD. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–69. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Murakami T, Kell CA, Restle J, Ugawa Y, Ziemann U. Left dorsal speech stream components and their contribution to phonological processing. J Neurosci. 2015;35:1411–22. doi: 10.1523/JNEUROSCI.0246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitani M, Bodart O, Canali P, Seregni F, Casali A, Laureys S, et al. Transcranial magnetic stimulation combined with high-density EEG in altered states of consciousness. Brain Inj. 2014;28:1180–9. doi: 10.3109/02699052.2014.920524. [DOI] [PubMed] [Google Scholar]
- Naro A, Leo A, Russo M, Cannavo A, Milardi D, Bramanti P, et al. Does Transcranial Alternating Current Stimulation Induce Cerebellum Plasticity? Feasibility, Safety and Efficacy of a Novel Electrophysiological Approach. Brain Stimul. 2016;9:388–95. doi: 10.1016/j.brs.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Huijbers W, Vrijlandt CM, Vlaskamp BN, Schutter DJ, Kenemans JL. TMS pulses on the frontal eye fields break coupling between visuospatial attention and eye movements. J Neurophysiol. 2007;98:2765–78. doi: 10.1152/jn.00357.2007. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, et al. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 2009;19:1654–65. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol. 2010;68:816–24. doi: 10.1002/ana.22221. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Kicic D, Kahkonen S, Ilmoniemi RJ. Modulation of electroencephalographic responses to transcranial magnetic stimulation: evidence for changes in cortical excitability related to movement. Eur J Neurosci. 2003;18:1206–12. doi: 10.1046/j.1460-9568.2003.02858.x. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007;26:2085–95. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Obeso I, Cho SS, Antonelli F, Houle S, Jahanshahi M, Ko JH, et al. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul. 2013;6:769–76. doi: 10.1016/j.brs.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Babiloni C, Filippi MM, Caltagirone C, Babiloni F, Cicinelli P, et al. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Exp Brain Res. 2003;149:214–21. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Panyakaew P, Cho HJ, Srivanitchapoom P, Popa T, Wu T, Hallett M. Cerebellar brain inhibition in the target and surround muscles during voluntary tonic activation. Eur J Neurosci. 2016;43:1075–81. doi: 10.1111/ejn.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang WH, Yoo WK, Shin YI, Kim ST, Kim YH. Brain topological correlates of motor performance changes after repetitive transcranial magnetic stimulation. Brain Connect. 2014;4:265–72. doi: 10.1089/brain.2013.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani S, Barchiesi G, Cattaneo L. The dorsal premotor cortex exerts a powerful and specific inhibitory effect on the ipsilateral corticofacial system: a dual-coil transcranial magnetic stimulation study. Exp Brain Res. 2015;233:3253–60. doi: 10.1007/s00221-015-4393-7. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–84. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phielipp NM, Saha U, Sankar T, Yugeta A, Chen R. Safety of repetitive transcranial magnetic stimulation in patients with implanted cortical electrodes. An ex-vivo study and report of a case. Clin Neurophysiol. 2017;128:1109–15. doi: 10.1016/j.clinph.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Mazzone P, Marciani MG, Rossini PM, Stefani A, et al. Deep brain stimulation of both subthalamic nucleus and internal globus pallidus restores intracortical inhibition in Parkinson's disease paralleling apomorphine effects: a paired magnetic stimulation study. Clin Neurophysiol. 2002;113:108–13. doi: 10.1016/s1388-2457(01)00694-0. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res. 2001;140:505–10. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Duchaine B, Walsh V. Combined TMS and FMRI reveal dissociable cortical pathways for dynamic and static face perception. Curr Biol. 2014;24:2066–70. doi: 10.1016/j.cub.2014.07.060. [DOI] [PubMed] [Google Scholar]
- Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–9. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, et al. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci. 2014;34:5603–12. doi: 10.1523/JNEUROSCI.5089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzoni A, Pirulli C, Veniero D, Feurra M, Cincotta M, Giovannelli F, et al. Vegetative versus minimally conscious states: a study using TMS-EEG, sensory and event-related potentials. PLoS One. 2013;8:e57069. doi: 10.1371/journal.pone.0057069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnev D, Nee DE, Riddle J, Larson AS, D'Esposito M. Causal evidence for frontal cortex organization for perceptual decision making. Proc Natl Acad Sci U S A. 2016;113:6059–64. doi: 10.1073/pnas.1522551113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V, Siebner HS, Morgante F, Mastroeni C, Girlanda P, Quartarone A. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex. 2009;19:907–15. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- Romero Lauro LJ, Rosanova M, Mattavelli G, Convento S, Pisoni A, Opitz A, et al. TDCS increases cortical excitability: direct evidence from TMS-EEG. Cortex. 2014;58:99–111. doi: 10.1016/j.cortex.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–85. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18:817–27. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge D, Cif L, Limousin P, Gonzalez V, Vasques X, Hariz MI, et al. Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain. 2011a;134:2106–15. doi: 10.1093/brain/awr122. [DOI] [PubMed] [Google Scholar]
- Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP, et al. Deep brain stimulation effects in dystonia: time course of electrophysiological changes in early treatment. Mov Disord. 2011b;26:1913–21. doi: 10.1002/mds.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, et al. Imaging the Brain Activity Changes Underlying Impaired Visuospatial Judgments: Simultaneous fMRI, TMS, and Behavioral Studies. Cereb Cortex. 2007;17:2841–52. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Sailer A, Cunic DI, Paradiso GO, Gunraj CA, Wagle-Shukla A, Moro E, et al. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology. 2007;68:356–63. doi: 10.1212/01.wnl.0000252812.95774.aa. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain. 2003;126:1883–94. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Bastian AJ, Celnik PA. Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci. 2012;32:11610–7. doi: 10.1523/JNEUROSCI.1609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Spampinato D, Celnik PA. Laterality Differences in Cerebellar-Motor Cortex Connectivity. Cereb Cortex. 2015;25:1827–34. doi: 10.1093/cercor/bht422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Friston KJ. Network discovery with large DCMs. Neuroimage. 2013;68:181–91. doi: 10.1016/j.neuroimage.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi MM, Vernet M, Klooster D, Chu CJ, Boric K, Barnard ME, et al. Physiological consequences of abnormal connectivity in a developmental epilepsy. Ann Neurol. 2015;77:487–503. doi: 10.1002/ana.24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J, Park JE, Srivanitchapoom P, Paine R, Thirugnanasambandam N, Kukke SN, et al. Probing the interaction of the ipsilateral posterior parietal cortex with the premotor cortex using a novel transcranial magnetic stimulation technique. Clin Neurophysiol. 2016;127:1475–80. doi: 10.1016/j.clinph.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara H, Shinozaki T, Takagishi K, Honda M, Hanakawa T. Time course and spatial distribution of fMRI signal changes during single-pulse transcranial magnetic stimulation to the primary motor cortex. Neuroimage. 2011;56:1469–79. doi: 10.1016/j.neuroimage.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A. Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. Neuroimage. 2001;14:883–90. doi: 10.1006/nimg.2001.0889. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–25. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Steel A, Song S, Bageac D, Knutson KM, Keisler A, Saad ZS, et al. Shifts in connectivity during procedural learning after motor cortex stimulation: A combined transcranial magnetic stimulation/functional magnetic resonance imaging study. Cortex. 2016;74:134–48. doi: 10.1016/j.cortex.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–15. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J. The functional importance of rhythmic activity in the brain. Curr Biol. 2012;22:R658–63. doi: 10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–32. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–85. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch S, Rothwell JC, Bhatia KP, Quinn N, Zrinzo L, Jahanshahi M, et al. Pallidal stimulation modifies after-effects of paired associative stimulation on motor cortex excitability in primary generalised dystonia. Exp Neurol. 2007;206:80–5. doi: 10.1016/j.expneurol.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Tononi G, Boly M, Massimini M, Koch C. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci. 2016;17:450–61. doi: 10.1038/nrn.2016.44. [DOI] [PubMed] [Google Scholar]
- Udupa K, Bahl N, Ni Z, Gunraj C, Mazzella F, Moro E, et al. Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson's Disease. J Neurosci. 2016;36:396–404. doi: 10.1523/JNEUROSCI.2499-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udupa K, Chen R. The mechanisms of action of deep brain stimulation and ideas for the future development. Prog Neurobiol. 2015;133:27–49. doi: 10.1016/j.pneurobio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–13. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, et al. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet M, Brem AK, Farzan F, Pascual-Leone A. Synchronous and opposite roles of the parietal and prefrontal cortices in bistable perception: a double-coil TMS-EEG study. Cortex. 2015;64:78–88. doi: 10.1016/j.cortex.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle Shukla A, Moro E, Gunraj C, Lozano A, Hodaie M, Lang A, et al. Long-term subthalamic nucleus stimulation improves sensorimotor integration and proprioception. J Neurol Neurosurg Psychiatry. 2013;84:1020–8. doi: 10.1136/jnnp-2012-304102. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Ohminami S, Tsutsumi R, Terao Y, et al. Bidirectional effects on interhemispheric resting-state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Hum Brain Mapp. 2014;35:1896–905. doi: 10.1002/hbm.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Tsutsumi R, Shimizu T, Hayashi T, et al. Effects of rTMS of pre-supplementary motor area on fronto basal ganglia network activity during stop-signal task. J Neurosci. 2015;35:4813–23. doi: 10.1523/JNEUROSCI.3761-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Taylor J, Ridding M, Meyer BU, Rothwell JC. Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. EEG Clin Neurophysiol. 1996;101:58–66. doi: 10.1016/0013-4694(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Ziluk A, Premji A, Nelson AJ. Functional connectivity from area 5 to primary motor cortex via paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2010;484:81–5. doi: 10.1016/j.neulet.2010.08.025. [DOI] [PubMed] [Google Scholar]