Summary
Stromal restraint of cancer growth and progression—emerging as a widespread phenomenon in epithelial cancers such as bladder, pancreas, colon, and prostate—appears rooted in stromal cell niche activity. During normal tissue repair, stromal niche signals, often Hedgehog-induced, promote epithelial stem cell differentiation as well as self-renewal, thus specifying regenerating epithelial pattern. In the case of cancerous tissue, stromal cell-derived differentiation signals in particular may provide a brake on malignant growth. Understanding and therapeutic harnessing of the role of stroma in cancer restraint may hinge on our knowledge of the signaling programs elaborated by the stromal niche.
Introduction
The support cells, or niches, that produce factors necessary for stem cell maintenance have been long studied and well characterized for C. elegans and Drosophila germline stem cells (Kiger et al., 2001; Kimble, 1981; Kimble and White, 1981; Spradling et al., 2011); these stem cells are localized in discrete structures, facilitating the identification and study of adjacent niche cells, also organized in morphologically discrete structures. Much less is known about how stem cell niches support the adult stem cells that maintain the integrity of vertebrate organs, in particular organs with complex epithelial architectures, encompassing extensive epithelial sheets or branching chambers and ductal systems. How is the proliferation and differentiation of stem cells regulated in these kinds of complex organs so that regenerating epithelial structures restore the pattern of the normal adult organ? Moreover, how does this signaling environment impact the initiation and progression of malignant growth?
Recent work has brought new light to bear on a role for the niche as a stromal template for epithelial organ maintenance and regeneration through the simultaneous production of proliferative and differentiative cues (Shin et al., 2011; Shin et al., 2014). Thus, it seems likely that the classical paradigm of the stem cell niche as a microenvironment required solely to maintain the “stemness” of a stem cell (Lander et al., 2012) may not be strictly applicable in the context of vertebrate organ regeneration. Instead, the existing paradigm should perhaps be expanded to include the possibility that niche cells also contribute factors required for stem cell differentiation, and that the production of differentiative cues is a key factor in the maintenance of adult tissue architecture.
At the same time, recent studies have identified a surprising role for stroma, which includes the niche cells for several populations of epithelial stem cells, in restraint of epithelial cancer growth and progression in multiple organs including bladder (Shin et al., 2014), pancreas (Lee et al., 2014; Özdemir et al., 2014; Rhim et al., 2014), colon (Gerling et al., 2016; Lee et al., 2016), and prostate (Yang et al., 2016). Taken together with our expanded understanding of the stromal niche as an active player in promoting differentiation of adult stem cells, these findings implicate niche-derived differentiative cues as potential barriers to malignant growth. Here, we review the current understanding of how stromal niches are specified, how they template regeneration of vertebrate epithelial organs, and how they might act as a brake on the excessive proliferation of malignant growth. One emerging theme is that the Hedgehog (Hh) signaling pathway is active in stromal niches and specifies the expression of secreted niche signals. We discuss the stromal niche signaling programs specified by Hedgehog response in both the urinary bladder (Shin et al., 2011; Shin et al., 2014) and the mammary gland (Zhao et al., 2017), two organs of very distinct developmental origin but with the best defined adult epithelial stem cells and corresponding stromal niches. For bladder, we review evidence that niche-derived differentiation factors play an important role in restraining bladder cancer progression (Shin et al., 2014). Finally, we connect these studies to recent work demonstrating a surprisingly general cancer-restraining effect of stromal Hedgehog response in organs where the stem cells or their stromal niches are less well defined (Gerling et al., 2016; Lee et al., 2014; Rhim et al., 2014; Shin et al., 2014; Yang et al., 2016).
We suggest a model whereby Hedgehog signal response is a general coordinator of stromal niche signaling activity, which includes the production of both proliferative and differentiative cues. In the context of cancer cells, which already have proliferation-promoting genetic lesions, obviating the need for stromally secreted proliferative signals, stromal niche-derived differentiation signals may play a critical role in restraining cancer growth and progression. Thus, despite initial emphasis on adult epithelial stem cells as the critical regenerative unit and the cancer cell-of-origin, stromal cells and their signaling programs may emerge as an additional focal point in future investigations of organ maintenance as well as control of cancer growth.
Specification of the stromal niche in bladder and mammary gland
Here, we review the stromal niche signaling programs in bladder and mammary gland, and their specification by Hedgehog pathway response. In later sections we will discuss the possibility that Hedgehog response may function similarly in other organs such as the pancreas, colon, and prostate, where stromal niche activity is less well defined, but where a similar cancer-restraining effect of stromal Hedgehog response has been observed.
Bladder
While essentially quiescent under normal conditions (Jost, 1989), the bladder epithelium (urothelium) mounts a robust Hedgehog signaling-dependent regenerative response following injury (Shin et al., 2011). Exposure of bladder epithelia to injurious compounds (such as protamine sulfate or cyclophosphamide) or uropathogenic bacteria causes exfoliation of umbrella cells, terminally differentiated urothelial cells that contact and form a barrier with the bladder lumen (Colopy et al., 2014; Gandhi et al., 2013; Mulvey et al., 1998; Mysorekar et al., 2009; Papafotiou et al., 2016; Shin et al., 2011). Such injuries induce massive proliferation of the remaining urothelium, followed by complete restoration of the umbrella layer within a few days.
Molecular cues essential both for urothelial proliferation and differentiation emanate from the underlying stroma and are upregulated in response to the Hedgehog signal. In response to injury, urothelially-derived Sonic hedgehog (Shh) induces stromal production of short-range mitogenic factors, including ligands for the Wnt pathway (Wnt2/4), which is required for robust epithelial proliferation (Papafotiou et al., 2016; Shin et al., 2011). Hedgehog also induces stromal expression of longer-range pro-differentiation Bmp ligands (Bmp4/5) (Mysorekar et al., 2009; Shin et al., 2014). In contrast to the mitogenic Wnt pathway, Bmp activity is essential for proper umbrella cell differentiation following bladder epithelial injury (Mysorekar et al., 2009). Thus, the stromal Hh response acts as a “master coordinator” of the stromal niche, inducing the production of signals that promote both epithelial proliferation and differentiation to template bladder epithelial regeneration (Fig. 1A). The distinct signaling ranges of Wnt and BMP signals may play a role in specifying epithelial architecture. Stem cell renewal and proliferation thus would be stimulated in nearby basal cells by lipid-modified, spatially-restricted Wnt signals (Alexandre et al., 2014; Janda et al., 2012; Takada et al., 2006; Willert et al., 2003), whereas BMP signals might then specify the differentiated umbrella cells lining the bladder lumen at a distance of several additional cell layers. In later sections, we will discuss the cancer-restraining effect of Bmp signals emanating from the bladder stromal niche, and how this phenomenon may apply to other cancers more broadly.
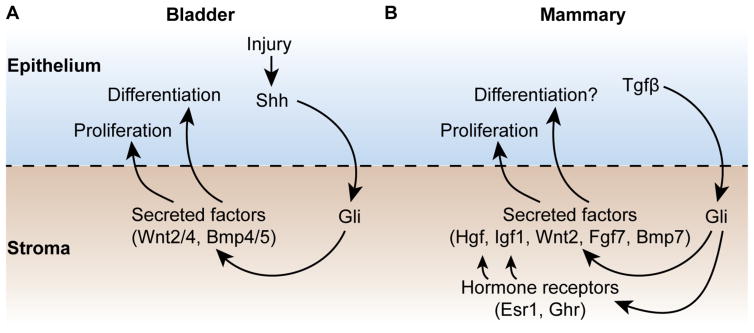
Figure 1. Gli activity is a coordinator of the stromal niche in bladder and mammary gland.
A. Bladder. Shh derived from bladder epithelium, upregulated in response to injury, activates Gli transcription factors in the bladder stroma, which induce transcription of secreted factors including proliferative factors (Wnt2/4) and differentiative factors (Bmp4/5) that regulate the activity of epithelial stem cells.
B. Mammary gland. Tgfβ produced by mammary epithelial cells induces expression in adjacent stromal cells of the major Hedgehog pathway transcriptional effector, Gli2. Gli2 in turn activates transcription of receptors for the mammatrophic hormones estrogen and growth hormone (Esr1, Ghr), thereby entraining the niche to regulation by mammatrophic hormones that control pubertal growth. The targets that respond to Gli2 and/or mammatrophic hormones include a set of secreted paracrine signals (Wnt2, Igf1, Hgf, Fgf7, Bmp7), some of which are known to support mammary epithelial stem cell proliferation/renewal, and others likely to play roles in differentiation and ductal morphogenesis.
Mammary gland
The mammary epithelial stem cell niche is particularly interesting as mammary stem cells and their progeny must respond to local signals that specify a branched pattern of outgrowth, but must also respond to systemic hormonal changes associated with the dramatic changes of puberty and lactation. Are paracrine and hormonal signals integrated by mammary stem cells directly, or is the stromal niche somehow entrained to systemic hormonal signals which are then relayed to epithelial stem cells by changes in the stromal niche signaling program?
During embryonic development, suppression of Hedgehog response by expression of the Gli3 repressor form is required in underlying somitic mesoderm for normal specification of the mammary bud and mammary mesenchyme (Chandramouli et al., 2013; Cowin and Wysolmerski, 2010; Hatsell and Cowin, 2006). Recent work, however, has shown that in the pubertal and adult mammary gland Hedgehog activity mediated by the transcriptional effector Gli2 is required to specify a stromal cell niche signaling program involving expression of both paracrine factors and hormone receptors. The Gli2-specified niche supports mammary epithelial stem cells in pubertal and virgin adult mice (Zhao et al., 2017). In the mammary gland, Gli2 expression is initially induced by crosstalk with TGFβ1 produced by adjacent epithelial cells. Stromal ablation of a conditional Gli2 allele (Gli2ΔS) causes delay and malformation in mammary ductal development, and differential gene expression analysis of stromal cells from Gli2ΔS or Gli2WT littermates reveals that Gli2 function is required for expression of genes encoding paracrine factors such as Igf1, Wnt2, Hgf, Fgf7, and Bmp7, as well as genes encoding receptors for mammatrophic hormones such as estrogen (Esr1) and growth hormone (Ghr) (Fig. 1B). Among the Gli2-dependent stromal paracrine factors identified, Wnt signals are known to sustain the activity of mammary epithelial stem cells in vivo and in ex vivo culture (Badders et al., 2009; Zeng and Nusse, 2010). Moreover, functional inactivation of Igf1 or its epithelially expressed receptor, Igf-1r, both lead to development of hypoplastic mammary glands with terminal end bud abnormalities (Bonnette and Hadsell, 2001; Kleinberg et al., 2000).
Importantly, Gli2-dependent expression of estrogen and growth hormone receptors entrains local stem cell niche activity to systemic hormonal regulation. The mammary ductal defects in Gli2ΔS mice thus are not rescued by growth hormone, but are rescued by local supplementation with the stromally-expressed paracrine factors induced by growth hormone (Igf1 and Wnt2b) (Zhao et al., 2017). This type of niche failure may explain the pathogenesis of certain human diseases sometimes associated with GLI2 mutation, including the deficient breast development and hormonal insensitivity associated with the human disorder combined pituitary hormone deficiency, (Darendeliler et al., 2011; Maghnie et al., 2006). How mammary niche function may contribute to breast cancer pathogenesis, however, remains unclear. Given reports that stromal gene expression is broadly altered in breast cancer (Finak et al., 2008), it will be interesting to see whether alterations in stromal niche signaling programs may play a role in breast cancer development and progression.
Loss of stromal niche specification facilitates malignant growth
Notably, whereas mutations leading to cell-autonomous activation of the Hedgehog pathway in primary tumor cells drive tumor growth in cancers such as basal cell carcinoma (BCC) and a subset of medulloblastoma, the majority of solid tumors do not have mutations in Hedgehog pathway genes, although they often do exhibit changes in Hedgehog pathway response or ligand expression. Furthermore, whereas pathway inhibition is regularly used in the treatment of BCC (Ruch and Kim, 2013; Sekulic et al., 2012; Tang et al., 2012), Hh pathway inhibition has either failed to show a benefit of or accelerated progression in pancreatic or colon cancer clinical trials (Berlin et al., 2013; Catenacci et al., 2015; Herter-Sprie et al., 2013).
Potentially explaining the disappointing results of these clinical trials, recent work has shown a surprisingly general effect of stromal Hedgehog response in restraining cancer growth and progression, most notably in bladder (Shin et al., 2014), pancreatic (Lee et al., 2014; Rhim et al., 2014), colon (Gerling et al., 2016; Lee et al., 2016), and prostate (Yang et al., 2016) cancers. From these studies, several common themes emerge: (1) Hh signaling occurs in a paracrine fashion, with epithelial cells producing Hedgehog ligand and pathway activity exclusively restricted to stromal cells; and (2) Hedgehog pathway activation in stromal cells restrains growth and progression of primary epithelial tumors. We propose that the cancer-restraining effect of stroma is at least partially mediated by Hedgehog-dependent specification of a niche program that includes differentiative cues (see Fig. 2, model). Here we discuss the recent findings in bladder, pancreas, colon, and prostate, and how they may be understood in light of this model.
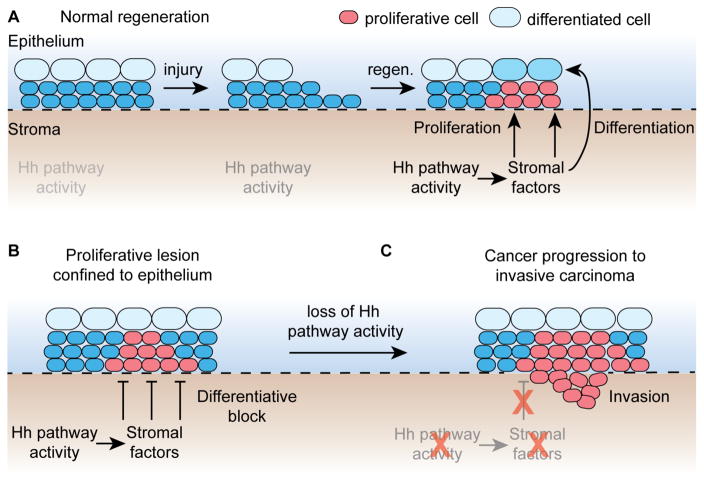
Figure 2. Model of niche dysfunction in cancer progression.
A. During normal homeostasis or injury-induced regeneration the stromal niche, specified by Hedgehog pathway activity, supplies both proliferative and differentiative signals to the overlying epithelium.
B. During the early stages of cancer formation genetic lesions with intrinsic proliferative effects in nascent tumor cells obviate the need for proliferative signals from the stromal niche. At this early stage, however, differentiation-promoting factors from the stromal niche are still able to restrain tumor growth/invasion.
C. Loss of Hedgehog pathway activity specifying the stromal niche disrupts production of the differentiation-inducing factors, allowing unrestrained cancer invasion and progression.
Bladder Cancer
Although stromal elements have been implicated in restraining cancer growth and/or progression in a number of organs, including bladder, pancreas, colon, and prostate, it remains difficult in many of these organs to distinguish between the stroma acting as a physical barrier to tumor growth vs. the importance of stromally secreted paracrine factors per se. Among these organs, the bladder represents a somewhat unique case in that the stroma does not become desmoplastic or act as a physical barrier to growth or invasion, allowing a more unambiguous dissection of the mechanism of stromal restraint of cancer progression. We therefore discuss the bladder as a case study for stromal niche factors providing a differentiative force that restrains cancer progression. Furthermore, we propose that a similar mechanism may apply in other cancer types.
The vast majority of bladder cancers derive from the urothelium (that is, are carcinomas) (Prasad et al., 2011). In muscle-invasive bladder cancer (MIBC) tumor cells infiltrate the lamina propria and muscle layers of the bladder (Alfred Witjes et al., 2017; Brierley, 2017). MIBCs are often metastatic and commonly lethal if left untreated (Prout and Marshall, 1956). A widely-accepted precursor to MIBC is an epithelially-confined lesion known as carcinoma in situ (CIS). Whereas Shh is expressed in the cell-of-origin for MIBC (basal urothelial cells), Shh expression is invariably lost in these tumors, both in mice and in human patients, before progression from CIS to MIBC (Shin et al., 2014). This finding led to the hypothesis that Hh signaling, elaborated by the stroma, restrains bladder cancer to the urothelium, and thus suppresses cancer progression. Indeed, conditional ablation of Hh signal response in bladder stroma results in rapid formation of MIBC and decreased survival rates of mice given the bladder-specific procarcinogen N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) in their drinking water (Shin et al., 2014).
One possibility is that Hedgehog response in stroma blocks progression of CIS to MIBC through the production of differentiation signals, as Hedgehog response specifies the bladder stromal niche signaling program that includes the differentiative signals Bmp4 and Bmp5 (Mysorekar et al., 2002; Shin et al., 2011; Shin et al., 2014). Consistent with this hypothesis, pharmacological activation of BMP signaling using the small molecule FK506, an immunosuppressant that activates BMP signaling at sub-immunosuppressive doses (Spiekerkoetter et al., 2013), suppressed MIBC formation in BBN treated mice, supporting a role of BMP signaling in blocking cancer progression (Shin et al., 2014).
Stromal production of urothelial differentiation-promoting BMP ligands in response to Hh signaling appears to be conserved in humans. Cultured human bladder stroma, but not urothelium, is responsive to Hedgehog pathway agonists (Shin et al., 2011). FK506 also induces upregulation of BMP target genes as well as markers for bladder epithelial differentiation in human bladder cancer cell lines (Shin et al., 2014). Moreover, The Cancer Genome Atlas (TCGA) gene expression data of MIBCs showed a decrease in both SHH and BMP pathway activities compared to non-cancerous tissue, especially in the most aggressive, “basal” MIBC subtype (Shin et al., 2014). These human data are fully consistent with data from the murine BBN-induced bladder cancer model, suggesting that loss of epithelial SHH and the consequent loss of stromal BMP is an essential feature of the progression of CIS to MIBC. Whether a sub-immunosuppressive dose of FK506 may block the progression of CIS to MIBC in human patients awaits clinical investigation.
Pancreatic cancer
Unlike bladder cancer, pancreatic ductal adenocarcinoma (PDAC) pathogenesis is associated with a striking desmoplastic reaction, including a dense extracellular matrix with abundant stromal fibroblasts (Bailey et al., 2008; Fendrich et al., 2011). Hedgehog (Hh) signaling was long thought to play a role in promoting PDAC desmoplasia and tumor progression, based on the following observations: (1) increased levels of Shh expression are observed in the setting of pancreatic injury and throughout the course of human and murine PDAC progression, beginning in the early epithelial precursor lesions, acinar-ductal metaplasia and pancreatic intraepithelial neoplasia (PanIN) (Greer et al., 2013; Hingorani et al., 2005; Thayer et al., 2003), and (2) Hedgehog antagonists decrease tumor desmoplasia and increase functional vascularity, allowing better tumor access of cytotoxic chemotherapy (Olive et al., 2009). However, clinical trials in pancreatic or colon cancers either failed to show a benefit of Hh pathway inhibition or actually accelerated cancer progression (Catenacci et al., 2015), suggesting that the Hedgehog pathway might even restrain rather than promote PDAC progression.
Confirming this hypothesis, three independent studies supported the cancer-restraining effect of stromal elements in a mouse model of pancreatic ductal adenocarcinoma (PDAC), driven by oncogenic Kras (Lee et al., 2014; Özdemir et al., 2014; Rhim et al., 2014). Demonstrating the overall importance of the stroma, conditional ablation of stromal cells expressing the myofibroblast marker α-smooth muscle actin in a genetically-engineered mouse model of PDAC resulted in formation of more aggressive and highly undifferentiated tumors and significantly reduced survival compared with controls (Özdemir et al., 2014). Similarly, in studies investigating more specifically the role for Hedgehog signaling in PDAC progression, genetic or pharmacologic reduction of Hh signaling increased pancreatic tumorigenesis and significantly decreased survival in several distinct oncogenic Kras models of PDAC, again resulting in formation of more aggressive and highly undifferentiated tumors. The stromal component of these tumors was also significantly reduced, and tumors that formed were more vascularized than in control animals (Lee et al., 2014; Rhim et al., 2014). In contrast, pathway activation using a small molecule agonist increased stromal hyperplasia and reduced epithelial proliferation (Lee et al., 2014).
Altogether, the findings in PDAC demonstrate convincingly that Hh pathway activity plays a key role in regulating stromal desmoplasia during PDAC, and that the stroma has some protective effect against progression of PDAC. However, the molecular and cellular basis of this effect remains unclear. It is possible that the effect of the desmoplastic stroma is mediated primarily by reduced tumor vascularity rather than through a direct effect of secreted stromal signals on cancer epithelial cell differentiation. Indeed, administration of a VEGFR blocking antibody, an inhibitor of angiogenesis, improved survival of mice bearing Shh-deficient tumors (Rhim et al., 2014). Several lines of evidence suggest, however, that stromal Hedgehog response has an effect on tumor cell differentiation beyond its effects on tumor vascularity. Notably, in a cerulein-enhanced mouse model of PDAC, pharmacological activation of the Hh pathway dramatically reduces expression of the progenitor marker Pdx1 to 0.2% of epithelial (EpCAM+) cells compared with 7.0% or 8.5%, respectively, of EpCAM+ cells in mice treated with vehicle or vismodegib, a Hedgehog pathway inhibitor (Lee et al., 2014). Hedgehog pathway activation thus is associated with reduced progenitor character in epithelial cells. Because exclusively stromal cells are responsive to Hedgehog in the adult pancreas, this argues for stromally-secreted factors induced by Hedgehog promoting epithelial differentiation as opposed to progenitor fate.
This phenomenon has precedent during embryonic development of the pancreas, where the absence of Hedgehog activity is required for normal specification of early pancreatic progenitor fate. Pharmacologic or antibody treatments that inhibit Hh pathway signaling during development cause expansion of pancreatic progenitor fate, indicated by ectopic expression of the pancreatic progenitor marker Pdx1 (Hebrok et al., 1998; Hebrok et al., 2000; Kim and Melton, 1998). Moreover, Hh pathway inhibition impairs normal recovery of the pancreas following cerulein-mediated injury, characterized by persistence of undifferentiated acinar-to-ductal metaplastic lesions staining positive for the progenitor markers Pdx1 and Nestin (Fendrich et al., 2008).
Further investigation is clearly required to tease apart the effects of a desmoplastic stroma as a physical impediment to angiogenesis vs. the role of secreted stromal differentiation signals in restraining progression of PDAC. However, it seems likely that, as in bladder, Bmp signals may play a key role. Indeed, the BMP/TGFβ transducer SMAD4 is lost in around 60% of PDAC, and its loss correlates with higher metastatic burden (Iacobuzio-Donahue et al., 2009), suggesting that SMAD4 loss is an important event in PDAC development. Thus, loss of epithelial Bmp response, perhaps normally triggered by stromal niche BMP signals in response to Hedgehog, may be a critical factor in restraining PDAC progression.
Colorectal cancer
Recent work suggests that a similar cancer-restraining effect of stromal Hedgehog response mediated by differentiation-promoting Bmp signaling occurs in colorectal cancer (CRC). Hh pathway activity (marked by Gli1 expression) is reduced in murine CRC, despite continued epithelial ligand expression (Gerling et al., 2016). Furthermore, genetic or pharmacologic blockade of Hh signaling promoted colitis-associated colonic tumorigenesis in mice (Gerling et al., 2016; Lee et al., 2016). In contrast, stroma-specific Hh pathway activation by deletion of the negative regulator Ptch1 restrained tumor development and resulted in reduced expression of the secreted BMP inhibitor, Gremlin 1 (Grem1), accompanied by reduced expression of colonic stem-cell associated genes Lgr5, Cdca7, and Cdk6 (Munoz et al., 2012). Although it is possible that the results of Hedgehog signaling modulation in colorectal cancer are partly due to anti-inflammatory effects of stromal Hedgehog response (Lee et al., 2016), the expression signatures identified suggest that stromal Hh activity exerts a pro-differentiative influence on intestinal epithelial stem cells, mediated at least in part by modulation of Bmp signaling factors secreted by stromal cells.
Hedgehog signaling as a pro-differentiative force has precedent in development and normal homeostasis of the intestinal epithelium. During development, Hedgehog ligands are concentrated at the tips of intestinal villi by the physical folding of the epithelial tissue (Shyer et al., 2015), leading to high stromal Hh response at the tips of villi, which induces the expression of Bmp4 (Shyer et al., 2015; Walton et al., 2012). Culturing chick embryonic intestinal explants in the presence of the Hedgehog inhibitor cyclopamine or the Bmp inhibitor Noggin leads to expansion of proliferation and expression of Lgr5 to the villus tips, whereas culturing explants in the presence of Shh or Bmp4 prevents proliferation (Shyer et al., 2015). Additionally, Wnt activity is decreased in the context of increased Bmp signaling, suggesting that Bmp signals may promote differentiation by suppressing Wnt activity (He et al., 2004; Shyer et al., 2015). During adult intestinal homeostasis, diminished Hh signaling also results in decreased differentiation and expansion of the intestinal stem cell compartment and expansion of Wnt activity, the main oncogenic driver of CRC (Buller et al., 2015; Kosinski et al., 2010; van Dop et al., 2010).
Prostate Cancer
In contrast to early reports that indicated a pro-cancer effect of Hh pathway activity in prostate cancer cell lines or xenografts (Fan et al., 2004; Karhadkar et al., 2004; Sanchez et al., 2004), more recent work in an autochthonous prostate tumor model demonstrated that Hh response in prostate cancer is restricted to stromal cells, and that increased Hh pathway activity restrains cancer progression (Yang et al., 2016).
One notable feature of the cancer-associated stroma of human prostate cancer is the depletion of mature smooth muscle cells (SMCs) (Tuxhorn et al., 2002), and SMCs are similarly depleted in advanced PB-MYC murine tumor models (Yang et al., 2016). Experimentally augmented stromal Hh pathway activity in the PB-MYC model resulted in an overall expansion of stromal cells, including an increase in both the number of SMCs and intact smooth muscle layers, as well as decreased progression from prostatic intraepithelial neoplasia to micro-invasive carcinoma (Yang et al., 2016). One possible explanation for the cancer-restraining effect of stromal Hh response is that more intact SM layers act as a physical barrier to prostate cancer invasion. Another possibility, in line with evidence for increased differentiation upon stromal Hh activation in bladder, pancreatic, and colon cancers, is that pro-differentiation factors secreted by stromal cells in response to Hh signaling help to restrain tumor progression. Interestingly, although stromal Hh response promotes prostate epithelial expansion and branching during embryonic development (Doles et al., 2006; Yu and Bushman, 2013), stromal Hh response restricts epithelial proliferation and branching during postnatal development (Yu and Bushman, 2013) and in the adult prostate (Lim et al., 2014), suggesting the possibility that stromal Hh response may similarly restrict epithelial proliferation in prostate cancer.
Conclusions
Tumor-associated fibroblast-like stromal cells have long been viewed as an enemy in cancer therapies, either by acting as a simple impediment to delivery of chemotherapeutic reagents or by supporting cancer cells through signaling programs promoting proliferation and metastasis. However, this view has been challenged by recent studies showing an unexpected cancer-restraining effect of stromal cells, and stromal Hedgehog response in particular. Work in the bladder has shown that both short-range proliferative cues and longer-range differentiative cues emanate from the stromal niche, and that the stromally-derived differentiation signals (Bmps) induced by Hedgehog response are central to the restraint of cancer progression. Indeed, the Hedgehog-BMP signaling axis, first identified in Drosophila development (Basler and Struhl, 1994; Capdevila and Guerrero, 1994; Tabata and Kornberg, 1994), appears to be employed rather broadly in control of vertebrate epithelial stem cells. In pancreatic and colon cancers, increasing progenitor character in epithelial cells and (in colon) decreasing levels of Bmp pathway activity following loss of stromal Hedgehog response suggest that a similar mechanism may be at play. Future understanding of the role of stroma in cancer-progression may thus hinge on our knowledge of the signaling programs elaborated by the stromal niche. In particular, it will be important to investigate how the stromal niche regulates differentiation, in addition to proliferation, to recapitulate the existing epithelial architectures of vertebrate organs.
Several important questions remain to be addressed, including: (1) What cell types define the stromal niche? Is the niche a heterogeneous compartment, including cells separately specified for the production of proliferative and differentiative cues? (2) What differentiation cues, including and in addition to Bmps, are necessary and/or sufficient for stem cell differentiation during normal homeostatic maintenance and injury repair in vertebrate epithelial organs? (3) Is activation of niche programs such as Bmp signaling sufficient to restrain the growth and/or progression of some epithelial cancers? (4) Finally, to what extent can combined therapies incorporating activation of differentiation pathways improve existing cancer therapeutics?
One thing is clear: fibroblast-like stromal cells, often ignored and poorly understood, are emerging as a critical signaling niche for vertebrate epithelial stem cell activities during normal tissue renewal and malignant growth. For now, the full complement of niche-derived signals, their cellular sources, and their roles in guiding proliferation and differentiation remains to be defined. However, with improved technologies for culturing stem cells in vitro, manipulation of signaling pathways, and transcriptional profiling at single-cell resolution, a more refined understanding of stem cell niche function in vertebrates now seems within reach.
Acknowledgments
We thank B. Kiss and G. Kingman for comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernandez V, Espinos EL, Dunn J, Rouanne M, Neuzillet Y, Veskimae E, van der Heijden AG, Gakis G, Ribal MJ. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. European urology. 2017;71:462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:4792–4801. [PubMed] [Google Scholar]
- Ayala GE, Muezzinoglu B, Hammerich KH, Frolov A, Liu H, Scardino PT, Li R, Sayeeduddin M, Ittmann MM, Kadmon D, Miles BJ, Wheeler TM, Rowley DR. Determining Prostate Cancer-Specific Death through Quantification of Stromogenic Carcinoma Area in Prostatectomy Specimens. The American journal of pathology. 2011;178:79–87. doi: 10.1016/j.ajpath.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM. The Wnt Receptor, Lrp5, Is Expressed by Mouse Mammary Stem Cells and Is Required to Maintain the Basal Lineage. PLoS ONE. 2009;4:e6594. doi: 10.1371/journal.pone.0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocrine-related cancer. 2012;19:R187–204. doi: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosopfiffa limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, Mulcahy MF, Zalupski MM, Mackey HM, Yauch RL, Graham RA, Bray GL, Low JA. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours 2017 [Google Scholar]
- Buller NV, Rosekrans SL, Metcalfe C, Heijmans J, van Dop WA, Fessler E, Jansen M, Ahn C, Vermeulen JL, Westendorp BF, Robanus-Maandag EC, Offerhaus GJ, Medema JP, D’Haens GR, Wildenberg ME, de Sauvage FJ, Muncan V, van den Brink GR. Stromal Indian hedgehog signaling is required for intestinal adenoma formation in mice. Gastroenterology. 2015;148:170–180. e176. doi: 10.1053/j.gastro.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. Embo j. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci DVT, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. Journal of Clinical Oncology. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli A, Hatsell SJ, Pinderhughes A, Koetz L, Cowin P. Gli Activity Is Critical at Multiple Stages of Embryonic Mammary and Nipple Development. PLOS ONE. 2013;8:e79845. doi: 10.1371/journal.pone.0079845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colopy SA, Bjorling DE, Mulligan WA, Bushman W. A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Developmental Dynamics. 2014;243:988–998. doi: 10.1002/dvdy.24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Wysolmerski J. Molecular Mechanisms Guiding Embryonic Mammary Gland Development. Cold Spring Harbor Perspectives in Biology. 2010;2:a003251. doi: 10.1101/cshperspect.a003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darendeliler F, Lindberg A, Wilton P. Response to growth hormone treatment in isolated growth hormone deficiency versus multiple pituitary hormone deficiency. Hormone research in paediatrics. 2011;76(Suppl 1):42–46. doi: 10.1159/000329161. [DOI] [PubMed] [Google Scholar]
- Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in Hedgehog signaling during mouse prostate development. Dev Biol. 2006;295:13–25. doi: 10.1016/j.ydbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- Fendrich V, Esni F, Garay MVR, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen JAN, Leach SD, Maitra A. Hedgehog Signaling Is Required for Effective Regeneration of Exocrine Pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich V, Oh E, Bang S, Karikari C, Ottenhof N, Bisht S, Lauth M, Brossart P, Katsanis N, Maitra A, Feldmann G. Ectopic overexpression of Sonic Hedgehog (Shh) induces stromal expansion and metaplasia in the adult murine pancreas. Neoplasia (New York, NY) 2011;13:923–930. doi: 10.1593/neo.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Franco OE, Hayward SW. Targeting the tumor stroma as a novel therapeutic approach for prostate cancer. Advances in pharmacology (San Diego, Calif) 2012;65:267–313. doi: 10.1016/B978-0-12-397927-8.00009-9. [DOI] [PubMed] [Google Scholar]
- Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, Laufer E, Metzger D, Liang F, Liao Y, Sun TT, Aronow B, Rosen R, Mauney J, Adam R, Rosselot C, Van Batavia J, McMahon A, McMahon J, Guo JJ, Mendelsohn C. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Developmental cell. 2013;26:469–482. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling M, Büller NVJA, Kirn LM, Joost S, Frings O, Englert B, Bergström Å, Kuiper RV, Blaas L, Wielenga MCB, Almer S, Kühl AA, Fredlund E, van den Brink GR, Toftgård R. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nature Communications. 2016;7:12321. doi: 10.1038/ncomms12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer RL, Staley BK, Liou A, Hebrok M. Numb regulates acinar cell dedifferentiation and survival during pancreatic damage and acinar-to-ductal metaplasia. Gastroenterology. 2013;145:1088–1097. e1088. doi: 10.1053/j.gastro.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell SJ, Cowin P. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development. 2006;133:3661. doi: 10.1242/dev.02542. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nature genetics. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes & Development. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- Herter-Sprie GS, Kung AL, Wong KK. New cast for a new era: preclinical cancer drug development revisited. The Journal of Clinical Investigation. 2013;123:3639–3645. doi: 10.1172/JCI68340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P, Herman JM, Cameron JL, Yeo CJ, Halushka MK, Eshleman JR, Raben M, Klein AP, Hruban RH, Hidalgo M, Laheru D. DPC4 Gene Status of the Primary Carcinoma Correlates With Patterns of Failure in Patients With Pancreatic Cancer. Journal of Clinical Oncology. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural Basis of Wnt Recognition by Frizzled. Science. 2012;337:59. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost SP. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Archiv B, Cell pathology including molecular pathology. 1989;57:27–36. doi: 10.1007/BF02899062. [DOI] [PubMed] [Google Scholar]
- Karhadkar SS, Steven Bova G, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kim SK, Melton DA. Pancreas development is promoted by cyclopamine, a Hedgehog signaling inhibitor. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13036–13041. doi: 10.1073/pnas.95.22.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol. 1981;87:286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981:81. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. Journal of mammary gland biology and neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- Kosinski C, Stange DE, Xu C, Chan AS, Ho C, Yuen ST, Mifflin RC, Powell DW, Clevers H, Leung SY, Chen X. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, Buckingham M, Calof AL, Trumpp A, Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biology. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, Nagle JM, Deshpande V, Boucher Y, Kato T, Chen JK, Willmann JK, Bardeesy N, Beachy PA. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proceedings of the National Academy of Sciences. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Rothenberg ME, Seeley ES, Zimdahl B, Kawano S, Lu WJ, Shin K, Sakata-Kato T, Chen JK, Diehn M, Clarke MF, Beachy PA. Control of inflammation by stromal Hedgehog pathway activation restrains colitis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E7545–e7553. doi: 10.1073/pnas.1616447113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A, Shin K, Zhao C, Kawano S, Beachy PA. Spatially restricted Hedgehog signalling regulates HGF-induced branching of the adult prostate. Nat Cell Biol. 2014;16:1135–1145. doi: 10.1038/ncb3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghnie M, Ambrosini L, Cappa M, Pozzobon G, Ghizzoni L, Ubertini MG, di Iorgi N, Tinelli C, Pilia S, Chiumello G, Lorini R, Loche S. Adult height in patients with permanent growth hormone deficiency with and without multiple pituitary hormone deficiencies. The Journal of clinical endocrinology and metabolism. 2006;91:2900–2905. doi: 10.1210/jc.2006-0050. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. The EMBO journal. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell host & microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. The Journal of biological chemistry. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- Özdemir Berna C, Pentcheva-Hoang T, Carstens Julienne L, Zheng X, Wu CC, Simpson Tyler R, Laklai H, Sugimoto H, Kahlert C, Novitskiy Sergey V, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses Harold L, Weaver Valerie M, Maitra A, Allison James P, LeBleu Valerie S, Kalluri R. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nature Communications. 2016;7:11914. doi: 10.1038/ncomms11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YC, Levine CM, Zahid S, Wilson EL, Joyner AL. Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. Proceedings of the National Academy of Sciences. 2013;110:20611–20616. doi: 10.1073/pnas.1315729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad SM, Decastro GJ, Steinberg GD. Urothelial carcinoma of the bladder: definition, treatment and future efforts. Nature reviews Urology. 2011;8:631–642. doi: 10.1038/nrurol.2011.144. [DOI] [PubMed] [Google Scholar]
- Prout GR, Marshall VF. The prognosis with untreated bladder tumors. Cancer. 1956;9:551–558. doi: 10.1002/1097-0142(195605/06)9:3<551::aid-cncr2820090319>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rhim Andrew D, Oberstein Paul E, Thomas Dafydd H, Mirek Emily T, Palermo Carmine F, Sastra Stephen A, Dekleva Erin N, Saunders T, Becerra Claudia P, Tattersall Ian W, Westphalen CB, Kitajewski J, Fernandez-Barrena Maite G, Fernandez-Zapico Martin E, Iacobuzio-Donahue C, Olive Kenneth P, Stanger Ben Z. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch JM, Kim EJ. Hedgehog signaling pathway and cancer therapeutics: progress to date. Drugs. 2013;73:613–623. doi: 10.1007/s40265-013-0045-z. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang ALS, Low JA, Mackey HM, Yauch RL, Graham RA, Reddy JC, Hauschild A. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. New England Journal of Medicine. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Lim A, Zhao C, Sahoo D, Pan Y, Spiekerkoetter E, Liao Joseph C, Beachy Philip A. Hedgehog Signaling Restrains Bladder Cancer Progression by Eliciting Stromal Production of Urothelial Differentiation Factors. Cancer Cell. 2014;26:521–533. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. Bending gradients: how the intestinal stem cell gets its home. Cell. 2015;161:569–580. doi: 10.1016/j.cell.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Fuller MT, Braun RE, Yoshida S. Germline Stem Cells. Cold Spring Harbor Perspectives in Biology. 2011;3:a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Developmental cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, Epstein EH. Inhibiting the Hedgehog Pathway in Patients with the Basal-Cell Nevus Syndrome. New England Journal of Medicine. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:2912–2923. [PubMed] [Google Scholar]
- van Dop WA, Heijmans J, Buller NV, Snoek SA, Rosekrans SL, Wassenberg EA, van den Bergh Weerman MA, Lanske B, Clarke AR, Winton DJ, Wijgerde M, Offerhaus GJ, Hommes DW, Hardwick JC, de Jonge WJ, Biemond I, van den Brink GR. Loss of Indian Hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology. 2010;139:1665–1676. 1676.e1661–1610. doi: 10.1053/j.gastro.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Walton KD, Kolterud Å, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15817–15822. doi: 10.1073/pnas.1205669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Yang Z, Peng Y-C, Gopalan A, Gao D, Chen Y, Joyner AL. Stromal Hedgehog signaling maintains smooth muscle and hampers micro-invasive prostate cancer. Disease Models & Mechanisms. 2016 doi: 10.1242/dmm.027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Bushman W. Differential stage-dependent regulation of prostatic epithelial morphogenesis by Hedgehog signaling. Developmental Biology. 2013;380:87–98. doi: 10.1016/j.ydbio.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng YA, Nusse R. Wnt proteins are self-renewing factors for mammary stem cells and promote their long-term expansion in culture. Cell stem cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Cai S, Shin K, Lim A, Kalisky T, Lu W-J, Clarke MF, Beachy PA. Stromal Gli2 activity coordinates a niche signaling program for mammary epithelial stem cells. Science. 2017 doi: 10.1126/science.aal3485. [DOI] [PubMed] [Google Scholar]