Abstract
Purpose/Background
The goal of this study was to determine whether pediatric serum concentration of riluzole is similar to that observed in adults, and to determine whether riluzole serum concentration is associated with adverse effects or efficacy in children and adolescents with treatment-refractory obsessive-compulsive disorder.
Methods/Procedures
Data were drawn from previously published studies: one open-label trial and one randomized controlled trial with an open-label extension phase. Serum was drawn at 24, 36, and 52 weeks in 37 patients who were taking approximately 100 mg riluzole daily (mean dose at 24 weeks, 99±28 mg).
Findings/Results
Across all samples, serum riluzole concentration ranged from 7 ng/mL to 963 ng/mL. At week 24 (n=37), the median concentration was 76 ng/mL (IQR=53–172). Concentration was relatively stable within-patient. One subject, who had the highest serum concentration levels during the study, developed pancreatitis after exiting the study. The patient had recently added fluvoxamine to the riluzole regimen. Controlling for concomitant fluvoxamine (in six participants) and time-of-draw, serum riluzole concentration was not associated with obsessive compulsive disorder symptom severity; nor was it associated with side effect profile.
Implications/Conclusions
The dose of riluzole used in these pediatric subjects appears to have achieved serum concentration levels similar to those observed in adults. However, as previously reported in adults, the serum concentration had no discernable relationship to efficacy or adverse effects. ClinicalTrials.gov: NCT00251303.
Keywords: riluzole, obsessive compulsive disorder, serum concentration
INTRODUCTION
Riluzole is a glutamatergic modulator that is used, off-label, to treat some psychiatric conditions in adults1. The adverse effects of riluzole are generally mild in physically healthy subjects2. The most commonly reported adverse effect is elevation of transaminases, especially alanine aminotransferase, but rarely to more than three times upper limits of laboratory normal3. The one clinical study that attempted to correlate adverse effects and benefits with riluzole serum levels found that diarrhea was more common at higher doses in adults4. No relationship was found between riluzole serum levels and survival of adults treated for amyotrophic lateral sclerosis (ALS) in another study5.
Peak serum levels in healthy adult volunteers is achieved after about sixty-to-ninety minutes, and trough levels at about twelve hours. Inter-individual variability of riluzole serum concentration is far greater than within-individual variability2,6. In one study of adults with ALS, the serum riluzole levels associated with a 50 mg twice-daily dose had a median trough value of 54 (range 20–343) and a median peak value of 183 (44–1,552) ng/ml5. However, within-subject variability is high; in another study of ALS patients, visit-to-visit variability (28%) was confirmed to be lower than between-subject variability7.
Plasma concentration may vary across patient population; a study of adults with acute spinal cord injury documented lower peak plasma concentrations than observed in ALS patients at the same dose8. In that study, free riluzole after ultrafiltration was measured in a subset and did not account for the differences noted in total drug9. Of particular relevance to the present report, a study of 14 young patients (ages 6–20 years) with spinal muscular atrophy documented maximum concentrations higher than observed in both healthy subjects and ALS patients; the authors speculate that the pathophysiology was responsible for the altered pharmacokinetics, rather than other variables such as age or weight10.
Intra-individual variability may be partially explained by metabolic activity. In vitro data suggest that riluzole is predominantly metabolized by CYP1A2 human hepatic microsomes to the N-hydroxy conjugate, and glucuronidation appears to be a “relatively minor metabolic route”11. However, among ALS patients, cytochrome P450 1A1 and 1A2 polymorphisms were not demonstrated to account for mean riluzole serum level differences12. Another study of ALS patients used serum caffeine metabolism as a probe of CYP1A2 activity; while the activity was significantly correlated with riluzole clearance, it only explained 37% of the variability13. The authors speculated that the large inter-individual variation is possibly attributable to glucuronidation and extra-hepatic CYP1A2 activity13. Following on this, glucuronide formation in vivo was confirmed in serum from ALS patients, and the metabolite concentration was positively associated with drug levels14. In the same publication, UGT1A1*28 genotype was not associated with peak or trough concentrations in a second larger group of ALS patients, leading the authors to conclude that glucuronidation is not a major contributor to inter-individual pharmacokinetic variability14.
Given the interest in glutamate as a therapeutic target in obsessive-compulsive disorder (OCD)15,16, we conducted an open-label trial of riluzole in six children with OCD17. In that study, riluzole was well-tolerated, and we hypothesized that a longer duration of treatment would result in symptom improvement. Thus, we completed a 12-week, randomized, placebo-controlled, double-blind trial (RCT) of riluzole in children and adolescents with treatment-refractory OCD and, in some cases, with co-occurring autism spectrum disorder18. To make study participation attractive, the RCT study design included open-label administration of riluzole from 12 weeks to 52 weeks. During the double-blind portion of that study, all participants experienced improvements in OCD symptoms, but there was no advantage of riluzole compared to placebo. Interpretation of the results was moderated by the fact that the children were treatment-refractory, and the majority received concomitant medications. Specifically, 71% of the sample was taking a selective serotonin reuptake inhibitor and at least one additional psychotropic medication. Further, given the documented variability in adults of riluzole serum concentration, it was considered possible that the study dose (100mg/day) was not adequate for some participants.
In this report, we combine data from the open-label pilot study17 and the open-label portion of the RCT18 to address the question of whether riluzole serum concentration is associated with clinical response or adverse effect profile in our young subjects.
METHODS
Participants
Data were drawn from two studies completed in the Pediatrics and Developmental Neuroscience Branch of the National Institute of Mental Health of the National Institutes of Health. Both studies were approved by the National Institute of Mental Health Institutional Review Board, and consent/assent was obtained for all participants. The full details of both reports are found elsewhere17,18.
Participants in the current investigation were all those who received open-label administration of riluzole, who attended follow-up visits at 24 weeks, 36 weeks, and/or 52 weeks post-baseline, and whose serum sample was tested on at least one of those occasions. The final sample, from the 66 eligible participants in the original studies, comprised 37 individuals (five from the pilot study and 32 from the RCT); 27 (73%) were male, and all were Caucasian. These participants ranged in age from 8.8 to 18.4 years (M±SD = 14.6±2.7) at the Week 24 visit. Body mass index (BMI) at the final study visit ranged from 15.1 to 37.2 (M±SD = 25.5±5.1).
Procedures
During the open label portion of the RCT study (starting at week 12 post-baseline), most participants were prescribed 50 mg of riluzole every 12 hours. Doses had been titrated during the transition to open label on a fixed schedule, with a target dose of 100 mg/day, barring any adverse effects. The open-label protocol did allow for dose adjustment, and the pilot study was partially dedicated to dose-finding, so the mean daily dose for all participants varied across visit (week 24, M±SD = 99±28; week 36, 108±28; week 52, 120±34). However, across all study visits, the median daily dose was 100 mg. Exact doses for each subject are recorded in Supplementary Table 1. Daily diaries were to be kept of doses and physical symptoms. For adverse effect monitoring, clinical laboratory tests were obtained at all study visits. Exact timing of the morning dose was not available, so a time of 8:00 AM was used to approximate the amount of time passed between the morning dose and venipuncture, the exact time of which was recorded. This value is referred to herein as time-to-draw (in hours). The serum samples were stored at −80°C until they were batched and sent for analysis.
Frontage Laboratories, Inc. (700 Pennsylvania Dr., Exton, PA 19341) measured the total riluzole in these serum samples, blind to subject and to riluzole administration. Because there were no published methods for the measurement of total riluzole in biological samples, the laboratory used a liquid chromatography/tandem mass spectrometry method developed specifically for this study. The method was developed and validated for the determination of riluzole in human serum using 2-amino-6-bromobenzothiazole as the internal standard (IS). The high performance liquid chromatography column (HPLC) was Agela Venusil XBP C18 100A, 2.1 × 50 mm, 5 micron. Mobile Phase A was 0.1% HCOOH in MeOH/H2O (2/98, V/V). Mobile Phase B was 0.1% HCOOH in MeOH. The retention time of riluzole was 1.6 minutes, and the retention time of the IS was 1.3 minutes. Sciex API 4000 with Interface TIS was used to monitor riluzole and IS in positive ionization mode. The transitions were m/z 235.1 → 138.1 for riluzole, and m/z 231.0 → 150.0 for IS. Human serum samples of 100 μL were used for analysis. The serum samples with 20 μL of IS (1 μg/mL of ABBT) were extracted with MTBE. The organic phase was dried down and reconstituted with 0.1% Formic Acid in MeOH/H2O (50/50, V/V), and 10 μL of the final solution was injected onto the HPLC column.
Behavioral data were obtained at each study visit; in this study, the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) total score was the primary outcome19.
Analysis
A mixed model with a random effect of subject, to account for repeated observations, was used for all analyses where serum concentration was the dependent variable. The covariance structure was compound symmetry. A generalized linear mixed model with random intercept was used to evaluate the relationship between concentration and (binary) side effects. Serum concentration was natural log-transformed to accommodate distributional assumptions. Alpha was set to .05. All analyses were performed in SAS 9.320.
RESULTS
Thirty-seven participants contributed serum data at the 24-week visit, 30 (81%) had additional data at the 36-week visit, and a further 22 (59%) had 52-week data. Across the 89 samples, 28% were collected before 12:00 PM, and 70% between 12:00 PM and 4:00 PM. Across all samples, serum riluzole concentration ranged from 7 ng/mL to 963 ng/mL. At week 24 (n=37), the median concentration was 76 ng/mL (interquartile range, IQR=53–172). Concentrations were similar at week 36 (n=30, median=81, IQR=44–128) and week 52 (n=22, median=95, IQR=59–192). Dose was not associated with serum concentration [F(1,51)=0.33, p=.57], but it was still retained in the remainder of the analyses as a covariate. See Figure 1 for serum riluzole levels by patient, over time. The full dataset, including time-of-draw.
Figure 1. Serum concentration of riluzole in 37 participants (89 samples).
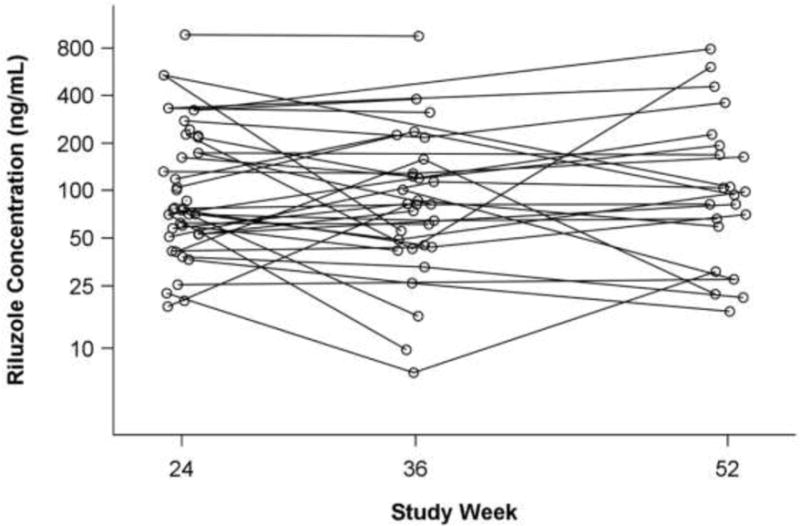
Median dose at all visits was 100 mg/day; mean doses were 99±28, 108±28, and 120±34 mg/day at weeks 24, 36, and 52, respectively. Y-axis is on the natural logarithmic scale for ease of viewing.
Six participants (16%) received fluvoxamine, a CYP1A2 inhibitor, during the open-label study. Controlling for riluzole dose, concomitant fluvoxamine use was significantly associated with higher riluzole concentration; the least squares mean estimate for individuals not taking fluvoxamine was 79 ng/ml, compared to 164 ng/ml in those taking fluvoxamine [F(1,50)=4.68, p=.035].
Riluzole concentration was not related to BMI [F(1,51)=0.36, p=.55]. Pharmacokinetic studies7 have documented an exponential decline of riluzole over time, but in this study, time of blood draw was linearly and significantly related to riluzole concentration, such that each additional hour was associated with a decrease of approximately 1 ng/mL (SE=1) [F(1,50) = 4.81, p=.03]. Thus, both concomitant fluvoxamine and time-of-draw were entered with riluzole dose as a covariate in the final analysis. Controlling for time-of-draw [F(1,47)=0.85, p=.36] and fluvoxamine [F(1,47)=0.39, p=.54], natural log-transformed serum riluzole concentration was not associated with CY-BOCS score [F(1,47)=1.36, p=.25] (see Figure 2).
Figure 2. Symptom severity (CY-BOCS) versus riluzole concentration.
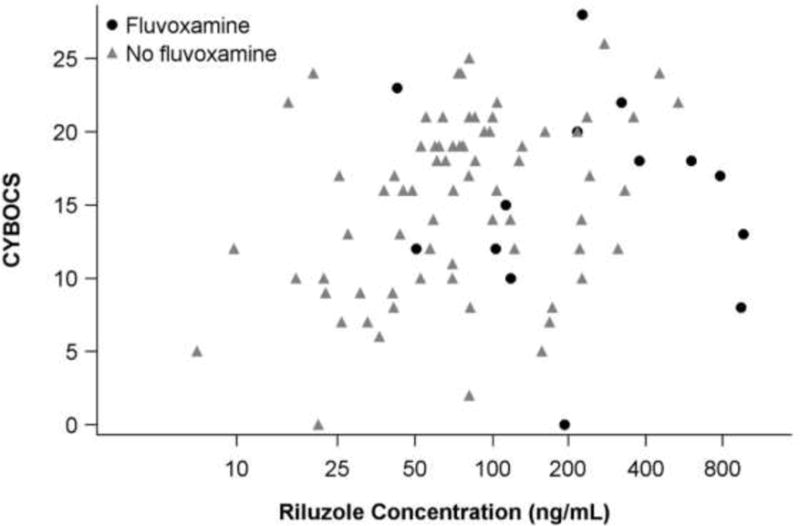
Eighty-nine samples from n=37 participants. X-axis is on the natural logarithmic scale for ease of viewing. Controlling for riluzole dose [F(1,47)=0.07, p=.80], time-of-draw [F(1,47)=0.85 p=.36] and fluvoxamine [F(1,47)=0.39, p=.54], natural log-transformed serum riluzole concentration was not associated with CY-BOCS score [F(1,47)=1.36, p=.25].
One female child developed pancreatitis after finishing the study and while still taking riluzole. Fluvoxamine, which is an inhibitor of the 1A2 enzyme, had recently been added to her regimen because of continuing symptoms. There had also been a seemingly mild abdominal injury prior to onset of pancreatitis. Riluzole was stopped, and she fully recovered. Although the pancreatitis developed after participation in the study ended (but before serum data were analyzed), we note that this child’s serum riluzole levels were the highest observed in the study (week 24, 963 ng/mL; week 36, 943 ng/mL; no data at week 52).
Elevated transaminases were the only frequent laboratory finding (in 10 patients at 12 visits). Controlling for dose, serum concentration did not predict the presence of elevated transaminases [F(1,50)=0.03, p=.85]. No other side effect occurred with enough frequency to determine a relationship with serum concentration.
DISCUSSION
In this report, we utilized data from two open-label studies of riluzole for the treatment of OCD in pediatric patients to investigate the variability of riluzole serum concentration and its association, if any, with symptom severity and adverse effects. There were several objectives in measuring serum riluzole levels in this sample. First, we wished to know if serum levels in younger subjects were comparable to those achieved in adults taking similar doses. Serum concentration among the children and adolescents in this study was similar in range and variability to what is reported in the adult data. Second, we wanted to know if adverse effects were associated with serum levels, and we found no evidence to support this connection. The interpretation of the adverse event data is complicated by the fact that many patients were taking concomitant medications during the open label administration of riluzole. This was not surprising, given that our studies deliberately recruited young people who had failed standard-of-care treatments and who remained quite impaired by their OCD. As one would predict, concomitant fluvoxamine, a CYP1A2 inhibitor, was associated with increased riluzole serum levels. The practical meaning of this is unclear, since ideal (or toxic) riluzole level is unknown. It is worthy of note that the child who developed pancreatitis during the open label phase had the highest riluzole serum levels. However, we were not able to determine if this was coincidence or causally related, as she had begun taking fluvoxamine prior to development of the pancreatitis. Third and finally, we sought to determine whether serum level of riluzole was associated with clinical effect. Based on these open label data, serum level of riluzole does not appear to have any bearing on severity of OCD symptoms. Thus, although the serum concentration was variable across participants, it did not appear to relate to symptom severity.
This study had several limitations. The sample size is relatively small. However, we note that there are no other available reports of riluzole use in pediatric patients in psychiatry. Serum samples were obtained without regard for timing of prior riluzole dose. Not only did this prevent us from examining peak and trough drug levels, but it may have confounded our observed serum concentrations. We did attempt to ameliorate this possibility by controlling for an approximated time between dose and venipuncture, and given the relative consistency of serum levels within subject, it is unlikely that these factors played a major role. Finally, and perhaps most importantly, peripheral concentration of drug is only a proxy for active drug in the central nervous system.
Supplementary Material
Clinical Significance.
Riluzole was well tolerated by our pediatric participants, at doses of approximately 100 mg per day. This dose, and the resulting serum drug levels, are comparable to those reported for adults using riluzole for treatment of ALS. Although serum riluzole concentration varied widely across participants, it was not associated with OCD symptomatic benefit or the occurrence of adverse effects. Although our study design limited use of riluzole to treatment-refractory patients, the results of this study suggest that drug might be appropriately tested in larger doses or as a single agent in a larger cohort of youth with OCD.
Acknowledgments
FUNDING
This work was supported by the Intramural Research Program of the National Institute of Mental Health of the National Institutes of Health (NCT00251303; 05-M-0225; ZIAMH002666).
Footnotes
DISCLOSURES
The authors have no financial interests to report.
References
- 1.Zarate CA, Jr, Manji HK. Riluzole in psychiatry: a systematic review of the literature. Expert opinion on drug metabolism & toxicology. 2008;4:1223–1234. doi: 10.1517/17425255.4.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liboux AL, Lefebvre P, Roux YL, et al. Single-and Multiple-Dose Pharmacokinetics of Riluzole in White Subjects. The Journal of Clinical Pharmacology. 1997;37:820–827. doi: 10.1002/j.1552-4604.1997.tb05630.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller RG, Mitchell J, Lyon M, et al. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotrophic lateral sclerosis and other motor neuron disorders. 2003;4:191–206. [PubMed] [Google Scholar]
- 4.Groeneveld G, Van Kan H, Kalmijn S, et al. Riluzole serum concentrations in patients with ALS Associations with side effects and symptoms. Neurology. 2003;61:1141–1143. doi: 10.1212/01.wnl.0000090459.76784.49. [DOI] [PubMed] [Google Scholar]
- 5.Groeneveld G, Kan H, Guchelaar HJ, et al. An association study of riluzole serum concentration and survival and disease progression in patients with ALS. Clinical Pharmacology & Therapeutics. 2008;83:718–722. doi: 10.1038/sj.clpt.6100382. [DOI] [PubMed] [Google Scholar]
- 6.Groeneveld G, Van Kan H, Toraño JS, et al. Inter-and intraindividual variability of riluzole serum concentrations in patients with ALS. Journal of the neurological sciences. 2001;191:121–125. doi: 10.1016/s0022-510x(01)00613-x. [DOI] [PubMed] [Google Scholar]
- 7.Bruno R, Vivier N, Montay G, et al. Population pharmacokinetics of riluzole in patients with amyotrophic lateral sclerosis. Clinical Pharmacology & Therapeutics. 1997;62:518–526. doi: 10.1016/S0009-9236(97)90047-3. [DOI] [PubMed] [Google Scholar]
- 8.Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow DS, Teng Y, Toups EG, et al. Pharmacology of riluzole in acute spinal cord injury. Journal of Neurosurgery: Spine. 2012;17:129–140. doi: 10.3171/2012.5.AOSPINE12112. [DOI] [PubMed] [Google Scholar]
- 10.Abbara C, Estournet B, Lacomblez L, et al. Riluzole pharmacokinetics in young patients with spinal muscular atrophy. Br J Clin Pharmacol. 2011;71:403–410. doi: 10.1111/j.1365-2125.2010.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderink G-J, Bournique B, Stevens J, et al. Involvement of human CYP1A isoenzymes in the metabolism and drug interactions of riluzole in vitro. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1465–1472. [PubMed] [Google Scholar]
- 12.Ajroud-Driss S, Saeed M, Khan H, et al. Riluzole metabolism and CYP1A1/2 polymorphisms in patients with ALS. Amyotroph Lateral Scler. 2007;8:305–309. doi: 10.1080/17482960701500650. [DOI] [PubMed] [Google Scholar]
- 13.van Kan HJ, Groeneveld GJ, Kalmijn S, et al. Association between CYP1A2 activity and riluzole clearance in patients with amyotrophic lateral sclerosis. Br J Clin Pharmacol. 2005;59:310–313. doi: 10.1111/j.1365-2125.2004.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Kan HJ, van den Berg LH, Groeneveld GJ, et al. Pharmacokinetics of riluzole: evidence for glucuronidation as a major metabolic pathway not associated with UGT1A1 genotype. Biopharm Drug Dispos. 2008;29:139–144. doi: 10.1002/bdd.594. [DOI] [PubMed] [Google Scholar]
- 15.Coric V, Milanovic S, Wasylink S, et al. Beneficial effects of the antiglutamatergic agent riluzole in a patient diagnosed with obsessive-compulsive disorder and major depressive disorder. Psychopharmacology (Berl) 2003;167:219–220. doi: 10.1007/s00213-003-1396-z. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger C, Kelmendi B, Wasylink S, et al. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a series of 13 cases, with long-term follow-up. Journal of clinical psychopharmacology. 2008;28:363–367. doi: 10.1097/JCP.0b013e3181727548. [DOI] [PubMed] [Google Scholar]
- 17.Grant P, Lougee L, Hirschtritt M, et al. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2007;17:761–767. doi: 10.1089/cap.2007.0021. [DOI] [PubMed] [Google Scholar]
- 18.Grant P, Joseph LA, Farmer CA, et al. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology. 2014;39:1453–1459. doi: 10.1038/npp.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scahill L, McDougle CJ, Williams SK, et al. Children’s Yale-Brown Obsessive Compulsive Scale modified for pervasive developmental disorders. J Am Acad Child Psy. 2006;45:1114–1123. doi: 10.1097/01.chi.0000220854.79144.e7. [DOI] [PubMed] [Google Scholar]
- 20.SAS. [computer program]. Version 9.3. Cary, NC: SAS Institute, Inc; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


