Abstract
Angiotensin II (AngII)-activated epidermal growth factor receptor (EGFR) has been implicated in abdominal aortic aneurysm (AAA) development. In vascular smooth muscle cells (VSMC), AngII activates EGFR via a metalloproteinase, a disintegrin and metallopeptidase domain 17 (ADAM17). We hypothesized that AngII-dependent AAA development would be prevented in mice lacking ADAM17 in VSMCs. To test this concept, control and VSMC ADAM17 deficient mice were co-treated with AngII and a lysyl oxidase inhibitor, β-aminopropionitrile, to induce AAA. We found that 52.4% of control mice did not survive due to aortic rupture. All other surviving control mice developed AAA and demonstrated enhanced expression of ADAM17 in the AAA lesions. In contrast, all AngII and β-aminopropionitrile-treated VSMC ADAM17 deficient mice survived and showed reduction in external/internal diameters (51%/28%, respectively). VSMC ADAM17 deficiency was associated with lack of EGFR activation, interleukin-6 induction, ER/oxidative stress and matrix deposition in the abdominal aorta of treated mice. However, both VSMC ADAM17 deficient and control mice treated with AngII and β-aminopropionitrile developed comparable levels of hypertension. Treatment of C57Bl/6 mice with an ADAM17 inhibitory antibody but not with control IgG also prevented AAA development. In conclusion, VSMC ADAM17 silencing or systemic ADAM17 inhibition appears to protect mice from AAA formation. The mechanism appears to involve suppression of EGFR activation.
Keywords: Aneurysm, Rupture, Hypertension, Angiotensin II, Signal Transduction
Introduction
Abdominal aortic aneurysm (AAA) is a significant cause of death in the elderly, whereas effective pharmacological intervention is not yet available 1. Activation of the renin angiotensin II (AngII) system has been implicated in AAA development in human and animal models of AAA 2, 3. While the detailed molecular mechanism by which AngII promotes AAA development remains unclear, it seems to involve multiple cell types (leukocytes, vascular smooth muscle cells (VSMC) and endothelial cells) and signaling responses such as oxidative stress, induction of inflammatory cytokines and activation of matrix metalloproteinases (MMPs) 4, 5. However, limited information is available regarding the cell type-specific mechanism critical for AAA development. Therefore, the aims of this study are to define an essential signaling mechanism for development of AAA in an AngII-dependent mouse model and to provide a novel therapeutic target in AAA.
We have demonstrated that a metalloproteinase, a disintegrin and metallopeptidase domain 17 (ADAM17), is required for AngII-induced epidermal growth factor receptor (EGFR) transactivation in VSMCs 6 and that the ADAM17/EGFR activation mediates vascular remodeling in mice infused with AngII 7, 8. Moreover, ADAM17 expression is enhanced in human AAA 9, 10 and ADAM17 silenced mice do not develop CaCl2-induced AAAs 9.
β-aminopropionitrile (BAPN) is an inhibitor for lysyl oxidase, which cross-links elastin fibers and collagen fibers and plays a critical role in maintaining homeostasis of the elastic lamina 11. While BAPN treatment alone does not promote AAA, it causes AAA development and rupture when combined with AngII via degeneration of elastic lamina 12. In VSMCs, both ADAM17 and EGFR co-localize at caveolae, and AAA formation induced by AngII plus BAPN was attenuated in caveolin-1 deficient mice 13. Moreover, we have recently reported that pharmacological inhibition of EGFR prevents AAA development induced by AngII plus BAPN, which was associated with suppression of ER stress, oxidative stress, and interleukin-6 and MMP-2 expression 10. However, whether VSMC specific ADAM17 expression is essential for signal transduction leading to AAA development or any pharmacological ADAM17 intervention prevents AAA development remains unclear. In the present study, we tested the hypothesis that genetic silencing of VSMC ADAM17 or systemic ADAM17 inhibitory antibody treatment prevents AAA formation induced by AngII plus BAPN.
Materials and Methods
Animal protocol
All animal procedures were performed with prior approval from Temple University Institutional Animal Care and Use Committee and in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice deficient in VSMC ADAM17 (ADAM17flox/flox Sm22α-Cre+/−) were generated as reported 14. At 8–10 weeks of age, male ADAM17flox/flox Sm22α-Cre+/− mice and littermate male control, ADAM17flox/flox Sm22α-Cre−/− mice, were infused with AngII (Bachem, 1 µg/kg/min) for 4 weeks and BAPN (TCI, 150 mg/kg/day) for the first 2 weeks or control saline for 4 weeks (Alzet, Durect Corp) 13. In addition, 8–10 week old male C57Bl6 mice (Jackson) were infused with AngII (1 µg/kg/min) for 4 weeks and received BAPN in drinking water (1 mg/mL) for the first 2 weeks together with human/mouse cross-reactive ADAM17 inhibitory antibody A9B8 15 or control human IgG2 (Athens Research & Technology) which was solubilized in PBS and administered at 10 mg/kg/day via intraperitoneal injection, at days 1, 7, 14 and 21. Control C57Bl6 mice were sham-operated.
The AngII plus BAPN-induced mouse model of aortic aneurysm reproducibly induces AAA with morphological and histological characteristics similar to human AAA, but without enhancing atherosclerosis, as seen in other AngII-dependent AAA models 12. Aortic luminal diameter at maximal dilation was measured using high-resolution two-dimensional imaging (B mode) with high frequency ultrasound (VisualSonics Vevo2100) on day 0, 14, 21 and 28 of the study. The treatment protocols were blinded to the evaluator. Despite the original manuscript reporting incidence of thoracic aortic aneurysm (TAA) in this model (38%) 12, we consistently observed much less TAA (0~10%) 10, 13. Therefore, quantitative evaluation was not performed in thoracic aortas. Blood pressure and heart rate were evaluated in the conscious state at day 28 by telemetry (DSI equipped with ADInstrument 6 software) via carotid catheter (PA-C10 transmitter). For animals that died before the completion of the study, necropsy was performed when possible to define the causes of death. The thoracic aorta or abdominal aorta rupture as a cause of death was defined with the presence of hematoma in thoracic or abdominal cavity, respectively. After 28 days, mice were euthanized, perfused with formalin, and dissected for tissue samples. Abdominal aortas were extracted and subjected to paraffin embedding. Sections were stained with a standard Masson’s trichrome protocol 8 to distinguish medial area from adventitia. Images were visualized on an Olympus IX81 inverted microscope using an Olympus SC30 high resolution camera and acquired with Olympus cellSens Entry 1.11 software.
Immunohistochemistry
Sections from abdominal aortas were deparaffinized and blocked in 5% goat serum and 1% BSA for 1h at room temperature, incubated with primary antibody in PBS containing 1% BSA and 0.1% Tween 20 overnight at 4 °C followed by biotinylated secondary antibody for 90 min at room temperature. The sources and dilutions of the primary antibodies used in this study are provided in Supplementary Table S1. Slides were incubated with avidin–biotin peroxidase complex for 30 min at room temperature and staining was visualized with the substrate diaminobenzidine (Vector), which produced a brown color, and counterstained with haematoxylin. An equal concentration of control IgG was used side-by-side with each antibody to ensure staining specificity. Quantification of the antibody staining was performed as reported previously with subtraction of IgG background staining 13. All images were visualized on Olympus SC30 high resolution camera and acquired with Olympus cellSens Entry 1.11 software using the same exposure time. Images were loaded into the ImageJ program (http://rsb.info.nih.gov/ij) for analysis. A region of interest was drawn around the entire aorta with the freehand selection tool. Adventitia was excluded from the quantification since the adventitia areas were quite limited in aortas except those with AAA. All images were set to the same hue, saturation, and brightness. The area and intensity (integrated density) in the region of interest were then measured and analyzed. Data were obtained from three to four non-overlapping fields per aortic cross-section for each antibody (n=4 aortas per treatment or genotype). Results are presented as fold increase over control, which was set at 1.
Statistical analysis
Data from the groups were analyzed by 1-way ANOVA with Tukey’s multiple comparison test (C57Bl6 mice with basal, AAA treatment or AAA treatment plus ADAM17 antibody), 2-way ANOVA with Bonferroni post-tests (ADAM17 wild type or deficient mice with or without AAA treatment) or log-rank (Mantel-Cox) test (Kaplan-Meier survival curves) using GraphPad Prism version 5.0C for Macintosh. Data were presented as mean±SEM. Statistical significance was taken at p<0.05.
Results
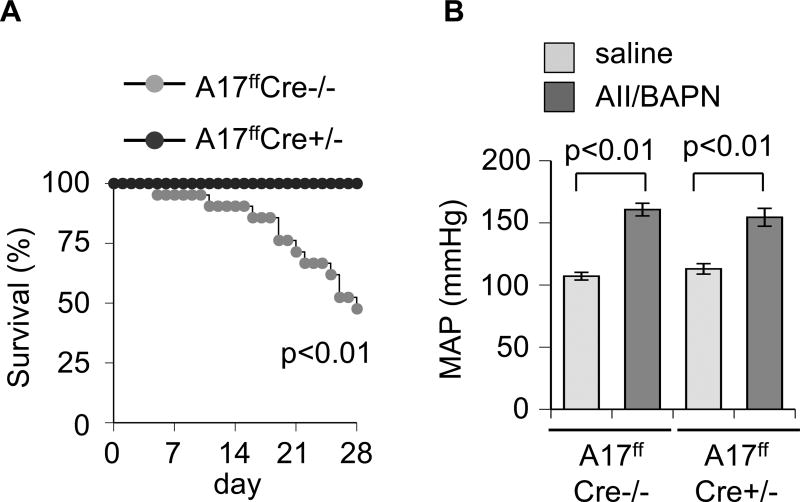
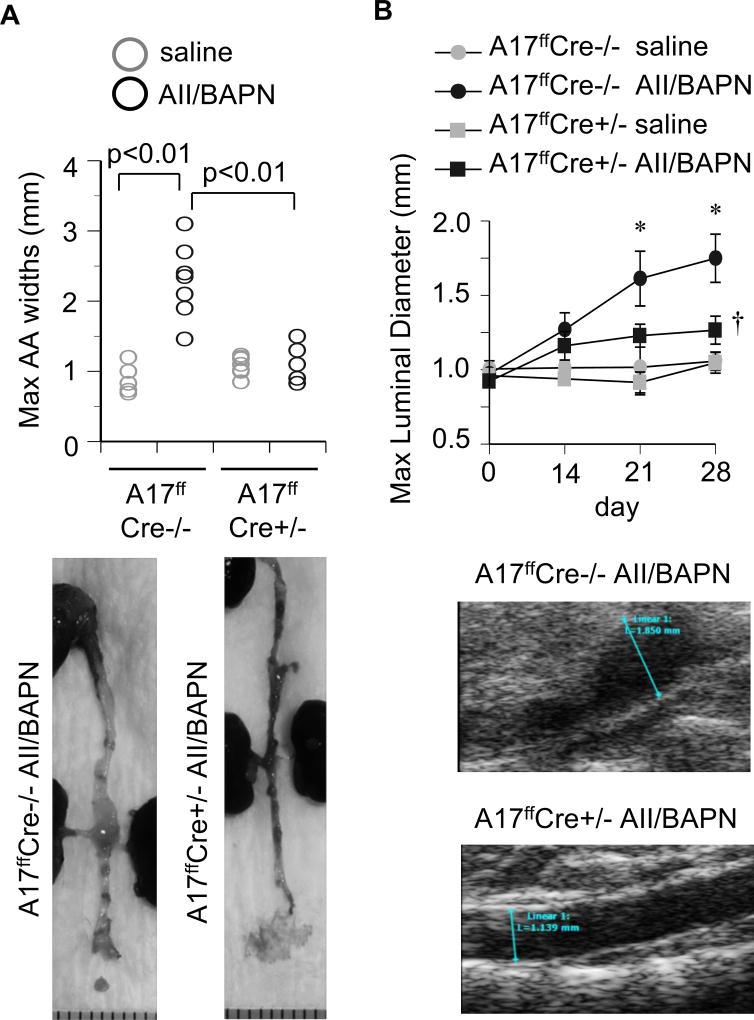
There was a significant difference in survival rates between mice deficient with VSMC ADAM17 (100%) and littermate control mice (47.6%) when treated with AngII plus BAPN over the 28-day observational period (Figure 1A). Based on necropsy, all confirmed deaths were caused by aortic rupture. Surviving control mice, as well as mice deficient in VSMC ADAM17, treated with AngII plus BAPN developed comparable degree of hypertension (Figure 1B and Supplementary Table S2). All surviving AngII plus BAPN treated control mice developed AAA with significant enlargements of the maximum diameter of abdominal aortas. In contrast, VSMC ADAM17 deficient mice with AngII plus BAPN treatment had significantly less aortic diameter enhancement compared to control mice (Figure 2 and Supplementary Figure S1 and S2).
Figure 1.
A. VSMC ADAM17 deficient mice survived from aortic rupture. 8 week old VSMC ADAM17 deficient mice (ADAM17flox/flox sm22fCre+/−, A17ffCre+/−) and control mice (ADAM17flox/flox sm22 Cre−/−, A17ffCre−/−) were infused with AngII (1 µg/kg/min, 4 weeks) and BAPN (150 mg/kg/day, first 2 weeks) or saline for 4 weeks. Percentage survival curve is shown (n=21). B. Telemetry recording of mean arterial blood pressure (MAP) upon 4 week infusion (n=5).
Figure 2.
VSMC ADAM17 deficient mice did not develop AngII-dependent AAA. VSMC ADAM17 deficient mice (A17ffsm22αCre+/−) and control littermate mice (Cre−/−) were infused with AngII plus BAPN or saline as in Figure 1. A. Measurements of maximal external width of abdominal aorta upon fixation at 4 weeks (Cre−/−: 2.29±0.50 mm vs Cre+/−: 1.12±0.23 mm with AngII plus BAPN, n=6). B. Weekly ultrasound evaluation of maximal abdominal aorta luminal diameter. The panels shown were at 4w. *, † p<0.05 compared with control saline or AngII/BAPN infusion, respectively (each n=5). The measurements were blinded and performed by two evaluators confirming the reproducibility.
Histological analysis demonstrated that AAAs induced by AngII plus BAPN in control mice were associated with vascular fibrosis/matrix deposition and disruption of medial layer structures. In addition, enhanced EGFR activation, increased expression of ADAM17, MMP-2, and interleukin-6, and enhanced ER stress (KDEL), oxidative stress (nitro-tyrosine) and leukocyte infiltration (CD45) were observed with semi-quantitative immuno-histochemical staining. These AAA associated responses were attenuated in VSMC ADAM17 deficient mice. However, tumor necrosis factor α (TNFα) expression did not show any statistical differences among the groups (Supplementary Figure S3, S4, S5 and S6).
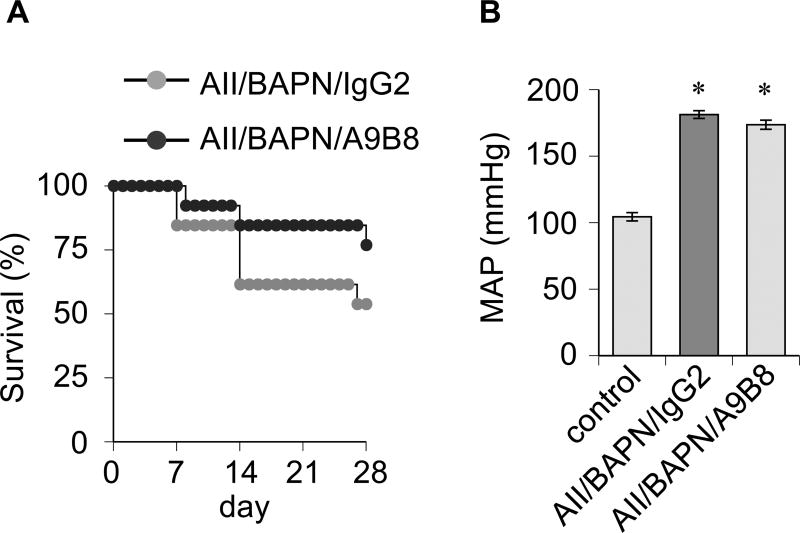
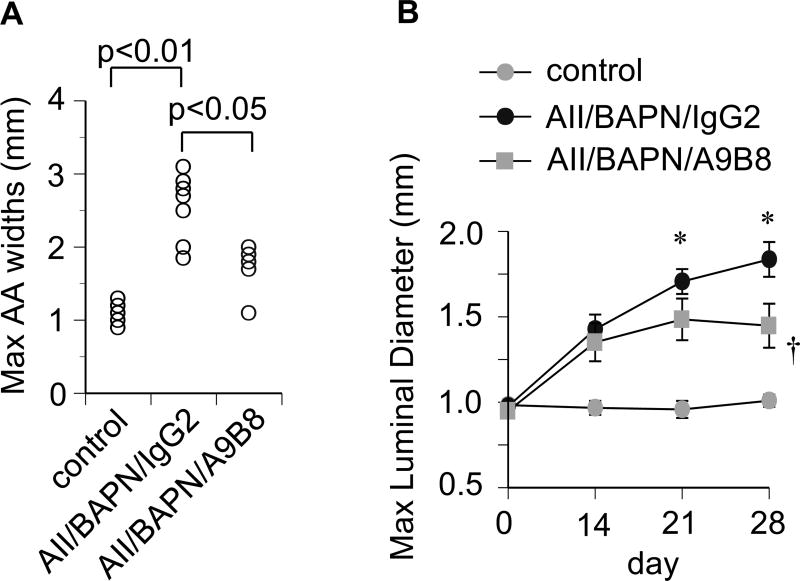
To ascertain that ADAM17 represents a novel therapeutic target to prevent AAA development, C57Bl6 mice treated with AngII plus BAPN were injected with a human/mouse cross-reactive ADAM17 inhibitory antibody. Distinct from VSMC ADAM17 silencing, there was no statistical difference in the survival rate with the ADAM17 antibody treatment (Figure 3A). The ADAM17 antibody treatment did not alter hypertension induced by AngII plus BAPN (Figure 3B and Supplementary Table S3). However, AAA development was significantly reduced in mice treated with the ADAM17 antibody compared with the control IgG treatment (Figure 4 and Supplementary Figure S7, S8 and S9).
Figure 3.
A. Survival data of C57Bl6 mice with ADAM17 antibody treatment. 8 week old C57Bl6 mice infused with AngII (1 µg/kg/min, 4 weeks) and received BAPN (1 mg/mL in drinking water, first 2 weeks) were intraperitoneally injected ADAM17 antibody A9B8 or control human IgG2 (10 mg/kg) at day 1, 7, 14 and 21. B. Telemetry recording of mean arterial blood pressure (MAP) upon 4 week infusion. Control C57Bl6 mice are sham-operated for minipump implantation (n=5).
Figure 4.
ADAM17 antibody attenuated AAA development. C57Bl6 mice were received AngII plus BAPN together with ADAM17 antibody A9B8 or control IgG2 as in Figure 3. A. Measurements of maximal external width of abdominal aorta upon fixation at 4 weeks (n=6). B. Weekly ultrasound evaluation of maximal abdominal aorta luminal diameter. *, † p<0.05 compared with control saline or AngII/BAPN infusion, respectively (each n=6~7).
Discussion
Enhanced ADAM17 expression 9 and down-stream EGFR activation 10 have been reported in human AAA samples. Requirement of ADAM17 in CaCl2-induced AAA has also been reported with inducible systemic ADAM17 deletion 9. However, our study provides new information that AngII-dependent AAA development and rupture are markedly prevented in mice lacking VSMC ADAM17 and that pharmacological intervention of ADAM17 can attenuate AAA in a mouse model. Limited VSMC-specific mechanisms are known to contribute to AAA in animal models, which includes decreased catalase 16 and activation of Notch1 17, whereas induction of hypoxia-inducible factor-1α in VSMCs appears protective 18.
In VSMCs stimulated with AngII, ADAM17-dependent shedding produces EGFR ligands such as heparin-binding EGF-like growth factor leading to EGFR transactivation 19. Since EGFR inhibition also prevents AngII plus BAPN-mediated AAA development and rupture 10, VSMC EGFR most likely mediates the ADAM17-dependent function in this mouse model of AAA. Upon activation, EGFR mediates several downstream responses in VSMCs including oxidative stress 20, ER stress 8 and intereukin-6 induction 21. These downstream effects were also evident in the present study, thus likely contributing to AAA development (Supplementary Figure S10) 16, 22, 23. In addition, enhanced vascular ER and oxidative stress further promote immune cell infiltration, which is also critical for AAA development 4. Therefore, the findings demonstrated in this manuscript suggest that all of these mechanisms are potentially mediated by a single but multifunctional metalloprotease ADAM17 specifically expressed in VSMCs. However, additional experiments are desired to track these mechanisms before the establishment of AAA. It is also ideal to utilize a distinct model of AAA to generalize our findings, which are limitations in this study.
While we confirmed a previously suggested EGFR-dependent mechanism as a downstream signal of VSMC ADAM17, our findings cannot exclude other and potentially new mechanism(s) through which ADAM17 contributes to AAA development. Other ADAM17 substrates including TNFα, Notch1 and angiotensin converting enzyme 2 may also participate in AAA pathology according to literature 17, 24, 25. Note that while the protein expression analysis of TNFα did not show any enhancement in AAA, the experiment did not measure the potential conversion of pro-TNFα to mature and active TNFα by ADAM17. Moreover, ADAM17 has additional diverse substrates and many regulatory mechanisms 26. Therefore, further research is required to identify potentially new pathways through which ADAM17 regulates AAA.
The AngII plus BAPN model consistently produces AAA associated with hypertension, but without enhancing atherosclerosis, as observed in other AngII-dependent AAA models including hyperlipidemic mice 12. Incidence of AAAs in normolipidemic mice with AngII infusion alone is very low 27 even though AngII infusion is sufficient for ADAM17 induction and EGFR activation in the vasculature, including the aorta 8. Therefore, we surmise that ADAM17/EGFR activation is required to advance AAA but insufficient to initiate AAA, which requires an additional signal or condition such as those primed by BAPN or hyperlipidemia.
In the present study, a discrepancy was observed in survival rates between VSMC ADAM17 deletion with mixed background mice and systemic ADAM17 inhibition with C57BL6 mice. This may be due to significantly higher blood pressure in C57BL6 mice regardless of the ADAM17 antibody or control IgG treatment. The discrepancy could also be due to distinct genetic background of the mice and/or distinct cell type specific roles (promoting vs preventing rupture in AAA) of ADAM17. In addition to the medial layer, enhanced ADAM17 expression was also observed in endothelium and adventitia of AAA, a finding that is in agreement with prior published work 13. Since the promoter used to target ADAM17 is relatively specific to smooth muscle 14, we assume that attenuation of AAA by silencing VSMC ADAM17 results in prevention of endothelial and adventitial ADAM17 induction by AngII plus BAPN. In addition to VSMC ADAM17, the ADAM17 antibody likely inhibited endothelial as well as adventitial ADAM17 in the present study. Therefore, the roles of endothelial and adventitial ADAM17 in AAA require further study.
Supplementary Material
Novelty and Significance.
What is new?
Systemic and VSMC specific ADAM17 inhibition established a role for ADAM17 in AngII-dependent AAA development independent of hypertension in mice.
The concept of vascular ADAM17 in mediating the EGFR pathway, oxidative stress, ER stress and inflammation was presented.
What is relevant?
Results indicating prevention of AAA but not hypertension by ADAM17 inhibition provide a foundation to seek a potential therapy to prevent AAA development.
The vascular dominant ADAM17 signal transduction highlights the importance of vascular signal transduction for AAA formation.
Perspective.
Our findings highlight the critical role of ADAM17 in mediating AAA development and rupture. We propose VSMC ADAM17 is a needed component for EGFR transactivation contributing to ER stress and oxidative stress in AAA. Our results also indicate ADAM17 inhibition can be a valuable treatment option for AAA. However, it remains to be explored how the VSMC ADAM17 signal communicates with other cell type-specific mechanisms presented before.
Summary.
In AngII plus BAPN treated VSMC ADAM17 deficient mice, AAA development and rupture were prevented compared with treated control mice. These aortas with AAA showed vascular EGFR activation and induction of ER stress and oxidative stress markers, which were attenuated in ADAM17 deficient mice. ADAM17 inhibitory antibody was utilized to confirm the contribution of ADAM17 in AAA pathology.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants HL128324 (S.E. and V.R.), HL133248 (S.E.), DK111042 (R.S. and S.E.), F31HL127971 (S.J.F.), American Heart Association grants 16GRNT30410007 (S.E.), 16GRNT30130013 (V.R.), 15POST25550083 (K.J.E.) and 16POST3051004 (T.K.), University of Macau grants SRG2014-0006-FHS (H.F.K) and MYRG2015-00025-FHS (H.F.K.) and Science and Technology Development Fund of Macau FDCT 018/2015/A1 (H.F.K.).
Footnotes
Disclosures
None.
References
- 1.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 2.Golledge J, Norman PE. Pathophysiology of abdominal aortic aneurysm relevant to improvements in patients' management. Curr Opin Cardiol. 2009;24:532–538. doi: 10.1097/HCO.0b013e328330c2d3. [DOI] [PubMed] [Google Scholar]
- 3.Lu H, Rateri DL, Bruemmer D, Cassis LA, Daugherty A. Involvement of the renin-angiotensin system in abdominal and thoracic aortic aneurysms. Clin Sci (Lond) 2012;123:531–543. doi: 10.1042/CS20120097. [DOI] [PubMed] [Google Scholar]
- 4.Davis FM, Rateri DL, Daugherty A. Abdominal aortic aneurysm: novel mechanisms and therapies. Curr Opin Cardiol. 2015;30:566–573. doi: 10.1097/HCO.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emeto TI, Moxon JV, Au M, Golledge J. Oxidative stress and abdominal aortic aneurysm: potential treatment targets. Clin Sci (Lond) 2016;130:301–315. doi: 10.1042/CS20150547. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 7.Takayanagi T, Forrester SJ, Kawai T, Obama T, Tsuji T, Elliott KJ, Nuti E, Rossello A, Kwok HF, Scalia R, Rizzo V, Eguchi S. Vascular ADAM17 as a Novel Therapeutic Target in Mediating Cardiovascular Hypertrophy and Perivascular Fibrosis Induced by Angiotensin II. Hypertension. 2016;68:949–955. doi: 10.1161/HYPERTENSIONAHA.116.07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park JY, Eguchi S. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65:1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko H, Anzai T, Horiuchi K, Kohno T, Nagai T, Anzai A, Takahashi T, Sasaki A, Shimoda M, Maekawa Y, Shimizu H, Yoshikawa T, Okada Y, Yozu R, Fukuda K. Tumor necrosis factor-alpha converting enzyme is a key mediator of abdominal aortic aneurysm development. Atherosclerosis. 2011;218:470–478. doi: 10.1016/j.atherosclerosis.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Obama T, Tsuji T, Kobayashi T, Fukuda Y, Takayanagi T, Taro Y, Kawai T, Forrester SJ, Elliott KJ, Choi ET, Daugherty A, Rizzo V, Eguchi S. Epidermal Growth Factor Receptor Inhibitor Protects Abdominal Aortic Aneurysm in a Mouse Model. Clin Sci (Lond) 2014 doi: 10.1042/CS20140696. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez C, Martinez-Gonzalez J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- 12.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayanagi T, Crawford KJ, Kobayashi T, Obama T, Tsuji T, Elliott KJ, Hashimoto T, Rizzo V, Eguchi S. Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin Sci (Lond) 2014;126:785–794. doi: 10.1042/CS20130660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106:932–940. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok HF, Botkjaer KA, Tape CJ, Huang Y, McCafferty J, Murphy G. Development of a 'mouse and human cross-reactive' affinity-matured exosite inhibitory human antibody specific to TACE (ADAM17) for cancer immunotherapy. Protein Eng Des Sel. 2014;27:179–190. doi: 10.1093/protein/gzu010. [DOI] [PubMed] [Google Scholar]
- 16.Parastatidis I, Weiss D, Joseph G, Taylor WR. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2013;33:2389–2396. doi: 10.1161/ATVBAHA.113.302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachdeva J, Mahajan A, Cheng J, Baeten JT, Lilly B, Kuivaniemi H, Hans CP. Smooth muscle cell-specific Notch1 haploinsufficiency restricts the progression of abdominal aortic aneurysm by modulating CTGF expression. PLoS One. 2017;12:e0178538. doi: 10.1371/journal.pone.0178538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imanishi M, Chiba Y, Tomita N, Matsunaga S, Nakagawa T, Ueno M, Yamamoto K, Tamaki T, Tomita S. Hypoxia-Inducible Factor-1alpha in Smooth Muscle Cells Protects Against Aortic Aneurysms-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36:2158–2162. doi: 10.1161/ATVBAHA.116.307784. [DOI] [PubMed] [Google Scholar]
- 19.Forrester SJ, Kawai T, O'Brien S, Thomas W, Harris RC, Eguchi S. Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System. Annu Rev Pharmacol Toxicol. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 21.Ichiki T. Role of cAMP response element binding protein in cardiovascular remodeling: good, bad, or both? Arterioscler Thromb Vasc Biol. 2006;26:449–455. doi: 10.1161/01.ATV.0000196747.79349.d1. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Wang Y, Liu O, Jia L, Fang W, Du J, Wei Y. Tauroursodeoxycholic Acid Attenuates Angiotensin II Induced Abdominal Aortic Aneurysm Formation in Apolipoprotein E-deficient Mice by Inhibiting Endoplasmic Reticulum Stress. Eur J Vasc Endovasc Surg. 2017;53:337–345. doi: 10.1016/j.ejvs.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Harrison SC, Smith AJ, Jones GT, Swerdlow DI, Rampuri R, Bown MJ, on behalf of the Aneurysm, C. Folkersen L, Baas AF, de Borst GJ, Blankensteijn JD, Price JF, van der Graaf Y, McLachlan S, Agu O, Hofman A, Uitterlinden AG, Franco-Cereceda A, Ruigrok YM, Van't Hof FN, Powell JT, van Rij AM, Casas JP, Eriksson P, Holmes MV, Asselbergs FW, Hingorani AD, Humphries SE. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2012;34:3707–3716. doi: 10.1093/eurheartj/ehs354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thatcher SE, Zhang X, Howatt DA, Yiannikouris F, Gurley SB, Ennis T, Curci JA, Daugherty A, Cassis LA. Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2014;34:2617–2623. doi: 10.1161/ATVBAHA.114.304613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Mukerjee S, Silva-Alves CR, Carvalho-Galvao A, Cruz JC, Balarini CM, Braga VA, Lazartigues E, Franca-Silva MS. A Disintegrin and Metalloprotease 17 in the Cardiovascular and Central Nervous Systems. Front Physiol. 2016;7:469. doi: 10.3389/fphys.2016.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remus EW, O'Donnell RE, Jr, Rafferty K, Weiss D, Joseph G, Csiszar K, Fong SF, Taylor WR. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2012;303:H1067–1075. doi: 10.1152/ajpheart.00217.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.