Abstract
Lupus nephritis is a common and severe manifestation of systemic lupus erythematosus that disproportionately affects non-Whites and those in lower socioeconomic groups. This review discusses recent data on the incidence, prevalence, and outcomes of patients with lupus nephritis with a focus on low-income US Medicaid patients. We also review recent guidelines on diagnosis, treatment, and screening for new onset and relapses of lupus nephritis. Finally, we discuss the management of lupus nephritis from a rheumatologist’s perspective, including vigilance for the common adverse events related to disease and treatment, and review prevention and new treatment strategies.
Keywords: lupus, glomerulonephritis
Introduction
Lupus nephritis (LN) affects approximately 40% of those with systemic lupus erythematous (SLE) (1–3). Over the past five decades, therapeutic advances, in particular immunosuppression, have significantly improved outcomes for patients with LN (4). However, recent evidence indicates that improvement in LN outcomes plateaued in the 2000s, perhaps because we have reached the limitations of our current therapies (4). With current induction and maintenance therapies, the risk of developing LN-related ESRD at five, ten, and fifteen years remains at 11, 17, and 22% (CI 10–12%, 16–18%, 20–23%) for the last decade (4).
The lack of sustained progress in LN outcomes may be due to poor healthcare access for some groups, limited efficacy of current therapies, and/or their adverse effects (4). Here, we discuss recent epidemiological studies addressing which patients develop LN and LN-related ESRD with an emphasis on the US given its diverse population. We also discuss the role of the rheumatologist in the management of LN, and review some recent management guidelines. We discuss patient- and medication-related obstacles that hinder progress, while highlighting several promising new ideas addressing these obstacles.
Rates of SLE and lupus nephritis, and barriers to healthcare access in the US
SLE and LN both occur disproportionately among non-Whites, which is well demonstrated in the heterogeneous population of the US. In recent studies from Centers for Disease Control, the overall prevalence of SLE was 72.2 and 74.4 per 100 000 persons in large patient cohorts from Georgia and Michigan, respectively (5, 6). However, the prevalence is several times greater in Blacks than Whites (116.1 vs. 34.8 per 100 000 persons), and a higher proportion of Black SLE patients developed renal disease and ESRD (40.5% and 15.3%) compared to Whites (18.8% and 4.5%) (5). Similarly, in a national cohort of patients enrolled from 2000 to 2004 in Medicaid, the US government-administered health insurance for the poor, incidence rates of LN were 3.8 times higher in Blacks, 3.7 in Asians, 2.3 in Native Americans, and 1.9 in Hispanics patients compared to White SLE patients (7).
While the majority of new SLE cases occur in individuals between 15 to 40 years of age, approximately 15% of cases arise in patients <16 years old (8). The proportion of SLE patients with LN is higher among those with childhood-onset compared to adult-onset SLE (approximately 37 vs. 20%) (8). As in adults, SLE occurs most frequently in girls, while boys and non-White children experience higher rates of LN (8, 9).
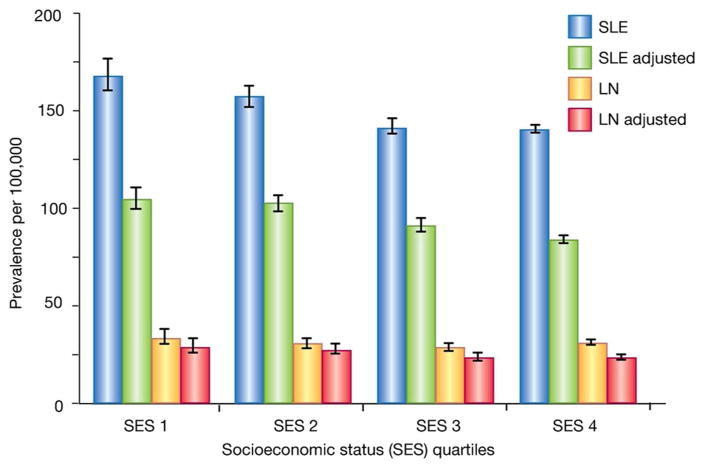
A socioeconomic gradient is apparent in the prevalence of LN, with increased prevalence in poorer geographic areas that persists after adjustment for age, sex, and ethnicity (Figure 1) (7). The reasons for this are unclear, but recent studies may shed some light. In many poor US urban areas, LN patients are more likely to be underinsured and to utilize emergency room care at higher rates than those in more affluent areas (10), possibly leading to suboptimal care. Additionally, some patients in the rural US reside >200 miles from the nearest rheumatologist, prohibiting timely access to medical care (11). When a LN patient in a resource-poor area does find appropriate care, immunosuppressive medication may be prohibitively expensive or unavailable (12). Finally, new evidence suggests the care itself may be substandard in some medically-underserved areas. In one study, less than half of Medicaid enrollees with new onset LN received standard antimalarial and renoprotective agents at 90 days, with little improvement at one year (13). These studies suggest high barriers to healthcare exist in resource-poor areas of the US, but barriers are probably higher in developing countries, which may contribute to increased rates of SLE-associated ESRD outside the US (4).
Figure 1.
The prevalence of systemic lupus erythematosus (SLE) and lupus nephritis (LN) is inversely related to socioeconomic status (SES). Depiction of the prevalence of SLE and LN per per 100,000 US Medicaid enrollees aged 18–65 years old. Data are stratified by SES quartile where SES 1 (lowest) is < −1.62, SES 2 above −1.62 to −0.74, SES 3 = above −0.74 through 0.26, SES 4 [highest] = above 0.26). The results of crude analyses and analyses are adjusted for age group, sex, and ethnicity. Bars represent 95% confidence intervals.
The Rheumatologist’s role in lupus nephritis: Screening, diagnosis, and coordinated treatment
Systemic manifestations of SLE precede renal involvement in the majority of patients (14). The rheumatologist is therefore positioned to recognize new onset or recurrent LN and to refer to a nephrologist for kidney biopsy, initiation of appropriate therapy, and co-management. The ACR recommends regular screening for new onset and recurrent kidney involvement. While the screening frequency depends on disease severity and prior history of LN, we are especially vigilant in the first six months of SLE diagnosis when new LN most often occurs (15). Otherwise, screening should occur every three to six months in patients with no past history or stable LN. Specifically, blood pressure, body weight, anti-double stranded DNA (dsDNA), C3 and C4 complement levels should be followed (16, 17). In addition, glomerular health should be regularly assessed with urinalysis and sediment analysis to identify new cellular casts (red cells, granular, tubular or mixed casts), active urinary sediment (>5 RBCs/HPF or >5WBC/HPF in the absence of infection), or persistent proteinuria (>0.5 mg/day) (17). These biomarkers are not infallible, but increases in anti-dsDNA antibody titers and decreases in C3 and C4 track with disease activity and the risk of LN relapse. Other novel biomarkers, including urinary biomarkers, are being investigated but are not used for routine clinical care.
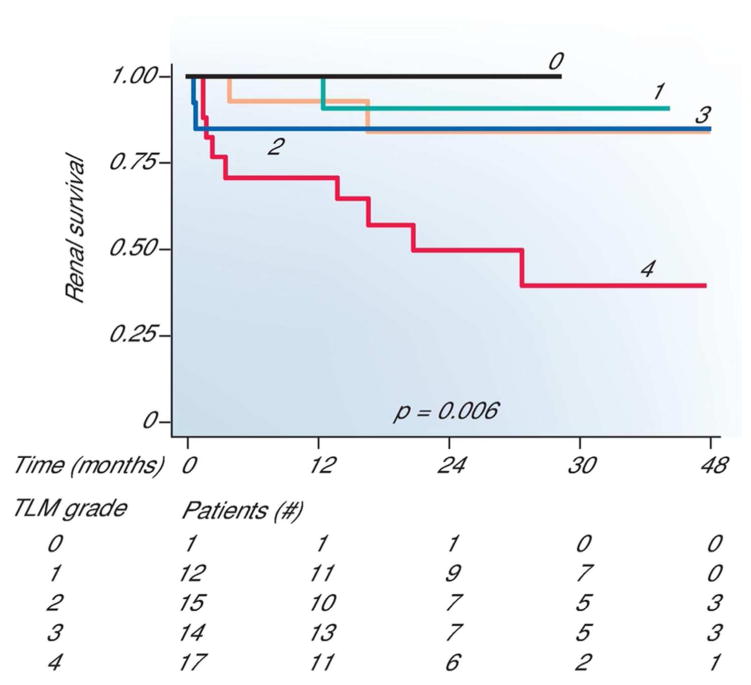
SLE patients with evidence of new onset LN should undergo a renal biopsy (unless contraindicated) to evaluate for histopathologic lesions of glomerulonephritis, as well as other renal injury. As discussed in the accompanying review by Rovin et al, lupus glomerulonephritis is categorized histologically into six classes by the International Society of Nephrology/Renal Pathology Society (ISN/RPS). Revised in 2003, this classification system has become the standard for renal biopsy interpretation because of interobserver reproducibility, and improved correlation with prognostic and therapeutic outcomes (17, 18). In addition to the ISN/RPS classes, other histological features affect treatment decisions and prognosis, although no guidelines exist for these (17, 18). For example, patients with high “activity” lesions are usually treated with immunosuppression, while those with “chronic” lesions may not receive therapy because of a poorer response prognosis (18, 19). Another concomitant biopsy finding, tubolointerstitial inflammation, confers more than two-fold risk on the doubling of serum creatinine or ESRD in some ethnic populations (Figure 2) (20). The significance of other biopsy findings, such as thrombotic microangiopathy, cryoglobulinemia, acute interstitial nephritis and collapsing glomerulonephropathy, in treatment responsiveness and outcomes is not yet well characterized.
Figure 2.
The extent of interstitial nephritis severity correlates with renal survival.
Applying a five-tier* measurement of interstitial inflammation Hsieh et al generate Kaplan-Meier curves of renal survival. Total conventional light microscopy (TLM) without subtracting areas containing interstitial fibrosis and tubular atrophy was used with standard histopathologic staining. The number of patients available for analysis in each grade is provided. P values were derived using a log rank trend test. *5 tier measure: none/grade 0=0%; minimal/grade 1= <10%, mild/grade 2= 10–25%, moderate/grade 3=26–50%; or severe/grade 4= >50%.
Treatment for Lupus Nephritis
Six separate LN management guidelines from the US, Europe, and international sources were recently published and generally agree on major treatment strategies (21). Here, we highlight recommendations from the American College of Rheumatology (ACR), and joint recommendations from the European League Against Rheumatism (EULAR) and European Renal Association-European Dialysis and Transplant Association (ERA-EDTA).
Adjunctive medications
The ACR and EULAR/ERA-EDTA recommend that all LN patients receive several adjunctive medications consisting of: (i) hydroxychloroquine (HCQ), (ii) a renoprotective agent, and (iii) a lipid-lowering statin. HCQ has many benefits and carries very low risk of side effects. HCQ reduces the rate of systemic relapses of SLE, and may be associated with slowing renal damage, thromboses, hyperglycemia, and hyperlipidemia. While rare, the most common and serious risk from HCQ is irreversible vision loss from retinal toxicity. Therefore, patients should have regular dilated retinal exams by a trained ophthalmologist. In cases of chronic renal insufficiency and ESRD, dosing guidelines for HCQ are not clear. Even reduced HCQ doses may increase the risk for retinal damage, as well as neurologic and muscle toxicities. Renin-angiotensin-aldosterone system (RAAS) inhibitors are recommended to patients with proteinuria >0.5 gm/day. Patients with SLE, even more so with LN, have increased risk of cardiovascular events (22). Therefore, lipid-lowering statin medications are also indicated for SLE and LN patients to maintain LDL <100 mg/dL (17, 23).
Immunosuppression
For class II lupus glomerulonephritis alone there is no ideal treatment strategy. RAAS inhibitors are indicated for proteinuria, but the role of immunosuppression is less clear. While the ACR does not recommend immunosuppression for class II LN, EULAR/ERA-EDTA endorses low to moderate oral glucocorticoids doses, plus azathioprine for individuals with proteinuria >1 gm/day or urinary acanthocytes.
For patients with class III or IV proliferative glomerulonephritis, the guidelines uniformly agree on induction therapy with moderate oral glucocorticoid doses plus intravenous (IV) cyclophosphamide or mycophenolate mofetil (MMF), with or without initial pulses of IV methylprednisolone. One of two schedules of IV cyclophosphamide is administered according to ethnic background and disease severity: the “low-dose” Eurolupus regimen is usually administered to Whites and consists of IV cyclophosphamide 500 mg every two weeks for three months; the “high-dose” NIH regimen is usually administered to Blacks and consists of 0.5 – 1 gm/m2 monthly for six doses (21). Given cyclophosphamide’s risk of bladder and hematologic malignancies, ovarian failure in women, and mounting evidence that MMF is more effective in Blacks, MMF may be the preferred induction therapy (24). Additionally, as both cyclophosphamide and MMF are potentially teratogenic, women of childbearing age should have negative pregnancy tests and receive contraceptive planning prior to starting therapy. The risk of ovarian failure associated with cyclophosphamide increases with age, and ovarian preserving regimens, such as GnRH agonists, should be considered (25).
For maintenance therapy, both the ACR and EULAR/ERA-EDTA recommend MMF or azathioprine, with or without low dose glucocorticoids. However, EULAR/ERA-EDTA recommends treatment only for “active lesions”. As is the case for induction therapy, MMF may be preferred in maintenance therapy given its superiority to azathioprine in preventing relapse (26). While the duration of maintenance therapy is not agreed upon, EULAR/ERA-EDTA recommends continuation for two years after complete remission (proteinuria <0.5 mg/day, near normal GFR). The National Institutes of Health is currently sponsoring a randomized trial of MMF continuation vs. withdrawal among LN patients who have been stable for two or more years to determine the ideal treatment duration and to identify those who could be tapered off earlier.
For pure class V lupus glomerulonephritis all guidelines endorse the use of RAAS inhibitors to suppress proteinuria. In patients with nephrotic range proteinuria both ACR and EULAR/ERA-EDTA recommend the addition of MMF.
Response to therapy and renal outcomes
Patients should be seen initially every few weeks and monitored closely for therapeutic response and adverse side effects. While a precise definition is not agreed upon, a “complete” renal response usually means significantly reduced proteinuria (<0.33 to 1.0 gm/day), improved or stable GFR (15 to 25% of baseline), and/or normalization of urinary sediment (<5 to 10 RBC/HPF without casts) (27–29). Complete-responders to induction therapy may thereafter be seen less frequently, while partial- and non-responders are seen more often. With current induction regimens, <60% of class III to V patients achieve a complete response (27). Among those who attain a complete response, nearly half relapse at a rate of five to fifteen per 100 patient years (30). The risk of developing LN-related ESRD at five, ten, and fifteen years is 11, 17, and 22% (CI 10–12%, 16–18%, 20–23%) (4), and patients with class IV LN are especially vulnerable: 44% develop ESRD at 15 years (4). The standardized incidence rate of LN-related ESRD in the US is higher among all non-Whites, and 7-fold greater among Blacks compared to Whites (31). Finally, survival rates at ten years are two- and threefold higher in complete-responders than partial- and non-responders (32). Not surprisingly, non-responders have the worst outcomes (33).
Risk factors to poor outcomes
Patient-related factors that contribute to poor outcomes
Poor adherence to treatment regimens and appointments is not uncommon among SLE and LN patients, and substantially negatively impacts outcomes. In a US publicly funded clinic, <25% of SLE patients had adherence rates >80% over two years (34). In a large multi-ethnic US Medicaid cohort from 2000 to 2006, poor adherence was associated with higher rates of systemic relapse, poor renal response, and increased hospitalizations (35). Assessing adherence is difficult in SLE and LN patients, but a recent study offers one possible solution. Serial serum measurements found that HCQ levels between 500 to 2 000 ng/mL were associated with modestly improved SLE disease activity (36). While serum HCQ assays are not routine clinical practice, they may offer a quantitative method to assess adherence and/or adjust dosing to achieve a therapeutic range. In addition, patient education and behavioral support are potentially effective interventions to increase adherence in chronic diseases, although they have not yet been vigorously studied in LN.
Recent work suggests that a genetic basis may partially account for poor renal outcomes. Genome wide association studies (GWAS) over the past decade identified >50 SLE risk loci (37). Although focused on SLE, a sizeable number of GWAS enrollees had LN, allowing for the identification of LN-associated risk loci such as BLK, STAT4, TNFS4, IKZF1, IRF5, TLR9, TNFAIP3, TNIP3, ACE, KLK, FCGR2A, FCGR3A, FCGR3B, ITGAM (37). Most participants were European and Asian, leaving open the questions of whether these or undiscovered risk alleles segregate along ethnicities, and to what extent they contribute to ethnic differences in disease severity and outcomes. For example, APOL1 risk alleles are associated with ESRD and are sixty times more frequent in Blacks than Whites. Among Black SLE patients, this risk genotype more than doubles the risk of developing ESRD and shortens the time to ESRD by two years (38). The population attributable risk for SLE-ESRD due to the APOL1 risk genotype is estimated at 26% in Blacks, and <1% in Whites (38).
Several clinical markers near the time of SLE diagnosis are also associated with renal outcomes. Among them, hypertension, elevated serum creatinine, proteinuria, and persistently elevated anti-dsDNA and low complements following immunosuppression all portend poor outcomes. However, early reduction in proteinuria is associated with renal preservation. In the ten-year follow-up of the MAINTAIN trial (azathioprine vs. MMF as maintenance therapy for proliferative LN), the positive predictive value for preserving renal function was approximately 90% if proteinuria was reduced to <0.5 gm/day at 3, 6, and 12 months (39).
Treatment-related factors that contribute to poor outcomes
LN treatment regimens have substantial side effects from glucocorticoids and prolonged immunosuppression (40). Infections in particular pose a significant risk to immunosuppressed SLE patients, and even more so to LN patients. In a multi-ethnic Medicaid cohort of more than 33 000 SLE and 7 100 LN patients, the incidence rate of serious infections was more than two-fold higher in LN compared to SLE patients (23.4 vs. 10.8 per 100 000 person years) (41). Among those with LN, the risk of serious infection was highest in those treated with glucocorticoids (HR 1.51; 95% CI: 1.43, 1.61) followed by immunosuppressive drugs (HR 1.11; 95% CI: 1.03, 1.20), compared to non-users. To reduce infectious risks, we follow EULAR vaccination guidelines for patients with autoimmune rheumatic diseases (42). Prior to starting immunosuppression, we screen all patients for tuberculosis mycobacterium using a T-cell based blood test, and hepatitis B and C. We avoid live vaccinations in immunosuppressed patients, defined as those taking (i) prednisone >20 mg/day or the equivalent, (ii) azathioprine >3.0 mg/kg/day, or (iii) any dose of cyclophosphamide or MMF (42). Killed vaccinations such as influenza, pneumococcus, hepatitis B, and human papillomavirus are also recommended during stable, quiescent periods. The EULAR guidelines do not recommend the Bacillus Calmette-Geurin (BCG) vaccination, as its efficacy against tuberculosis is not proven in adults (42).
Prolonged use of glucocorticoids is a major challenge in the treatment of LN. There are many glucocorticoid-associated adverse effects, including osteoporotic fractures, avascular necrosis, diabetes mellitus, cataracts, glaucoma and premature mortality (43). Unfortunately, mainly for their rapidity of action, glucocorticoids are still a mainstay therapy for LN induction and relapses. There is a current impetus to quickly taper the maintenance glucocorticoid dose, although the optimal taper rate is unknown. In addition, an ACR expert panel has made several recommendations to prevent bone-related complications (44). All patients starting glucocorticoids should be evaluated for fall risk and counseled on smoking cessation, reducing excessive alcohol, performing modest weight-bearing exercises, and consuming adequate amounts of calcium and vitamin D. Clinical testing should include a 25-hydroxy vitamin D level, and baseline and follow-up bone mineral density scans. The ACR recommends anti-osteoporotic medication for patients who have a prior fragility fracture, are at high risk for fracture, or who will be on prolonged glucocorticoids. The choice of anti-osteoporotic medications is not always straightforward. For example, bisphosphonates are contraindicated in patients with renal insufficiency or ESRD, and there is currently no consensus about use in women of childbearing age given bisphosphonates’ long half-life and potentially adverse effects on a developing fetal skeleton.
A future without oral glucocorticoids?
Although glucocorticoid-sparing immunosuppressive agents (i.e. azathioprine, MMF, cyclosporine, and cyclophosphamide) have allowed for lower glucocorticoid doses and less toxicity, many LN patients still require prolonged low-doses of glucocorticoid for remission maintenance. As a result, glucocorticoid-free maintenance therapy for LN is currently under investigation (45). Rituximab, a monoclonal antibody targeting the B-cell surface protein CD20, was initially examined in the “Lupus Nephritis Assessment with Rituximab” (LUNAR) trial, which included 144 patients with class III or IV LN, In that trial, rituximab did not statistically affect the outcomes in patients who also received MMF and glucocorticoid.
However, in a more recent prospective observational study, fifty LN patients, 44% with pure class V, were treated without any oral glucocorticoids (46). Subjects received only two pulses of methylprednisolone, and two one-gram doses of rituximab separated by two weeks, plus daily MMF. At one year, 52% of patients achieved complete renal remission (urine protein:creatinine <50mg/mmol, Cr <15% above baseline) with low rates of adverse events. Although small and unblinded, this study laid the foundation for a larger ongoing trial, “Trial of Rituximab and MMF without Oral Steroids for Lupus Nephritis” (RITUXILUP). In this randomized non-inferiority multi-center trial, both arms of LN patients are receiving two pulses of methylprednisolone and daily MMF. In addition, the experimental arm receives two one-gram doses of rituximab, and the control group receives daily weight-based oral glucocorticoids. Comparing renal response rates and glucocorticoid toxicities between the two arms over time will address the question of whether combining rituximab and MMF can replace oral glucocorticoids for maintenance therapy.
Conclusion
LN affects approximately 40% of SLE patients over their lifetime and disproportionately burdens non-White women from lower socioeconomic groups (1–3). The ACR and EULAR/ERA-EDTA recommend close monitoring for new onset or recurrent LN in SLE patients by routinely screening for kidney involvement at least every three to six months, and obtaining a kidney biopsy for persistent evidence of LN barring any contraindication (17). They also recommend high doses of glucocorticoid plus cyclophosphamide or MMF to induce a complete renal response within six months. After remission, maintenance therapy with either MMF or azathioprine should be continued for some time, although the duration of therapy is not agreed upon. Of these medications, MMF may be preferred due fewer associated toxicities compared to cyclophosphamide and superiority over azathioprine in preventing relapse (24, 26). All LN patients should receive additional medications that include HCQ, a RAAS inhibitor for proteinuria, a statin for goal LDL <100 mg/dL, and anti-hypertensives to maintain blood pressure <130/80 mmHg. Women planning or anticipating pregnancy should be counseled regarding potential complications related to LN and medications, as many are toxic to the developing fetus. Finally, glucocorticoid-induced osteoporosis and other complications are ongoing challenges (44).
Less than 60% of class III to V LN patients achieve a complete response with current induction regimens (27), and the risk of LN-related ESRD at five, ten, and fifteen years remains at 11, 17, and 22% (CI 10–12%, 16–18%, 20–23%) for the last decade (4). The lack of progress in LN outcomes is not due to lack of effort. In fact, alongside government funding, private agencies have emerged as important drivers of LN research through their own investigations and funding mechanisms.
Improved outcomes in LN could come about in several ways. First, increasing access to specialty care and adherence rates in resource-poor areas would help close the socioeconomic and ethnicity gap in LN. This is no easy feat, but studying behavioral interventions while longitudinally monitoring serum HCQ for adherence might be a starting point. Furthermore, disease-specific pathways must be identified and targeted in order to reduce and/or eliminate long-term glucocorticoids and their toxicities. LN is a severe complication of SLE that too often leads to ESRD, especially in non-Whites and patients from resource-poor areas. Although outcomes plateaued over the last decade (4), new interventions have the potential to positively impact LN patients.
Acknowledgments
Grant support: KHC: NIH R01 AR057327 and K24 066109, PJH: T32AR007530-31
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ward MM. Prevalence of physician-diagnosed systemic lupus erythematosus in the United States: results from the third national health and nutrition examination survey. J Womens Health (Larchmt) 2004;13(6):713–8. doi: 10.1089/jwh.2004.13.713. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tektonidou MG, Dasgupta A, Ward MM. Risk of End-stage Renal Disease in Patients with Lupus Nephritis, 1970 to 2015 A systematic review and Bayesian meta-analysis. Arthritis Rheumatol. 2016 doi: 10.1002/art.39594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66(2):357–68. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014;66(2):369–78. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraki LT, Feldman CH, Liu J, Alarcon GS, Fischer MA, Winkelmayer WC, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum. 2012;64(8):2669–76. doi: 10.1002/art.34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker LB, Uribe AG, Fernandez M, Vila LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII) Lupus. 2008;17(4):314–22. doi: 10.1177/0961203307087875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37(6):1158–63. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Rheumatology Committee on Rheumatology T Workforce I. FitzGerald JD, Battistone M, Brown CR, Jr, Cannella AC, et al. Regional distribution of adult rheumatologists. Arthritis Rheum. 2013;65(12):3017–25. doi: 10.1002/art.38167. [DOI] [PubMed] [Google Scholar]
- 12.Oyoo GO, Mody GM. Report on the Fifth African League Against Rheumatism Congress in Nairobi, Kenya. Clin Rheumatol. 2007;26(7):1033–5. doi: 10.1007/s10067-007-0620-3. [DOI] [PubMed] [Google Scholar]
- 13.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken) 2014;66(4):617–24. doi: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balow JE. Clinical presentation and monitoring of lupus nephritis. Lupus. 2005;14(1):25–30. doi: 10.1191/0961203305lu2055oa. [DOI] [PubMed] [Google Scholar]
- 15.Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2015 doi: 10.1093/rheumatology/kev311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 1999;42(9):1785–96. doi: 10.1002/1529-0131(199909)42:9<1785::AID-ANR1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64(6):797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markowitz GS, D’Agati VD. The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years. Kidney Int. 2007;71(6):491–5. doi: 10.1038/sj.ki.5002118. [DOI] [PubMed] [Google Scholar]
- 19.Hiramatsu N, Kuroiwa T, Ikeuchi H, Maeshima A, Kaneko Y, Hiromura K, et al. Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology (Oxford) 2008;47(5):702–7. doi: 10.1093/rheumatology/ken019. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 2011;63(6):865–74. doi: 10.1002/acr.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelmus S, Bajema IM, Bertsias GK, Boumpas DT, Gordon C, Lightstone L, et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv102. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum. 2013;43(1):77–95. doi: 10.1016/j.semarthrit.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7(7):399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 24.Muangchan C, van Vollenhoven RF, Bernatsky SR, Smith CD, Hudson M, Inanc M, et al. Treatment algorithms in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015 doi: 10.1002/acr.22589. [DOI] [PubMed] [Google Scholar]
- 25.Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, et al. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. J Womens Health (Larchmt) 2009;18(3):311–9. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med. 2011;365(20):1886–95. doi: 10.1056/NEJMoa1014460. [DOI] [PubMed] [Google Scholar]
- 27.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Group AT. Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 2014;66(11):3096–104. doi: 10.1002/art.38790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–26. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 30.Grootscholten C, Berden JH. Discontinuation of immunosuppression in proliferative lupus nephritis: is it possible? Nephrol Dial Transplant. 2006;21(6):1465–9. doi: 10.1093/ndt/gfl208. [DOI] [PubMed] [Google Scholar]
- 31.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63(6):1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J, Rohde RD. Factors predictive of outcome in severe lupus nephritis. Lupus Nephritis Collaborative Study Group. Am J Kidney Dis. 2000;35(5):904–14. doi: 10.1016/s0272-6386(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ Collaborative Study G. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3(1):46–53. doi: 10.2215/CJN.03280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marengo MF, Waimann CA, de Achaval S, Zhang H, Garcia-Gonzalez A, Richardson MN, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus. 2012;21(11):1158–65. doi: 10.1177/0961203312447868. [DOI] [PubMed] [Google Scholar]
- 35.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication Nonadherence Is Associated With Increased Subsequent Acute Care Utilization Among Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2015;67(12):1712–21. doi: 10.1002/acr.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine Blood Levels in Systemic Lupus Erythematosus: Clarifying Dosing Controversies and Improving Adherence. J Rheumatol. 2015;42(11):2092–7. doi: 10.3899/jrheum.150379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol. 2015;11(6):329–41. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
- 38.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014;66(2):390–6. doi: 10.1002/art.38220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houssiau FA, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–9. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz N, Goilav B, Putterman C. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr Opin Rheumatol. 2014;26(5):502–9. doi: 10.1097/BOR.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67(6):1577–85. doi: 10.1002/art.39070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70(3):414–22. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012;51(7):1145–53. doi: 10.1093/rheumatology/ker410. [DOI] [PubMed] [Google Scholar]
- 44.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62(11):1515–26. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 45.Lightstone L. Minimising steroids in lupus nephritis--will B cell depletion pave the way? Lupus. 2013;22(4):390–9. doi: 10.1177/0961203313476155. [DOI] [PubMed] [Google Scholar]
- 46.Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280–6. doi: 10.1136/annrheumdis-2012-202844. [DOI] [PubMed] [Google Scholar]