SUMMARY
Pupil size is collectively controlled by the sympathetic dilator and parasympathetic sphincter muscles. Locus coeruleus (LC) activation has been shown to evoke pupil dilation, yet how the sympathetic and parasympathetic pathways contribute to this dilation remains unknown. We examined pupil dilation elicited by LC activation in lightly anesthetized rats. Unilateral LC activation evoked bilateral, yet lateralized pupil dilation, i.e, the ipsilateral dilation was significantly larger than the contralateral dilation. Surgically blocking the ipsilateral, but not contralateral, sympathetic pathway significantly reduced lateralization, suggesting lateralization is mainly due to sympathetic contribution. Moreover, we found that sympathetic, but not parasympathetic, contribution is correlated with LC activation frequency. Together, our results unveiled the frequency-dependent contributions of the sympathetic and parasympathetic pathways to LC-activation-evoked pupil dilation, and suggest that lateralization in task-evoked pupil dilations may be used as a biomarker for autonomic tone.
eTOC BLURB
Liu et al. show that unilateral LC activation evokes bilateral yet lateralized pupil dilation. This lateralization is dependent upon the frequency of LC activation, which results from sympathetic but not parasympathetic contributions. This suggests a non-invasive technique for indexing autonomic imbalances in disorders involving the autonomic nervous system.
INTRODUCTION
Mounting experimental data from humans, non-human primates, and rodents show that nonluminance-induced changes in pupil size are tightly correlated with arousal and various cognitive factors (Ebitz et al., 2014; Eldar et al., 2013; Hong et al., 2014; McCormick et al., 2015; McGinley et al., 2015a; Nassar et al., 2012; Reimer et al., 2014; Vinck et al., 2015). Recent work demonstrated a consistent difference in pupil dilation across two eyes in an attentional task, and suggested that the level of this lateralization was collectively modulated by the attentional load and arousal (Wahn et al., 2017). Activity of the locus coeruleus (LC) has also been related to arousal and cognitive processing (Clayton et al., 2004; Nassar et al., 2012; Sara and Bouret, 2012; Sara et al., 1994), leading many to hypothesize that the LC mediates the dilations seen during cognitive processing (Aston-Jones and Cohen, 2005). The LC is also the primary source of norepinephrine (NE) to the forebrain (Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003; Sara, 2009; Szabadi, 2013). NE release results in a spectrum of modulatory effects on neural representation and computations, through its action on adrenergic receptors (Ego-Stengel et al., 2002; Hirata et al., 2006; Martins and Froemke, 2015; McCormick and Pape, 1990; McGinley et al., 2015b; Moxon et al., 2007; Wekselblatt and Niell, 2015). The LC fires in two modes: tonic, i.e. long-term, continuous spiking at a rate of 1–5 Hz, and phasic which manifests as sparsely-occurring, transient, bursting events (Aston-Jones and Cohen, 2005). These modes are thought to have important behavioral relevance (Aston-Jones et al., 1994; Aston-Jones et al., 1996; Bouret and Richmond, 2015; Clayton et al., 2004; Kalwani et al., 2014; Rajkowski et al., 2004; Usher et al., 1999) and are hypothesized to separately affect pupil size, with tonic and phasic activation influencing baseline pupil size and transient pupil dilations, respectively, both of which were shown to reflect different cognitive factors (de Gee et al., 2014; Nassar et al., 2012).
Pupil size is determined by a balancing act between two smooth muscles: the sphincter and dilator pupillae muscles of the iris (Andreassi, 2006). The dilator muscle is innervated by sympathetic neurons in the superior cervical ganglion (SCG) which is in turn innervated by the intermediolateral cell column (IML) of the spinal cord. The sphincter muscle is innervated by parasympathetic neurons in the ciliary ganglion (CG), which is in turn innervated by the Edinger-Westphal nucleus (EWN). Thus, either excitation of the sympathetic SCG neurons or inhibition of the parasympathetic EWN neurons results in pupil dilation. Recent work established a causal link between LC activation and pupil size by showing that a brief phasic electrical microsimulation of the LC elicited transient dilation, when measured unilaterally, in monkeys and rodents (Joshi et al., 2016; Reimer et al., 2016). However, little is known about the dynamics of bilateral pupil dilation in response to different modes of LC activation. In addition, the functional contributions of the sympathetic and parasympathetic systems to LC-activation-evoked dilations remain unclear.
Here we investigated the pupil dilations elicited by tonic and phasic activation of the LC in lightly-isoflurane-anesthetized rats, and present several findings that substantially elucidate the neural circuitry mediating the relationship between LC activation and pupil size. We show that unilateral LC activation evoked dilations for both pupils. Furthermore, the dilations were lateralized, i.e. ipsilateral dilation was significantly larger than contralateral dilation, and tonic LC stimulation resulted in more lateralization than phasic LC stimulation, indicating that the difference between the dilations of the two pupils depends upon the frequency of LC activation. Next, by pharmacologically and surgically blocking the EWN and SCG we demonstrated that LC control of pupil size must only involve the parasympathetic EWN and sympathetic SCG, respectively. Finally, through removal of either the ipsilateral or contralateral SCG, i.e. superior cervical ganglionectomy (SCGx), we found that the LC influenced the ipsilateral pupil through both parasympathetic and sympathetic pathways, but influenced the contralateral pupil only through the parasympathetic pathway. Therefore, differences between bilateral pupil dilations were attributed to sympathetic contribution, suggesting these differences as a possible index for autonomic tone.
RESULTS
Pupil dilation in response to phasic LC activation and pupillary light reflex does not significantly differ between lightly-isoflurane-anesthetized rats and awake rats
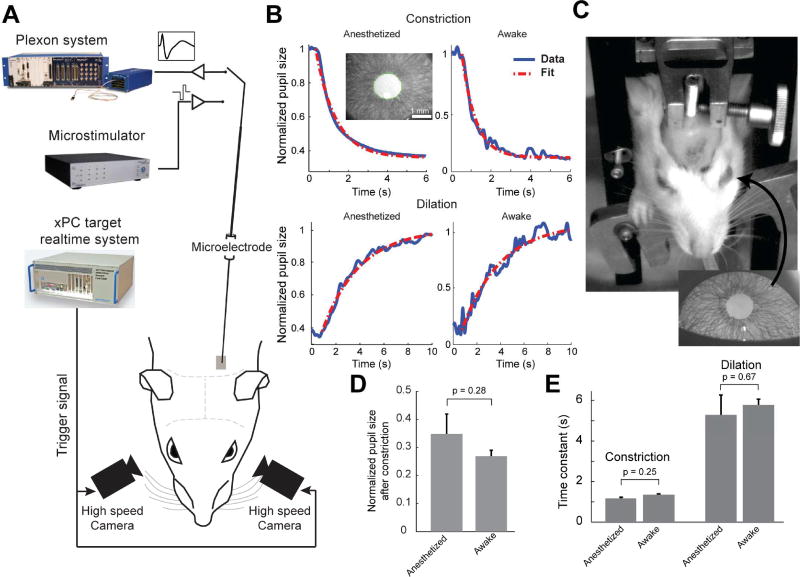
All pupillometry data showing responses to tonic or phasic LC stimulation were recorded from rats lightly anesthetized with isoflurane (approximately 1% during recording) (Figure 1A). Since pupil dilation and constriction are mediated by the dilator and sphincter muscles, respectively (Lowenstein and Loewenfeld, 1962), we first tested whether isoflurane anesthesia significantly altered the response properties of these muscles by comparing the pupillary light reflex properties of the lightly-isoflurane-anesthetized rats (Figure 1B, n=4) with those of awake rats (Figure 1C, n=3). For both anesthetized and awake animals, switching ambient luminance from 15 to 150 lux resulted in a rapid pupil constriction (with pupil constricting to 35.5±5.8% of the baseline for the anesthetized animals, and 26.9±1.5% for the awake animals, p=0.28, Mann-Whitney U-test, Mean ± SEM, Figure 1D), with the pupil gradually relaxing back to the baseline once the ambient luminance was switched back to 15 lux (Figure 1B). Changes in pupil size were fit with exponential decay or growth curves, respectively (Figure 1B). Time constants of the curves fit to pupil contraction and dilation were not significantly different between anesthetized and awake rats (Figure 1E; constriction: 1.135±0.094 s vs. 1.32±0.098 s; p = 0.248, Mann-Whitney U-test; dilation: 5.25±1.01 s vs 5.74±0.41 s; p = 0.668, Mann-Whitney U-test. Mean ± SEM). To further test whether LC mediated pupil dilation is affected by anesthesia, we measured pupil dilation evoked by 50 Hz phasic LC stimulation in three awake, head-constrained animals (Figure S1A). In our awake animals, although the ambient illuminance was constant during each session and there was no behavioral task involved, pupil size tended to fluctuate both across and within trials (Joshi et al., 2016). LC stimulation with an amplitude of 60 or 100 µA failed to elicit a distinguishable pupil dilation from background fluctuation for the animals, presumably due to scar tissue formation around the implanted electrodes (Ersen et al., 2015). Thus we used an amplitude of 150 µA for these awake animals (see Methods). LC phasic stimulation with this amplitude evoked dilation for both pupils (37.3±6.1% for the ipsilateral pupil and 31.6±5.5% for contralateral pupil, Mean ± SEM, Figure S1B). The time course of the dilations was not significantly different between the anesthetized and awake animals (ipsilateral rising stage: 1.38+0.16 s vs. 1.47±0.10 s, p = 0.76; contralateral rising stage: 1.38±0.18 s vs. 1.22±0.23 s, p = 0.57; ipsilateral decaying stage: 4.66±0.89 s vs. 4.48±2.17 s, p = 0.93; contralateral decaying stage: 4.84±0.61 vs. 4.06±1.1 s, p = 0.52; Mann-Whitney U-test, Figure S1C). Importantly, the lateralization ratio was similar between the anesthetized and awake animals (1.25+0.038 vs. 1.21+0.085, p = 0.62, Mann-Whitney U-test, Figure S1D). Therefore, these results suggest that light isoflurane anesthesia does not significantly disrupt LC mediated changes in pupil size, and that the pupil size changes in response to LC stimulation presented in this study approximate the dynamic changes seen in awake animals.
Figure 1. Pupillometry and pupillary light reflex in both lightly-isoflurane-anesthetized and awake rats.
A) Experimental setup to measure bilateral pupil response to LC activation. Both pupils in lightly anesthetized rats were imaged during electrical microstimulation of the LC. Single-unit extracellular recordings of the LC were obtained prior to stimulation to ensure correct microelectrode placement. B) Example pupillary light reflex (PLR) in anesthetized and awake animals. Top, pupil constriction following ambient lighting being switched from 15 to 150 lux. Bottom, pupil dilation following ambient lighting being switched from 150 to 15 lux. Inset: example pupil image with green circle depicting automatically-segmented pupil contour. C) An awake, head-constrained rat during PLR measurement and a close up view of the easily segmented, reflective pupil. D) Normalized constriction amplitude of PLR for anesthetized and awake animals. E) Time constants of PLR in lightly anesthetized rats are not significantly different from those in awake rats. Error bars: SEM.
Electrophysiology: identification of LC neurons
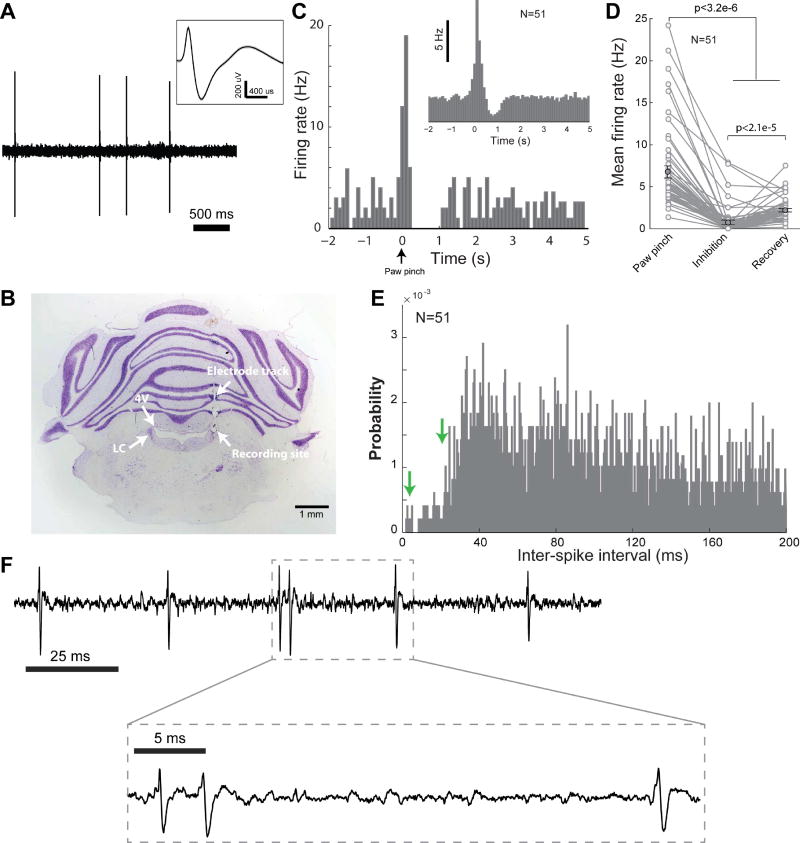
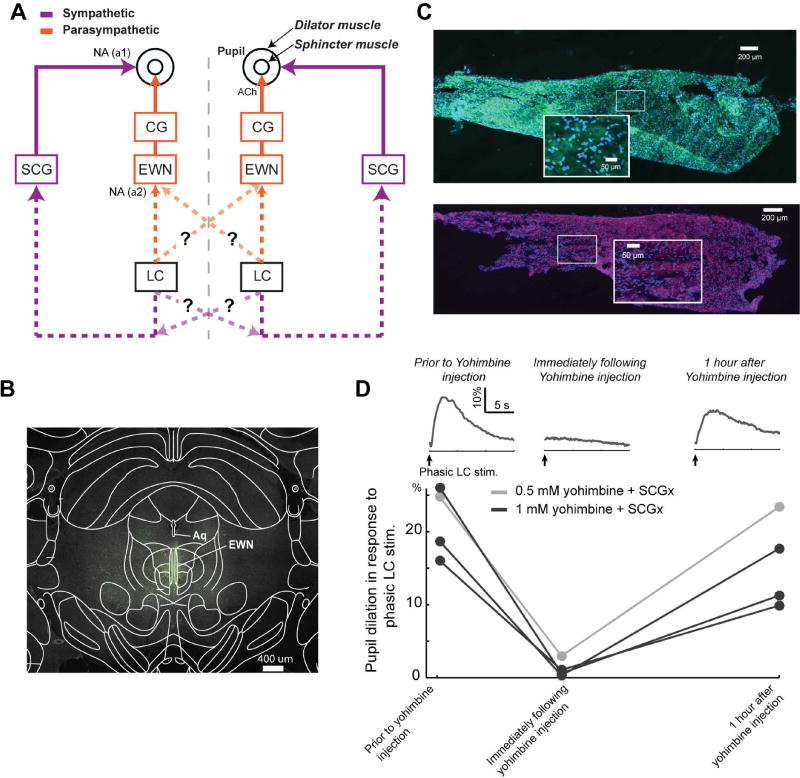
Single-unit recordings of LC cells were obtained using sharp tungsten microelectrodes with an impedance of approximately 2 MΩ (Figure 2A). LC cells were reliably found 5.5–6.2 mm below the brain surface. All recordings contained either a single large-amplitude unit, or two units that could be isolated without ambiguity using PCA sorting techniques. Expected anatomical location and electrophysiological characteristics of LC neurons, including a low, spontaneous firing rate, wide waveform (>1.8 ms), and phasic response to paw pinch, were used to confirm microelectrode placement within the LC (Aston-Jones et al., 1991; Vazey and Aston-Jones, 2014). For all experiments, electrode placement in the LC was further confirmed with post-experiment histological analysis (Figure 2B, see Methods). In response to a paw/tail pinch, LC cells typically fire a brief burst of 3–6 spikes with short interspike intervals (ISI), which is usually followed by sustained suppression (>500 ms) of firing activity (Aston-Jones and Bloom, 1981; Clayton et al., 2004; Devilbiss and Waterhouse, 2011; Kalwani et al., 2014). The mean firing rate during the suppression period (500–1000 ms after the paw/tail pinch) was significantly lower than both the baseline firing rate and mean firing rate within the 500 ms immediately following the paw/tail pinch (Figures 2C and 2D, p < 0.001, paired Wilcoxon signed-rank test). A paw pinch also evoked pupil dilation. Although the paw-pinch-evoked-pupil-dilation may engage other brain structures besides the LC (Chapman et al., 1999), pupil dilation in response to the paw pinch exhibit a lateralization with a ratio of 1.68±0.23 (n=2, Figures S2A & S2B), suggesting that lateralized pupil dilation is a general phenomenon. We also found no significant correlation between the changes in pupil size and LC activity in response to paw pinch (n=2, p > 0.25). However, this lack of significance could be due to the involvement of other circuitry (Chapman et al., 1999) or the small sample size. Future work is necessary to further examine this.
Figure 2. Electrophysiology of LC neurons.
A) Example single-unit recording of spontaneous LC activity. Inset shows typical wide waveform of LC neurons. B) A Nissl-stained brain slice confirming correct placement of microelectrode in the LC. C) A single-unit example of the characteristic phasic firing of LC neurons in response to a paw pinch (bin size 100 ms). Time zero indicates the time of the paw pinch. Inset shows the population PSTH before and after the tail/paw pinch for 51 different LC neurons. D) Mean firing rate of the same 51 LC neurons during the 500 ms following the tail/paw pinch, the following suppression period, and finally the recovery period. E) The distribution of inter-spike-intervals for all phasic LC responses to the tail/paw pinch, binned at 0.5 ms. The arrows on the plot are at the inter-spike-intervals which our phasic microstimulation patterns were designed to mimic (left arrow, 3 ms i.e. 333 Hz; right arrow, 20 ms i.e. 50 Hz). Bin width: 0.5 ms. F) Example phasic LC activity containing spikes with an ISI of approximately 3 ms. Error bars: SEM.
The mean firing rate, during the period of 500 ms following the paw/tail pinch, when phasic firing is present, was approximately 7 Hz. However, the ISIs of single-unit activity during this time period were widely distributed, ranging from several milliseconds to a couple hundreds of milliseconds. The peak of the ISI distribution was at approximately 40 ms; yet, a small number of spikes had ISIs shorter than 20 ms (Figures 2E and S2C). We also observed single unit spikes with ISIs as short as ~3 ms during phasic responses (Figure 2F). Although, the evoked firing of neurons next to a stimulating electrode is dependent upon many factors including electrode properties and relative position (Ranck, 1975), when attempting to phasically activate the LC through microstimulation, we used stimulation patterns with an inter-stimulus-interval of both 20 (n=10) and 3 ms (n=32) which we found allowed us to examine the extent to which the frequency of LC activation affects the contribution of the sympathetic and parasympathetic pathway.
LC stimulation desynchronizes cortical EEG
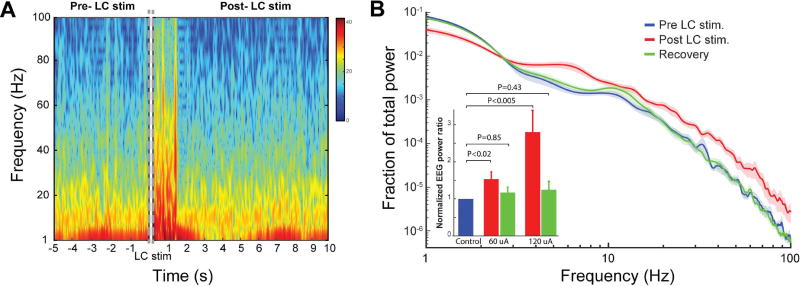
We first examined if activation of the LC-NE neurons using electrical microstimulation would alter cortical arousal by shifting the power spectrum of cortical EEG, as had been previously shown using pharmacological compounds (Berridge and Foote, 1991; Steriade et al., 1993; Vazey and Aston-Jones, 2014) and photostimulation (Li et al., 2016). Consistent with previous work, brief electrical microstimulation (50 Hz, 6 biphasic current pulses, 200 µs per phase, 60 or 120 µA amplitude) of the LC induced the EEG power spectrum to shift towards higher frequencies (Figure 3A), quantifiable by an increase in the ratio of 10–100 Hz to 1–10 Hz power (p < 0.02, Wilcoxon signed-rank test, Figure 3B). 60 µA LC stimulation caused less desynchronization than 120 µA LC stimulation (Figure 3B), suggesting that the observed desynchronization of cortical EEG was due to the activation of the LC.
Figure 3. Shift in EEG power following phasic LC stimulation.
A) Example spectrogram of cortical EEG before and after phasic LC stimulation. Dashed, gray line indicates the time of phasic LC stimulation. B) Average fraction of EEG total power vs frequency around LC stimulation. LC stimulations with amplitude of 60 uA or 120 uA both significantly increased the ratio of EEG power in high frequencies (10–100 Hz) to low frequencies (1–10 Hz). Error bars: SEM.
Tonic and phasic LC stimulation elicits bilateral pupil dilation with different temporal characteristics
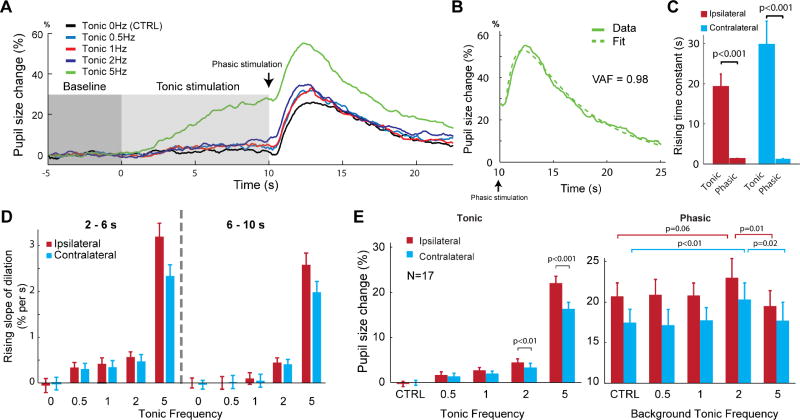
Phasic LC stimulation (6 biphasic pulses, 200 µs per phase, 60 µA, 3 ms or 20 ms inter-pulse-interval, see Methods) was delivered following tonic LC stimulation (biphasic pulses with either 0, 0.5, 1, 2 or 5 Hz for 10 s, 200 µs per phase, 60 µA) meant to mimic the natural tonic firing patterns of the LC. Pupil dilation was measured as the change in pupil size following LC stimulation divided by the pupil size at resting state. Both tonic and phasic unilateral LC microstimulation (n=15 for left LC, n=2 for right LC) elicited pupil dilation for both the ipsilateral and contralateral eyes. The temporal characteristics of dilation in response to phasic LC stimulation were distinct from those in response to tonic stimulation. Tonic LC stimulation generally induced an extended, gradual dilation whereas phasic LC stimulation induced a rapid, steep increase in pupil size followed by a quick constriction back to the baseline size (Figures 4A and 4B). The lags of the pupil dilation evoked by phasic LC stimulation are 0.83±0.038 and 0.91±0.056 s for ipsilateral and contralateral pupils, respectively, which are comparable to the latency between pupil microdilations and the associated depolarization of transmembrane potentials as reported in previous work (McGinley et al., 2015a) and the latency between pupil dilation and activation of cortical NE axons (Reimer et al., 2016).
Figure 4. Bilateral pupil dilation in response to tonic and phasic unilateral LC stimulation.
A) Example change in pupil size (normalized to baseline pupil size) in response to LC activation, with tonic LC stimulation occurring from 0 to 10 s and phasic stimulation, indicated by the arrow, occurring immediately following tonic stimulation. Each color represents a different tonic stimulation condition. B) Pupil dilation following phasic LC stimulation can be well fit with a bi-exponential function. C) Average time constant for the rising phase of either ipsilateral or contralateral pupil dilation following either tonic or phasic LC stimulation. D) Average rising slope of dilation during each tonic stimulation condition for the ipsilateral and contralateral pupil for the 2–6 s and 6–10 s period of the tonic stimulation period. E) Left: Percent of pupil size change during tonic LC stimulation for each tonic stimulation condition in the ipsilateral and contralateral pupils. Both 2 and 5 Hz tonic activation showed a significant across-eye difference. Right: Percent of pupil size change following phasic LC stimulation with different background LC activation for the ipsilateral and contralateral pupils. Error bars: SEM.
Further, to quantify the time course of the pupil dilations, we calculated time constants for phasic pupil dilations and 5 Hz tonic pupil dilations by fitting an exponential growth curve (see Methods). Although pupil dilations elicited by 50 Hz phasic LC stimulation were smaller than those by 333 Hz phasic LC stimulation (Ipsilateral: 15±1% vs. 22±0.9%, p < 4.8×10−5; contralateral: 12±0.7% vs. 20±0.9%, p < 3.2×10−4, Student’s t-test, Figures S3A and S3B), their time courses were not significantly different (Ipsilateral rising: 1.38±0.16 vs. 1.31±0.06 s, p = 0.68; contralateral rising: 1.38±0.18 vs. 1.26±0.07, p = 0.48; ipsilateral decaying: 4.66±0.62 vs. 5.0±0.27, p=0.64; contralateral decaying: 4.84 ±0.89 vs. 5.12±0.33, p=0.71; Student’s t-test, Figure S3C). Pupil dilation in response to 5 Hz tonic LC stimulation increased exponentially with time constants of 19.25±3.16 and 29.76±5.80 s for the ipsilateral and contralateral eyes, respectively. In contrast, pupil dilation in response to phasic LC stimulation progressed relatively rapidly. The rising time constant of pupil dilation in response to 5 Hz tonic stimulation was approximately 15-fold larger than that of phasic dilation for both the ipsilateral (19.25±3.16 vs. 1.32±0.058 s, p < 2×10−21, Student’s t-test) and contralateral pupil (29.76±5.80 vs. 1.28±0.068 s, p < 5×10−16, Student’s t-test, Figure 4C). The average time constants of the decaying phases of phasic pupil dilation are 4.95±0.26 and 5.07±0.29 s for the ipsilateral and contralateral eyes, respectively. Since pupil dilations in response to LC tonic stimulation with frequencies of 0.5, 1, and 2 Hz didn’t typically exhibit an exponential growth curve, we calculated the rising linear slope during 2–6 and 6–10 s periods for all tonic stimulation conditions (Figure 4D). In general, the rising slope and size of the evoked dilation was positively correlated with the frequency of tonic stimulation, i.e. higher tonic frequency stimulations generally produce quicker and larger pupil dilations (Figures 4D and 4E).
We next examined what effect background tonic LC activity might have on pupil dilations elicited by phasic LC activity. The percent change of pupil dilation evoked by phasic LC stimulation for both ipsilateral and contralateral eyes exhibited an inverted-U profile, relative to the increasing frequencies of background tonic LC stimulation. For both the ipsilateral and contralateral pupils, the increase in pupil size after phasic LC stimulation was significantly greater when preceded by 2 Hz tonic LC stimulation than by 5 Hz (p = 0.01, Student’s t-test, for ipsilateral pupil, p < 0.03, Student’s t-test, for contralateral pupil, n=17). Moreover, contralateral pupil dilations evoked by phasic LC stimulation were significantly larger in the presence of 2 Hz tonic stimulation than when following no background tonic stimulation (p < 0.01, Student’s t-test). However, this facilitative effect of tonic stimulation on phasic stimulation-induced dilation was only marginally present (p = 0.06 Student’s t-test) for the ipsilateral eye (Figure 4E). Finally, we compared the results from both left (n=15) and right LC stimulation (n=2) and found they were similar (Figure S4), ruling out the possibility that unilateral-LC-stimulation-induced-lateralized-pupil-dilation was dependent upon which LC was stimulated.
LC controls pupil size only through the parasympathetic EWN and sympathetic SCG
Although previous tracing works have suggested that the LC projects to the EWN, a parasympathetic nucleus controlling the pupillary sphincter muscle via the CG (Breen et al., 1983), and the SCG, a sympathetic nucleus controlling the pupillary dilator muscle (Hancock and Fougerousse, 1976), these works didn’t rule out the possibility that the LC affects pupil size through additional pathways (Figure 5A). To test whether the LC modulates pupil size through nuclei other than the EWN and SCG, we pharmacologically blocked the influence of the LC on the EWN by injecting ~1 uL yohimbine, an alpha-2 receptor antagonist, into the ipsilateral EWN. Florescent dye mixed with the yohimbine solution confirmed that the spread of yohimbine solution was limited to ~250 um from the injection site (Figure 5B). At the same time, we performed a SCGx, i.e. surgical removal of the SCG. Immunohistochemistry confirmed the identity of the removed tissue by showing its cells expressed both neurofilaments and tyrosine hydroxylase, hallmarks of sympathetic neurons (Savastano et al., 2010) (Figure 5C). In concert with SCGx, the pharmacological blockage of alpha-2 receptors in the EWN with 1 mM yohimbine eliminated the pupil dilation in response to phasic LC activation (Figure 5D, dark traces), while 0.5 mM yohimbine only partially diminished pupil dilation in response to LC activation (Figure 5D, grey trace). Pupillary responses to LC stimulation started to reappear one hour after the injection. Taken together, the dose-dependent reduction and re-appearance of LC-activation-evoked pupillary responses following a recovery period suggest that the observed inactivation was due to the injection of yohimbine. Thus, this confirmed that the EWN and SCG were essential to the pupillary response to LC activation.
Figure 5. The sympathetic SCG and parasympathetic EWN nuclei are essential for the LC to modulate pupil size.
A) Although literature suggests that the LC modulates ipsilateral pupil size through both a sympathetic (LC→IML→SCG→dilator muscle) and parasympathetic (LC→EWN→CG→sphincter muscle) pathway, the pathway through which the LC modulates contralateral pupil size remains unclear (question marks indicate other possible pathways). B) Histological confirmation that the yohimbine and dye injection spread throughout the EWN (overlaid brain atlas aligned by aqueduct location). C) Immunohistochemical identification of an SCG that was surgically removed through SCGx. Neurofilaments and tyrosine hydroxylase are labeled green and purple, respectively, and blue DAPI labels cell nuclei. The area within the white box is shown at 40× magnification to show labeling at a cellular scale. D) The effects of blocking alpha-2 receptors in the EWN with 0.5 and 1 mM yohimbine on phasic-LC-stimulation-induced pupil dilations.
Lateralization of pupil dilation in response to LC stimulation is due to a unilateral sympathetic pathway
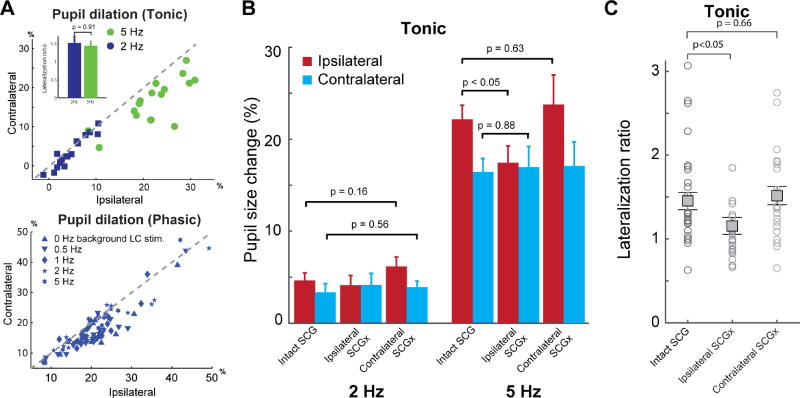
Although unilateral LC stimulation induced bilateral pupil dilation, normalized dilation of the ipsilateral pupil was significantly larger than that of the contralateral pupil in response to both 2 and 5 Hz tonic, and phasic LC stimulation (tonic 5 Hz: 0.22±0.015 vs. 0.16±0.014, p < 0.001, paired Student’s t-test; tonic 2 Hz: 0.05±0.008 vs. 0.034±0.009, p = 0.002, paired Student’s t-test; phasic: 0.211±0.008 vs. 0.181±0.008, p < 3×10−13, paired Student’s t-test. Figures 4E and 6A). This suggests that LC activity may affect the ipsilateral and contralateral pupils differently through the sympathetic and parasympathetic pathways, producing this pronounced lateralization.
Figure 6. Ipsilateral or contralateral SCGx allowed us to tease apart the individual contributions of the sympathetic and parasympathetic pathways.
A) Top: Percent of pupil size change during 2 and 5 Hz tonic LC stimulation in the ipsilateral versus contralateral pupil. Inset shows the mean lateralization ratio of pupil dilation in response to 2 and 5 Hz tonic LC stimulation. The dashed line is the unity line. Bottom: Percent of pupil size change during phasic LC stimulation following various tonic LC stimulation conditions in the ipsilateral versus contralateral pupil. B) Contralateral SCGx had no effect on bilateral pupil dilation in response to 2 and 5 Hz tonic LC stimulation, whereas ipsilateral SCGx resulted in significantly-diminished 5 Hz-evoked ipsilateral, but not contralateral dilation. C) Ipsilateral SCGx resulted in a decrease in the mean lateralization ratio for 2 and 5 Hz LC stimulation whereas contralateral SCGx did not affect the lateralization ratio. Error bars: SEM.
The difference between the dilation of the ipsilateral and contralateral pupils was quantified as a lateralization ratio, defined as the normalized change in pupil size for the ipsilateral pupil divided by that for the contralateral pupil. We only analyzed the lateralization ratios for 2 and 5 Hz tonic stimulation, as the marginal pupil dilations induced by stimulations at <2 Hz resulted in unreliable estimation of the lateralization ratios.
For almost all animals, tonic LC stimulation induced a significantly larger ipsilateral than contralateral pupil dilation, resulting in a lateralization ratio of 1.45±0.1 (p < 2×10−4, Student’s t-test, mean ± SEM). When percent change in pupil size was plotted for the ipsilateral eye versus the contralateral eye, the vast majority of the data points fell below the unity line (Figure 6A). Interestingly, the lateralization ratios for 2 Hz was slightly but not significantly larger than 5 Hz (p = 0.91, Student’s t-test test), although pupil dilation was larger for 5 Hz than 2 Hz (Figure 6A, inset).
We next examined the extent to which the sympathetic and parasympathetic pathways contribute to the pupil dilation evoked by LC activation. We found that unilateral LC stimulation evoked bilateral pupil dilation with lateralization. Yet, it is unclear whether this lateralization was due to bilateral LC projections to either the sympathetic or parasympathetic pathways, or through a combination of both. Moreover, as pupil size has been shown to tightly co-vary with level of arousal (McGinley et al., 2015a; Nassar et al., 2012; Reimer et al., 2014; Vinck et al., 2015), which involves both the sympathetic and parasympathetic nervous systems, we wanted to further tease apart the contribution of these systems to pupil dilation in response to LC activation. To this end, we performed a SCGx to remove either the ipsilateral or contralateral SCG, a part of the sympathetic pathway from the LC to the dilator pupillae muscle. To our surprise, contralateral SCGx did not induce any significant change in pupil dilations in response to tonic LC stimulation for either the ipsilateral or contralateral pupils (p > 0.16 for the ipsilateral pupil and p > 0.56 for the contralateral pupil, Mann-Whitney U-test, Figure 6B). Nor did it induce any significant change in the lateralization ratio, when compared to data collected with an intact SCG (1.45±0.1 vs. 1.51±0.11, p = 0.66, Mann-Whitney U-test, Figure 6C). This suggests LC activation affects the size of the contralateral pupil only through lateral projections to the parasympathetic pathway. In line with this hypothesis, ipsilateral SCGx significantly reduced pupil dilation in response to tonic LC stimulation for ipsilateral pupils (0.22±0.015 vs. 0.17±0.016, p = 0.026, Mann-Whitney U-test), but this change was not seen in contralateral pupils (0.16±0.014 vs. 0.16±0.018, p = 0.88, Mann-Whitney U-test) (Figure 6B). Moreover, ipsilateral SCGx resulted in the mean lateralization ratio for 2 and 5 Hz LC stimulation to significantly diminish from 1.45±0.1 to 1.15±0.1 (p = 0.048 Student’s test, Figure 6C), indicating that the sympathetic pathway substantially contributed to pupil dilation induced by unilateral LC activation only for the ipsilateral eye.
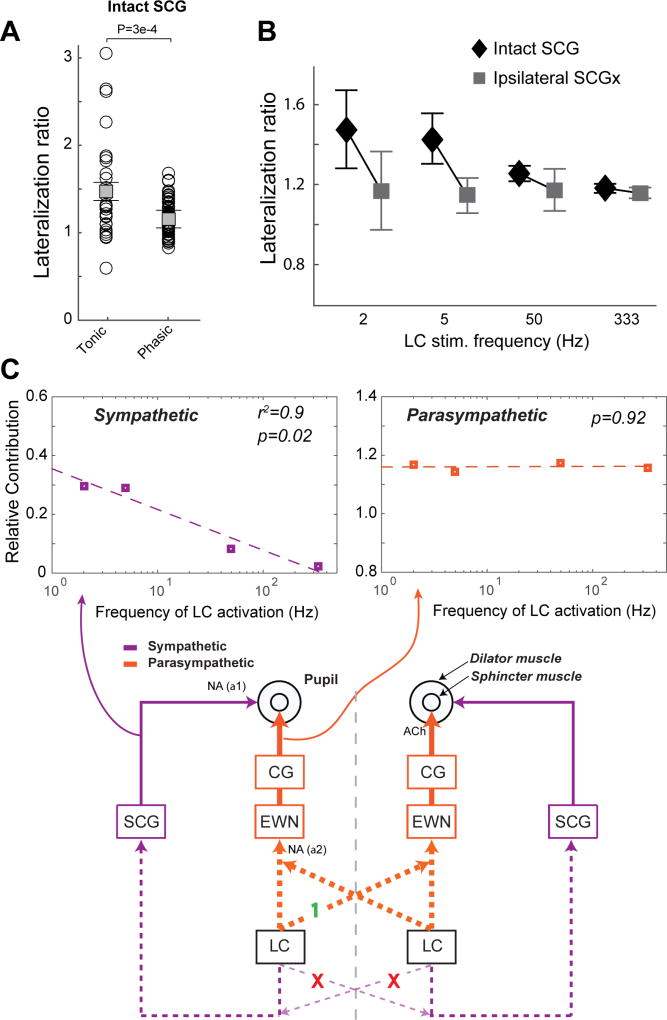
Contribution of the sympathetic pathway is dependent on the frequency of LC activation, whereas parasympathetic contribution is not
Our data have suggested that the lateralization of pupil dilation in response to LC stimulation is mainly due to unilateral projection of the LC through the sympathetic pathway. In addition, the lateralization ratio of pupil dilation following phasic LC stimulation was significantly less than that following tonic LC stimulation (Figure 7A, p < 0.001, Student’s t-test). Interestingly, for phasic LC stimulation, although contralateral SCGx had no effect on lateralization ratio, ipsilateral SCGx also failed to significantly diminish the lateralization ratio (Figure 7B). Taken together, this leads us to hypothesize that the relative contribution of the ipsilateral sympathetic pathway to LC-activation-evoked pupil dilation was dependent upon the frequency of LC activation. Indeed, when we compared the lateralization ratio of pupil dilation across different LC stimulation frequencies in animals with an intact SCG, we observed that the lateralization ratio decreased as the frequency of LC stimulation increased. This contrasts with the relatively constant lateralization ratio seen across the same stimulation frequencies in rats with ipsilateral SCGx (Figure 7B). We then could examine the contribution of the ipsilateral sympathetic and parasympathetic pathways relative to the contralateral parasympathetic pathway as ipsilateral SCGx eliminated the sympathetic influence of LC stimulation on the ipsilateral pupil’s dilation (Figure 7C), allowing us to isolate the contribution of the parasympathetic pathway to the pupils’ dilations. As we have confirmed that the LC modulates pupil dilation only through the sympathetic SCG and parasympathetic EWN (Figure 5D), we were able to determine the relative contribution of the sympathetic pathway by subtracting the mean lateralization ratio in rats that received ipsilateral SCGx from that of rats with an intact SCG. This calculation unveiled the logarithmic relationship between the contribution of the ipsilateral sympathetic pathway and LC activation frequency, as evidenced by a linear relationship when plotted in a logarithmic graph (p = 0.02, r2 = 0.9, Figure 7C, top left). Of note, the relative contribution of the ipsilateral parasympathetic pathway was insensitive to the frequency of LC stimulation (p = 0.92, Figure 7C, top right).
Figure 7. The relative contribution of the sympathetic, but not parasympathetic, pathway depends on the frequency of LC activation.
A) The lateralization ratio for tonic LC stimulation was larger than that for phasic LC stimulation in animals with intact SCGs. B) Lateralization ratio of pupil dilation evoked by different LC stimulations in both animals with intact SCGs and with ipsilateral SCGx’s. The differences in the lateralization ratio between SCG-intact animals and ipsilateral SCGx animals reflects the relative contribution of the sympathetic pathway. C) The LC modulates the size of the contralateral pupil only through a lateral projection to the contralateral parasympathetic system (x’s indicate disproved potential pathways). The relative contribution of the sympathetic pathway exponentially decayed with increasing frequency of LC activation, while the relative contribution of the parasympathetic pathway was insensitive to the frequency. All contributions were normalized by the contribution from the LC to contralateral, parasympathetic pathway (green 1). Error bars: SEM
DISCUSSION
Although the LC has been hypothesized, for decades, to modulate changes in pupil size during behavioral tasks, very little is known about how LC activity influences pupil size besides recent work conclusively establishing the causal link between LC activation and pupil dilation (Joshi et al., 2016). We show that unilateral LC activation evoked bilateral dilation. Further, we found an across-eye difference in the size of the evoked bilateral dilation, indicating a lateralization in the pathways between the LC and both pupils. The degree of this lateralization was found to be greater for dilations in response to tonic LC stimulation when compared with phasic LC stimulation evoked dilations, indicating that the across-eye difference depends upon the frequency of LC activation. Next, through pharmacological and surgical blocking of the EWN and SCG, we demonstrate that the LC influences pupil size only through two pathways, a parasympathetic pathway through the EWN and a sympathetic pathway through the SCG. Finally, by performing ipsilateral or contralateral SCGx, we found that the LC influences the ipsilateral pupil through both parasympathetic and sympathetic pathways, but influences the contralateral pupil only through the parasympathetic pathway. Therefore, differences between bilateral pupil dilations were mainly attributed to sympathetic contribution, suggesting that a measurement of across-eye differences in bilateral pupil dilation may present itself as a biomarker for abnormal autonomic activities, such as those observed in neuropsychiatric disorders (Eilam-Stock et al., 2014; Fujibayashi et al., 2009). Here, we discuss these findings in the context of previous studies in which pupil size was used to index neural computation and cognitive processing.
In this project, we used an anesthetized rat model, instead of an awake animal model, for several reasons. First, our data suggested that there is no significant difference in LC mediated changes between the light-isoflurane-anesthetized animals and head-constrained, idle (i.e. no behavioral tasks) animals (Figure S1). Second, the anesthesia allowed us to carefully characterize pupil dilation in response to different LC activation frequencies (five tonic frequencies in combination with a phasic frequency), which entails prolonged pupil recording procedures (~3–5 hours). To the best of our knowledge, this duration is well beyond the limits for which the animals can tolerate head-fixation. Finally, to tease apart the contributions of the sympathetic pathway, we performed SCGx to block the sympathetic pathway. SCGx, however, typically results in a blepharoptosis, causing the eyelid to occlude the pupil. In our anesthetized setup, we used eyelid retractors to gently hold open the eyelids after SCGx. However, in an awake setup, there is no humane ways to open animals’ eyelids during blepharoptosis.
Pupil dilation may reflect activity of an arousal network affecting multiple brain structures (Aston-Jones and Cohen, 2005; Eldar et al., 2013; Hermans et al., 2011; Joshi et al., 2016). For example, Joshi et al. (2016) unexpectedly found that the activation of multiple brain structures, including LC, superior colliculus, inferior colliculus, and cingulate cortex, elicited pupil dilation. In this work, we focused on elucidating only the mechanisms through which LC mediates pupil dilations. Although anesthesia has allowed us to do so with minimal background noises from the other pupil-dilation-evoking brain structures, it remains important to characterize the complex interplay between the LC and other arousal-related brain structures in collectively mediating pupil dilation. This will necessitate further work in awake behaving animals.
Consistent with previous work, our data showed that LC stimulation evoked pupil dilation. Moreover, unilateral LC stimulation evoked pupil dilation for both eyes, but with lateralization. One possible explanation for this result may be found in previous work which has shown that electrical stimulation of a single LC nucleus results in activation of the corresponding contralateral LC (Marzo et al., 2014). Although this finding is very interesting and the existence of lateral projections between the two LCs remains unclear (Li et al., 2016; Luppi et al., 1995), for the following reasons, we believe indirect activation of the contralateral LC is unlikely the cause of bilateral pupil dilation observed in our experiments. First, the intensity we used for electrical microstimulation was substantially lower than the current threshold necessary for eliciting contralateral LC activity, as reported in previous work (Marzo et al., 2014). Second, if bilateral dilation resulting from unilateral LC stimulation was due to bilateral LC activation, surgically blocking the sympathetic pathway to the contralateral pupil would result in a decrease in dilation for the contralateral pupil. However, our data demonstrated that this is not the case. The LC has been shown to make bilateral projections into many brain regions (Simpson et al., 1997; Steindler, 1981). For example, Simpson et al (1997) found that each LC bilaterally projects to different stages along the somatosensory pathway, with a relatively small difference in projection density in the brainstem and a large difference in the cortex. Consistent with this notion, our data suggests that the LC exhibits bilateral influence over both EWNs in the brainstem, with little difference in relative strength, given our calculated lateralization ratio for the parasympathetic pathway (~1.15) is close to unity.
Lateralization is ubiquitous in sensory processing and executive functions. For instance, information about tactile grating orientations is processed in the left hemisphere while the information about grating locations is processed in the right hemisphere (Van Boven et al., 2005). Similarly, visuospatial attentional tasks dominantly engage the right hemisphere (Thiebaut de Schotten et al., 2011). Although the LC is interconnected with various brain regions, it remains unclear whether the lateralized information processing differentially involves the bilateral LCs. As our data suggested, if there is a lateralization in the involvement of the bilateral LCs in any process, it should be evidenced by lateralized pupil dilation. Indeed, a recent work reported that there was a systematic lateralization in pupil sizes in human subjects performing an attentional task. This lateralization became evident in the 2nd and 3rd session of the task and increased with attentional load, suggesting that it was co-modulated by the attentional load and arousal associated with task experience (Wahn et al., 2017). Consistent with this work, electrodermal-activity-linked arousal also exhibits a lateralization (Picard et al., 2016). Future non-invasive neural imaging work remains to be done to test if this lateralization in changes in pupil size is due to bilateral differences in LC activation (de Gee et al., 2017; Murphy et al., 2014; Payzan-LeNestour et al., 2013).
“Microdilations”, with similar properties to our phasic-LC-activation-induced dilations, have been observed to frequently occur in the pupils of awake behaving rodents (McGinley et al., 2015a). These microdilations follow an archetypical temporal shape: an initial, rapid increase in pupil size with an approximately 2 s time course followed by a slower constriction back to baseline size. We found phasic-LC-activation-evoked dilations exhibited a qualitatively similar temporal shape, with time constants of 1.28 and 1.32 s for rise (ipsilateral and contralateral pupil, respectively), and 4.95 and 5.07 s for decay (ipsilateral and contralateral pupil, respectively). These similarities suggest that microdilations may be a good indicator of LC phasic firing, providing a physiological relevance to their correlation with arousal. An intriguing property of the microdilations was their high correlation with a transient depolarization of membrane potentials of cortical neurons, with microdilations occurring approximately 1 s after depolarizations (McCormick et al., 2015). Similarly, in our data, pupil dilation had an average onset time of 0.83 and 0.91 s (ipsilateral and contralateral pupil, respectively) following phasic activation of the LC. Previous work has shown NE release from LC projections has proven to depolarize the membrane potential of neurons by reducing resting potassium conductance (McCormick and Pape, 1990; McCormick and Prince, 1988). Therefore, consistent with previous experimental results, our data suggest that the correlation between microdilations and the depolarization of cortical membrane potentials may result from common LC input.
Slow fluctuations in baseline pupil size and rapid, transient pupil dilations are thought to encode non-redundant information about cognitive processes. For instance, de Gee et al. (2014) demonstrated that, in a protracted perceptual decision process, in addition to a transient pupil dilation associated with the final behavioral choice, there were sustained, relatively slow changes in pupil size throughout the decision formation period. In a separate study, in a predictive-inference task, Nassar et al. (2012) found that transient pupil dilation correlated with the level of unexpected uncertainty while average pupil size correlated with the level of expected uncertainty, leading them to hypothesize that these important properties of internal models may be represented by the tonic and phasic modes of LC activation, respectively. In strong support of this idea, our data indicated that tonic and phasic LC activation resulted in pupil dilations with disparate characteristics. For both eyes, tonic LC activation induced relatively-weak, slow, pupil dilations, while phasic LC activation resulted in strong, but transient, pupil dilations.
Attempts have previously been made to infer the individual contributions of the parasympathetic and sympathetic pathways to task-evoked pupil dilations by pharmacologically blocking the dilator or sphincter muscles separately during cognitive tasks (Steinhauer et al., 2004). Blocking the dilator muscle was found to have a weaker effect on pupil dilations as compared to blocking the sphincter muscle, suggesting the sympathetic pathway less strongly modulates pupil size. Our data quantified the contribution of the sympathetic pathway to pupil dilation relative to the parasympathetic pathway, with a ratio of approximately 0.36:1 and 0.09:1 for tonic and phasic LC activation, respectively. This supports the notion that phasic pupil dilation is mainly due to central inhibition of parasympathetic preganglionic neurons through the EWN in the midbrain.
The LC remains a challenge to study because it is relatively inaccessible due to its small size and deep location in the brainstem. A model by which LC activation can be inferred from non-luminance-induced changes in pupil size may benefit the community studying this important brain structure. Revealing the neural mechanisms by which the LC modulates pupil size is a step toward the development of such a model.
EXPERIMENTAL PROCEDURES
Surgery & Electrophysiology
All procedures were approved by Columbia University Institutional Animal Care and Use Committee and were in agreement with NIH guidelines. 56 Sprague-Dawley rats (225–300 g, 53 females; n=50 for acute experiments; n=6 for behavior) were used for these experiments. Acute procedures were similar to those previously described in detail (Wang et al., 2012; Zheng et al., 2015). Animals were anesthetized with isoflurane (1–2%). After craniotomy, a tungsten microelectrodes (1–2 MΩ, FHC) was advanced to the LC using a hydraulic microdrive (Wang et al., 2010). LC activity was determined based on wide spike waveform, response to a paw pinch, and other criteria used in previous studies (see Supplemental text). EEG signals between a contralateral frontal cortex screw and a screw over the contralateral occipital cortex were differentially amplified and recorded along with extracellular neural signals using a Plexon system.
For chronic implantations, a sterile platinum/iridium microelectrode (~1 MΩ, FHC) was advanced to the LC, bonded to a head-plate using dental cement, and connected to a connector cemented in the headcap (Figure S1).
Superior cervical gangiionectomy (SCGx)
The SCG was removed on either the right (n=12) or the left (n=13) side before craniotomy using a procedure described in detail previously (Savastano et al., 2010). A vertical incision along the midline was made to expose the mandibular glands. The sternohyoid and omohyoid muscles were carefully separated to expose the lymph node and carotid artery. At the bifurcation of the carotid artery, the external and internal carotid artery were carefully separated from the SCG underneath. The pre and post-ganglion branches of the SCG were cut to allow for its removal, followed by its fixation in 4% paraformaldehyde for immunohistochemistry.
Pupillometry recording
Recordings of both pupils were made using two pupillometry systems triggered by a real-time system with data streamed to hard drives. For LC stimulation experiments, eyelids of the animal were gently held open by eyelid retractors, and artificial eye drops were used to hydrate the corneas. Images of both pupils were collected at 50 Hz.
Pupillary light reflex was measured in 4 acute experiments, in which left pupillary responses to switching of ambient illuminance between 15 and 150 lux every 20 s were recorded. The same measurement was also performed in three awake, head-fixed animals after they became habituated to head-fixation (Bari et al., 2013; Ollerenshaw et al., 2012; Ollerenshaw et al., 2014).
LC microstimuiation
Biphasic current pulses (cathode-leading, 200 µs per phase) were delivered by a calibrated electrical microstimulator (Multi Channel Systems, or PSIU6/S88, Grass Instrument) to activate the LC. In acute setups, current pulses (60 µA) were used to evoke pupil dilation. Each stimulus block consisted of 5 s of baseline followed by 10 s of tonic LC stimulation with different frequencies (0, 0.5, 1, 2, and 5 Hz), which was then followed by a phasic LC stimulation (50 Hz or 330 Hz, 6 pulses). These stimulus blocks (Figure 3A) were delivered in an interleaved fashion with 60 s between each block. In awake setups, current pulses (50 Hz, 6 pulses, 150 µA) were delivered to an implanted LC electrode every 60–120 seconds, with a random delivery of a drop of Kool-aid solution occurring 20–40 seconds prior.
Immunohistochemistry
The animal’s brain was sectioned coronally at 20 µm using a freezing microtome (Leica Microsystems). Standard Nissl staining was used to verify placement of the electrode tip in the LC. Sections were examined using an Olympus CKX41 microscope.
SCG cells were labeled with tyrosine hydroxylase and neurofilament antibodies to confirm their sympathetic and neuronal identity, respectively. SCGs were sectioned in the longitudinal direction at 10 µm using the freezing microtome and sections were mounted onto two separate microscope slides for immunodetection of neurofilament-containing cells and tyrosine hydroxylase-containing neurons using different antibodies (see Supplemental text). Sections were imaged on an Olympus FV-1000 confocal microscope.
Data analysis
To quantify the shift of EEG power, Welch’s power spectral density estimate was used to calculate the ratio of power between 10–100 Hz to power between 1–10 Hz. This ratio was calculated for the 4 s segment before LC stimulation, the 4 s segment after LC stimulation, and the 4 s second segment lasting from 4 to 8 s after LC stimulation, referred to as the recovery period.
Pupil contour was segmented by estimating a histogram of pixel intensity which allows for calculation of the optimal threshold to extract pupil contour (Otsu, 1979). In acute setups, a small subset of trials (<10% of the total trials) during which pupil size exceeded 250% of the baseline or fell beneath 40% of the baseline for >2 s were excluded from the analysis. In awake setups, pupil size during blinks (<2% of frames) was linearly interpolated using values from before and after each blink. Pupil size was low-pass filtered (cutoff frequency: 3.75 Hz). The change in pupil size was then calculated for each trial by subtracting the mean baseline pupil size and subsequently dividing by the mean pupil size.
The time constants of the pupillary light reflex were evaluated by fitting exponential rising and decaying curves, respectively, as:
where τd and τc are the time constants of the rising and decaying curves, respectively. A is the baseline pupil size and B is the amplitude of change in pupil size induced by switching ambient illuminance.
To estimate the time constant of transient pupil dilation in response to LC stimulation, the change in pupil size following phasic stimulation was fit with a bi-exponential curve as:
where τr and τd are the time constants of the rising and decaying phases, respectively, y(0) is the initial value of the bi-exponential curve, and A is the amplitude. Only estimated time constants with a variance accounted for (VAF) > 0.9 were used in the comparison between phasic and 5 Hz tonic stimulation (Figure 3D). Phasic pupil dilation amplitude was defined as the difference between the max pupil size within 5 s post phasic stimulation and the pupil size at the onset of the phasic dilation.
To estimate the time constant of pupil dilation in response to tonic 5 Hz LC stimulation, the change in pupil size was fit with an exponential rise curve as:
where τt is the curve’s time constant. Only estimated time constants with a VAF > 0.9 were used in the comparison between phasic and 5 Hz tonic stimulation. The pupil size change evoked by 2 and 5 Hz tonic stimulation was defined as the mean normalized change in pupil size during the last 2 s.
Statistics
A one-sample Kolmogorov-Smirnov test (Matlab function kstest) was used to assess the normality of data before performing statistical tests. If the samples were normally distributed, a Student’s t-test was used. Otherwise, the Mann-Whitney U-test was used for unpaired samples, or the Wilcoxon signed-rank test for paired samples. Tukey’s posthoc test was performed for all multiple comparisons.
Supplementary Material
HIGHTLIGHTS.
Unilateral LC activation evokes lateralized, bilateral pupil dilation
Lateralization is dependent upon the frequency of LC activation
Dynamic lateralization arises from sympathetic not parasympathetic contributions
Across-eye differences in dilation may provide a possible index of sympathetic tone
Acknowledgments
We would like to thank Dr. J. M. Alonso and Dr. R. Bruno for their comments at various points of this work. This work is supported by NIH R01MH112267.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Q.W. conceived the study. Y.L. and Q.W. designed the experiments. Y.L., C.R., B.S., and Q.W. performed the experiments. Y.L. and N.M. performed histological analysis. Q.W. and C.R. analyzed the data, and Y.L., C.R. and Q.W. wrote the paper. All authors commented on the manuscript.
References
- Andreassi JL. Psychophysiology: Human Behavior & Physiological Response. Psychology Press; 2006. Pupillary response and behavior. [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Bari BA, Ollerenshaw DR, Millard DC, Wang Q, Stanley GB. Behavioral and Electrophysiological Effects of Cortical Microstimulation Parameters. PLoS ONE. 2013;8:e82170. doi: 10.1371/journal.pone.0082170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ. Sensitivity of Locus Ceruleus Neurons to Reward Value for Goal-Directed Actions. The Journal of Neuroscience. 2015;35:4005–4014. doi: 10.1523/JNEUROSCI.4553-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen LA, Burde RM, Loewy AD. Brainstem connections to the Edinger-Westphal nucleus of the cat: a retrograde tracer study. Brain Res. 1983;261:303–306. doi: 10.1016/0006-8993(83)90633-9. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Oka S, Bradshaw DH, Jacobson RC, Donaldson GW. Phasic pupil dilation response to noxious stimulation in normal volunteers: Relationship to brain evoked potentials and pain report. Psychophysiology. 1999;36:44–52. doi: 10.1017/s0048577299970373. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gee JW, Colizoli O, Kloosterman NA, Knapen T, Nieuwenhuis S, Donner TH. Dynamic modulation of decision biases by brainstem arousal systems. Elife. 2017;6:e23232. doi: 10.7554/eLife.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gee JW, Knapen T, Donner TH. Decision-related pupil dilation reflects upcoming choice and individual bias. Proceedings of the National Academy of Sciences. 2014;111:E618–E625. doi: 10.1073/pnas.1317557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J Neurophysiol. 2011;105:69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Pearson JM, Piatt ML. Pupil size and social vigilance in rhesus macaques. Frontiers in Neuroscience. 2014;8:100. doi: 10.3389/fnins.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Bringuier V, Shulz DE. Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study. Neuroscience. 2002;111:275–289. doi: 10.1016/s0306-4522(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Eilam-Stock T, Xu P, Cao M, Gu X, Dam NT, Anagnostou E. Abnormal autonomic and associated brain activities during rest in autism spectrum disorder. Brain. 2014;137:153–171. doi: 10.1093/brain/awt294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16:1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersen A, Elkabes S, Freedman DS, Sahin M. Chronic tissue response to untethered microelectrode implants in the rat brain and spinal cord. J Neural Eng. 2015;12:016019. doi: 10.1088/1741-2560/12/1/016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi M, Matsumoto T, Kishida I, Kimura T, Ishii C, Ishii N, Moritani T. Autonomic nervous system activity and psychiatric severity in schizophrenia. Psychiatry and clinical neurosciences. 2009;63:538–545. doi: 10.1111/j.1440-1819.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- Hancock MB, Fougerousse CL. Spinal projections from the nucleus locus coeruleus and nucleus subcoeruleus in the cat and monkey as demonstrated by the retrograde transport of horseradish peroxidase. Brain Research Bulletin. 1976;1:229–234. doi: 10.1016/0361-9230(76)90072-1. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marie HJF, Ossewaarde L, Henckens MJAG, Qin S, van Kesteren MTR, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G. Stress-Related Noradrenergic Activity Prompts Large-Scale Neural Network Reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4426–4436. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L, Walz JM, Sajda P. Your eyes give you away: prestimulus changes in pupil diameter correlate with poststimulus task-related EEG dynamics. PLoS One. 2014;9:e91321. doi: 10.1371/journal.pone.0091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani Rishi M, Gold Joshua I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron. 2016;89:221–234. doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwani RM, Joshi S, Gold JI. Phasic Activation of Individual Neurons in the Locus Ceruleus/Subceruleus Complex of Monkeys Reflects Rewarded Decisions to Go But Not Stop. The Journal of Neuroscience. 2014;34:13656–13669. doi: 10.1523/JNEUROSCI.2566-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hickey L, Perrins R, Werlen E, Patel AA, Hirschberg S, Jones MW, Salinas S, Kremer EJ, Pickering AE. Retrograde optogenetic characterization of the pontospinal module of the locus coeruleus with a canine adenoviral vector. Brain Res. 2016;1641:274–290. doi: 10.1016/j.brainres.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein O, Loewenfeld IE. The Pupil. In: Davson H, editor. The Eye. New York: Academic Press; 1962. [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Martins ARO, Froemke RC. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci. 2015;18:1483–1492. doi: 10.1038/nn.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A, Totah NK, Neves RM, Logothetis NK, Eschenko O. Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J Neurophysiol. 2014;111:2570–2588. doi: 10.1152/jn.00920.2013. [DOI] [PubMed] [Google Scholar]
- McCormick DA, McGinley MJ, Salkoff DB. Brain state dependent activity in the cortex and thalamus. Current Opinion in Neurobiology. 2015;31:133–140. doi: 10.1016/j.conb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988;59:978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- McGinley Matthew J, David Stephen V, McCormick David A. Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron. 2015a;87:179–192. doi: 10.1016/j.neuron.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley Matthew J, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell Cathryn R, Tolias Andreas S, Cardin Jessica A, McCormick David A. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron. 2015b;87:1143–1161. doi: 10.1016/j.neuron.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon KA, Devilbiss DM, Chapin JK, Waterhouse BD. Influence of norepinephrine on somatosensory neuronal responses in the rat thalamus: A combined modeling and in vivo multichannel, multi-neuron recording study. Brain Research. 2007;1147:105–123. doi: 10.1016/j.brainres.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Human brain mapping. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci. 2012;15:1040–1046. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerenshaw DR, Bari BA, Millard DC, Orr LE, Wang Q, Stanley GB. Detection of tactile inputs in the rat vibrissa pathway. J Neurophysiol. 2012;108:479–490. doi: 10.1152/jn.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerenshaw Douglas R, Zheng He JV, Millard Daniel C, Wang Q, Stanley Garrett B. The Adaptive Trade-Off between Detection and Discrimination in Cortical Representations and Behavior. Neuron. 2014;81:1152–1164. doi: 10.1016/j.neuron.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N. A Threshold Selection Method from Gray-Level Histograms. Systems, Man and Cybernetics, IEEE Transactions on. 1979;9:62–66. [Google Scholar]
- Payzan-LeNestour E, Dunne S, Bossaerts P, O’Doherty John P. The Neural Representation of Unexpected Uncertainty during Value-Based Decision Making. Neuron. 2013;79:191–201. doi: 10.1016/j.neuron.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard RW, Fedor S, Ayzenberg Y. Multiple Arousal Theory and Daily-Life Electrodermal Activity Asymmetry. Emotion Review. 2016;8:62–75. [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–371. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Research. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell Cathryn R, Yatsenko D, Denfield George H, Tolias Andreas S. Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron. 2014;84:355–362. doi: 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, Tolias AS. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun. 2016;7:13289. doi: 10.1038/ncomms13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Savastano LE, Castro AE, Fitt MR, Rath MF, Romeo HE, Muñoz EM. A standardized surgical technique for rat superior cervical ganglionectomy. Journal of Neuroscience Methods. 2010;192:22–33. doi: 10.1016/j.jneumeth.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RCS, Waterhouse BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. The Journal of Comparative Neurology. 1997;385:135–147. [PubMed] [Google Scholar]
- Steindler DA. Locus coeruleus neurons have axons that branch to the forebrain and cerebellum. Brain Res. 1981;223:367–373. doi: 10.1016/0006-8993(81)91149-5. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Szabadi E. Functional neuroanatomy of the central noradrenergic system. Journal of Psychopharmacology. 2013;27:659–693. doi: 10.1177/0269881113490326. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The Role of Locus Coeruleus in the Regulation of Cognitive Performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Ingeholm JE, Beauchamp MS, Bikle PC, Ungerleider LG. Tactile form and location processing in the human brain. Proc Natl Acad Sci U S A. 2005;102:12601–12605. doi: 10.1073/pnas.0505907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proceedings of the National Academy of Sciences. 2014;111:3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin Jessica A. Arousal and Locomotion Make Distinct Contributions to Cortical Activity Patterns and Visual Encoding. Neuron. 2015;86:740–754. doi: 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahn B, Ferris DP, Hairston WD, König P. Pupil Size Asymmetries Are Modulated By An Interaction Between Attentional Load And Task Experience. bioRxiv 2017 [Google Scholar]
- Wang Q, Millard DC, Zheng HJV, Stanley GB. Voltage-sensitive dye imaging reveals improved topographic activation of cortex in response to manipulation of thalamic microstimulation parameters. Journal of Neural Engineering. 2012;9:026008. doi: 10.1088/1741-2560/9/2/026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Webber R, Stanley GB. Thalamic Synchrony and the Adaptive Gating of Information Flow to Cortex. Nat Neurosci. 2010;13:1534–1541. doi: 10.1038/nn.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekselblatt Joseph B, Niell Cristopher M. Behavioral State—Getting “In The Zone”. Neuron. 2015;87:7–9. doi: 10.1016/j.neuron.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HJV, Wang Q, Stanley GB. Adaptive shaping of cortical response selectivity in the vibrissa pathway. Journal of Neurophysiology. 2015;113:3850–3865. doi: 10.1152/jn.00978.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.