Abstract
The energy available from the diet, which affects fat deposition in vivo, is a major factor in the expression of genes regulating fat deposition in the longissimus dorsi muscle. Providing high-energy diets to yaks might increase intramuscular fat deposition and fatty acid concentrations under a traditional grazing system in cold seasons. A total of fifteen adult castrated male yaks with an initial body weight 274.3 ± 3.14 kg were analyzed for intramuscular adipose deposition and fatty acid composition. The animals were divided into three groups and fed low-energy (LE: 5.5 MJ/kg), medium-energy (ME: 6.2 MJ/kg) and high-energy (HE: 6.9 MJ/kg) diets, respectively. All animals were fed ad libitum twice daily at 08:00–09:00 am and 17:00–18:00 pm and with free access to water for 74 days, including a 14-d period to adapt to the diets and the environment. Intramuscular fat (IMF) content, fatty acid profile and mRNA levels of genes involved in fatty acid synthesis were determined. The energy levels of the diets significantly (P<0.05) affected the content of IMF, total SFA, total MUFA and total PUFA. C16:0, C18:0 and C18:1n9c account for a large proportion of total fatty acids. Relative expression of acetyl-CoA carboxylase (ACACA), fatty acid synthase (FASN), stearoyl-CoA desaturase (SCD), sterol regulatory element-binding protein-1c (SREBP-1c), peroxisome proliferator-activated receptor γ (PPARγ) and fatty acid-binding protein 4 (FABP4) was greater in HE than in LE yaks (P<0.05). Moreover, ME yaks had higher (P<0.05) mRNA expression levels of PPARγ, ACACA, FASN, SCD and FABP4 than did the LE yaks. The results demonstrate that the higher energy level of the diets increased IMF deposition and fatty acid content as well as increased intramuscular lipogenic gene expression during the experimental period.
Introduction
Yaks (Bos grunniens), one of the world’s most extraordinary herbivores, are a multifunctional principal livestock species unique to the Qinghai-Tibetan Plateau, where altitudes range from 3,000 to 5,000 m and which is characterized by hypoxia, low annual average temperatures, a short forage growing season (approximately 90–120 days) and a shortage of forage nutrients [1, 2]. There are approximately 15 million yaks on the Qinghai-Tibetan Plateau, accounting for more than 90% of total yak population in the world. These yaks provide many major resources (e.g., meat, milk, hair, hides and dung), and yak meat is the major economic resource for local herders [3, 4].
As quality life improves worldwide, people increasingly expect that the meat they consume should be healthy and high quality (low in fat; high in protein, vitamins and minerals). The intramuscular fat (IMF) and intramuscular fatty acid content play an important role in meat quality [5, 6]. However, yak meat has a low IMF content because intramuscular adipose deposition is difficult under the conditions of long-term malnutrition in a traditional grazing system in cold seasons [7]. Meat quality, including palatability, tenderness and juiciness, could be improved by increasing the IMF content [8], and increasing the IMF content and fatty acid profile may improve the quality of yak meat. Many studies have demonstrated that the IMF content and fatty acid levels are influenced by age [9], breed [10] and diets [11–15]. In vivo, lipogenesis, lipolysis and fatty acid transport lead to IMF deposition. A higher-energy content diet could contribute to lipogenesis [16]. The concentration of intramuscular fatty acids is mainly regulated by inducing and inhibiting genes encoding specific metabolic enzymes associated with lipid metabolism or transcription factors [17]. Sterol regulatory element-binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor γ (PPARγ) are the most important genes involved in lipid metabolism in muscle tissue [18, 19]. Recently, effects of different dietary energy levels on IMF deposition and fatty-acid composition in beef cattle [20], double-muscled Belgian Blue bulls [21] and Angus × Chinese Xiangxi yellow cattle [22] have been reported, as being affected by diets with different protein levels on intramuscular fat deposition in yaks [23]. However, little information is available regarding the influence of different energy diets on IMF deposition and fatty acid profiles in yaks.
The effects of different dietary energy content on IMF deposition and fatty acid composition in yaks are unclear. Therefore, the objective of this study was to analyze the relationship between dietary energy and fat deposition and fatty-acid profile, as well as expression of the regulatory genes PPARγ, SREBP-1c, stearoyl-CoA desaturase (SCD), acetyl CoA carboxylase (ACACA), lipoprotein lipase (LPL), fatty acid-binding protein 4 (FABP4) and fatty acid synthase (FASN) in the muscle of adult yaks.
Materials and methods
Ethics statement
This feeding experiment was conducted between February and May 2016 at Hongtu Yak Breeding Cooperatives (located in Qinghai-Tibetan Plateau, at 35°08′38″N, 102°99′36″E and with average altitude 3,230 m) of Tibetan Autonomous Prefecture of Gannan, Gansu Province, China. Before the experiment, all animal studies and the barn environment were examined and approved by the Institutional Animal Care and Use Committee of Lanzhou Institute of Husbandry and Pharmaceutical Sciences, China. The yaks were provided for use as experimental animals by the owner, and all animals were cared for according to the Guide for the Care and Use of Laboratory Animals, Lanzhou Institute of Husbandry and Pharmaceutical Sciences, China.
Animals, diets and management
A total of fifteen adult castrated male yaks with similar body conditions (body weight 274.3 ± 3.14 kg) were included in a single-factor completely randomized experimental design. The animals were randomly divided into three treatment groups with five replicates each. Three diets with different levels of energy and a concentrate-to-forage ratio of 30:70 (DM basis) were formulated to be isonitrogenous, containing similar roughage mixtures (40% oats silage, 40% microbial corn stalk silage and 20% highland barley hay) and different energy concentrates: low energy level (LE: 5.5 MJ/kg), medium energy level (ME: 6.2 MJ/kg) and high energy level (HE: 6.9 MJ/kg). The ingredients and nutrient composition of the three diets are shown in Table 1.
Table 1. Ingredients and nutrient composition of the diets during the experiment.
| Item | LE | ME | HE |
|---|---|---|---|
| Ingredient (%) | |||
| Corn | 29.00 | 44.80 | 56.00 |
| Corn germ | 30.00 | 20.00 | 12.00 |
| Wheat bran | 4.00 | 4.00 | ─ |
| DDGS | 15.00 | 7.00 | 6.30 |
| Prickly ash seed | 10.00 | 2.00 | 4.00 |
| Cottonseed meal | 6.00 | 12.00 | 16.00 |
| Soybean meal | ─ | 5.00 | ─ |
| Salt | 0.80 | 0.80 | 0.80 |
| White stone powder | 2.00 | 2.00 | 2.00 |
| Dicalcium phosphate | 0.60 | 0.60 | 0.60 |
| Urea | 0.80 | ─ | 0.50 |
| Sodium bicarbonate | 1.00 | 1.00 | 1.00 |
| Premixa | 0.80 | 0.80 | 0.80 |
| Nutrient composition, % of DM | |||
| Crude protein | 16.53 | 16.74 | 17.21 |
| Crude fat | 3.73 | 4.18 | 5.57 |
| NEgb (MJ/kg) | 5.5 | 6.2 | 6.9 |
| Neutral detergent fiber | 15.93 | 13.15 | 12.32 |
| Acid detergent fiber | 4.54 | 4.14 | 3.72 |
| Calcium | 0.64 | 0.84 | 0.75 |
| Phosphorus | 0.31 | 0.34 | 0.36 |
a Premix was provided per kilogram of total diet DM, and the composition was as follows: 22,520 IU of vitamin A, 1,920 IU of vitamin D3, 18 IU of vitamin E, 0.36 IU of vitamin K3, 5.28 mg of vitamin B2, 0.008 mg of vitamin B12, 21.2 mg of D-calcium pantothenate, 9 mg of Cu, 132.8 mg of Zn, 240 mg of Fe and 8 mg of Mn, 0.28 mg of Co.
b NEg, net energy for gain; DDGS, dried distillers grains with solubles; LE, low energy level; ME, medium energy level; HE, high energy level.
The experiment lasted for 60 d, and the animals were given a 14-d adaptation period to familiarize them with the diets, facilities and staff before the experiment. All animals were weighed, labeled before feeding in the morning and then individually housed in tie-stalls; each animal had 9 m2 for normal activities with bedding, and the barn was cleaned every day. The yaks were fed twice a day at 08:00–09:00 am and 17:00–18:00 pm with a total of 2.45 kg concentrates and 5.75 kg roughage mixtures; they were given free access to water and mineral blocks. The final weights of the animals were determined by weighing them after fasting them for 12 h. They were transferred to a slaughter house and all yaks received a jugular vein injection of sierra oxazine hydrochloride injection (Shengda Animal Pharmaceutical Co. Ltd., Dunhua, Jilin, China) with 0.2 mL/kg liveweight to ease their pain before slaughter, and exsanguination via the carotid artery was executed after animals completely under anesthesia.
Sample collection
After slaughter, the carcasses were immediately washed and divided into halves. Subsequently, two samples of the longissimus dorsi muscle between 12th and 13th ribs were collected from each animal. All instruments used to collect the tissue were sterilized in advance. The muscle samples were washed in 0.9% NaCl solution, and the first samples were stored in Ziploc bags at -20°C for proximate nutrient and fatty acid analysis. The second were placed in 2 mL cryogenic vials (Corning Incorporated, New York, USA), transported in liquid nitrogen and stored at -80°C for total RNA extraction.
Intramuscular fat content analysis
A 5-g meat sample was used to measure intramuscular fat content in each animal. Sea sand was added to evaporate moisture in a water bath. Lipids were extracted using the petroleum ether of Soxhlet apparatus (AOAC method) and expressed as grams per 100 g of fresh muscle tissue.
Fatty acid profile analysis
Samples of yak muscles (100 mg) were trimmed of connective tissue and finely chopped. Intramuscular fat was extracted in 3 mL chloroform-methanol 1:1 [24] and methylated in 2 mL 4% methanol solution in HCl [25] containing 100 μL C19:0 (methyl nonadecanoate) as an internal standard, and then the mixtures were heated in a water bath at 85°C for 1 h. Subsequently, 1 mL n-hexane was added to the mixture after it reached room temperature and shaking extraction was performed for 2 min. The mixture was allowed to stand for 1 h until layering occurred; following this, 100 μL supernatant was transferred to a new centrifuge tube and diluted with n-hexane to 1 mL. The extract was then filtered through filter membrane with an aperture of 0.45 μm. Fatty acid profiles were determined by gas chromatograph-mass spectrometer (ThermoFisher Trace 1310, Thermo Scientific, MA, USA) with a flame ionization detector and a 30-m-long capillary column 0.25 mm in internal diameter and 0.25 μm thick (TG-5MS, Thermo Scientific, MA, USA). Nitrogen was used as a carrier gas at a flow rate of 1.2 mL/min. The chromatographic conditions were as follows: the capillary column was incubated at 80°C for 1 min, and the temperature was increased by 10°C/min until it reached 200°C. Subsequently, the temperature was increased by 4°C/min until it reached 250°C and then increased at 2°C/min to 270°C. Following this, the temperature was held for three minutes at 270°C. During analysis, the temperature of injector was kept at 290°C and splitless injection was performed with a 1-min opening valve time. Next, the capillary column was directly subjected to mass spectrum analysis under the following conditions: the interface temperature set at 290°C and the ion source maintained at 280°C. Otherwise, 70 eV ionization energy of impact ionization (EI) was used to fragment the eluents from the capillary gas chromatograph. The scanned area was from 30 to 400 amu (atomic mass units). Individual fatty acid contents were determined through comparison to the mixed fatty acid standard product and the internal standard, and the fatty-acid levels were calculated by following formula:
where Xi is the level of each fatty acid, expressed as mg/kg muscle tissue; m is the intramuscular fat content of the sample; Asi represents the peak area of all types of fatty acids in the sample; A ∑ si is the sum of the peak area of all fatty acids in the sample.
RNA extraction and quantitative RT-PCR
The target and reference gene primers were designed using integrated mRNA sequences based on sequences published by the National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov) primers were designed using Premier Primer 5 software (PREMIER Biosoft International, CA, USA), and sequences of each are shown in Table 2.
Table 2. Gene names, primer sequences, accession, product size of the used genes.
| Gene name* | Primer sequence (5’→3’) | Accession | Product size | |
|---|---|---|---|---|
| β-actin | F | ACCATCGGCAATGAGCG | XM_005887322.2 | 150bp |
| R | CACCGTGTTGGCGTAGAG | |||
| LPL | F | TCCTGGAGTGACCGAATC | XM_005902304.2 | 125bp |
| R | AGGCAGCCACGAGTTTT | |||
| ACACA | F | AAGCAATGGATGAACCTTCTTC | XM_005888165.2 | 197bp |
| R | GATGCCCAAGTCAGAGAGC | |||
| PPARγ | F | CATTTCCACTCCGCACTA | XM_005902845.2 | 122bp |
| R | GGGATACAGGCTCCACTT | |||
| FASN | F | GACGGTCGCATCATCTTCC | XM_005905364.2 | 156bp |
| R | GAGCACAATCCCTGTCTTCG | |||
| FABP4 | F | TGAGATTTCCTTCAAATTGGG | XM_014478668.1 | 101bp |
| R | CTTGTACCAGAGCACCTTCATC | |||
| SCD | F | TACTGCGGTCCAAGTCGTT | NM_173959.4 | 165bp |
| R | CAGCCTTGTCTGGAGTCATC | |||
| SREBP-1c | F | AGCTCAAGGACCTGGTGGTG | XM_014477492.1 | 140bp |
| R | GACAGCAGTGCGCAGACTCA | |||
* LPL, lipoprotein lipase; ACACA, acetyl-CoA carboxylase; PPARγ, peroxisome proliferator-activated receptors gamma; FASN, fatty acid synthase; FABP4, adipocyte fatty acid binding protein 4; SCD, stearoyl-CoA desaturase; SREBP-1c, sterol regulatory element-binding protein-1c.
The total RNA of the longissimus dorsi muscle was extracted by RNAiso Plus (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The extracted RNA was dissolved in 30 μL diethylpyrocarbonate (DEPC)-treated water (Solarbio LIFE SCIENCES, Beijing, China), and the concentration was measured by spectrophotometer (NanoDrop 2000, Thermo Scientific, MA, USA). Purity and integrity were checked using agarose-gel electrophoresis with ethidium bromide. Total RNA was reverse transcribed into five copies using a PrimeScript™ RT reagent kit (TaKaRa, Dalian, China) in accordance with the manufacturer’s instructions, and the RT-PCR products were stored at -20°C for quantitative real-time PCR.
Quantitative real-time PCR was performed in triplicate to determine mRNA relative expression using a SYBR® Premix Ex Taq™ II (TaRaKa, Dalian, China). Each 25-μL real-time reaction contained 12.5 μL SYBR Premix Ex Taq II (2×), 1 μL each of 10 μM primers, 2.0 μL cDNA and 8.5 μL RNase Free dH2O. Reactions were run on a fluorescence thermal cycler (CFX96, Bio-Rad, CA, USA), and the program was as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, annealing at 60°C for 30 s and a melting curve with a temperature increase of 0.5°C every 5 s starting at 65°C. The RT-PCR analyses for each studied gene were performed using cDNA from five biological replicates with three technical replicates per biological replicate. The threshold cycle (CT) resulting from quantitative RT-PCR was analyzed using the 2-ΔΔCt method, and all data were normalized with the reference gene beta-actin (β-actin) gene [26].
Statistical analysis
The fatty acid content and mRNA abundance in the longissimus dorsi muscle were processed by one-way ANOVA analysis using the LSD procedure to perform multiple comparisons in SPSS 19.0 (SPSS Inc., Chicago, USA). The Pearson correlation analyses were performed to calculate correlation coefficients among gene expression, as well as correlation coefficients between gene expression and intramuscular fat content. Furthermore, the correlations between gene expression and fatty acid concentrations were also determined. Values at P<0.05 were considered significant difference.
Results
Intramuscular fatty acid composition
The effect of diets with different energy contents on the fatty acid profile of yaks is shown in Table 3. Intramuscular fat content was highly affected (P = 0.001) by dietary energy levels. The fatty acids content varied significantly among diets and was positively associated with higher-energy diets. Significant (P<0.001, P = 0.011 and P = 0.001) differences were found between HE and LE yaks regarding saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA), and there were also significant (P = 0.037 and P = 0.005) differences between HE and ME yaks in terms of SFA and PUFA content. However, ME yaks were not significantly different from LE and HE yaks (P = 0.227 and P = 0.111) in terms of MUFA, and the level of PUFA was similar (P = 0.363) between the ME and LE yaks.
Table 3. IMF content and fatty acid composition in longissimus dorsi of yaks fed diets supplying different energy levels.
| Item* | LE | ME | HE | SEM | P-value |
|---|---|---|---|---|---|
| IMF (g/100 g) | 0.56c | 0.92b | 1.34a | 0.102 | 0.001 |
| Fatty acid (mg/kg) | |||||
| C14:0 Myristic acid | 15.35c | 21.55b | 25.11a | 1.138 | <0.001 |
| C14:1 Myristoleic acid | 3.33c | 4.14b | 5.73a | 0.291 | <0.001 |
| C15:0 Pentadecanoic acid | 2.08b | 3.01ab | 4.03a | 0.308 | 0.020 |
| C16:0 Palmitic acid | 1951.06c | 2748.31b | 3367.25a | 174.237 | <0.001 |
| C16:1 Palmitoleic acid | 69.80c | 81.91b | 118.14a | 5.60 | <0.001 |
| C17:0 Margaric acid | 6.31b | 7.81b | 10.26a | 0.595 | 0.010 |
| C17:1 Heptadecanoic acid | 5.20b | 6.02ab | 8.44a | 0.566 | 0.038 |
| C18:0 Stearic acid | 1044.74c | 1242.88b | 1588.32a | 63.775 | <0.001 |
| C18:1n9c Oleic acid | 1952.68b | 2441.40ab | 3059.18a | 186.659 | 0.038 |
| C18:1n9t trans oleic acid | 37.37b | 44.45b | 75.05a | 5.866 | 0.008 |
| C18:2n6c Linolenic acid | 64.49b | 66.39b | 80.41a | 2.846 | 0.029 |
| C20:1 Eicosenoic acid | 1.22b | 1.82a | 2.11a | 0.136 | 0.012 |
| C20:3n3 Eicosatrienoic acid | 0.71b | 1.14a | 1.31a | 0.085 | 0.002 |
| C20:4n6 Arachidonic acid | 8.61c | 11.81b | 18.81a | 1.185 | <0.001 |
| C20:5n3 EPA | 1.84b | 2.22ab | 3.08a | 0.212 | 0.036 |
| C22:6n3 DHA | 1.26b | 1.54ab | 2.17a | 0.157 | 0.038 |
| ∑ SFA | 3019.55c | 4023.57b | 4994.97a | 232.906 | <0.001 |
| ∑ MUFA | 2069.60b | 2579.74ab | 3268.65a | 195.509 | 0.027 |
| ∑ PUFA | 76.91b | 83.09b | 105.78a | 4.134 | 0.002 |
a,b,c Means in a row with different small letter superscripts differ significantly (P<0.05).
* IMF, intramuscular fat; ∑ SFA, saturated fatty acid (without any double bonds, C14:0-C18:0); ∑ MUFA, monounsaturated fatty acid, all fatty acids with a single double bond (C14:1-C20:1); ∑ PUFA, polyunsaturated fatty acid, all fatty acids with 2 or more double bonds (including C18:2n6c, C20:3n3, C20:4n6 and C20:5n3); EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
LE, low energy level; ME, medium energy level; HE, high energy level.
The fatty acids C16:0, C18:0 and C18:1n9c stand out, accounting for a large proportion of total fatty acids. HE yaks had the highest levels of C14:0, C14:1, C16:0, C16:1, C17:0, C18:0, C18:1n9t, C18:2n6c and C20:4n6, and HE yaks had significantly higher (P<0.001) concentrations of C14:0, C14:1, C15:0, C16:0, C16:1, 17:0, C17:1, C18:0, C18:1n9c, C18:1n9t, C18:2n6c, C20:1, C20:3n3, C20:4n6, C20:5n3 and C22:6n3 than did the LE yaks. Otherwise, significant (P<0.001, P = 0.022, P = 0.003, P = 0.001, P = 0.005, P = 0.034, P = 0.008 and P = 0.003) differences were found between ME and LE yaks in the concentrations of C14:0, C14:1, C16:0, C16:1, C18:0, C20:1, C20:3n3 and C20:4n6.
mRNA abundance of longissimus dorsi muscle
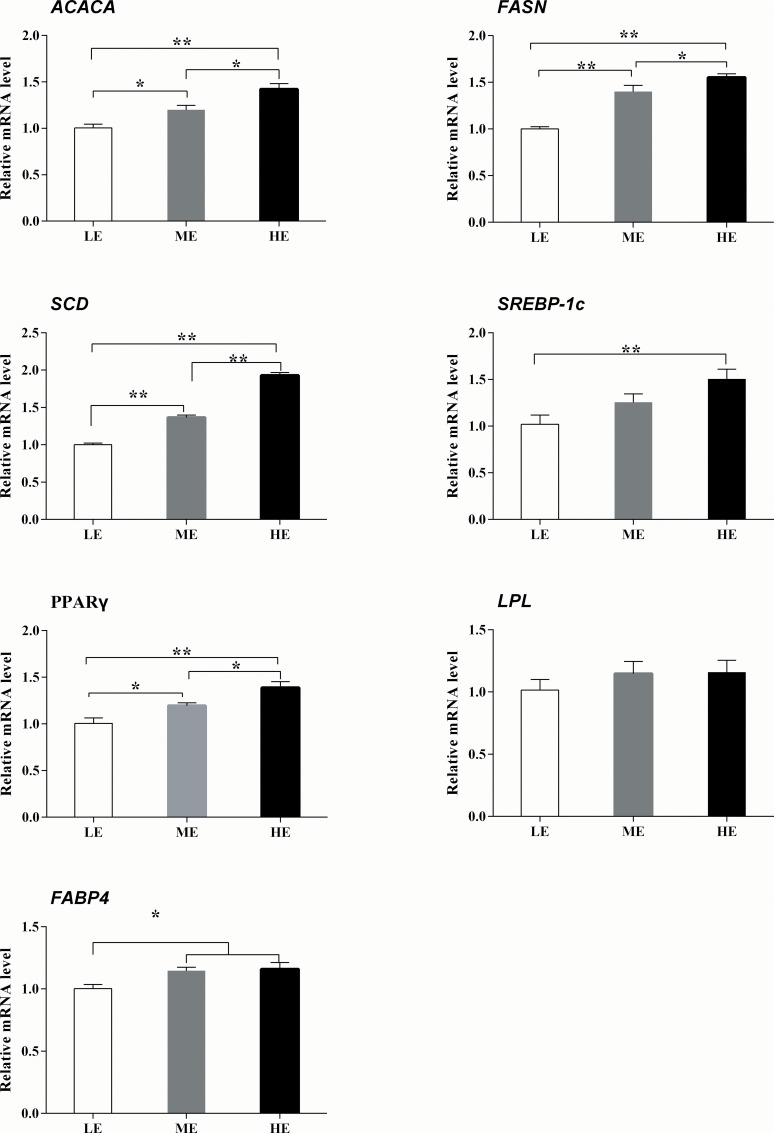
The mRNA abundance of the candidate genes in each diet is shown in Fig 1. The mRNA levels of ACACA, FASN, SCD, SREBP-1c, PPARγ and FABP4 increased (P<0.05) with increasing dietary energy; HE and ME yaks had higher (P<0.05) mRNA abundance of above genes (except for SREBP-1c) than did the LE yaks. In addition, significant (P = 0.025, P = 0.01, P = 0.041 and P<0.001) differences were found in gene expression between HE and ME yaks for PPARγ, ACACA, FASN and SCD; ME yaks had higher (P<0.001, P<0.001, P<0.001, P<0.001 and P = 0.016) expression levels of PPARγ, ACACA, FASN, SCD and FABP4 than did the LE yaks. Furthermore, no significant (P = 0.508) differences were found of LPL among three treatments, and HE and ME yaks had similar (P>0.05) expression of SREBP-1c and FABP4.
Fig 1. Relative expression levels of seven candidate genes in longissimus dorsi muscle of yaks fed diets supplying different energy levels.
ACACA: acetyl-CoA carboxylase, FASN: fatty acid synthase, SCD: stearoyl-CoA desaturase, SREBP-1c: sterol regulatory element-binding protein-1c, PPARγ: peroxisome proliferator-activated receptors gamma, LPL: lipoprotein lipase, FABP4: fatty acid binding protein 4. Values are represented as the Mean ± SEM. * and ** indicate P < 0.05 and P < 0.01, respectively, between two groups.
Relationships among the expression levels of genes involved in lipid metabolism in longissimus dorsi muscle
The expression of PPARγ was positively correlated with those of ACACA (P<0.01), FABP4 (P<0.01), SCD (P<0.01), SREBP-1c (P<0.05) and FASN (P<0.01). The expression of SREBP-1c was also positively correlated with those of ACACA (P<0.01), SCD (P<0.05) and FASN (P<0.01). In addition, FASN expression was positively correlated with those of ACACA (P<0.01), FABP4 (P<0.05) and SCD (P<0.01). There was a significant (P<0.01) correlation between SCD and ACACA and a positive correlation (P<0.05) between SCD and FABP4 (Table 4).
Table 4. Pearson correlation coefficients among gene expression in longissimus dorsi muscle of yaks.
| Gene a | ACACA | LPL | FABP4 | SCD | SREBP-1c | FASN |
|---|---|---|---|---|---|---|
| PPARγ | 0.682** | 0.075 | 0.730** | 0.812** | 0.534* | 0.663** |
| FASN | 0.684** | 0.246 | 0.571* | 0.850** | 0.765** | |
| SREBP-1c | 0.741** | 0.220 | 0.267 | 0.631* | ||
| SCD | 0.748** | 0.286 | 0.621* | |||
| FABP4 | 0.351 | -0.213 | ||||
| LPL | 0.407 |
Pearson correlation coefficients are across all treatments. Number of observations = 15.
*P<0.05
** P<0.01,and
*** P<0.001.
a LPL, lipoprotein lipase; ACACA, acetyl-CoA carboxylase; PPARγ, peroxisome proliferator-activated receptors gamma; FASN, fatty acid synthase; FABP4, adipocyte fatty acid binding protein 4; SCD, stearoyl-CoA desaturase; SREBP-1c, sterol regulatory element-binding protein-1c.
Relationships among gene expression levels, intramuscular fat content and fatty acid content in longissimus dorsi muscle
IMF content was positively correlated with the expression levels of PPARγ (P<0.05), ACACA (P<0.01), FASN (P<0.01), SCD (P<0.01) and SREBP-1c (P<0.01), but no significant correlation was found between IMF content and the expression of other genes (Table 5).
Table 5. Pearson correlation coefficients among gene expression, IMF and fatty acid content in longissimus dorsi muscle of yaks.
| Item a | PPARγ | FASN | ACACA | LPL | SREBP-1c | SCD | FABP4 |
|---|---|---|---|---|---|---|---|
| IMF (g/100 g) | 0.549* | 0.791** | 0.743** | 0.339 | 0.785** | 0.786** | 0.216 |
| Fatty acid b (mg/kg) | |||||||
| C14:0 Myristic acid | 0.735** | 0.959** | 0.753** | 0.356 | 0.736** | 0.887** | 0.566* |
| C14:1 Myristoleic acid | 0.693** | 0.819** | 0.788** | 0.212 | 0.743** | 0.889** | 0.422 |
| C16:0 Palmitic acid | 0.805** | 0.911** | 0.710** | 0.129 | 0.668** | 0.851** | 0.588* |
| C16:1 Palmitoleic acid | 0.724** | 0.812** | 0.825** | 0.244 | 0.730** | 0.920** | 0.481 |
| C18:0 Stearic acid | 0.762** | 0.838** | 0.830** | 0.175 | 0.754** | 0.886** | 0.584* |
| C18:1n9c Oleic acid | 0.446 | 0.896** | 0.524* | 0.177 | 0.224 | 0.651** | 0.313 |
| C18:1n9t trans oleic acid | 0.489 | 0.727** | 0.600* | -0.055 | 0.500 | 0.666** | 0.267 |
| C18:2n6c Linolenic acid | 0.586* | 0.672** | 0.313 | 0.036 | 0.400 | 0.670** | 0.309 |
| C20:1 Eicosenoic acid | 0.434 | 0.869** | 0.447 | 0.404 | 0.360 | 0.750** | 0.373 |
| C20:3n3 Eicosatrienoic acid | 0.776** | 0.780** | 0.466 | 0.187 | 0.449 | 0.853** | 0.673** |
| C20:4n6 Arachidonic acid | 0.822** | 0.778** | 0.744** | 0.282 | 0.589* | 0.961** | 0.581* |
| C20:5n3 EPA | 0.624* | 0.779** | 0.382 | 0.337 | 0.396 | 0.727** | 0.291 |
| C22:6n3 DHA | 0.629* | 0.776** | 0.379 | 0.358 | 0.378 | 0.723** | 0.289 |
| ∑ SFA | 0.696** | 0.913** | 0.641* | 0.096 | 0.544* | 0.785** | 0.524* |
| ∑ MUFA | 0.443 | 0.891** | 0.532* | 0.165 | 0.248 | 0.652** | 0.304 |
| ∑ PUFA | 0.711** | 0.757** | 0.472 | 0.140 | 0.488 | 0.819** | 0.419 |
Pearson correlation coefficients are across all treatments. Number of observations = 15.
*P<0.05
** P<0.01, and
*** P<0.001.
a ACACA, acetyl-CoA carboxylase; FASN, fatty acid synthase; SCD, stearoyl-CoA desaturase; SREBP-1c, sterol regulatory element-binding protein-1c; PPARγ: peroxisome proliferator-activated receptors gamma; LPL, lipoprotein lipase; FABP4, adipocyte fatty acid binding protein 4.
b IMF, intramuscular fat; ∑ SFA, total saturated fatty acid (without any double bonds, C14:0-C18:0); ∑ MUFA, total monounsaturated fatty acid, all fatty acids with a single double bond (C14:1-C20:1); ∑ PUFA, total polyunsaturated fatty acid, all fatty acids with 2 or more double bonds (including C18:2n6c, C20:3n3, C20:4n6 and C20:5n3); EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Considering the correlations between gene expression and fatty acid content, FASN and SCD are notable (Table 5). These genes were positively correlated with levels of C14:0 (P<0.01), C14:1 (P<0.01), C16:0 (P<0.01), C16:1 (P<0.01), C18:0 (P<0.01), C18:1n9c (P<0.01), C18:1n9t (P<0.01), C18:2n6c (P<0.05 and P<0.01), C20:1 (P<0.01), C20:3n3(P<0.01), C20:4n6 (P<0.01), C20:5n3 (P<0.01), C22:6n3 (P<0.01), SFA (P<0.01), MUFA (P<0.01) and PUFA (P<0.01). The expression levels of ACACA and PPARγ were positively correlated with C14:0 (P<0.01), C14:1 (P<0.01), C16:0 (P<0.01), C16:1 (P<0.01), C18:0 (P<0.01), C20:4n6 (P<0.01) and SFA (P<0.05 and P<0.01), and ACACA was positively correlated with C18:1n9c (P<0.05), C18:1n9t (P<0.05) and MUFA (P<0.05). PPARγ had positive correlation with C18:2n6c (P<0.05), C20:3n3 (P<0.01), C20:5n3 (P<0.05), C22:6n3 (P<0.05) and PUFA (P<0.01). In addition, SREBP-1c was positively correlated with C14:0 (P<0.01), C14:1 (P<0.01), C16:0 (P<0.01), C16:1 (P<0.01), C18:0 (P<0.01), C20:4n6 (P<0.05) and SFA (P<0.05). Significant correlations were found between the expression level of FABP4 and the concentrations of C14:0, C16:0, C18:0, C20:4n6, SFA (P<0.05) and C20:3n3 (P<0.01).
Discussion
This paper describes for the first time the effects of diets supplying different energy levels on fat deposition and the fatty acid profile of the longissimus dorsi muscle in the domesticated yak and the role of genes involved in lipid metabolism in changing the fatty acid composition across three treatments. Non-structural carbohydrate (starch) was priority to support skeletal and muscle growth resulting in low rate of fat deposition during the “growing phase” in beef cattle. Conversely, the intake of starch, first and foremost, contributed to fat deposition during the “finishing phase” of beef cattle [27]. Consistently, several studies have demonstrated that IMF content increases with increased energy content in finishing cattle [20] and buffalo cattle [28], which was also found in current study. Bovine intramuscular fat is characterized by its abundant saturated fatty acids (SFA), especially palmitic acid and stearic acid [29], as we found for the yaks. Moreover, previous research showed that the level of SFA depends on the degree of fat deposition [30, 31] and that SFA content increased with increasing IMF content. It is, therefore, possible that higher energy content regulates SFA levels by enhancing IMF content. Consistent with our data, Smet et al [21] reported that the levels of C14:0, C15:0, C16:0, C16:1 and C18:1 increased with higher-energy diets in Belgian Blue bulls. By contrast, the level of C18:0 decreased in previous studies [32–34] but increased in the current research when higher-energy diets were provided. Pentadecanoic acid (C15:0) and heptadecanoic acid (17:0) belong to odd-chain fatty acids (OCFAs) and only have a small proportion of total saturated fatty acids in ruminant meat. De novo synthesis of linear OCFAs is different from palmitic acid which used propionyl-CoA as primer, instead of acetyl-CoA and a large portion of OCFAs in ruminants was synthesized by rumen bacteria, and it also originates from diets including maize silage, grass silage and other plants [35, 36]. Therefore, the synthesis of OCFAs may either not occur in adipose tissue or regulate by lipogenic genes.
Fatty acid composition of the longissimus dorsi muscle is affected by diets, including protein content, energy content [37] and fatty acid profile [17, 38, 39]. Abundant MUFA and PUFA not only improve meat characteristics, such as flavor, cholesterol content and nutritional benefits, but are also beneficial for human health. Contrary to our expectations, dietary MUFA and PUFA can’t be directly deposited in muscle due to the biohydrogenation of the rumen, and a portion of unsaturated fatty acids (UFA) become saturated [40]. The concentrations of MUFA and PUFA were greater (P<0.05) in the HE group than in the LE group, which might be influenced by two factors: On one hand, the diets contained different amounts of dried distillers grains with solubles (DDGS), and a previous study found that high-fat DDGS is a good source of UFA and to some extent, protects the UFA from ruminal biohydrogenation [41]. On the other hand, high dietary energy level may affect the normal function of rumen microbes associated with biohydrogenation, allowing more UFA to reach the small intestine, where they can be absorbed by the intestinal epithelium and deposited in muscle [42]. There were small amounts of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in yak muscle, as described by Qin et al.; however, EPA and DHA were not detected in finishing cattle [22, 43]. To some degree, yak meat is more nutritious than cattle, as EPA and DHA contribute to reduced serum levels of fat and cholesterol and improved brain function [44]. In the HE condition, the SFA content was higher than those of MUFA and PUFA, and the meat therefore does not meet human nutritional needs. Therefore, future research should focus on developing yak meat with higher MUFA and PUFA levels and lower SFA levels.
IMF deposition is the result of a balance between dietary energy content and animal’s maintenance requirements. The net energy for gain (NEg) provided in the three diets was 5.5 MJ/kg, 6.2 MJ/kg and 6.9 MJ/kg. As a result, the high-energy diet had the highest IMF content. It is possible that more available substrate (glucose or acetate) involved in fat synthesis resulted in a higher degree of intramuscular fat deposition [45]. Additionally, the fatty acids profile of bovines is not strongly affected by diet because most dietary unsaturated fatty acids are saturated by microbial biohydrogenation in the rumen [46, 47]. Our results differed because higher-energy diets were provided. Fatty acid composition may be regulated by genes involved in lipid synthesis and fatty acid metabolism. Nevertheless, no studies have evaluated in detail the molecular mechanism underlying changes in fatty acid composition in finishing yaks. We found that expression of lipogenic and fatty acid metabolic genes affect fatty acid concentrations. Therefore, more attention should be paid to transcription factors and other key proteins related to lipid metabolism in the longissimus dorsi muscle.
The process of IMF deposition can be regulated by diets (including different energy or protein levels) through the potentially complex regulation mechanism. PPARγ, which belongs to the nuclear receptor superfamily, plays a vital role in regulating the expression of some genes encoding proteins involved in fat accumulation and adipocyte differentiation in adipose and muscle tissue. It has been reported that PPARγ was identified as an important candidate gene which has positive role in the several lipogenesis-related pathways of intramuscular fat using protein-protein interaction networks [48]. In detail, PPARγ promotes the expression of some adipocyte proteins or enzymes, such as fatty acid binding protein (FABP4), fatty acid synthase (FASN) and lipoprotein lipase (LPL) [49]. Few studies examined the effects of dietary energy levels on PPARγ gene expression in longissimus dorsi muscle of yak. We observed that the mRNA expression levels of PPARγ, FABP4 and FASN were increased with increasing IMF content in the HE diets as compared to LE diet, but no expression changes occurred for the LPL gene. The result was in line with a previous study where the expressions of PPARγ, FABP4 and FASN were activated with an increase in IMF content in Angus×Simmental cattle fed high-starch or low-starch diets [50], and in vitro study also found that bovine perimuscular pre-adipocytes cultured with insulin and dexamethasone to stimulate the differentiation had greater mRNA expression of PPARγ, FABP4 and FASN compared with control [51]. It was expected that the expression of PPARγ was positively (P<0.05) correlated with the expression of FABP4 and FASN as well as IMF content, however, had no correlation with LPL in our study. Interestingly, the expression of LPL was not significantly (P>0.05) influenced by dietary energy content in this study, indicating that the rate of lipogenesis was greater than that of lipolysis due to adequate energy intake and that little triglyceride was hydrolyzed into non-esterified fatty acids in the muscle to provide energy for the peripheral tissues. In brief, our results indicated that the IMF deposition in yaks fed high dietary energy was attributed to increased expression of PPARγ, FABP4 and FASN which accelerate the rate of lipogenesis and low expression level of LPL during the finishing stage.
Peroxisome proliferator-activated receptors (PPARs), which belong to the nuclear receptor superfamily, are responsible for nutrient metabolism and energy homeostasis [52, 53]. All isoforms of PPARs can be activated or inhibited by the agonists (long-chain fatty acids or chemically associated derivatives) binding in the area of nutrition stems, and C16:0, C18:0, C18:1, C18:2 and C20:5n3 can indirectly activate PPARγ [54]. Long-chain fatty acids (LCFAs) such as C18:1 and C18:2, provided ligand to activate PPARγ and increased its mRNA expression level, can stimulate porcine adipocyte differentiation [55]. The significant positive correlations were found between the mRNA expression of PPARγ and the concentrations of C16:0, C18:2, C20:3n3, C20:4n6, C20:5n3 and C22:6n3 in our study. We speculated that these LCFAs activate the PPARγ to up-regulate its downstream genes and expedite the process of lipogenesis in intramuscular fat. FABP4 is a carrier protein binding intracellular LCFAs into nucleus to activate peroxisome proliferator-activated receptors. The mRNA expression level of FABP4 positively correlated with C16:0, C20:3n3 and C20:4n6 may be due to its greater affinity for these LCFAs.
It is well known that FASN produces a multipurpose enzyme that catalyzes the entire pathway of palmitate synthesis from malonyl-CoA [56]. In agreement with our results, Saburi et al [57] observed that FASN affected the synthesis of C14:0, C16:0, C16:1, C18:0, C18:1 and C18:2 as well as the ratio of monounsaturated to saturated fatty acids in Japanese black cattle and its TW haplotype enhanced C18:0, C18:1 content and decreased C14:0, C14:1, C16:0 and C16:1 content. The ‘AA’ genotype of the FASN SNP was dramatically related to higher concentrations of C14:0, C14:1, C16:1 and C18:2, but lower concentrations of 18:1n9c and C20:3n6 in Canadian commercial cross-bred beef steers [58]. However, we found the mRNA expression of FASN was positively (P<0.05) correlated with the content of large proportion of fatty acids which detected in current study. It suggested that the mechanism of FASN to regulate fatty acid synthesis may be influenced by different diets and genotypes or by the regulation of transcription factors (PPARγ and SREBP-1c).
In IMF accumulation, SREBP-1c is a critical transcription factor that regulates the expression of lipogenic genes (ACACA, FASN and SCD), and positive (P<0.05) correlations were found among these genes in current study (Table 4). The expression of FASN, ACACA, SREBP-1c and SCD was positively (P<0.05) correlated with IMF content, in agreement with Jeong and Kwon [59], who observed that expression of ACACA and FASN were significantly positively correlated (P<0.05) with IMF content and that higher IMF content up-regulated the expression of FASN, ACACA, SREBP-1c and SCD [37]. By contrast, no clear correlation between these genes and the IMF content of beef cattle fed soybeans or rumen-protected fat [17] may be due to higher dietary energy up-regulating the expression of genes related to lipid metabolism to enhance the rate of fat synthesis in our study. Acetyl CoA carboxylase (ACACA), a crucial rate-limiting enzyme in the mammalian cytosol, catalyzes the first step in de novo fatty-acid synthesis in the short term [60], resulting in the biosynthesis of long-chain fatty acids, principally palmitic acid. In this study, C16:0, C18:1n9t and C20:4n6 were positively associated with ACACA, but ACACA was not positively associated with C16:0 or other long-chain fatty acids in beef cattle fed high- or low-silage diets [61]. Two closely synonymous mutations in the ACACA gene significantly affected the polyunsaturated/saturated fatty acid ratio in the meat [62]. Therefore, the effect of ACACA on fatty acid composition may have been not evident because of single-nucleotide polymorphisms. Stearoyl-CoA desaturase (SCD) is the rate-limiting enzyme and catalyzes biosynthesis of MUFA from SFA, as well as conjugated linoleic acid from vaccenic acid. SCD increased the concentrations of C16:1 and C18:1 via C16:0 and C18:0 as substrates [63], which is similar to our results. However, SCD was negatively correlated with α-linolenic and arachidonic acid (C20:4n6) concentrations in beef cattle, and feeding linolenic acid (C18:2n6c), eicosapentaenoic acid (C20:5n3), and docosahexaenoic acid (C22:6n3) reduced SCD expression in rats by 50% [17, 64]. The results of this study, in which the expression of SCD was positively correlated with levels of C18:2n6c, C20:4n6 and C20:1, indicate that gene expression and fatty acids content might be primarily affected by dietary energy. SREBP-1c affects fatty acid composition through binding the sterol-regulatory-element sequences in the SCD promoter region [65], but the positive correlation between the expression of SREBP-1c and C20:4n6 levels may also be attributed to the diet. This suggested that the increased IMF content by high dietary energy level probably is due to increased expression of SREBP-1c, FASN, ACACA and SCD, and the above genes may further regulate the biosynthesis of fatty acids.
Conclusions
The present study investigated the impact of dietary energy on the IMF deposition, partial fatty acid content and the mRNA expression of lipogenic genes in the longissimus dorsi muscle during the experimental period in detail. The results indicated that the HE diets promoted the deposition and partial fatty acid content of longissimus dorsi muscle mainly by up-regulation of mRNA expression of ACACA, SCD, FASN, SREBP-1c, PPARγ and FABP4. The data might be used to manipulate or provide useful information to improve IMF deposition and fatty acids accumulation in muscle.
Supporting information
(PDF)
(DOCX)
Acknowledgments
The authors thank Animal Husbandry Sciences Institute of Tibetan Autonomous Prefecture of Gannan for their help, and we are also grateful to the staff of Hongtu Specialized Yak Breeding Cooperative for their assistance in sample collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by the International cooperation and exchange program of the National Natural Science Foundation of China (NO.314691143020) and International cooperation projects in Gansu province (No.1504WKCA053) to XD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ding XZ, Guo X, Yan P, Liang CN, Bao PJ, Chu M. Seasonal and nutrients intake regulation of lipoprotein lipase (LPL) activity in grazing yak (Bos grunniens) in the Alpine Regions around Qinghai Lake. Livest Sci. 2012;143(1):29–34. doi: 10.1016/j.livsci.2011.08.004 [Google Scholar]
- 2.Zhong CL, Kang JP, Stewart GS, Zhou JW, Huang XD, Mi JD, et al. Comparison of aquaporin-1 expression between yak (Bos grunniens) and indigenous cattle (Bos taurus) in the Qinghai-Tibetan Plateau. Anim Prod Sci. 2016. doi: 10.1071/an15702 [Google Scholar]
- 3.Long RJ, Ding LM, Shang ZH, Guo XH. The yak grazing system on the Qinghai-Tibetan plateau and its status. Rangeland J. 2008;30(2):241 doi: 10.1071/rj08012 [Google Scholar]
- 4.Qiu Q, Zhang G, Ma T, Qian W, Wang J, Ye Z, et al. The yak genome and adaptation to life at high altitude. Nat Genet. 2012;44(8):946–9. doi: 10.1038/ng.2343 . [DOI] [PubMed] [Google Scholar]
- 5.Gerbens F, Verburg FJ, Van Moerkerk HTB, Engel B, Buist W, Veerkamp JH, et al. Associations of heart and adipocyte fatty acid-binding protein gene expression with intramuscular fat content in pigs. J Anim Sci. 2001;79:347–54. [DOI] [PubMed] [Google Scholar]
- 6.Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, et al. The biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87(4):1218–46. doi: 10.2527/jas.2008-1427 [DOI] [PubMed] [Google Scholar]
- 7.Qin W, Liang CN, Guo X, Chu M, Pei J, Bao PJ, et al. PPARα signal pathway gene expression is associated with fatty acid content in yak and cattle longissimus dorsi muscle. Genet Mol Res. 2015;14(4):14469–78. doi: 10.4238/2015.November.18.9 [DOI] [PubMed] [Google Scholar]
- 8.Fernandez X, Monin G, Talmant A, Mourot J, Lebret B. Influence of intramuscular fat content on the quality of pig meat—1. Composition of the lipid fraction and sensory characteristics of m. longissimus lumborum. Meat Sci. 1999;53(1):59–65. [DOI] [PubMed] [Google Scholar]
- 9.Bednárová A, Mocák J, Gössler W, Velik M, Kaufmann J, Staruch L. Effect of animal age and gender on fatty acid and elemental composition in Austrian beef applicable for authentication purposes. Chem Pap. 2012;67(3):274–83. [Google Scholar]
- 10.Enser M, Hallett K, Hewitt B, Fursey GA, Wood JD. Fatty acid content and composition of english beef, lamb and pork at retail. Meat Sci. 1996;42(4):443–56. [DOI] [PubMed] [Google Scholar]
- 11.Katsumata M, Kobayashi SI, Matsumoto M, Tsuneishi E, Kaji Y. Reduced intake of dietary lysine promotes accumulation of intramuscular fat in the Longissimus dorsi muscles of finishing gilts. Anim Sci J. 2005;76(3):237–44. [Google Scholar]
- 12.Da CN, Mcgillivray C, Bai Q, Wood JD, Evans G, Chang KC. Restriction of dietary energy and protein induces molecular changes in young porcine skeletal muscles. J Nutr. 2004;134(9):2191–9. [DOI] [PubMed] [Google Scholar]
- 13.Gondret F, Lebret B. Feeding intensity and dietary protein level affect adipocyte cellularity and lipogenic capacity of muscle homogenates in growing pigs, without modification of the expression of sterol regulatory element binding protein. J Anim Sci. 2002;80(12):3184–93. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, DongXianwen, Wang Z, Zhou A, Peng Q, Zou H, et al. Dietary conjugated linoleic acids increase intramuscular fat deposition and decrease subcutaneous fat deposition in Yellow Breed × Simmental cattle. Anim Scie J. 2015. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Dong X, Wang Z, Zhou A, Peng Q, Zou H, et al. Dietary conjugated linoleic acids increase intramuscular fat deposition and decrease subcutaneous fat deposition in Yellow Breed × Simmental cattle. Anim Sci J. 2015;87(4):517–24. doi: 10.1111/asj.12447 [DOI] [PubMed] [Google Scholar]
- 16.Jurie C, Cassarmalek I, Bonnet M, Leroux C, Bauchart D, Boulesteix P, et al. Adipocyte fatty acid-binding protein and mitochondrial enzyme activities in muscles as relevant indicators of marbling in cattle. J Anim Sci. 2007;85(10):2660–9. doi: 10.2527/jas.2006-837 [DOI] [PubMed] [Google Scholar]
- 17.Oliveira DM, Chalfun-Junior A, Chizzotti ML, Barreto HG, Coelho TC, Paiva LV, et al. Expression of genes involved in lipid metabolism in the muscle of beef cattle fed soybean or rumen-protected fat, with or without monensin supplementation. J Anim Sci. 2014;92(12):5426–36. doi: 10.2527/jas.2014-7855 . [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Teran-Garcia M, Park JH, Nakamura MT, Clarke SD. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J Biol Chem. 2001;276(13):9800–7. doi: 10.1074/jbc.M008973200 . [DOI] [PubMed] [Google Scholar]
- 19.Takahashi N, Goto T, Kusudo T, Moriyama T, Kawada T. The structures and functions of peroxisome proliferator-activated receptors (PPARs). Nippon Rinsho Jpn J Clin Med. 2005;63(4):557–64. [PubMed] [Google Scholar]
- 20.Peng QH, Wang ZS, Tan C, Zhang HB, Hu YN, Zou HW. Effects of different pomace and pulp dietary energy density on growth performance and intramuscular fat deposition relating mRNA expression in beef cattle. J Food Agric Environ. 2012;10(1):404–7. [Google Scholar]
- 21.Smet SD, Webb EC, Claeys E, Uytterhaegen L, Demeyer DI. Effect of dietary energy and protein levels on fatty acid composition of intramuscular fat in double-muscled Belgian Blue bulls. Meat Sci. 2000;56(1):73–9. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Zhu Y, Wang X, He Y, Cao B. Effects of different dietary energy and protein levels and sex on growth performance,carcass characteristics and meat quality of F1 Angus x Chinese Xiangxi yellow cattle. J Anim Sci Biotechnol. 2014;5(1):485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Zhang X, Wang Z, Dong X, Tan C, Zou H, et al. Effects of dietary energy level on lipid metabolism-related gene expression in subcutaneous adipose tissue of Yellow breed × Simmental cattle. Anim Sci J. 2015;86(4):392–400. doi: 10.1111/asj.12316 [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, SLOANE STANLEY GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 25.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anall Biochem. 1978;90(1):420–6. [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 27.Vernon RG. Lipid metabolism in the adipose tissue of ruminant animals. Prog Lipid Res. 1980;19(1–2):23–106. [DOI] [PubMed] [Google Scholar]
- 28.Luccia AD, Satriani A, Barone CMA, Colatruglio P, Gigli S, Occidente M, et al. Effect of dietary energy content on the intramuscular fat depots and triglyceride composition of river buffalo meat. Meat Sci. 2003;65(4):1379–89. doi: 10.1016/S0309-1740(03)00060-3 [DOI] [PubMed] [Google Scholar]
- 29.Scheeder MRL, Casutt MM, Roulin M, Escher F, Dufey PA, Kreuzer M. Fatty acid composition, cooking loss and texture of beef patties from meat of bulls fed different fats. Meat Sci. 2001;58(3):321–8. [DOI] [PubMed] [Google Scholar]
- 30.Fiego DPL, Santoro P, Macchioni P, Leonibus ED. Influence of genetic type, live weight at slaughter and carcass fatness on fatty acid composition of subcutaneous adipose tissue of raw ham in the heavy pig. Meat Sci. 2005;69(1):107–14. doi: 10.1016/j.meatsci.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Knight TJ, Stalder KJ, Goodwin RN, Lonergan SM, Beitz DC. Effects of breed, sex, and halothane genotype on fatty acid composition of pork longissimus muscle. J Anim Sci. 2007;85(3):583–91. doi: 10.2527/jas.2006-239 [DOI] [PubMed] [Google Scholar]
- 32.Westerling DB, Hedrick HB. Fatty Acid Composition of Bovine Lipids as Influenced by Diet, Sex and Anatomical Location and Relationship to Sensory Characteristics. J Anim Sci. 1979;48(6):1343–8. [Google Scholar]
- 33.Mandell IB, Buchanansmith JG, Campbell CP. Effects of forage vs grain feeding on carcass characteristics, fatty acid composition, and beef quality in Limousin-cross steers when time on feed is controlled. J Anim Sci. 1998;76(10):2619–30. [DOI] [PubMed] [Google Scholar]
- 34.Rosa HJ, Rego OA, Silva CC, Alves SP, Alfaia CM, Prates JA, et al. Effect of corn supplementation of grass finishing of Holstein bulls on fatty acid composition of meat lipids. J Anim Sci. 2014;92(8):3701–14. doi: 10.2527/jas.2013-6982 [DOI] [PubMed] [Google Scholar]
- 35.Vlaeminck B, Fievez V, Arj C, Ajm F, Dewhurst RJ. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim Feed Sci Technol. 2007;131(4):389–417. [Google Scholar]
- 36.Pfeuffer M, Jaudszus A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv Nutr. 2016;7(4):730 doi: 10.3945/an.115.011387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Zhao SM, Song XL, Pan HB, Li WZ. Low protein diet up-regulate intramuscular lipogenic gene expression and down-regulate lipolytic gene expression in growth–finishing pigs. Livest Sci. 2012;148(1–2):119–28. [Google Scholar]
- 38.Ladeira MM, Santarosa LC, Chizzotti ML, Ramos EM, Neto ORM, Oliveira DM, et al. Fatty acid profile, color and lipid oxidation of meat from young bulls fed ground soybean or rumen protected fat with or without monensin. Meat Sci. 2014;96(1):597–605. doi: 10.1016/j.meatsci.2013.04.062 [DOI] [PubMed] [Google Scholar]
- 39.Renaville B. Eicosapentaenoic acid and 3,10 dithia stearic acid inhibit the desaturation of trans-vaccenic acid into cis-9, trans-11-conjugated linoleic acid through different pathways in Caco-2 and T84 cells. Br J Nutr. 2006;95(4):688–95. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery SP, Drouillard JS, Nagaraja TG, Titgemeyer EC, Sindt JJ. Effects of supplemental fat source on nutrient digestion and ruminal fermentation in steers. J Anim Sci. 2008;86(3):640–50. doi: 10.2527/jas.2006-812 [DOI] [PubMed] [Google Scholar]
- 41.Pol KJV, Luebbe MK, Crawford GI, Erickson GE, Klopfenstein TJ. Performance and digestibility characteristics of finishing diets containing distillers grains, composites of corn processing coproducts, or supplemental corn oil. J Anim Sci. 2009;87(2):639–52. doi: 10.2527/jas.2008-1036 [DOI] [PubMed] [Google Scholar]
- 42.Beam TM, Jenkins TC, Moate PJ, Kohn RA, Palmquist DL. Effects of Amount and Source of Fat on the Rates of Lipolysis and Biohydrogenation of Fatty Acids in Ruminal Contents. J Dairy Sci. 2000;83(11):2564–73. doi: 10.3168/jds.S0022-0302(00)75149-6 [DOI] [PubMed] [Google Scholar]
- 43.Oliveira DM, Ladeira MM, Chizzotti ML, Machado Neto OR, Ramos EM, Gonçalves TM, et al. Fatty acid profile and qualitative characteristics of meat from zebu steers fed with different oilseeds. J Anim Sci. 2011;89(8):2546–55. doi: 10.2527/jas.2010-3553 [DOI] [PubMed] [Google Scholar]
- 44.Mcmanus S, Tejera N, Awwad K, Vauzour D, Rigby N, Fleming I, et al. Differential effects of EPA versus DHA on postprandial vascular function and the plasma oxylipin profile in men. J Lipid Res. 2016;57(9):1720–7. doi: 10.1194/jlr.M067801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert CD, Lunt DK, Miller RK, Smith SB. Carcass, sensory, and adipose tissue traits of Brangus steers fed casein-formaldehyde-protected starch and/or canola lipid. J Anim Sci. 2003;81(10):2457–68. doi: 10.2527/2003.81102457x [DOI] [PubMed] [Google Scholar]
- 46.Jenkins T. C. Lipid metabolism in the rumen. J Dairy Sci. 1993;76(12):205–19. [DOI] [PubMed] [Google Scholar]
- 47.Cabezas MT, Hentoes JF, Moore JE, Olson JA. Effect of diet on fatty acid composition of body fat in steers. J Anim Sci. 1965;24(1):57–61. [DOI] [PubMed] [Google Scholar]
- 48.Lim D, Kim NK, Park HS, Lee SH, Cho YM, Oh SJ, et al. Identification of Candidate Genes related to Bovine Marbling using Protein-Protein Interaction Networks. Int J Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boone C, Mourot J, Grégoire F, Remacle C. The adipose conversion process: regulation by extracellular and intracellular factors. Reprod Nutr Dev. 2000;40(4):325 [DOI] [PubMed] [Google Scholar]
- 50.Graugnard DE, Piantoni P, Bionaz M, Berger LL, Faulkner DB, Loor JJ. Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post-weaning growth in Angus and Angus × Simmental cattle fed high-starch or low-starch diets. BMC Genet. 2009;10(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taniguchi M, Guan LL, Zhang B, Dodson MV, Okine E, Moore SS. Adipogenesis of bovine perimuscular preadipocytes. Biochem Biophys Res Commun. 2008;366(1):54–9. doi: 10.1016/j.bbrc.2007.11.110 [DOI] [PubMed] [Google Scholar]
- 52.König B, Koch A, Spielmann J, Hilgenfeld C, Hirche F, Stangl GI, et al. Activation of PPARα and PPARγ reduces triacylglycerol synthesis in rat hepatoma cells by reduction of nuclear SREBP-1. Eur J Pharmacol. 2009;605(1–3):23–30. doi: 10.1016/j.ejphar.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 53.Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61(4):393–416. doi: 10.1007/s00018-003-3216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadegowda AKG, Bionaz M, Piperova LS, Erdman RA, Loor JJ. Peroxisome proliferator-activated receptor-γ activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J Dairy Sci. 2009;92(9):4276 doi: 10.3168/jds.2008-1932 [DOI] [PubMed] [Google Scholar]
- 55.Ding ST, Wang JC, Mersmann HJ. Effect of unsaturated fatty acids on porcine adipocyte differentiation. Nutr Res. 2003;23(8):1059–69. [Google Scholar]
- 56.Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res. 2003;42(4):289–317. [DOI] [PubMed] [Google Scholar]
- 57.Abe T, Saburi J, Hasebe H, Nakagawa T, Misumi S, Nade T, et al. Novel mutations of the FASN gene and their effect on fatty acid composition in Japanese Black beef. Biochem Genet. 2009;47(5):397–411. [DOI] [PubMed] [Google Scholar]
- 58.Li C NA, Vinsky M, Dugan MER, Mcallister TA. Association analyses of single nucleotide polymorphisms in bovine stearoyl-CoA desaturase and fatty acid synthase genes with fatty acid composition in commercial cross-bred beef steers. Anim Genet. 2012;43(1):93–7. doi: 10.1111/j.1365-2052.2011.02217.x [DOI] [PubMed] [Google Scholar]
- 59.Jeong J, Kwon EG, Im SK, Seo KS, Baik M. Expression of fat deposition and fat removal genes is associated with intramuscular fat content in longissimus dorsi muscle of Korean cattle steers. J Anim Sci. 2012;90(6):2044–53. doi: 10.2527/jas.2011-4753 [DOI] [PubMed] [Google Scholar]
- 60.Jeukendrup AE. Regulation of Fat Metabolism in Skeletal Muscle. Ann N Y Acad Sci. 2002;967(1):217–35. [DOI] [PubMed] [Google Scholar]
- 61.Costa ASHD, Pires VMR, Prates JAM. Expression of genes controlling fat deposition in two genetically diverse beef cattle breeds fed high or low silage diets. BMC Vet Res. 2013;9(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumoto H, Sasaki K, Bessho T, Kobayashi E, Abe T, Sasazaki S, et al. The SNPs in the ACACA gene are effective on fatty acid composition in Holstein milk. Mol biol Rep. 2012;39(9):8637–44. doi: 10.1007/s11033-012-1718-5 [DOI] [PubMed] [Google Scholar]
- 63.Yao D, Luo J, He Q, Shi H, Li J, Wang H, et al. SCD1 Alters Long-Chain Fatty Acid (LCFA) Composition and Its Expression Is Directly Regulated by SREBP-1 and PPARgamma 1 in Dairy Goat Mammary Cells. J cell physiol. 2016. doi: 10.1002/jcp.25469 . [DOI] [PubMed] [Google Scholar]
- 64.Bellinger L. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92(3):513–20. [DOI] [PubMed] [Google Scholar]
- 65.Strange H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res 2001;40(6):439–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.