Abstract
A series of wound membranes of polyvinyl alcohol and recombinant spider silk protein (pNSR16) was prepared by electrospinning. The membrane was analyzed by scanning electron microscopy, atomic force microscopy, and Fourier transform infrared spectroscopy. The result showed that the three factors that affected average fiber diameter from high to low were, voltage, flow speed, and solidification distance; and the three factors that affected fiber uniformity from high to low were, flow speed, solidification distance, and voltage. The fibers adhered together after being dealt with alcohol. pNSR16 transformed from random coil into β-sheet after being immersed in alcohol. Additionally, the porosity of the electrospun membrane was 84.85%, which was higher than that of cast membrane prepared with the same composition. Experiments of applying electrospun membranes as wound dressing for Sprague Dawley rat wound healing showed that it could promote wound healing and basic fibroblast growth factor expression.
Keywords: recombinant spider silk protein, polyvinyl alcohol, electrospinning, characterization, wound dressing
Introduction
Biomaterial, applied as a human tissue substitute, plays an important role in tissue engineering. With increased surface and porosity rate for cell attachment, nano-materials have gained more interest as biomaterials in recent years. As nano-materials and biomaterials research progresses, nano-biomaterials have been applied in a wide range of biomedicines, such as drug delivery, wound dressing, and tissue engineering of tendon and ligament.1–3
Trauma is common in clinics since skin is the first barrier for humans. In the last few decades, various medical materials such as ointments and wound dressings have been developed for treating severe skin wounds or ulcers including bedsores and burn wounds. Beyond providing physical protection and an optimal moisture environment for the wound, new types of wound dressing also perform many other functions for wound healing like recent advances in the understanding of the etiology, pathogenesis, and microbiology of various wounds.4 Furthermore, to improve the poor biological performances and mechanical characteristics of wound dressings made of synthetic polymers, a new class of specifically designed material, bioartificial polymeric material, has been introduced.5 As a result, there have been many studies on improving the properties of wound dressings,6 but very few on wound dressings which contain cytokine.
Electrospinning is a simple and effective nanofabrication method for preparing nanofibers with diameters ranging from 5 to 500 nm, 102–104 times smaller than the fibers prepared by traditional methods of solution or melt spinning.7 Non-woven membrane prepared by electrospinning can be used as sensors, wound dressings, drug control release carriers, and tissue engineering scaffolds.8 Wound dressing from electrospun nanofibrous membrane (NFM) has many advantages over conventional processes. With its large surface area and microporous structure, NFM could quickly start signaling pathway and attract fibroblasts to the dermal layer, which can excrete important extracellular matrix components, like several cytokines, to repair damaged tissue. The electrospun membrane is also important for cell attachment and proliferation in wound healing.9
Spider silk is a natural polymer made up of repeated amino acid motifs that are composed of fibroin proteins. Spider silk has attracted the attention of the scientific community mainly due to its excellent mechanical and biochemical properties (such as biocompatibility and biodegradability).10 These features have aroused great interest in cloning and expressing the materials as well as mimicking these properties in polymeric systems. Recombinant spider silk protein has broad prospects in tissue repair, nerve damage repair, artificial joints, artificial tendons, and artificial ligaments.11 In previous studies, a recombinant spider silk protein, including RGD cell-binding domain, was cloned and expressed by our laboratory staff, and it was termed pNSR16.12–14 Polyvinyl alcohol (PVA) is a water-soluble synthetic polymer with excellent membrane forming property. It has been widely applied in biomedicine such as artificial cornea, medicinal sponges, and artificial cartilage.15
In this study, pNSR16/PVA electrospinning membranes were used as dressing for curing Sprague Dawley (SD) rat skin burns. The aim was to test the applicability of the pNSR16/PVA membrane as the wound dressing. There is one advantage in using this membrane as wound dressing. RGD contained in pNSR16 could be released slowly from the membrane during the healing process, which could accelerate wound healing.
Materials and methods
Materials
pNSR16 was prepared in our laboratory.14 PVA was bought from Daicel Chemical Reagent Company (Osaka, Japan). Medical collagen sponge was purchased from Qisheng Bio Company (Shanghai, People’s Republic of China). Gauze was bought from the Yuhua Biochemical Company (Fuzhou, People’s Republic of China). The kit for hydroxyproline (Hyp) detection was purchased from Jiancheng Bio Technology Co Ltd (Nanjing, People’s Republic of China), and that for basic fibroblast growth factor (bFGF) detection was bought from Zhongshan Golden Bridge Biotechnology Co Ltd (Beijing, People’s Republic of China). Other materials were purchased from Fuchen Chemical Reagent Company (Tianjin, People’s Republic of China).
Preparation of pNSR16/PVA membrane
Acidic solution (98% formic acid) of pNSR16 and PVA was prepared with total polymer concentrations fixed at 15% (w/w). The pNSR16/PVA mass ratio was 1/1. By using the orthogonal design experiment, three factors including voltage, extrusion speed, and reception distance which affected the characteristics of the electrospinning fiber structure were selected as influence factors to optimize process conditions of preparing composite nanofibers electrospun pNSR16/PVA. Each factor took three levels, according to the L18 (35) orthogonal experimental design for three factors and three levels; the selected factors and levels are shown in Table 1. In the electrospinning process, a high electric potential was applied onto the syringe, while grounded. When the electric force from the applied field became stronger than the surface tension of the droplet, a charged jet of the solution was formed and ejected. The nonwoven mats were collected on a collecting plate (stannum foil). The internal diameter of the syringe tip was 0.6 mm. The electrospinning process was carried out at 45°C.
Table 1.
Factors and levels of orthogonal test
| Levels | Factors
|
||
|---|---|---|---|
| Voltage (kV) | Flow speed (mL/h) | Solidification distance (cm) | |
| 1 | 80 | 3 | 18 |
| 2 | 90 | 5 | 20 |
| 3 | 100 | 7 | 22 |
Characterizations
The morphology of the pNSR16/PVA membrane was examined with a KYKY AMRAY-1000B scanning electron microscope (SEM; Chinese Academy of Sciences Co, Beijing, People’s Republic of China) at 10 kV and 3,000 times magnification and an SPA300HV atomic force microscope (AFM; NSK Ltd, Tokyo, Japan). The average fiber diameter and uniformity coefficient were obtained by measuring 25 fibers selected randomly from the SEM images. The pH of the electrospinning membrane was tested by the following method: the membrane with an area of 5×5 cm was immersed in deinonized water for 24 hours, and then the pH of water was detected.
Fourier transform infrared spectroscopy (FTIR) spectrum of pNSR16/PVA membrane was observed with a Nicolet 5700 FTIR spectrometer (Nicolet Co, Madison, WI, USA).
The pNSR16/PVA membrane was dried in an oven at 10°C for 2 hours before its porosity was measured. The membrane was placed in a pycnometer that was filled with degassed alcohol afterwards. The membranes were taken out, and the pycnometer with the alcohol left was weighed. The porosity of the membrane was calculated as follows:
W1 referred to the weight of the pycnometer filled with alcohol, W2 referred to the weight of the pycnometer with alcohol and membranes, W3 referred to the weight of the pycnometer with the membranes taken out and alcohol left, and WS referred to the weight of the membranes in dry state.
Wound healing test
SD rats (about 250 g) were purchased from Fujian Medical University. Before the test, the rats were anesthetized with pentobarbital. After the dorsal hair was removed with a razor, 8% (m/v) sodium sulfide dissolved in 75% alcohol was used to sterilize the dorsal area. After 1 day, three full-thick wounds (2×2 cm) were created in the dorsum. The wound was covered with pNSR16/PVA membrane, with gauze and collagen sponge as the negative and positive groups, respectively. To fix the wound dressings, a piece of gauze was attached on their top. The healing wounds were observed on days 3, 5, 7, 14, 21, and 28 with a digital camera. In wound healing process, the SD rats were observed everyday. The healing degree was calculated by the remaining wound area ratio (RWAR):
A0 and At represent, respectively, the initial area and the wound area at time t. Wound tissue was dissected, fixed with 10% phosphate-buffered formalin, and stained with hematoxylin and eosin reagents for histological observations. Meanwhile, immunohistochemistry staining was carried out for bFGF in wound skins. All animal procedures were approved by the Animal Care Committee and in accordance with the regulations for the administration of affairs concerning experimental animals in Fujian Normal University; and the study was approved by the Ethics Commitee of Fujian Normal University.
Water ratio of skin
In wound healing, a microthrombus would be formed when microcirculation was blockaded by the edema. Detumescence would contribute to the regeneration of tissue. On days 3, 5, 7, 14, 21, and 28, wound tissue was dissected, weighed (W1), dried in an oven for 72 hours at 80°C, and weighed (W0). The water ratio of skin was formulated as (W1 − W0)/W1 ×100%.
Hyp content in skins
Since the percentage of Hyp in collagen is stable, we could get to know the amount of collagen in the skin according to it. With SPSS statistics software (version 11.5; SPSS Inc, Chicago, IL, USA), the data measured by an Hyp assay kit would be analyzed, and significance was determined at P<0.05.
Neogenesis capillary statistics
Granulation, young connective tissue, is composed of capillaries, fibroblast, and extracellular matrix. It is crucial for the generation of new skin tissue. Thus, the wound healing process can be evaluated by neogenesis capillary statistics. With a light microscope, the amount of neogenesis capillaries can be calculated by randomly selecting six fields from the wound tissue at every definite interval of time. With SPSS statistics software (version 11.5), the data would be analyzed and significance was determined at P<0.05.
Results and discussion
Effects of processing parameters on the morphology of electrospun fibers
The electrospinning parameters are important influence factors for physicochemical properties of vascular scaffolds. When pNSR16/PVA composite nanofibrous scaffolds are prepared by electrospinning, the fiber surface morphology and diameter change due to different blending liquid composition, concentration, voltage, solidification distance, and flow speed. The orthogonal test was used to optimize and control the microstructure of spider silk protein composite nanofibrous scaffolds, so as to get bionic extracellular matrix three-dimensional porous scaffolds with even fiber diameter (300–500 nm) and smooth surface. Specific test and results are displayed in Table 3; the diameter in the table was the average value of 25 nanofiber diameters.
Table 3.
The orthogonal experimental design and results
| Test | Voltage (kV) | Flow speed (mL/h) | Solidification distance (cm) | Average diameter (nm) | Uniformity coefficient |
|---|---|---|---|---|---|
| 1 | 80 | 3 | 18 | 270.51±14.68 | 0.054 |
| 2 | 80 | 5 | 20 | 330.46±20.75 | 0.063 |
| 3 | 80 | 7 | 22 | 380.03±15.86 | 0.042 |
| 4 | 90 | 3 | 20 | 336.27±25.23 | 0.075 |
| 5 | 90 | 5 | 22 | 444.57±18.79 | 0.042 |
| 6 | 90 | 7 | 18 | 384.49±27.37 | 0.071 |
| 7 | 100 | 3 | 22 | 427.19±31.68 | 0.074 |
| 8 | 100 | 5 | 18 | 444.38±23.69 | 0.053 |
| 9 | 100 | 7 | 20 | 468.02±29.37 | 0.063 |
| ∑K1 | 981 | 1,033.97 | 1,099.38 | Sequence of influence factors for fiber diameter A>B>C |
|
| ∑K2 | 1,165.33 | 1,219.41 | 1,134.75 | ||
| ∑K3 | 1,339.59 | 1,232.54 | 1,251.79 | ||
| K1 | 327 | 344.66 | 366.46 | ||
| K2 | 388.44 | 406.47 | 378.25 | ||
| K3 | 446.53 | 410.85 | 417.26 | ||
| Variation R | 119.73 | 66.19 | 50.8 | ||
| ∑K1 | 0.159 | 0.203 | 0.178 | Sequence of influence factors for fiber uniformity B>C>A |
|
| ∑K2 | 0.188 | 0.158 | 0.201 | ||
| ∑K3 | 0.19 | 0.176 | 0.158 | ||
| K1 | 0.053 | 0.068 | 0.059 | ||
| K2 | 0.063 | 0.053 | 0.067 | ||
| K3 | 0.063 | 0.059 | 0.053 | ||
| Variation R | 0.01 | 0.015 | 0.014 | ||
Note: A, voltage; B, flow speed; C, solidification distance.
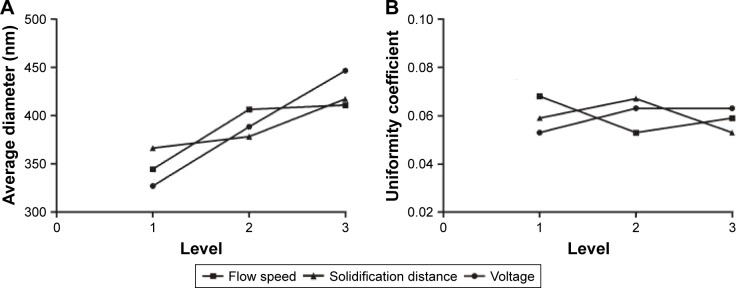
Variation R reflects the range of the experiment index when the levels of factors change. The more larger the R, the greater is the factor’s influence on the experimental index.16 The three factors that affected the average fiber diameter from high to low were voltage, flow speed, and solidification distance, and the three factors that affected fiber uniformity from high to low were flow speed, solidification distance, and voltage. The trend of average fiber diameter and uniformity coefficient affected by each factor is displayed in Figure 1.
Figure 1.
Fiber range analysis. (A) Diameter; (B) uniformity coefficient.
According to the above studies, parameters such as polymer concentration, voltage, solidification distance, and flow speed were found to play a significant role in controlling the morphology and size of pNSR16/PVA membranes. It was found that fibers would adhere together when the solvent did not evaporate completely because of low temperature, and 45°C was appropriate for the electrospinning of pNSR16/PVA. One of the crucial issues in electrospinning is how to obtain submicron scale fibers without bead. According to a study by Ma et al, with a decrease of the solution concentration, viscosity tended to decrease.17 When the concentration of the spinning solution reduced to a certain degree, however, beaded fibers would appear. The size of the fibers would shrink when the polymer concentration reduced. However, this method of obtaining finer fibers was compromised by the change of the fiber uniformity.
AFM characterization of electrospun fibers
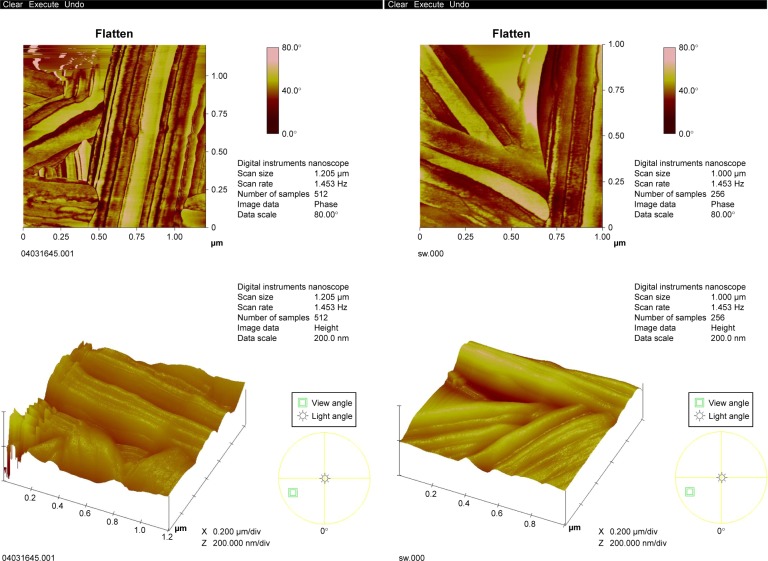
The surfaces of pNSR16/PVA electrospun fibers prepared at 80 kV, 20 cm, 5 mL/h were smooth without beads. The average fiber diameters were detected to be 200–400 nm, as demonstrated in Figure 2.
Figure 2.
AFM of pNSR16/PVA membrane.
Abbreviations: AFM, atomic force microscopy; PVA, polyvinyl alcohol.
Effects of alcohol on the morphology of fiber
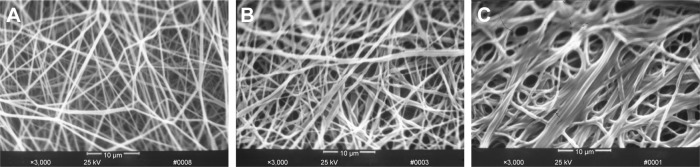
According to the current theory, after being dealt with alcohol, the mechanical property of protein fiber can be enhanced when its secondary structure changed. Figure 3 displays the SEM images of pNSR16/PVA membranes before and after being dealt with alcohol. It can be seen that the smooth electrospun fibers turned to adhere together with alcohol treatment (Figure 3B and C). This phenomenon was evident for fibers without thermal treatment before soaking in alcohol (Figure 3C). Thus, we assumed that the formic acid residue on fibers would evaporate completely after thermal treatment. Without this step, the remaining formic acid made the surface of fibers dissolve again and adhere together.
Figure 3.
SEM images of pNSR16/PVA membrane with/without alcohol treatment (3,000×). (A) Without alcohol treatment; (B) with alcohol treatment after thermal treatment (80°C, 3 hours); (C) with alcohol treatment.
Abbreviations: SEM, scanning electron microscopy; PVA, polyvinyl alcohol.
Alcohol’s effects on fiber structure
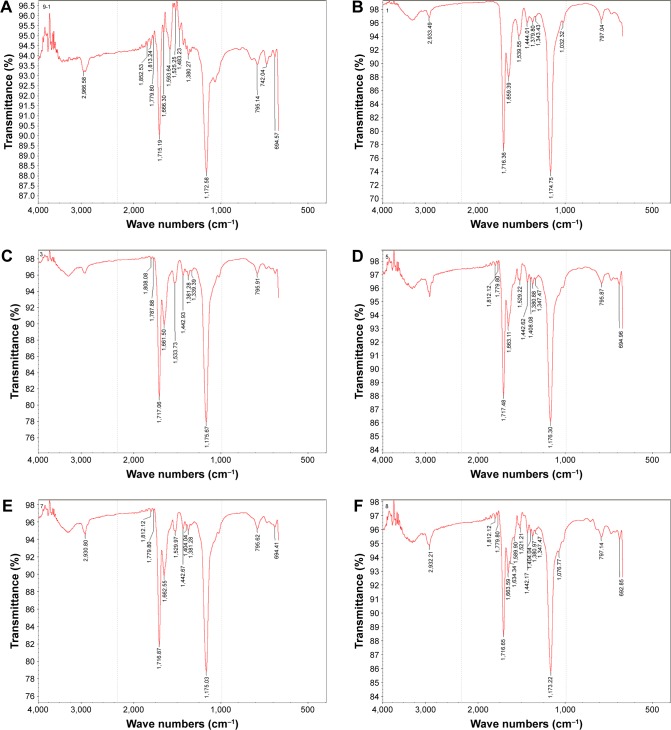
Fiber property is determined by structure and crystallinity. For proteins, there are at least three kinds of structure, that is, random coil, β-sheet, and α-helix. Since there are limited data about the FTIR spectra of spider silk protein, the FTIR resulting in this work was compared with that of the silk-worm silk protein. Table 4 shows the FTIR spectra of the typical bands of silkworm silk protein. Figure 4 shows the FTIR spectra of pNSR16/PVA membranes prepared in different conditions. Random coils showed strong absorption bands at 1,659.39 (amide I) and 1,539.55 cm−1 (amide III). After the random coils were dealt with alcohol, there were significant differences in the major bands, which indicated changes in the secondary structure. The absorption peak of amide I and amide II in pNSR16 was located at 1,659.39 and 1,539.55 cm−1, respectively, without ethanol treatment. However, the absorption peaks shifted to 1,663.59 and 1,521.21 cm−1 after being treated with ethanol, which showed that the molecular conformation of pNSR16 was transformed from random coil to β-sheet after being treated with ethanol. The absorption peaks of membranes prepared in different electrospinning conditions remained the same.
Table 4.
FTIR characteristic spectrum bands of silkworm silk (cm−1)
| Structure | Amide I, cm−1 | Amide II, cm−1 | Amide III, cm−1 | Amide IV, cm−1 |
|---|---|---|---|---|
| β-Sheet | 1,625–1,640 | 1,515–1,525 | 1,265 | 700 |
| Random coil | 1,650–1,660 | 1,535–1,545 | 1,235 | 650 |
| α-Helix | 1,650 | 1,545 | 1,240 | 600 |
Abbreviation: FTIR, Fourier transform infrared spectroscopy.
Figure 4.
FTIR spectra of pNSR16/PVA membrane with/without alcohol treatment. (A) PVA, 80 kV, 20 cm, 5 mL/h. (B) 15% pNSR16/PVA, 80 kV, 20 cm, 5 mL/h. (C) 15% pNSR16/PVA, 100 kV, 20 cm, 5 mL/h. (D) 15% pNSR16/PVA, 100 kV, 22 cm, 5 mL/h. (E) 15% pNSR16/PVA, 80 kV, 20 cm, 7 mL/h. (F) 15% pNSR16/PVA, 80 kV, 20 cm, 5 mL/h, alcohol treatment.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; PVA, polyvinyl alcohol.
Other properties of pNSR16/PVA electrospun membrane
The structure of the scaffold is helpful for cell attachment, proliferation, and differentiation. Tissue scaffolds should have excellent mechanical properties, such as morphologic flexibility, optimal pore size, and porosity. Previous work by Li et al showed that only scaffolds with porosity >70% can supply a sound environment for cell proliferation and metabolism.18 Our experiments showed that the porosity of the pNSR16/PVA electrospun membrane was 84.85%, higher than 65.2% of cast membrane prepared by the same composition. It is obvious that electrospinning can increase the porosity of pNSR16/PVA membrane. Therefore, a proper fluid balance on the wound was achieved so as to facilitate cellular migration and enhance reepithelialization.2 The appropriate thickness of the membrane was about 1±0.05 mm, and its pH fell between 6.5 and 7.0.
Wound healing performance
By electrospinning, membranes comprising PVA and pNSR16 were prepared at 80 kV, 20 cm, 5 mL/h. All electrospun membranes were conserved in vacuum for at least 24 hours to ensure that the formic acid vaporized completely. PVA guaranteed that blend membrane had the mechanical strength for wound dressing, and the release of RGD peptide would accelerate wound healing.
By measuring the wound area at definite intervals of time, the reduction in wound defect area was calculated. All SD rats survived throughout the postoperative period. In the group of pNSR16/PVA electrospun membranes, healing was quick and wound closure was achieved within 28 days. In contrast, it took 30 days for the positive control, and 32 days for the negative control. Figure 5 reveals that the healing of the electrospun membrane-treated wound is the fastest, with the positive control group coming second, and the negative control group being the last. RWAR of the electrospun group was the smallest, and for the negative control it was the largest, demonstrating that the wound healing ability is related to the amount of pNSR16. It was supposed that the cytokine RGD included in pNSR16 can accelerate the generation of new skin tissue for wound healing. This hypothesis was supported by the studies of Chen et al.19 It was concluded that the electrospun membranes containing pNSR16 and PVA were comparable with the medical collagen sponge.
Figure 5.
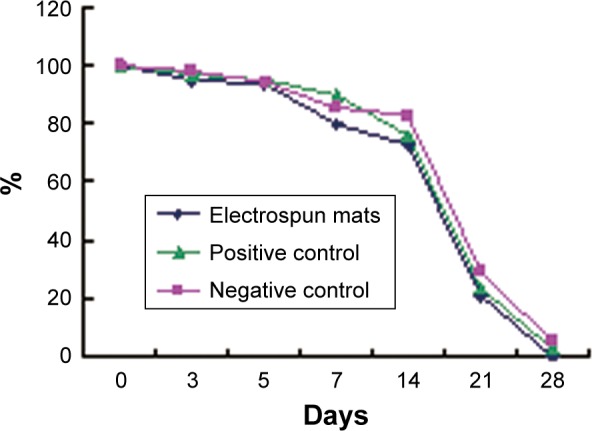
RWAR variation trend of SD rats.
Abbreviations: SD, Sprague Dawley; RWAR, remaining wound area ratio.
Water absorption ratio of skins
Microcirculation will be oppressed by tissue edema after skin scald, forming microthrombi. Edema regression contributes to skin repair. The water ratio in wound skin increased after being burned in all groups. The details are provided in Table 5. At day 3, the water ratios of skins in negative control and electrospinning group were significantly different from that of the normal group. The water ratio of the electrospinning group was significantly different from that of the positive control. Medical collagen sponge (positive group) as a wound dressing could promote fast fading of edema, while the experimental group still demonstrated the phenomenon of edema on day 3 compared with the normal group, and the edema phenomenon of the negative group was more obvious. The collagen sponge group has the best absorption capacity, the gauze group takes the second place, and the electrospun membrane was the worst. We supposed that the hydrophobicity of recombinant spider silk protein contributed to this.
Table 5.
Statistical analysis for water ratio of SD rat skin
| Groups | 3 Days, % | 7 Days, % | 14 Days, % | 21 Days, % | 28 Days, % |
|---|---|---|---|---|---|
| Negative | 70.88±0.57** | 66.52±2.76 | 65.29±1.16 | 65.85±1.00 | 65.44±3.10 |
| Positive | 67.70±0.41ΔΔ | 64.96±3.15 | 65.86±2.34 | 65.43±0.25 | 65.62±1.96 |
| Electrospun | 69.15±0.78Δ,☆,* | 65.15±3.10 | 65.49±1.84 | 66.24±0.69 | 65.26±0.86 |
Notes: Water ratio of normal SD rat skin: 64.89%±2.07%; compared with normal group,
P<0.05,
P<0.01; compared with negative group,
P<0.05,
P<0.01; compared with positive group,
P<0.05.
Abbreviation: SD, Sprague Dawley.
Hyp content in SD rats skin
Collagen, the principal structural element of the native extracellular matrix, exists in a three-dimensional network structure. Hyp, seldom appearing in other proteins, is the major and relatively constant amino acid in collagen. Through the determination of Hyp, the content of collagen could be detected so as to evaluate the wound healing ability.20 As shown in Figure 6 and Table 2, Hyp content of skin in the electrospinning group was higher than that in the negative control on days 3, 5, 7, and 14, which were different (P<0.05) or significantly different (P<0.01) in statistics. At 5 days, the Hyp content in skin approached the normal level in the electrospinning group. However, it took as long as 21 days for the negative group. It was evident that fibroblast proliferation and collagen formation were quicker in the electrospinning group than in the negative control. It was supposed that fibroblast proliferation would speed up due to the promotion by RGD contained in pNSR16/PVA membranes.
Figure 6.
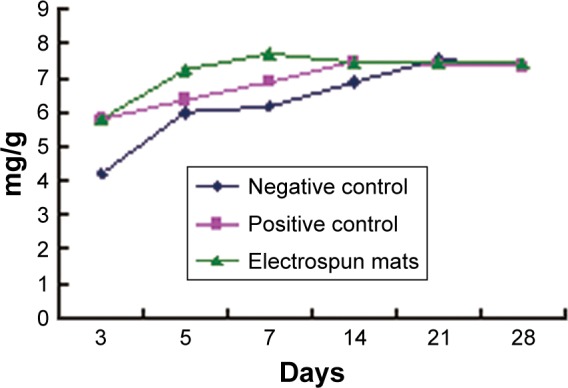
Hyp content of SD rat skin.
Abbreviations: SD, Sprague Dawley; Hyp, hydroxyproline.
Table 2.
Statistical analysis for Hyp content of SD rat skin
| Group | 3 Days, μg/mg | 5 Days, μg/mg | 7 Days, μg/mg | 14 Days, μg/mg | 21 Days, μg/mg | 28 Days, μg/mg |
|---|---|---|---|---|---|---|
| Negative | 4.21±0.60** | 5.98±0.14** | 6.13±0.11** | 6.88±0.06** | 7.54±0.10 | 7.35±0.16 |
| Positive | 5.81±0.33** | 6.36±0.23** | 6.89±0.81 | 7.48±0.05ΔΔ | 7.42±0.10 | 7.35±0.12 |
| Electrospun | 5.80±0.24**,Δ | 7.24±0.79ΔΔ | 7.73±0.29ΔΔ | 7.43±0.10ΔΔ | 7.45±0.06 | 7.45±0.07 |
Notes: The Hyp content of skins in normal SD rats is 7.44±0.06 μg/mg.
P<0.01 compared with normal control.
P<0.05,
P<0.01 compared with negative control (N=9).
Abbreviations: SD, Sprague Dawley; Hyp, hydroxyproline.
Histological examination
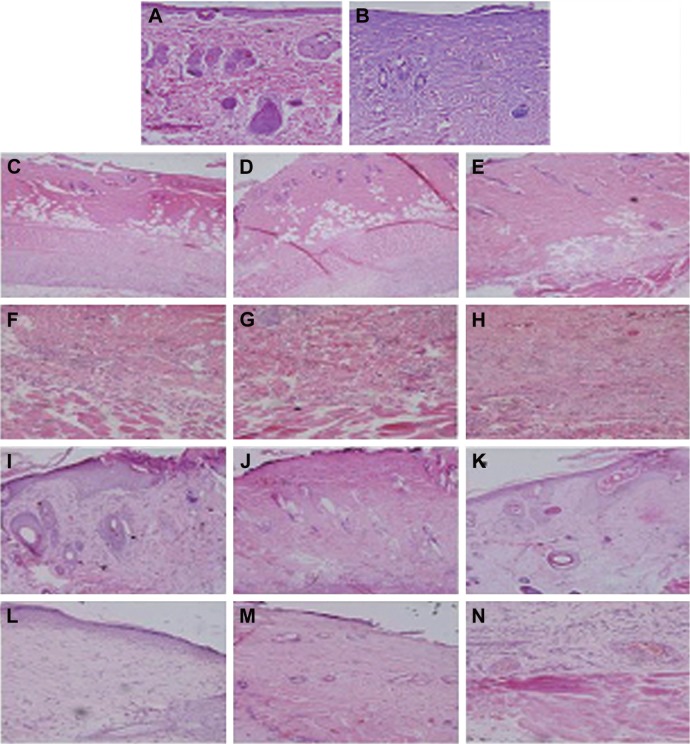
Figure 7 shows the healing process of wounds treated with gauzes, medical collagen sponges, and electrospun pNSR16/PVA membranes after 3, 5, 7, 14, 21, and 28 days, respectively. After 3 days, the wounds were in the inflammatory phase, which was essential for wound healing. There were many granulation tissues and angiogenesis was observed in dermis in the positive control and the electrospun groups. A large number of exudates and neutrophil were found in all of the three groups. After 7 days, the wound evolved into the proliferation phase. The capillaries disappeared and were replaced by blood vessels. The neogenesis epidermis proliferated from the edge of the wound. After 14 days, inflammatory cells, granulation tissues, and blood vessels disappeared. At the same time, gauze-treated wounds showed inflammation in the new epidermis and lightly on the dermis. Twenty-one days later, many kinds of glandular organs, such as hair follicle and sebaceous follicles, regenerated. The wounds except those treated by gauze were almost covered with a layer of epidermis at 4 weeks. In addition, cicatrix was formed in the wounds treated with medical collagen sponge. In conclusion, the electrospinning membrane group and the positive control group show positive effect in comparison with the negative control. It is supposed that PVA is the biodegradable and nontoxic polymer and the RGD contained in pNSR16 was helpful for tissue regeneration in wound healing.
Figure 7.
HE staining of SD rat skin. (A) SD rat skin (200×); (B) SD rat skin with wound (200×); (C) 3 days, negative control (100×); (D) 3 days, positive control (100×); (E) 3 days, electrospun group (100×); (F) 5 days, negative control (200×); (G) 5 days, positive control (200×); (H) 5 days, electrospun group (200×); (I) 7 days, negative control (100×); (J) 7 days, positive control (100×); (K) 7 days, electrospun group (100×); (L) 14 days, negative control (200×); (M) 14 days, positive control (200×); (N) 14 days, electrospun group (200×); (O) 21 days, negative control (200×); (P) 21 days, positive control (200×); (Q) 21 days, electrospun group (200×); (R) 28 days, negative control (100×); (S) 28 days, positive control (100×); (T) 28 days, electrospun group (100×).
Abbreviations: SD, Sprague Dawley; HE, hematoxylin and eosin.
Table 6 shows the changes of neogenesis capillaries in wound skins at different time points using gauze (negative control), collagen sponges (positive control), and electrospinning membranes. In 5 days, the number of neogenesis capillaries in the electrospun membrane group was significantly higher than that of the positive control group and negative control group, while the negative group could not reach the maximum amount of neogenesis capillaries before 14 days. Electrospun membranes were found to have a better performance than gauze (P<0.01) and collagen sponge (P<0.01) in promoting granulation tissue formation, although the difference with the latter was more evident. It was supposed that the scaffolds with pNSR16 could promote cell adhering, proliferation, and migration.
Table 6.
Statistical analysis for variation of micrangium in SD rat skin (n=6)
| Groups | 3 Days | 5 Days | 7 Days | 14 Days |
|---|---|---|---|---|
| Negative | 0.5±0.408 | 3.167±1.169 | 3±0.894 | 11.5±3.728 |
| Positive | 0.167±0.408 | 2.5±0.837** | 2.667±0.817 | 1.5±0.548** |
| Electrospun | 0.167±0.408 | 7±1.673**,ΔΔ | 1.5±0.548Δ | 0.667±0.817** |
Notes:
P<0.01, compared to negative group;
P<0.05,
P<0.01, compared to positive group.
Abbreviation: SD, Sprague Dawley.
Expression of bFGF in wound healing
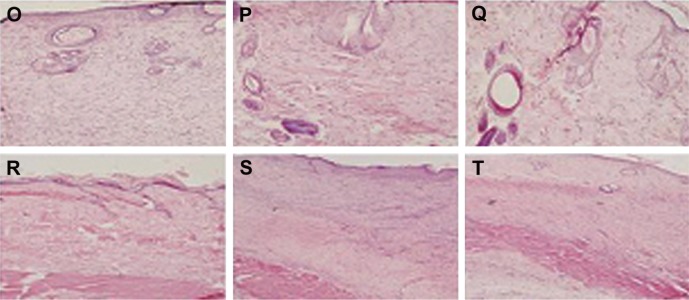
FGF family proteins, such as aFGF and bFGF, are expressed in specific areas of the skin. These proteins are suggested to mediate proliferation and differentiation of fibroblasts. They could also promote the formation of vessels, granulations, and epidermis. In immunohistochemistry experiments, the main assessment method is to observe changes of the organizational structure and bFGF expression. bFGF expression is weakly positive in normal skin tissue, but strongly positive in inflammatory cells, impaired glands, and epidermis around the wound.21 On day 5, bFGF showed strong positive expression, and the expression level of the positive control group and the experimental group was both higher than that of the negative control group. On day 7, the bFGF expression of the three groups was significantly lower than those of days 3 and 5, which reflected that the amount of inflammatory cells significantly decreased. At 7 days, the bFGF expression of the three groups focused on epidermis and newborn skin glands, and bFGF expression near the blood vessels reduced obviously, which indicated that the capillaries had gradually developed into blood vessels. At 14 days, the bFGF expression level of the three groups continued to decrease. At 21 days, bFGF expression was strongly positive in the newborn epidermis, especially in hypertrophic scar of the positive group. At 28 days, bFGF expression level of the experimental group decreased to the level of normal tissue, which showed that the wound nearly closed up. However, in the new epidermis layer and hair follicles of the negative control group, bFGF expression was strongly positive, indicating that the wound had not yet healed fully (Figure 8).
Figure 8.
Immunohistochemistry results of bFGF for SD rat skin. (A) 3 Days, negative control (100×); (B) 3 days, positive control (100×); (C) 3 days, electrospun group (100×); (D) 5 days, negative control (200×); (E) 5 days, positive control (200×); (F) 5 days, electrospun group (200×); (G) 7 days, negative control (100×); (H) 7 days, positive control (100×); (I) 7 days, electrospun group (100×); (J) 14 days, negative control (200×); (K) 14 days, positive control (200×); (L) 14 days, electrospun group (200×); (M) 21 days, negative control (200×); (N) 21 days, positive control (200×); (O) 21 days, electrospun group (200×); (P) 28 days, negative control (100×); (Q) 28 days, positive control (100×); (R) 28 days, electrospun group (100×).
Abbreviations: SD, Sprague Dawley; bFGF, basic fibroblast growth factor.
Conclusion
The electrospun pNSR16/PVA membrane was successfully prepared. The three factors which affected average fiber diameter from high to low were voltage, flow speed, and solidification distance, and the three factors which affected fiber uniformity from high to low were flow speed, solidification distance, and voltage. With alcohol treatment, the molecular conformation of pNSR16 transformed from random coil to β-sheet. The porosity of the membrane can be improved by electrospinning. We conclude that pNSR16/PVA electrospun membranes can be used as wound membrane.
Although the water absorption ratio of electrospun membranes was inferior to commercial wound dressings, it may be applicable for wound healing since RGD-pNSR16 may be not covalently bonded with PVA, and it could be released and induce adherence, proliferation, and migration of cells. In the wound healing of SD rats, the contraction of wound can be better accelerated by electrospun membranes in comparison with the other two groups. The recovering time in the positive group was 28 days, 4 days less than that in the negative group. The formation of granulous tissue was faster in electrospun membrane-treated wounds than in the other two groups. pNSR16/PVA membrane can be used as a wound dressing to accelerate wound healing.
Acknowledgments
This research was supported by grants from the Science and Technology Department of Fujian Province, People’s Republic of China (2010Y0020).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Allmeling C, Jokuszies A, Reimers K, Kall S, Vogt PM. Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J Cell Mol Med. 2006;10(3):770–777. doi: 10.1111/j.1582-4934.2006.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman GH, Jakuba DF, Calabro C, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen JP, Chang GY, Chen JK. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf A Physicochem Eng Asp. 2008;313–314:83–188. [Google Scholar]
- 4.Lin WC, Lien CC, Yeh HJ, Yu CM, Hsu SH. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr Polym. 2013;94(1):603–611. doi: 10.1016/j.carbpol.2013.01.076. [DOI] [PubMed] [Google Scholar]
- 5.Shimpo A, Manabu K, Hiromi M, et al. Application of poly-l-lactic acid nanosheet as a material for wound dressing. Plast Reconstr Surg. 2013;131(2):236–240. doi: 10.1097/PRS.0b013e3182789c79. [DOI] [PubMed] [Google Scholar]
- 6.Thakur RA, Florek CA, Kohn J, Michniak BB. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int J Pharm. 2008;364(1):87–93. doi: 10.1016/j.ijpharm.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Deizel JM, Kleinmeyer JD, Hirvonen JK, Beck Tan NC. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer. 2001;42(19):8163–8170. [Google Scholar]
- 8.Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16(14):1151–1170. [Google Scholar]
- 9.Chong EJ, Phan TT, Lim IJ, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3(3):321–330. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Vladimir GB, Olga SS, Lyubov ID, et al. A novel model system for design of biomaterials based on recombinant analogs of spider silk proteins. J Neuroimmune Pharmacol. 2009;4(1):17. doi: 10.1007/s11481-008-9129-z. [DOI] [PubMed] [Google Scholar]
- 11.Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Spider silks and their applications. Trends Biotechnol. 2008;26(5):244–251. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhao L, Chen DL, Wei MH, Yao QH, Li M. Preparation of a recombinant spider silk protein/PCL blend submicrofibrous mat and its cytocompatibility. Polym Polym Compos. 2013;21(2):85–86. [Google Scholar]
- 13.Zhao L, Zhang WX, Chen DL, Qiu H, Li M. Function model construction on the question of defining optimal Ag-concentration in silver-containing biological wound dressing. Compos Interface. 2013;20(2):139–153. [Google Scholar]
- 14.Ruan CR, Huang JX, Wei MH, Li M. Construction, fermentation and purification of high polymer spider dragline silk protein containing RGD peptide. Chin J Biotechnol. 2007;23(5):858–861. Chinese. [PubMed] [Google Scholar]
- 15.Xu F, Li Y, Deng Y, Xiong J. Porous nano-hydroxyapatite/poly(vinyl alcohol) composite hydrogel as artificial cornea fringe: characterization and evaluation in vitro. J Biomater Sci Polym Ed. 2008;19(4):431–439. doi: 10.1163/156856208783719473. [DOI] [PubMed] [Google Scholar]
- 16.Gu SY, Wang ZM, Ren J, Zhang CY. Electrospinning of gelatin and gelatin/poly(L-lactide) blend and its characteristics for wound dressing. Mater Sci Eng C. 2009;29(6):1822–1828. [Google Scholar]
- 17.Ma YL, Zhu JX, Shao HL, Hu XC. Methanol induced changes in structure and properties of dry spinning fibers of regenerated silk fibroin. Adv Mater Res. 2011;335–336:908–911. [Google Scholar]
- 18.Li GY, Liang CY, Zheng Y, et al. Establishing a tube foam scaffold for tracheal cartilage tissue engineering by using solvent casting/particulate leaching method. J Clin Rehabil Tissue Eng Res. 2007;11(31):6278–6281. [Google Scholar]
- 19.Chen J, Altman GH, Karageorgiou V, et al. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67(2):559–570. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 20.Lu BY, Zheng J, Chen DL, Li M. Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns. 2010;36(6):891–896. doi: 10.1016/j.burns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Aoyagi S, Onishi H, Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm. 2007;330(1–2):138–145. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]