ABSTRACT
Severe dengue cases have increased in Brazil since 2001, with the first records in Maranhão dating back to 2002. The aim of this study was to determine the prevalence of severe dengue cases by age group and the possible risk factors. This was a study of secondary data on dengue in residents of São Luís, Maranhão, Brazil, using probable cases notified to the National Mandatory Reporting System (SINAN) from 2002 to 2011. The diagnosis and classification of dengue were based on the Brazilian Ministry of Health criteria: dengue fever (DF), dengue hemorrhagic fever (DHF) and dengue fever with complications (DWC). DHF and DWC were considered severe dengue, and DF was classified as non-severe dengue. A logistic regression analysis was performed with severe dengue as the outcome. During the study period, 1,229 cases of severe dengue were reported; of these, 812 in patients under the age of 15 (66%). Among the risk factors evaluated, age under 15 years old (OR = 3.10, 95% CI = 2.69-3.57, p-value = 0.001) was associated with severe dengue. The prevalence of severe dengue in children under the age of 15 was higher, and only this age group was associated with the occurrence of severe dengue.
Keywords: Severe dengue, Children, Public health surveillance, Risk factors, Dengue complications, Dengue clinical severity
INTRODUCTION
The World Health Organization (WHO) estimates that 40% of the world’s population lives in areas endemic to dengue virus 1 . The clinical spectrum of the disease varies from asymptomatic infection to severe conditions 2 . Dengue infection is a serious public health problem both because of the spread of the disease, on a worldwide scale, and because of the increase in severe cases and deaths 3 .
Occurrence of severe dengue cases is associated with factors related to the host (age, phenotype, presence of comorbidities, immunogenetic profile, sequential infection), to the etiological agent (serotype, strain, genotype), and to environmental aspects favoring the vector proliferation 4 - 7 .
In Americas, Brazil and Mexico account for approximately 14% of the severe dengue cases, which is equivalent to the proportion of the entire African continent 8 . During the 2007 epidemic in Brazil, a change in the age distribution of dengue was reported, with an increase in the burden on children that year. Of the 2,706 dengue hemorrhagic fever cases in 2007, 1,710 (63.2%) were reported from the Northeast region; 1,119 (65.4%) of these were in children <15 years old 9 .
In São Luís, the capital of the State of Maranhão, dengue cases hemorrhagic fever and dengue deaths were detected for the first time in 2002 10 . In Maranhão, the number of severe cases increased from 50 in 2009 to 185 in 2010 11 .
The present study analyzed the prevalence of severe dengue by comparing the number of dengue cases in two age groups, patients <15 years old and patients >15 years in São Luís, Maranhão, from 2002 to 2011. This study aimed to answer the following question: in São Luís, was the occurrence of dengue more frequent and more severe in children under 15 years old?
MATERIALS AND METHODS
This population-based study used an analytical and descriptive approach of secondary data on probable dengue cases notified to the National Mandatory Reporting System (SINAN) in residents of São Luís, Maranhão, Brazil. All probable dengue cases from the period of 2002 to 2011 were included.
Comparing data from the last two census of the Brazilian Institute of Geography and Statistics (IBGE) - 2000 and 2010, São Luís population increased from 857,387 inhabitants to 1,015,837; the urban population decreased from 96,17% to 94,45%; the population of inhabitants under 15 years old decreased from 260,426 (30.37%) to 240,467 (23,70%) and the proportion of people with monthly per capita household income inferior to US$ 44. dropped from 34.90% in 2000 to 13,81% in 2010 12 . In the period of this study, the Brazilian Ministry of Health adopted the WHO dengue case classification: dengue fever and dengue hemorrhagic fever 13 , adapted with the inclusion of an additional category: dengue fever with complications 14 . Dengue fever is defined as a suspected case - an acute febrile illness (fever up to 7 days of duration) accompanied by at least two of the following clinical findings: nausea, vomiting, headache, arthralgia, retro-orbital pain, rash, myalgia, hemorrhagic manifestations and leukopenia confirmed by laboratory tests or, during an epidemic, confirmed by clinical-epidemiological criteria. The definition of dengue hemorrhagic fever consists in the presence of all of the five following criteria: fever (up to 7 days of duration), evidence of bleeding (spontaneous bleeding or a positive tourniquet test), thrombocytopenia < 100,000 cells/mm3, plasma leakage evidence (pleural effusion, ascites, hypoalbuminemia or hemoconcentration greater than 20% from baseline) and at least one positive test for dengue: detection of dengue virus nonstructural protein 1 (NS1) by using ELISA, IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA), viral isolation or reverse transcription-polymerase chain reaction (RT-PCR). Dengue fever with complications is defined as any severe case that does not fit the WHO criteria for dengue hemorrhagic fever and when the classification of dengue fever is unsatisfactory, that is, when one of the following complications is found: severe nervous system disorders, cardiorespiratory dysfunction, liver failure, gastrointestinal bleeding, cavitary effusion, thrombocytopenia = 20,000/mm3, leukopenia = 1,000/mm3, and suspected dengue with progression to death that does not fit completely the dengue hemorrhagic fever criteria 14 .
The tests used for laboratory confirmation of the cases were: MAC-ELISA, RT-PCR, NS1 ELISA, immunohistochemistry and histopathology, the last two used in death cases.
STATA® version 14.0 (Stata Corporation, College Station, TX, USA) was used for the statistical analysis. In the descriptive analysis, the qualitative variables were presented as absolute frequencies and proportions. For the quantitative variables, measures of central tendency and dispersion were calculated. Two categorical variables were created in the statistical analysis: severe dengue and non-severe dengue based on the final case classification in the SINAN form. Dengue hemorrhagic fever and dengue fever with complications were classified as severe dengue, and dengue fever was classified as non-severe dengue. To test the association between the variables, the chi-square test was used. To analyze the associated factors, we used a logistic regression model. The outcome was severe dengue and the co-variables included were gender, race/skin color, area of residence and age group (<15 years old and >15 years old). The inclusion of these variables on the model derives from the fact that in the literature female gender 15 , caucasian phenotype 16 , 17 and children <15 years old 18 are reported as risk factors to severe dengue. The odds ratios and respective 95% confidence intervals were estimated.
The case-fatality rate over a year was calculated by dividing the number of deaths by the number of severe cases.
RESULTS
Notification of dengue cases is based on clinical suspicion. After investigation of the notified suspected cases, they are classified as discarded or probable. Although this study data refer to probable cases from 2002 to 2011, on SINAN, only 397 records from 2007 to 2010 related to the number of discarded cases were available regarding the residents of São Luís.
Severe dengue cases of São Luís residents were first reported in 2002. From 2002 to 2011, there were 14,780 probable dengue cases, of which 1,229 (7.09%) were severe. Of these, 812 (66.07%) were in children under 15 years old (6.9 ± 3.4 years) and 417 (33.93%) were in patients >15 years old (33.1 ± 14.5 years). There was a predominance of females, people with mixed ethnicity and residents in the urban area, regardless of age and severity. The lowest values for platelet count were found in patients with severe dengue, irrespective of the age group (Table 1).
Table 1. Sociodemographic, clinical and laboratory characteristics of dengue cases, according to age group and clinical severity. São Luís, Maranhão, 2002 to 2011.
| Characteristics | < 15 years old | ≥ 15 years old | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Severe dengue | Non-severe dengue | Severe dengue | Non-severe dengue | |||||
|
| ||||||||
| n | % | n | % | n | % | n | % | |
| Gender | 812 | 100.00 | 5,316 | 100.00 | 417 | 100.00 | 8,235 | 100.00 |
| Male | 380 | 46.80 | 2,657 | 49.98 | 190 | 45.45 | 3,771 | 45.79 |
| Female | 432 | 53.20 | 2,659 | 50.02 | 228 | 54.55 | 4,464 | 54.21 |
| Race/skin color | 673 | 100.00 | 3,823 | 100.00 | 334 | 100.00 | 5,813 | 100.00 |
| White | 148 | 22.00 | 591 | 15.50 | 69 | 20.65 | 1,099 | 18.90 |
| Black | 37 | 5.49 | 184 | 4.80 | 22 | 6.60 | 493 | 8.46 |
| Asian | 2 | 0.30 | 68 | 1.77 | 8 | 2.40 | 110 | 1.90 |
| Mixed | 485 | 72.06 | 2,975 | 77.80 | 235 | 70.35 | 4,095 | 70.44 |
| Indigenous | 1 | 0.15 | 5 | 0.13 | 0 | 0.00 | 16 | 0.30 |
| Area | 755 | 100.00 | 5,097 | 100.00 | 391 | 100.00 | 7,941 | 100.00 |
| Urban | 667 | 88.34 | 4,710 | 92.41 | 370 | 94.63 | 7,458 | 93.92 |
| Rural/Periurban | 88 | 11.66 | 387 | 7.59 | 21 | 5.37 | 483 | 6.08 |
| Final classification | 812 | 100.00 | 5,316 | 100.00 | 417 | 100.00 | 8,235 | 100.00 |
| DF | 0 | 0.00 | 5,316 | 100.00 | 0 | 0.00 | 8,235 | 100.00 |
| DWC | 618 | 76.11 | 0 | 0.00 | 326 | 78.18 | 0 | 0.00 |
| DHF | 194 | 23.89 | 0 | 0.00 | 91 | 21.27 | 0 | 0.00 |
| Confirmation criterion | 589 | 100.00 | 4,944 | 100.00 | 267 | 100.00 | 7,745 | 100.00 |
| Laboratorial | 349 | 59.25 | 1,475 | 29.83 | 193 | 72.28 | 2,174 | 28.07 |
| Clinical-epidemiological | 240 | 40.75 | 3,469 | 70.17 | 74 | 27.72 | 5,571 | 71.93 |
|
| ||||||||
| Platelets/mm 3, n | 499 | 72 | 174 | 116 | ||||
| Mean±SD | 51,255.78±45,375.01 | 67,258.33±39,044.30 | 45,199.48±39,445.23 | 84,421.55±53,175.76 | ||||
| Hematocrit (%), n | 62 | 136 | 77 | 313 | ||||
| Mean±SD | 39.17±4.64 | 38.23±5.67 | 40.73±6.26 | 40.12±5.82 | ||||
DF = dengue fever, DWC = dengue fever with complications, DHF = dengue hemorrhagic fever. Source: SINAN.
Among the cases classified as dengue fever with complications, the most frequent criteria were cavitary effusions and platelet count <20,000/mm3 in both age groups; gastrointestinal bleeding was found in 11 children under 15 years old and in seven people who were >15 years old; cardiorespiratory complications were found in four cases in each age group and neurological complications were found in seven children under 15 years old and in one in the group >15. In the criterion “does not fit dengue hemorrhagic fever”, there were 62 cases among the patients <15 years old and 23 among patients >15.
There were 532 (43.29%) severe dengue cases and 9,040 (66.71%) non-severe dengue cases (Table 1) confirmed by laboratory criterion. It was not possible to establish a proportion of positive/reagent results for each exam, because frequently the information was missing when the exam was not made or the result was negative/non-reagent.
The viral serotype was identified in 47 patients (Table 2). Among the severe dengue cases, there was a higher occurrence of isolation of DENV-1 (12.50%) and DENV-2 (87.50%) serotypes in patients < 15 years old and of DENV-3 (57.14%) and DENV-2 (28.57%) serotypes in patients > 15. There was no viral isolation in 2005, 2006 or 2009. In the years 2007 and 2011, the simultaneous circulation of three serotypes was detected: DENV-1, DENV-2 and DENV-3 in 2007 and DENV-1, DENV-2 and DENV-4 in 2011. DENV-1 was not isolated in individuals >15 with severe dengue. DENV-2 was detected in both age groups, regardless of clinical severity. DENV-3 was not isolated in children <15 years old with severe dengue. DENV-4 was not isolated in children <15 years old.
Table 2. Distribution of the 47 dengue virus serotypes according clinical severity and year of occurrence. São Luís, Maranhão, 2002 to 2011.
| Serotype | Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Total | |
| Non-severe dengue | |||||||||||
| DENV-1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 12 | 2 | 17 |
| DENV-2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 |
| DENV-3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| DENV-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 |
| Severe dengue | |||||||||||
| DENV-1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| DENV-2 | 0 | 0 | 0 | 0 | 0 | 8 | 1 | 0 | 0 | 0 | 9 |
| DENV-3 | 1 | 0 | 3 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 7 |
| DENV-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
|
| |||||||||||
| Total | 5 | 2 | 3 | 0 | 0 | 14 | 2 | 0 | 12 | 9 | 47 |
Source: SINAN.
There was a higher frequency of severe cases in 2007, with 630 cases (51.26%), and a particularly high proportion in children under 15 years old (60.71%). Likewise, there were 225 severe cases (20.35%) in 2011. Most cases occurred in the period from April to August. Most hospitalizations of severe cases occurred in May whereas most hospitalizations of non-severe cases occurred in August, regardless of age. The monthly distribution of hospitalization of the total study period follows the distribution of hospitalization months in the years with the highest absolute numbers of cases: 2005, 2007, 2010 and 2011 (Table 3).
Table 3. Distribution of dengue cases by year and month of occurrence and of hospitalization, according to age group and clinical severity. São Luís, Maranhão, 2002 to 2011.
| Characteristics | < 15 years old (n=6,128) | ≥ 15 years old (n=8,652) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Severe dengue | Non-severe dengue | Severe dengue | Non-severe dengue | |||||
|
| ||||||||
| n | % | n | % | n | % | n | % | |
| Year of occurrence | 812 | 100.00 | 5,316 | 100.00 | 417 | 100.00 | 8,235 | 100.00 |
|
| ||||||||
| 2002 | 6 | 0.74 | 182 | 3,42 | 16 | 3.83 | 351 | 4.26 |
| 2003 | 4 | 0.49 | 120 | 2.26 | 30 | 7.18 | 448 | 5.44 |
| 2004 | 2 | 0.25 | 25 | 0.47 | 5 | 1.20 | 129 | 1.57 |
| 2005 | 24 | 2.96 | 134 | 2.52 | 43 | 10.29 | 248 | 3.01 |
| 2006 | 35 | 4.31 | 57 | 1.07 | 3 | 0.72 | 215 | 2.61 |
| 2007 | 493 | 60.71 | 1,187 | 22.33 | 137 | 32.78 | 1,541 | 18.71 |
| 2008 | 17 | 2.09 | 277 | 5.21 | 22 | 5.26 | 813 | 9.87 |
| 2009 | 7 | 0.86 | 22 | 0.41 | 8 | 1.91 | 29 | 0.35 |
| 2010 | 111 | 13.67 | 1,242 | 23.36 | 41 | 9.81 | 1,174 | 14.26 |
| 2011 | 113 | 13.92 | 1,946 | 36.61 | 112 | 26.79 | 2,855 | 34.67 |
|
| ||||||||
| Month of occurrence | 701 | 100.00 | 3,430 | 100.00 | 307 | 100.00 | 5,436 | 100.00 |
|
| ||||||||
| Missing | 111 | 13.67 | 1,886 | 35.48 | 110 | 26.38 | 2,799 | 33.99 |
| January | 24 | 2.96 | 45 | 0.85 | 28 | 6.71 | 144 | 1.75 |
| February | 20 | 2.46 | 85 | 1.60 | 22 | 5.28 | 241 | 2.93 |
| March | 58 | 7.14 | 177 | 3.33 | 33 | 7.91 | 455 | 5.53 |
| April | 78 | 9.61 | 444 | 8.35 | 37 | 8.87 | 819 | 9.95 |
| May | 105 | 12.93 | 553 | 10.40 | 58 | 13.91 | 965 | 11.72 |
| June | 145 | 17.86 | 375 | 7.05 | 42 | 10.07 | 660 | 8.01 |
| July | 106 | 13.05 | 406 | 7.64 | 43 | 10.31 | 505 | 6.13 |
| August | 83 | 10.22 | 622 | 11.70 | 24 | 5.76 | 786 | 9.54 |
| September | 55 | 6.77 | 480 | 9.03 | 9 | 2.16 | 461 | 5.60 |
| October | 17 | 2.09 | 165 | 3.10 | 5 | 1.20 | 210 | 2.55 |
| November | 8 | 0.99 | 45 | 0.85 | 5 | 1.20 | 118 | 1.43 |
| December | 2 | 0.25 | 33 | 0.62 | 1 | 0.24 | 71 | 0.86 |
|
| ||||||||
| Year of hospitalization | 538 | 100.00 | 1,347 | 100.00 | 227 | 100.00 | 1,577 | 100.00 |
|
| ||||||||
| 2002 | 2 | 0.37 | 25 | 2.00 | 10 | 4.40 | 37 | 2.35 |
| 2003 | 3 | 0.55 | 39 | 3.00 | 25 | 11.01 | 108 | 6.85 |
| 2004 | 2 | 0.37 | 12 | 1.00 | 4 | 1.76 | 40 | 2.54 |
| 2005 | 20 | 3.75 | 72 | 5.20 | 31 | 13.69 | 86 | 5.46 |
| 2006 | 32 | 5.95 | 46 | 3.40 | 3 | 1.32 | 61 | 3.86 |
| 2007 | 336 | 62.45 | 0 | 0.00 | 66 | 29.07 | 0 | 0.00 |
| 2008 | 14 | 2.60 | 0 | 0.00 | 14 | 6.16 | 0 | 0.00 |
| 2009 | 5 | 0.92 | 0 | 0.00 | 5 | 2.20 | 0 | 0.00 |
| 2010 | 66 | 12.26 | 680 | 50.00 | 21 | 9.25 | 452 | 28.66 |
| 2011 | 58 | 10.78 | 473 | 35.40 | 48 | 21.14 | 793 | 50.28 |
|
| ||||||||
| Month of hospitalization | 524 | 100.00 | 1,282 | 100.00 | 206 | 100.00 | 1,466 | 100.00 |
|
| ||||||||
| Missing | 14 | 2.60 | 65 | 4.83 | 21 | 9.25 | 111 | 7.04 |
| January | 8 | 1.49 | 18 | 1.34 | 8 | 3.52 | 42 | 2.66 |
| February | 18 | 3.35 | 41 | 3.04 | 10 | 4.41 | 54 | 3.42 |
| March | 44 | 8.18 | 38 | 2.82 | 15 | 6.61 | 73 | 4.63 |
| April | 68 | 12.64 | 62 | 4.60 | 29 | 12.78 | 54 | 3.42 |
| May | 94 | 17.47 | 98 | 7.28 | 48 | 21.15 | 152 | 9.64 |
| June | 93 | 17.29 | 111 | 8.24 | 28 | 12.33 | 208 | 13.19 |
| July | 76 | 14.13 | 207 | 15.37 | 32 | 14.10 | 168 | 10.65 |
| August | 60 | 11.15 | 382 | 28.36 | 21 | 9.25 | 450 | 28.54 |
| September | 39 | 7.25 | 194 | 14.40 | 8 | 3.52 | 113 | 7.17 |
| October | 15 | 2.79 | 69 | 5.12 | 3 | 1.32 | 57 | 3.61 |
| November | 7 | 1.30 | 44 | 3.27 | 3 | 1.32 | 53 | 3.36 |
| December | 2 | 0.37 | 18 | 1.34 | 1 | 0.44 | 42 | 2.66 |
Source: SINAN.
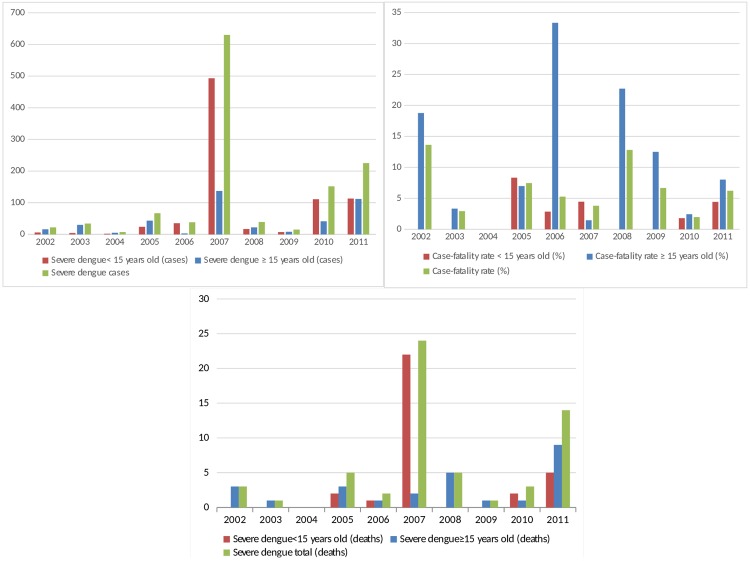
The first deaths occurred in 2002, concomitantly with the first records of severe dengue. In total, there were 58 deaths, 32 of which were of patients under 15 years old. There was an abrupt increase in the number of deaths in children under 15 years old in 2007, and there were high case-fatality rates, particularly in 2005 and in 2007 in children under 15 (Figure 1). Among the 47 patients in whom the serotype was identified, 8 (17.02%) died.
Figure 1. Distribution of dengue cases according to case-fatality rate, age group and clinical severity. São Luís, Maranhão, 2002 to 2011.
The most frequent clinical manifestations were severe abdominal pain (72.86%), petechia (66.43%) and gastrointestinal bleeding (25.93%); these symptoms were predominant in children <15 years old (Table 4).
Table 4. Hemorrhagic manifestations and plasma extravasation according to the age group and clinical severity. São Luís, Maranhão, 2002 to 2011.
| Clinical characteristics | < 15 years old | ≥ 15 years old | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Severe | Non-severe | Severe | Non-severe | |||||
|
| ||||||||
| n/total | % | n/total | % | n/total | % | n/total | % | |
| Hemorrhagic manifestations | 357/428 | 74.07 | 1/1 | 100.00 | 111/142 | 78.17 | 1/1 | 100.00 |
| Epistaxis | 49/428 | 11.45 | 24/620 | 3.87 | 35/208 | 16.83 | 39/1,713 | 2.23 |
| Gum bleeding | 29/428 | 6.78 | 8/623 | 1.28 | 37/209 | 17.70 | 18/1,762 | 1.02 |
| Uterine bleeding | 11/229 | 4.80 | 3/254 | 1.18 | 8/123 | 14.63 | 17/744 | 2.28 |
| Petechia | 285/428 | 66.43 | 66/621 | 10.63 | 109/208 | 52.40 | 120/1,753 | 6.85 |
| Hematuria | 22/428 | 5.14 | 3/500 | 0.60 | 19/207 | 9.18 | 15/1,331 | 1.13 |
| Gastrointestinal bleeding | 111/428 | 25.93 | 7/500 | 1.40 | 32/207 | 15.46 | 14/1,331 | 1.05 |
| Positive tourniquet test | 152/349 | 43.55 | 1/1 | 100.00 | 40/207 | 37.38 | 1/1 | 100.00 |
| Plasma extravasation | 408/428 | 95.32 | 23/620 | 3.70 | 85/148 | 57.43 | 16/16 | 100.00 |
Source: SINAN.
In the univariate analysis, there was a significant association between severe dengue and age <15 years old, caucasian skin color and urban area of residence, but the association with gender was not significant. In the multivariate analysis, there’s only the association with age <15 years old (Table 5).
Table 5. Univariate and multivariate analysis of factors associated with severe dengue. São Luís, Maranhão, 2002 to 2011.
| Variable | Non-adjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male | 1 | 1 | ||||
| Female | 1.04 | 0.93-1.17 | 0.477 | 1.05 | 0.91-1.20 | 0.516 |
| ≥15 years old | 1 | 1 | ||||
| <15 years old | 3.01 | 2.66-3.41 | 0.001 | 3.11 | 2.70-3.60 | <0.001 |
| Rural area | 1 | 1 | ||||
| Urban area | 0.68 | 0.55-0.83 | 0.001 | 0.82 | 0.65-1.03 | 0.091 |
| Race/skin color (black) | 1 | 1 | ||||
| Race/skin color (caucasian) | 1.47 | 1.09-1.99 | 0.012 | 1.30 | 0.95-1.78 | 0.103 |
| Race/skin color (Asian) | 0.64 | 0.32-1.28 | 0.213 | 0.60 | 0.30-1.21 | 0.153 |
| Race/skin color (mixed) | 1.17 | 0.88-1.54 | 0.271 | 0.96 | 0.72-1.28 | 0.769 |
| Race/skin color (indigenous) | 0.55 | 0.07-4.13 | 0.558 | 0.64 | 0.08-4.94 | 0.667 |
OR = odds ratio. CI = confidence interval. Source: SINAN.
DISCUSSION
In 2009, the WHO issued the revised dengue classification: dengue without warning signs, dengue with warning signs (abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, restlessness, liver enlargement, increasing hematocrit with decreasing platelets) and severe dengue (severe plasma leakage, severe bleeding or organ failure) 19 . It was only from 2014 that the Brazilian Ministry of Health started to use the revised WHO dengue classification. In the first study in Brazil to evaluate the revised WHO classification criteria, Lima et al. 20 conducted a cross-sectional survey to evaluate the ability of the 1997 and 2009 WHO classification systems to detect severe dengue cases based on the medical records of dengue patients who were admitted to the University Hospital of the Federal University of Grande Dourados, Mato Grosso do Sul State, in the summers of 2009 and 2010. They reported that, of the 150 patients classified as having dengue fever, 105 (70%) were re-classified as having dengue with warning signs or severe dengue. They did not consider the Brazilian category dengue fever with complications, whose several criteria are included in dengue with warning signs or in severe dengue. Otherwise, Cavalcanti et al. 21 evaluated the revised WHO dengue classification in a retrospective cross-sectional study of the dengue hemorrhagic fever patients who were admitted to São José Hospital in Fortaleza, Ceará State, Northeastern Brazil. They also reported 52 patients classified as dengue fever with complications, of whom 17 (32.7%) were re-classified as dengue with warning signs and 32 (61.5%) as severe dengue. In the present study the term “severe dengue” included dengue hemorrhagic fever and dengue fever with complications.
We found an association between severe dengue with being caucasian/skin color only in the univariate analysis. In 1981, in Havana, Kouri et al. 5 identified that the majority (80.4%) of adults and children who died due to severe dengue fever were of white phenotype; this association was statistically significant. An investigation conducted by Ayllón et al. 17 in 1989 showed that girls of the caucasian phenotype were more susceptible to develop severe forms even with secondary dengue fever.
There was a three-fold increased chance of developing severe dengue in children younger than 15. In Thailand, Gamble et al. 22 found that 97.4% of severe dengue cases occurred in children.
In this study, severe dengue was more frequent in females, including those under 15 years old; however, there was no significant association between gender and severe dengue. A study conducted in Southeast Asia by Ooi et al. 23 showed that female patients were more likely to develop severe forms of dengue. In another study also conducted in Southeast Asia in 1970, Halsted et al. 4 noted that males predominated among patients with milder disease, but the disease was more severe in females. In a Nicaraguan study 15 of adults and children who developed severe forms of dengue fever, the female/male ratio was 3:2 among adults and 1:1 among children.
As predicted by Vasconcelos et al. 24 in the classic sero-epidemiological study of the dengue outbreak that occurred in 1995 and 1996 in São Luís, there was a high risk of the development of severe forms in the city due to the isolation and sensitization of the population to DENV-1. Starting in 2002, this prediction materialized, and there were epidemics with severe presentations and the first deaths 10 .
The most common criteria for classifying the case as dengue fever with complications were cavitary effusions and thrombocytopenia. The onset of complications in individuals affected by dengue, such as cavitary effusions and thrombocytopenia, characterize the clinical severity. Consequently, accurate diagnosis of the complications presented by the patient requires not only a basis in clinical criteria but also the performance of complementary tests, including laboratory analyses and ultrasound examinations 25 , 26 . Thrombocytopenia can be caused by direct or indirect interaction of the virus with platelets, which is a constant finding in patients with severe forms of dengue 27 . In a study performed in Recife, the capital of Pernambuco State, platelet counts averaged 48,538/mm3 in patients who died 28 . In a study conducted in Ceará, a State in Northeastern Brazil, in 2003, patients who died had lower platelet counts than those with favorable outcomes, and their platelet count decreased progressively until death 29 .
In the 10-year period analyzed in this study, the viral serotype was isolated only in 47 cases. In a study 30 conducted for over 2 years in Manaus, the capital of Amazonas State, the serotype was identified in 41 cases, which demonstrates the difficulty of laboratory support for dengue epidemiological surveillance in São Luís. Additionally, the presence of three serotypes that were simultaneously isolated in 2007 (DENV-1, DENV-2 and DENV-3) has possibly contributed to the abrupt increase of severe cases, particularly in children <15 years old.
The simultaneous circulation of three serotypes (DENV-1, DENV-2 and DENV-4) was observed again in 2011, and there was a new increase in the number of cases and an increase in cases with clinical severity in children <15 years old. The circulation of three serotypes (DENV-1, DENV-2 and DENV-3) in Brazil since 2000 and, more recently, the reintroduction of DENV-4, which is associated with the spread of its main vector, Aedes aegypti, in more than two-thirds of the country’s municipalities, has contributed to the worsening of the epidemiological situation of the disease 11 .
The DENV-2 serotype was detected in all age groups, both in patients with severe dengue and in those who did not progress to severe dengue. According to Watts et al. 31 , an American genotype of the DENV-2 serotype isolated in Peru in 1995 was not related to the emergence of severe forms. However, a DENV-2 genotype from Southeast Asia was related to epidemics of more severe forms in 2008 in Brazil.
Together with two other serotypes, the DENV-2 serotype was isolated in the years with large increases in the number of cases, namely, in 2007 and 2011, both among severe and non-severe cases. In a study conducted in Rio de Janeiro in 2001/2002, individuals affected by the DENV-3 serotype exhibited dengue with a greater clinical severity 32 . In Manaus, there was simultaneous circulation of serotypes DENV-1 and DENV-2 in 2006/2007, which facilitated the emergence of severe cases in children under 15 30 . In Ceará, DENV-2 became the predominant serotype in 2007 (84%) and 2008 (76.1%), followed by the increasing of dengue hemorrhagic fever incidence and hospitalization rate due to dengue among for children <10 years old 33 .
Most of the hospitalizations occurred in the first semester of each year. Studies conducted in 1999 in São Luis, Maranhão 34 , from 2002 to 2006 in Teresina, Piauí 35 , and from 2001 to 2002 in São Sebastião, São Paulo 36 , demonstrated that most dengue cases had also occurred in the first semester of each year.
Fever defervescence in dengue can last up to 7 days and, during this period, the patient may present signs that characterize complications and/or severity, which may explain the hospitalizations in the same period as the notifications. Kouri et al. 37 reconstructed the natural history of dengue in children under 15 who died in Cuba in 1981 and found that the most important hemorrhagic manifestations occurred on the third day of disease, followed by shock on the fourth day and death on the fifth day.
Most hospitalizations were concentrated in the 2005/2007 and 2010/2011 periods. Dengue severity was related to the consecutive/simultaneous circulation of two or more serotypes 38 .
In Brazil from 2000 to 2010, the co-circulation of multiple DENV serotypes and high dengue disease endemicity may be responsible for the increased occurrence of severe forms of dengue disease and increases in the numbers of dengue disease-related hospitalizations. In addition, increase in the number of severe dengue disease cases and a shift in age group predominance of severe forms observed during 2007/08 confirm that dengue disease must remain a public health priority 18 .
The high case-fatality rates found in this study may have been caused by the underreporting of severe cases, including the year 2005. Notably, the acceptable case-fatality rate of dengue fever should be less than 1% according to the WHO 19 . We found high case-fatality rates in children under 15 years in this study. In a study conducted in the city of Rio de Janeiro, most dengue deaths occurred in the <15 age group 39 . However, in a study conducted in the State of Bahia from 2001 to 2009, the majority of deaths occurred in the group >15 years old 40 .
It is imperative to diagnose dengue during the early phase in order to provide information for the appropriate management of cases and to avoid complications 41 . Sensitivity and specificity tests are essential for the accurate laboratory diagnosis of DENV-infected patients 41 . RT–PCR has become a primary tool to detect the virus early in the course of illness; DENV can be detected in the blood (serum) of patients during the first 5 days of symptoms; current tests are between 80-90% sensitive, and more that 95% specific 42 . Solanke et al. 41 found that on days 1-3 of the acute phase, the sensitivity and specificity of NS1 ELISA were 66.6% and 89.1%, while sensitivity and specificity of rapid NS1 antigen were 55.5% and 92%, respectively. MAC-ELISA has a sensitivity and specificity of approximately 90% and 98%, respectively, but only when used five or more days after the onset of fever 42 . The main limitations of the study are inherent to the use of secondary data; not all cases were confirmed by the laboratory criterion and there is no information in the SINAN form about primary vs. secondary infection.
The following are strengths of this study: it included a large number of cases; it is one of the few studies on severe dengue in children in Brazil and it provides an analysis of secondary data from SINAN that may serve as a management tool, including the patient’s care.
ACKNOWLEDGMENTS
EPBM received a master’s degree grant from the National Counsel for Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq). We thank the Research Scientific and Development Support Foundation of Maranhão (Fundação de Amparo à Pesquisa e Desenvolvimento Científico do Maranhão – FAPEMA) for the support program to article publication.
REFERENCES
- 1.Word Health Organization . Dengue control. Geneva: WHO; 2016. [cited 2017 Apr 04]. http://www.who.int/denguecontrol/en/ [Google Scholar]
- 2.Word Health Organization . Weekly epidemiological record. Dengue vaccine: WHO position paper – July 2016. Geneva: WHO; 2016. [cited 2017 Apr 04]. http://www.who.int/wer/2016/wer9130.pdf?ua=1. [DOI] [PubMed] [Google Scholar]
- 3.Singhi S, Kissoon N, Bansal A. J Pediatr. Suppl 2. Vol. 83. Rio J: 2007. Dengue and dengue hemorrhagic fever: management issues in an intensive care unit; pp. S22–S35. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB, Nimmannitya S, Cohen SN. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970;42:311–328. [PMC free article] [PubMed] [Google Scholar]
- 5.Kourí GP, Guzmán MG, Bravo JR. Why dengue hemorrhagic fever in Cuba? 2. An integral analysis. Trans R Soc Trop Med Hyg. 1987;81:821–823. doi: 10.1016/0035-9203(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 6.Guzmán G, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 7.Guzmán MG. Thirty years after the Cuban hemorrhagic dengue epidemic of 1981. MEDICC Rev. 2012;14:46–51. doi: 10.37757/MR2012V14.N2.11. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt S, Gething PW, Brady OJ, Messina PJ, Farlow AW, Moyes LC, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira MG, Costa MC, Coelho G, Barreto ML. Recent shift in age pattern of dengue hemorrhagic fever, Brazil. 1663Emerg Infect Dis. 2008;14 doi: 10.3201/eid1410.071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves VS, Neto, Rebêlo JM. Aspectos epidemiológicos do dengue no município de São Luís, Maranhão, Brasil, 1997-2002. Cad Saude Publica. 2004;20:1424–1431. doi: 10.1590/s0102-311x2004000500039. [DOI] [PubMed] [Google Scholar]
- 11.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Sistema nacional de vigilância em saúde: relatório de situação: Maranhão. 5ª. Brasília: Ministério da Saúde; 2011. [cited 2017 Mar 30]. http://bvsms.saude.gov.br/bvs/publicacoes/sistema_nacional_vigilancia_saude_ma_5ed.pdf. [Google Scholar]
- 12.Atlas do desenvolvimento humano no Brasil: São Luis, MA. Rio de Janeiro: PNUD: Fundação João Pinheiro: IPEA; 2013. [cited 2017 Jun 27]. http://www.atlasbrasil.org.br/2013/pt/perfil_m/sao-luis_ma. [Google Scholar]
- 13.World Health Organization . Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 2nd. Geneva: WHO; 1997. [cited 2017 Jun 24]. http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/ [Google Scholar]
- 14.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Diretrizes nacionais para prevenção e controle de epidemias de dengue. Brasília: Ministério da Saúde; 2009. [cited 2017 Aug 29]. http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_nacionais_prevencao_controle_dengue.pdf. [Google Scholar]
- 15.Hammond SN, Balmaseda A, Pérez L, Tellez Y, Saborío SI, Mercado JC, et al. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 16.Kouri G, Guzmán MG, Bravo J. Hemorrhagic dengue in Cuba: history of an epidemic. Bull Pan Am Health Organ. 1986;20:24–30. [PubMed] [Google Scholar]
- 17.Ayllón L, Martinez E, Kouri G, Guzmán MG, Paradoa ML. Factores del huésped en la fiebre hemorrágica dengue - sindrome de shock por dengue en el niño. Rev Cubana Pediatr. 1989;61:498–517. [Google Scholar]
- 18.Teixeira MG, Siqueira JB, Jr, Ferreira GL, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. e2520PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Dengue guidelines for diagnosis, treatment, prevention and control. new. Geneva: WHO; 2009. [cited 2017 Aug 29]. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. [PubMed] [Google Scholar]
- 20.Lima FR, Croda MG, Muniz DA, Gomes IT, Soares KR, Cardoso MR, et al. Evaluation of the traditional and revised World Health Organization classifications of dengue cases in Brazil. Clinics. 2013;68:1299–1304. doi: 10.6061/clinics/2013(10)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalcanti LP, Mota LA, Lustosa GP, Fortes MC, Mota DA, Lima AA, et al. Evaluation of the WHO classification of dengue disease severity during an epidemic in 2011 in the state of Ceará, Brazil. Mem Inst Oswaldo Cruz. 2014;109:93–98. doi: 10.1590/0074-0276140384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamble J, Bethell D, Day NP, Loc PP, Phu NH, Gartside IB, et al. Clin Sci. Vol. 98. Lond: 2000. Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? pp. 211–216. [PubMed] [Google Scholar]
- 23.Ooi EE, Hart TJ, Tan HC, Chan SH. Dengue seroepidemiology in Singapore. Lancet. 2001;357:685–686. doi: 10.1016/S0140-6736(00)04137-4. [DOI] [PubMed] [Google Scholar]
- 24.Vasconcelos PF, Lima JW, Raposo ML, Rodrigues SG, Rosa JF, Amorim SM, et al. Inquérito soro-epidemiológico na ilha de São Luís durante epidemia de dengue no Maranhão. Rev Soc Bras Med Trop. 1999;32:171–179. doi: 10.1590/s0037-86821999000200009. [DOI] [PubMed] [Google Scholar]
- 25.Thulkar S, Sharma S, Srivastava DN, Sharma SK, Berry M, Pandey RM. Sonographic findings in grade III dengue hemorrhagic fever in adults. J Clin Ultrasound. 2000;28:34–37. doi: 10.1002/(sici)1097-0096(200001)28:1<34::aid-jcu5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Vabo KA, Torres G, Neto, Santos AA, Vabo TP, Santos ML, Marchiori E. Achados ultra-sonográficos abdominais em pacientes com dengue. Radiol Bras. 2004;37:159–162. [Google Scholar]
- 27.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montenegro D, Lacerda HR, Lira TM, Oliveira DS, Lima AA, Guimarães MJ, et al. Aspectos clínicos e epidemiológicos da epidemia de dengue no Recife, PE, 2002. Rev Soc Bras Med Trop. 2006;39:9–13. doi: 10.1590/s0037-86822006000100002. [DOI] [PubMed] [Google Scholar]
- 29.Cavalcanti LP, Coelho IC, Vilar DC, Holanda SG, Escóssia KN, Souza-Santos R. Clinical and epidemiological characterization of dengue hemorrhagic fever cases in northeastern, Brazil. Rev Soc Bras Med Trop. 2010;43:355–358. doi: 10.1590/s0037-86822010000400003. [DOI] [PubMed] [Google Scholar]
- 30.Rocha LA, Tauil PL. Dengue em criança: aspectos clínicos e epidemiológicos, Manaus, estado do Amazonas, no período de 2006 e 2007. Rev Soc Bras Med Trop. 2009;42:18–22. doi: 10.1590/s0037-86822009000100005. [DOI] [PubMed] [Google Scholar]
- 31.Watts DM, Porter KR, Putvatana P, Vasquez B, Calampa C, Hayes CG, et al. Failure of secondary infection with American genotype dengue 2 to cause dengue hemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 32.Passos MN, Santos LM, Pereira MR, Casali CG, Fortes BP, Valencia LI, et al. Diferenças clínicas observadas em pacientes com dengue causadas por diferentes sorotipos na epidemia de 2001/2002, ocorrida no município do Rio de Janeiro. Rev Soc Bras Med Trop. 2004;37:293–295. doi: 10.1590/s0037-86822004000400001. [DOI] [PubMed] [Google Scholar]
- 33.Cavalcanti LP, Vilar D, Souza-Santos R, Teixeira MG. Change in age pattern of persons with dengue, northeastern Brazil. Emerg Infect Dis. 2011;17:132–134. doi: 10.3201/eid1701.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebêlo JM, Costa JM, Silva FS, Pereira YN, Silva JM. Distribuição de Aedes aegypti e do dengue no estado do Maranhão, Brasil. Cad Saude Publica. 1999;15:477–486. doi: 10.1590/s0102-311x1999000300004. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro ES, Coelho ME, Cunha IS, Cavalcante MA, Carvalho FA. Aspectos epidemiológicos e vetoriais da dengue na cidade de Teresina, Piauí - Brasil, 2002 a 2006. Epidemiol Serv Saúde. 2009;18:365–374. [Google Scholar]
- 36.Ribeiro AF, Marques GR, Voltolini JC, Condino ML. Associação entre incidência de dengue e variáveis climáticas. Rev Saude Publica. 2006;40:671–676. doi: 10.1590/s0034-89102006000500017. [DOI] [PubMed] [Google Scholar]
- 37.Kouri GP, Guzmán MG, Bravo JR. Why dengue hemorrhagic fever in Cuba? 2. An integral analysis. Trans R Soc Trop Med Hyg. 1987;81:821–823. doi: 10.1016/0035-9203(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 38.Morens DM, Marchette NJ, Chu MC, Halstead SB. Growth of dengue type 2 virus isolates in human peripheral blood leukocytes correlates with severe and mild dengue disease. Am J Trop Med Hyg. 1991;45:644–651. doi: 10.4269/ajtmh.1991.45.644. [DOI] [PubMed] [Google Scholar]
- 39.Vita WP, Nicolai CC, Azevedo MB, Souza MF, Baran M. Dengue: alertas clínicos e laboratoriais da evolução grave da doença. Rev Bras Clin Med. 2009;7:11–14. [Google Scholar]
- 40.Passos MC, Figueiredo MA. Mortalidade por dengue no estado da Bahia. Rev Baiana Saude Publica. 2011;35:687–694. [Google Scholar]
- 41.Solanke VN, Karmarkar MG, Mehta PR. Early dengue diagnosis: role of rapid NS1 antigen, NS1 early ELISA, and PCR assay. Trop J Med Res. 2015;18:95–99. [Google Scholar]
- 42.Centers for Disease Control and Prevention Laboratory guidance and diagnostic testing. [cited 2017 Aug 10]. https://www.cdc.gov/dengue/clinicallab/laboratory.html.