ABSTRACT
Considering the widespread popular use of Morus nigra and the amount of scientific information on its antioxidant and anti-inflammatory activity, the effectiveness of this phytotherapeutic compound in the parasitemia progression during the acute phase of Chagas disease and its role in the development of the inflammatory process as well as its effects on the oxidative damage in the chronic phase of infection were evaluated. Thus, 96 male Swiss mice were randomly divided into eight groups, four groups were uninfected controls, and four groups were intraperitoneally infected with 5.0 x 104 blood trypomastigotes forms of T. cruzi QM2 strain. Four batches composed of one uninfected and one infected group were respectively treated with 70% alcohol solution and 25 μL, 50 μL and 75 μL of the phytotherapeutic compound. Levels of antioxidant elements (TBARS, FRAP, GSH and Sulfhydryl groups) were measured in plasma samples. The phytotherapeutic compound’s antioxidant activity was measured by polyphenol and total flavonoid quantification, DPPH, NO, and FRAP method. Our results showed that the vehicle influenced some of the results that may have physiological relevance in Chagas disease. However, an important action of M. nigra tincture was observed in the progression of Chagas disease, since our results demonstrated a reduction in parasitemia of treated groups when compared to controls, especially in the group receiving 25 µL. However, in the chronic phase, the 50-µL dosage presented a better activity on some antioxidant defenses and minimized the tissue inflammatory process. Results indicated an important action of M. nigra tincture on the Chagas disease progression.
Keywords: Phytotherapy, Herbal medicine, Herbal compounds, Phytotherapeutic compound, Plant extracts, Parasitic diseases, Blackberry, Oxidative stress
INTRODUCTION
Chagas disease is a parasitic and inflammatory infection caused by the protozoan Trypanosoma cruzi (T. cruzi) discovered in 1909. It is estimated that, from 2000 to 2011, more than 1,200 cases were recorded, 70% of infections were acquired by oral transmission, 7% by vectorial transmission and 22% did not have the transmission mode identified 1 .
The vectorial transmission occurs with penetration of metacyclic trypomastigote forms in the host leading to the development of the disease’s acute phase. This initial phase can last from thirty to ninety days, with presence of high levels of parasites in bloodstream (parasitemia) and tissue, which may go unnoticed in most infected individuals due to insufficient clinical signs and symptoms 2 . Subsequently, the chronic phase of disease may be asymptomatic or symptomatic when cardiac and digestive clinical manifestations are observed. Symptomatic chronic phase of Chagas disease is characterized by low parasitemia and high level of antibodies, which makes the laboratory diagnosis possible through several different techniques 3 , 4 .
During the life cycle of T. cruzi, free radicals resulting from oxidative stress caused by both host immune response and parasite’s aerobic metabolism and, occasionally, by drugs used to treat the disease, are associated with the appearance of tissue damage 5 .
In this sense, the excessive production of free radicals can trigger oxidative stress and, consequently, the loss of primordial cellular functions, which can lead to apoptosis and / or cell necrosis 6 . Studies have shown that increased oxidative stress is associated with disease progression and the use of antioxidant elements has been effective in attenuating the oxidative damage caused by disease and may influence its course 7 , 8 .
In this context, several studies have demonstrated the efficacy of different phytotherapeutic compounds as effective antioxidant agents against many diseases, such as Alzheimer’s, thyroid disorders, hypertension and obesity 9 - 11 .
Morus nigra (M. nigra), popularly known as mulberry or blackberry, stands out for its edible fruits with bittersweet flavor and also for the wide popular use of its leaves as an hypoglycemic agent in the complementary treatment of diabetes mellitus and in menopause by attenuating climacteric symptoms 12 . Other studies have also highlighted its use as an analgesic, anti-inflammatory, antipyretic, antiseptic, healing, depurative and diuretic, anthelmintic and emetic 13 - 15 . Several chemical compounds such as alkaloids, coumarins, flavonoids, triterpenes and steroids have been investigated in the Morus genus and researches have been carried out to prove these compounds’ action on specific receptors, especially polyphenolic ones present in leaves and fruits of M. nigra, due to the presence of a wide spectrum of biochemical activities, such as antioxidant, antimicrobial, antimutagenic and immunoregulatory properties 16 , 17 .
Although the role of free radicals in inflammatory processes is widely discussed, their interactions in the body deserve extensive investigations 18 . In this context, the activity of M. nigra in the parasitemia progression during the acute phase of Chagas disease, as well the influence of this phytotherapeutic compound in the development of the inflammatory process in the chronic phase of infection and its effects on the oxidative damage originated from infection in a murine model were evaluated, considering its widespread popular use and the amount of scientific information on its antioxidant, anti-inflammatory and anti-aging activities.
MATERIALS AND METHODS
Phytotherapy compound’s antioxidant activity
The phytotherapy compound used in this research was a 20% M. nigra (blackberry) leaf dye purchased from the Panizza laboratory (batch Nº 16013B124, reference MAP012), produced in May/2012 with expiration date in May/2016.
This phytotherapy compound was diluted in different concentrations, which were later used in in vitro antioxidant activity tests, as well as in the characterization of polyphenols and flavonoids. As for the animal’s treatment, the phytotherapy compound was administered in different dosages according to the distribution of study groups. The Ethics Committee on the Use of Animals of the Medical School of Marília/CEUA-FAMEMA has approved the experimental study under the protocol Nº 178/14.
Antioxidant activity test (DPPH)
The phytotherapy compound’s antioxidant activity was determined by the H + donor ability to the stable radical 1,1-diphenyl-2-picrylhydrazyl, according to the in vitro methodology proposed by Blois 19 . The phytotherapy compound reacted with DPPH radical for a period of thirty minutes under low light and then subjected to a UV-vis Femto® spectrophotometer at a 517-nm wavelength. The antioxidant activity of the phytotherapy compound can be seen by the reagent’s degree of discoloration required for the reaction to attain a plateau, and also by the low IC50 value translating the phytotherapy compound’s ability to inhibit radical oxidation by 50%. Gallic acid was used as the standard.
Ferric Reducing the Antioxidant Power (FRAP)
The FRAP assay was performed as previously described by Benzie and Strain 20 . Sample or Trolox standard was mixed with distilled water to prepare the FRAP reagent extemporaneously, which was subsequently incubated at 37 ºC for 30 min. Maximum absorbance values were read at 595 nm UV-vis Femto® spectrophotometer. Results were expressed as micromoles of Trolox Equivalents (TE) per mL of extract.
Antioxidant ability assessment through the nitric oxide (NO) scavenging assay
The method used to measure this phytotherapy compound’s antioxidant ability was based on the Griess et al. 21 methodology with adaptations. The experiment was performed by transferring a 320-μL aliquot of each extract into test tubes, adding 360 μL of 25 mM NPS solution and 215 μL of Griess reagent. They were kept in a water bath for 150 min at 37 ºC and were then allowed to stand for an additional 60 min at room temperature. For the preparation of the standard curve, solutions ranging in concentrations from 5 to 60 μM/mL were prepared from the standard sodium nitrite solution of 500 μM/mL. Absorbance readings were performed using UV-vis Femto® spectrophotometer at 540 nm and the mean and nitrite concentration in μM/mL were calculated from the absorbances obtained.
Total phenol content
The Folin-Ciocalteu method was used to determine the total phenol content in the extracts, with gallic acid as the comparative standard 22 . Phytotherapy compound’s samples at different concentrations, distilled water and the Folin-Ciocalteu reagent were added. Then, the absorbance was measured at 725 nm using a UV-vis Femto® spectrophotometer. All measurements were performed in triplicate and results were expressed in μg of gallic acid/mL of the phytotherapy compound.
Flavonoid content
The total flavonoid content of the phytotherapy compound was determined by a UV-vis spectrophotometer, and samples were prepared according to the methodology proposed by Yao et al. 23 , based on the flavonoid complexation with AlCl3, which dislocates the absorption bands to higher wavelengths. Samples were shaken in a vortex shaker and the absorbance was measured at 510 nm using UV-vis Femto® spectrophotometer. All the tests were performed in triplicate and the results were expressed in μg of rutin/mL of the phytotherapy compound.
Animal setting and infection methodology
A total of 96 male Swiss mice with 20 days old were randomly divided into eight groups of 12 animals, consisting of 4 (-) groups of uninfected animals and 4 (+) groups of infected animals. The infected groups (+) received 5.0 x 10 4 blood trypomastigotes forms of T. cruzi QM2 strain intraperitoneally, characterized by Martins et al. 24 , from another infected mouse. One (C (+)) and one (C (-)) groups were used as controls and received 50 μl of 70% alcohol solution because this solution was the basis of the M. nigra phytotherapy compound.
The other 6 groups were treated with the phytotherapy compound and received 25 μL, 50 μL and 75 μL, these groups were named 25(+), 25(-), 50(+), 50(-), 75(+) and 75(-). All treatments were carried out every day in the morning and the mice received the alcohol solution or the phytotherapy compound orally (in their mouth) through a Gilson® automatic pipette tip for a period of 180 days. Treatment, care and euthanasia of the mice followed CEUA/FAMEMA standards (protocol: 178/14).
Parasitemia analysis
Parasitemia was investigated during the acute phase of infection (60 days), according to Brener’s method 25 . Twice a week we collected 5 µl of blood from the tails of five animals from each infected group, starting on the 7th day after infection, totaling 17 counts to each animal. These animals were randomly chosen and kept in isolated cages until the end of the experiments and treatment was administered normally until the end of the chronic phase.
Euthanasia, samples collection and preparation
For the histopathological and biochemical study, animals were euthanized with 100% CO2 on the 180th day after infection. The blood sample of each mouse was collected by cardiac puncture in tubes containing heparin, and the hemolysate was immediately performed to determine glutathione reduction according to the methodology used. The remainder of the blood sample was centrifuged at 1,110 g for 5 min at 4 ºC and the collected plasma was stored at -20 °C for use in other biochemical tests.
Histopathological study
For histopathological analysis, fragments of heart, colon and skeletal muscle tissue were collected from all mice that survived until the 180th post-infection day. The tissues were embedded in paraffin and 5 µm sections, were stained with hematoxylin-eosin and examined under a light microscope with 400x magnification. For each fragment, five sequential histological sections were performed, which were analyzed and graded according to the intensity of the inflammation process and the amount of amastigotes nests. A total of 10 high magnification fields were analyzed for each type of tissue. A semiquantitative scale varying from zero to three was used to grade the inflammatory process, the amastigote nests and the necrosis. “Zero” was considered as the absence of inflammation, necrosis and amastigote nests, “+” - mild inflammation, and rare amastigote nests and necrosis, “++” - moderate inflammation, moderate number of amastigote nests and necrosis, and “+++” - intense inflammation, frequent amastigote nests and necrosis.
Antioxidant activity and oxidative stress assessment in vitro
FRAP
The antioxidant ability was determined using the technique described by Benzie and Strain 20 . It is based on the ability of the plasma to reduce the FeIII to FeII ions in the presence of 2,4,6-tripyridyltriazine at low pH, which results in the formation of a blue color read in a spectrophotometer at 593 nm. Plasma antioxidant ability was determined for each sample by comparing the absorbance test with the absorbance of FeII solutions of known concentrations (100-1000 mmol/L) tested in parallel.
TBARS
The thiobarbituric acid reactive species (TBARS), used as biomarkers of lipid peroxidation, was measured in plasma using a method adopted from Yagi 26 . TBARS samples concentrations were determined by comparing their absorbance with a standard malondialdehyde solution.
GSH
This method relies on the reduction of 5,5’-dithiobis acid (2-nitrobenzoic acid) (DTNB). The concentration of erythrocyte glutathione (GSH) was determined by the colorimetric method developed by Beutler 27 . The DTNB in the presence of glutathione produces a yellow compound, the optical density of which is measured at 412 nm. The concentration of GSH was expressed in mmol/g of Hb.
Total sulfhydryl groups (Ellman’s test protocol)
The methodology proposed by Sedlak and Lindsay 28 using the Ellman reagent DTNB (5,5´-dithio-bis 2-nitrobenzoic acid) was employed. The thiol groups of the sample react with DTNB, forming a complex that absorbs light at 412 nm. The concentration of the total sulfhydryl groups was calculated by the equation {[(Absorbance2 - Absorbance1] - White)/ Ɛ* DTNB} x dilution x 1000 with the molar absorption coefficient used for DTNB Ɛ =13,600 cm-1 M-1.
Statistical Analysis
Data normality was verified using the Shapiro-Wilk test (W). The ANOVA test was used for the comparisons between three or more groups complemented by the Tukey test. The Games-Howell test was used when the homogeneity of the variances was unidentified. Kruskal-Wallis non-parametric analysis of variance (KW), complemented by Dunn’s test for multiple comparisons, was used when data normality was not identified in at least one of the groups in comparison 29 . The level of significance was set at 5%.
RESULTS
Phytotherapy compound’s antioxidant activity: polyphenol and total flavonoid quantification, DPPH, NO and FRAP method
The assessment of the phytotherapy compound’s polyphenol and total flavonoid concentrations showed 261.66 μg of gallic acid equivalent/mL and 361.83 μg of equivalent routine/mL of the phytotherapy compound, respectively.
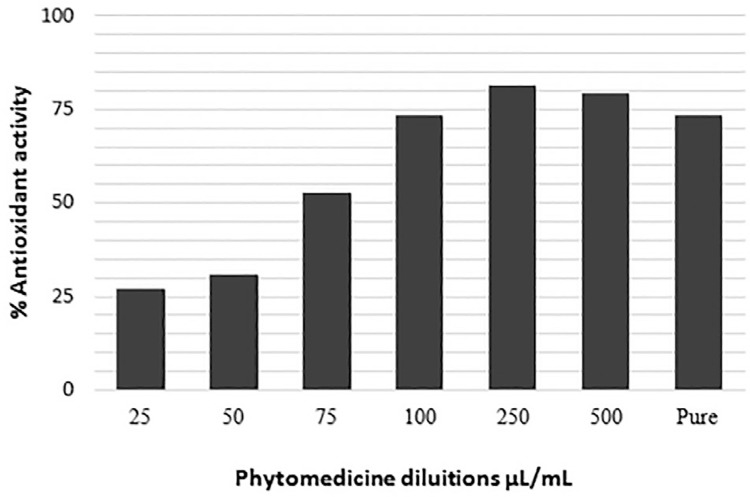
To determine the antioxidant activity by the FRAP method, 32.09 μM of trolox/g equivalent of the phytotherapy compound was used. Similarly to the DPPH test, Figure 1 shows that once different dilutions and pure phytotherapy compound were used, the antioxidant activity was progressively increased with increasing concentrations and the highest antioxidant activity was observed at 250 μL/mL of concentration presenting 81% of reduction, as shown in Table 1, and EC50= 72.28 μL/mL (Figure 1).The potential for NO sequestration has also been tested. For this assessment, it was possible to verify a concentration of 13.64 μM/mL of nitrite formed, values close to those obtained with the gallic acid control that presented 10.11 μM/mL.
Figure 1. Free radical scavenging activity DPPH in M. nigra - phytotherapy compound.
Table 1. Free radical DPPH and nitric oxide (NO) scavenging activity, polyphenols, total flavonoid and FRAP test in M. nigra - phytotherapy compound.
| Total Polyphenolsa | Total Flavonoidsb | FRAPc | DPPH EC50% (µl/mL) | Nitric Oxided Control: 10.11e | |
|---|---|---|---|---|---|
| Phytomedicine | 261.66 | 361.83 | 32.09 | 72.2 | 13.64 |
aµg of gallic acid quivalent/mL of the phytomedicine, bµg of rutine equivalent/mL of phytomedicine. cµM trolox equivalent/g of phytomedicine. dµM/mL of nitrite concentration formed by the antioxidant capacity of NO capture. e µM/mL of gallic acid.
Assessment of parasitemia in the acute phase
It is possible to observe that, in Figure 2, since the first count, the group C(+) presented with higher parasitemia when compared to the groups treated with the phytotherapy compound, remaining superior throughout the analyzed period.
Figure 2. Parasitemia curve by logarithmic mean of the number of trypomastigotes/5 μL of blood in the different experimental groups during the acute phase from the 7th to the 60th day of infection.
It was observed that the different concentrations were statistically different when compared to control groups, which exhibited the highest levels of parasitemia. It was verified that the 25(+) group exhibited the lowest parasitemia with p <0.05, when compared to 50(+), 75(+) and C(+). The groups that received 50(+) and 75(+) showed p <0.05 when compared to the C(+)
Assessment of antioxidant ability in plasma
The antioxidant capacity of the phytotherapy compound was performed in the animals that survived the studied period, as can be observed in Table 2.
Table 2. Number of animals that survived in each group during the chronic phase of infection.
| Group | Number of animals | |
|---|---|---|
| Uninfected | Control(-) | 11 |
| 25μL(-) | 11 | |
| 50μL(-) | 11 | |
| 75μL(-) | 9 | |
| Infected | Control(+) | 8 |
| 25μL(+) | 10 | |
| 50μL(+) | 8 | |
| 75μL(+) | 12 |
FRAP
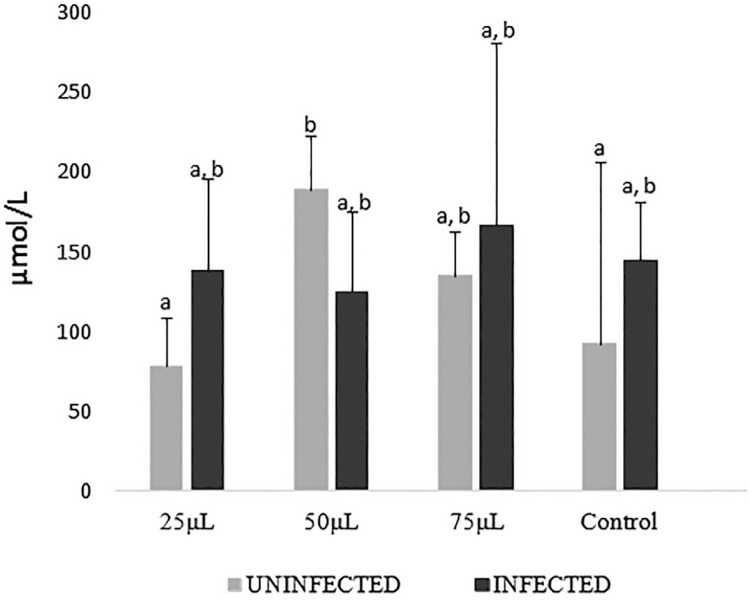
Taking into account the absence of infection, it is possible to observe that in Figure 3 the phytotherapy compound administration increased the concentrations of FRAP at the doses of 50(-) and 75 (-) in a similar way (p=0.05), but these results differed from the one obtained for 25(-) (p<0.05). These values did not differ statistically from group C(-).
Figure 3. FRAP results in different concentrations of the phytotherapy compound of M. nigra expressed in mean and standard deviation. Averages followed by the same designation do not differ from one another by the Dunn test at the 5% probability level.
When comparing FRAP concentrations in the different dosages with C(-), although increments can be observed, these differences did not reach statistical significance (p> 0.05). This may suggest an effect of the alcoholic vehicle acting in a dose dependent manner, with a maximum effect in the 50(-) group.
To evaluate the animals, compared with the C (+) group, only the group 75 (+) showed a statistically significant difference (p <0.05) with increasing FRAP concentrations.
When the effect of different dosages of each uninfected group was compared with its infected counterpart, differences (p<0-.05) were observed in the group that ingested 50 µL, which, when under infection, had their FRAP values decreased.
When comparing the controls with their infected counterparts, the 50 µL group showed a greater reduction of approximately 30% when under infection condition (p<0.05). The effects of the different dosages of each uninfected group were compared with –the infected counterparts and significant differences (p<0-.05) were observed in the group that ingested 50 µL, which, when under infection, had their FRAP values decreased in approximately 30% (p<0.05). .
TBARS
It was observed that, in Figure 4, the C(-) group produced TBARS levels which were relatively higher than those obtained in the other groups with or without infection, not differing only from the 25(-) group, and this dosage showed the highest level of peroxidation in the absence of infection.
Figure 4. TBARS results in different concentrations of the phytotherapy compound of M. nigra expressed in mean and standard deviation. Averages followed by the same designation do not differ from one another by the Dunn test at the 5% probability level.
However, in relation to the 50(-) and 75(-) groups, when compared to C(-), there was a statistically significant reduction in TBARS levels of approximately 80%. This may indicate that the presence of the phytotherapy compound at these concentrations minimizes the possible alcohol-induced lipid peroxidation.
The C(+) group did not differ statistically from the other infected groups. The animals of 75(+) presented higher levels of TBARS when compared to C(+), but without statistical significance. Between the 25(+) and 50(+) groups, there were also no statistically significant differences.
GSH
Regarding GSH concentrations, as seen in Figure 5, the group’s 25(-), 50(-) and 75(-) presented higher concentrations of GSH when compared to C(-) group receiving the alcoholic solution, indicating that the phytotherapy compound induced effects mainly among animals receiving 50 µL and 75 µL of tincture.
Figure 5. GSH results in different concentrations of the phytotherapy compound of M. nigra expressed in mean and standard deviation. Averages followed by the same designation do not differ from one another by the Dunn test at the 5% probability level.
When comparing C(+) and C(-), there was no statistically significant difference, evidencing that, in the chronic phase, the infection is not acting as an inducer of GSH synthesis.
It is also possible to observe that, among the infected groups, differences were not statistically significant among them, nor when they were compared to C(+), suggesting that the phytotherapy compound does not increase glutathione in the chronic phase infection.
However, for the groups that received 50 µL and 75 µL, the values are relevant when compared to the respective uninfected groups, because there is a statistically significant decrease, suggesting that the glutathione decrement is inherent to the its consumption during the infection process. When comparing 50(-) and 75(-) with each other, they were not statistically different, inferring a maximal inducing effect at 50 µL.
Total Sulfhydryl Groups
Figure 6 shows that the 25(-) dosage decreased the total sulfhydryl groups in relation to 50(-) (p<0.05). This dosage, however, was not different from the concentration observed in 75(-), differing, however, from C(-) (p<0.05). This may evidence a possible phytotherapeutic effect at this concentration in increasing this type of defense mechanism.
Figure 6. Sulfhydryl groups results in different concentrations of the phytotherapy compound of M. nigra expressed in mean and standard deviation. Averages followed by the same designation do not differ from one another by the Dunn test at the 5% probability level.
Among the animals in the infected groups, no statistically significant difference was observed, a fact that has also occurred when they were compared to C(+) (p=0.05). When comparing the uninfected groups with their respective infected counterparts, the differences were also not statistically significant.
Histopathological study
Considering uninfected animals that received tincture, no inflammation, necrosis or amastigote nests were observed in the histopathological analysis. Table 3 demonstrates exclusively the animals that presented any change in one or more of the analyzed parameters.
Table 3. Histopathological analysis performed in skeletal muscle, cardiac muscle and colon during the chronic phase of infection, in mice experimentally infected by Trypanosoma cruzi QM2 strain and treated with alcoholic solution (control), 25, 50 and 75 μL of Morus nigra phytotherapy compound.”%” indicates the percentage of mice with inflammation or necrosis in each group, and ( ) * indicates the absolute number of animals in each group.
| Groups | Histological Analysis | Skeletal muscle | Cardiac muscle | Colon | |
|---|---|---|---|---|---|
| C(+) | Inflammation %(8)* | + | 50.0 (4) | 87.5 (7) | 62.5 (5) |
| ++ | 37.5 (3) | --- | --- | ||
| +++ | --- | --- | --- | ||
| Amastigote %(8)* | + | --- | 25.0 (2) | 12.5 (1) | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| Necrosis %(8)* | + | 25.0 (2) | --- | --- | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| 25(+) | Inflammation %(10)* | + | 90.0 (9) | 40.0 (4) | 10.0 (1) |
| ++ | 10.0 (1) | 40.0 (4) | --- | ||
| +++ | --- | --- | --- | ||
| Amastigote %(10)* | + | 8.3 (1) | --- | 30.0 (3) | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| Necrosis %(10)* | + | 20.0 (2) | --- | --- | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| 50(+) | Inflammation %(8)* | + | 62.5 (5) | 25.0 (2) | 37.5 (3) |
| ++ | 12.5 (1) | 12.5 (1) | --- | ||
| +++ | --- | --- | --- | ||
| Amastigote % (8)* | + | 12.5 (1) | --- | --- | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| Necrosis %(8)* | + | 12.5 (1) | --- | --- | |
| ++ | 12.5 (1) | --- | --- | ||
| +++ | --- | --- | --- | ||
| 75(+) | Inflammation %(12)* | + | 83.3 (10) | 58.3 (7) | 24.9 (3) |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| Amastigote %(12)* | + | 8.3 (1) | --- | --- | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- | ||
| Necrosis %(12)* | + | 16.6 (2) | --- | --- | |
| ++ | --- | --- | --- | ||
| +++ | --- | --- | --- |
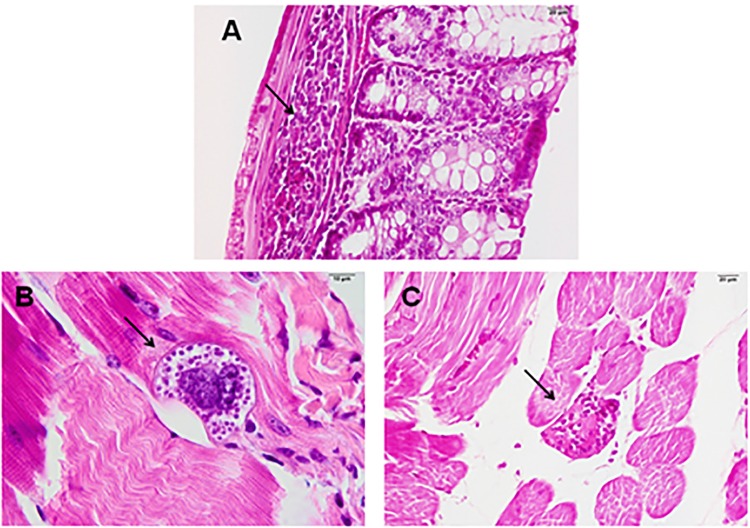
It was observed that the highest levels of inflammation were found in the skeletal muscle, heart and colon (Figure 7A), respectively, in all study groups.
Figure 7. – Histological preparations of infected animals stained with hematoxylin-eosin: A) presence of inflammation in the control animal’s colon; B) presence of amastigotes nests in the skeletal musculature of animals from the 50 μL group; C) myocardial necrosis in a sample from a 25 μL group animal.
In skeletal muscle, all animals in the 25 (+) group presented inflammatory process, being 90% discrete ad 10% moderate; however, 37.5% of the animals in group C (+) presented moderate inflammation. Regarding the heart, although C (+) presented a greater number of animals (87.5%) with mild inflammatory process in the heart, in the group 25 (+) 40% of the animals presented moderate inflammation. As for the colon, the group C (+) had a higher number of animals with inflammatory process.
However, as shown in Figure 7B, the 25 (+) group had a higher number of animals with amastigote nests. In the case of necrosis, all groups had necrotic processes in skeletal tissues in 2 animals from each group, as shown in Figure 7C.
DISCUSSION
Several studies have demonstrated the relationship between oxidative stress and antioxidant defenses during the course of infection and progression of Chagas disease in humans and experimental models 7 , 30 .
The intense pharmacological potential of phenolic compounds and the presence of flavonoids can result in the ability to act on inflammation and on the immune system 31 . In addition, the antioxidant activity of these compounds is predominantly due to their ability to act as reducing agents of reactive oxygen species (ROS), in addition of reducing or chelating ferric ions that catalyze lipid peroxidation 32 .
Thus, the in vitro results obtained in this research (Table 1) corroborated several studies demonstrating that M. nigra leaves constitute a rich source of phenolic compounds capable of positively impacting human health, in addition to presenting a higher content of phenolic compounds and antioxidant activity when compared to its fruits 33 , 34 .
Our results showed that the 25 (+) group exhibited a lower parasitemia when compared to the other groups (Figure 2). This decrease could be related to the anti-inflammatory activity of M. nigra leaves that contain germanicol among other compounds, which is described as an important natural anti-inflammatory, since flavonoids act modulating cells involved with inflammation 16 .
In addition, this decrease may be related to the availability of NO, since the ability to inhibit NO synthesis by leaf extracts of the Morus genus is described in a dose-dependent manner 35 . Thus, results could indicate that the lower dose of tincture used in the treatment induced a greater control of parasitemia (Figure 2) by allowing a greater availability of nitric oxide, which plays an important role in the defense system to combat the parasite.
However, animals from the C (+) group showed patent parasitemia for a longer period of time probably due to the chronic use of ethanol, which according to Gomes and Pereira 36 can induce alterations in the nonspecific defense mechanisms or in immune responses. Thus, the chronic use of alcohol could explain the results disagreements reported by Gusmão et al. 37 when studying the parasitemia infected with the QM2 strain of T. cruzi receiving only water.
Although some studies have shown that the decrease of parasites influences the reduction of cardiac damage and the increase of animals’ survival, our results showed that although the 25(+) group presented lower parasitemia in the acute phase, the reduction of the inflammatory process in histopathological studies was observed in the 50(+) group (Table 3). These differences may be related to the levels of active compounds present in the different dosages administered, since they may inhibit oxidation processes in certain systems, but this does not mean that they can protect cells and tissues from all types of oxidative damages, since they may present pro-oxidant activity under certain conditions 38 , 39 .
Our results evidenced complex interactions in the defense systems according to the infection status, as well as the possible effect of the phytotherapy compound’s vehicle itself on the evaluated parameters.
Thus, administration of the phytotherapy compound showed a maximal effect on FRAP at the 50 µL dosage (Figure 3) that could translate a beneficial protection effect for cells and tissues from the oxidative damage, since this method mainly reflects the reducing action of these phenolic compounds 40 .
This dosage was still effective in increasing another defense mechanism, such as total sulfhydryl groups reaction (Figure 6) that represent the first line of defense, and the most ready source of antioxidants in plasma that can act in situations in which vitamins C and E are not effective, acting also as savers of these vitamins 41 . This parameter relates to all thiols found in GSH and plasma proteins, such as albumin, and low molecular weight compounds consisting of free sulfhydryl, reduced cysteine residues in particular, cystenyl glycine and others which are targeted during the oxidative attack 38 , 42 .
The reduction of sulfhydryl groups concentration could reflect the oxidative alteration of proteins resulting in a decrease in the arsenal of antioxidant defenses, which could be observed in the FRAP result of the infected groups which would be used to neutralize the free radicals generated, minimizing their damage on cellular structures.
In the present study, it is possible that T. cruzi infection has induced a minimized oxidative stress by mobilizing a defense arsenal that also combated the damage produced by alcohol. Unexpectedly, the presence of infection also appears to decrease TBARS levels (Figure 4), as can be observed in the C(+) group which had a 50% reduction when compared to C(-) (p <0.05).
However, when comparing the uninfected groups with their infected counterparts, it was evidenced that the 25µL dosage resulted in a statistically significant reduction of parasitemia. Considering that this group presented, in absence of infection, a high concentration of TBARS, this expressive reduction in the presence of infection may suggest, once again, that the mobilized antioxidant defenses during infection has also acted in this situation.
It was observed that the phytotherapy compound was able to increase glutathione concentrations (Figure 5) in the absence of infection, which provided a possible defense against the oxidative stress caused by infection, as can be observed by the reduction of glutathione concentration in infected groups.
The possible regulatory role of some flavonoids on the level of glutathione γ-SGC (γ-glutamylcysteine synthetase) was investigated in incubated cells that received different treatments. Flavonoids usually limit the rate of protein synthesis and regulate intracellular GSH levels, capable of increasing the intracellular concentration of glutathione γ-GCS by approximately 50% 43 .
The metabolism of glutathione and the interaction with the chronic use of ethanol is not well defined and may be related to the time of exposure. Studies have reported that chronic ethanol administration may increase the activity of glutathione peroxidase enzymes by 45% as a consequence of their action on peroxidation and glutathione reductase activities leading to a 15% reduction as a result of increased GSH demand for their oxidized form 44 , 45 .
Molina et al. 46 reported that ethanol and its metabolites facilitate biomolecules’ oxidation, acting directly as oxidants reducing antioxidant levels or by combining both phenomena, increasing the levels of molecules with antioxidant properties such as flavonoids. These molecules have been increasingly investigated as possible therapeutic agents for the processes of tissue injury resulting from the excessive ethanol intake, demonstrating the protective effect of these substances against the oxidative stress caused by the excess consumption of ethanol. In our study, the increase of glutathione observed in the groups treated with the phytotherapy compound , has demonstrated a beneficial effect in the process of T. cruzi infection, more specifically regarding the level of tissue inflammation observed in the 50(+) group (Table 3). Apparently, the phytotherapy compound could induce a response and this response may have been relevant in the context of infection.
The presence of this response to the phytotherapy compound administration may be important, since previous research has reported that, in the chronic phase of disease, there is a depletion of the antioxidant defense system involving glutathione, which no longer responds to up regulation mechanisms as occurs in the acute phase 47 , 48 .
Thus, the results of this study corroborate other researches, making it evident that natural products may be a source of new drugs with high activity and low toxicity of their secondary metabolites and that the M. nigra leaves are a promising source of natural compounds that should be considered for future studies, which could provide alternatives for Chagas disease treatment.
However, more detailed studies are needed mainly regarding the mechanisms of action and bioavailability, as well as the isolation and characterization of the phytotherapy compounds detected or not in this work, in order to collaborate with possible pharmacological applications of this M. nigra phytotherapy compound. herbal medicine.
REFERENCES
- 1.Cruz Fundação Oswaldo. Doença de Chagas. Rio de Janeiro: FIOCRUZ; 2013. [cited 2016 Dec 08]. https://agencia.fiocruz.br/doen%C3%A7a-de-chagas. [Google Scholar]
- 2.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 3.Portela-Lindoso AA, Shikanai-Yasuda MA. Doença de Chagas crônica: do xenodiagnóstico e hemocultura à reação em cadeia da polimerase. Rev Saude Publica. 2003;37:107–115. doi: 10.1590/s0034-89102003000100016. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Research priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. Geneva: WHO; 2012. [cited 2017 Aug 21]. http://apps.who.int/iris/bitstream/10665/77472/1/WHO_TRS_975_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 5.Turrens JF. Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med. 2004;25:211–220. doi: 10.1016/j.mam.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 7.Maçao LB, Wilhelm D, Filho, Pedrosa RC, Pereira A, Backes P, Torres MA, et al. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol. 2007;123:43–49. doi: 10.1016/j.ijcard.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira TB, Pedrosa RC, Wilhelm D., Filho Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol. 2007;116:357–363. doi: 10.1016/j.ijcard.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 9.El-Sayyad HI, Elmansi AA, Bakr EH. Hypercholesterolemia-induced ocular disorder: Ameliorating role of phytotherapy. Nutrition. 2015;31:1307–1316. doi: 10.1016/j.nut.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Mezzomo TR, Nadal J. Efeito dos nutrientes e substâncias alimentares na função tireoidiana e no hipotireoidismo. Demetra. 2016;11:427–443. [Google Scholar]
- 11.Bai M, Yao GD, Liu SF, Wang D, Liu QB, Huang XX, et al. Lignans from a wild vegetable (Patrinina villosa) able to combat Alzheimer’s disease. J Funct Foods. 2017;28:106–113. [Google Scholar]
- 12.Miranda MA, Vieira GD, Alves MA, Yamamoto CH, Pinho JJ, Sousa OV. Uso etnomedicinal do chá de Morus nigra L. no tratamento dos sintomas do climatério de mulheres de Muriaé, Minas Gerais, Brasil. HU Rev. 2010;36:61–68. [Google Scholar]
- 13.Naderi GA, Asgary S, Sarraf-Zadegan N, Oroojy H, Afshin-Nia F. Antioxidant activity of three extracts of Morus nigra. Phytother Res. 2004;18:365–369. doi: 10.1002/ptr.1400. [DOI] [PubMed] [Google Scholar]
- 14.Quer PF. Plantas medicinales: el discórides renovado. 6ª. Barcelona: Labor; 2007. [Google Scholar]
- 15.Padilha MM, Moreira LQ, Morais FF, Araújo TH, Silva GA. Estudo farmacobotânico das folhas de amoreira-preta, Morus nigra L., Moraceae. Ver Bras Farmacogn. 2010;20:621–626. [Google Scholar]
- 16.Simões CA, Schenkel EP, Gosmann G, Mello JC, Mentz LA, Petrovick PR, organizadores, compilers. In: Farmacognosia da planta ao medicamento. 6ª. Florianópolis: Editora da UFSC; 2007. [Google Scholar]
- 17.Ozgen M, Serce S, Kaya C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci Hortic. 2009;119:275–279. [Google Scholar]
- 18.Jiang T, Sun Q, Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 20.Benzie IF, Strain JJ. The reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Griess P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Eur J Inorg Chem. 1879;12:426–428. [Google Scholar]
- 22.Stagos D, Portesis N, Spanou C, Mossialos D, Aligiannis N, Chaita E, et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem Toxicol. 2012;50:4115–4124. doi: 10.1016/j.fct.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Yao X, Zhu L, Chen Y, Tian J, Wang Y. In vivo and in vitro antioxidant activity and α-glucosidase, α-amylase inhibitory effects of flavonoids from Cichorium glandulosum seeds. Food Chem. 2013;139:59–66. doi: 10.1016/j.foodchem.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Martins LP, Marcili A, Castanho RE, Therezo AL, Oliveira JC, Suzuki RB, et al. Rural Triatoma rubrovaria from Southern Brazil Harbors Trypanosoma cruzi of Lineage IIc. Am J Trop Med Hyg. 2008;79:427–434. [PubMed] [Google Scholar]
- 25.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- 26.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 27.Beutler E. Red cell metabolism: a manual of biochemical methods. 3rd. Orlando: Grune & Stratton; 1984. [Google Scholar]
- 28.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and non protein sulfhy dry groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 29.Armitage P, Berry G. Estadística para la investigación biomédica. 3ª. Madrid: Harcourt Brace; 1997. [Google Scholar]
- 30.Budni P, Pedrosa RC, Garlet TR, Dalmarco EM, Dalmarco JB, Lino MR, et al. Carvedilol atenua o estresse oxidativo na cardiopatia chagásica crônica. Arq Bras Cardiol. 2012;98:218–224. doi: 10.1590/s0066-782x2012005000015. [DOI] [PubMed] [Google Scholar]
- 31.Coutinho MA, Muzitano MF, Costa SS. Flavonoides: potenciais agentes terapêuticos para o processo inflamatório. Rev Virtual Quim. 2009;1:241–256. [Google Scholar]
- 32.Delazar A, Talischi B, Nazemiyeh H, Rezazadeh H, Nahar L, Sarker SD. Chrozophorin: a new acylated flavone glucoside from Chrozophora tinctoria (Euphorbiaceae) Rev Bras Farmacogn. 2006;16:286–290. [Google Scholar]
- 33.Sánchez-Salcedo EM, Mena P, García-Viguera C, Martínez JJ, Hernández F. Phytochemical evaluation of white (Morusalba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J Funct Foods. 2015;12:399–408. [Google Scholar]
- 34.Tallini LR, Pedrazza GP, Bordignon SA, Costa AC, Steppe M, Fuentefria A, et al. Analysis of flavonoids in Rubus erythrocladus and Morus nigra leaves extracts by liquid chromatography and capillary electrophoresis. Rev Bras Farmacogn. 2015;25:219–227. [Google Scholar]
- 35.Dkhil MA, Bauomy AA, Diab MS, Al-Quraishy S. The antioxidant effect of Morus nigra leaves extract on kidney, testes, spleen and intestine of mice. Pakistan J Zool. 2015;47:393–397. [Google Scholar]
- 36.Gomes NG, Pereira FE. Efeitos da intoxicação crônica com o etanol na evolução da trípanosomíase cruzi experimental no camundongo. Rev Soc Bras Med Trop. 1989;22:191–197. [PubMed] [Google Scholar]
- 37.Gusmão AS, Castanho RE, Andrade RF, Farsetti CM, Mathias AB, Therezo AL, et al. Vitamin C effects in mice experimentally infected with Trypanosoma cruzi QM2 strain. Rev Soc Bras Med Trop. 2012;45:51–54. doi: 10.1590/s0037-86822012000100010. [DOI] [PubMed] [Google Scholar]
- 38.Decker EA. Phenolics: prooxidants or antioxidants? Nutrit Rev. 1997;551:396–407. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 39.Lo Presti MS, Bazán C, Strauss M, Báez AL, Rivarola W, Paglini-Oliva PA. Trypanothione reductase inhibitors: overview of the action of thioridazine in different stages of Chagas disease. Acta Trop. 2015;145:79–87. doi: 10.1016/j.actatropica.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 41.Wayner DD, Burton GW, Ingold KU, Barclay LR, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidante activity of human blood plasma. Biochim Biophys Acta. 1987;924:408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 42.Silva RA. Avaliação do quadro de estresse metabólico em ratos Wistar expostos à aflatoxina B1. 311Rev Inst Adolfo Lutz. 2007;66 [Google Scholar]
- 43.Myhrstad MC, Carlsen H, Nordström O, Blomhoff R, Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32:386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 44.Jordão AA, Jr, Chiarello PG, Bernardes MS, Vannucchi H. Peroxidação lipídica e etanol: papel da glutationa reduzida e da vitamina E. Med Ribeirao Preto. 1998;31:434–449. [Google Scholar]
- 45.MacDonald CM. The effects of ethanol on hepatic lipid peroxidation and on the activities of glutathione reductase and peroxidase. FEBS Lett. 1973;35:227–230. doi: 10.1016/0014-5793(73)80291-1. [DOI] [PubMed] [Google Scholar]
- 46.Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects agaistetanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26:1398–1402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- 47.Wen JJ, Garg N. Oxidative modification of mitochondrial respiratory complexes in response to the stress of Trypanosoma cruzi infection. Free Radic Biol Med. 2004;37:2072–2081. doi: 10.1016/j.freeradbiomed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Wen JJ, Vyatkina G, Garg N. Oxidative damage during chagasic cardiomyopathy development: Role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]