Abstract
Purpose
Although cisplatin-based adjuvant chemotherapy improves the survival of patients with resected non-small-cell lung cancer, not all patients show a survival benefit, and some patients experience severe toxicity. Therefore, identifying biomarkers is important for selecting subgroups of patients who may show improved survival with platinum-based adjuvant chemotherapy. S100A16 is thought to play key roles during different steps of tumor progression. The aim of this study was to evaluate the use of S100A16 expression as a prognostic marker in patients with completely resected lung adenocarcinoma receiving platinum-based adjuvant chemotherapy.
Methods
S100A16 expression was immunohistochemically studied in 65 consecutive lung adenocarcinoma patients who underwent complete resection and received platinum-based adjuvant chemotherapy. Kaplan–Meier survival analysis and Cox proportional hazards models were used to estimate the effect of S100A16 expression on disease-free survival (DFS) and overall survival (OS).
Results
S100A16 expression was detected in 26 of the 65 (40.0%) lung adenocarcinoma patients. Although S100A16 expression was not correlated with DFS (P=0.062), it was significantly correlated with OS (P=0.009). In addition, multivariable analysis revealed that S100A16 expression independently predicted a poorer survival (HR =4.79; 95% CI =1.87–12.23; P=0.001).
Conclusion
The present study revealed that S100A16 is a promising candidate as a prognostic marker for platinum-based adjuvant chemotherapy in resected lung adenocarcinoma. A further large-scale study is needed to confirm the present results.
Keywords: S100A16, lung adenocarcinoma, platinum-based adjuvant chemotherapy, immunohistochemistry, prognostic marker
Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related mortality worldwide, and lung adenocarcinoma is the most common histological type of NSCLC.1,2 Despite advances in surgical techniques, the 5-year survival rate for patients with surgically resectable NSCLC has only slightly improved over the last few decades.3
Recently, several randomized controlled trials such as the International Adjuvant Lung Cancer Trial,4 the National Cancer Institute of Canada Clinical Trials Group JBR.10,5 and the Adjuvant Navelbine International Trialist Association6 have indicated that cisplatin-based adjuvant chemotherapy significantly improves the survival of patients with resected NSCLC. However, not all patients show a survival benefit, and some patients experience severe toxicity. Therefore, identifying biomarkers is important for selecting subgroups of patients who may show improved survival with platinum-based adjuvant chemotherapy.
The S100 protein family comprises calcium-binding protein with the EF-hand motif that consists of at least 25 distinct members, and it plays roles including intracellular and extracellular functions involved in the cell cycle, growth, migration, and protein phosphorylation.7,8 Recently, the S100 protein family was implicated in multiple stages of tumor progression such as cell proliferation, migration, invasion, and apoptosis. Moreover, the expression of the S100 protein family is detected in many human cancers, and it has been reported to be correlated with a poorer prognosis.9 S100A16 was recently identified as a new member of the S100 protein family,10 and S100A16 expression is upregulated in tumors of the bladder, lung, thyroid gland, pancreas, and ovary.11 Our previous study indicated that S100A16 expression is correlated with vessel invasion and a poor prognosis in patients with completely resected lung adenocarcinoma. In addition, S100A16 expression is an independent prognostic marker in patients with resected lung adenocarcinoma.12 However, it is unclear whether S100A16 contributes to the chemoresistance of cancer cells, and no report is available concerning the significance of S100A16 expression and efficacy of systemic chemotherapy. Recently, epithelial–mesenchymal transition (EMT) was reported to be correlated with more aggressive tumor behavior and the chemoresistance of malignant tumors.13 Zhou et al reported that S100A16 promotes EMT through the Notch1 signaling pathway in breast cancer.14 Therefore, the aims of the present study were to immunohistochemically examine S100A16 expression in tumor cells of lung adenocarcinoma patients and to estimate the prognostic impact of S100A16 expression on the survival of resected lung adenocarcinoma patients receiving platinum-based adjuvant chemotherapy. Furthermore, we performed immunohistochemical staining of the EMT-related markers E-cadherin and vimentin to explore the correlation between S100A16 and EMT in lung adenocarcinomas.
Methods
Patients and tissue specimens
A total of 65 consecutive lung adenocarcinoma patients with pathological stages IIA through IIIA who underwent complete resection and received platinum-based adjuvant chemotherapy between January 2002 and December 2012 at Kitasato University Hospital participated in this retrospective study. None of the patients had received preoperative chemotherapy and/or radiotherapy. The histological diagnosis was based on the criteria of the World Health Organization/International Association for the Study of Lung Cancer.15 The postoperative pathological stage was determined according to the 7th edition of the TNM classification.16 Clinical and pathological parameters including the age at surgical resection, sex, smoking habits, pathological TNM (p-TNM) stage, surgical procedures, adjuvant chemotherapy regimen and cycles, viability status, disease-free survival (DFS), and overall survival (OS) were extracted from the medical records. DFS was defined as the time from resection to first recurrence or death by any cause. OS was defined as the time from resection to death by any cause. The study was approved by the Ethics Committee of Kitasato University School of Medicine (KMEO B15-149). Written informed consent was obtained from all the patients.
Immunohistochemical staining
In immunohistochemical staining, 10% formalin-fixed and paraffin-embedded tissues were processed into 3-µm-thick sections. The sections were reacted with 1,000-times-diluted anti-S100A16 polyclonal antibody (Abcam, Cambridge, UK) for 2 hours, anti-vimentin antibody (clone V9; DAKO, Glostrup, Denmark) for 2 hours, and 200-times-diluted anti-E-cadherin antibody (clone HECD-1; Takara, Kusatsu, Japan) for 1 hour at room temperature. The details of the procedure were described previously by our laboratory.17
Evaluation of immunohistochemical staining
In accordance with our previous report,12 membranous staining of the tumor cells was considered to be positive for S100A16. The staining intensity was categorized into 4 groups: 0= negative; 1= weak; 2= moderate; and 3= strong. The tumors with a staining score of 2 or 3 were judged as positive. A tissue consisting of >5% positive tumor cells was considered to show significant S100A16 staining. Membranous staining of the tumor cells was considered to be a positive result for E-cadherin, and cytoplasmic staining of the tumor cells was considered to be a positive result for vimentin. According to previous studies,18,19 the evaluation of the stainability was performed in accordance with the following immunoreactive score (IRS): IRS = staining intensity (SI) × percentage of positive tumor cells (PP). SI was defined as 0, negative; 1, weak; 2, moderate; and 3, strong. PP was defined as 0, negative; 1, 1%–10% positive tumor cells; 2, 11%–50% positive tumor cells; 3, 51%–80% tumor cells; and 4, >80% positive tumor cells. An IRS value ≥4 was considered to be a positive staining result. All the immunostained sections were reviewed by two investigators (KK and YS) without any knowledge of the clinical data. Discordant cases were reviewed and discussed until a consensus was reached.
Statistical analysis
The relationships between S100A16 expression and clinicopathological parameters were analyzed by using Fisher’s exact test. Survival curves were estimated by the Kaplan–Meier method, and the log rank test was used to evaluate the significance of the survival differences between S100A16-positive and S100A16-negative groups. The Cox proportional hazards regression model was used for multivariable analysis to estimate independent prognostic factors. In the present study, sample size is small (n=65). Therefore, selecting many independent variables may statistically induce bias. We selected independent variables based on a preceding study.20 A P-value <0.05 was considered as significant. All reported P-values are two-sided. All statistical analyses were performed with EZR Version 1.33 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
S100A16 expression in lung adenocarcinoma
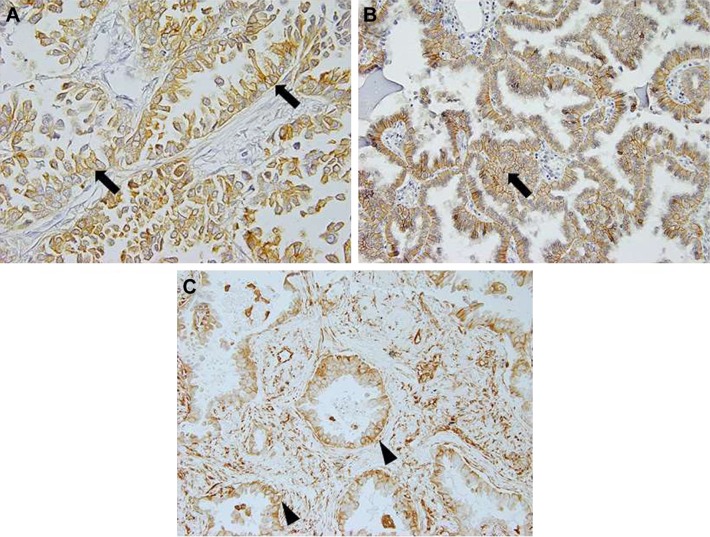
S100A16 staining was mostly observed in the plasma membrane of tumor cells, but cytoplasmic and nuclear staining was detected in some cases (Figure 1A). S100A16-positive expression was observed in 26 of the 65 (40.0%) cases. S100A16 expression was also detected in vascular endothelial cells and fibroblasts in the tumor stroma. S100A16 was not detected in normal alveolar epithelial cells.
Figure 1.
S100A16, E-cadherin, and vimentin expression in lung adenocarcinoma. (A) The majority of the tumor cells showed membranous S100A16 expression (arrows) in lung adenocarcinoma (×400 magnification). (B) Membranous staining of the tumor cells (arrows) was considered to be a positive result for E-cadherin (arrow, ×200 magnification). (C) Cytoplasmic staining of the tumor cells (arrow heads) was considered to be a positive result for vimentin (×200 magnification).
Clinicopathological characteristics of patients
Table 1 summarizes the clinicopathological characteristics of the 65 lung adenocarcinoma patients. The overall follow-up durations ranged from 5.8 to 149.7 months (median =49.6 months). A total of 30 patients were alive at the end of the follow-up, 27 patients died of lung cancer, 3 patients died from other causes, and 5 patients were lost to follow-up. The causes of the 3 non-lung-cancer deaths were multiple myeloma, endometrial cancer, and acute myocardial infarction. Loss to follow-up of the 5 patients was due to discontinuing hospital attendance, and the durations of these patients’ follow-up ranged from 14.7 to 59.5 months (median =58.2 months).
Table 1.
Characteristics of the patients
| Characteristics | Patients, n (%) (n=65) |
|---|---|
| Age, years | |
| Median age (range) | 60 (41–74) |
| ≤60 | 31 (47.7) |
| >60 | 34 (52.3) |
| Sex | |
| Male | 35 (53.8) |
| Female | 30 (46.2) |
| Smoking habit | |
| Never smoked | 36 (55.4) |
| Smoker | 29 (44.6) |
| p-TNM stagea | |
| Stage IIA/IIB | 16 (24.6) |
| Stage IIIA | 49 (75.4) |
| Surgical procedure | |
| Lobectomy | 63 (96.9) |
| Pneumonectomy | 2 (3.1) |
| Chemotherapy regimen | |
| Carboplatin-based | 18 (27.7) |
| Cisplatin-based | 47 (72.3) |
| Chemotherapy cycle | |
| 1–2 | 9 (13.8) |
| 3–4 | 56 (86.2) |
| Vital status | |
| Alive | 30 (46.2) |
| Lung cancer-related death | 27 (41.5) |
| Other causes of death | 3 (4.6) |
| Unknown | 5 (7.7) |
Note:
Each case was reassigned to a pathological stage on the basis of the IASLC Lung Cancer Staging Project (7th edition).
Abbreviations: IASLC, International Association for the Study of Lung Cancer; p-TNM, pathological TNM.
Relationship between S100A16 expression and clinicopathological characteristics
Table 2 summarizes the relationships between S100A16 expression and clinicopathological characteristics. There was no significant correlation between S100A16 expressions and the age, sex, smoking habits, tumor differentiation, or p-TMN stage.
Table 2.
Relationships between S100A16 expression and clinic-opathological parameters
| Clinicopathological parameters | S100A16 expression
|
Total | P-value | |
|---|---|---|---|---|
| Positive (n=26) |
Negative (n=36) |
|||
| Age, years; n (%) | 0.613 | |||
| ≤60 | 11 (35.5) | 20 (64.5) | 31 | |
| >60 | 15 (44.1) | 19 (55.9) | 34 | |
| Sex; n (%) | 0.623 | |||
| Male | 13 (37.1) | 22 (62.9) | 35 | |
| Female | 13 (43.3) | 17 (56.7) | 30 | |
| Smoking habits; n (%) | 0.455 | |||
| Never smoked | 10 (34.5) | 19 (65.5) | 29 | |
| Smoker | 16 (44.4) | 20 (55.6) | 36 | |
| Tumor differentiation; n (%) | 1.000 | |||
| Well | 4 (44.4) | 5 (55.6) | 9 | |
| Moderately/poorly | 22 (39.3) | 34 (60.7) | 56 | |
| p-TNM stagea; n (%) | 1.000 | |||
| Stage IIA/IIB | 6 (37.5) | 10 (62.5) | 16 | |
| Stage IIIA | 20 (40.8) | 29 (59.2) | 49 | |
Note:
Each case was reassigned to a pathological stage on the basis of the IASLC Lung Cancer Staging Project (7th edition).
Abbreviations: IASLC, International Association for the Study of Lung Cancer; p-TNM, pathological TNM.
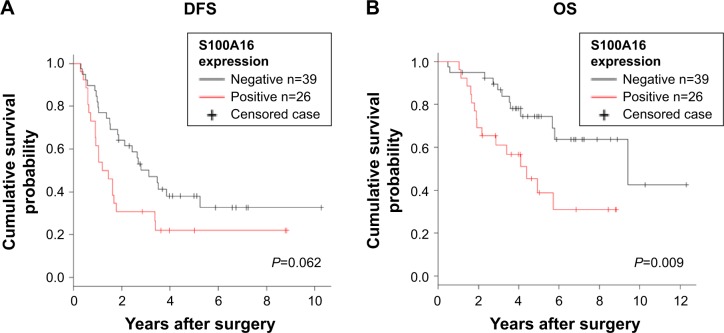
Kaplan–Meier estimates of DFS and OS in S100A16-positive and S100A16-negative patients
All the 65 patients with lung adenocarcinoma were included in the survival analysis. Although S100A16 expression was not correlated with DFS (Figure 2A), the S100A16-positive group showed significantly shorter OS than the S100A16-negative group (median =53.4 vs 114.5 months, respectively, P=0.009; Figure 2B).
Figure 2.
Survival analysis. (A) DFS; (B) OS.
Abbreviations: DFS, disease-free survival; OS, overall survival.
Effect of S100A16 expression on patients’ survival with univariable and multivariable analyses
Univariable analysis performed according to the Cox proportional hazard model indicated that the p-TNM stage (HR =10.22; 95% CI =1.38–75.45; P=0.02) and S100A16 expression (HR =2.73; 95% CI =1.25–5.96; P=0.01) were significantly correlated with shorter OS. Furthermore, multivariable analysis confirmed that S100A16 expression (HR =4.79; 95% CI =1.87–12.23; P=0.001) was significantly correlated with a shorter OS independent of other factors (Table 3).
Table 3.
Univariable and multivariable analyses of the effects of S100A16 expression on OS
| Factors | Univariable analysis
|
Multivariable analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| S100A16 expression | ||||||
| Positive vs negative | 2.73 | 1.25–5.96 | 0.01 | 4.79 | 1.87–12.23 | 0.001 |
| Age, years | ||||||
| ≤60 vs >60 | 2.01 | 0.89–4.51 | 0.09 | 1.83 | 0.75–4.45 | 0.18 |
| Sex | ||||||
| Male vs female | 0.93 | 0.43–2.03 | 0.87 | 0.39 | 0.11–1.35 | 0.14 |
| Smoking habits | ||||||
| Smokers vs nonsmokers | 1.20 | 0.56–2.60 | 0.64 | 0.49 | 0.15–1.64 | 0.25 |
| p-TNM stage | ||||||
| Stage III vs stage IIA/IIB | 10.22 | 1.38–75.45 | 0.02 | 21.44 | 2.62–175.00 | 0.004 |
| Tumor differentiation | ||||||
| Poorly/moderately vs well | 1.54 | 0.46–5.13 | 0.48 | 1.09 | 0.46–2.58 | 0.84 |
| Platinum agent | ||||||
| CDDP vs CBDCA | 0.47 | 0.22–1.01 | 0.05 | 0.40 | 0.16–1.02 | 0.06 |
| Chemotherapy cycle | ||||||
| 3–4 cycles vs 1–2 cycles | 0.65 | 0.24–1.73 | 0.39 | 1.04 | 0.28–3.92 | 0.94 |
Note: Analyses were performed using Cox proportional hazard regression model.
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; OS, overall survival; p-TNM, pathological TNM.
Relationship between S100A16 expression and EMT-related markers, E-cadherin and vimentin expressions
Immunohistochemical staining of E-cadherin and vimentin was not performed in one case because a tissue block was not available. Figure 1B and C shows positive cases of E-cadherin and vimentin. Positive S100A16 expression was not correlated with the loss of E-cadherin (P=0.945) or gain of vimentin (P=0.772; Table 4).
Table 4.
Relationships between S100A16 expression and EMT markers E-cadherin and vimentin
| Clinicopathological parameters | S100A16 expression
|
Total | P-value | |
|---|---|---|---|---|
| Positive (n=26) |
Negative (n=38) |
|||
| E-cadherin; n (%) | 0.771 | |||
| Positive | 16 (35.5) | 22 (64.5) | 38 | |
| Negative | 10 (44.1) | 16 (55.9) | 26 | |
| Vimentin; n (%) | 0.945 | |||
| Positive | 8 (37.1) | 12 (62.9) | 20 | |
| Negative | 18 (43.3) | 26 (56.7) | 44 | |
Abbreviation: EMT, epithelial–mesenchymal transition.
Discussion
In the present study, we demonstrated that S100A16 expression is correlated with a poorer prognosis and is an independent prognostic factor for survival in patients with resected lung adenocarcinoma who receive platinum-based adjuvant chemotherapy. Several studies reported that chemoresistance and metastasis were closely correlated in some molecules.21,22 We previously reported that S100A16 expression was correlated with vascular and lymphatic invasion in lung adenocarcinomas.12 Because local cancer invasion and migration are the initial steps of metastasis,23 this result raises the possibility that S100A16-overexpressing cancer cells show a more aggressive phenotype with metastatic ability, and S100A16 expression is correlated with resistance to platinum-based adjuvant chemotherapy.
The biological functions of S100A16 in cancer cells are still unclear. Zhu et al reported that, in prostate cancer, S100A16 promotes cell invasion, migration, and proliferation through the activation of the AKT signaling pathway.24 The AKT signaling pathway promotes cell survival, suppresses apoptosis, and promotes proliferation in various cancers including lung adenocarcinoma.25 Furthermore, activated AKT plays an important role in cisplatin resistance of lung cancer.26,27 Thus, our results lead to the following hypothesis: S100A16 induces the metastatic potential and resistance to platinum-based adjuvant chemotherapy of lung adenocarcinoma through the activation of the AKT signaling pathway. EMT is a process whereby epithelial cells lose polarity and cell–cell adhesion and progress to invasive mesenchymal cells.28 EMT is correlated with not only the acquisition of invasive and metastatic ability, but also the chemoresistance of several cancers.13 Zhou et al reported that S100A16 promotes EMT through the Notch1 signaling pathway in breast cancer.14 We evaluated the relationship between S100A16 expression and the EMT-related markers E-cadherin and vimentin. In the present study, there was no significant correlation between S100A16 expression and EMT markers. Therefore, these results raise the possibility that the role of S100A16 in the EMT process is different between lung adenocarcinoma and breast cancer.
Lung squamous cell carcinoma patients were excluded from the present study because of the possibility that the S100A16 function is different between lung adenocarcinoma and squamous cell carcinoma. Sapkota et al reported that, in oral squamous cell carcinoma patients, a high S100A16 expression is correlated with a less aggressive tumor phenotype and good prognosis.29 These findings suggest that the function associated with tumorigenesis and tumor progression may be different between adenocarcinoma and squamous cell carcinoma.
Our study had several limitations. First, the sample size was small, and a retrospective single-center study may involve some selection bias. Second, the platinum-based chemotherapy regimen included cisplatin and carboplatin in the present study. Evidence for the efficacy of cisplatin-based adjuvant chemotherapy is being accumulated, but evidence for the efficacy of carboplatin-based adjuvant chemotherapy from large-scale clinical trials is still insufficient.30
Conclusion
In conclusion, we revealed that S100A16 expression is a prognostic indicator of a poorer survival probability in lung adenocarcinoma patients receiving platinum-based adjuvant chemotherapy. A large-scale prospective study and elucidation of the biological functions of S100A16 are necessary to confirm the utility of S100A16 as a prognostic marker for platinum-based adjuvant chemotherapy and a promising therapeutic target for lung adenocarcinoma.
Acknowledgments
The authors are grateful to Professor Yukitoshi Satoh and Dr Kazu Shiomi (Department of Thoracic and Cardiovascular Surgery, School of Medicine, Kitasato University) for contributing to the acquisition and interpretation of the data. The authors are also grateful to Keita Saito and Kazuo Hachimura for contributing to the analysis and interpretation of the data. This study was supported in part by a Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (JSPS) KAKENHI Grant numbers JP23590414, JP26460441.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Salgia R. Mutation testing for directing upfront targeted therapy and post-progression combination therapy strategies in lung adenocarcinoma. Expert Rev Mol Diagn. 2016;16(7):737–749. doi: 10.1080/14737159.2016.1181545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50(2):227–234. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 7.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33(7):637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 8.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322(4):1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Xu C, Jin Q, Liu Z. S100 protein family in human cancer. Am J Cancer Res. 2014;4(2):89–115. [PMC free article] [PubMed] [Google Scholar]
- 10.Sturchler E, Cox JA, Durussel I, Weibel M, Heizmann CW. S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem. 2006;281(50):38905–38917. doi: 10.1074/jbc.M605798200. [DOI] [PubMed] [Google Scholar]
- 11.Marenholz I, Heizmann CW. S100A16, a ubiquitously expressed EF-hand protein which is up-regulated in tumors. Biochem Biophys Res Commun. 2004;313(2):237–244. doi: 10.1016/j.bbrc.2003.11.115. [DOI] [PubMed] [Google Scholar]
- 12.Saito K, Kobayashi M, Nagashio R, et al. S100A16 is a prognostic marker for lung adenocarcinomas. Asian Pac J Cancer Prev. 2015;16(16):7039–7044. doi: 10.7314/apjcp.2015.16.16.7039. [DOI] [PubMed] [Google Scholar]
- 13.Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21(7) doi: 10.3390/molecules21070965. pii:E965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Pan H, Xia T, et al. Up-regulation of S100A16 expression promotes epithelial-mesenchymal transition via Notch1 pathway in breast cancer. J Biomed Sci. 2014;21:97. doi: 10.1186/s12929-014-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 16.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 17.Katono K, Sato Y, Jiang SX, et al. The clinicopathological significance of S100A10 expression in lung adenocarcinomas. Asian Pac J Cancer Prev. 2016;17(1):289–294. doi: 10.7314/apjcp.2016.17.1.289. [DOI] [PubMed] [Google Scholar]
- 18.Sung R, Lee SH, Ji M, et al. Epithelial-mesenchymal transition-related protein expression in biliary epithelial cells associated with hepatolithiasis. J Gastroenterol Hepatol. 2014;29(2):395–402. doi: 10.1111/jgh.12349. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Liu J, Yue D, et al. Clinical significance of E-cadherin, beta-catenin, vimentin and S100A4 expression in completely resected squamous cell lung carcinoma. J Clin Pathol. 2013;66(11):937–945. doi: 10.1136/jclinpath-2013-201467. [DOI] [PubMed] [Google Scholar]
- 20.Ryuge S, Sato Y, Nagashio R, et al. Prognostic significance of nestin expression in patients with resected non-small cell lung cancer treated with platinum-based adjuvant chemotherapy; relationship between nestin expression and epithelial to mesenchymal transition related markers. PLoS One. 2017;12:e0173886. doi: 10.1371/journal.pone.0173886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Yue CF, Zhou WH, et al. Aurora-A contributes to cisplatin resistance and lymphatic metastasis in non-small cell lung cancer and predicts poor prognosis. J Transl Med. 2014;12:200. doi: 10.1186/1479-5876-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011;728(1–2):23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W, Xue Y, Liang C, et al. S100A16 promotes cell proliferation and metastasis via AKT and ERK cell signaling pathways in human prostate cancer. Tumour Biol. 2016;37(9):12241–12250. doi: 10.1007/s13277-016-5096-9. [DOI] [PubMed] [Google Scholar]
- 25.Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J Thorac Oncol. 2012;7(8):1315–1326. doi: 10.1097/JTO.0b013e31825493eb. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Bao C, Mu Q, et al. Reversal of cisplatin resistance by inhibiting PI3K/Akt signal pathway in human lung cancer cells. Neoplasma. 2016;63(3):362–370. doi: 10.4149/304_150806N433. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Wang Z, Liu L, Chen L. Akt is the downstream target of GRP78 in mediating cisplatin resistance in ER stress-tolerant human lung cancer cells. Lung Cancer. 2011;71(3):291–297. doi: 10.1016/j.lungcan.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Sung WJ, Park KS, Kwak SG, Hyun DS, Jang JS, Park KK. Epithelial-mesenchymal transition in patients of pulmonary adenocarcinoma: correlation with cancer stem cell markers and prognosis. Int J Clin Exp Pathol. 2015;8(8):8997–9009. [PMC free article] [PubMed] [Google Scholar]
- 29.Sapkota D, Bruland O, Parajuli H, et al. S100A16 promotes differentiation and contributes to a less aggressive tumor phenotype in oral squamous cell carcinoma. BMC Cancer. 2015;15:631. doi: 10.1186/s12885-015-1622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou W, Sun HB, Ye X, et al. Adjuvant carboplatin-based chemotherapy in resected stage IIIA-N2 non-small cell lung cancer. J Thorac Oncol. 2010;5(7):1033–1041. doi: 10.1097/JTO.0b013e3181d95db4. [DOI] [PubMed] [Google Scholar]