Abstract
The loss of muscle mass and strength with aging results in significant functional impairment. Creatine supplementation has been used in combination with resistance training as a strategy for increasing lean tissue mass and muscle strength in older adults, but results across studies are equivocal. We conducted a systematic review and meta-analysis of randomized controlled trials of creatine supplementation during resistance training in older adults with lean tissue mass, chest press strength, and leg press strength as outcomes by searching PubMed and SPORTDiscus databases. Twenty-two studies were included in our meta-analysis with 721 participants (both men and women; with a mean age of 57–70 years across studies) randomized to creatine supplementation or placebo during resistance training 2–3 days/week for 7–52 weeks. Creatine supplementation resulted in greater increases in lean tissue mass (mean difference =1.37 kg [95% CI =0.97–1.76]; p<0.00001), chest press strength (standardized mean difference [SMD] =0.35 [0.16–0.53]; p=0.0002), and leg press strength (SMD =0.24 [0.05–0.43]; p=0.01). A number of mechanisms exist by which creatine may increase lean tissue mass and muscular strength. These are included in a narrative review in the discussion section of this article. In summary, creatine supplementation increases lean tissue mass and upper and lower body muscular strength during resistance training of older adults, but potential mechanisms by which creatine exerts these positive effects have yet to be evaluated extensively.
Keywords: muscle, age, sarcopenia, exercise, nutrition, bench press, leg press
Introduction
The European Working Group on Sarcopenia in Older People characterizes sarcopenia as a loss of both muscle mass and muscle function (ie, strength and performance),1 whereas the International Working Group on Sarcopenia defines sarcopenia as a decline in muscle mass and walking speed.2 Janssen et al3 estimated that health-care costs associated with sarcopenia were $18.5 billion per year in the USA in 2000; this projects to $26.3 billion for 2017. Independent of pharmacological interventions, resistance training is effective for increasing lean tissue mass and muscular strength in older adults, with nutritional interventions (ie, creatine, proteins, and omega-3 fatty acids), further augmenting these beneficial effects on muscle.4
Creatine monohydrate is the most popular nutritional supplement used by athletes5 and increasingly used in combination with resistance training to preserve or increase lean tissue mass and muscle strength in older adults.4–6 Creatine is a compound synthesized from three amino acids, with the first steps of synthesis from arginine and glycine in the kidney and subsequent steps involving methionine in the liver.7 Creatine is consumed in the diet, mainly from beef, pork, and fish.8 The majority of creatine is taken up by skeletal muscle where it combines with phosphate to form phosphorylcreatine (PCr). PCr buffers adenosine triphosphate (ATP) levels to improve high-intensity exercise capacity,7 potentially allowing one to train with higher volumes during resistance training sessions.9 Creatine may also lead to cell swelling, through increased water via osmosis, and this may activate protein synthesis within muscle fibres.10
Previous meta-analyses have determined that creatine supplementation during resistance training is effective for improving lean tissue mass and some muscular strength measures compared with resistance training without creatine supplementation in older adults.11,12 Lean tissue mass and upper body (ie, chest press) strength were increased with high probability with creatine supplementation (p<0.01); however, the meta-analyses were mixed as to whether creatine supplementation increased lower body (ie, leg press) strength, with one meta-analysis failing to reach statistical significance (p=0.1)11 and another reaching statistical significance at p=0.02.12 It is important to determine with certainty whether creatine supplementation during resistance training can augment lower body strength since it is more negatively affected with aging than upper body strength.13 Since the publication of these meta-analyses, the number of studies on creatine supplementation during resistance training in older adults has almost doubled. The primary purpose of this review was to systematically review the literature on creatine supplementation and resistance training in older adults and perform updated meta-analyses on outcomes of lean tissue mass and muscular strength in the upper and lower body (ie, chest press and leg press strength). The latter part of this review is a narrative review of the potential physiological mechanisms by which creatine supplementation might increase muscle mass in older adults.
Meta-analysis methods
The population we chose to study was older adults; therefore, we included only studies where the mean age of participants was ≥50 years. This is the approximate age at which lean tissue mass and muscle strength begin to precipitously decline.14 The intervention we assessed was creatine monohydrate supplementation during resistance training programs that lasted for at least 5-week duration with training at least twice per week. We included studies that combined creatine monohydrate with other nutritional supplements but also ran our meta-analyses without these studies to find whether they affected the meta-analyses results. We included studies of healthy older adults and older adults with specific disease conditions and again ran our meta-analyses with and without these studies to determine how they influenced the results. We included only studies combining creatine supplementation (with or without other nutritional supplements) with resistance training as creatine is minimally effective for enhancing cellular responses, leading to muscle hypertrophy if muscle loading is not present.15 The comparator was resistance training without creatine supplementation. The outcomes we assessed were whole-body lean tissue mass, determined with dual-energy X-ray absorptiometry, hydrostatic weighing, or air displacement plethysmography, and chest press and leg press muscular strength, representing global measures of upper and lower body strength, respectively. Adverse events were assessed descriptively, as a secondary outcome measure. We included only randomized controlled studies.
PubMed and SPORTDiscus databases were searched using key terms and Boolean phrases used in previous meta-analyses11,12 – (creatine OR creatine monohydrate OR creatine supplementation OR creatine loading) AND (weight lifting OR weight training OR resistance training, OR resistance exercise OR strength training) AND (age OR middle-age OR older adults OR elderly). Abstracts and manuscripts retrieved were reviewed by at least two investigators for inclusion. A third investigator was consulted when there was disagreement about inclusion. Authors were contacted for any missing information. Databases were searched up until June 2017. There were no language restrictions. Jadad scores, based on five questions, were used to assess the quality of studies.16
Data extracted included pre- and posttraining means and SDs or change scores for outcome variables and SDs for the change scores. When pre- and posttraining means were extracted, change scores were calculated as pretraining mean subtracted from posttraining mean. SDs for the change scores were estimated from pre- and posttraining SDs (SDpre and SDpost) using the following equation derived from the Cochrane Handbook for Systematic Reviews of Interventions:17
In this equation, we used 0.8 as the assumed correlation between pre- and post-scores.
Meta-analyses were run by using RevMan 5 software (Cochrane Community, London, UK). Heterogeneity was evaluated using χ2 and I2 tests where heterogeneity was indicated by either χ2 p-value ≤0.1 or I2 test value >75%. When heterogeneity was present, we used a random-effects model, and when heterogeneity was not present, we used a fixed-effects model for our meta-analysis. Weighted mean difference was calculated for lean tissue mass, along with the 95% CI. As units of measurement differed across studies for measurements of strength, we calculated standardized mean differences (SMDs) and 95% CIs for leg press and chest press strength. Forest plots were generated for study-specific effect sizes along with 95% CIs and pooled effects. A p-value ≤0.05 was considered statistically significant. Funnel plots were generated and inspected for publication bias.
Results
A total of 321 abstracts were retrieved. After the review of abstracts, 26 full-length manuscripts were retrieved. Three manuscripts were excluded because they involved interventions of creatine supplementation without resistance training,18–20 and one study presented lean tissue mass and strength results from the same participants across two manuscripts;21,22 therefore, 22 unique studies were included. Of these studies, five combined creatine supplementation with other nutritional supplements (ie, protein or conjugated linoleic acid). Table 1 shows the description of the included studies.
Table 1.
Study characteristics and outcomes of research examining the influence of creatine in older adults with a resistance training program
| Study | Study population | Intervention | Duration | Outcome measure | Adverse events | Jadad score |
|---|---|---|---|---|---|---|
| Aguiar et al23 | n=18 (healthy women) Mean age =64.9 years |
CR (5 g/day) or PLA RT =3 days/wk |
12 wks | CR ↑ bench press, knee extension, bicep curl, fat-free mass, and muscle mass | Not reported and/or not collected | 4 |
| Alves et al24 | n=22 (healthy women) Mean age =66.8 years |
CR (4×5 g/day for 5 days, 5 g/day thereafter) or PLA RT =2 days/wk |
24 wks | ↔ leg press or chest press strength | No adverse events | 5 |
| Bemben et al21 and Eliot et al22 | n=42 (healthy men) Age =48–72 years |
CR (5 g/day), protein (35 g/day), protein (35 g/day) + CR (5 g/day) or PLA RT =3 days/wk |
14 wks | ↔ upper or leg press strength, lean tissue mass | No adverse events | 4 |
| Bermon et al25 | n=32 (16 men, 16 women, healthy) Age =67–80 years |
CR (20 g/day for 5 days followed by 3 g/day) or PLA RT =3 days/wk |
52 days | ↔ leg press and chest press strength | No adverse events | 3 |
| Brose et al26 | n=28 (15 men; 13 women, healthy) Mean age = men, 68.7 years; women, 70.8 years |
CR (5 g/day) or PLA RT=3 days/wk | 14 wks | CR ↑ lean tissue mass, isometric knee extension strength, isometric dorsiflexion strength in men ↔ sit to stand in 30 s; 30 m walking time, time to climb 14 stairs, type I, IIa, IIx muscle fiber area |
One gastrointestinal adverse event in the CR group, no kidney or liver adverse events | 3 |
| Candow et al27 | n=35 (healthy men) Age =55–77years |
CR (0.1 g/kg/day) or CR + protein (0.3 g/kg/day) or PLA RT =3 days/wk |
10 wks | CR + CR + PRO conditions ↑ muscle thickness. Participants in both CR groups had a decrease in muscle protein catabolism compared with PLA ↔ bench press or leg press strength |
No adverse events | 4 |
| Candow et al28 | n=39 (17 men, 22 women, healthy) Age =50–71 years |
CR (0.1 g/kg) before RT, CR (0.1 g/kg) after RT, or PLA RT =3 days/wk |
32 wks | CR after RT ↑ lean tissue mass CR before and after RT ↑ leg press and chest press strength |
No adverse events | 4 |
| Chilibeck et al29 | n=33 (healthy women) Mean age =57 years |
CR (0.1 g/kg/day) or PLA | 52 wks | CR ↑ chest press strength ↔ lean tissue mass or hack squat strength |
Gastrointestinal adverse events, CR =5 vs PL =2 Muscle cramps, CR =2 No adverse events related to liver or kidney function with CR |
5 |
| Chrusch et al9 | n=30 (healthy men) Age =60–84 years |
CR (0.3 g/kg/day for 5 days followed by 0.07 g/kg/day) or PLA RT =3 days/wk |
12 wks | CR ↑ lean tissue mass and leg press strength, leg press endurance, and average power (knee extension). ↔ chest press strength, chest press endurance |
CR during loading phase increased gastrointestinal adverse events CR increased muscle cramping and muscle pulls/strains |
3 |
| Collins et al30 | n=16 (frail men and women) Mean age =70 years |
CR (4 g/day) and whey protein (20 g/day) or whey protein RT =2 days/wk |
14 wks | ↔ lean tissue mass, handgrip strength, timed up and go test, number of stands in 30 s | No adverse events | 4 |
| Cooke et al31 | n=20 (healthy men) Age =55–70 years |
CR (20 g/day for 7 days; 0.1 g/day thereafter on training days) or PLA RT =3 days/wk |
12 wks | ↔ lean tissue mass, bench press or leg press strength, myofibrillar protein, type I or II muscle fiber area, serum IGF-1 | No reporting of adverse events and/or not collected | 4 |
| Cornelissen et al32 | n=70 (66 men, 4 women with coronary artery disease or chronic heart failure) Mean age =57.5 years |
CR (5 g/day) or PLA RT =3 days/wk |
3 months | ↔ isometric or isokinetic knee extension strength | No adverse events including no reports of liver or kidney adverse events | 5 |
| Deacon et al33 | n=80 (50 men, 30 women with COPD) Mean age =68.2 years |
CR (22 g/day for 5 days followed by 3.76 g/day) RT =3 days/wk |
7 wks | ↔ shuttle walk distance, knee extensor work, isometric or isokinetic strength, lean tissue mass | No reporting of adverse events and/or not collected | 5 |
| Eijnde et al34 | n=46 (healthy men) Age =55–75 years |
CR (5 g/day) or PLA RT + cardiorespiratory=2–3 days/wk |
26 wks | ↔ lean tissue mass, isometric strength | One CR group participant reported overuse trauma of the shoulder | 3 |
| Gualano et al35 | n=25 (9 men, 16 women with type 2 diabetes) Mean age =57 years |
CR (5 g/day) or PLA RT =3 days/wk |
12 wks | ↔ lean tissue mass, chest press, leg press, handgrip, and low back strength, chair stands in 30 s, timed up and go. CR decreased glycosylated hemoglobin levels, area under the blood glucose curve during oral glucose tolerance test, and increased muscle membrane glucose transport protein 4 levels |
No adverse events | 5 |
| Gualano et al36 | n=30 (“vulnerable” women) Mean age =65.4 years |
CR (20 g/day for 5 days; 5 g/day thereafter) or PLA RT =2 days/wk |
24 wks | CR ↑ lean tissue mass and chest press strength ↔ leg press strength, timed up and go test, chair stands in 30 s |
No adverse events including markers of kidney and liver function | 5 |
| Hass et al37 | n=20 (17 men, 3 women with idiopathic Parkinson’s disease) Mean age =62 years |
CR (20 g/day for 5 days followed by 5 g/day); PLA RT =2 days/wk |
12 wks | CR ↑ chest press strength, chair rise performance. ↔ 1 repetition maximum leg extension, muscular endurance |
No adverse events | 3 |
| Johannsmeyer et al38 | n=31 (17 men, 14 women, healthy) Mean age =58 years |
CR (0.1 g/kg/day) or PLA RT =3 days/wk |
12 wks | CR ↑ lean tissue mass, men on CR reduced protein catabolism ↔ leg press or bench press strength, handgrip strength, 80 m walking time, balance |
No adverse events | 5 |
| Neves et al39 | n= 24 (postmenopausal women with knee osteoarthritis) Age =55–65 years |
CR (20 g/day for 1 wk followed by 5 g/day) or PLA RT =3 days/wk |
12 wks | CR ↑ physical function, stiffness subscales, lower limb lean mass, quality of life ↔ leg press strength, whole-body lean tissue mass |
No adverse events | 5 |
| Pinto et al40 | n=27 (men and women, healthy) Age =60–80 years |
CR (5 g/day) or PLA RT =3 days/wk |
12 wks | CR ↑ lean tissue mass ↔ leg press or chess press strength |
No adverse events | 4 |
| Tarnopolsky et al41 | n=39 (19 men; 20 women, healthy) Age =65–85 years |
CR (5 g/day) + CLA (6 g/day) or PLA RT =2 days/wk |
6 months | CR + CLA ↑ muscular endurance, isokinetic knee extension strength, lean tissue mass ↔ 30 s sit to stand test, 30 m walking, tandem balance testing, timed up and go test |
One individual in the CR + CLA group reported gastrointestinal distress; no liver or kidney abnormalities | 5 |
| Villanueva et al42 | n=14 (healthy men) Mean age =68.7 years |
CR (0.3 g/kg/day for 5 days, 0.07 g/kg/day thereafter + 35 g whey protein or PLA RT =3 days/wk |
12 wks | ↔ lean tissue mass, chest press or leg press strength, chest press or leg press muscular endurance, Margaria power test, 400 m walking time | No adverse events | 2 |
Notes: ↑ represents significant increase compared with the placebo condition; ↔ represents no difference between the creatine and placebo conditions. Jadad scores range from 0 to 5 with 0 being poor quality and 5 being optimal quality.
Abbreviations: CR, creatine; IGF-1, insulin-like growth factor 1; PLA, placebo; PRO, protein; RT, resistance training; wk, week.
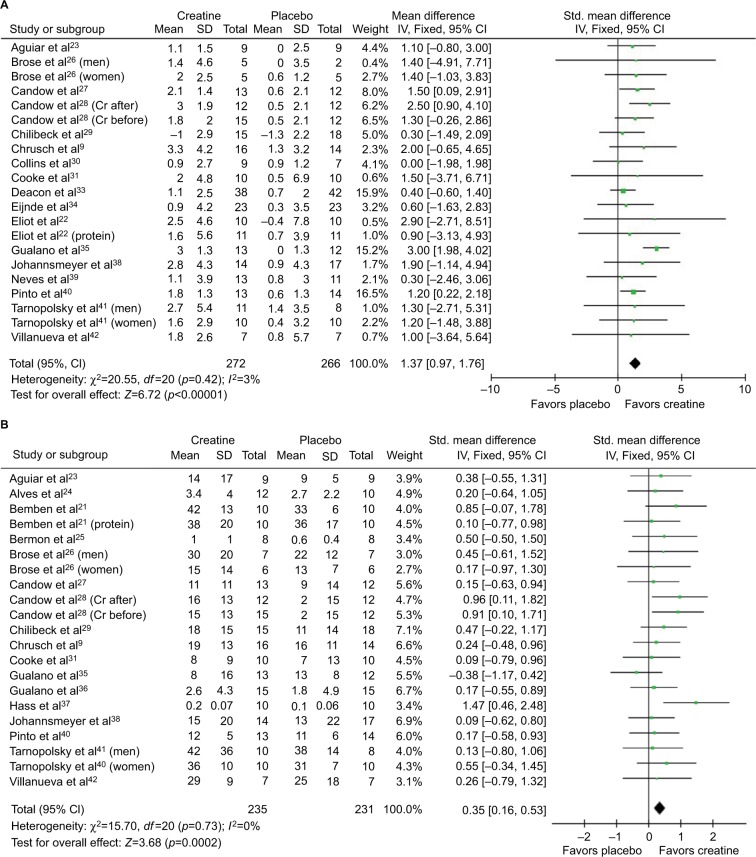
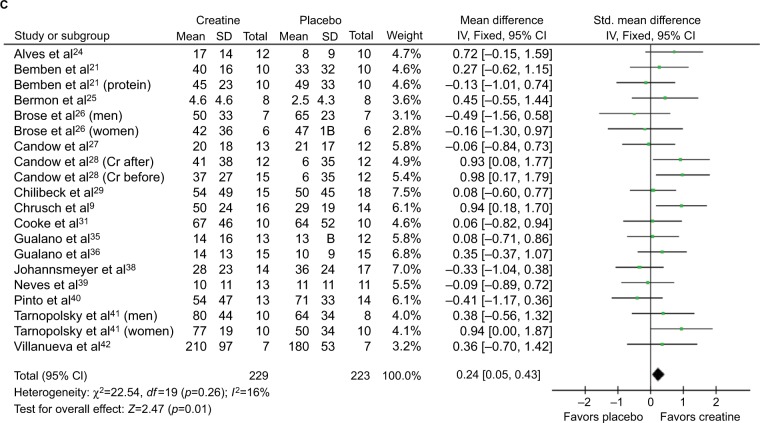
Participants (n=721) across studies were randomized to receive creatine or placebo during resistance training programs. Resistance training was performed 2–3 days per week. Participants included healthy older adults (n=15 studies); frail or vulnerable older adults (n=2); and older adults with heart disease, Parkinson’s disease, chronic obstructive pulmonary disease, type 2 diabetes, and osteoarthritis (n=1 for each). Mean ages ranged from 57 to 70 years across individual studies. Study duration was 7–52 weeks. Eight studies included a creatine “loading” phase, where a creatine dosage of 20 g/day for 5–7 days was consumed. Dosing thereafter and dosing in other studies ranged from 3 to 5 g/day. Twelve studies reported that creatine supplementation increased either lean tissue mass or muscle function, whereas ten studies showed no effect from creatine. Most studies reported no adverse effects related to creatine, but four studies reported gastrointestinal adverse events, and two studies reported muscle cramping. These adverse events did not cause participants to withdraw from these studies. Importantly, five studies with duration ranging from 3 to 52 weeks that evaluated liver or kidney function through blood or urine testing found no adverse effects. Figure 1 presents the forest plots from the meta-analyses.
Figure 1.
Forest plots for lean tissue mass (A), chest press strength (B), and leg press stress (C).
Notes: Some studies presented data on men and women separately26,41 and on creatine and creatine + protein groups separately;21,22 therefore, these studies are entered twice in the meta-analysis for these separate subgroups. One study also presented data on participants who received creatine before versus after resistance training programs;28 therefore, these subgroups are entered separately in the meta-analysis.
Abbreviations: IV, inverse variance; Std, standardized; Cr, creatine.
Creatine-supplemented groups had significantly greater increases in lean tissue mass (p<0.00001), chest press strength (p=0.0002), and leg press strength (p=0.01) compared with placebo. When studies that combined creatine supplementation with other nutritional supplements (ie, conjugated linoleic acid or protein21,22,30,41,42) were excluded one at a time, the increases in creatine-supplemented groups were still greater than that in placebo groups for all measures. When all of these studies were excluded, the creatine-supplemented groups had significantly greater increases for lean tissue mass (mean difference =1.44 [95% CI =1.02–1.86] kg; p<0.00001), chest press strength (standardized mean difference [SMD] =0.36 [0.16–0.57]; p=0.0004), and leg press strength (SMD =0.21 [0.01–0.42]; p=0.04). When studies of individuals with chronic conditions (eg, osteoarthritis, chronic obstructive pulmonary disease, Parkinson’s disease, and type 2 diabetes33,35,37,39) were excluded one at a time, creatine-supplemented groups still had greater increases than that of placebo groups for all measures. When all of these studies were excluded, the creatine-supplemented groups had significantly greater increases for lean tissue mass (mean difference =1.26 [0.77–1.74] kg; p<0.00001), chest press strength (SMD =0.35 [0.16–0.55]; p=0.0004), and leg press strength (SMD =0.27 [0.07–0.47]; p=0.009). When studies that combined other nutritional supplements with creatine and studies of individuals with chronic conditions were excluded, creatine supplementation still resulted in greater increases than placebo for lean tissue mass (mean difference =1.35 [0.83–1.88]; p<0.00001), chest press strength (SMD =0.37 [0.16–0.58]; p=0.0007), and leg press strength (SMD =0.25 [0.03–0.47]; p=0.03). Inspection of Funnel plots did not reveal any publication bias.
Discussion
The important outcome from this meta-analysis is that creatine supplementation during resistance training results in ~1.4 kg greater increase in lean tissue mass than when placebo is consumed (Figure 1A), and this translates to significantly greater increases in upper body (ie, chest press) and lower body (ie, leg press) strength (Figure 1B and C) in older adults. Meta-analyses of creatine supplementation interventions in older adults are important because individual studies are equivocal as to whether creatine supplementation is effective in older adults, with just more than half of the studies showing significant effects from creatine supplementation on lean tissue mass or muscular strength (Table 1). Variability in lean tissue mass and muscle strength measurements is quite high in older adults,13 and therefore, it is difficult to obtain adequate statistical power to detect differences with creatine supplementation in many individual studies. Meta-analyses allow one to address this lack of statistical power by assessing large numbers of individuals.
Our review included over 700 participants, which is approximately double the number of participants from previous meta-analyses.11,12 These previous meta-analyses found that creatine increased lean tissue mass and upper body strength, but either had no effect on lower body strength (ie, leg press)11 or a statistically significant effect.12 Increasing lower body strength is clinically significant because it is affected more than upper body strength with aging, and lower leg strength is related to a reduction in performance of moderate- to high-intensity activities in older individuals.13
Evidence for adverse effects from creatine is scarce. Some studies show altered kidney and liver function with high doses of creatine in animal models.43 In a couple of case studies in young men (18–24 years) who took resistance training frequently and intensely, it was suggested that creatine or a creatine-containing supplement contributed to impaired kidney function;44,45 however, no evidence from randomized controlled trials has shown that creatine has serious adverse effects. Studies included in our review (Table 1) or others directly assessing kidney function with creatine in older adults46 have not detected adverse effects on the kidney or liver with creatine supplementation. Further, a recent position paper by the International Society of Sports Nutrition concludes that creatine supplementation does not have any adverse effects on older adults.5 Very large numbers of individuals are usually required to adequately detect whether an intervention increases chances of serious adverse events;47 therefore, even a review of the existing studies with ~700 older adults might be underpowered to detect adverse events with creatine supplementation.
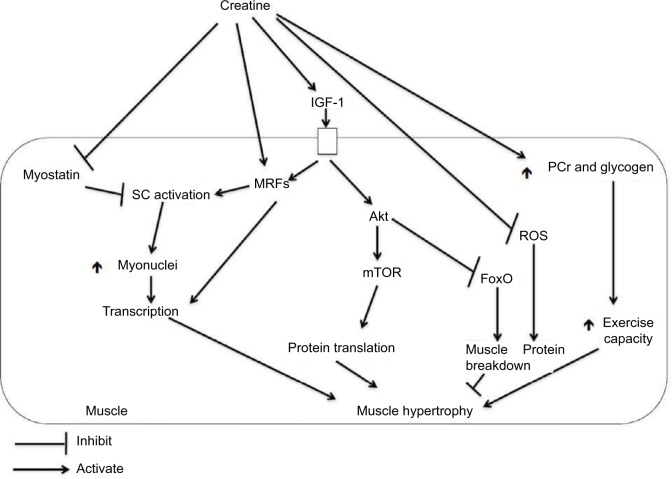
A number of mechanisms are involved by which creatine supplementation leads to an increase in lean tissue mass and muscular strength. Figure 2 summarizes these mechanisms, which are discussed below.
Figure 2.
Potential mechanisms by which creatine supplementation leads to muscle hypertrophy.
Abbreviations: IGF-1, insulin-like growth factor 1; MRFs, myogenic regulatory factors; mTOR, mammalian target of rapamycin; PCr, phosphorylcreatine; ROS, reactive oxygen species; SC, satellite cells.
Creatine supplementation increases intramuscular creatine stores in older adults, which may result in greater levels of PCr.26,34,48–50 Increased PCr would provide greater buffering of ATP during high-intensity exercise (ie, resistance training), allowing one to train with a greater volume.7 Furthermore, elevated intramuscular creatine allows for greater rates of PCr recovery following exercise in older adults49 as the creatine kinase reaction would be driven in the direction of enhanced PCr resynthesis (ie, creatine + ATP → PCr + ADP), which would improve the performance on repeated bouts of high-intensity exercise. Most51–54 but not all55 studies of short-term creatine supplementation (ie, 5–14 days) indicate that creatine can improve high-intensity exercise performance in older adults.
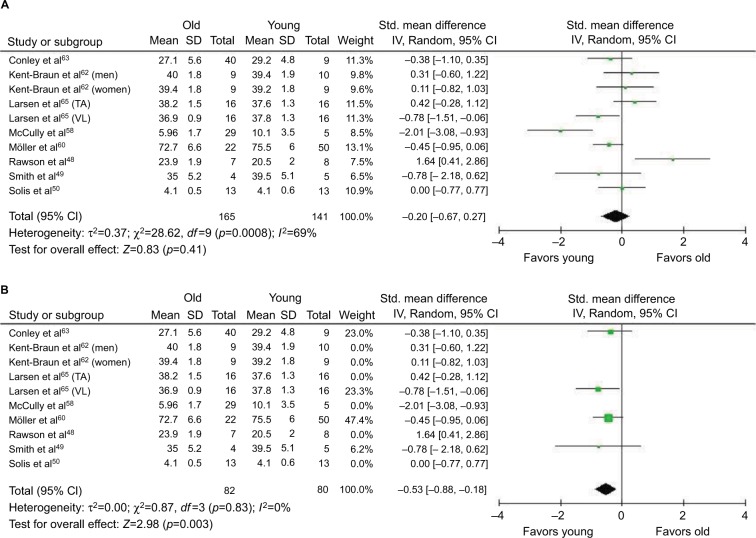
Individuals with low intramuscular creatine stores (ie, vegetarians) are more responsive to creatine supplementation.56 Controversy exists as to whether creatine stores are reduced and PCr metabolism is affected with aging. Some studies show reduced stores of creatine in older adults and slow PCr kinetics during or following exercise,49,57–60 whereas others indicate no difference between young and older adults61–63 or even higher PCr stores in older adults.64 Differences between studies may be related to muscle groups evaluated. PCr levels might be similar between young and older adults in the lower leg (ie, gastrocnemius or tibialis anterior), which is used to a greater extent during daily lower-intensity activities (ie, walking), but PCr might be lower in the vastus lateralis of older adults.65 The vastus lateralis would be used to a greater extent during higher intensity activities such as running or cycling,65 activities that are lower in older versus younger adults.13 Using the same methodology as our meta-analyses in the earlier section, we conducted a meta-analysis to assess PCr differences between young and older adults. When all studies were considered, no difference was shown in PCr stores between muscles of young and older adults (p=0.41; Figure 3A); however, when only studies of vastus lateralis were included (ie, studies of gastrocnemius and tibialis anterior were excluded), there was lower intramuscular PCr in the muscles of older adults (p=0.003; Figure 3B).
Figure 3.
Forest plots for phosphorylcreatine content in muscle of young and older adults for all studies (A) and for studies of only vastus lateralis (ie, studies of gastrocnemius or tibialis anterior were excluded from the analysis) (B).
Notes: One study presented data on men and women separately;62 therefore, these subgroups are entered separately in the meta-analysis. One study presented data for two different muscle groups;65 therefore, these are entered separately in the meta-analysis.
Abbreviations: IV, inverse variance; Std, standardized; TA, tibialis anterior; VL, vastus lateralis.
Lower creatine stores in the vastus lateralis of older adults may be related to the effects of aging on type II muscle fibers (ie, preferential atrophy66), as these generally have higher PCr stores than in type I fibers.67 Alternatively, dietary changes with aging may be the cause as older adults typically have low meat intake68 and, therefore, lower dietary creatine intake.50 Physical activity levels are generally reduced with aging,13 and the level of physical activity is related to changes in PCr stores, with higher physical activity increasing PCr stores and immobilization (no physical activity) decreasing PCr stores.69 The lower PCr levels in the vastus lateralis of older adults (Figure 3B) may suggest that older adults are more responsive to creatine supplementation when considering muscle performance that involves the activation of the larger muscles of the leg (ie, knee extensors).
A direction for future research is to further determine the effects of creatine on aging cellular biology. A couple of studies in our review27,38 showed that a whole-body marker of muscle protein catabolism (ie, urinary excretion of 3-methylhistidine) is reduced in older men (but not in older women) after supplementing with creatine. This positive effect of creatine in older men is in agreement with research in younger adults.70 Only a couple of studies included in our review26,31 performed muscle biopsies to assess changes in muscle fiber area. No significant effects of creatine supplementation on muscle fiber area were evident, despite an increase in lean tissue mass and strength found in one of these studies.26 Muscle fiber area measurements are subject to high variability; therefore, larger sample sizes are needed to detect whether creatine supplementation has effects on the muscle fiber level.
In this section, we discuss the effects of creatine supplementation on the cellular level from studies of cell cultures, animals, and younger humans and how this might translate to the enhancement of muscle mass in older individuals. Ingwall et al71,72 were the first to show that creatine added to muscle cell cultures could stimulate myosin heavy chain and actin protein synthesis, myofibrillar proteins important in the muscle contractile process. Important, however, is that these cells were from the breast muscles of chick embryos and, therefore, were muscle cells that were undergoing rapid differentiation (ie, development) and that they would most likely respond differently than human adult muscle cells. To address this limitation, Willoughby and Rosen73 assessed the effect of creatine supplementation during 12 weeks of resistance training in young adult men and showed that creatine increased myosin heavy chain type I, IIa, and IIx mRNA expression (indicating greater transcription for these proteins) and myosin heavy chain type I and IIx (but not IIa) protein levels compared with resistance training without creatine supplementation. These changes occurred in conjunction with increased muscle mass and strength in the creatine-supplemented versus placebo group. These results are in contrast, however, to other studies that indicated that 5–9 days of creatine supplementation with or without an acute session of resistance training failed to increase the synthetic rate of myofibrillar (ie, myosin and actin) or sarcoplasmic proteins as assessed by the incorporation of radio-labeled leucine into muscle biopsies of young men or women.70,74 Longer periods of creatine supplementation may be needed for the stimulation of protein synthesis.
In order to determine in detail which proteins are upregulated with creatine supplementation, Safdar et al10 assessed a global array of mRNAs (to assess which proteins are transcribed) and proteins in the vastus lateralis in response to 10 days of creatine supplementation (ie, 20 g/day for 3 days; 5 g/day for 7 days) in young men. Proteins involved in sensing changes in osmolarity and signal transduction were upregulated with creatine supplementation, along with proteins involved in satellite cell proliferation and differentiation. Satellite cells sit between the basal lamina and sarcolemma (ie, muscle fiber membrane) of muscle fibers and are involved in the development of new muscle fibers or, in adult muscle, repair of damaged muscle fibers.75 Satellite cells donate their nuclei to muscle fibers, increasing the capacity for protein synthesis within the muscle fibers.75 Safdar et al10 proposed that the cellular swelling induced by the increased water content from creatine supplementation facilitated the production of “myogenic regulatory factors” (MRFs) that are involved in stimulating satellite cells to proliferate and fuse with muscle fibers. There are four major MRFs involved in proliferation and differentiation of satellite cells, expressed at different time points:75 1) Myf5 is expressed at the earliest and involved in satellite cell activation and proliferation; 2) MyoD is involved in the migration of satellite cells to the muscle fibers and differentiation of the satellite cells; 3) myogenin is involved in the fusion of the satellite cells with the muscle fibers; and 4) Mrf4 is involved in maturation of the newly repaired or newly formed muscle fibers. Insulin-like growth factor-1 (IGF-1), produced by muscle fibers, is thought to regulate MRF expression, so that the sequence of events is increased IGF-1 production, followed by increased expression of MRFs simultaneous with increased satellite cell activation, proliferation, and differentiation to form new myonuclei within muscle fibers to increase capacity for protein synthesis.75 In contrast to the effect of MRFs on satellite cells, myostatin, another signaling protein or “myokine” seems to have the opposite effect; it suppresses satellite cell activation. In addition to their effect on satellite cell activation, proliferation, and differentiation, the MRFs also bind to DNA and activate genes involved in the expression of proteins important for muscle contraction such as myosin heavy chains, myosin light chains (ie, important components of the myosin contractile protein), actin (another important contractile protein), and creatine kinase (an enzyme involved in breakdown of PCr which plays a major role in buffering ATP during high-intensity exercise).76
Creatine supplementation may stimulate the steps from IGF-1 production to satellite cell activation and differentiation. During 72 hours of incubating differentiating murine cell culture (ie, derived from rats and mice) with creatine, mRNA for IGF-1 was upregulated during the entire 72-hour period; whereas MRFs were upregulated in their expected sequence (ie, mRNA for Myf5 was increased by 24 hours and MyoD, myogenin, and Mrf4 by 72 hours).76 As noted earlier, cultures of rapidly developing muscle fibers from animals may not respond the same way as adult human muscle fibers. During 8 weeks of resistance training, young men and women who supplemented with creatine had greater increases in muscle-specific IGF-1 compared with those who supplemented with placebo.77 The response of MRFs to creatine supplementation is however mixed. Hespel et al78 supplemented young adults with creatine or placebo during 2 weeks of leg immobilization followed by 10 weeks of knee extension exercise. Creatine supplementation increased Mrf4 expression, and this was correlated with an increase in muscle fiber size; however, myogenin decreased with creatine compared with placebo by Week 10. As Mrf4 is expressed after myogenin, it may be that the sequence by which MRFs were expressed was faster with creatine supplementation, and myogenin may have been decreasing, whereas Mrf4 was increasing in the creatine group by the end of the 10-week intervention. In contrast, Willoughby and Rosen79 found that creatine supplementation during 12 weeks of heavy resistance training in young men was superior to placebo for increasing mRNA and protein levels of myogenin and Mrf4, with no effect on the other MRFs (ie, Myf5 and MyoD). The lack of effect on these two MRFs may be due to the timing of the assessment (ie, Myf5 and MyoD are expressed first, and by 12 weeks, their expression is likely decreased compared with the other MRFs).
As mentioned above, myostatin has an opposite effect when compared with the MRFs, in that it suppresses satellite cell activation. A limited number of studies on the effect of creatine supplementation on myostatin levels have shown that creatine supplementation in pigs decreased mRNA expression of myostatin,80 and creatine supplementation during 8 weeks of resistance training in young men decreased serum levels of myostatin.81 This latter study is limited in that myostatin was not assessed in muscle. The effect of creatine supplementation on MRF and myostatin expression may translate to increased satellite cell activation and differentiation. For example, over 8 weeks of resistance training in young men, creatine supplementation resulted in a faster increase in satellite cell number, myonuclei per muscle fiber, and muscle fiber size.82 The effect of creatine on the stimulation of satellite cell proliferation may have important implications for older individuals, as satellite cell number is reduced with aging.83 Following an acute bout of resistance training exercise, compared with younger men, older men have a delayed increase in satellite cell number, a smaller increase in satellite cells expressing MyoD, a smaller decrease in satellite cells expressing myostatin, smaller increases in myogenin mRNA, and greater increases in myostatin protein expression.84 Creatine supplementation, therefore, has the potential, in older adults, to offset these changes in myostatin and MRF expression and their effects on satellite cells. This has not however been directly assessed in older adults and is an avenue for future research.
Along with the stimulation of MRFs, IGF-1 stimulates signaling pathways (ie, phosphatidylinositol 3-kinase [PI3K]-Akt/protein kinase B [PKB]-mammalian target of rapamycin [mTOR]) within muscle that are involved in translation of proteins (ie, synthesis of proteins at ribosomes from codes on mRNA delivered from the nucleus).75 Controversy exists as to whether creatine supplementation is effective for stimulating these signaling pathways. Creatine added to murine cell culture enhanced differentiation by activating these pathways involved in translation;85 however, when creatine was supplemented for 5 days before an acute resistance training session in young human adults, there was either enhanced activation of only specific components of this pathway and only at 24 hours postexercise86 or no enhanced activation of these pathways.87 These pathways involved in translation may be negatively affected by aging.88 The total amount of mTOR is upregulated in older adults when a nutritional supplement containing creatine is consumed;20 however, there was no effect of creatine on the phosphorylation (ie activation) of mTOR or other components of the translation-signaling pathway. The nutritional supplement used in this study also contained additional components besides creatine (ie, L-carnitine, leucine, and vitamin D); thus, the direct effect of creatine could not be determined. The effect of creatine on the activation of translation-signaling pathways in older adults remains another area of future research. Along with translation initiation that is activated by the PI3K-Akt/PKB-mTOR signaling pathway, muscle proteolysis (via FoxO3a) is also inactivated by Akt.75 As mentioned earlier, some research, albeit at the whole-body level (ie, urinary excretion of 3-methylhistidine), indicates that muscle protein catabolism is reduced with creatine supplementation during resistance training in older men,27,38 suggesting activation of Akt and inhibition of proteolysis; therefore, creatine may have anabolic and anti-catabolic effects on muscle.
Other mechanisms by which creatine supplementation may enhance the adaptations to resistance training session in older adults include reducing oxidative stress and enhancing energy stores aside from PCr. Creatine supplementation prevents oxidative stress and inflammation and could protect against tissue and mitochondrial DNA damage.89 Mitochondrial defects with aging may lead to increased production of reactive oxygen species, which leads to inflammation and muscle atrophy.90 With aging, there is a loss of efficiency in transfer of electrons along the electron transport chain during mitochondrial oxidative phosphorylation, resulting in increased proportion of electrons transferred to oxygen, leading to formation of reactive oxygen species, which damages mitochondrial DNA. This leads to alterations in genes encoding electron transport chain proteins and further defects in the electron transport chain.90 Reactive oxygen species produced by defective transfer of electrons along the electron transport chain include hydrogen peroxide and hydroxl radicals.90 When creatine is added to cell cultures that have been oxidatively damaged, it has direct effects for reducing these reactive oxygen species.91 It is hypothesized that creatine is oxidized to C4H9NO4 during the scavenging of these reactive oxygen species.91
Regarding energy stores, creatine supplementation upregulates genes encoding proteins involved in increased glycogen synthesis and decreases the expression of genes encoding proteins involved in glycogen breakdown.10 In older diabetic adults, creatine supplementation during 12 weeks of resistance training increased the amount of glucose transport protein 4 in muscle fiber membranes35 that would enhance glucose uptake into muscle to favor glycogen storage. This may provide an important additional energy source during resistance training because resistance training exercise lowers muscle glycogen.92
In summary, our meta-analyses show that creatine supplementation during resistance training is effective for increasing lean tissue mass and upper and lower body strength in older adults. Creatine might enhance energy stores, including PCr and glycogen to allow better buffering (ie, resynthesis) of ATP during intense exercise. This might allow for resistance training with a greater volume and translate into superior adaptation to training. Creatine supplementation might enhance protein synthesis, possible by stimulating signaling pathways activated by the osmotic effect of creatine. Although study results are mixed, creatine supplementation could stimulate myogenic regulator factors, which activate transcription of contractile proteins and enhance satellite cell activation, proliferation, and differentiation. Satellite cells donate their nuclei to adjacent muscle fibers, increasing capacity for protein synthesis. Creatine might activate pathways within muscle fibers involved in protein translation and might also reduce oxidative stress and inflammation. These mechanisms could explain the superior adaptation to resistance training when creatine is supplemented in older adults; however, further study is needed to confirm whether these mechanisms are actually involved in older adults.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 4.Candow DG, Forbes SC, Little JP, Cornish SM, Pinkoski C, Chilibeck PD. Effect of nutritional interventions and resistance exercise on aging muscle mass and strength. Biogerontology. 2012;13(4):345–358. doi: 10.1007/s10522-012-9385-4. [DOI] [PubMed] [Google Scholar]
- 5.Kreider RB, Kalman DS, Antonio J, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017;14:18. doi: 10.1186/s12970-017-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candow DG, Chilibeck PD. Effect of creatine supplementation during resistance training on muscle accretion in the elderly. J Nutr Health Aging. 2007;11(2):185–188. [PubMed] [Google Scholar]
- 7.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 8.Clark JF. Creatine: a review of its nutritional applications in sport. Nutrition. 1998;14(3):322–324. doi: 10.1016/s0899-9007(97)00482-6. [DOI] [PubMed] [Google Scholar]
- 9.Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001;33(12):2111–2117. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics. 2008;32(2):219–228. doi: 10.1152/physiolgenomics.00157.2007. [DOI] [PubMed] [Google Scholar]
- 11.Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Endocrine. 2014;45(3):354–361. doi: 10.1007/s12020-013-0070-4. [DOI] [PubMed] [Google Scholar]
- 12.Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2014;46(6):1194–1203. doi: 10.1249/MSS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 13.Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci. 2005;60(2):148–156. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- 14.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol (1985) 1997;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 15.Dangott B, Schultz E, Mozdziak PE. Dietary creatine monohydrate supplementation increases satellite cell mitotic activity during compensatory hypertrophy. Int J Sports Med. 2000;21(1):13–16. doi: 10.1055/s-2000-8848. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org. [Google Scholar]
- 18.Lobo DM, Tritto AC, da Silva LR, et al. Effects of long-term low-dose dietary creatine supplementation in older women. Exp Gerontol. 2015;70:97–104. doi: 10.1016/j.exger.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson TJ, Lemmey AB, Jones JG, et al. Can creatine supplementation improve body composition and objective physical function in rheumatoid arthritis patients? A randomized controlled trial. Arthritis Care Res (Hoboken) 2016;68(6):729–737. doi: 10.1002/acr.22747. [DOI] [PubMed] [Google Scholar]
- 20.Evans M, Guthrie N, Pezzullo J, Sanli T, Fielding RA, Bellamine A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled study. Nutr Metab (Lond) 2017;14:7. doi: 10.1186/s12986-016-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bemben MG, Witten MS, Carter JM, Eliot KA, Knehans AW, Bemben DA. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. 2010;14(2):155–159. doi: 10.1007/s12603-009-0124-8. [DOI] [PubMed] [Google Scholar]
- 22.Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging. 2008;12(3):208–212. doi: 10.1007/BF02982622. [DOI] [PubMed] [Google Scholar]
- 23.Aguiar AF, Januário RS, Junior RP, et al. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur J Appl Physiol. 2013;113(4):987–996. doi: 10.1007/s00421-012-2514-6. [DOI] [PubMed] [Google Scholar]
- 24.Alves CR, Merege Filho CA, Benatti FB, et al. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: a randomized double-blind study. PLoS One. 2013;8(10):e76301. doi: 10.1371/journal.pone.0076301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bermon S, Venembre P, Sachet C, Valour S, Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol Scand. 1998;164(2):147–155. doi: 10.1046/j.1365-201X.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 26.Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2003;58(1):11–19. doi: 10.1093/gerona/58.1.b11. [DOI] [PubMed] [Google Scholar]
- 27.Candow DG, Little JP, Chilibeck PD, et al. Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc. 2008;40(9):1645–1652. doi: 10.1249/MSS.0b013e318176b310. [DOI] [PubMed] [Google Scholar]
- 28.Candow DG, Vogt E, Johannsmeyer S, Forbes SC, Farthing JP. Strategic creatine supplementation and resistance training in healthy older adults. Appl Physiol Nutr Metab. 2015;40(7):689–694. doi: 10.1139/apnm-2014-0498. [DOI] [PubMed] [Google Scholar]
- 29.Chilibeck PD, Candow DG, Landeryou T, Kaviani M, Paus-Jenssen L. Effects of creatine and resistance training on bone health in postmenopausal women. Med Sci Sports Exerc. 2015;47(8):1587–1595. doi: 10.1249/MSS.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 30.Collins J, Longhurst G, Roschel H, Gualano B. Resistance training and co-supplementation with creatine and protein in older subjects with frailty. J Frailty Aging. 2016;5(2):126–134. doi: 10.14283/jfa.2016.85. [DOI] [PubMed] [Google Scholar]
- 31.Cooke MB, Brabham B, Buford TW, et al. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur J Appl Physiol. 2014;114(6):1321–1332. doi: 10.1007/s00421-014-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelissen VA, Defoor JG, Stevens A, et al. Effect of creatine supplementation as a potential adjuvant therapy to exercise training in cardiac patients: a randomized controlled trial. Clin Rehabil. 2010;24(11):988–999. doi: 10.1177/0269215510367995. [DOI] [PubMed] [Google Scholar]
- 33.Deacon SJ, Vincent EE, Greenhaff PL, et al. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(3):233–239. doi: 10.1164/rccm.200710-1508OC. [DOI] [PubMed] [Google Scholar]
- 34.Eijnde BO, Van Leemputte M, Goris M, et al. Effects of creatine supplementation and exercise training on fitness in men 55-75 yr old. J Appl Physiol (1985) 2003;95(2):818–828. doi: 10.1152/japplphysiol.00891.2002. [DOI] [PubMed] [Google Scholar]
- 35.Gualano B, DE Salles Painneli V, Roschel H, et al. Creatine in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Med Sci Sports Exerc. 2011;43(5):770–778. doi: 10.1249/MSS.0b013e3181fcee7d. [DOI] [PubMed] [Google Scholar]
- 36.Gualano B, Macedo AR, Alves CR, et al. Creatine supplementation and resistance training in vulnerable older women: a randomized double-blind placebo-controlled clinical trial. Exp Gerontol. 2014;53:7–15. doi: 10.1016/j.exger.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007;21(2):107–115. doi: 10.1177/1545968306293449. [DOI] [PubMed] [Google Scholar]
- 38.Johannsmeyer S, Candow DG, Brahms CM, Michel D, Zello GA. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp Gerontol. 2016;83:112–119. doi: 10.1016/j.exger.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Neves M, Jr, Gualano B, Roschel H, et al. Beneficial effect of creatine supplementation in knee osteoarthritis. Med Sci Sports Exerc. 2011;43(8):1538–1543. doi: 10.1249/MSS.0b013e3182118592. [DOI] [PubMed] [Google Scholar]
- 40.Pinto CL, Botelho PB, Carneiro JA, Mota JF. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J Cachexia Sarcopenia Muscle. 2016;7(4):413–421. doi: 10.1002/jcsm.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarnopolsky M, Zimmer A, Paikin J, et al. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One. 2007;2(10):e991. doi: 10.1371/journal.pone.0000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villanueva MG, He J, Schroeder ET. Periodized resistance training with and without supplementation improve body composition and performance in older men. Eur J Appl Physiol. 2014;114(5):891–905. doi: 10.1007/s00421-014-2821-1. [DOI] [PubMed] [Google Scholar]
- 43.Tarnopolsky MA, Bourgeois JM, Snow R, et al. Histological assessment of intermediate- and long-term creatine monohydrate supplementation in mice and rats. Am J Physiol Regul Integr Comp Physiol. 2003;285(4):R762–R769. doi: 10.1152/ajpregu.00270.2003. [DOI] [PubMed] [Google Scholar]
- 44.Taner B, Aysim O, Abdulkadir U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus. 2011;4(1):23–24. doi: 10.1093/ndtplus/sfq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorsteinsdottir B, Grande JP, Garovic VD. Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr. 2006;16(4):341–345. doi: 10.1053/j.jrn.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Neves M, Jr, Gualano B, Roschel H, et al. Effect of creatine supplementation on measured glomerular filtration rate in postmenopausal women. Appl Physiol Nutr Metab. 2011;36(3):419–422. doi: 10.1139/h11-014. [DOI] [PubMed] [Google Scholar]
- 47.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 48.Rawson ES, Clarkson PM, Price TB, Miles MP. Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol Scand. 2002;174(1):57–65. doi: 10.1046/j.1365-201x.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 49.Smith SA, Montain SJ, Matott RP, Zientara GP, Jolesz FA, Fielding RA. Creatine supplementation and age influence muscle metabolism during exercise. J Appl Physiol (1985) 1998;85(4):1349–1356. doi: 10.1152/jappl.1998.85.4.1349. [DOI] [PubMed] [Google Scholar]
- 50.Solis MY, Artioli GG, Otaduy MCG, et al. Effect of age, diet and tissue type on PCr response to creatine supplementation. J Appl Physiol (1985) 2017;123(2):407–414. doi: 10.1152/japplphysiol.00248.2017. [DOI] [PubMed] [Google Scholar]
- 51.Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc. 2002;34(3):537–543. doi: 10.1097/00005768-200203000-00023. [DOI] [PubMed] [Google Scholar]
- 52.Gotshalk LA, Kraemer WJ, Mendonca MA, et al. Creatine supplementation improves muscular performance in older women. Eur J Appl Physiol. 2008;102(2):223–231. doi: 10.1007/s00421-007-0580-y. [DOI] [PubMed] [Google Scholar]
- 53.Rawson ES, Clarkson PM. Acute creatine supplementation in older men. Int J Sports Med. 2000;21(1):71–75. doi: 10.1055/s-2000-8859. [DOI] [PubMed] [Google Scholar]
- 54.Stout JR, Sue Graves B, Cramer JT, et al. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64–86 years) J Nutr Health Aging. 2007;11(6):459–464. [PubMed] [Google Scholar]
- 55.Jakobi JM, Rice CL, Curtin SV, Marsh GD. Neuromuscular properties and fatigue in older men following acute creatine supplementation. Eur J Appl Physiol. 2001;84(4):321–328. doi: 10.1007/s004210000373. [DOI] [PubMed] [Google Scholar]
- 56.Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnopolsky M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med Sci Sports Exerc. 2003;35(11):1946–1955. doi: 10.1249/01.MSS.0000093614.17517.79. [DOI] [PubMed] [Google Scholar]
- 57.Forsberg AM, Nilsson E, Werneman J, Bergström J, Hultman E. Muscle composition in relation to age and sex. Clin Sci (Lond) 1991;81(2):249–256. doi: 10.1042/cs0810249. [DOI] [PubMed] [Google Scholar]
- 58.McCully KK, Forciea MA, Hack LM, et al. Muscle metabolism in older subjects using 31P magnetic resonance spectroscopy. Can J Physiol Pharmacol. 1991;69(5):576–580. doi: 10.1139/y91-084. [DOI] [PubMed] [Google Scholar]
- 59.McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol (1985) 1993;75(2):813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- 60.Möller P, Bergström J, Fürst P, Hellström K. Effect of aging on energy-rich phosphagens in human skeletal muscles. Clin Sci (Lond) 1980;58(6):553–555. doi: 10.1042/cs0580553. [DOI] [PubMed] [Google Scholar]
- 61.Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Thompson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol. 1998;83(1):107–117. doi: 10.1113/expphysiol.1998.sp004087. [DOI] [PubMed] [Google Scholar]
- 62.Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol (1985) 2000;89(3):1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- 63.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kan HE, van der Graaf M, Klomp DW, Vlak MH, Padberg GW, Heerschap A. Intake of 13C-4 creatine enables simultaneous assessment of creatine and phosphocreatine pools in human skeletal muscle by 13C MR spectroscopy. Magn Reson Med. 2006;56(5):953–957. doi: 10.1002/mrm.21068. [DOI] [PubMed] [Google Scholar]
- 65.Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab. 2012;37(1):88–99. doi: 10.1139/h11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lexell J. Ageing and human muscle: observations from Sweden. Can J Appl Physiol. 1993;18(1):2–18. doi: 10.1139/h93-002. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi H, Kuno SY, Katsuta S, et al. Relationships between fiber composition and NMR measurements in human skeletal muscle. NMR Biomed. 1996;9(1):8–12. doi: 10.1002/(SICI)1099-1492(199602)9:1<8::AID-NBM387>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 68.Gualano B, Rawson ES, Candow DG, Chilibeck PD. Creatine supplementation in the aging population: effects on skeletal muscle, bone and brain. Amino Acids. 2016;48(8):1793–1805. doi: 10.1007/s00726-016-2239-7. [DOI] [PubMed] [Google Scholar]
- 69.MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):700–703. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- 70.Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol (1985) 2001;91(3):1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- 71.Ingwall JS, Morales MF, Stockdale FE. Creatine and the control of myosin synthesis in differentiating skeletal muscle. Proc Natl Acad Sci U S A. 1972;69(8):2250–2253. doi: 10.1073/pnas.69.8.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ingwall JS, Weiner CD, Morales MF, Davis E, Stockdale FE. Specificity of creatine in the control of muscle protein synthesis. J Cell Biol. 1974;62(1):145–151. doi: 10.1083/jcb.62.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willoughby DS, Rosene J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc. 2001;33(10):1674–1681. doi: 10.1097/00005768-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Louis M, Poortmans JR, Francaux M, et al. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003;285(5):E1089–E1094. doi: 10.1152/ajpendo.00195.2003. [DOI] [PubMed] [Google Scholar]
- 75.Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013;70(21):4117–4130. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Louis M, Van Beneden R, Dehoux M, Thissen JP, Francaux M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C(2)C(12) cells. FEBS Lett. 2004;557(1–3):243–247. doi: 10.1016/s0014-5793(03)01504-7. [DOI] [PubMed] [Google Scholar]
- 77.Burke DG, Candow DG, Chilibeck PD, et al. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab. 2008;18(4):389–398. doi: 10.1123/ijsnem.18.4.389. [DOI] [PubMed] [Google Scholar]
- 78.Hespel P, Op’t Eijnde B, Van Leemputte M, et al. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536(Pt 2):625–633. doi: 10.1111/j.1469-7793.2001.0625c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc. 2003;35(6):923–929. doi: 10.1249/01.MSS.0000069746.05241.F0. [DOI] [PubMed] [Google Scholar]
- 80.Young JF, Bertram HC, Theil PK, et al. In vitro and in vivo studies of creatine monohydrate supplementation to Duroc and Landrace pigs. Meat Sci. 2007;76(2):342–351. doi: 10.1016/j.meatsci.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 81.Saremi A, Gharakhanloo R, Sharghi S, Gharaati MR, Larijani B, Omidfar K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrinol. 2010;317(1–2):25–30. doi: 10.1016/j.mce.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 82.Olsen S, Aagaard P, Kadi F, et al. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol. 2006;573(Pt 2):525–534. doi: 10.1113/jphysiol.2006.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;29(1):120–127. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- 84.Snijders T, Verdijk LB, Smeets JS, et al. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age (Dordr) 2014;36(4):9699. doi: 10.1007/s11357-014-9699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deldicque L, Theisen D, Bertrand L, Hespel P, Hue L, Francaux M. Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am J Physiol Cell Physiol. 2007;293(4):C1263–C1271. doi: 10.1152/ajpcell.00162.2007. [DOI] [PubMed] [Google Scholar]
- 86.Deldicque L, Louis M, Theisen D, et al. Increased IGF mRNA in human skeletal muscle after creatine supplementation. Med Sci Sports Exerc. 2005;37(5):731–736. doi: 10.1249/01.mss.0000162690.39830.27. [DOI] [PubMed] [Google Scholar]
- 87.Deldicque L, Atherton P, Patel R, et al. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol (1985) 2008;104(2):371–378. doi: 10.1152/japplphysiol.00873.2007. [DOI] [PubMed] [Google Scholar]
- 88.Li M, Verdijk LB, Sakamoto K, Ely B, van Loon LJ, Musi N. Reduced AMPK-ACC and mTOR signaling in muscle from older men, and effect of resistance exercise. Mech Ageing Dev. 2012;133(11–12):655–664. doi: 10.1016/j.mad.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sestili P, Martinelli C, Colombo E, et al. Creatine as an antioxidant. Amino Acids. 2011;40(5):1385–1396. doi: 10.1007/s00726-011-0875-5. [DOI] [PubMed] [Google Scholar]
- 90.Johnston AP, De Lisio M, Parise G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl Physiol Nutr Metab. 2008;33(1):191–199. doi: 10.1139/H07-141. [DOI] [PubMed] [Google Scholar]
- 91.Sestili P, Martinelli C, Bravi G, et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med. 2006;40(5):837–849. doi: 10.1016/j.freeradbiomed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 92.Haff GG, Koch AJ, Potteiger JA, et al. Carbohydrate supplementation attenuates muscle glycogen loss during acute bouts of resistance exercise. Int J Sport Nutr Exerc Metab. 2000;10(3):326–339. doi: 10.1123/ijsnem.10.3.326. [DOI] [PubMed] [Google Scholar]