Abstract
There is substantial evidence that the ovarian sex hormones, estrogen and progesterone, which vary considerably over the course of the human female lifetime, contribute to changes in brain structure and function. This structured, quantitative literature reviews aims to summarize neuroimaging literature addressing physiological variation in brain macro- and microstructure across an array of hormonal transitions including the menstrual cycle, use of hormonal contraceptives, pregnancy, and menopause. Twenty-five studies reporting structural neuroimaging of women, addressing variation across hormonal states, were identified from a structured search of PUBMED and were systematically reviewed. Although the studies are heterogenous with regard to methodology, overall the results point to overlapping areas of hormone related effects on brain structure particularly affecting the structures of the limbic system. These findings are in keeping with functional data that point to a role for estrogen and progesterone in mediating emotional processing.
Keywords: Hormones, Neuroimaging, Sex differences, Women, Menstrual cycle, Neuroplasticity, Voxel-based morphometry, Estrogen, Progesterone, Neuroendocrinology
Introduction
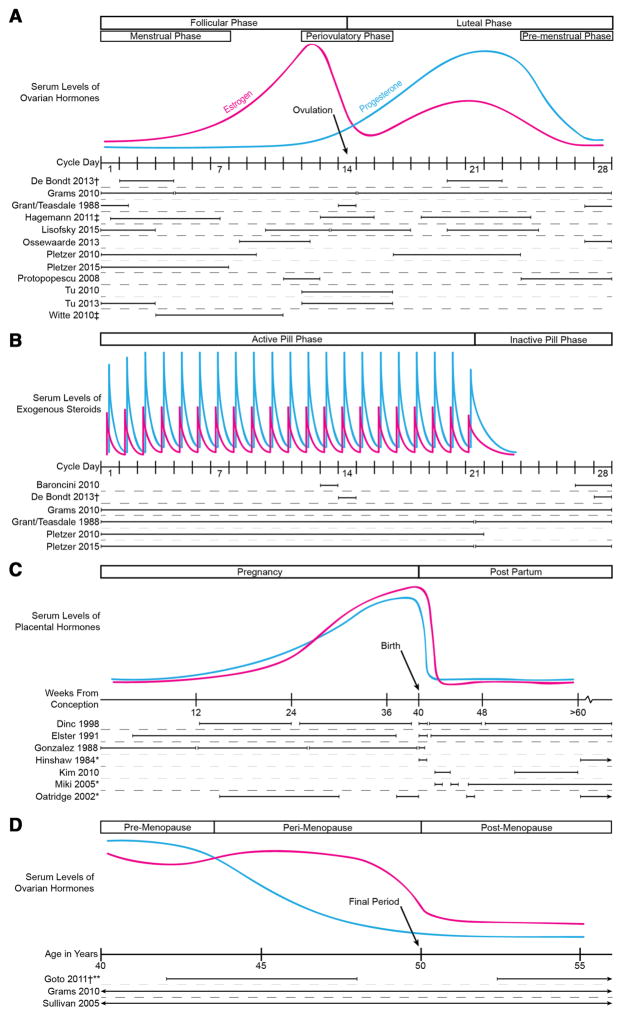
Across the lifespan, women transition sequentially through states modulated by relative levels of the ovarian sex hormones, estrogen and progesterone. These include the pre-pubertal years, menarche, menstruation, pregnancy, lactation, and menopause. Additionally, exogenous sex hormones, including hormonal contraceptives and hormone replacement therapy (HRT), may further modify normal hormonal states. Time courses of hormone level fluctuation include higher frequency effects such as the menstrual cycle or oral contraceptive pill (OCP) use, as well as lower frequency effects that span many years, such as menopause or long-lasting hormonal birth control methods such as progesterone implants (e.g. Nexplanon) (Fig. 1).
Fig. 1.
Variation of relative levels of estrogen (pink) and progesterone (blue) levels across a the menstrual cycle, b the oral contraceptive pill cycle, c pregnancy and the postpartum period, and d the menopause transition. Timing of structural MRI scans of reviewed studies shown based on an idealized 28-days cycle menstrual cycle, gestation length of 40 weeks, and menopause at 50 years. Menstrual cycle length may vary physiologically from 21 to 35 days. †Study resulted in more than 1 publication. ‡Specific day ranges of scan timing was not reported, so ranges are estimated based on text descriptions. *Subjects were scanned beyond 6 months postpartum. Oatridge et al. (2002) also scanned two subjects before conception. **Women were scanned up to age 77
It is important to characterize and account for natural physiological variation to distinguish it from pathology. The impact of cyclical variation of endogenous or exogenous hormones on brain structure and function may also introduce bias to studies comparing men and women or different groups of women. This effect may be direct, such as hormone-mediated modulation of gene transcription in central nervous system (CNS) cells, or indirect, through the enhancement or mitigation of systemic processes that affect brain structure and function, such as inflammation. The impact of ovarian sex hormones on the female brain is an important area of ongoing investigation.
Functional neuroimaging has been employed to assess the impact of ovarian sex hormones on cortical function in healthy female animals (Chen et al. 2009), healthy women (Dreher et al. 2007; Schoning et al. 2007), and in human disease states such as premenstrual dysphoric disorder (PMDD) (Poromaa 2014; Protopopescu et al. 2008b). Functional, but not structural, imaging studies of women across hormonal transitions have recently been extensively reviewed (Peper et al. 2011; Sacher et al. 2013; Toffoletto et al. 2014), and will therefore provide context, but not be the focus of this review. Brain areas exhibiting hormone-related functional effects are diverse, but tend to show a concentration of putatively hormone-mediated functional changes in areas related to emotional processing, memory, and cognition.
Given the short time course over which ovarian sex hormones fluctuate, it might seem that the changes detectable by neuroimaging would be purely functional in nature. Since brain networks underlying behavior depend on structural network connections, functional neuroimaging effects related to changes in behavior implicate underlying changes in the structural components of brain networks: network nodes (grey matter, GM) and network connections (white matter, WM). In other contexts, neuroimaging has demonstrated rapid changes in both brain function and structure in response to extrinsic stimuli. For example, both brain macro- and microstructure are enhanced following motor training. Novice jugglers exhibit increases in GM volume and in WM anisotropy over 3 months of training, which regress to baseline after cessation of practice (Draganski et al. 2004; Scholz et al. 2009).
Multiple studies, in fact, support the notion that structural foundations underlay the functional alterations associated with hormonal fluctuations in healthy women. However, these studies of structural plasticity have not been comprehensively synthesized or integrated with knowledge from functional imaging studies to identify salient insights regarding the impact of the ovarian sex hormones on the brain. This structured literature review aims to summarize macro- and microstructural changes associated with different hormonal states and transitions across the female lifespan.
Background
Endocrinology of endogenous and exogenous ovarian sex hormones
Estrogen and progesterone are steroid hormones derived from enzymatic modifications of cholesterol. In women, production of estrogen and progesterone occurs primarily in the ovary, but also in the adrenal gland, and at other locations, such as adipose tissue. The gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH), modulate ovarian sex hormone synthesis and secretion. FSH and LH are thus released from the pituitary gland under CNS control, mediated predominantly by the hypothalamus, which is in turn extensively connected to other CNS areas.
Different stages of the female lifespan may be characterized according to the relative levels of the ovarian sex hormones (Fig. 1). Estrogen and progesterone levels are low throughout childhood, but increase dramatically at the onset of puberty under the influence of pulsatile gonadotropin release from the pituitary (Gillies and McArthur 2010). This process typically leads to the onset of regular menstrual cycling, divided into two phases: the follicular phase when serum levels of estrogen are high and progesterone low, and the luteal phase, during which the progesterone level is high relative to the estrogen level (Abraham et al. 1972). The late luteal phase is associated with a spectrum of premenstrual symptoms including headaches, abdominal bloating, cramping, breast tenderness, weight changes, irritability, decreased concentration, depression, and anxiety (Biggs and Demuth 2011). The two major biologically active estrogens in non-pregnant women are estrone and estradiol, while pregnant women also produce significant quantities estriol (Torrealday et al. 2000). Estradiol circulates at higher concentrations and has greater biological potency than estrone (Blaustein 2008; Gillies and McArthur 2010).
Combined estrogen/progesterone OCP most commonly create daily spikes in estrogen and progesterone levels over a three-week period (active pill phase), followed by a period of low estrogen and progesterone levels during a week when no hormones are administered (inactive pill phase). This “placebo week” is comparable to the early follicular phase of the normal menstrual cycle in terms of serum hormone levels (De Bondt et al. 2013a). The predominant form of estrogen comprising OCP is ethinyl estradiol (EE), rather than the endogenously produced estradiol (Stanczyk et al. 2013). Combination birth control pills—those that contain both EE as well as a progestin—may be administered with different cyclical patterns, termed monophasic, biphasic, triphasic, or multiphasic, based on the degree of variation in the magnitude of the estrogen and progesterone dose across the cycle. Other hormonal methods of birth control alternate modes of delivery for a combination of estrogen and progesterone (transdermal patch, intravaginal ring), progesterone-only methods (pills, injections, intradermal implants) and the hormone eluting intra-uterine device (IUD).
During pregnancy, both estrogen and progesterone increase steadily across the three trimesters and then return rapidly to baseline following parturition (Torrealday et al. 2000). During the postpartum phase, the estrogen level is relatively low, a fact that has been implicated in the pathophysiology of postpartum depression (Skalkidou et al. 2012). The typically high prolactin level present during lactation suppresses the release of gonadotropin-releasing hormone (GnRH), the synthesis of estrogen and progesterone, and ovulation (Heinrichs et al. 2001).
During the years preceding menopause, the progesterone level declines more quickly than estrogen. Finally, menopause ultimately results in a permanent decline in the levels of both estrogen and progesterone. Symptoms of menopause are associated with low levels of estrogen. Hence, estrogen supplementation (HRT) has been used to treat symptoms and physiological consequences of menopause including vasomotor symptoms (hot flashes) and decreased bone mineral density (Soares and Frey 2010).
Neurosteroids
Various types of steroid hormones are synthesized in multiple organs as well as within the CNS, either from serum precursors or de novo. Due to their lipophilic properties both estrogen and progesterone, cross the blood brain barrier (Banks 2012). They may either exert their effects directly or undergo further modifications. Within the CNS, a potent neuroactive metabolite of progesterone is 3-alpha, 5-alpha-TH-progesterone, also called allopregnanolone (ALLO), which directly modulates gamma-aminobutyric acid type A (GABA-A) receptors affecting neuronal and glial excitability (Pluchino et al. 2006). Additionally, levels of peripheral ovarian sex hormones are known to affect de novo neurosteroidgenesis within the CNS (Pluchino et al. 2013). ALLO concentrations, for example, vary with estrus phase in rodents (Palumbo et al. 1995). Synthetic progestins, such as those used in hormonal birth control, frequently are not metabolized to ALLO (Herson et al. 2009) and may therefore be less neuroactive (Pluchino et al. 2006).
The limbic system
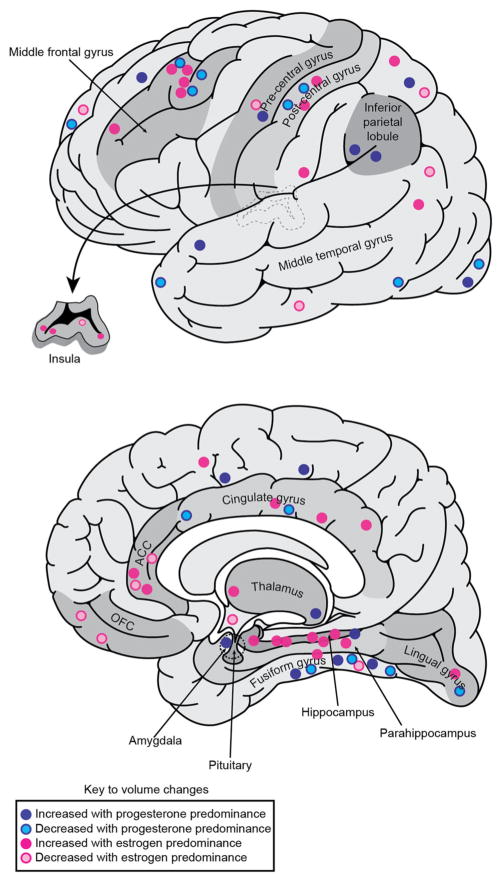
Studies of the effects of ovarian sex hormones on brain structure and function have frequently focused on components of the limbic system (Fig. 2). In rodents, limbic structures are crucial for sexual behavior. For example, the male rat amygdala mediates sexual arousal (Kondo et al. 1997), while the female rat hypothalamus coordinates lordosis (back-arching) behavior which indicates sexual receptivity (Cooke and Woolley 2005). In humans, the limbic system comprises an extended set of structures including both subcortical regions (olfactory bulb, thalamus, hypothalamus, amygdala, mammillary bodies, nucleus accumbens, and septum) and cortical regions (hippocampal formation, parahippocampal gyrus, insula, orbitofrontal cortex (OFC), medial prefrontal cortex (PFC) and cingulate gyrus) (Braun 2011). These areas mediate reproductive function and neuroendocrine homeostasis (hypothalamus), as well as a diverse set of additional functions, including memory (hippocampal formation) and emotional processing (amygdala, nucleus accumbens). The OFC and other prefrontal regions provide top-down modulation of limbic system function and are involved in emotional learning and decision-making (Protopopescu et al. 2005; Rolls 2000). Many of the symptoms associated with menstrual cycling represent impairments of these functions including irritability, impulsivity, decreased concentration, anger, and anxiety (Biggs and Demuth 2011).
Fig. 2.
Areas of the limbic system that show structural variation with predominance of either estrogen (pink) or progesterone (blue). Circles are based on approximate locations of peak MNI coordinates of clusters identified from the reviewed studies. Anterior cingulate cortex ACC; orbitofrontal cortex OFC
Several white matter tracts link the diverse structures comprising the limbic system, which can be considered as nodes on a “limbic network”. The fornix projects from the hippocampus, to the mammillary bodies, anterior thalamic nuclei, nucleus accumbens, septum, and hypothalamus; the uncinate fasciculus projects from the anterior temporal lobe to the OFC;1 the cingulum projects from the amgydala and the parahippocampal gyrus to the OFC; and the anterior thalamic radiations project from the thalamus to the OFC and anterior cingulate cortex (ACC) (Catani et al. 2013). Through these complex connections the limbic system components modulate hypothalamic and pituitary function and hormonal state (Harris 1970; Herman et al. 2005).
CNS receptors for progesterone
The classic nuclear receptor for progesterone has been localized in the rat brain to the frontal cortex, hypothalamus, thalamus, amygdala, hippocampus and cerebellum as well as other areas (Brinton et al. 2008; Pluchino et al. 2006). Additionally, a transmembrane G-protein-coupled receptor (GPCR) for progesterone is widely distributed in the CNS (Brinton et al. 2008). Progesterone receptor distribution is sexually dimorphic and is responsive to variations in estrogen and progesterone levels in female and, to a lesser extent, male rats (Guerra-Araiza et al. 2002).
CNS receptors for estrogen
Estrogen receptors (ERs) in the CNS include the classic nuclear ER-alpha and ER-beta nuclear receptors as well as a GPCR that inserts into the plasma membrane (Srivastava et al. 2013). Areas in the rat brain showing the highest concentrations of ER messenger ribonucleic acid (mRNA) expression include the amygdala, septum, thalamus, hypothalamus, and the dentate nucleus of the cerebellum (Simerly et al. 1990). Studies in both animals and humans demonstrate expression of ER-alpha in the ventromedial nucleus of the hypothalamus and amygdala, and expression of both ER-alpha and ER–beta at high concentrations in the hippocampus, with ER-beta expression dominating in the subiculum [for review see (Gillies and McArthur 2010)]. Although there are sex differences in ER concentration in the hypothalamus (Kruijver et al. 2002), overall both classic nuclear ERs are similarly widely distributed throughout the rest of the brain in adult males and females (Gillies and McArthur 2010). Aromatase, the enzyme responsible for aromatizing androgens to estrogens in estrogen biosynthesis, is expressed both pre- and post-synaptically in CNS neurons suggesting that neuroactive forms of estrogen may act as neuromodulators at the synapse (Srivastava et al. 2013).
Given the range of sites at which ovarian sex hormones may be neuroactive, both progesterone and estrogen are poised to induce and modulate neuroplasticity, thereby influencing neuroendocrine states, emotional processing, learning, and memory, among other domains. These effects, described below, lead to modification of CNS structure through both classic genomic and non-genomic (membrane-based, synaptic) signaling and across a spectrum of time scales ranging from seconds to days.
Mechanisms of ovarian sex hormone modulation of structural neuroplasticity
Changes in brain structure occur through a variety of mechanisms and across a range of scales that can affect GM, WM, and/or extra-neuronal tissue. Volumetric changes in GM may represent axon sprouting, dendritic branching, synaptogenesis, neurogenesis, myelination or changes in neural morphology (Zatorre et al. 2012). The relative contributions of these different mechanisms to volumetric changes will vary with developmental stage and brain region—e.g. neurogenesis is well characterized in the hippocampus (Eriksson et al. 1998), but its contribution in other areas remains uncertain and controversial. In WM, changes may occur in axon fiber number, density, diameter, branching, and myelination (Zatorre et al. 2012). Uniquely to humans compared to other mammals, volume changes in WM may also reflect plasticity of interstitial neuronal cell bodies located in cerebral WM that persist beyond the completion of embryonic brain development (Meyer et al. 1992). Inferences drawn from pre-clinical or animal models and applied to non-invasive imaging techniques in humans may thus be challenging. Changes in structures that are composed of a mixture of GM and WM, such as the thalamus and basal ganglia, will be driven by both sets of mechanisms. Extra-neuronal tissue changes may represent angiogenesis or glial cell proliferation or growth. Each of these mechanisms has differential effects on magnetic resonance imaging (MRI) signal intensity—for example, myelination affects lipid content and thus relaxation times (Laule et al. 2006) and increases fractional anisotropy (FA) by creating barriers to water diffusion perpendicular to the fiber orientation.
Historically, the effects of sex steroids on brain development and function were divided into permanent organizational/structural effects early in development and transient activational/functional effects that continued after brain maturation (Arnold and Gorski 1984; Phoenix et al. 1959). A wealth of animal studies in the 1960s and 1970s investigated the effects of prenatal testosterone and estradiol exposure on adult CNS structure and sexual behaviors and characterized the locations, timing, and molecular mechanisms of steroid hormone effects (Breedlove and Arnold 1980; Lieberburg and McEwen 1975; McEwen et al. 1975) [for review see (Arnold and Gorski 1984; McEwen et al. 2015)]. Eventually, evidence accumulated supporting the presence of ongoing organizational/structural effects of sex steroid hormones long into adult life.
In mammalian adulthood, the ovarian sex hormones are thought to induce neuroplasticity in the CNS primarily by modulating dendritic spine and synapse density in areas such as the hippocampus, hypothalamus, nucleus accumbens, and amygdala (Cooke and Woolley 2005; Micevych and Christensen 2012). This plasticity may also have important consequences for WM volume, such as enhancement of myelination. Over the course of the five-day estrous cycle in rats, lower serum levels of estradiol are correlated with lower hippocampal synapse density and higher serum estradiol levels with higher hippocampal synapse density (Woolley and McEwen 1992). Significant changes in synapse density may occur over as little as a 24-h period. These fluctuations may correspond to hippocampal volume variation over the estrous cycle visualized by MRI (Qiu et al. 2013). In the rat hypothalamus, specifically the ventromedial nucleus, estrogen positively modulates dendritic spine density of hypothalamic neurons (Madeira et al. 2001). In the ventrolateral subdivision of the ventromedial nucleus, dendritic spine density doubles during the proestrus phase (high estrogen) compared to diestrus (low estrogen). Changes in dendritic spine density in this region were accompanied by increases in dendrite length and terminal branch number. The effects of estradiol on spine density in the hypothalamus are mediated by a GPCR, the metabotropic glutamate receptor 1a (Christensen et al. 2011). In the nucleus accumbens estradiol exposure decreases dendritic spine density, similarly via a GPCR, the metabotropic glutamate receptor subtype five (Peterson et al. 2015).
Although the literature on sex steroid mediated structural plasticity in the rat amygdala has focused on male rats, it is worth noting that, at least in the amygdala, the effects of androgens are mediated by ERs; androgens are converted to estradiol by aromatase expressed by amygdala neurons (Cooke et al. 2003; Cooke and Woolley 2005). Additionally, exposure to both estradiol and testosterone increase cell proliferation in the medial portion of the amygdala of castrated meadow voles, but exposure to a non-aromatizable androgen does not (Fowler et al. 2003). In the adult rat brain, androgens have a trophic effect on cell size rather than cell number (Cooke et al. 1999).
The mechanisms of progesterone-mediated neuroplasticity have been less extensively characterized, but experimental treatment of rat cortical neurons with progesterone and synthetic progestin has similarly been shown to increase dendritic spine number and density (Sanchez et al. 2013). Additionally, progesterone has been implicated as a potent neuroprotectant that reduces inflammation and restores blood–brain-barrier function in response to a wide variety of insults including traumatic brain injury and cerebral ischemia (Herson et al. 2009).
Structural neuroimaging
MRI and computed tomography (CT) both non-invasively delineate brain structure and have been used to quantify brain volume in both normal individuals and disease states (Dastidar et al. 1999; Lai 2013). Neuroimaging methods for quantifying regional brain volumes can be divided into three broad categories: manual, semi-automated, and fully automated segmentation/parcellation methods. Manual parcellation requires segmentation of brain regions, based on anatomic landmarks, by an expert rater. This approach is, in expert hands, considered the gold standard, but is extremely labor intensive, demands specific neuroanatomical knowledge, and requires validation to demonstrate adequate intra- and inter-rater reliability (Bergouignan et al. 2009). Semi-automated and fully automated segmentation methods can reduce the burden of manual parcellation, but are subject to limitations of the tissue segmentation and image registration techniques they depend on. Additionally, these techniques are computationally intensive and statistically complex.
Voxel-based morphometry (VBM) is one fully automated technique designed to support inferences regarding group-level variation of brain structure. Measurements made by VBM correlate well with manual parcellation (Davies et al. 2009). VBM, however, is not a truly quantitative technique, as it does not directly measure volume. In VBM, a series of statistical tests are performed, comparing signal intensity across all image voxels of the T1-weighted MRI volumes of multiple subjects. Because GM, WM, and cerebrospinal fluid (CSF) exhibit distinct signal intensities, contrast differences between groups of subjects can be leveraged to infer, but not to actually quantify, the location of volume differences (Ashburner and Friston 2000). Images from all subjects are spatially normalized (or registered) either to a published or custom template brain volume, segmented by tissue class (GM, WM, CSF), and scaled to correct for changes in tissue volume during spatial normalization (modulation). Finally, smoothing is performed, a process that replaces the intensity of a voxel with an average of the intensities of a neighborhood of voxels, thereby improving the signal-to-noise ratio and moderating variance induced by the spatial transformation procedures. Global VBM is useful in that it is unbiased and operator-independent. However, hypothesis-driven region of interest (ROI) analyses are more sensitive than whole brain-comparisons and many studies using VBM take both approaches (Bergouignan et al. 2009). ROIs may be delineated in an operator-independent fashion, based on published atlases of brain structures. Interpreting the results of VBM across multiple studies may be challenging because of potential variability in the image processing steps and in statistical analyses including correction for multiple comparisons (Whitwell 2009).
The issue of type I error is particularly important in whole brain analyses because of the sheer number of statistical tests performed and appropriate correction for multiple comparisons is necessary. Some potential approaches include the Bonferroni correction, false discovery rate (FDR) estimation and family-wise error rate correction. However, because VBM is frequently performed on small samples, many studies report results both before (uncorrected) and after accounting for multiple comparisons (Ridgway et al. 2008). Additionally, while VBM provides information about alterations of tissue volume, its results do not address underlying mechanisms of volume change.
Methods
We developed a search strategy in consultation with a research librarian and utilized both medical subject headings (MeSH) terms and keywords in article titles/abstracts in PUBMED. Terms and keywords searched included “menstrual cycle”, “hormonal contraception”, “pregnancy”, “menopause”, “neuroimaging”, “magnetic resonance imaging”, “diffusion weighted imaging”, “diffusion tensor imaging”, “brain”, and relevant variations and abbreviations. Functional MRI (fMRI) studies have been reviewed recently and were not included (Sacher et al. 2013; Toffoletto et al. 2014). A total of 532 unique citations were identified and reviewed. Off-target studies were eliminated on the basis of the following exclusion criteria: papers with use of non-structural imaging techniques (fMRI, electroencephalography, magnetoencephalography, positron emission tomography), papers with a focus on HRT or a focus on diseases other than PMDD, premenstrual-syndrome (PMS) or primary dysmenorrhea (PD), reviews, case reports, case series, editorials, abstracts, or posters. This process yielded 20 relevant papers. Five additional papers were identified from the cited articles’ references and using the “cited by” feature in the Web of Science database. We included studies that examined the pituitary gland because, although it is not part of CNS, it acts a key hormonal bridge between the brain and the body and directly modulates sex hormone production under the influence of both centrally and peripherally originating signals.
A structured review of each study was performed to extract methodological details across a predefined set of parameters including imaging modality, experimental design, control group assignment, method for assessing menstrual cycle phase, image analysis method, and areas of brain examined (Table 1). Where criteria were not clearly reported (1 study), attempts were made to reach authors for clarification, but were not answered.
Table 1.
Methodological characteristics of the reviewed studies group by hormonal transition assessed
| Study | Imaging modality | Design | Subject group(s) | Ages | Hormone status determination | Analysis method | Areas examined |
|---|---|---|---|---|---|---|---|
| Menstrual cycle and OCPs | |||||||
| Baroncini et al. (2010) | dMRI | LO | 10 OCP ♀, 10 ♂ | 19–25 | N/A | Manual ROI | ROI |
| De Bondt et al. (2013a, b) | sMRI, DTI | LO | 15 MC ♀, 15 OCP ♀ | 18–28 | Serum | VBM, tractography | WB, ROI |
| Grant et al. 1988/Teasdale (1988) | sMRI | LO | 10 MC ♀, 10 OCP ♀, 10 post-menopause ♀, 14 ♂ | 18–64 | Self-report | Manual ROI | WB |
| Hagemann et al. (2011) | sMRI | LO | 8 MC ♀, 8 ♂ | NR | Serum, TVUS | Automated segmentation volumtery | WB |
| Lisofsky et al. (2015b) | sMRI, DTI | LO | 21 MC ♀ | 22–31 | Serum, urine | VBM, tractography | WB, ROI |
| Ossewaarde et al. (2013) | sMRI | LO | 28 MC ♀ | 18–38 | Saliva, urine | VBM, atlas-based ROI | WB, ROI |
| Pletzer et al. (2010) | sMRI | LO | 14 MC ♀, 14 OCP ♀, 14 ♂ | 19–31 | Urine | VBM, atlas-based ROI | WB, ROI |
| Pletzer et al. (2015) | sMRI | CS, LO | 20 MC ♀, 40 OCP ♀ | 20–33 | NR | VBM, atlas-based ROI | WB, ROI |
| Protopopescu et al. (2008a) | sMRI | LO | 21 MC ♀ | 22–35 | Urine | VBM, atlas-based ROI | WB, ROI |
| Tu et al. (2010) | sMRI | CS | 32 MC♀ with PD, 32 MC ♀ | 20–27 | Urine | VBM, atlas-based ROI | WB, ROI |
| Tu et al. (2013) | sMRI | LO | 32MC ♀ with PD, 32 MC ♀ | 21–28 | Urine | VBM, atlas-based ROI | WB, ROI |
| Witte et al. (2010) | sMRI | CS | 17 MC ♀, 17 ♂ | 21–36 | Serum | VBM | WB |
| Pregnancy and postpartum | |||||||
| Dinc et al. (1998) | sMRI | CS | 78 pregnant/postpartum ♀, 18 non-pregnant ♀ | 20–38 | NR | Manual ROI | Pituitary |
| Elster et al. (1991) | sMRI | CS, LO | 68 pregnant/postpartum ♀, non-pregnant ♀ | NR | NR | Manual ROI | Pituitary |
| Gonzalez et al. (1988) | sMRI | CS | 32 pregnant ♀, 20 nulliparous ♀ | 16–30 | Menstrual dating, TVUS | Manual ROI | Pituitary |
| Hinshaw et al. (1984) | CT | CS, LO | 8 pregnant/postpartum ♀ | NR | NR | Manual ROI | Pituitary |
| Kim et al. (2010) | sMRI | LO | 19 pregnant/postpartum ♀ | 27–40 | NR | VBM | WB |
| Miki et al. (2005) | sMRI | LO | 13 pregnant/postpartum ♀ | 26–32 | NR | Manual ROI | Pituitary |
| Oatridge et al. (2002) | sMRI | LO | 9 pregnant/postpartum ♀ | 20–38 | NR | Automated segmentation volumetry | WB |
| Menopause | |||||||
| Goto et al. (2011a, b) | sMRI | CS | 59 pre-menopause ♀, 46 post-menopause ♀ | 40–70 | Self-report | VBM, atlas-based ROI | WB, ROI |
| Sullivan et al. (2005) | sMRI | CS | 17 pre-menopause ♀, 27 post-menopause ♀, 84 ♂ | 20–85 | Self-report | Manual ROI | ROI |
| Multiple categories | |||||||
| Grams et al. (2010) | sMRI | CS | Menstrual: 47 MC ♀ OCP: 18 OCP ♀, 31 MC ♀ Menopause: 49 pre, 45 post ♀ |
18–80 | Self-report | Semi-automated segmentation volumetry | Pituitary |
Scan timing is reported in Fig. 1. For hormone status determination—”serum” refers to measurement of serum estrogen and/or progesterone, “urine” refers to the serial measurement of luteinizing hormone in the urine, and self-report refers to women providing confirmation about the beginning or ending of menses
♀ female, ♂ male
CS cross sectional, dMRI diffusion magnetic resonance imaging, DTI diffusion tensor imaging, LO longitudinal, MC menstrual cycling, N/A not applicable, NR not reported, OCP oral contraceptive pills, PD primary dysmenorrhea, ROI region of interest, sMRI structural magnetic resonance imaging, TVUS transvaginal ultrasound, VBM voxel-based morphometry, WB whole brain
Results and discussion
Papers included
Of the 25 papers we identified: 14 papers examined structural brain changes associated with natural menstrual cycling and use of hormonal birth control (Baroncini et al. 2010; De Bondt et al. 2013a, b; Grant et al. 1988; Hagemann et al. 2011; Lisofsky et al. 2015b; Ossewaarde et al. 2013; Pletzer et al. 2010, 2015; Protopopescu et al. 2008a; Teasdale et al. 1988; Tu et al. 2010, 2013; Witte et al. 2010), seven examined structural brain changes associated with pregnancy and the postpartum period (Dinc et al. 1998; Elster et al. 1991; Gonzalez et al. 1988; Hinshaw et al. 1984; Kim et al. 2010; Miki et al. 2005; Oatridge et al. 2002), 3 papers examined structural brain changes associated with the menopause transition (Goto et al. 2011a, b; Sullivan et al. 2005), and 1 paper examined structural pituitary changes across multiple categories (Grams et al. 2010). We identified instances where study subjects were included across two publications: Teasdale et al. (1988) and Grant et al. (1988), Goto et al. (2011a, b), and De Bondt et al. (2013a, b). Reporting of subject overlap was unclear between two studies by Tu et al. (2010), (2013), but the authors did not respond to requests for clarification. The total number of unique subjects across all studies was approximately 1321, with 383 male subjects and 938 female subjects. Subjects’ ages ranged from 18 to 85.
Studies’ sample, measurement and design characteristics
Results of the structured assessment of study methods are presented in Table 1. Timing of MRI scans during the menstrual cycle or OCP cycle varied considerably across studies and is depicted along with estimated relative serum hormone levels in Fig. 1. Additionally, use of menstrual cycle terminology was inconsistent across studies. For clarity, the following terms will be used to describe an idealized 28 day menstrual cycle with menses beginning on day one: follicular (days 1–14), luteal (days 15–28). The following phases are also included because they are frequently used comparatively in the reviewed studies: menstrual (days 1–7), periovulatory (days 12–16) and premenstrual (days 24–28). For OCP, the first 3 weeks during which hormonally active pills are administered will be referred to as the “active pill phase” while the remaining week of placebo pills or skipped doses will be referred to as the “inactive pill phase”. These phase designations are indicated in Fig. 1.
Of those studies including menstruating women, two assessed patients with PD (Tu et al. 2010, 2013), and one study enrolled both healthy women and patients with PMDD so as to include a range of menstrual symptoms (Protopopescu et al. 2008a). Menopause status, for papers addressing this transition, was usually assigned based on participant age (probable menopause status) or self-report; no studies determined menopause based on hormone levels. Sullivan et al. (2005) did not report the proportion of pre- vs. post-menopausal women in their sample. Of the four papers that enrolled pregnant patients, only one described how patients were assigned to gestational age groups, employing a consensus approach based on menstrual dating and ultrasound (Gonzalez et al. 1988).
Almost all studies employed volumetric structural MRI and compared the same subjects across two hormones states (e.g. follicular phase versus luteal phase or menstrual phase versus peri-ovulatory phase). Findings of these studies are summarized separately for each hormonal phase comparison (Tables 2, 3, 4, 5) and discussed in detail below. Only three studies employed any type of diffusion MRI (Baroncini et al. 2010; De Bondt et al. 2013b; Lisofsky et al. 2015b). Four studies reported associations of imaging measures with serum hormone levels (De Bondt et al. 2013a, b; Hagemann et al. 2011; Lisofsky et al. 2015b; Witte et al. 2010). These findings are summarized in Table 6. Seven studies examined the relationship between GM volume and either cognitive or affective changes in participants (Kim et al. 2010; Lisofsky et al. 2015b; Ossewaarde et al. 2013; Pletzer et al. 2015; Protopopescu et al. 2008a; Tu et al. 2010, 2013). These findings are discussed in the section “(Structure–function relationships in brain areas affected by ovarian sex hormones)”.
Table 2.
Volumetric changes associated with the menstrual cycle
| Menstrual cycle—volumetric changes | ||
|---|---|---|
|
| ||
| Study | Analysis | Regions |
| Follicular > luteal | ||
| Protopopescu et al. (2008a)a | VBM | L lingual g., R hippocampus/parahippocampal g., L mid frontal g. |
| ROI | R hippocampus | |
| Pletzer et al. (2010) | VBM | R fusiform/parahippocampal g. |
| De Bondt et al. (2013a) | VBM | R mid frontal g., b/l BA 6, L cingulate g., R ACC, L mid temporal g., L insula |
| Lisofsky et al. (2015b) | VBM | L hippocampus/parahippocampal g., b/l cerebellum |
| Luteal > follicular | ||
| Grant et al. 1988/Teasdale et al. (1988) | ROI | CSF volume |
| Protopopescu et al. (2008a)a | VBM | L sup parietal lobule, R dorsal basal ganglia, R medial frontal g./ACC, R thalamus |
| ROI | R dorsal basal ganglia | |
| Pletzer et al. (2010) | VBM | None |
| Ossewaarde et al. (2013) | ROI | L amygdala |
| De Bondt et al. (2013a) | VBM | R sup temporal g. |
| Lisofsky et al. (2015b) | VBM | R cerebellum |
| Follicular = luteal | ||
| Pletzer et al. (2010) | VBM | Total GM |
| Grams et al. (2010) | ROI | Pituitary gland |
| Hagemann et al. (2011) | Volumetry | Total GM, total WM, total CSF |
| Peri-ovulatory > menstrual | ||
| Hagemann et al. (2011) | Volumetry | Total GM |
| Tu et al. (2013)a | ROI |
(PD patients): L secondary somatosensory cortex, L ACC/dPCC (Healthy controls): none |
| Lisofsky et al. (2015b) | VBM | R cerebellum, R insula, L inf parietal lobe, b/l post hippocampib, b/l thalamib |
| Menstrual > peri-ovulatory | ||
| Hagemann et al. (2011) | Volumetry | CSF volume |
| Tu et al. (2013)a | ROI |
(PD patients): L medial OFC, L precentral g., L inf temporal g., R hypothalamus (Healthy controls): none |
| Lisofsky et al. (2015b) | VBM | R inf parietal lobe |
| Peri-ovulatory = menstrual | ||
| Tu et al. (2013)a | VBM | Total GM |
| Grams et al. (2010) | ROI | Pituitary Gland |
| Hagemann et al. (2011) | Volumetry | Total WM |
| Primary dysmenorrhea patients > healthy controls | ||
| Tu et al. (2010)a | ROI | R post hippocampus/parahippocampal g., ACC/dPCC, periaqueductal grey, hypothalamus, L ventral precuneus, L sup/mid temporal g., R cerebellar tonsil |
| Healthy controls > primary dysmenorrhea patients | ||
| Tu et al. (2010)a | ROI | R medial frontal g./PFC, R central and ventral precuneus, b/l secondary somatosensory cortices, insula, R culmen, L cerebellar tonsil |
| Primary dysmenorrhea patients = healthy controls | ||
| Tu et al. (2010)a | VBM | Total GM |
ACC anterior cingulate cortex, CSF cerebrospinal fluid, dPCC dorsal posterior cingulate cortex, GM grey matter, g gyrus, inf inferior, L left, med medial, mid middle, NA not applicable, NR not reported, OCP oral contraceptive pills, OFC orbitofrontal cortex, PFC prefrontal cortex, post posterior, R right, ROI region of interest, sup superior, VBM voxel-based morphometry
Includes or focuses on patients with menstrual cycle related symptoms (PMDD or PD)
These two regions were found in a comparison of the early follicular (menstrual) vs. late follicular phases, but the late follicular phase as defined by Lisofsky et al. (2015b) (days 10–13) shows considerable overlap with our designation of the peri-ovulatory phase (days 12–16) and thus is grouped with the other menstrual vs. peri-ovulatory results for simplicity
Table 3.
Volumetric changes associated with hormonal contraceptive use
| Hormonal contraceptive use—volumetric changes | ||
|---|---|---|
|
| ||
| Study | Analysis | Regions |
| Menstrual cycling women > OCP users | ||
| Pletzer et al. (2010) | VBM | None |
| Grams et al. (2010) | ROI | Pituitary volume |
| De Bondt et al. (2013a) | VBM | L Fusiform gyrus |
| Pletzer et al. (2015) | ROI | (vs. androgenic OCP users): b/l mid frontal g., L sup frontal g. |
| OCP users > menstrual cycling women | ||
| Pletzer et al. (2010) | VBM | B/l PFC, b/l ACC, b/l pre/post-central g., b/l SMA, R fusiform g., R parahippocampal g., R lingual g., R sup/inf temporal g., b/l cerebellum |
| De Bondt et al. (2013a) | VBM | R BA 6, R sup frontal g., b/l fusiform g., R cingulum |
| Pletzer et al. (2015) | ROI | (vs. anti-androgenic OCP users): b/l fusiform g., b/l fusiform face areas, b/l parahippocampal place area, cerebellum |
| Menstrual cycling women = OCP users | ||
| Grant et al. 1988/Teasdale (1988) | ROI | Total CSF |
| Pletzer et al. (2010) | VBM | Total GM |
| De Bondt et al. (2013a) | VBM | Total GM |
| Pletzer et al. (2015) | VBM | Total GM |
| Active pill > inactive pill | ||
| De Bondt et al. (2013a) | VBM | L ACC, L insula |
| Inactive pill > active pill | ||
| De Bondt et al. (2013a) | VBM | L BA 6, R post-central g., L caudate (ACC) |
| Pletzer et al. (2015) | ROI | L fusiform g., b/l fusiform face areas, L parahippocampal place area, R cerebellum |
ACC anterior cingulate cortex, b/l bilateral, CSF cerebrospinal fluid, dPCC dorsal posterior cingulate cortex, g gyrus, GM grey matter, inf inferior, L left, mid middle, OCP oral contraceptive pills, PFC prefrontal cortex, post posterior, R right, ROI region of interest, sup superior, SMA supplementary motor area, VBM voxel-based morphometry
Table 4.
Volumetric changes associated with pregnancy and the postpartum period
| Pregnancy and postpartum—volumetric changes | ||
|---|---|---|
|
| ||
| Study | Analysis | Regions |
| Pregnant > not pregnant | ||
| Gonzalez et al. (1988), Elsteret al. (1991), Dinc et al. (1998) | ROI | Pituitary gland volume |
| Pregnant > postpartum | ||
| Oatridge (2002) | Volumetry | Whole brain volume (corresponding decrease in CSF) |
| Gonzalez et al. (1988), Elsteret al. (1991), Dinc et al. (1998) | ROI | Pituitary volumea |
| Late postpartum > early postpartum | ||
| Oatridge et al. (2002) | Volumetry | Whole brain volume (corresponding decrease in CSF) |
| Kim et al. (2010) | VBM | PFC, pre/post-central g., sup/inf parietal lobe, insula, thalamus |
CSF cerebrospinal fluid, g gyrus, inf inferior, PFC prefrontal cortex, ROI region of interest, sup superior, VBM voxel-based morphometry
Pituitary gland volume peaked 0–6 days postpartum and then decreased in size
Table 5.
Volumetric changes associated with menopause
| Menopause—volumetric changes | ||
|---|---|---|
|
| ||
| Study | Analysis | Regions |
| Pre-menopausal > post-menopausal | ||
| Goto et al. (2011a, b) | VBM/ROI | B/l hippocampus |
| Pre-menopausal = post-menopausal | ||
| Sullivan et al. (2005) | ROI | B/l hippocampus |
| Grams et al. (2010) | ROI | Pituitary |
Table 6.
Structural variation correlated with serum hormone levels
| Study | Serum hormones—imaging correlations |
|---|---|
| Estrogen—positive correlations | |
| Hagemann et al. (2011) | None |
| Witte et al. (2010) | Follicular: L sup parietal g. |
| De Bondt et al. (2013a) | Follicular: BA 8, cingulum, post-central g., insula Luteal: none |
| Lisofsky et al. (2015b) | L parahippocampal g., L mid frontal g., R cerebellum |
| Estrogen—negative correlations | |
| Hagemann et al. (2011) | None |
| Witte et al. (2010) | Follicular: none |
| De Bondt et al. (2013a) |
Follicular: Fusiform g. Luteal: ACC, sup frontal g., mid temporal g. |
| De Bondt et al. (2013b) | MD in the fornix |
| Lisofsky et al. (2015b) | None |
| Progesterone—positive correlations | |
| Hagemann et al. (2011) | Luteal (vs. menstrual): Total CSF |
| Witte et al. (2010) | Follicular: R mid temporal pole |
| De Bondt et al. (2013a) |
Follicular: Fusiform g., lingual/parahippocampal g., pre-central g. Luteal: BA 8, SMA |
| Progesterone—negative correlations | |
| Hagemann et al. (2011) | Luteal (vs. menstrual): Total GMa |
| Witte et al. (2010) | Follicular: none |
| De Bondt et al. (2013a) |
Follicular: none Luteal: Fusiform g. |
| Follicle-stimulating hormone—positive correlations | |
| De Bondt et al. (2013a) | Follicular/Luteal: none |
| Follicle-stimulating hormone—negative correlations | |
| De Bondt et al. (2013a) | Follicular/Luteal: none |
| Luteinizing hormone—positive correlations | |
| De Bondt et al. (2013a) | Follicular/Luteal: none |
| Luteinizing hormone—negative correlations | |
| De Bondt et al. (2013a) | MD in the fornix |
ACC anterior cingulate cortex, CSF cerebrospinal fluid, FSH follicle stimulating hormone, g gyrus, GM grey matter, L left, LH luteinizing hormone, MD mean diffusivity, mid middle, R right, ROI region of interest, sup superior, SMA supplementary motor area, VBM voxel-based morphometry
Excluding one outlier subject (n = 6)
Menstrual cycle
Global brain changes identified over the course of the menstrual cycle include variation in brain size, total CSF volume, and total GM volume. Hagemann et al. (2011) used automated segmentation to partition images into GM, WM, and CSF and then summed the number of voxels for each tissue compartment to calculate total tissue volumes at three different points in the menstrual cycle (menstrual, peri-ovulatory, and luteal). No differences were found in total GM volume between the menstrual and luteal phases, a result supported by those of Pletzer et al. (2010). Lisofsky et al. (2015b), who focused on hippocampal volume changes, did not report on total GM, WM, or CSF volume changes across the four time points at which they scanned subjects. Additionally, Hagemann et al. did not find differences between menstrual and luteal phases in WM or CSF volume. However, there was a statistically significant 1.81 % increase in GM volume and a corresponding 4.4 % decrease in CSF volume between the luteal and peri-ovulatory phases with no change in WM volume. The increase in brain volume is generally consistent with the results of Grant et al. (1988) and Teasdale et al. (1988) who similarly found lesser CSF volume (mean decrease of 11.3 %) during the peri-ovulatory phase compared to the pre-menstrual phase. Since Grant and Teasdale did not report on changes in GM or WM, however, it is not possible to infer whether the implicit change in tissue volume is exclusively due to GM volume increase as found by Hagemann et al. A follow up analysis by the same authors as Hagemann et al. demonstrated that the peri-ovulatory increase in total GM could have a significant impact on estimates of brain age that model normal age related brain atrophy with women appearing to be younger at time of ovulation (Franke et al. 2015).
Structural plasticity has also been localized to specific brain regions. Areas that exhibit follicular/luteal structural plasticity include hippocampus, the parahippocampal gyrus, the fusiform gyrus, the cingulate cortex (in particular, ACC), the insula, the middle frontal gyrus, the thalamus and the cerebellum. Overall, estrogen seems to have a trophic effect on the hippocampus. Protopopescu et al. (2008a) identified lower volume in the right anterior hippocampus during the pre-menstrual (luteal) phase. The association of lesser hippocampal volume with the low-estrogen luteal phase is consistent with findings in rodents that identify decreases in hippocampal spine density associated with declining estrogen levels during the low estrogen estrus phase (Woolley and McEwen 1992). Lisofsky et al. specifically examined the relationship between serum estrogen and hippocampal volume by comparing volume during the late follicular phase (high estrogen) to the menstrual phase (low estrogen), thereby minimizing the potentially confounding effects of progesterone. Lisofsky et al. found regions of significantly increased volume in the posterior portions of both right and left hippocampi during the late follicular phase. Furthermore, in these regions, greater GM volume was associated with lower mean diffusivity (MD) in the same region—lower MD is associated with higher GM density that restricts the free diffusion of water. As discussed above, higher GM density may be the result of increases in dendritic spines and synapses.
Both Pletzer et al. and Protopopescu et al. identified lower volume in the right parahippocampal/fusiform gyrus during the progesterone dominated pre-menstrual (luteal) phase compared with the estrogen dominated follicular phase. De Bondt et al. (2013a) found that fusiform gyrus volume during the luteal phase was smaller in women with higher serum progesterone levels, an apparent extension of the effect observed by Pletzer et al. and Protopopescu et al. During the follicular phase, however, fusiform volume trended positively with serum progesterone, but negatively with serum estrogen levels. Lisofsky et al. identified a follicular increase in parahippocampal volume on the left side and a positive correlation between left hippocampal/parahippocampal volume and serum estrogen levels.
Three studies identified changes to the middle frontal gyrus—De Bondt et al. on the left, and Protopopescu et al., and Lisofsky et al. on the right. Both De Bondt et al. and Protopopescu et al. identified greater GM volume during the follicular phase compared with the luteal phase. Lisofsky et al. identified a positive correlation between serum estradiol levels and left middle frontal gyrus volume. Two studies—De Bondt et al. and Lisofsky et al.—discovered increased GM volume of the insula during the follicular phase but in opposite hemispheres.
Other areas including the cingulate gyrus, ACC, amygdala, thalamus, cerebellum, and parietal and temporal cortical areas also exhibit follicular vs. luteal structural differences, but these locations have each been reported in individual studies only. De Bondt et al. identified decreased ACC volume in the mid-luteal phase compared with the early follicular phase. Moreover, across subjects ACC volume was negatively correlated with serum estradiol concentration during the luteal phase. Ossewaarde et al. (2013) identified luteal phase increases in left amygdala volume, a finding in keeping with evidence from animals (Cooke 2006; Cooke et al. 2003; Cooke and Woolley 2005; Fan et al. 2008). Witte et al. (2010), studying women in the mid-follicular phase, found that higher serum estradiol is associated with greater GM volume in the left superior parietal lobule and that higher serum progesterone is associated with lower GM volume in the right temporal pole. Ossewaarde et al. found increased GM in the left superior parietal lobule during the luteal phase—as Witte et al. did not image subjects during the luteal phase, it cannot be determined if the results of these two studies are in conflict. De Bondt et al. also investigated the association of GM volume with serum hormone levels, but did not detect similar structural variations. Inconsistencies between the results reported by Witte et al. and De Bondt et al. may be attributed to slight differences in the timing of imaging during the follicular phase; De Bondt et al. imaged subjects during the early follicular phase, when both estrogen and progesterone levels are low, while Witte et al. imaged subjects during the mid-follicular phase when estrogen rises rapidly. Alternatively, results of the studies may differ because the design of Witte et al. included comparison to men, whereas De Bondt et al. did not.
Two papers by Tu et al. (2010, 2013) report on peri-ovulatory vs. menstrual phase effects and specifically examine the trait- and state-dependent effects of PD. The first paper compares women with PD to healthy controls in the pain-free peri-ovulatory phase. Women with PD exhibit higher GM volume in areas related to endocrine function (hypothalamus) and emotional processing (parahippocampus and ACC), and lower GM volume in areas related to pain transmission and sensory processing (insula) and in areas that regulate affective responses to negative stimuli (medial PFC). Unlike most changes associated with the menstrual cycle, which revert to baseline over the course of the cycle, the signature brain volumetric changes of PD persist throughout the pain-free peri-ovulatory period. The enduring nature of the structural changes associated with PD distinguishes the disorder as an entity beyond the normal spectrum of PMS and menstrual symptoms. Although the volumetric changes could be attributed to the effects of chronic cyclic pain rather than purely to the effects of hormone variability, they are likely mediated at least in part by estrogen and progesterone. As discussed by the authors, PD has been associated with increased estrogen levels during the later stages of the menstrual cycle (Ylikorkala et al. 1979), and with menstrual cramping due to increased estrogen-mediated prostaglandin synthesis (Ham et al. 1975).
Tu et al. next examined state-dependent structural changes within the same ROIs identified above, but comparing the pain-free peri-ovulatory phase with the symptomatic menstrual phase. During menses, PD patients exhibit increased GM in the medial OFC and hypothalamus and decreased GM in the secondary somatosensory cortex and ACC, compared to the period surrounding ovulation. In the control subjects, no changes were found in global GM volume or in regional volume of the ROIs. This is in contrast to the study by Hagemann et al. that found total GM volume to increase in the peri-ovulatory compared to the menstrual phase and to the study by Lisofsky et al. which identified regional changes between the peri-ovulatory and menstrual phases in the right insula, bilateral inferior parietal lobes, bilateral posterior hippocampi, bilateral thalami, and right cerebellum. Notwithstanding the divergent nature of some findings, the unique structural changes associated with PD support the phenomenon of rapid neuroplasticity in association with a time-limited stimulus (1–3 days of menstrual cramping pain).
No menstrual cycle dependent effects were found on pituitary size (Grams et al. 2010). As pituitary size did not change in response to menstrual fluctuations in estrogen, the degree of hormone variation may need to be quite large in order for pituitary size to be affected in a detectable way. During puberty, for example, when there is a rapid and substantial increase in ovarian sex hormones and testosterone, the pituitary gland also experiences a physiological volume increase (Wong et al. 2014).
Hormonal contraceptives
Global brain changes have not been identified between menstrual cycling women and OCP users, despite assessments of CSF volume (Grant et al. 1988; Teasdale et al. 1988) and total GM volume (De Bondt et al. 2013a; Pletzer et al. 2010, 2015). Even when different formulations of OCPs have been considered (i.e. androgenic vs. anti-androgenic), no total GM volume differences between menstrual cycling women and OCP users were detected (Pletzer et al. 2015).
Despite the lack of global differences, structural differences have been identified in specific brain regions between menstrual cycling women and OCP users. Pletzer et al. (2010) identified greater GM volume in the PFC, ACC, parahippocampal and fusiform gyri, and cerebellum in OCP users compared to menstrual cycling women. De Bondt et al. (2013a) similarly identified GM volume effects in the fusiform gyri and ACC and an additional finding in the superior frontal gyrus, but no cerebellar findings. Pletzer et al. (2015) found that compared with menstrual cycling women, users of anti-androgenic OCPs had relatively larger GM volumes in the bilateral fusiform gyri, parahippocapmus, and cerebellum, while users of androgenic OCPs had relatively smaller volumes in the bilateral middle and superior frontal gyri.
De Bondt et al. (2013b), one of the few diffusion-MRI studies, also found increased MD in the fornix, a WM tract connecting the hippocampus and mammillary body, key elements of the limbic system, in the OCP group compared to the naturally cycling group. The authors hypothesize that increased MD may represent lower synapse number in the OCP group compared to the naturally cycling group. In a study correlating histological findings with diffusion imaging, rats who had undergone Morris Water Maze training showed histological evidence of increased astrocyte processes and synaptic markers and a decreased apparent diffusion coefficient (ADC; equivalent to MD measured with diffusion tensor imaging), representing greater tissue density, on imaging (Blumenfeld-Katzir et al. 2011). Additionally, in De Bondt et al., MD in the fornix was significantly negatively correlated with serum LH and estradiol concentrations, implying that LH and estradiol may up-regulate synaptogenesis or preserve existing synapses as has also been demonstrated in animal literature (Naftolin et al. 2007).
Both De Bondt et al. (2013a, b) and Pletzer et al. (2015) compared the active and inactive pill phases, but did not have overlapping findings. De Bondt et al., however, did not characterize OCP type. Baroncini et al. (2010) focused on changes in the hypothalamus between the active and inactive phases using a ROI-based diffusion-weighted MRI analysis. The ADC within the hypothalamus was higher during the inactive pill phase than during the active pill phase. Again, this may represent higher tissue density during the active pill phase as the result of synaptogenesis associated with higher hormone levels. No changes were observed in a control ROI placed in the thalamus, a region chosen as unrelated to reproductive neuroendocrine function. Structural changes within the hypothalamus as a result of exogenous hormone administration suggest a possible mechanism for hormonal birth control-mediated ovulation suppression through effects on synapse and astrocyte morphology.
Additionally, women taking OCPs were found to have slightly smaller pituitary volumes than naturally cycling women (Grams et al. 2010).
Pregnancy and the postpartum period
Almost all the studies examining pregnant women report exclusively on changes of the pituitary (Dinc et al. 1998; Elster et al. 1991; Gonzalez et al. 1988; Hinshaw et al. 1984; Miki et al. 2005) even though this period, during which progesterone significantly dominates over estrogen, would provide an exceptional window into the unique contributions of progesterone relative to estrogen. Overall the findings from these papers showed considerable overlap—pituitary gland volume increased throughout pregnancy up until delivery and the first postpartum week and then declined throughout the postpartum period until reaching normal size by about 6 months after delivery. Pituitary volume changes associated with pregnancy result from physiologic hyperplasia of lactotrophic cells under the influence of placental estrogen (Karaca et al. 2010).
Oatridge et al. (2002) investigated changes in total maternal brain volume from pre-conception to postpartum. Total brain volume decreased leading up to delivery and then increased during the postpartum phase. This change was complemented by an increase in ventricular size during pregnancy and subsequent decrease postpartum. All pregnancy-related changes returned to pre-conception baseline by 24 weeks postpartum. Kim et al. (2010) used VBM and demonstrated that GM volume of a number of regions increased significantly from the early to the late postpartum period, including the PFC, pre- and post-central gyri, superior/inferior parietal lobule, insula, and thalamus.
These findings are in agreement with evidence of maternal brain structural plasticity in animals. Virgin rats exposed to an artificial approximation of the hormone milieu of parturition will begin to respond to pups in a similar manner as pregnant/maternal rats (Siegel and Rosenblatt 1975). Parturient rats exhibit a greater number of hypothalamic astrocytes in proportion to the number of interactions they have with their pups (Featherstone et al. 2000). It is likely that many of these postpartum functional and structural changes are related to other hormones, including oxytocin and vasopressin, as well as estrogen and progesterone. In lactating rats, glial processes in the hypothalamus withdraw allowing direct neuronal contact (neuro-juxtaposition) between oxytocinergic neurons (Montagnese et al. 1987). These changes return to baseline after lactation ends.
Menopause
Most studies we identified that compared pre-menopausal and post-menopausal women focused on the effects of HRT and thus are beyond the scope of this review. We identified two studies that reported structural brain changes associated with physiologic menopause, both of which specifically focused on the effect of menopause on the hippocampus. Sullivan et al. (2005) used a semi-automated segmentation method and manual ROI placement to look at age- and menopause-related hippocampal volume changes and failed to find evidence of either. It should be noted that Sullivan et al. did include some women who took HRT, but also failed to find an effect of HRT on hippocampal volume. Goto et al. (2011a, b) made two separate comparisons, women in their fourth decade vs. women in their fifth decade and pre-menopausal vs. post-menopausal women. Both comparisons showed age- and menopause-related decline in hippocampal volume. In a follow-up investigation, the authors performed an atlas-based ROI analysis to determine absolute changes in hippocampal volume. This study confirmed the prior results and demonstrated a greater decrement in hippocampal volume when comparing women in their fifties to women in their forties than in similar age groups of men.
The results from these two studies investigating the hippocampus during menopause are in direct conflict. This may be attributable to differences in sample size—Sullivan et al. enrolled 17 premenopausal women, 16 post-menopausal women not receiving HRT, and 11 post-menopausal women receiving HRT, while Goto et al. enrolled 54 pre- and 54 post-menopausal women not receiving HRT. Thus, Sullivan et al., who reported no effect of menopause, may not have been powered to detect change in hippocampal volume. However, another study of 210 post-menopausal women that measured serum estradiol levels and used manual tracing to quantify hippocampal volume also failed to find an association between estrogen and hippocampal size or memory performance (den Heijer et al. 2003). Overall, despite multiple studies, the effects of aging, menopause, and HRT on cognition and memory remain controversial (Fischer et al. 2014). In part this may be due to the particular difficulty of disentangling the confounding factors of menopausal status and age.
Additionally, although pituitary volumes decreased with age, no effect of menopause duration on pituitary size was found (Grams et al. 2010).
Hormone mediated structural neuroplasticity in humans
We identify a salient theme of GM volume changes associated with variation in the ovarian sex hormones in women. These effects are prominent among many components of the limbic system, including the hippocampus, parahippocampal gyrus, fusiform gyrus, cingulate gyrus, insula, amygdala, thalamus, and hypothalamus, as well as in the middle frontal gyrus, basal ganglia, and cerebellum (Fig. 2). Although much fewer in number, studies of WM microstructure have also revealed plasticity of the limbic system structures, such as the fornix, related to ovarian sex hormone transitions. Notably, the structures exhibiting volumetric change include those exhibiting high concentrations of estrogen and progesterone receptors including the hypothalamus, amygdala, and hippocampus.
Variations in brain structure among women may represent a segment of the larger spectrum of structural variability among humans; the findings we have reviewed in fact do overlap known male–female dimorphisms of brain structure. A meta-analysis of studies examining sex differences of brain structure found larger volume and higher density of GM in the amygdala, hippocampus, parahippocampus, insula, and putamen in men compared with women (Ruigrok et al. 2014). It is unclear, however, if the meta-analysis controlled for overall brain size in these comparisons; evidence supports that, as a fraction of brain size, the hippocampus may in fact be larger in women (Filipek et al. 1994). Other areas identified in the meta-analysis overlapped with areas associated with hormonal transitions, including anterior and posterior cingulate cortices, OFC, left temporal pole, right frontal pole, right and left middle frontal gyri, and right and left thalamus. Notably, many of the studies included in the meta-analysis did not characterize menstrual cycle phase of their female participants. Another analysis of sexually dimorphic brain structures in humans found that the magnitude of the dimorphism was proportional to the expression of sex hormone receptors during development in homologous structures in other animals (Andreano and Cahill 2009; Goldstein et al. 2001). Additionally, as demonstrated by Witte et al. (2010), serum hormone levels correlate with GM volumes in sexually dimorphic areas across both male and female adult subjects.
Effects of estrogen and progesterone are of course not limited to the limbic system. A second concentration of structural changes associated with variation in ovarian sex hormones may be found in somatosensory processing areas. The “pain network” as identified by neuroimaging and neurophysiology investigations includes the primary and secondary somatosensory cortices as well as the insula, ACC, and thalamus (Nakata et al. 2014). Menstrual cycle disorders, such as PD, have been reported in association with both trait- and state-dependent changes in limbic areas (hippocampus/parahippocampal gyrus) as well as somatosensory and pain pathway areas (post-central gyrus, secondary somatosensory cortex, and insula) (Tu et al. 2010, 2013). These areas as well as the thalamus (Lisofsky et al. 2015b) and ACC (De Bondt et al. 2013a) may play a role in mediation of somatic menstrual cycle complaints including headaches, fatigue, nausea, menstrual cramps, and increased pain sensitivity. OCPs also affect volume of the insula (De Bondt et al. 2013a), as does the transition from the early to late postpartum period (Kim et al. 2010).
Structural variability also plays a role in the patho-physiology of PMDD. A VBM study comparing PMDD patients with healthy controls found higher GM density in the hippocampus and lower GM density in the parahippocampal gyrus (Jeong et al. 2012). PMDD is thought to be a disorder of hypersensitivity to estrogen and progesterone; serum levels of these hormones in PMDD patients are normal (Backstrom et al. 2003). In this regard, the volume of GM in ER-rich structures could be a structural modulator of estrogen sensitivity.
Findings regarding the effect of OCPs do not represent a mere extension of the effects of endogenous sex hormones. The impact of OCPs was stronger in frontal areas including the PFC and weaker, though still present, in the hippocampus and parahippocampus. Additionally, differences between OCP users and menstrual cycling women were for the most part much larger than the differences within either group, in terms of cluster size. Moreover, OCP use enhanced differences found between the sexes, in the PFC in particular (Pletzer et al. 2010). Variable cerebral uptake of endogenous ovarian sex hormones compared with exogenous artificial hormones may explain structural and functional divergence between naturally cycling women and users of hormonal contraception. Moreover, as demonstrated by Pletzer et al. (2015), variable androgenic activity of different progestins used in OCPs may also explain the range of effects.
Structure–function relationships in brain areas affected by ovarian sex hormones
Functional domains that display variation in behavior and in activation patterns on functional neuroimaging in association with ovarian sex hormone transitions, such as emotion—e.g. viewing erotic images (Abler et al. 2013), emotionally valenced pictures (Goldstein et al. 2005), or facial expressions (Ossewaarde et al. 2010)—and cognition—affective response inhibition (Amin et al. 2006) and working memory (2-back task) (Konrad et al. 2008)—are extensively linked to limbic regions. Limbic structures play a central role in mood and emotion, functional domains that underpin menstrual symptoms such as irritability, depression, and anxiety (Biggs and Demuth 2011). Although a detailed summary of the functional effects of estrogen and progesterone is beyond the scope of this review,2 preliminary structure–function correlations support the concept that structural alteration of limbic components represents a key substrate of this functional variability.
The hippocampus was the region that most consistently exhibited neuroplasticity in association with variation in hormonal factors and these findings were present across multiple hormone transitions, including menstrual cycle, OCP use, and menopause. As suggested by animal studies, estrogen exhibits a trophic effect on the hippocampus in humans. We found, with relative consistency across the reviewed studies, that the hippocampus was larger during estrogen dominated phases (i.e. the follicular phase of the menstrual cycle and before the onset of menopause). We would therefore expect verbal and visuospatial memory performance would improve during the follicular phase compared with the luteal phase; there is some evidence from functional and cognitive studies that this is the case (Rosenberg and Park 2002; Sundstrom Poromaa and Gingnell 2014). Contrary to this expectation, however, Lisofsky et al. (2015b) did not find any reliable variation in cognitive tasks across the menstrual cycle and so did not correlate hippopcampal structural changes with cognitive outcomes. They did, however, identify increased functional connectivity between the hippocampi and bilateral superior parietal lobes during the late follicular phase.
The right hippocampus has traditionally been implicated in visuospatial memory formation (Smith and Milner 1981) while the left has been associated with verbal or narrative memories in connection with left sided language structures (Burgess et al. 2002; Frisk and Milner 1990). However, Protopopescu et al. found that improved verbal memory during the follicular phase (versus the luteal phase) positively correlated with increased GM volume in the right anterior hippocampus (Protopopescu et al. 2008a). A systematic review of studies examining cognitive effects of combined oral contraceptives found inconclusive and contradictory effects overall, though improved verbal memory in OCP users has been demonstrated (Warren et al. 2014). The positive effect of estrogen on the hippocampus and memory within women is also in keeping with the well-established finding of superior verbal memory performance in women compared with men (Andreano and Cahill 2009).
Volume changes associated with menstrual cycling and OCP use were found in the parahippocampal and fusiform gyri. The parahippocampal gyrus has been ascribed a diverse set of functions, but it is most prominently related to episodic memory and visuospatial processing (Aminoff et al. 2013). The robust response of the parahippocampus to viewing spatial scenes, but not objects or faces, led to the designation of the parahippocampal place area (Aminoff et al. 2013; Epstein and Kanwisher 1998). The fusiform gyrus plays a role in the processing of both faces (Kanwisher et al. 1997) and words (Harris et al. 2015). Pletzer et al. (2015) found that users of anti-androgenic OCPs had greater GM volumes in both the parahippocampal place area and the fusiform face area compared to menstrual cycling women as well as better performance on a facial recognition task that was correlated with GM volume.
Surprisingly, the amygdala, which has a well-established sexual dimorphism, with respect to its size, and has been found to be functionally variable across the menstrual cycle across multiple functional imaging studies (Lisofsky et al. 2015a; Toffoletto et al. 2014), only demonstrated menstrual cycle related volume change in a single ROI analysis (Ossewaarde et al. 2013). Ossewaarde et al. found that, during the pre-menstrual period, increasing GM volume in the amygdala correlates with heightened negative affect in the context of stressful visual stimuli. The amygdala is involved in generating affective and behavioral responses to both aversive and rewarding stimuli (Janak and Tye 2015). Frequently, amygdala hyperactivity is invoked as an example of failure of top-down modulation of limbic activity by frontal areas. This is exemplified in PMDD patients who demonstrate decreased OFC and increased amgydala activity in the context of negatively valenced emotional stimuli during the pre-menstrual (luteal) phase (Protopopescu et al. 2008b). Additionally, during the postpartum period, greater GM volume in the maternal amygdala and hypothalamus was associated with mothers endorsing a more positive perception of their babies (Kim et al. 2010)—this effect may reflect changes in a subpopulation of valence-selective neurons within the amygdala that respond to reward rather than to fear (Paton et al. 2006).
Tu et al. (2010), (2013) identified correlations between the experience of menstrual pain measured by the McGill Pain Questionnaire and GM volume. During the pain-free peri-ovulatory phase, patients’ ratings of menstrual pain were negatively correlated with GM volume in the bilateral PFC and positively correlated with GM volume in the bilateral OFC and ACC (Tu et al. 2010). As with Protopopescu et al. (2008b), this result suggests a failure of top-down modulation of limbic structures. During menses, in women with dysmenorrhea, greater GM volume in the right caudate nucleus and the hypothalamus was positively correlated with a higher pain score, while GM volume in the left thalamus was negatively correlated with pain score. As suggested by Tu et al., both the caudate and the hypothalamus (via the bulbospinal loop) play a role in the regulation of both pain processing and the negative emotional processing associated with the experience of pain (Scott et al. 2006; Suzuki et al. 2004); changes in these areas, therefore, may represent maladaptive plasticity underlying the hyperalgesia associated with PD (Tu et al. 2013).
Although speculating on the evolutionary advantages or disadvantages of hormone mediated neuroplasticity in detail is beyond the scope of this review, various theories regarding the origins of PMS and other menstrual cycle related cognitive changes have been put forth (Gillings 2014). One theory suggests that PMS symptoms, such as irritability, functioned to dissolve sexual partnerships that did not result in pregnancy. However, given that for much of human history women may have had far fewer cycles than modern women, due to earlier and more frequent pregnancy and prolonged lactation-induced amenorrhea, menstrual cycle related changes and symptoms may instead represent an evolutionary byproduct. If frequent menstrual cycling is, in fact, a relatively recent physiologic phenomenon, it may only now begin to be exposed to natural and sexual selection pressures.
Methodological considerations
Limitations of the individual structural neuroimaging studies reviewed here are similar to those affecting fMRI studies on this topic and include variable timing of scans during hormone cycles, small individual study sample sizes and lack of a standard method for menstrual cycle phase determination (Sacher et al. 2013; Toffoletto et al. 2014).
The majority of the studies we have reviewed utilized VBM to assess structural changes. However, due to small sample sizes, many studies were insufficiently powered to identify effects across the whole brain and many reported whole brain findings did not survive correction for multiple comparisons. When hypothesis-driven atlas-based ROI approaches were used to supplement the whole brain VBM analyses, as in Protopopescu et al., De Bondt et al., and Pletzer et al., areas that were identified in whole brain analysis at an uncorrected significance level, were found to be significant. Most of these hypothesis-driven ROI analyses specifically targeted limbic structures implicated by the underpowered whole brain analyses. While this approach suggests that a specific subset of brain regions demonstrate structural plasticity related to ovarian sex hormone levels, it may also be the case that the whole-brain approaches, which drove the analyses, selectively captured regions exhibiting the most dramatic degree of variability (i.e. limbic structures). Studies employing larger sample sizes might detect a greater diversity of effects of ovarian sex hormones in other brain regions.
A critical question for researchers preparing structural neuroimaging studies examining hormonal effects or that include women with variable hormonal status is the magnitude of detected changes in volume that are attributable to hormone-based neuroplasticity, in light of the variance across the sample. This effect size (e.g. Cohen’s d) was not reported in a systematic way across the studies reviewed here. However, for studies where the means and standard deviations of volume measurements or the degrees of freedom from t tests are provided, it may be possible to calculate the effect size as the mean difference between two conditions (e.g. menstrual phases, pregnant vs. non-pregnant) divided by the pooled standard deviation or from the t-statistic (Rosenthal and DiMatteo 2001). Sufficient information was only available, however, for four of the 25 papers included in our review.
Although effect size calculations were possible in only a minority of the studies, these few examples suggest that hormone associated volume changes have a broad range of effect sizes.3 Goto et al. (2011b) demonstrated moderate to large effect sizes (d = 0.90 for the right, d = 0.61 for the left) for hippocampal volume differences observed between pre and post-menopausal women using atlas-based morphometry. For the difference in pituitary volume between menstrual cycling women and women taking OCPs, the effect size was small (d = 0.34) (Grams et al. 2010). There was only one instance in which effect sizes could be reasonably compared between two studies. In their comparison of the luteal and follicular phases in menstrually cycling women, Protopopescu et al. (2008a) demonstrated a small effect size (d = 0.12) for GM volume increase in the right anterior hippocampus and for GM volume decrease in the right dorsal basal ganglia (d = 0.24). However, Lisofsky et al. (2015b) found a large effect size (d = 0.99) for average hippocampal GM volume increase from the menstrual to the peri-ovulatory phase. One possible explanation for this discrepancy in the size of the effect is that the relative difference in serum estrogen between the luteal and early follicular (menstrual) phases (Protopopescu et al.) is much smaller than the difference between the early follicular (menstrual) and peri-ovulatory phases (Lisofsky et al.). Given the theoretical trophic effect of estrogen on the hippocampus, we might expect a greater degree of hippocampal volume change in response to the peri-ovulatory estrogen peak (Lisofsky et al.). Future studies that examine hormone-associated structural plasticity should report effect sizes in a standardized manner to facilitate comparison across studies and ultimately rigorous meta-analysis.
Additionally, VBM does not provide information regarding the degree of volume change—it will be important to confirm these intriguing results with more detailed assessments of cortical thickness, as was done by Goto et al. in their atlas-based analysis. Only two studies employed diffusion MRI to assess microstructural features; the relationship between hormones and brain microstructure remains an opportune area for deeper investigation. Finally, as functional network and connectivity fMRI investigations become increasingly popular, the underlying structural framework for connectivity between disparate brain regions will be an important focus area.
In light of the temporal variation of both endogenous and exogenous hormone exposure among women and the evidence that these variations impact brain structure and function, researchers planning neuroimaging studies that will enroll women should consider characterizing female subjects’ hormonal status. Variation in menstrual cycle effects could be addressed in future studies by imaging female participants at a specific menstrual cycle phase (Witte et al. 2010), or by exclusively enrolling either women who are or those who are not taking oral contraceptives. Alternatively, counterbalanced, longitudinal crossover designs, where subjects serve as their own controls, could be employed to address this issue (Poromaa 2014). Most practical, however, might be determination of menstrual cycle phase at the time of imaging, which can then be considered as a regressor during data analysis.
Confirmation of menstrual cycle phase must be balanced between practical and logistical concerns associated with more accurate approaches such as daily measurement of serum progesterone, estrogen, LH, or FSH levels or transvaginal ultrasound, and less accurate ascertainment when relying on subject reporting of menstrual cycle pattern. One study comparing women’s self report of menstrual cycle phase, urine LH kits (which approximate ovulation by the LH surge), and serum progesterone levels found that when counting forward from the first day of menses to determine ovulation, only 35–42 % had a positive urinary test between 10 and 14 days and only 18 % had a serum progesterone >2.0 ng/mL, an indication that ovulation has occurred (Wideman et al. 2013). A combined approach using urine ovulation kits that measure luteinizing hormone levels and subject self-report may be an effective and less invasive option.
Variation due to birth control type should also be considered; different OCP formulations confer different estrogen and progesterone levels and some formulations (biphasic, triphasic, or multiphasic) may vary the relative doses over the course of a 1-month cycle. However, one study reported that while patients were taking different formulations of monophasic combined (estrogen and progesterone) OCPs there were no significant differences in measurements of hormonal concentrations of serum samples (De Bondt et al. 2013b). Alternative methods of birth control such as the estrogen/progesterone patch or vaginal ring are associated with lower, but more constant serum levels of estrogen and progesterone, (van den Heuvel et al. 2005) and both the copper and Mirena (levonorgestrel-releasing) intrauterine devices may leave normal ovarian hormone cycles intact (Faundes et al. 1980; Xiao et al. 1995).
In studies involving older women, establishing reproductive senescence may be more amenable to subject self-report measures since, in a clinical setting, menopause is diagnosed after 12 months of amenorrhea in the absence of other causes. Alternatively, ascertaining elevation of FSH may be confirmatory and could be correlated with the degree of structural change.
Conclusions
Multiple studies in young healthy women demonstrate the impact of menstrual cycle phase and hormonal contraceptives on brain macrostructure as well as similar structural effects related to menopause in older healthy women. Generalizing the findings of the studies reviewed here is constrained by sample and methodological heterogeneity across studies, but overall they support the notion that ovarian sex hormones drive neuroplasticity. Cyclical and gradual hormonal variation throughout the female lifespan may also be important in a myriad of disorders including traumatic brain injury, stroke, multiple sclerosis, and migraine. Neuroimaging is an appropriate and informative tool for exploring hormone-related structural plasticity and pathology. The interaction of hormone exposure and neuroimaging findings can also serve to develop hypotheses regarding structure–function relationships, especially when considering menstrual cycle specific disorders such as PD, PMS, and PMDD.
Abbreviations
- ACC
Anterior cingulate cortex
- ADC
Apparent diffusion coefficient
- ALLO
Allopregnanolone
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- EE
Ethinyl estradiol
- ER
Estrogen receptor
- FDR
False discovery rate
- fMRI
Functional magnetic resonance imaging
- FSH
Follicle stimulating hormone
- GABA
Gamma-aminobutryic acid
- GM
Grey matter
- GnRH
Gonadotropin releasing hormone
- GPCR
G-protein coupled receptor
- HRT
Hormone replacement therapy
- IUD
Intrauterine device
- LH
Luteinizing hormone
- MD
Mean diffusivity
- MNI
Montreal Neurological Institute
- MRI
Magnetic resonance imaging
- mRNA
Messenger ribonucleic acid
- OCP
Oral contraceptive pills
- OFC
Orbitofrontal cortex
- PD
Primary dysmenorrhea
- PFC
Prefrontal cortex
- PMDD
Premenstrual dysphoric disorder
- PMS
Premenstrual syndrome
- ROI
Region of interest
- VBM
Voxel based morphometry
- WM
White matter
Footnotes
Despite the relatively late maturation of the uncinate fasciculus, a review of DTI imaging examining the uncinate fasciculus across the lifespan did not identify sex differences in white matter microstructure (Hasan et al. 2009). This is at least suggestive that there may not be a robust relationship between myelination of this structure and the sex hormones.
For further review of functional neuroimaging studies investigating the menstrual cycle and OCP use see Sacher et al. (2013) and Toffoletto et al. (2014). For further review of the putative roles of neurosteroids in symptomatology of PMS, pregnancy, the postpartum period, and menopause, see Pluchino et al. (2013).
Cohen’s d is commonly used to classify the effect size as small (<00.4), medium (0.4–0.7), or large (>0.7) (Rose and Donohoe 2013).
References
- Abler B, Kumpfmuller D, Gron G, Walter M, Stingl J, Seeringer A. Neural correlates of erotic stimulation under different levels of female sexual hormones. PloS one. 2013;8:e54447. doi: 10.1371/journal.pone.0054447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham GE, Odell WD, Swerdloff RS, Hopper K. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17 beta during the menstrual cycle. J Clin Endocrinol Metabol. 1972;34:312–318. doi: 10.1210/jcem-34-2-312. [DOI] [PubMed] [Google Scholar]
- Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage. 2006;32:457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends in cognitive sciences. 2013;17:379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Backstrom T, et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology. 2012;153:4111–4119. doi: 10.1210/en.2012-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncini M, Jissendi P, Catteau-Jonard S, Dewailly D, Pruvo JP, Francke JP, Prevot V. Sex steroid hormones-related structural plasticity in the human hypothalamus. NeuroImage. 2010;50:428–433. doi: 10.1016/j.neuroimage.2009.11.074. [DOI] [PubMed] [Google Scholar]
- Bergouignan L, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? NeuroImage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Biggs WS, Demuth RH. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2011;84:918–924. [PubMed] [Google Scholar]
- Blaustein JD. An estrogen by any other name. Endocrinology. 2008;149:2697–2698. doi: 10.1210/en.2008-0396. [DOI] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PloS One. 2011;6:e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K. The prefrontal-limbic system: development, neuroanatomy, function, and implications for socioemotional development. Clin Perinatol. 2011;38:685–702. doi: 10.1016/j.clp.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Brinton RD, et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Chen W, Shields J, Huang W, King JA. Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav Brain Res. 2009;201:8–13. doi: 10.1016/j.bbr.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci Off J Soc Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Nat Acad Sci USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Dastidar P, et al. Volumes of brain atrophy and plaques correlated with neurological disability in secondary progressive multiple sclerosis. J Neurol Sci. 1999;165:36–42. doi: 10.1016/s0022-510x(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR. Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology. 2009;51:491–503. doi: 10.1007/s00234-009-0521-z. [DOI] [PubMed] [Google Scholar]
- De Bondt T, Jacquemyn Y, Van Hecke W, Sijbers J, Sunaert S, Parizel PM. Regional gray matter volume differences and sex-hormone correlations as a function of menstrual cycle phase and hormonal contraceptives use. Brain Res. 2013a;1530:22–31. doi: 10.1016/j.brainres.2013.07.034. [DOI] [PubMed] [Google Scholar]
- De Bondt T, et al. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Prelim Results DTI Tract Study. Eur Radiol. 2013b;23:57–64. doi: 10.1007/s00330-012-2572-5. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hofman A, de Jong FH, Launer LJ, Pols HA, Breteler MM. Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol. 2003;60:213–220. doi: 10.1001/archneur.60.2.213. [DOI] [PubMed] [Google Scholar]
- Dinc H, Esen F, Demirci A, Sari A, Resit Gumele H. Pituitary dimensions and volume measurements in pregnancy and post partum. MR Assess Acta (Radiol Stockholm Sweden, 1987) 1998;39:64–69. doi: 10.1080/02841859809172152. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elster AD, Sanders TG, Vines FS, Chen MY. Size and shape of the pituitary gland during pregnancy and post partum: measurement with MR imaging. Radiology. 1991;181:531–535. doi: 10.1148/radiology.181.2.1924800. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fan L, Hanbury R, Pandey SC, Cohen RS. Dose and time effects of estrogen on expression of neuron-specific protein and cyclic AMP response element-binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology. 2008;88:111–126. doi: 10.1159/000129498. [DOI] [PubMed] [Google Scholar]
- Faundes A, Segal SJ, Adejuwon CA, Brache V, Leon P, Alvarez-Sanchez F. The menstrual cycle in women using an intrauterine device. Fertil Steril. 1980;34:427–430. doi: 10.1016/s0015-0282(16)45131-9. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS, Ivy GO. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behav Neurosci. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex (New York NY 1991) 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fischer B, Gleason C, Asthana S. Effects of hormone therapy on cognition and mood. Fertil Steril. 2014;101:898–904. doi: 10.1016/j.fertnstert.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Franke K, Hagemann G, Schleussner E, Gaser C. Changes of individual BrainAGE during the course of the menstrual cycle. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28:349–359. doi: 10.1016/0028-3932(90)90061-r. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings MR. Were there evolutionary advantages to premenstrual syndrome? Evol Appl. 2014;7:897–904. doi: 10.1111/eva.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex (New York NY 1991) 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci Off J Soc Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JG, Elizondo G, Saldivar D, Nanez H, Todd LE, Villarreal JZ. Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med. 1988;85:217–220. doi: 10.1016/s0002-9343(88)80346-2. [DOI] [PubMed] [Google Scholar]
- Goto M, et al. 3 Tesla MRI detects accelerated hippocampal volume reduction in postmenopausal women. J Magn Reson Imaging JMRI. 2011a;33:48–53. doi: 10.1002/jmri.22328. [DOI] [PubMed] [Google Scholar]
- Goto M, et al. Accelerated hippocampal volume reduction in post-menopausal women: an additional study with Atlas-based method. Radiol Phys Technol. 2011b;4:185–188. doi: 10.1007/s12194-011-0120-7. [DOI] [PubMed] [Google Scholar]
- Grams AE, Gempt J, Stahl A, Forschler A. Female pituitary size in relation to age and hormonal factors. Neuroendocrinology. 2010;92:128–132. doi: 10.1159/000314196. [DOI] [PubMed] [Google Scholar]
- Grant R, Condon B, Lawrence A, Hadley DM, Patterson J, Bone I, Teasdale GM. Is cranial CSF volume under hormonal influence? An MR study. J Comput Assist Tomogr. 1988;12(1):36–39. doi: 10.1097/00004728-198801000-00005. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59:105–109. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, Gaser C. Changes in brain size during the menstrual cycle. PloS One. 2011;6:e14655. doi: 10.1371/journal.pone.0014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham EA, Cirillo VJ, Zanetti ME, Kuehl FA., Jr Estrogen-directed synthesis of specific prostaglandins in uterus. Proc Natl Acad Sci USA. 1975;72:1420–1424. doi: 10.1073/pnas.72.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Effects of the nervous system on the pituitary-adrenal activity. Prog Brain Res. 1970;32:86–88. [PubMed] [Google Scholar]
- Harris RJ, Rice GE, Young AW, Andrews TJ. Distinct but overlapping patterns of response to words and faces in the fusiform gyrus. Cereb Cortex (New York, NY 1991) 2015 doi: 10.1093/cercor/bhv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metabol. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamopituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Sem Reprod Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw DB, Jr, Hasso AN, Thompson JR, Davidson BJ. High resolution computed tomography of the post partum pituitary gland. Neuroradiology. 1984;26:299–301. doi: 10.1007/BF00339774. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HG, Ham BJ, Yeo HB, Jung IK, Joe SH. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140:260–267. doi: 10.1016/j.jad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci Off J Soc Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F. Pregnancy and pituitary disorders. Eur J Endocrinol Eur Fed Endocr Soc. 2010;162:453–475. doi: 10.1530/EJE-09-0923. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 2010;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1997;88:153–160. doi: 10.1016/s0166-4328(97)02287-0. [DOI] [PubMed] [Google Scholar]
- Konrad C, et al. The functional anatomy of semantic retrieval is influenced by gender, menstrual cycle, and sex hormones. J Neural Transm. 2008;115:1327–1337. doi: 10.1007/s00702-008-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454:115–139. doi: 10.1002/cne.10416. [DOI] [PubMed] [Google Scholar]
- Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Laule C, Leung E, Lis DK, Traboulsee AL, Paty DW, MacKay AL, Moore GR. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler. 2006;12:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Lieberburg I, McEwen BS. Estradiol-17beta: a metabolite of testosterone recovered in cell nuclei from limbic areas of adult male rat brains. Brain Res. 1975;91:171–174. doi: 10.1016/0006-8993(75)90479-5. [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Lindenberger U, Kuhn S. Amygdala/hippocampal activation during the menstrual cycle: evidence for lateralization of effects across different tasks. Neuropsychologia. 2015a;67:55–62. doi: 10.1016/j.neuropsychologia.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Martensson J, Eckert A, Lindenberger U, Gallinat J, Kuhn S. Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage. 2015b;118:154–162. doi: 10.1016/j.neuroimage.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Pfaff DW, Chaptal C, Luine VN. Brain cell nuclear retention of [3H]estradiol doses able to promote lordosis: temporal and regional aspects. Brain Res. 1975;86:155–161. doi: 10.1016/0006-8993(75)90649-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gray JD, Nasca C. 60 years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol. 2015;226:T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Wahle P, Castaneyra-Perdomo A, Ferres-Torres R. Morphology of neurons in the white matter of the adult human neocortex. Exp Brain Res. 1992;88:204–212. doi: 10.1007/BF02259143. [DOI] [PubMed] [Google Scholar]
- Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33:331–341. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, et al. The pituitary gland: changes on MR images during the 1st year after delivery. Radiology. 2005;235:999–1004. doi: 10.1148/radiol.2353040243. [DOI] [PubMed] [Google Scholar]
- Montagnese CM, Poulain DA, Vincent JD, Theodosis DT. Structural plasticity in the rat supraoptic nucleus during gestation, post-partum lactation and suckling-induced pseudogestation and lactation. J Endocrinol. 1987;115:97–105. doi: 10.1677/joe.0.1150097. [DOI] [PubMed] [Google Scholar]
- Naftolin F, et al. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- Nakata H, Sakamoto K, Kakigi R. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol. 2014;5:1489. doi: 10.3389/fpsyg.2014.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, Bydder GM. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. AJNR Am J Neuroradiol. 2002;23:19–26. [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, Fernandez G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, van Wingen GA, Rijpkema M, Backstrom T, Hermans EJ, Fernandez G. Menstrual cycle-related changes in amygdala morphology are associated with changes in stress sensitivity. Hum Brain Mapp. 2013;34:1187–1193. doi: 10.1002/hbm.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo MA, et al. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Invest. 1995;18:853–856. doi: 10.1007/BF03349832. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36:1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2015;220:2415–2422. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Kerschbaum H. Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volume and face recognition performance. Brain Res. 2015;1596:108–115. doi: 10.1016/j.brainres.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Pluchino N, et al. Progesterone and progestins: effects on brain, allopregnanolone and beta-endorphin. J Steroid Biochem Mol Biol. 2006;102:205–213. doi: 10.1016/j.jsbmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Santoro A, Casarosa E, Wenger JM, Genazzani AD, Petignat P, Genazzani AR. Advances in neurosteroids: role in clinical practice. Climact J Int Menopause Soc. 2013;16(Suppl 1):8–17. doi: 10.3109/13697137.2013.809647. [DOI] [PubMed] [Google Scholar]
- Poromaa IS. Physiological correlates of premenstrual dysphoric disorder (PMDD) Curr Topics Behav Neurosci. 2014 doi: 10.1007/7854_2014_296. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008a;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008b;108:87–94. doi: 10.1016/j.jad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Qiu LR, Germann J, Spring S, Alm C, Vousden DA, Palmert MR, Lerch JP. Hippocampal volumes differ across the mouse estrous cycle, can change within 24 hours, and associate with cognitive strategies. NeuroImage. 2013;83:593–598. doi: 10.1016/j.neuroimage.2013.06.074. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Henley SM, Rohrer JD, Scahill RI, Warren JD, Fox NC. Ten simple rules for reporting voxel-based morphometry studies. NeuroImage. 2008;40:1429–1435. doi: 10.1016/j.neuroimage.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb cortex (New York, NY: 1991) 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Donohoe G. Brain vs. behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull. 2013;39:518–526. doi: 10.1093/schbul/sbs056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroen-docrinology. 2002;27:835–841. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Okon-Singer H, Villringer A. Evidence from neuroimaging for the role of the menstrual cycle in the interplay of emotion and cognition. Front Human Neurosci. 2013;7:374. doi: 10.3389/fnhum.2013.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AM, Flamini MI, Genazzani AR, Simoncini T. Effects of progesterone and medroxyprogesterone on actin remodeling and neuronal spine formation. Mol Endocrinol. 2013;27:693–702. doi: 10.1210/me.2012-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoning S, et al. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45:3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci Off J Soc Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Estrogen-induced maternal behavior in hysterectomized-overiectomized virgin rats. Physiol Behav. 1975;14:465–471. doi: 10.1016/0031-9384(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Skalkidou A, Hellgren C, Comasco E, Sylven S, Sundstrom Poromaa I. Biological aspects of postpartum depression. Women’s Health. 2012;8:659–672. doi: 10.2217/whe.12.55. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Soares CN, Frey BN. Challenges and opportunities to manage depression during the menopausal transition and beyond. Psychiatr Clin N Am. 2010;33:295–308. doi: 10.1016/j.psc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Penzes P. Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev. 2013;65:1318–1350. doi: 10.1124/pr.111.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ, Archer DF, Bhavnani BR. Ethinyl estradiol and 17beta-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception. 2013;87:706–727. doi: 10.1016/j.contraception.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Sundstrom Poromaa I, Gingnell M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 2014;8:380. doi: 10.3389/fnins.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Grant R, Condon B, Patterson J, Lawrence A, Hadley DM, Wyper D. Intracranial CSF volumes: natural variations and physiological changes measured by MRI. Acta neurochir Suppl (Wien) 1988;42:230–235. doi: 10.1007/978-3-7091-8975-7_45. [DOI] [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundstrom-Poromaa I, Comasco E. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology. 2014;50:28–52. doi: 10.1016/j.psyneuen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Torrealday S, Taylor HS, Burney RO, Mooney SB, Giudice LC. Endocrinology of Pregnancy. In: De Groot LJ, et al., editors. Endotext. South Dartmouth, MA: 2000. [Google Scholar]
- Tu CH, et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Tu CH, et al. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013;154:1718–1724. doi: 10.1016/j.pain.2013.05.022. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168–174. doi: 10.1016/j.contraception.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Warren AM, Gurvich C, Worsley R, Kulkarni J. A systematic review of the impact of oral contraceptives on cognition. Contraception. 2014;90:111–116. doi: 10.1016/j.contraception.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Whitwell JL. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J Neurosci Off J Soc Neurosci. 2009;29:9661–9664. doi: 10.1523/JNEUROSCI.2160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman L, Montgomery MM, Levine BJ, Beynnon BD, Shultz SJ. Accuracy of calendar-based methods for assigning menstrual cycle phase in women. Sports Health. 2013;5:143–149. doi: 10.1177/1941738112469930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. NeuroImage. 2010;49:1205–1212. doi: 10.1016/j.neuroimage.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Wong AP, et al. Estimating volumes of the pituitary gland from T1-weighted magnetic-resonance images: effects of age, puberty, testosterone, and estradiol. NeuroImage. 2014;94:216–221. doi: 10.1016/j.neuroimage.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci Off J Soc Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Zeng T, Wu S, Sun H, Xiao N. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception. 1995;51:359–365. doi: 10.1016/0010-7824(95)00102-g. [DOI] [PubMed] [Google Scholar]
- Ylikorkala O, Puolakka J, Kauppila A. Serum gonadotrophins, prolactin and ovarian steroids in primary dysmenorrhoea. Br J Obstet Gynaecol. 1979;86:648–653. doi: 10.1111/j.1471-0528.1979.tb10829.x. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]