Abstract
Adoptive cellular therapy (ACT) with the Th17 subset of CD4+ T cells can cure established melanoma in preclinical models and holds promise for treating human cancer. However, little is known about the growth factors necessary for optimal engraftment and anti-tumor activity of Th17 cells. Due to the central role of IL-2 receptor gamma chain (IL2Rγ-chain) cytokines (IL-2, IL-7, and IL-15) in the activity and persistence of many T cell subsets after adoptive transfer, we hypothesized that these cytokines are important for Th17 cells. We found that Th17 cells proliferated in response to IL-2, IL-7, and IL-15 in vitro. However, in contrast to many other T cell subsets, including conventionally activated CD8+ T cells, we found that Th17 cells were resistant to apoptosis in the absence of IL2Rγ-chain cytokines. To determine whether Th17 cells utilize IL2Rγ-chain cytokines in vivo, we tracked Th17 cell engraftment after adoptive transfer with or without cytokine depletion. Depletion of IL-7 and/or IL-2 decreased initial engraftment, while depletion of IL-15 did not. Supplementation of IL-2 increased initial Th17 engraftment. To assess the clinical relevance of these findings, we treated melanoma-bearing mice with Th17 cell adoptive transfer and concurrent cytokine depletion or supplementation. We found that simultaneous depletion of IL-2 and IL-7 decreased therapeutic efficacy, depletion of IL-15 had no effect, and IL-2 supplementation increased therapeutic efficacy. Our results show that Th17 cells are responsive to IL2Rγ-chain cytokines, and provide insight into the application of these cytokines for Th17-based therapeutic strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-1965-3) contains supplementary material, which is available to authorized users.
Keywords: ACT, Th17 cells, IL-2, IL-7, IL-15
Introduction
Adoptive T-cell therapy (ACT) has the potential to generate an effective anti-tumor response in select cancer patients who have failed the standard of care treatments [1, 2]. However, response rates are low [1]. Preclinical studies suggest that efficacy of ACT may be further improved through the use of polarized T cell subsets in place of the more traditional bulk unpolarized T cells. In particular, CD4+ T cells that are polarized to the Th17 subset by exposure to IL-6 and TGFβ during activation outperform unpolarized controls in murine anti-tumor adoptive transfer models [3, 4]. The mechanistic basis of Th17 cells’ improved efficacy is not yet fully understood. Previous studies have suggested roles for increased replicative capacity [5], pluripotency of cytokine production [6], improved recruitment of host effector cells to tumor [4], and direct killing of tumor cells by CD4+ cells [7, 8]. Following adoptive transfer, most donor Th17 cells undergo a transition from production of IL-17 to IFNγ [9], which is critical for Th17-mediated anti-tumor immunity [5]. For most T cell populations, it is thought that lymphodepletion using cytotoxic chemotherapy or irradiation prior to adoptive transfer improves therapeutic efficacy. The benefit of lymphodepletion is thought to occur through several mechanisms, including increased IL2Rγ-chain cytokine availability [10]. However, the efficacy of this intervention in the context of adoptive transfer of Th17 cells has not been evaluated.
IL2Rγ-chain cytokines (IL-2, IL-7, and IL-15) are important for maintaining T-cell homeostasis and contribute to the persistence and functionality of adoptively transferred T cells [11, 12]. Binding of these cytokines to their receptors leads to proliferation, enhanced effector function, and decreased apoptosis by multiple T-cell subsets [12–18]. Recognition of the ability of IL-2 to support tumor-specific T cells and other lymphocyte populations has led to its use both as a single agent and concurrently with adoptive transfer in clinical protocols [1, 2]. Previous studies have shown that IL2Rγ-chain cytokines can impede Th17 polarization via STAT5 activation [19] but, in CD4+ memory cells, can induce IL-17 production via Akt-mediated suppression of the transcription factors FoxO1 and Klf2 [20]. One report has shown that human PBMCs initially enriched for Th17 cells through IL23R+ positive selection were able to expand in the presence of IL-2, IL-7, or IL-15 with concurrent TCR stimulation [21]. While these reports suggest that IL2Rγ-chain cytokines may contribute to the anti-tumor efficacy of mature Th17 cells, such studies have yet to be conducted.
Given that IL-2, IL-7, and IL-15 play a critical role in the persistence and function of both CD4+ and CD8+ T-cell subsets [12, 13, 22, 23], we sought to determine how these cytokines might be involved in Th17 cell activity both in vitro and in vivo. We now show that Th17 cells proliferate in response to IL2Rγ-chain cytokines in vitro and, in contrast to reports on many other T cell subsets, are resistant to IL2Rγ-chain cytokine withdrawal-induced apoptosis. We also show that IL-2 and IL-7 contribute to donor Th17 cell engraftment after adoptive transfer and the ability of Th17 cells to mediate anti-tumor immunity. In addition to their relevance for cancer immunotherapy, these new insights may contribute to our understanding of the role of IL2Rγ-chain cytokines in Th17-mediated autoimmune disease processes.
Materials and methods
Th17 in vitro polarization
TRP-1 mice, which express an MHC class II-restricted TCR specific for the melanocyte antigen tyrosinase-related peptide, on a RAG-1 knockout background, were used as a source of CD4+ T cells [3]. For activation, 1.5 × 106 TRP-1 cells were cultured in a 48 well flat-bottom tissue culture plate with 3 × 105 10 Gy irradiated B6 splenocytes along with peptide (TRP-1, SGHNCGTCRPGWRGAACNQKILTVR, 1 uM, Genemed Synthesis). Pmel-1 TCR transgenic mice were used as a source of CD8+ T cells [24]. These were activated by hgp100 (KVPRNQDWL, 1 ug/ml, American peptide). B6 mice were used as a source of polyclonal T cells. These were activated by plate bound anti-CD3 mAb (145-2C11, 1 ug/ml, Bioxcell) and anti-CD28 mAb (37.51, 5 ug/ml, Bioxcell). For Th17/Tc17 polarization, the following polarizing cytokines were added prior to activation: human (h)TGFβ1 (30 ng/ml), hIL-6 (100 ng/ml), hIL-1β (10 ng/ml), and hIL-21 (100 ng/ml) as well as blocking antibodies against IFNγ (XMG1.2, Bioxcell), IL-4 (11B.11, NCI Biorepository), and IL-2 (JES6-1A12, Bioxcell), all at 10 ug/ml. Polarizing cytokines were removed immediately prior to IL2Rγ-chain cytokine stimulation (culture day 5–6). Some replicates (3/8 in Fig. 1b/7 in Figs. 1d, 2/3 in Fig. 1f, 1/2 in Fig. 3a, 2/6 in supplementary Fig. 2c, 1/2 in supplementary Fig. 5a, and 1/1 in supplementary Figs. 6a and 6b) utilized slightly different polarizing cytokines, including hTGFβ3 instead of hTGFβ1, 100 ng/ml mouse (m)IL-1β instead of 10 ng/ml hIL-1β, and mIL-21 instead of hIL-21. Cells polarized by these two methods performed similarly in all assays in which they were compared, including cytokine-induced signaling (Fig. 1), cytokine-induced proliferation (Fig. 1), cytokine receptor expression (supplementary Fig. 2), and engraftment in lymphodepleted vs non-lymphodepleted hosts (Fig. 3). Unpolarized cells were activated in the same way as Tc17/Th17 cells but received no polarizing cytokines. Cells were supplemented daily with mIL-23 (20 ng/ml, Th17/Tc17 only) and hIL-2 (50–100 IU/ml, all cells) beginning on day 3 of culture and were split as necessary to maintain growth. Cytokines were obtained from Shenandoah Biotechnology unless otherwise noted.
Fig. 1.
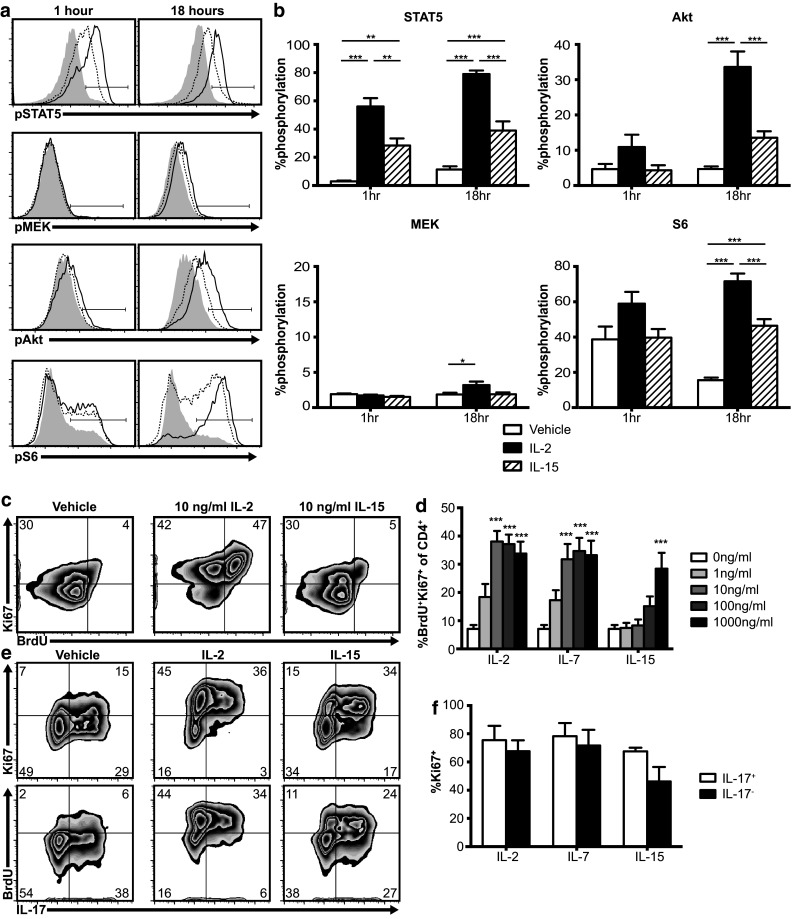
Th17 cells respond to IL2Rγ-chain cytokines in vitro. a, b TRP-1 Th17 cells phosphorylate STAT5, MEK, Akt, and S6 following IL-2 (100 ng/ml, solid lines) or IL-15 (100 ng/ml, dotted lines) stimulation (gray histograms = no cytokine). Graphs show mean plus standard error of eight independent experiments, *p < .05, ** p < .01, *** p < .001 by one-way ANOVA with Tukey’s multiple comparison test. c, d TRP-1 Th17 proliferate following 2 day stimulation with IL-2, IL-7, or IL-15 (100 ng/ml) in vitro. Graph shows mean plus standard error of seven independent experiments, *p < .05, ** p < .01, *** p < .001 for the indicated dose vs vehicle by one-way ANOVA using Dunnett’s multiple comparison correction. e, f IL-17+ and IL-17− populations of Th17 polarized TRP-1 cells proliferate similarly in response to IL-2, IL-7, or IL-15 (100 ng/ml). Graph shows mean plus standard error of three independent experiments, IL17+ vs IL17− p > .05 for all cytokines by two-tailed t test
Fig. 2.

Th17 cells are resistant to apoptosis in the absence of IL2Rγ-chain cytokines. a Gating strategy for apoptosis assays. b, c Apoptosis of Th17 cells (TRP-1), unpolarized CD4+ T cells (TRP-1), Tc17 cells (pmel-1), or unpolarized CD8+ T cells (pmel-1) was measured after culture with IL-2, IL-7, IL-15, or vehicle. Panel b shows representative samples from day 2. Panel c shows mean plus standard error of three independent experiments for the days indicated. d, e Antibody-mediated depletion of IL2Rγ-chain cytokines does not induce apoptosis of Th17 (TRP-1) cells over 2 day culture. Triangles represent independent experiments. p > .05 for all depletion conditions vs control by two-tailed t test. f Expression of Bcl-2 protein is greater in Th17 cells (dashed line) than in unpolarized CD8+ cells (solid line). Gray histogram is FMO control. Representative of two independent experiments
Fig. 3.

IL-2 and IL-7 contribute to Th17 engraftment but IL-15 does not. a Host lymphodepletion improves Th17 engraftment and persistence. Donor Th17 cell (CD4+Vb14+CD45.2+) persistence in peripheral blood (left panel) and spleen at 1 month (right panel) after adoptive transfer into CD45.1+ mice with or without 6 Gy total body irradiation 1 day prior to adoptive transfer. Graphs show mean plus/minus standard error of 3–4 mice per group, representative of two independent experiments. ***p < .001 by two-tailed t test assuming equal variance. b IL2Rγ-chain cytokine depletion affects Th17 engraftment. Th17 persistence in the spleen and peripheral blood approximately 1 week (early) or 1 month (late) after adoptive transfer into a lymphodepleted host, with IL2Rγ-chain cytokines being depleted from day 0 to 26 post-adoptive transfer. *p < .05,** p < .01, *** p < .001 for each condition vs vehicle by two-tailed t test. c IL-17 and IFNγ production by Th17 cells before adoptive transfer and after recovery from host spleens (with no cytokine depletion) 1 month post-adoptive transfer. d Graphs show mean plus standard error of cytokine production by donor cells from five mice (Day 27 data), representative of two independent experiments. e Effects of IL2Rγ-chain cytokine depletion on donor Th17 IL-17 and IFNγ production. Mean plus standard error of 4–5 mice per group, representative of two independent experiments
Flow cytometry
Flow cytometry was performed as previously described [25]. Antibodies used include CD8 (53–6.7), CD4 (RM4-5), IL7Rα (A7R34), CD45.1 (A20), and CD62L (MEL-14) from eBioscience; Vβ14 (14–2), IL2Rγ (4G3), STAT5 pY694 (47), MEK1/2 pS218/222 (024–836), and CD44 (IM7) from BD Biosciences; IL2Rα (PC61), IL2Rβ (5H4), CD45.2 (104), IFNγ (XMG1.2), IL-17 (TC11-18H10.1), and Bcl-2 (BCL/10C4) from Biolegend; Akt pS473 (D9E) and S6 pS240/244 (D68F8) from Cell Signaling Technologies; and biotinylated polyclonal IL15Rα (BAF551, R&D Systems), which was detected by secondary stain with streptavidin (eBioscience). Cytokine production was measured after 5 h incubation with PMA (100 ng/ml, Sigma), Ionomycin (1 uM, Sigma), and Golgistop (BD) unless otherwise noted. Analysis of pSTAT5, Akt, S6, and MEK phosphorylation followed the manufacturer’s protocol using lyse/fix buffer and perm buffer III (BD). For analysis of apoptosis, cells were stained for 15 min with AnnexinV and Propidium Iodide according to manufacturer’s instructions (BD). Flow cytometry was performed on a BD FACS Accuri. Data were analyzed using the FlowJo software.
Proliferation assays
Two different proliferation assays were performed; cells were washed prior to addition of IL2Rγ-chain cytokines in both assays. In the standard proliferation assay (Fig. 1c, d, supplementary Figs. 3c, 3d, 4a, 4b, 4c), cells were exposed to 5-bromo-2′-deoxyuridine (BrdU; 10 mM, BD) for 2 h beginning 2 days after the addition of IL2Rγ-chain cytokines, then fixed and permeabilized according to the manufacturer’s protocol for Cytofix/Cytoperm (BD), and stained with anti-Ki67 mAb (SolA15, eBioscience) and anti-BrdU mAb (Bu20a, Biolegend) as previously described [25]. For the simultaneous proliferation/cytokine production assay (Fig. 1e f), cells were exposed to BrdU for 8 h beginning 1 day after the addition of IL2Rγ-chain cytokines. Cells were then washed and soluble anti-CD3 mAb (10 ug/ml) or vehicle was added, along with the protein transport inhibitor Golgistop (BD), for 5 h to stimulate cytokine production and intracellular accumulation. Cells were then fixed and stained for CD4, IL-17, Ki67, and BrdU.
Adoptive transfer experiments
B6 and CD45.1 recipient mice were purchased from the Jackson Laboratory and Charles River and were housed under specific pathogen–free conditions in accordance with institutional and federal guidelines. Th17 cells were adoptively transferred by tail vein injection. Where indicated, mice received 6 Gy total body irradiation 1 day prior to transfer. Cytokine depletion was performed by intraperitoneal injection of anti-IL-7 mAb (M25, Bioxcell, 200 ug), anti-IL-15mAb (M96, Amgen, 10 ug), or anti-IL-2 mAbs (S4B6 and JES6-1A12, Bioxcell, 100 ug each) on days 0, 2, 5, 8, 12, 16, 21, and 26 post-adoptive transfer [26, 27]. We confirmed the efficacy of IL-15 depletion by measuring recovery of NK cells after lymphodepletion (supplementary Fig. 1). IL-2 complexes [28] were formed by incubating human IL-2 with anti-IL-2 mAb [clone 5355, R&D Systems (Fig. 5) or clone 5344.111, BD (supplementary Fig. 8)] for 15 min at 37° at a 2:1 molar ratio. 1.5 ug IL-2 with 7.5 ug mAb in 200 ul PBS (IL-2/mAb) was injected intraperitoneally on days 0, 2, 4, and 6 post-adoptive transfer. Peripheral blood was obtained by retromandibular venipuncture. For tumor experiments, mice received subcutaneous abdominal injections of 4 × 105 B16F10 melanoma cells (American Type Culture Collection). Tumors were allowed to establish for at least 1 week. Mice then received 6 Gy total body irradiation and, on the following day, adoptive transfer of 5 × 105 TRP-1 Th17 cells. Tumor surface area was measured three times per week by blinded personnel. We found during data analysis that injection site lesions smaller than 5 mm2 at the time of treatment initiation frequently did not grow, even in the absence of treatment; therefore, we retrospectively excluded these from analysis. These exclusions did not result in significant intergroup differences in average tumor size at treatment initiation.
Fig. 5.
IL-2 administration enhances Th17 engraftment and tumor control. a, b Administration of IL-2/mAb complexes augments the expansion of Th17 cells adoptively transferred into irradiated recipients. Donor Th17 cell (CD4+Vb14+CD45.2+) persistence in peripheral blood (a) and spleen (b) after adoptive transfer into 6 Gy irradiated CD45.1+ mice which received IL-2/mAb complexes or vehicle after adoptive transfer. Graphs show mean plus/minus standard error of 4–5 mice per group, representative of two independent experiments (a), ** p < .01 by two-tailed t test assuming equal variance (b). c, d IL-17/IFNγ production and CD62L expression of donor Th17 cells following adoptive transfer with or without IL-2/mAb complexes. Graphs show mean plus standard error of 4–5 mice per group, representative of two independent experiments (cytokine production), **p < .01 by 2-tailed t test assuming unequal variance (CD62L expression). e IL-2/mAb complex administration improves efficacy of Th17 adoptive transfer. B6 mice-bearing established B16 melanomas received adoptive transfer of 5 × 105 TRP-1 Th17 cells plus IL-2/mAb complex or vehicle and tumor size was measured at the indicated timepoints. Each line represents one mouse. f Survival of tumor bearing mice receiving adoptive transfer with or without IL-2/mAb complexes. Survival curves for deaths due to any cause (top) or only deaths attributable to tumor burden (tumor area > 400mm2) (bottom), survival data was pooled from two independent experiments with a total of 13–16 mice or 5–8 mice per group for all cause and tumor-specific mortality, respectively, *p < .05, ** p < .01, ***p < .001 by log-rank test
Statistics
T tests were conducted assuming equal or unequal variance based on a preceding F test. For tests between multiple groups of interest, ANOVA was utilized, unless otherwise noted, with appropriate multiple comparison corrections. Survival data are displayed using Kaplan–Meier curves and tested using log-rank tests. In Fig. 3b, persistence was normalized to the mean of the vehicle group for each experiment and multiple experiments were then pooled (four experiments for early blood and two experiments each for all other plots). Each cytokine depletion condition is compared to injection of vehicle alone by two-sample t tests within each group assuming equal or unequal variance based on the corresponding F tests (i.e., 4 t tests per group). For IL-2, IL-7, IL-15, and IL-2/7/15, respectively, the p values for early spleen are p = .003, 0.005, 0.1625, and 0.00; for late spleen, p = .94, 0.45, 0.0279, and 0.42; for early blood, p = .0009, 0.0208, 0.38, and 0.001; and for late blood, p = .02, 0.112, 0.13, and 0.09. Accounting for four comparisons made within each group, a Bonferroni significance level of 0.0125 can be used for statistical significance. As indicated in “Results”, the number of mice lost between the early and late timepoints in each condition was: vehicle: 4/12 mice, IL2 depletion: 1/11 mice, IL7 depletion: 4/11 mice, IL15 depletion: 2/11 mice, and IL2/7/15 depletion: 1/11 mice.
Results
Th17 cells are responsive to IL2Rγ-chain cytokines in vitro
IL2Rγ-chain cytokines signal through the cell surface receptor IL2Rγ (CD132), also known as the common gamma chain, which is incorporated into a unique multimeric receptor for each member of the cytokine family [11–13]. These receptors are composed of IL2Rα (CD25) + IL2Rβ (CD122) + IL2Rγ for IL-2, IL7Rα (CD127) + IL2Rγ for IL-7, and IL15Rα + IL2Rβ + IL2Rγ for IL-15 [11–13]. We found that Th17 cells generated from TRP-1 TCR transgenic mice (supplementary Fig. 2a) express all these IL2Rγ-chain cytokine receptor subunits (supplementary Fig. 2b, 2c). Binding of IL2Rγ-chain cytokines to their receptors induces signaling through the JAK3/STAT5, Akt, and MAPK pathways [11–13]. To assess the activity of these pathways in Th17 cells, we measured phosphorylation of STAT5, Akt, S6, and MEK by flow cytometry after 1 or 18 h of in vitro IL-2 stimulation. We observed robust activation of STAT5 and Akt signaling in Th17 cells with cytokine treatment (Fig. 1a, b). In contrast, signaling through the Ras->Raf->MAPK pathway in Th17 cells was minimal. IL-15 also activated STAT5 and Akt signaling, but to a lesser degree than IL-2. We next assessed the functional consequences of IL2Rγ-chain cytokine signaling in Th17 cells, starting with proliferation, which is known to be induced in CD8+ T cells by IL2Rγ-chain cytokines [11–13]. We found that IL-2, IL-7, and IL-15 each induced proliferation of Th17 cells (Fig. 1c, d) and that this proliferation was dependent on STAT5, but not Akt signaling (supplementary Fig. 3). Proliferation was less pronounced with IL-15 than with IL-2 and IL-7, which we confirmed using both human (Fig. 1d) and murine (supplementary Fig. 4a) cytokines. We observed no difference in proliferation between the IL-17 positive and IL-17 negative populations (Fig. 1e, f), confirming that the observed proliferation was by Th17 polarized cells.
While the conventional signaling functions of the IL2Rγ-chain cytokine receptors are mediated by IL2Rβ, IL2Rγ, and IL7Rα [12], IL15Rα contributes by mediating trans-presentation and both IL2Rα and IL15Rα contribute by increasing the affinity and duration of interactions between IL2Rγ-chain cytokines and their receptors [11–13, 29, 30]. In assessing the importance of the IL2Rα and IL15Rα subunits in IL-2- and IL-15-mediated proliferation of Th17 cells, we found that monoclonal antibody (PC61) blockade of IL2Rα had minimal effect on IL-2-mediated proliferation (supplementary Fig. 4b), with IL-2 still stimulating proliferation at significantly lower doses than IL-15. Similarly, polyclonal antibody blockade of IL15Rα caused little change in IL-15-mediated proliferation (supplementary Fig. 4c). To address the possibility of deficient trans-presentation of IL-15, we incubated Th17 cells with IL-15 that was pre-associated with soluble IL15Rα, which also did not restore IL-15 responsiveness to the same level as IL-2 (supplementary Fig. 4c). Finally, we tested whether IL2Rα could maintain signaling after cytokine withdrawal in Th17 cells by monitoring STAT5 phosphorylation over time after exposure to IL-2 and subsequent wash. We found that STAT5 signaling was maintained through 6 h post-IL-2 withdrawal in an IL2Rα-dependent manner (supplementary Fig. 4d). Interestingly, Th17 cells still showed less short-term responsiveness to IL-15 (at 15 min), relative to Tc1 controls (supplementary Fig. 4e).
Th17 cells do not require IL2Rγ-chain cytokines to prevent apoptosis in vitro
In addition to supporting Th17 engraftment by inducing proliferation, IL2Rγ-chain cytokines may protect donor cells from apoptosis. To measure this effect in vitro, we cultured Th17 cells with IL-2, IL-7, IL-15, or vehicle, and then quantified the apoptotic fraction (Fig. 2a, b, c). We found that Th17 cells were resistant to apoptosis, even in the absence of added IL2Rγ-chain cytokines, while unpolarized (activated) CD8+ (pmel-1) cells underwent significant apoptosis when IL2Rγ-chain cytokines were not added. We next sought to determine whether this resistance to apoptosis was a specific feature of type 17 polarization or a general feature of TRP-1 CD4+ T cells. To do this, we subjected CD4+ (TRP-1) and CD8+ (pmel-1) T cells to activation with or without Th17 polarizing cytokines then repeated the above apoptosis assay on the four resulting cell populations. We found that neither of the CD4+ subsets showed significant apoptosis upon IL2Rγ-chain cytokine withdrawal. Type 17 polarization appeared to have a protective effect on CD8+ cells, as Tc17 cells underwent less apoptosis than unpolarized CD8+ cells. To investigate the possibility that Th17 cells were being protected from apoptosis through autocrine cytokine signaling, we repeated the assay with the addition of IL2Rγ-chain cytokine-blocking antibodies but still observed no increase in apoptosis (Fig. 2d, e). Finally, in investigating the mechanism of Th17 cells’ apoptotic resistance, we found that they express higher levels of the anti-apoptotic protein Bcl-2, than do unpolarized CD8+ cells (Fig. 2f).
IL2Rγ-chain cytokines contribute to initial engraftment of Th17 cells
Next, we used an adoptive transfer model to investigate what support donor Th17 cells require from the host. We found that lymphodepletion by 6 Gy total body irradiation greatly increased the efficiency of donor Th17 cell engraftment (Fig. 3a, supplementary Fig. 5a). To determine the role of IL2Rγ-chain cytokines in Th17 engraftment, we used monoclonal antibodies to deplete these cytokines from lymphodepleted hosts following adoptive transfer. We found that depletion of IL-7 and/or IL-2, but not IL-15, decreased the initial engraftment of donor Th17 cells (Fig. 3b). Efficacy of IL-15 depletion was confirmed by measuring depletion of NK cells (supplementary Fig. 1). In control mice, the frequency of donor cells that were IL-17+ declined and the frequency of donor cells that were IFNγ+ increased over the month following adoptive transfer (Fig. 3c, d). Mice receiving IL-2 depletion had reduced frequency of IFNγ+ cells among donor cells (Fig. 3e), but the transition of donor cells to a central memory phenotype was not affected by depletion of IL-2, IL-7, or IL-15 (supplementary Figs. 5b, 5c). We confirmed Th17 cells’ lack of requirement for IL-15 by assessing engraftment and persistence in IL15Rα knockout mice (supplementary Fig. 6), in which donor cells receive no IL-15 stimulation due to the inability of host cells to trans-present this cytokine [31]. In several of our adoptive transfer experiments, we observed unexpected toxicities during the second week after T-cell transfer, necessitating exclusion of some mice prior to analysis as outlined in the methods. To investigate the possibility that these toxicities were related to elevation of inflammatory cytokines caused by adoptively transferred Th17 cells [32, 33], we measured the serum levels of several inflammatory cytokines in separate cohorts of mice with or without adoptive transfer of Th17 cells. Mice that received Th17 cells had elevated levels of several inflammatory cytokines, including IL-6 which was has been associated with cytokine-release syndrome in humans receiving CD19-reactive CAR-modified T cells [33] (supplementary Fig. 7).
IL2Rγ-chain cytokines contribute to efficacy of Th17 adoptive transfer
To determine whether the observed dependence of Th17 engraftment on IL2Rγ-chain cytokine availability impacts Th17-mediated tumor control, we performed adoptive transfer of Th17 cells with or without cytokine depletion into mice-bearing established B16 melanomas. We used a dose of Th17 cells which caused a significant delay of tumor growth but was not curative (Fig. 4a, b), creating a therapeutic window which could potentially be affected by cytokine availability. Simultaneous depletion of IL-2 and IL-7 negated the benefit of Th17 adoptive transfer, while depletion of IL-15 had no significant effect. These results were consistent with those in our engraftment experiments in that only those cytokines which contributed to engraftment (IL-2 and IL-7 but not IL-15) were needed for optimal anti-tumor efficacy of donor Th17 cells.
Fig. 4.
IL2Rγ-chain cytokines affect the ability of adoptively transferred Th17 cells to control tumor growth. a Depletion of IL-2 and IL-7 decreases efficacy of Th17 adoptive transfer. B6 mice bearing established B16 melanomas received adoptive transfer of 5 × 105 TRP-1 Th17 cells. The indicated IL2Rγ-chain cytokines were then depleted from the mice, and tumor area was measured three times per week. Each line represents one mouse. b Survival curves for deaths due to any cause (top) or only deaths attributable to tumor burden (tumor area > 400 mm2) (bottom). Survival data was pooled from two independent experiments with a total of 14–16 mice or 8–12 mice per group for all cause and tumor-specific mortality respectively, *p < .05, ** p < .01, *** p < .001 by log-rank test
IL2Rγ-chain cytokine supplementation improves Th17 anti-tumor efficacy
The responsiveness of Th17 cells to IL2Rγ-chain cytokines demonstrated thus far raised the possibility that cytokine supplementation could improve their ability to control tumor. To test this, we performed adoptive transfer of Th17 cells with or without administration of IL-2/anti-IL-2 monoclonal antibody (IL-2/mAb) complexes (clone 5355), which have improved half-life and biological activity relative to free cytokine [28]. We found that IL-2/mAb complex supplementation significantly improved short-term Th17 engraftment in lymphodepleted hosts (Fig. 5a, b). IL-2/mAb complex supplementation decreased the frequency of donor cells that were IL-17+ (Fig. 5c) and decreased expression of CD62L on donor cells (Fig. 5d), indicating a possible shift from central memory to effector memory phenotype [34] as a result of IL-2/mAb complex supplementation. In a separate experiment, use of a different IL-2/mAb complex (clone 5344.111) that preferentially expands IL2Rαhi cells (IL-2/mAbCD25) allowed Th17 cells to engraft at detectable levels in lymphoreplete hosts (supplementary Fig. 8), where engraftment would otherwise be undetectable. Finally, to determine whether the increased engraftment observed in Fig. 5a and b would translate to improved tumor control, we adoptively transferred Th17 cells into lymphodepleted, tumor bearing mice with or without IL-2/mAb complex (clone 5355) supplementation. We found that IL-2/mAb complexes significantly improved the ability of Th17 cells to control tumor growth (Fig. 5e, f).
Discussion
Adoptive cellular therapy with Th17 cells represents a promising new strategy for cancer patients not benefiting from current treatments. Appreciation of the integral role played by IL2Rγ-chain cytokines both in normal T-cell biology and as adjuvants to adoptive cellular therapy led us to investigate their potential roles in cellular therapy with Th17 cells. We sought to determine whether Th17 cells are responsive to IL2Rγ-chain cytokines and whether these cytokines contribute to the ability of Th17 cells to control tumor in an adoptive transfer model. We found significant proliferative responses by Th17 cells to IL2Rγ-chain cytokines in vitro, which correlated with induction of STAT5 and Akt signaling by these cytokines. Surprisingly, we found that Th17 cells are highly resistant to IL2Rγ-chain cytokine withdrawal-induced apoptosis, a finding which distinguishes them from the effector CD8+ T cells commonly used for adoptive cellular therapy. Finally, we found that host lymphodepletion significantly improved engraftment of Th17 cells and that IL2Rγ-chain cytokines contributed to engraftment and were required for Th17-mediated tumor control.
We found that Th17 cells express all of the IL2Rγ-chain cytokine receptor subunits and that they proliferate in response to these cytokines in vitro. Interestingly, a 100-fold higher dose of IL-15 was required to induce proliferation relative to IL-2 or IL-7, regardless of IL2Rα or IL15Rα subunit availability. IL2Rγ-chain cytokine-induced proliferation was not dependent on Akt signaling, as proliferation was not decreased by the PI3K/mTOR inhibitor PI-103, in contrast to prior studies with this inhibitor in naïve CD8+ T cells [35]. The similarity in IL2Rγ-chain cytokine-induced in vitro proliferation between IL-17 positive and negative subsets may indicate that Th17 polarization confers neither an advantage nor a disadvantage for proliferation or that all the cells tested were polarized, even though they did not all produce IL-17 in our assay.
IL2Rγ-chain cytokines down-regulate the intrinsic apoptosis pathway by driving expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL [36]. Previous studies of apoptosis in various populations of T cells, including activated CD4+ cells [14], resting CD4+ cells [15, 16], activated CD8+ cells [17], resting CD8+ cells [15], and Tc1 polarized CD8+ cells [18], have shown that these cells undergo apoptosis in the absence of IL2Rγ-chain cytokines. While Th17 cells are resistant to activation-induced cell death [5, 37, 38], cytokine withdrawal-induced apoptosis in this subset has not been examined. We found that Th17 cells were resistant to apoptosis in vitro in the absence of added IL2Rγ-chain cytokines. We validated this finding through the parallel use of apoptosis-sensitive activated CD8+ cells in the same assay. In seeking to determine the relative contributions of CD4 lineage and type 17 polarization to this resistant phenotype, we found that unpolarized CD4+ cells were significantly more resistant than unpolarized CD8+ cells. Because these results reflect a comparison between two distinct TCR transgenic T cell populations, it will be important to further investigate the role played by variations in TCR signaling during activation in resistance to cytokine withdrawal-induced apoptosis. Type 17 polarization also appears to play a role in apoptotic resistance, as Tc17 cells were more resistant than unpolarized CD8+ cells. Further experiments with CD4+ subsets polarized from other TCR transgenic mouse strains could help to clarify the relative contributions of TCR specificity, CD4 lineage, and type 17 polarization to the apoptosis-resistant phenotype that we observed. Importantly, the addition of IL2Rγ-chain cytokine-blocking antibodies did not reverse this resistance, suggesting that autocrine cytokine signaling is not responsible for this effect. Resistance of Th17 cells to activation-induced cell death is mediated by elevated expression of c-FLIP, which acts on the extrinsic apoptosis pathway [37, 38]. However, since cytokine withdrawal-induced apoptosis involves the intrinsic apoptosis pathway [39], the resistance we observed is more likely due to elevated expression by Th17 cells of proteins that inhibit this pathway, such as Bcl-xl [37] and Bcl-2 [5]. As Bcl-2 expression is driven by Akt signaling [40], our finding that cytokine-mediated proliferation of Th17 cells is Akt-independent, in contrast to CD8+ cells [26], is consistent with our finding that Th17 cells constitutively overexpress Bcl-2, potentially contributing to their apoptotic resistance.
Because persistence of donor T cells after adoptive transfer can be important for anti-tumor efficacy [41, 42], we were particularly interested in the effects of IL2Rγ-chain cytokines on Th17 engraftment and persistence. In accordance with reports on other subsets, including Tc17 cells [43], we found that host lymphodepletion substantially increases Th17 cell engraftment, which led us to conduct our cytokine depletion experiments in lymphodepleted hosts. We found small but significant contributions of IL-2 and IL-7, but not IL-15, to the initial engraftment of Th17 cells in lymphodepleted hosts. This is notable, since it contrasts with the cytokine requirements of conventionally activated CD8+ cells, which are more similar to the T cells currently being used in adoptive transfer clinical trials. Specifically, IL-7, but not IL-2 or IL-15, contributes to the initial engraftment of Tc1 CD8+ T cells [26] and homeostatic proliferation of memory CD8+ T cells after adoptive transfer requires the presence of either IL-7 or IL-15 [44]. Overall, we found that Th17 engraftment was less dependent on IL2Rγ-chain cytokine availability than previous observations with other subsets. This may be because Th17 cells are intrinsically resistant to apoptosis in the absence of IL2Rγ-chain cytokines, which would be consistent with our in vitro apoptosis data. Alternatively, Th17 cells may lose resistance to apoptosis under in vivo conditions but then receive compensatory anti-apoptotic signals through the TCR or costimulatory receptors in the absence of cytokines [45].
Unlike IL-2 and IL-7, depletion of IL-15 had no significant effect on Th17 engraftment in our model. This may be a result of Th17 cells not localizing to areas, where IL-15 is abundant, or it may be because Th17 cells proliferate less in response to IL-15 than to IL-2 or IL-7 in vivo, which would be consistent with our in vitro data. While this comparative unresponsiveness to IL-15 represents a contrast to effector CD8+ T cells, further investigation will be needed to determine whether Th17 cells are unique among CD4+ subsets in this respect. The degree to which memory CD4+ cells require IL-15 in vivo seems to depend on whether or not the host is lymphodepleted and on whether the cells being studied are of the naturally occurring memory phenotype or an antigen-specific population [44, 46, 47]. Recent studies have shown that engraftment by naive CD4+ cells is enhanced in IL-15 knockout hosts [48]. Studies have also shown that deprivation of IL-15 may affect CD4+ cells indirectly through other populations [49]. Thus, our finding that the absence of IL-15 has no effect on Th17 engraftment in a lymphodepleted host represents a contrast with other populations of CD4+ T cells. Previous reports have shown that IL-17-producing NKT cells [50] and γδ T cells [51] do not depend on IL-15, indicating that type 17 polarization may contribute to IL-15 independence, consistent with our results.
In contrast to IL-7 and IL-15, we did not expect IL-2 to be important for engraftment in a lymphodepleted host, as IL-2-producing host lymphocytes should be removed by lymphodepletion. Our finding that IL-2 contributes to Th17 engraftment may indicate that donor Th17 cells are aiding their own engraftment, either directly or indirectly, through IL-2 production [5]. While the fact that we did not observe elevated serum levels of IL-2 following adoptive transfer of Th17 cells makes this possibility less likely, donor cell-produced IL-2 might only be detectable in specific lymphoid tissues. Since engraftment of NK cells in a host receiving 5 Gy irradiation can depend on host production of IL-2 [52], we also cannot completely rule out host IL-2 production in our model. In addition to assessing the requirements of Th17 cells for endogenous cytokines, we also demonstrated that provision of exogenous IL-2 could drive Th17 cell persistence and anti-tumor immunity. IL-2 supplementation also appeared to modify the phenotype of donor Th17 cells, which may have contributed to the improved anti-tumor immunity we observed. These findings suggest that cytokine supplementation could increase the therapeutic benefit of adoptively transferred Th17 cells.
Carl June and colleagues have found that anti-tumor efficacy of CD19-reactive CAR T cells can be accompanied by cytokine-release syndrome which can be reversed with IL-6-targeted therapies [33]. As the toxicities we observed in mice that received Th17 cells were consistent with cytokine-release syndrome and were associated with elevated serum levels of several inflammatory cytokines, including IL-6, our model may be useful for differentiating the role of inflammatory cytokines in efficacy vs toxicity of Th17 therapy. While our findings support the further development of Th17 cells as a human cancer therapy, previously noted differences between human and murine Th17 cells in terms of extracellular phenotype and cytokines required for polarization [53] necessitate further studies of the effects of IL2Rγ-chain cytokines on human T cell populations.
The ability of Th17 cells to not undergo apoptosis in the absence of IL2Rγ-chain cytokines may represent an advantage of Th17 cells over conventionally activated CD8+ T cells used in adoptive cellular therapy. Further defining the extrinsic and intrinsic factors differentially driving cytokine dependence will be useful in developing more efficacious T cell-based therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Casey White for assistance with tumor measurement. Support for this project was provided by the following grants and fellowships from the National Institutes of Health and National Cancer Institute: F30CA200272 (Jacob Bowers), T32GM008716 (Daniel Neitzke and Jacob Bowers), 5P01CA186866 (Zihai Li), 5R01CA188419 (Zihai Li), and 5R01CA175061 (Chrystal Paulos). We are also grateful to the Melanoma Research Alliance for grant funding (Mark Rubinstein). This work was also supported in part by the Biostatistics and the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30CA138313). We are grateful to Amgen for kindly providing anti-IL-15 mAb (clone M96).
Abbreviations
- Akt
Protein kinase B
- B6
C57BL/6
- Bcl-2
B-cell lymphoma 2
- Bcl-xl
B-cell lymphoma extra-large
- BrdU
5-bromo-2′-deoxyuridine
- c-FLIP
c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein
- FoxO1
Forkhead box protein O1
- γδ T cells
Gamma delta T cells
- hgp100
Human melanoma antigen gp100
- IL2Rγ-chain cytokines
Cytokines which signal through the common gamma chain (CD132) – here:IL-2, IL-7, and IL-15
- Ki67
Ki67 protein (proliferation marker)
- Klf2
Kruppel-like factor 2
- MEK
Mitogen activated protein kinase (MAPK) kinase
- mTOR
Mammalian target of rapamycin
- NK
Natural killer
- NKT
Natural killer T
- PMA
Phorbol 12-myristate 13-acetate
- RAG-1
Recombination activating gene 1
- S6
Ribosomal protein S6
- Tc1
T cytotoxic 1 subset
- Tc17
T cytotoxic 17 subset
- Th17
T helper 17 subset
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution where conducted.
References
- 1.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4(127):128. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35(6):972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei S, Zhao E, Kryczek I, Zou W. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology. 2012;1(4):516–519. doi: 10.4161/onci.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207(3):651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overwijk WW, Schluns KS. Functions of gammaC cytokines in immune homeostasis: current and potential clinical applications. Clin Immunol. 2009;132(2):153–165. doi: 10.1016/j.clim.2009.03.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 13.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9(7):480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95(7):3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, Kay TW, Starr R, Alexander WS. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278(25):22755–22761. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- 16.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999;163(4):1817–1826. [PubMed] [Google Scholar]
- 17.Bosque A, Marzo I, Naval J, Anel A. Apoptosis by IL-2 deprivation in human CD8+ T cell blasts predominates over death receptor ligation, requires Bim expression and is associated with Mcl-1 loss. Mol Immunol. 2007;44(6):1446–1453. doi: 10.1016/j.molimm.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Hao S, Li F, Ye Z, Yang J, Xiang J. CD4+ Th1 cells promote CD8+ Tc1 cell survival, memory response, tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007;120(2):148–159. doi: 10.1111/j.1365-2567.2006.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Wan Q, Kozhaya L, ElHed A, Ramesh R, Carlson TJ, Djuretic IM, Sundrud MS, Unutmaz D. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J Exp Med. 2011;208(9):1875–1887. doi: 10.1084/jem.20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nady S, Ignatz-Hoover J, Shata MT. Interleukin-12 is the optimum cytokine to expand human Th17 cells in vitro. Clin Vaccine Immunol. 2009;16(6):798–805. doi: 10.1128/CVI.00022-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19(3):320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Gasper DJ, Tejera MM, Suresh M. CD4 T-cell memory generation and maintenance. Crit Rev Immunol. 2014;34(2):121–146. doi: 10.1615/CritRevImmunol.2014010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112(9):3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson CB, Riesenberg BP, May BR, Gilreath SC, Li G, Staveley-O’Carroll KF, Garrett-Mayer E, Mehrotra S, Cole DJ, Rubinstein MP. Effector CD8+ T-cell engraftment and antitumor immunity in lymphodepleted hosts Is IL7Ralpha dependent. Cancer Immunol Res. 2015;3(12):1364–1374. doi: 10.1158/2326-6066.CIR-15-0087-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebrec H, Horner MJ, Gorski KS, Tsuji W, Xia D, Pan WJ, Means G, Pietz G, Li N, Retter M, Shaffer K, Patel N, Narayanan PK, Butz EA. Homeostasis of human NK cells is not IL-15 dependent. J Immunol. 2013;191(11):5551–5558. doi: 10.4049/jimmunol.1301000. [DOI] [PubMed] [Google Scholar]
- 28.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 29.Ring AM, Lin JX, Feng D, Mitra S, Rickert M, Bowman GR, Pande VS, Li P, Moraga I, Spolski R, Ozkan E, Leonard WJ, Garcia KC. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol. 2012;13(12):1187–1195. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su EW, Moore CJ, Suriano S, Johnson CB, Songalia N, Patterson A, Neitzke DJ, Andrijauskaite K, Garrett-Mayer E, Mehrotra S, Paulos CM, Doedens AL, Goldrath AW, Li Z, Cole DJ, Rubinstein MP. IL-2Ralpha mediates temporal regulation of IL-2 signaling and enhances immunotherapy. Sci Transl Med. 2015;7(311):311ra170. doi: 10.1126/scitranslmed.aac8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17(5):537–547. doi: 10.1016/S1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 32.Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer MJ, Mahajan VS, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8(+) T cells. Immunol Cell Biol. 2011;89(5):581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996;26(2):294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Iclozan C, Yamazaki T, Yang X, Anasetti C, Dong C, Yu XZ. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114(5):1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang Y, Yu S, Ellis JS, Sharav T, Braley-Mullen H. Comparison of sensitivity of Th1, Th2, and Th17 cells to Fas-mediated apoptosis. J Leukoc Biol. 2010;87(6):1019–1028. doi: 10.1189/jlb.0509352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis–immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 40.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275(15):10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 41.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowers JS, Nelson MH, Kundimi S, Bailey SR, Huff LW, Schwartz KM, Cole DJ, Rubinstein MP, Paulos CM. Dendritic cells in irradiated mice trigger the functional plasticity and antitumor activity of adoptively transferred Tc17 cells via IL12 signaling. Clin Cancer Res. 2015;21(11):2546–2557. doi: 10.1158/1078-0432.CCR-14-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 46.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204(4):951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc Natl Acad Sci USA. 2004;101(25):9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen XL, Bobbala D, Cepero Donates Y, Mayhue M, Ilangumaran S, Ramanathan S. IL-15 trans-presentation regulates homeostasis of CD4(+) T lymphocytes. Cell Mol Immunol. 2014;11(4):387–397. doi: 10.1038/cmi.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson KA, Goding SR, Neely HR, Harris KM, Antony PA. Depletion of B220 + NK1.1 + cells enhances the rejection of established melanoma by tumor-specific CD4+ T cells. Oncoimmunology. 2015;4(8):e1019196. doi: 10.1080/2162402X.2015.1019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster KE, Kim HO, Kyparissoudis K, Corpuz TM, Pinget GV, Uldrich AP, Brink R, Belz GT, Cho JH, Godfrey DI, Sprent J. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 2014;7(5):1058–1067. doi: 10.1038/mi.2013.122. [DOI] [PubMed] [Google Scholar]
- 51.Corpuz TM, Stolp J, Kim HO, Pinget GV, Gray DH, Cho JH, Sprent J, Webster KE. Differential responsiveness of Innate-like IL-17- and IFN-gamma-producing gammadelta T cells to homeostatic cytokines. J Immunol. 2016;196(2):645–654. doi: 10.4049/jimmunol.1502082. [DOI] [PubMed] [Google Scholar]
- 52.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20(11):1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




