Abstract
Background
Evidence supports the benefits of exercise for cancer patients, however, specific guidance for clinical decision making regarding exercise timing, frequency, duration and intensity is lacking. Efforts are needed to optimize clinical recommendations for exercise in the cancer population.
Objectives
To aggregate information regarding the benefit of exercise through a systematic review of existing systematic reviews in the cancer exercise literature.
Data Sources
PubMed, CINAHL Plus, Scopus, Web of Science, EMBASE.
Study Eligibility Criteria
Systematic reviews and meta-analyses of the impact of movement-based exercise on the adult cancer population.
Methods
Two author teams reviewed 302 abstracts for inclusion with 93 selected for full text review. 53 studies were analyzed. A Measurement Tool to Assess Systematic Reviews (AMSTAR©) was used as a quality measure of the reviews. Information was extracted using the PICO format (participants, intervention, comparison, outcomes). Descriptive findings are reported.
Results
Mean AMSTAR© score = 7.66 / 11 (±2.04) suggests moderate quality of the systematic reviews. Exercise is beneficial before, during, and after cancer treatment, across all cancer types, and for a variety of cancer-related impairments. Moderate to vigorous exercise is the best level of exercise intensity to improve physical function and mitigate cancer-related impairments. Therapeutic exercises are beneficial to manage treatment side effects, may enhance tolerance to cancer treatments, and improve functional outcomes. Supervised exercise yielded superior benefits versus unsupervised. Serious adverse events were not common.
Limitations
Movement-based exercise intervention outcomes are reported. No analysis of pooled effects was calculated across reviews due to significant heterogeneity within the systematic reviews. Findings do not consider exercise in advanced cancers or pediatric populations.
Conclusions
Exercise promotes significant improvements in clinical, functional, and in some populations, survival outcomes and can be recommended regardless of the type of cancer. Although generally safe, patients should be screened and appropriate precautions taken. Efforts to strengthen uniformity in clinical trial reporting, develop clinical practice guidelines, and integrate exercise and rehabilitation services into the cancer delivery system are needed.
Introduction
Exercise interventions are well-established as safe and beneficial for individuals receiving cancer treatment.1 Exercise contributes to improved health and functional outcomes in the cancer population.2,3 Although most national guidelines recommend that cancer survivors meet the public health guidelines for physical activity, exercise prescription is nuanced and requires consideration of many factors to positively and safely impact individuals with a cancer diagnosis.4,5 Different types of exercise interventions have been studied in the cancer population and have resulted in general recommendations for increasing overall physical activity and including specific resistive or aerobic exercise regimens to the cancer care plan.1,6,7 Therapeutic exercise is additionally recommended as a rehabilitative approach for individuals experiencing more specific functional impairments and disability.8
Oncology care providers are challenged to identify and synthesize the significant volume of relevant literature around exercise prescription. The complexities of the health status, clinical history, and functional abilities of the individual being treated for cancer introduce a spectrum of considerations that further challenge exercise recommendations.4 Models of care that provide access to exercise and rehabilitation professionals have been developed but are not broadly utilized and the workforce supporting them is still developing.9 As a result, exercise prescription is frequently overlooked in cancer care planning.10,11 Although recommendations have urged greater integration of exercise into the cancer care continuum, active integration will require more precise guidelines to support provider decision making.12
The cancer exercise research generally demonstrates significant and positive impact on variables of interest however most studies have focused on exercise within specific types of cancer (breast, colorectal, etc.) or on a single cancer-related impairment (cancer-related fatigue, muscle weakness, etc.) using widely variable modes of exercise. Further complicating the ability to harmonize information around exercise prescription is the variability across studies regarding optimal timing, frequency, duration, and intensity for exercise prescription. Systematic reviews, while prevalent in the cancer exercise literature, tend to follow a disease-specific or impairment-specific focus (e.g., systematic review of strength training in androgen deprived prostate cancer patients) whereas in the clinical setting, providers see a wide range of oncologic patients with varying disease stages often experiencing multiple comorbidities and functional impairments. A review of the existing literature is needed to compile and synthesize evidence from the numerous and varied systematic reviews in order to aggregate the most meaningful literature with a broad perspective on exercise and rehabilitation interventions for individuals with cancer.13
The purpose of this report is to present the results of a systematic review of published systematic reviews on exercise interventions for the cancer population in order to identify key common features of exercise programs in the cancer population. The aggregate findings provide a comprehensive resource of current evidence that support health care providers in selecting exercise-based interventions for the individual being treated for or with a history of cancer.
Methods
The methodology for conducting a systematic review of systematic reviews is supported by the Cochrane group and articulated by Smith et al.13 This approach is recommended when attempting to apprise, summarize, and aggregate research findings from separate systematic reviews in order to compare and contrast results to provide clinical decision makers with relevant evidence.13
Search
The search strategy was designed to identify existing, published systematic reviews and meta-analyses. Search terms were formulated using the PICO structure. Participants (P) included adults (18 – 80 years old) with any type of cancer who were not considered to have advanced cancer or were not receiving palliative care. Intervention (I) included exercise and its various forms including therapeutic exercise, physical activity, strength training, aerobic conditioning, rehabilitative exercise, stretching, etc. Comparisons (C) broadly addressed exercise intervention versus none, supervised versus unsupervised, varied frequency and duration of exercise interventions as well as comparison of different types of exercise. Outcomes (O) included functional gains such as neuromusculoskeletal and cardiometabolic function, improvement in physical impairment, functional measures, overall quality of life, blood count and biomarker improvements and psychological and psychosocial gains.
The search terms and strategy were developed by an Informationist at the National Institutes of Health, NIH Library in consultation with the author team. The comprehensive search strategy is provided in Table 1. Five databases were searched: PubMed, CINAHL Plus, Web of Science, EMBASE, and Scopus with date range from 2000 to 2017.
Table 1.
Search Terms and Yield
| Search Criteria | ||||
|---|---|---|---|---|
| (neoplasms[majr] OR cancer[tiab] OR cancers[tiab] OR carcinoma[tiab] OR carcinomas[tiab] OR leukemia[tiab] OR lymphoma[tiab] OR neoplasm[tiab] OR neoplasms[tiab]) AND (exercise[majr] OR exercise movement techniques[majr] OR exercise therapy[majr] OR rehabilitation[majr] OR activity[tiab] OR activities[tiab] OR aerobic[tiab] OR aerobics[tiab] OR exercise[tiab] OR exercises[tiab] OR exercising[tiab] OR exertion[tiab] OR “occupational therapy”[tiab] OR “physical therapy”[tiab] OR physiotherapy[tiab] OR recreation[tiab] OR recreational[tiab] OR reflexology[tiab] OR rehabilitate[tiab] OR rehabilitated[tiab] OR rehabilitation[tiab] OR rehabilitative[tiab] OR stretch[tiab] OR stretching[tiab] OR strengthen[tiab] OR strengthened[tiab] OR strengthening[tiab] OR “tai chi”[tiab] OR train[ti] OR trained[ti] OR training[ti] OR walk[tiab] OR walks[tiab] OR walked[tiab] OR walking[tiab] OR yoga[tiab]) AND (activities of daily living[majr] OR emotions[majr] OR pain management[majr] OR physical fitness[majr] OR exercise test[majr] OR recovery of function[majr] OR mobility limitation[majr] OR “activities of daily living”[tiab] OR “aerobic capacity”[tiab] OR “aerobic endurance”[tiab] OR anemia[tiab] OR anorexia[tiab] OR anxiety[tiab] OR anxious[tiab] OR balance[tiab] OR balancing[tiab] OR “body image”[tiab] OR biomarker[tiab] OR biomarkers[tiab] OR “blood count”[tiab] OR “blood counts”[tiab] OR “body mass index”[tiab] OR “body strength”[tiab] OR “bone density”[tiab] OR breathless[tiab] OR “breathlessness”[tiab] OR “cardiopulmonary strength”[tiab] OR depressed[tiab] OR depression[tiab] OR discomfort[tiab] OR distress[tiab] OR distressed[tiab] OR drowsy[tiab] OR drowsiness[tiab] OR dyspnea[tiab] OR edema[tiab] OR edematous[tiab] OR endurance[tiab] OR energy[tiab] OR “exercise capacity”[tiab] OR fall[tiab] OR falls[tiab] OR falling[tiab] OR fatigue[tiab] OR fatigued[tiab] OR fitness[tiab] OR flexible[tiab] OR flexibility[tiab] OR fracture[tiab] OR fractures[tiab] OR fractured[tiab] OR frailty[tiab] OR function[tiab] OR functions[tiab] OR functioning[tiab] OR functional[tiab] OR happiness[tiab] OR “heart failure”[tiab] OR immobile[tiab] OR immobility[tiab] OR impairment[tiab] OR impairments[tiab] OR insomnia[tiab] OR “lean mass”[tiab] OR lymphedema[tiab] OR lymphoedema[tiab] OR mental[tiab] OR mobile[tiab] OR mobility[tiab] OR mood[tiab] OR moods[tiab] OR morbidity[tiab] OR “muscle strength”[tiab] OR nausea[tiab] OR neuropathy[tiab] OR neuropathies[tiab] OR “quality of life”[tiab] OR pain[tiab] OR “physical performance”[tiab] OR “physical strength”[tiab] OR “psychological stress”[tiab] OR “range of motion”[tiab] OR relax[tiab] OR relaxed[tiab] OR relaxing[tiab] OR relaxation[tiab] OR “self-care”[tiab] OR “self-concept”[ tiab] OR “self-esteem”[tiab] OR “shortness of breath”[tiab] OR “sit to stand”[tiab] OR sleep[tiab] OR swelling[tiab] OR symptom[tiab] OR symptoms[tiab] OR vigor[tiab] OR vigorous[tiab] OR vomiting[tiab] OR “walk test”[tiab] OR “walk tests”[tiab] OR weakness[tiab] OR weight[tiab] OR “well-being”[tiab] OR wellbeing[tiab]) AND (randomized controlled trial[ptyp] OR systematic[sb] OR “randomized controlled trial”[tiab] OR “randomised controlled trial”[tiab] OR “systematic review”[tiab]) | ||||
| Search Yield = 9337 | ||||
| PubMed: 2526 | CINAHL Plus: 544 | EMBASE: 4313 | Scopus: 827 | Web of Science: 1127 |
Study Identification and Selection
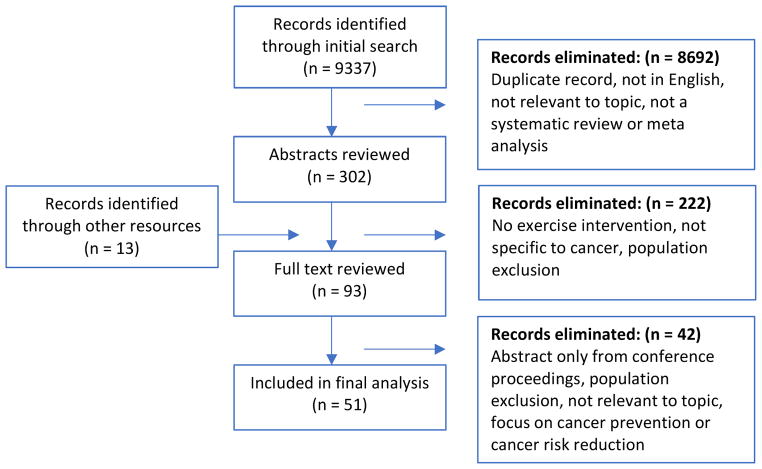
Figure 1 presents the PRISMA flow diagram. The initial search yielded 9337 results. Additional filters were then added for systematic reviews, and meta-analyses only, yielding 5453 records. After removing duplicate records and abstracts not available in English, as well as those not relevant to the topic of interest, 302 abstracts were agreed upon by the author team for screening. Authors worked in paired teams for the initial abstract screening reviews (JB/KWS and AS/NS) and each team reviewed half of the abstracts. In instances of disagreement by the team, the co-lead authors (NS and JB) made a final determination of inclusion.
Figure 1.
A priori, the authors agreed that reviews focusing on movement-based exercise, such as yoga, qigong, etc., would be included as well as studies that used various traditional forms of exercise including aerobic and resistive conditioning, flexibility, and muscle retraining activities. Studies that reviewed behavioral interventions to promote exercise or to encourage lifestyle behaviors to increase exercise engagement were excluded. Reviews of exercise in the pediatric population were excluded. The pediatric population was defined as study participants who were under the age of eighteen when the exercise intervention took place. Studies of exercise in individuals receiving palliative care or those with advanced cancer were also excluded.
Eighty abstracts were approved for full text review and an additional 13 abstracts were self-identified by the author team for inclusion resulting in 93 abstracts retrieved for full text review. After final full text review, 51 articles were included in this analysis. Data were extracted from the full text articles by one author and reviewed and confirmed by their paired counterpart. All authors reviewed and approved the final inclusion list and extracted content.
Summary Measures and Study Quality Assessment
Due to significant heterogeneity within the various systematic reviews, pooled effects were not assessed and rather descriptive findings are provided. Each author scored her respective articles using A Measurement Tool to Assess Systematic Reviews (AMSTAR©). AMSTAR© is a validated qualitative tool that evaluates the quality of systematic reviews.13,14 The AMSTAR© on-line calculator queries 11 items of relevance that provide insight on the quality of the systematic review methodology. The authors scored each of the included articles using the online AMSTAR© calculator.*
Results
Information was extracted and synthesized from 51 articles. Table 2 provides a summary of the included studies. Quality analysis, revealed a mean score of 7.7 (± 2.0), and median of 8 with a range of 3 – 11. Descriptive findings are provided as pooled effects were not calculated.
Table 2.
Synopsis of Findings
| Reference | Review characteristics | Participants | Intervention | Comparison | Outcomes |
|---|---|---|---|---|---|
| Babatunde O. et al.* 201616 | 2 intervention trials 5 observational studies Evaluating physical activity levels. AMSTAR Score 6/11 |
Endometrial cancer survivors with cross sectional, self-report. | Self-report of PA Intensity Moderate Duration 150 minutes/week at least 30 min day Session Frequency 5 days/week Complemented with computer technology/accelerometer and intervention with computer-based or mobile app |
Cross sectional and single arm intervention (one with baseline data from prospective lifestyle intervention trials) | Increased physical activity contributes to improved QOL Higher BMI correlated with lower QOL. |
| Bergenthal, N. et al 201440 | 9 RCT’s Evaluating the efficacy, safety or feasibility of aerobic physical exercise. Moderate selection bias. High bias in patient-reported outcomes. AMSTAR Score 9/11 |
n = 818 adults with hematological cancers including; ALL, AML, malignant lymphoma, and multiple myeloma. | AT programs mostly walking programs. Duration and Intensity: Variable |
No exercise intervention or ‘usual care’. |
Quality of Life outcomes: Significant improvements but small effect size (SMD 0.26; 95% CI 0.03 to 0.49; P = 0.03). Physical functioning: Significant improvements but small effect size (SMD 0.33; 95% CI 0.13 to 0.52; P = 0.0009) Depression: Significant improvements but small effect size (SMD 0.25; 95% CI −0.00 to 0.50; P = 0.05) Anxiety: No significant changes Fatigue: Significant improvement but small effect size (SMD 0.24; 95% CI 0.08 to 0.40; P = 0.003) Physical Performance: Individual trials demonstrated significant improvements favoring exercise intervention vs none, however results could not be pooled. Serious Adverse Events: No significant difference in events between exercise intervention vs none. (RR 1.44; 95% CI 0.96 to 2.18; P = 0.06) |
| Bourke, L. et al. 201331 | 14 RCTs Cochrane review AMSTAR Score 10/11 Low selection and reporting bias. Moderate attrition bias. |
n = 648 Various cancer types including breast, colorectal, prostate and others. |
AT with or without RT RT alone Only “6 trials would meet current recommendations for aerobic exercise” Questionnaires or exercise log reported 2 – 5 times/week |
Control group with the same type of cancer * standard care did include physiotherapy in at least one trial |
Aerobic exercise tolerance improved at 8 –12 weeks’ post intervention with large effect size. (SMD 0.73, 95% CI 0.51–0.95) and at 6 months with large effect size. (SMD 0.84, 95% CI 0.45–0.94) |
| Bradt J. et al.* 201452 | 1 quasi-experimental RCT 1 RCT * Cochrane review AMSTAR Score 9/11 |
n= 68 Women with breast cancer within 5 years of treatment |
Dance/movement therapy | Wait-list control group |
Body Image No significant effect Individual studies reviewed trend towards significance in QOL and fatigue, but no pooled effects analyzed. No effect on shoulder ROM and arm circumference, but large variability was reported in these measures. |
| Buffart, L. et al. 201736 | 34 RCTs AMSTAR Score 9/11 |
n = 4,519 Various types of cancers including; Breast, male GU, hematologic, GI, GYN, respiratory, and other. Post completion of active cancer treatment. |
AT and RT exercise programs. Supervised and unsupervised exercise programs. Session Frequency 2 – 5x/week |
Control groups varied; usual care, wait-list, attention. |
QOL Significantly improved with small effect size. (0.15, 95% CI 0.10; 0.20) Physical Function Signifiantly improved with exercise but with small effect size. (0.18, 95% CI 0.13; 0.23) Effects of supervised exercise twice as large as unsupervised exercise. Suggested that impact of attention from physiotherapist, better equipment, more challenging prescriptions, or better adherence from supervised programs needed further investigation. No significant effect on BMI. Studies may not adequately measure and reflect adiposity. |
| Capozzi, L.C. et al.* 201637 | 16 observational studies 8 experimental trials Moderate selection bias. Low to moderate outcomes reporting bias. AMSTAR score 8/11 |
Various cancers of the head and neck including: Hypopharynx, Larynx, Oropharynx, Lip, Oral Cavity, Tonsil, Salivary glands, Nasopharynx, Nasal cavity, Paranasal sinus, and middle ear. During and after cancer treatment. |
RT, Hydrotherapy, Walking, Walking + exercise. Exercise frequency was highly variable Intensity Moderate to vigorous Duration was highly variable Supervised and unsupervised trials. |
4 trials with control groups of usual care. Remaining trials with no control comparison group. |
Significant improvement in lean body mass, strength, physical function, QOL, fatigue management. *75% of patients reported “possibly” or “definitely” interested in physical activity counseling. |
| Carvahlo, A.P. et al. 201253 | 3 controlled trials Low selection, attrition and reporting bias. * Cochrane review AMSTAR score 9/11 |
n = 104 Head and neck cancer survivors (primarily oropharynx) with shoulder dysfunction Range 2 – 180 months post-surgery |
PRE with ROM and stretching. Frequency Average 3x/week Program Duration 12 weeks Intensity Variable |
Control groups with “standard care,” some of which included shoulder ROM exercises (but not progressive) | Progressive resistive training was more effective than standard physiotherapy for restoring shoulder function however effect is small. (−6.26, 95% CI −12.2;−0.31) |
| Cheema, B. et al. 200833 | 5 RCT 4 uncontrolled trials 1 non-randomized intervention trial AMSTAR score 5/11 |
Women only, during or after chemotherapy and radiation, Variable disease stage Variable extent of surgery No males |
Various AT and RT programs with PRE. Duration 8 – 24 weeks Supervision 6 trials with complete supervision 3 with partial supervision 1 with no supervision. Progressive resistive exercise was referred to but parameters were not defined. |
“Non-exercise” control group | PRE significantly improved: endurance, strength, flexibility, lean mass, cardiorespiratory fitness, immune system, mood, self-esteem. Large effect size seen with change in grip strength. Moderate effect size with peak power and VO2 improvements. Chemotherapy dose tolerance significantly improved. Immune Function: Increased % T-helper lymphocytes. Increased total activated CD-4 cells. Increased lymphocyte proliferation. Improved IFN gamma to IL-6 ratio. Increased circulating IGF-II. |
| Cheifetz, O. et al.* 201055 | 10 trials focusing on the role of exercise in lymphedema High selection and outcomes measurement bias. AMSTAR score: 4/11 |
Breast cancer | Early physiotherapy RT Primarily supervised exercise programs. Frequency or duration not defined. |
“Non-intervention” group | Exercise is beneficial and safe for secondary lymphedema. Post-operative rehabilitation improves shoulder ROM. Supervised PRE does not worsen lymphedema. |
| Chipperfield, K. et al.* 201415 | 4 interventional trials 2 pilot studies 1 cross-sectional survey High selection and outcomes reporting bias. AMSTAR score 6/11 |
Prostate cancer patients during ADT administration. | Variable RT and AT programs 1 cross-sectional of PA Program Duration 12 weeks - 6 months. Intensity Most trials moderate intensity Most trials supervised intervention. |
Two pilot studies and one cross-sectional without a control group “considerable variability in sample sizes” |
Significant improvement in QOL. Inconclusive findings regarding impact on cognitive changes, depression, and anxiety. * only 45% of reported PA met guideline standards. |
| Cramer, H. et al. 201766 | 24 RCTs of yoga interventions * Cochrane review Moderate attrition and reporting bias. AMSTAR score 9/11 |
n = 2,166 Breast cancer patients. During or after cancer treatment. |
Program duration Range of 6 sessions to 6 months. Session Frequency 1 – 3 x week Session Duration 20 – 120 min |
Wait list controls. One trial with exercise intervention control |
Significant improvements in: QOL with small effect size (SMD = 0.22, 95% CI 0.04; 0.40) Fatigue with medium effect size (SMD = −0.48, 95% CI − 0.75; − 0.40) Sleep disturbance with small effect size (SMD = − 0.25, 95% CI −0.40; − 0.09) Depression with very small effect size (SMD = −0.13, 95% CI −0.31; 0.05) Anxiety with medium effect size (SMD = − 0.53, 95% CI −1.10; 0.04) |
| Cramer, H. et al 201420 | 5 RCTs AMSTAR score 7/11 |
n = 238 Colorectal cancer patients from 3 – 60 months post cancer treatment |
AT Intensity Low vs Moderate Duration 2 –16 weeks 3 trials with supervision 2 trials home-based |
Usual care or different exercise program One trial with attention control with phone calls at same interval as program interventions. |
Significant short-term improvement of overall physical fitness. (SMD = 0.59, 95% CI 0.25; 0.93, P < 0.01) No evidence for significant effects on QOL or fatigue biomarkers. Inflammatory profile Significantly improved with moderate exercise. Greater DNA damage noted with moderate exercise. |
| Cramp, F. et al. 201221 | 56 controlled trials on cancer-related fatigue High detection bias * Cochrane review AMSTAR score 9/11 |
n = 4,068 Various types of cancer with the majority including breast cancer. During and after completion of cancer treatment. |
AT Frequency and duration Variable Mode Walking or cycling |
Usual care or wait-list. At least two trials controlled with psychotherapy interventions. |
Significant improvement in cancer-related fatigue with AT but with small effect size. (SMD = −0.27, 95% CI −0.37; −0.17) |
| D’Souza, V. et al.* 201638 | 8 studies reviewing 2 studies reviewed use of PA 5 trials included but were not limited to RCTs. AMSTAR score 7/11 |
Various types of cancers | Physical activity, various modes, | Variable |
Body composition Significant reduction in BMI Endurance Significant increase in peak O2 consumption and peak power. Fatigue Significantly less with greater levels of reported PA. QOL Improved with greater levels of PA. |
| Davies, N.J. et al.* 201164 | Review of studies with varied methodology including: 4 RCTs with biomarker of recurrence as outcome 4 Prospective cohort studies 2 Cross-sectional studies 3 Systematic reviews/meta-analysis Significant heterogeneity in included studies. High risk of selection bias. AMSTAR: 3/11 |
Breast, prostate, and colorectal cancer patients both during and after completion of cancer treatment |
Observational Studies: Self-reported physical activity RCTs: 1 Moderate intensity AT 2 AT + RT Program Duration: 12–36 weeks |
For observational studies & systematic reviews: Active PA group compared to inactive/lowest PA group; For RCTs: Exercise vs usual care |
Physical Activity Participation Improved survival and reduced risk of recurrence, mostly based on observational studies; Threshold of moderate intensity may be necessary to achieve positive impact on survival. Dose response improved with longer or more intense exercise. |
| De Backer, I.C. et al.* 200926 | 24 trials post chemotherapy 10 RCTs 4 Controlled intervention trials 10 Uncontrolled trials High risk of attrition and outcomes reporting bias. AMSTAR Score: 7/11 |
All trials post-chemotherapy 13 Breast 3 Prostate 6 various types of cancer 1 post-stem cell transplant |
RT with or without additional AT. RT was mostly machine based; total body program. Frequency 2 – 3 days/week Program Duration 3 – 24 weeks (median =12 weeks) Intensity Moderate to vigorous Detailed reporting of exercise parameters of included studies. |
Not reported |
Body composition No effect of resistance exercise on adiposity. Trend towards significance in improved lean body mass. Cardiopulmonary Function Increased Muscle function Improved muscle strength and muscle endurance. Lymphedema Exercise at any level had no impact on swelling. Immune Function No significant impact from exercise. Endocrine Function Decrease in insulin family proteins. Hematologic Function No influence on hemoglobin levels. |
| Egan, M.Y. et al.* 201342 | Exercise interventions post cancer treatment only 13 systematic reviews 6 RCTs AMSTAR Score: 6/11 |
Various types of cancers | Mixed AT and RT PA Supervised settings |
Low levels of PA or no PA or Usual care |
Physical Function Moderate improvements in overall function. Fatigue Significant improvements Depression Small effect trending towards positive impact. |
| Fong, D.T. et al. 201224 | 34 RCTs Evaluating the effects of PA after cancer treatment. AMSTAR Score: 9/11 |
22 trials breast cancer. 3 trials colorectal cancer 1 trial endometrial cancer. 8 trials including various cancer types. Average age 55 years (range = 39 – 74 years) |
27 trials AT 6 trials AT + RT Duration Average 13 weeks (range = 3 – 60 weeks) Intensity 11 trials: moderate 2 trials: vigorous |
Sedentary comparisons or assigned no exercise |
Physiological markers: Significant reduction in insulin-like growth factor-I (95% CI −23.3 to −0.5; P = 0.04) No effect on insulin, glucose, and homeostatic model assessment. Body composition: Slightly reduced BMI (−0.4, 95% CI, −0.6 to −0.2; P<0.01) and body weight (−1.1kg, 95% CI, −1.6 to − 0.6kg; P<0.001) No effect on waist: hip ratio Physical functions: Significant increase in peak oxygen consumption (2.2mL/kg/min, 1.0 to 3.4; P<0.01) peak power output (21W, 13.0 to 29.1; P<0.01) distance walked in six minutes (29m, 4 to 55; P = 0.03) bench press weight (6kg, 4 to 8; P<0.01) leg press weight (19kg, 9 to 28; P<0.01) right hand grip strength (3.5 kg, 0.3 to 6.7; P= 0.03) Psychological outcomes: Reduced depression using Beck depression inventory (−4.1, −6.5 to −1.8; P<0.01) Reduced fatigue using Piper Fatigue scale (−1.0, −1.8 to − 1.0: P=0.03) Quality of Life outcomes: Significant improvement on SF-36 physical function, social function, and mental health functions. |
| Fontein, D.B. et al.* 201363 | 14 Prospective observational studies 2 RCTs 2 Retrospective case control studies AMSTAR Score 7/11 |
Breast cancer only | Self-report levels of PA | Inactive or low self-reported PA |
Cancer specific survival and all-cause mortality: 36%–67% decrease in rate of disease-specific mortality of highest PA levels vs. lowest PA levels. Significant benefit on all-cause mortality in the highest PA group ranging from 14%–56% decrease compared to low PA. |
| Fu, M.R. et al.* 201460 | 9 RCTs 2 Uncontrolled trials 3 systematic reviews AMSTAR Score: 5/11 |
Various types of cancers | “Full body exercise” not characterized. Some reported use of resistance training. | Not described |
Full Body Exercise Does not worsen lymphedema and may improve shoulder mobility. Resistive Training Safe if progressive, starting with low intensity. |
| Granger, C.L. et al.* 201127 | 9 Case series 2 RCTs 3 Cohort studies AMSTAR: 11/11 |
Non-small cell lung cancer at any phase of treatment. | All studies included aerobic 54% added RT 31% added stretching Intensity Moderate to vigorous Program Duration 4–12 weeks Session Frequency 2–7 days/week |
Not described |
Pre-operative Exercise Improvements in 6-minute walk distance post treatment. No change in HRQOL. Post-operative Exercise Improvement in 6MWD but only small significance as compared to usual care. Conflicting evidence for HRQOL between trials. |
| Guinan, E.M. et al.* 201328 | 7 RCTs 2 non-randomized trials Moderate attrition bias AMSTAR Score: 8/11 |
Early stage, post-adjuvant treatment breast cancer survivors | 7 trials AT with or without RT 1 trail RT only Intensity Moderate to vigorous Program Duration 8–36 weeks |
Non-exercise control group |
Body Composition Mixed findings for impact on % body fat, BMI, and waist and hip circumferences. Insulin Resistance Markers No effect on insulin or FBG Decreased levels of IGF-1. Mixed results for IGF II or IGFBP3 levels. |
| Hackshaw-McGeagh et al. 201544 | 4 RCTs with exercise only 6 RCTs with Exercise + Diet AMSTAR: 10/11 |
Prostate cancer survivors at various stages of disease and phases of treatment. | AT + RT 1 trial RT only 3 aerobic only Program Duration 13 – 104 weeks |
Non-exercise control group in most studies. | No impact from exercise on disease progression markers, e.g., PSA, testosterone. |
| Harder, H. et al.* 201249 | 18 RCTs Moderate bias in randomization, attrition, and blinding. AMSTAR Score: 8/11 |
Breast cancer survivors at various phases of treatment and with stages of disease | Yoga Program Duration 4 – 36 weeks (most were between 4–12 weeks) Program Frequency 1–2 sessions per week + home practice |
Education only Rehabilitation intervention Wait-list control |
Psychological/symptom distress: Significantly reduced depression (ES: 0.24 to 0.33) anxiety (ES: 0.31) and negative affect (ES: 0.59 to 0.84) HRQOL: Significantly improved function scales: Social well-being (ES: 0.22) physical functioning (ES: 0.44) and emotional function (ES: 0.71) Significantly improved symptom or single-item symptom measures were 0.47 or below (insomnia and appetite loss). Fatigue (ES: .33–1.5) |
| Keogh, J.W. et al.* 201130 | 12 intervention trials AMSTAR Score: 7/11 |
Prostate cancer survivors | RT, AT or RT + AT Intensity Moderate to vigorous Frequency 2 – 5 days/week, Program Duration 8 – 25 weeks |
Not described |
Resistance Training: Grade A evidence for improves fatigue, QOL and muscle endurance; Grade C for body composition impact, muscle strength and general function. Aerobic Training: Grade B evidence for aerobic endurance, sit to stand time, fatigue, QOL; Grade C evidence for body composition and strength. Resistance + Aerobic Training: Grade B evidence for muscle mass, muscle strength & endurance, walk speed, QOL Grade C evidence for aerobic endurance, and fatigue. |
| Kwan, M.L. et al.* 201129 | 13 RCTs 2 Case series 4 Cohort studies AMSTAR Scores: 7/11 |
Breast cancer survivors | RT or RT + AT Intensity Low to moderate Frequency 2–3 days/week Program Duration Up to 39 weeks; Also included physiotherapy directed programs |
Usual care | Resistive Training is safe and does not increase risk of lymphedema in breast cancer. Aerobic + Resistive Training trends towards positive but results are inconclusive due to limited studies. |
| Larkin, D. et al. 201443 | 5 interventional trials AMSTAR Score: 9/11 |
Prostate cancer survivors on androgen depravation therapy (ADT) and/or radiation therapy | Mix of RT, AT, and RT + AT. Program Duration 8 – 16 weeks |
Not described | Significant effect of exercise on reducing fatigue. |
| Lof, M. et al*. 201251 | 9 RCTs AMSTAR Score: 3/11 |
Breast cancer survivors mostly early stage | Tai chi, AT, AT + RT Intensity Moderate Session Duration 30 – 60 minutes Frequency 3 – 5 days/week Program Duration 8 – 36 weeks |
Usual care or support group | No conclusive evidence for positive effect on insulin axis proteins or interleukins. |
| McNeely, M. et al. 200635 | 14 RCTs High risk of blinding bias in methodology and reporting. AMSTAR Score: 9/11 |
n = 717 Women with a history of breast cancer stage 0 – III. Surgery ± adjuvant treatment. |
Mixed AT + RT and AT alone. | Placebo, controlled comparison, or standard care. |
QOL Significant improvement using FACT-B (6.62, 95% CI 1.21 to 33.64) Endurance Significant improvement in peak oxygen consumption. Body composition Non-significant reduction in body weight and BMI. Fatigue Significant improvement with exercise after active treatment with moderate effect size. (SMD 0.46, 95% CI 0.23 to 0.70) but not significant during active treatment (SMD 0.28, 95% CI −0.02 to 0.57) |
| McNeely, M. et al 201056 | 24 RCTs evaluating interventions for breast cancer-related upper limb dysfunction. * Cochrane Review AMSTAR score: 10/11 |
n = 2132 Women with breast cancer receiving therapeutic exercise for upper limb recover after breast cancer treatment. |
Targeted upper limb exercises, AT, RT, and mixed AT + RT. Supervised vs unsupervised exercise. Timing: Early post-surgical exercise and delayed exercise during cancer treatment. |
Usual care control group |
Early versus Delayed Post-Operative Upper Limb Exercise Significant increase in return to ROM post-operatively with early exercise. (WMD 10.6; 95% CI, 4.51 to 16.6) Significant increase in wound drainage volume (SMD 0.31, 95% CI, 0.13 to 0.49) and in duration of drain placement (WMD 1.15, 95% CI, 0.65 to 1.65) with early exercise. Supervised vs unsupervised exercise Significant improvement with physical therapy supervised exercise in shoulder ROM post-operatively (WMD 12.92, 95% CI, 0.69 to 25.16) in shoulder function following intervention (SMD:0.77; 95% CI, 0.33 to 1.21) and at 6 months follow up (SMD: 0.75; 95% CI: 0.32 to 1.19) |
| Meneses-Echavez, J.F. et al 201525 | 9 RTCs examining impact of exercise on CRF AMSTAR Score: 9/11 |
n = 772 Various types of cancer during adjuvant cancer treatment. Average time since diagnosis 8.2 months (SD ± 10.7) Adults mean age 55.5 years (SD ± 7.2) |
Supervised, multi-modal exercise interventions including AT, RT, and stretching for CRF. | Controls with no intervention | 61.3 % adherence rate Significant improvement in CRF (SMD = −0.23; 95% CI −0.37 to −0.09, P = 0.001) Gains maintained at average 12 weeks, 24 weeks, and 6 months. Subsets
|
| Meneses-Echavez, J. F., et al. 201661 | 9 trials Evaluating inflammatory mediators in breast cancer patients. AMSTAR Score: 9/11 |
n = 478 (253 exercise/225 control) Age mean 54 ± 4 (range 49 – 56) Breast Cancer stage 0 – IIIb Majority of patients were postmenopaus al. |
AT +/− RT, yoga, Tai-chi Program Duration Mean 19 weeks (±13 weeks) Frequency Mean 3 (± 1) sessions/week Session Duration 69 (± 34) minutes |
No exercise or ‘usual care’ |
Inflammatory Markers Interleukin 6 Significant reduction in concentration (WMD −0.55 pg/ml, 95% CI −1.02 to − 0.09) Tumor Necrosis Factor α Significant reduction in concentration (WMD −0.64 pg/ml, 95% CI - −1.21 to − 0.06) Interleukin 8 Significant reduction in concentration (WMD −0.49 pg/ml, 95% CI −0.89 to − 0.09) Interleukin 2 Significant reduction in concentration (WMD 1.03 pg/ml, 95% CI 0.04 to 1.67) CRP No significant effect Interleukin 10 No significant effect |
| Mishra, S.I. et al. 201232 | 56 RCTs or quasi-randomized trials evaluating the effectiveness of exercise on HRQOL and HRQOL domains. * Cochrane Review AMSTAR Score: 9/11 |
n = 4826 Various types of cancers both during and after active cancer treatment. |
Mode: Walking, cycling, RT, strength training, mixed AT + RT, yoga, and Qigong |
Controls with no exercise intervention, or education only as an intervention. |
HRQOL Overall improvement with exercise from baseline to 12 week follow up (SMD = 0.33, 95% CI 0.12; 0.55) Improvement at 12 weeks in Physical functioning (SMD = 0.69, 95% CI 0.16; 1.22) Role function (SMD = 0.48, 95% CI 0.07; 0.9) Social function Improvement at 6 months in physical functioning Fatigue: Significant difference in fatigue levels favoring the exercise group at 12 weeks. Subset Disease State: Breast Cancer Significant reduction in anxiety as compared to other cancer types. Cancers other than breast Greater reduction in depression, fatigue, sleep disturbance as compared to breast cancer. Greater improvement in HRQOL, emotional wellbeing, physical functioning and role function as compared to breast cancer. Subset Exercise Intensity: Greater improvements in HRQOL and physical functioning, and significant reductions in fatigue, anxiety, and sleep disturbance with moderate or vigorous exercise versus mild or none. |
| Mustian, K.M. et al. 201739 | 113 trials comparing exercise, psychological, and pharmaceutical interventions to treat cancer-related fatigue AMSTAR Score: 11/11 |
n = 11,525 Various types of cancer. 78 % female 22 % male Mean age 54 years (range, 35 – 72 years) |
AT, RT, and mixed AT + RT. Program Duration Average 43 sessions (range = 1 – 364) over 14 weeks (range = 1 – 60 weeks) Session Duration Average 60 minutes (range = 16 – 150) |
68 % used standard care, no intervention or wait-list control. 31% used placebo, time attention or education control. |
Significant moderate improvement in CRF from pre to post treatment with exercise intervention (WES, 0.30; 95% CI, 0.25 – 0.36, P<0.001) and with psychological intervention (WES, 0.27; 95% CI, 0.21 – 0.33; P < 0.001) and with exercise + psychological intervention (WES, 0.26; 95% CI, 0.13 – 0.38; P < 0.001) Exercise, psychological, exercise + psychological interventions were superior to pharmaceutical interventions in improving CRF. |
| Otto, S.J. et al. 201562 | 7 observational studies examining self-reported levels of PA and impact on QOL and survival. AMSTAR Score: 10/11 |
n = 4487 colorectal cancer patients (2089 examining QOL end points and 2398 examining survival end points) Self-reported change in physical activity during cancer treatment. |
Patient self-reported recall regarding levels of physical activity pre-diagnosis, during treatment, and post-treatment. Variety of Patient Reported Outcomes Measures used to quantify level of PA. Assessment time points varied among trials. |
None |
QOL Increasing levels of PA during or post treatment associated with improved QOL (SMD = 0.74 (CI = 0.66–0.82)) Survival Increasing physical activity levels post diagnosis improved survival. (HR = 0.70 95% CI, 0.55; 0.85) * Weight gain did not affect disease-related mortality. |
| Pan, Y. et al 201548 | 16 RCTs AMSTAR Score: 6/11 |
n = 538 yoga/493 control Breast cancer patients Stage 0–III. +/− Hormonal therapy |
Supervised, guided yoga interventions. Program Duration Average 3 weeks to 6 months. Session Frequency Average 1 – 3 session(s)/week Session Duration Average 60 – 90 minutes. Yoga interventions included:
|
Waitlisted control group |
Depression: Significant improvement for yoga cohort. (SMD: −0.17, 95% CI: −0.32 to −0.01; P=0.00) Anxiety: Significant reduction for yoga cohort. (SMD: −0.98, 95% CI: −1.38 to −0.57; P<0.00) Physical Well-being No significant improvement for yoga cohort. (SMD: 0.23, 95% CI: −0.04, 0.52; P = 0.10) Overall Health-related Quality of Life Significant improvement for yoga cohort. (SMD: 0.85, 95% CI: 0.37, 1.34; P = 0.001) Fatigue No significant reduction in yoga cohort. (SMD: −0.22, 95% CI: −0.53, −0.09; P = 0.17) Sleep Quality No significant improvement in yoga cohort (SMD: −0.19, 95% CI: −0.39, 0.00; P=0.05) Gastrointestinal symptoms Significant improvement in yoga cohort (SMD: −0.09, 95% CI: −0.64, 0.46; P=0.74) Duration of Intervention Significantly improved effects with yoga program duration of > 3 months. (SMD: 0.40, 95% CI: 0.00, 0.79; P=0.04) |
| Schmid, D. et al. 201459 | 23 prospective longitudinal studies 16 studies breast cancer 7 studies colorectal cancer AMSTAR Score: 9/11 |
n = 49,095 Breast and colorectal cancer patients self-reported levels of physical activity pre-diagnosis, during cancer treatment, and post diagnosis. |
Patient self-reported level of physical activity converted to METS. Used pooled RRs to compare high vs. low categories of PA at each time point. Duration/Intensity Estimated at 150 minutes of moderate physical activity per week. |
Breast Cancer Survivors: High vs Low PA pre-diagnosis Associated with decreased risk of total mortality (RR = 0.77: 95% CI= 0.69–0.88) and decreased risk of disease mortality (RR = 0.77): 95% CI= 0.66–0.90) Each 5, 10, or 15 MET-h/week increase from pre-diagnosis PA level was associated with 7%, 13%, or 19% reduced mortality. High vs Low PA post-diagnosis Associated with decreased risk of total mortality (RR = 0.52: 95% CI = 0.42 – 0.64) and decreased risk of disease mortality (RR = 0.72; 95% CI = 0.60 – 0.85) Each 5, 10, or 15 MET-h/week increase in post-diagnosis PA levels was associated with 13%, 24%, or 34% reduced mortality. Colorectal Cancer Survivors: High vs Low PA pre-diagnosis Associated with decreased risk of total mortality (RR = 0.74; 95% CI = 0.63 –0.86) and decreased risk of disease mortality (RR = 0.75; 95% CI = 0.62 – 0.91) Each 5, 10, or 15 MET-h/week increase in pre-diagnosis PA levels was associated with 7%, 14%, or 20% reduction in total mortality. High vs Low PA post-diagnosis Associated with strong risk reduction for total mortality (RR = 0.58; 95% CI = 0.48 – 0.70) and colorectal cancer mortality (RR = 0.61; 95% CI = 0.40 – 0.92) Each 5, 10, or 15 MET-h/week increase in post-diagnosis PA levels was associated with a 15%, 28%, or 38% lower risk of mortality. |
|
| Scott, D.A. et al 201357 | 12 RCT’s AMSTAR Score: 4/11 |
n = 1669 Various types of cancers. All participants had completed primary cancer treatments. |
Multidimensional rehabilitation program (MDRP): Inclusive of a physical (exercise, dietary regime) and psychosocial (counseling, cognitive behavior therapy) component carried out on 2 or more occasions. Individual supervised Group supervised Unsupervised |
No intervention or lower-level intensity program, or different mode of administration. | Significant improvement in the SF-36 physical health component score (Mean Difference = 2.22 (95% CI 0.12 to 4.31, P = 0.04)) MDRP most successful when focusing on one behavior area (exercise or stress management) rather than focusing on several different behaviors at the same time. Significant improvements noted in supervised vs unsupervised settings, but the type of provider delivering services had no impact on improvements. Maximum benefit to MDRP was noted by 6 months. |
| Sebio Garcia, R. et al. 201634 | 21 controlled trials evaluating the impact of pre-operative exercise interventions. AMSTAR Score: 8/11 |
n = 1189 (595 intervention/594 controls) Lung cancer Stage I – IIIA during adjuvant or neoadjuvant treatment. 62 % male Average age 64.8 years (±5.28)/64.3 years (± 6.3) |
Outpatient-based exercise programs. AT, RT, or mixed AT + RT with or without breathing or incentive spirometry intervention. Duration Average 4 weeks (range = 1 week to 10 weeks) Intensity Moderate to Vigorous |
No exercise |
Pulmonary Function: Significant increase post operatively in FEV1 (SMD = 0.27, 95% CI 0.11, 0.42) and in FVC (SMD = 0.38, 95% CI 0.14, 0.63). Trend towards significance in VO2peak. Improvement noted but pooled effects were not possible. Functional Recovery: Significant reduction in post-operative hospital length of stay (mean difference = − 4.83, 95% CI −5.90, −3.67) Significant reduction in post-operative complications (RR = 0.45, 95% CI 0.28, 0.73) HRQOL: No significant improvements. Breathing Exercises: No evidence to support that adding breathing exercises or incentive spirometry provides additional benefit. |
| Shneerson, C. et al. 201346 | 5 RCTs Evaluating the effect of yoga 4/5 studies had high risk for selection and outcome reporting bias. AMSTAR score 7/11 |
n = 66 Breast cancer, after completion of active treatment. Age range 50–63 |
Yoga programs 3 trials of hatha 1 trial restorative 1 trial Iyengar) Program Duration 7 weeks - 6 months Frequency At least twice a week Session Duration 1 – 1.5 hours |
All RCTs, with waitlist controls | Very small effect sizes overall. QOL Improved in only 1 study vs controls Emotional subscale of FACT-B improved in only 1 study (ES 0.51, 95 % CI 0.18 – 0.84) for overall QOL at 3 months, no difference at 6 months. Physical QOL no difference at 3 months. Mental QOL better than controls at 3 months (ES 0.46, 95% CI 0.14 – 0.77) |
| Singh, F. et al.* 201358 | 18 controlled trials evaluating prehabilitation or pre-operative exercise programs. (10 RCT’s) AMSTAR Score: 7/11 |
n = 966 Lung, prostate, Abdominal & GI cancers receiving exercise training or intervention prior to surgery. Age range 54.1 years (± 8.53) to 71.1 years (± 6.3) |
AT, RT, and mixed forms AT + RT +/− muscle re-education exercises. Supervised and unsupervised programs. Timing of intervention prior to surgery Median 21 days (range = 7 – 52 days) Frequency 5 – 7 x/week Intensity Aerobic: Range 40% – 80% max capacity. Resistance: 60 % to 80 % 1RM Or Repetitions as a proxy for intensity Session Duration 15 minutes to up to 3 hours/session. |
Education-only or No intervention or Different training program |
Functional walking capacity: Trend towards significance, only 2 studies showed significance. Pooled effects not calculated. Cardiorespiratory fitness: Significant increases (8% to 32%) Pooled effects not calculated. Quality of Life: Mixed results. Significant variability in measurement tools prevented pooled calculations. 3/5 studies measuring QOL showed no improvement. Rate of Return to Continence: Trend towards significance, study heterogeneity prevented pooled calculations. Length of Hospital Stay: Significant improvements noted, pooled calculations not possible. |
| Smits, A. et al.* 201565 | 8 controlled trials (3 RCTs) AMSTAR score 7/11 |
n = 413 Endometrial and Ovarian cancers. Following completion of active cancer treatment. |
Predominately walking, and unspecified physical activity home-based program. Program Duration 4 weeks to 6 months Frequency 5x/week Session Duration 30 minutes |
Mixed controlled and single-arm trials. Comparisons not specified. |
Endurance 12-min walk and aerobic capacity improved at 3 and 6 months post intervention. Strength Improved at 6 months. QOL No improvement noted at 3 or 6 months. |
| Speck, R. et al 201017 | 82 studies 66/82 ‘high quality’ controlled studies included in meta-analysis. AMSTAR score 7/11 |
n = 6838 Breast (83%), colon, lung, ovarian, leukemia, lymphoma, prostate, sarcoma, stomach, testicular, and other cancer types. 40% during active cancer treatment. 60% post treatment. |
80% had combined exercise AT+RT programs. Mode was primarily AT. Intensity Not specified. Assessed frequency during vs. after treatment. Program Duration Most interventions > 5 weeks Session Frequency Average 3 – 5x/week. |
All studies included comparison groups but were unspecified. |
Exercise during active cancer treatment Significant WMES improvement in Overall physical activity level (0.38, p = 0.001) Aerobic fitness (0.33, p = 0.009) Upper body strength (0.39, p = 0.005) Lower body strength (0.24, p= 0.006) Body weight (−0.25, p = 0.05) Body fat percentage (−0.25, p = 0.04) Functional quality of life (0.28, p = 0.04) Positive mood (0.39, p = 0.002) Anxiety (−0.21, p = 0.02) Self-esteem (0.25, p = 0.02) No significant adverse effects were reported (e.g. blood counts) Exercise after completion of cancer treatment Significant WMES improvement in Physical activity level (0.38, p < 0.0001) Aerobic fitness (0.32, p = 0.03) Upper body strength (0.99, p<0.0001) Lower body strength, (0.90, p = 0.024) Body weight (−0.18, p = 0.004) Body fat percentage (−0.18, p = 0.006) BMI (−0.14, p = 0.002) Overall quality of life (0.29, p = 0.03) Breast cancer-specific concerns (0.62, p = 0.003) Perception of physical condition (0.57, p = 0.04) Mood disturbance (−0.39, p = 0.04) Confusion (−0.57, p = 0.05) Body image (−0.26, p = 0.03) Fatigue (−0.54, p = 0.003), General symptoms and side effects (−0.30, p = 0.03) IGF-1 (−0.31, p = 0.03) |
| Spence, R.R. et al.* 201018 | 10 studies (4 RCTs, 3 controlled non-randomized, 2 intervention, non-controlled, 1 single group design) AMSTAR score 8/11 |
n = 483 4 trials included breast cancer only. 3 trials included mostly breast cancer. 2 trials included only colorectal cancer. Age range 16 – 71 years |
AT and RT Program Duration 2 – 26 weeks Intensity Moderate Frequency 3x/week during ‘rehab period’ up to 12 months after adjuvant treatment |
Current activity Stretching 3 trials with no comparison group. |
Physical Function and Endurance Significantly improved VO2peak and strength. Fatigue Reduced Physiological Biomarkers Trend towards improvement but somewhat mixed. Improvements immune cell function, lower reported neutropenia, lower inflammatory markers. Modest improvements in body composition. |
| Steel, J. et al.* 201422 | 2 studies Both trials in hospital-based settings immediately after surgery. AMSTAR score 8/11 |
n = 58 GI cancers primarily stomach and colorectal. |
Arm and leg cycling exercises. Intensity Moderate Frequency 5x/week Program Duration 2 weeks Session Duration 40 minutes |
Lower intensity exercise or no exercise controls. |
Immune function Significant improvement in NK cell activity. Lower antagonist/cytokine ratio at end of program vs controls. * Initially exercise induced a decrease in NK cell activity. |
| Van Dijck, S. et al.* 2016 23 | 13 RCTs AMSTAR score 4/11 |
n = 2,180 Breast cancer patients during and after cancer treatment. |
AT Program Duration 1 – 12 months Unspecified duration, intensity, and frequency Primarily unsupervised (as part of ‘physical self-management’ program) |
Usual physical activity, usual care or written materials |
During cancer treatment QOL was modestly improved or no change was identified. Fatigue modestly improved. Physical function improved. After cancer treatment Consistent improvement in QOL. No significant difference for fatigue levels Mixed results on endurance measures (6MWD, VO2peak) |
| van Vulpen, J.K. et al. 201641 | 5 RCTs (784 patients) High risk of performance and attention bias. AMSTAR score 8/11 |
n = 784 Breast cancer patients during adjuvant cancer treatment. (defined as either chemotherapy or radiation therapy) Mean age 50 – 56 years |
RT and AT Session Frequency 2 – 5x/week Session Duration 30 – 60 minutes Intensity AT: Moderate RT: > 60 % of 1RM Supervised |
Usual care or sham |
Fatigue Small to medium effect sizes (ES 0.20–0.50) for general fatigue and physical fatigue improvements vs controls during chemotherapy. No significant effect on cognitive fatigue Supervised programs had larger effect sizes than unsupervised. |
| Visser, W. et al.* 201454 | 5 studies (2 prospective cohort, 2 retrospective cohort, 1 case control) AMSTAR score 7/11 |
n = 321 Rectal cancer Mean age 55 – 67 years |
Pelvic floor and core muscle training Program Duration 7 – 15 sessions Supervised |
2 trials pre-post comparison. 3 trials compared to no rehabilitation. |
QOL Significantly improved Improved incontinence and pelvic floor muscle function. |
| Winters Stone, K. M. et al.* 201019 | 8 studies investigating impact of exercise on bone density. (5 RCT, 5 uncontrolled intervention) AMSTAR score 9/11 |
n = 567 7 trials breast 1 trial prostate During survivorship period. Mean age range 48 – 55 years. |
50% AT 50% RT Program Duration 12 – 52 weeks Session Frequency 2 – 7x/week Intensity Predominately moderate 50% supervised 50% unsupervised |
Usual care or drug therapy without exercise | Most exercise groups maintained BMD while controls experienced decline in levels of BMD. Modest increase in BMD in some exercise groups. Trend towards positive improvement in BMD with exercise. |
| Zhu G. et al. 201646 | 33 RCTs Moderate allocation and reporting bias. AMSTAR 7/11 |
n = 2,659 Breast cancer survivors |
AT with or without RT, Tai-chi, yoga Frequency and duration not reported |
Usual care, wait-list, brief supportive therapy | Significant improvement in QOL (I2 = 0% P = 0.006, 95% CI: 0.11, 0.62) General health (I2 = 95%, P = 0.02, 95% CI: 0.70, 8.48) Emotional well-being (I2 = 2%, P = 0.0006, 95% CI: 0.12, 0.43) Social well-being (I2 = 0%, P = 0.01, 95% CI: 0.19, 1.69) No significant improvement in fatigue. Muscle strength significantly improved. (I2= 48%, P = 0.0009, 95% CI: 1.76, 6.78) BMI significantly improved (I2 = 0%, P = 0.00001, 95% CI: −1.09, −0.47) Significant reduction in Insulin (I2 = 95%, P = 0.05, 95% CI: −13.64, 0.06) and Insulin-like growth factor binding protein (IGFBP)-1 (I2 = 46%, P = 0.00001, 95% CI: −4.40, −1.91) |
| Zimmer, P. et al* 201647 | 14 studies (6 RTCs, 1 non-randomized, 2 prospective non-controlled, 1 case series, 1 observational study, 3 cross sectional studies) AMSTAR score 7/11 |
Mostly breast and some prostate cancer survivors. | 11 trials yoga of various forms 1 trial AT 1 trial RT 1 trial tai chi Program Duration 4 weeks - 6 months Session Frequency 1 – 3x/week Session Duration 60 – 90 minutes |
Most with no comparison group. 2 trials with usual care comparison. |
Cognitive Function Significant improvement with yoga. Significant improvement with other exercise types (AT, RT, and tai chi) Inflammatory Markers Profile improved in both yoga and other exercise groups. |
Table Abbreviations: 6MWD- 6 minute walk distance, ALL – Acute leukocytic leukemia, AML – Acute myeloid leukemia, AT – Aerobic training, BMD – Bone mineral density, BMI – Body mass index, CI – Confidence interval, CRC – colorectal cancer, CRF – Cancer-related fatigue, ES – Effect size, FEV – Forced expiratory volume, FVC – Forced vital capacity, GI – Gastrointestinal, HR – Hazzard ratio, IFN – Interferon IGF-BP3 – Insulin-like growth factor binding protein 3, ILGF-I – Insulin-like growth factor I, ILGF- II – Insulin-like growth factor II, HRQOL – Health related quality of life, MET – Metabolic equivalent of task, MDRP – multidimensional rehabilitation program, PA – Physical activity, PRE – progressive resistive training, PSA- Prostate-specific antigen, QOL- Quality of life, RCT – Randomized controlled trial, ROM – Range of motion, RR – Risk ratio, RT – Resistance training, SD – Standard deviation, SF-36 – Short form 36, SMD – Standard mean difference, VO2max – Maximal oxygen consumption, WES – Weighted effect size, XRT – Radiation therapy.
Effect size calculations not provided in the review
In general, findings demonstrate an overall positive benefit of exercise interventions among a variety of cancer types using various forms of movement-based exercise. There was significant variability regarding frequency, duration, and intensity of commonly prescribed exercise regimens. Some reviews cited that many of the studies examined failed to meet the definition of physical activity,15 while others reported well defined, if disparate, exercise parameters.16–19 The mode of exercise varied widely in reports, spanning both aerobic and resistive training protocols16,17,19–30 as well as described mixed (aerobic + resistance training) interventions15,18,24,31–44, yoga32,45–50, tai chi47,50,51, dance52, progressive resistive exercise26,34,53, and therapeutic exercises (focused on targeted body region impairments).34,53–56 Exercise programs were structured in various settings (home-based, outpatient ambulatory clinic, hospital-based) and provided various levels of provider supervision. A general trend towards improved outcomes was noted when exercise was conducted in a supervised setting.25,36,41,56,57
The reviews included in this analysis identified exercise intervention across the cancer care continuum including exercise interventions prior to the initiation of oncologic treatment34,54,58,59, during active oncology-directed treatment33,40,43,56, and following the completion of oncologic treatment.18–20,24,26,28 The results suggest that timing and type of exercise may impact various biological and physiological markers, psychosocial factors, and functional impairments differently15,17,23,27,32,59, and suggest overall improvements in tolerance to cancer treatment and functional outcomes when exercise is initiated before or during cancer treatment.33,34,54,58 Reviews included a wide sampling of various types of cancers with breast, prostate, and colorectal cancers most commonly studied.
Some reviews focused on exercise interventions targeting one specific cancer treatment-related impairment, such as cancer-related fatigue (CRF)18,21,25,39,41,43 or lymphedema29,60, and many reported on the impact of exercise on common treatment-related side effects such as body weight and body mass index18,24,26,28,35,36,38, depression15,40,45,49, anxiety24,32,40,45,48,49, bone density19, other physical and functional impairments23,26,27,29,30,33,42,48,52–54,56, and various biomarkers associated with cancer progression.17,18,22,24,28,33,50,51,61
Several large observational cohort study reviews examined patient self-reported levels of physical activity at various points in the cancer care continuum and offered longitudinal perspective on the association with meaningful endpoints such as disease free survival and mortality risks.59,62–64 While these reviews do not reflect comparisons of exercise intervention trials, they do provide substantive support for the impact of physical activity on meaningful endpoints such as disease progression and overall mortality.
Overall, across all reviews, there was poor reporting of trial and intervention adherence, adverse events, and a lack of specific characterization of exercise interventions.
Exercise Intensity
A general theme emerged regarding the intensity of aerobic exercise, favoring moderate to vigorous exercise, as compared to controls who did not exercise or who exercised at a lower level of intensity.27,28,30–32,36,64 This effect was noted in trials both during and after cancer treatment and was supported by observational study reviews that identified high vs low self-reported levels of physical activity.59,63,64 Results differ regarding the superiority of vigorous versus moderate intensity exercise with no clear evidence to demonstrate more significant or longer term carry over of positive outcomes based on the level of intensity. In general, exercise interventions at moderate and vigorous intensity are safe in supervised settings with small numbers of adverse events noted.17,40 Moderate and vigorous exercise resulted in improvements in measures of fitness including: VO2peak24,26,35, VO2 max38, muscle strength and endurance26,30, and in measures of function including 6 and 12 minute walk distance outcomes27,65, as well as improved measures of immune function.17,24,28,61 While moderate to vigorous exercise interventions significantly improved various physical and functional indicators, the impact on cognitive recovery, depression, and anxiety was mixed in several reports with some noting no significant impact from exercise.15,27,32,41,49 Low intensity exercise interventions demonstrated improvements for more deconditioned populations over time and positively impacted cancer-related fatigue, depression, anxiety and overall physical functioning.20,31,40,48
Reviews that looked specifically at therapeutic exercises, targeting one body region or specific impairment, frequently did not characterize intensity of the intervention. These interventions focused on a set of rehabilitative exercises based on a practice protocol and frequently included progressive forms of exercise. Although progressive resistive exercises (PRE) were frequently identified as a therapeutic exercise intervention, rarely was the specific intensity, number of repetitions, or activity duration defined. Many of the PRE interventions were targeted therapeutic exercises designed for impairment rehabilitation.53,55 In general, these interventions were supervised by a health care provider in a structured care setting and resulted in significant improvements in various domains of physical and functional status as compared to controls.33,53–56
Reviews of yoga, tai chi, and qigong exercise interventions frequently identified the type of yoga or specific tai chi exercises, program duration, and frequency.46–51,61,66 Although the intensity of these programs was not frequently defined most are characteristically lower intensity exercises as defined by the level of energy demand produced by the activity.67,68 The benefits from yoga were stronger with a greater duration of yoga practice (>3 months) and yoga tended to have greater impact on affective and psychosocial domains with mixed positive benefits on physical domains and inflammatory biomarkers.46–48,61
Exercise program structure
Most reviews examined exercise interventions in ambulatory settings, with some including a home-based component. One review, exclusive to hematological cancers, examined exercise interventions in hospital-based settings and demonstrated positive impact on various physical, functional, and psychological outcomes.40 Of importance, this review identified no significant adverse events reported with exercise in this controlled study population.40
Several reviews reported that supervised exercise interventions yielded superior benefits as compared to non-supervised exercise programs in a variety of outcome measures including health-related quality of life (HRQOL) and adherence to exercise, as well as other physical and psychosocial outcomes.15,36,41,57 Unsupervised programs were found to be useful in promoting adherence to exercise recommendations over time.57 Structured group exercise programs such as yoga, qigong, and other group movement-based classes demonstrated outcomes superior to controls.32,46,48,49,51,61 The question was raised in one report as to whether the impact of supervision by a health care provider creates an environment where more attention is given to the participant and therefore positive outcomes are attributable to the individualized experience rather than to the physiological impact of the exercise intervention.36 In the context of therapeutic exercise interventions, supervision was regarded as necessary due to the targeted nature of the prescribed exercise and the need to correct a physical or functional deficit. Supervision of therapeutic exercise interventions yielded significant improvements in overall functional outcomes.34,41,53,54,56
No evidence was found in these reviews to suggest superior impact of one setting over another on outcomes; however, considering that supervised exercise programs exceeded unsupervised in effect, supervision should be considered regardless of the setting. There were several reviews that included aspects of computer aided technology and telehealth as supportive adjuncts to the exercise intervention and suggest positive outcomes were enhanced when technology complemented the exercise intervention.16
Aside from setting and supervision, an additional factor considered in the structure of the exercise program was highlighted in a Cochrane review regarding multidimensional rehabilitation programs.57 Multidimensional rehabilitation programs (MDRP) were defined as addressing both a physical and a psychosocial component through the same intervention. Interestingly, while MDRPs contributed to greater improvements in physical health, the greatest successes were notable when the program focused on a single physical domain (e.g., exercise or dietary change) rather than when trying to impact multiple domains at once.
Time Course
The timing of exercise interventions spanned pre-treatment, active cancer treatment, and post treatment through survivorship. Exercise and physical activity interventions demonstrated beneficial effects regardless of the specific timing of exercise; however, introducing exercise at different time points in the cancer care continuum demonstrated different magnitude of effects on cancer treatment tolerance33, overall function17, mitigation of side effects21,35, and improvements in QOL.23,36,46,65 Effect sizes suggest that the impact of exercise on QOL, upper body and lower body strength, and physical function may be somewhat greater when exercise is introduced after the completion of cancer treatment, but the effect of exercise has greater impact on cancer-related fatigue during treatment.23,27,46 This suggests that the timing of the intervention is important in the context of individual factors including cancer type32,59 and adjuvant treatment phase.23,33,35,62
A small number of systematic reviews explored pre-treatment or prehabilitation exercise interventions.27,34,54,58 The prehabilitation and pre-surgical exercise reviews demonstrated improvements in adherence to exercise, tolerance to active cancer treatment specifically to chemotherapy, and mitigation of functional decline after the initiation of active cancer treatment.34,58 The concept of prehabilitation is relatively new in oncology rehabilitation practice, and although the body of evidence is maturing, a robust systematic review has not yet been conducted to inform broad intervention recommendations. The existing qualitative reviews identified improvements in meaningful endpoints related to post-treatment functional recovery54,58 and demonstrated reductions in post-operative hospital length of stay34,58, post-operative complications34, and return to pre-operative functional status.58
Numerous reviews highlighted the benefits of exercise programs during active cancer treatment with notable positive impact on a variety of side effects of cancer treatment including: cancer-related fatigue21,23,25,32,37–41,43,49,66, depression32,40,49,66, anxiety32,49,66, sleep32, HRQOL15,17,23,27,32,37,40,57,66, and physical function.23,27,32,33,37,40,57 Additionally, support for early targeted therapeutic exercises to alleviate impairments of specific body structures and function was identified for upper limb and shoulder in both the breast and head and neck cancer populations37,53 as well as for the pelvic floor in the gynecological and prostate cancer populations.54 These reviews support early therapeutic exercise to restore upper limb ROM53,55,56 and to prevent or reverse incontinence.54
Importantly, two reviews noted no adverse events associated with blood counts when the exercise intervention was undertaken during active cancer treatment.17,40 Additionally, several reviews cited improved immune function33,61 and tolerance to chemotherapy33 with exercise during cancer treatment. Reviews suggest that timing of exercise interventions should consider the phase of treatment in order to maintain blood counts.24,32,40 This may be beneficial to improving tolerance to treatment and may mitigate the risk for adverse events related to blood counts such as neutropenia and thrombocytopenia. Several reviews identified no adverse events associated with either the onset or progression of lymphedema as a result of exercise interventions both during and after breast cancer treatment.17,26,29,52,55,56,60
Cancer Type
The majority of reviews examined exercise across various types of cancer and demonstrated overall positive results from exercise regardless of the primary cancer diagnosis.17,20,21,24–26,31,32,36,38,39,42,47,57–59 Some reviews provided breakout comparisons that demonstrated slightly different nuanced outcomes from exercise interventions based on the type of cancer.32,59 For example, one report identified that breast cancer patients experienced greater reduction in anxiety with exercise as compared to other cancer types but made notably fewer gains in physical functioning and role function as compared to other cancer types.32 Table 3 outlines the clinical implications of exercise across different types of cancer.
Table 3.
Findings by Cancer Type
| Review synopsis | Intervention | Average duration | Clinical pearls |
|---|---|---|---|
|
| |||
| BREAST CANCER | |||
|
| |||
| 33 trials, 25 trials post completion of cancer treatment and 8 during cancer treatment | 11 trials with aerobic exercise only 8 trials with aerobic and resistance components 1 trial resistance exercise only |
16 weeks | Exercise across all groups improved quality of life and reduced insulin, ILGF- II, ILGF-I. |
|
| |||
| 24 trials | Therapeutic exercise for upper limb: aerobic, resistive, and mixed 4 trials were supervised by a physiotherapist |
Early vs delayed exercise Early= post-op day 1 to day 3 Late= post-op day 4 or later |
Early exercise is beneficial but may increase time to wound healing. Concerns raised that studies may overestimate wound protection from delayed exercise. Significant improvement with supervised vs unsupervised |
|
| |||
| 24 trials during or after treatment | Yoga | Total = 1,205 minutes (frequency X duration of session X duration of treatment) | Improvements in quality of life, depression, anxiety and fatigue and GI symptoms. |
| 18 trials during or after treatment | Yoga | Median = 8 weeks Mean = 9 weeks |
Improvements in mood and quality of life. |
| 16 trials | Yoga | Mean = 9.8 weeks | *significant improvement when duration of yoga > 3 months. |
|
| |||
| 14 trials | 6 aerobic and resistance 1 resistance alone 1 Tai Chi 9 trials conducted in supervised settings |
13 weeks | Improvements in peak oxygen consumption and quality of life. Improvement in cancer related fatigue with exercise after cancer treatment was completed. |
|
| |||
| 13 trials 8 trials during cancer treatment 5 after completion of cancer treatment |
Predominately aerobic exercise, some with walking program as recommended exercise intervention Intensity: moderate to vigorous in 3 studies |
1–12 months | Improvements in fatigue, endurance, physical function, and quality of life. *threshold identified for seated exercise: > 3-month exercise duration may have more significant effect on outcomes. |
|
| |||
| 13 trials all conducted exercise after completion of cancer treatment | 7 resistance exercises only 2 weight lifting 1 moderate intensity progressive resistive exercise, supervised 2 ROM and strength supervised by physiotherapist 1 supervised by exercise trainer |
30 weeks (including supervised and unsupervised portions) | Resistance training is safe in breast cancer survivors and does not increase risk of lymphedema. Education for unsupervised exercise supported adherence following supervised portion of program. |
|
| |||
| 10 trials during or after cancer treatment | 9 aerobic and resistance 1 trial resistance only |
14 weeks | Progressive resistance exercise may improve endurance, strength, flexibility, lean mass, cardiorespiratory fitness, immune system, mood, self-esteem, and chemotherapy dose tolerance. |
|
| |||
| 9 trials conducted after cancer completion of cancer treatment | 8/9 trials had at least one supervised exercise component. 4 aerobic and resistance 4 aerobic (1 with weight belt) 1 progressive resistive exercises |
16 weeks | 24 weeks of aerobic or resistance training may decrease body fat. Aerobic training may decrease ILGF-1 and resistance training may decrease ILGF-2. |
|
| |||
| 9 trials | 4 aerobic and resistance combined 3 aerobic alone 1 weight training alone 1 Tai Chi |
19 weeks | Exercise may favorably affect insulin levels in obese or sedentary women. *tamoxifen was found to lower ILGF-levels, it was inconclusive as to whether exercise impacts this effect. |
|
| |||
| 2 trials conducted within 5 years of treatment | Dance and movement therapy | Total program duration = 1035 minutes | May benefit quality of life in survivors. |
|
| |||
| GASTROINTESTINAL CANCERS | |||
|
| |||
| 5 studies | 4 pelvic floor muscle retraining and activation with biofeedback and/or rectal balloon 1 pelvic floor muscle and movement exercise. |
8 weeks | Improvements in quality of life and reduced incontinence rates in exercise group. |
|
| |||
| 5 studies all after completion of cancer treatment | 2 home-based aerobic exercise programs 1 supervised high intensity aerobic 1 supervised moderate intensity aerobic 1 partial supervised aerobic and resistance |
9 weeks | Improvements in short-term physical fitness. Supervised participants demonstrated greater adherence. |
|
| |||
| 2 studies after completion of cancer treatment | 1 Arm and cycle ergometers twice daily, 5 days/week. 1 Forty minutes of individualized moderate intensity exercise daily. |
2 weeks | Improvement in immune function overall. Initially exercise induced a decrease in natural killer cell activity in 1st week and improvement was noted after 2nd week. *Only two weeks of exercise may favorably affect immune function. |
|
| |||
| HEAD AND NECK CANCERS | |||
|
| |||
| 8 of 24 trials during or after completion of cancer treatment | 4 resistance exercise 1 walking program 1 brisk walking and active exercises 1 hydrotherapy 1 aerobic and resistance |
9 weeks | Improvements in lean body mass, strength, physical function, QOL, and fatigue. 75% of patients reported “possibly” or “definitely” interested in physical activity counseling following completion of the trial. |
|
| |||
| 3 trials acutely following cancer surgery | Progressive resistive exercise with ROM and stretching. Supervised initially and educated for unsupervised following initial therapy. |
3× week for 12 weeks | Progressive resistive training was more effective than standard physiotherapy stretching for shoulder dysfunction in head and neck cancer. |
|
| |||
| ENDOMETRIAL AND OVARIAN CANCERS | |||
|
| |||
| 8 trials during and after completion of cancer treatment | 3 Multimodal including exercise intervention and nutrition counseling, education for health behaviors, and cognitive therapies. 2 Multimodal including only physical activity and nutrition counseling 1 walking program 1 physical activity program 1 dietary intervention and education alone |
17 weeks | Improvements in fatigue, cardiovascular fitness, strength, and physical function Improvements in weight (in multimodal studies when nutrition intervention was provided) |
|
| |||
| 7 trials after completion of cancer treatment | 5 studies reported only cross sectional self-report of physical activity levels | Self-reported “moderate intensity” exercise defined as: at least 150 minutes/week at least 30 minutes per day, 5 days per week Intervention with computer/accelerometer and intervention with computer/mobile app to support supervised contact 3 studies: “60 minutes strenuous or 150 minutes moderate exercise weekly” 3 studies: “moderate intensity exercise for at least 30 minutes per day, 5 days per week” 1 study: Moderate intensity cardiorespiratory exercise training ≥ 150 minutes/week, or Vigorous exercise for ≥ 40 minutes/week, and resistance exercises for major muscle groups |
Increased physical activity can contribute to improved quality of life. Greater benefit seen in the obese/overweight population. |
|
| |||
| PROSTATE CANCER | |||
|
| |||
| 12 trials after completion of cancer treatment | Primarily aerobic exercise training 7 group-based programs and 5 home-based programs 5 study groups included resistive exercise 4 home-based programs also included some group intervention |
17 weeks | Resistance training may improve fatigue, QOL and muscle endurance. Aerobic training may improve endurance, sit to stand, fatigue and quality of life. Combined forms of exercise may improve muscle mass, muscle strength and endurance, walk speed, and QOL. *Group-based training programs were overall more effective than home-based programs |
|
| |||
| 7 trials during androgen deprivation therapy administration | 6 trials included exercise interventions: 4 aerobic and resistance exercise interventions 2 resistive training only 4 supervised programs 2 unsupervised programs |
13 weeks | Exercise may improve quality of life |
|
| |||
| 5 trials during and after cancer treatment | 2 combined resistance and aerobic exercise training 1 resistance only 1 aerobic only 1 aerobic compared to resistance |
14 weeks | Both aerobic and resistance exercise significantly mitigate cancer-related fatigue. Resistance exercise demonstrates longer term improvement in positive outcomes and improved QOL to a greater degree. |
|
| |||
| LUNG CANCER | |||
|
| |||
| 21 trials before the initiation of cancer treatments. | 20 supervised exercise programs 1 home-based exercise programs 16 pre-operative exercise programs 5 pre and post-operative exercise programs |
4.2 weeks | Improvements in post-operative pulmonary function. Reduced length of hospital stay. Reduced post-operative complications. |
|
| |||
| 14 trials, 5 pre-operative, 7 post-operative, 2 advanced disease | All trials included some form of aerobic exercise 54% included component of resistance exercise 6 conducted in an inpatient setting 6 conducted in an outpatient setting 2 home-based exercise programs |
7 weeks | Exercise may improve pre-operative and post-operative aerobic exercise tolerance. Exercise improves overall mortality rates in the lung cancer population. |
|
| |||
| HEMATOLOGICAL CANCERS | |||
|
| |||
| 9 trials during cancer treatment | 3 aerobic and resistance training programs 2 walking programs 1 aerobic exercise and resistance exercise and stretching 1 aerobic exercise and resistance exercise and sensorimotor training 1 cycle ergometer and activity of daily living training program 1 cycle ergometer program alone All programs in a supervised inpatient setting |
10 weeks | Improvements in physical performance and function, quality of life, fatigue and depression. No serious adverse events reported, no adverse events related to blood counts. |
|
| |||
| VARIOUS CANCERS | |||
|
| |||
| 113 trials with 53 of the trials specific to breast cancer treatment. Trials were both during and after cancer treatment | 69 studies included exercise interventions 10 studies included combined exercise and psychological interventions |
Average 14 weeks Average 43 sessions Average 60 minutes |
Exercise with or without a psychological intervention improves fatigue and is superior to pharmaceuticals or psychologic intervention alone. Internet delivery was the most effective form of treatment delivery as compared to telephone, print, or in-person. Combination of two modalities yielded inconsistent results. |
|
| |||
| 82 trials in breast, colon, lung, ovarian, leukemia, lymphoma, prostate, sarcoma, stomach, testicular, and other cancers. | 80 % of trials included aerobic exercise 60 % of aerobic programs were at moderate to vigorous intensity 59 % of programs were conducted 3–5 × per week 40 % of trials were of 30–45 minutes per session duration 60 % of trials were conducted post cancer treatment and 40 % during cancer treatment |
48 % of programs were 5–12 weeks in duration | Improvements in strength, fatigue, fitness, cancer-related treatment symptoms, quality of life, reduced confusion, and reduction in ILGF-1. Recommended duration of 8–12 weeks. |
|
| |||
| 56 trials with 28 specific to breast cancer | 37 exercise programs were supervised and institution-based 43 trials included various types of aerobic exercise: 23 general aerobic 14 walking program 6 cycling exercises 6 yoga exercises 4 trials included resistance training only 2 trials used Qigong exercises 1 seated exercise program |
Various | Walking and cycling significantly reduced fatigue during and after cancer treatment, especially in breast and prostate cancers. |
|
| |||
| 34 trials including breast, male genitourinary, hematologic, gastrointestinal, gynecological, respiratory, and other types of cancer both during and after completion of cancer treatments | 15 programs included both resistance and aerobic components 12 programs were aerobic exercise alone 7 programs included resistance alone with or without weight bearing components |
21 weeks | Improvements in HRQOL No effect was noted on BMI *concern was raised that measures of adiposity were not either not used or were incorrectly used. |
|
| |||
| 56 trials across various cancer populations. | 32 trials included aerobic exercise: 22 included walking alone or in combination with another form of exercise training. 8 included cycling alone or in combination with another form of exercise training. 9 yoga trials 2 Qigong trails 18 programs were facility-based 18 programs included facility-based exercise and a home component 16 programs were home-based only |
Modal exercise intervention of 12 weeks | Improvements in health-related quality of life, fatigue, sleep disturbance, mood disorder, and physical function. Greater benefit with moderate or vigorous exercise |
|
| |||
| 34 trials 22 breast only 3 colorectal only 1 endometrial only 8 various cancers |
27 aerobic exercise only 6 aerobic and resistive training Intensity: moderate to vigorous in most studies |
13 weeks | Exercise significantly reduced ILGF-1, BMI, and weight. Significant gains in physical function, depression, fatigue, and overall physical performance. |
|
| |||
| 14 trials including breast, colorectal, prostate cancer populations | 6 trials aerobic exercise only 6 trials aerobic and resistance exercise 1 trial with aerobic and water and land resistance 1 with aerobic with Greek traditional dances and upper body training and cool down |
Variable duration of exercise programs 4–24 weeks | Improvements in overall measures of aerobic exercise tolerance and endurance. |
|
| |||
| 14 trials primarily in the breast cancer population but inclusive of other cancer types | Yoga or yoga-type exercises, aerobic programs, resistance training, and Tai Chi exercises 3 physical activity alone Otherwise 7 different interventions (physical activity behavior change) |
4 weeks – 6 months 1–3 x/week 60–90 minutes |
Improvements in cognitive function. Decreased levels of inflammatory markers. |
|
| |||
| 10 trials including 4 breast cancer, 3 predominantly breast, 2 colorectal cancer populations | 7 trials included aerobic exercise alone 3 trials included aerobic and resistance exercises |
Average 1,334 minutes total exercise program | Exercise interventions improve VO2max, strength, quality of life, fatigue, immune function, and body composition. |
|
| |||
| 9 trials including various cancer populations investigating impact of exercise on cancer-related fatigue. Interventions were supervised and multi-modal. |
5 trials included aerobic and resistance exercise 3 trials include aerobic, resistance, and stretching exercise 1 trial resistance exercises only |
Average program duration: 16 weeks Average session duration: 50 minutes Average frequency: 2.7 x/week |
Significant improvements in fatigue. Stronger effect from exercise when the intervention included aerobic, resistance, and stretching. |
|
| |||
| 8 trials including 7 breast and 1 prostate cancer populations. | 3 trials resistance exercise only 3 trials aerobic exercise only 1 trial walking with weight belt 1 trail resistance and aerobic exercise 50% supervised 50% unsupervised |
Program duration: 12–52 weeks Session frequency: 2–7×/week Intensity of exercise was predominately ‘moderate’. |
Weak support for exercise improving bone density. Questionable whether sufficient load was utilized to impact bone density. Noted as potential mitigating factor for exercise impact. Questionable benefit over and above bisphosphonates if the patient is doing both for bone health. |
Table abbreviations: BMI – Body mass index, ILGF-I – Insulin-like growth factor I, ILGF- II – Insulin-like growth factor II, HRQOL – Health related quality of life, QOL-Quality of life, ROM – Range of motion, VO2max – maximal oxygen consumption.
Cancer treatment-related side effects and functional impairments
Systemic and local antineoplastic treatments often include similar treatment modalities, chemotherapeutic drugs, and poly-pharmacy administered in different doses and combinations based on the disease and disease severity introducing anticipated side effects that contribute to greater risk for cardiovascular and neuromuscular impairments. Several reviews focused on the effect of exercise on a single physical impairment19,25,29,39,60 while the majority aggregated findings and provided sub-analyses data on specific impairments.
Cancer-related Fatigue
Cancer-related fatigue (CRF) was the most commonly identified physical impairment in systematic reviews examining the impact of exercise in the cancer population. The evidence presented in these reviews overwhelmingly supports a significant benefit from exercise in reducing cancer-related fatigue.17,18,21,24,25,30,32,37–43,49,52,66 Two reviews reported greater magnitude of effect from exercise during active cancer treatment than after.23,35 Yoga interventions demonstrated mixed results regarding impact on CRF.47,48,66
Most notably a recent, large, high-quality systematic review by Mustian and colleagues identified exercise as the most impactful intervention to reduce CRF when compared to pharmaceutical intervention or psychological intervention alone.39 The elements of effective exercise programs identified by Mustian et al. averaged 14 weeks in duration with 60-minute average session length and included aerobic, resistive, and mixed aerobic and resistive forms of exercise.
Physical Fitness
Exercise interventions demonstrated a strong positive impact on physical fitness measures including: VO2peak18,23,24,35,38, aerobic exercise tolerance31,34,65, peak power,24,38 strength,17,24,26,30,33,37,65, flexibility33, and various measures of cardiorespiratory fitness.17,20,26,33,38,58 These trials included aerobic, resistance, and mixed forms of exercise interventions with the majority of positive outcomes and larger effects associated with moderate to vigorous exercise intensity.
Psychological Function
Reviews demonstrated variable impact of exercise on psychological functioning and ranged from positive benefits17,24,33,48,49,66 to inconclusive15,40,42 results regarding mood, depression and anxiety. Rationale for the disparity in findings among was attributed to the use of varied and disparate measurement tools in the trials of interest.15,66 Cognitive improvements with exercise were also reported to be absent or only moderate in effect, though the volume of studies that included cognitive outcomes were more limited in our sample.41,47
Physical Function
Measures of physical function were positively impacted by exercise interventions.18,23,32,37,40,42,57,58 The outcome measures varied widely among studies and included positive gains in measures of endurance and general physical function.23,24,26,27,30,34,40,65 Therapeutic exercises, targeting restoration of function in specific body regions, including flexibility and progressive resistive exercises for the upper extremity53,55,56 and pelvic floor strengthening were beneficial.54
Body composition
Reviews regarding the impact of exercise on various measures of body composition varied in the type of measures used as well as the body tissue compartment measured (e.g., fat, lean mass, bone mass etc.). Body mass index (BMI) and body weight ranged from positive impact from exercise,17,38 to mixed results from exercise24,28,30 to null findings18,26,35,36. Weight gain was not found to impact mortality in one cohort study62; however, subset analysis by one review suggested that individuals with higher BMI experienced less benefit from exercise interventions in both physical and psychosocial measures.17 Disparity was reported in the use of measures of adiposity as well as in measurement methodology which may account for the variation in findings. Exercise positively impacted lean mass33,37 and weak evidence supported benefits of exercise on bone mineral density.19
QOL and HRQOL
QOL and HRQOL were defined and delineated by the specific research report reviewed and based on the measurement tools used in the trial. Generally, the impact of exercise on both QOL and HRQOL measures was positive15–17,24,30,32,35,37,38,40,48,50,54,57,66 or showed an overall positive trend towards significance although effect size was relatively small in many of the QOL outcomes.23,49,52,58 A small number of reviews failed to demonstrate significant impact on QOL.20,27,34,46,65
One report identified that higher BMI was associated with lower reported QOL.16 One review reported the effect of supervised exercise on HRQOL to be twice as high compared to unsupervised exercise.36
Biomarkers associated with cancer progression
Exercise positively impacted biomarkers, specifically immune and inflammatory markers, both during and after cancer treatment. Significant improvements in biomarker profiles were noted with exercise interventions including: improved IGF-I17,22,24,50 and IGF-II24,28,33, increased CD-4 cells33, improved immune function18,22, and decreased inflammatory markers.20,47,61 No effect was noted on prostate specific antigen nor on testosterone in prostate cancer survivors.44 Reviews on the effects of exercise on circulating insulin levels were mixed, with some reporting an exercise-lowering effect26,50 and others reporting mixed results or no response on insulin and insulin-like markers.18,28,51 Exercise interventions that supported positive outcomes favored moderate or vigorous exercise versus low intensity or non-exercising controls.24,26
Observational studies
Several systematic reviews aggregated information from longitudinal, observational studies.59,62,64 These studies relied on patient self-reported levels of physical activity during and after treatment. Although no specific exercise interventions are articulated through these reports, the results provide consistent epidemiological evidence of the positive association between reported levels of physical activity and meaningful endpoints such as overall mortality59,62,64, disease-specific mortality59, and quality of life62 in individuals with breast, prostate, or colorectal cancers. Additionally, one study demonstrated incremental improvements in mortality with increasing intensity of self-reported physical activity.59
Limitations
A significant limitation of this report is the inability to pool results and calculate effects across systematic reviews regarding specific exercise interventions and exercise parameters. Within individual systematic reviews, heterogeneity was often reported as significant which challenged valid calculation of effects. Based on the AMSTAR© scores of the selected studies, there was also significant concern about the lack of data extraction and disparity in reported outcomes. To maintain the integrity of the findings, the authors decided against pooled effects and instead provide descriptive findings. Additionally, there is significant variability in the type of studies analyzed in the included systematic reviews. Many reviews included uncontrolled, observational, or case series design studies. Those that identified as controlled studies often poorly identified the control group intervention. These methodological shortcomings have the potential to introduce significant bias into these findings.
This review is limited in that it focused only on movement-based forms of exercise. As a result, some therapeutic exercise interventions that restore and support vital aspects of function, such as swallowing and daily task retraining, were not included. Additionally, behavioral interventions designed to increase exercise participation and attitude towards physical activity were not included. While behavioral interventions are an important supportive element of exercise prescription, the inclusion of these strategies was beyond the scope of the stated goals of this manuscript.
Additionally, these systematic reviews largely assess findings from controlled trials that are likely to have exclusion criteria that aim to optimize safety. In the relatively controlled setting of a study protocol fewer safety issues and adverse events would be anticipated compared to an uncontrolled clinical setting. Therefore, safety considerations should not be overlooked in exercise the prescription and ongoing vigilance for safety during training is necessary.
Discussion and Clinical Considerations
This systematic review of existing cancer-related exercise-specific systematic reviews is the first of its kind to aggregate outcomes associated with movement-based exercise across cancer types, cancer treatment time course, and cancer-related impairments. The overall quality of the included systematic reviews was moderate, limiting the ability to draw decisive conclusions about specific elements of exercise prescription. The evidence presented in this review strongly supports a multitude of physical and functional benefits from exercise at any time point before, during, or after cancer treatment with consideration for the cancer type, presenting or anticipated side effects of treatment, and the presence of physical impairments. Moderate to vigorous intensity exercise is safe and appears to provide greater benefit than lower intensity exercise. However, low intensity exercise benefits deconditioned individuals and promotes a dose response that positively impacts physical function and fitness.
The impact of exercise interventions was better when the program was supervised versus unsupervised. This may be attributed to greater individualized attention from the health care provider. The actual dose of exercise may be greater in supervised settings where effort and volume are better controlled, thereby enabling greater impact of exercise effects.
These findings are a useful beginning to guide health care providers in exercise prescription and planning. Any health care provider interfacing with individuals before, during, and after cancer treatment should encourage exercise as a part of the cancer care plan and should work to incorporate specific recommendations for exercise. It is important to recognize however, that these findings were elucidated through controlled trials that possibly excluded participants deemed unsafe based on exclusion criteria and therefore these results should be interpreted with appropriate caution in a clinical setting where exercise capacity among patients could vary widely. The individual with cancer does require different attention to their exercise recommendations and a plan should be developed in the context of their known and anticipated risk for disease treatment-related side effects. This is ideally guided by a health care provider who is an exercise specialist such as a physical therapist, occupational therapist, exercise physiologist, or other medical rehabilitation professional with robust knowledge of cancer and its treatment. It is of critical importance that providers understand the limitations of exercise training to alleviate more complex, underlying neuromusculoskeletal conditions as well as recognize that exercise prescription, when incorrectly applied, may magnify such conditions. Rehabilitative, i.e. therapeutic, interventions may be more appropriate to manage these underlying conditions and discipline-specific triage is warranted. Exercise and rehabilitation disciplines should work collaboratively to ensure that safe and effective exercise training is implemented. The exercise prescription should ultimately seek to optimize an individuals’ ability to independently perform their exercise program while affording them a supportive care interface if complications or physical impairments limit their ability to complete the exercise program or disrupt their ability to function. For example, a physical therapist could prescribe therapeutic exercises to address balance impairments associated with chemotherapy-induced neuropathy to better prepare the individual to safely work with an exercise physiologist to engage in a moderate to vigorous intensity exercise conditioning program to restore aerobic fitness.
This review supports exercise prescription for the cancer population should follow the principles of exercise training as they are applied to other impaired or chronic disease populations. Consideration for specificity of exercise based on the individual’s initial fitness and functional levels, treatment-related side effects and personal health goals should guide recommended exercise interventions.5 Initial values and baseline measures should be obtained and repeated over time to gauge meaningful change and assure the effectiveness of the program. Exercise precautions / contraindications, and safety monitoring should be readily observed specific to cancer treatment-related side effects.4
Our findings demonstrate that the elements of exercise prescription should be relatively controlled and guided by a health care provider to optimize benefit and overall safety. This review, however, demonstrates that positive outcomes can be achieved using widely variable exercise frequency, intensity, duration and mode, suggesting that recommendations can be flexible while still enabling overall benefits. The significant heterogeneity in exercise interventions exposed by these systematic reviews is both a shortcoming and an opportunity for providers seeking optimal exercise prescription guidance and enables providers to use broad license in recommending exercise. This is also beneficial for individuals as it enables them to engage in activities that may be meaningful and enjoyable to them, rather than constraining them to highly specified parameters.
This review demonstrates a significant challenge regarding clinical measurement. One primary barrier identified by many systematic reviews was the significant variability in the clinical measurement tools, both objective and patient-reported, across trials and studies. The differences between trials prevent strong statistical analysis to support definitive recommendations. Specifically, robust analysis is impeded by: disparity in domain-specific outcomes measurement tools, disparate methods of quantifying exercise dose, lack of reporting of specific elements of the exercise prescription including frequency, duration, and intensity, and dissimilarity in terminology used to quantify and qualify exercise interventions. Outcome reporting could be markedly improved if the exercise and medical rehabilitation communities collaborated towards a common lexicon to define the various modalities and interventions that comprise this body of knowledge. Exercise, physical activity, therapeutic exercise, aerobic conditioning, physical fitness, physical functioning and other terminology are often used interchangeably and without clear delineation among them. Standardized reporting of intervention parameters in exercise trials would also be helpful to researchers and would enable aggregation of findings across trials. Peer-review journals and entities such as the Cochrane Rehabilitation group† could encourage standardized exercise reporting to include the specific intervention protocol as well as basic parameters such as frequency, intensity, and duration. This would enable significant contribution to the evidence base to guide intervention recommendations in the future.
Few studies, outside of population-based cohort studies, reported long-term follow up regarding survival and disease-specific endpoints. Controlled intervention trials rarely reported the long-term impact of exercise and often failed to investigate any carry over of the positive outcomes achieved through the intervention. While it is widely accepted that exercise is positive under controlled circumstances, uncertainty persists regarding adherence to exercise and long term impact. Future research should seek to better understand the long-term impact of exercise on endpoints such as time to disease recurrence, duration of overall survival, and overall mortality rates.
While not illustrated in the included systematic reviews, there is a body of evidence that speaks to the importance of exercise preferences and individual personality and attitudinal preferences of significant factors impacting the effectiveness of exercise interventions.69 These preferences should be considered along with other factors that are known to underlie adherence and intention. Although behavioral modification studies were excluded from our review, strong support for the theory of planned behavior is evident in the cancer literature as a driver of motivation towards exercise.70 Behavioral models such as this should be considered by health care providers who are prescribing exercise and encouraging exercise behavior carry over in the cancer population. One review did identify a significant increase in participants willingness to seek physical activity counseling as a result of their participation in the exercise intervention study.37 This suggests that exercise interventions present an opportunity to initiate behavior change. Critical questions for future research should include long term adherence to exercise and carry over of physiological and psychological changes following a trial of supervised exercise as well as the impact an exercise trial has on patient attitudes and self-activation towards health behavior changes. This will require the use of research paradigms that follow a more protracted timeline and will encourage longitudinal studies to closely examine and document exercise interventions, in addition to patient self-report, to track survival endpoints.
The findings reported in this review suggest that timing of an exercise intervention may impact the overall benefit for some populations. The prehabilitation model of care shows promise in promoting a proactive approach to introducing exercise and rehabilitation into the cancer care plan from the point of diagnosis. Important findings regarding improved recovery and tolerance to treatment are reported and suggest not only functional improvements but economic benefits. Accelerated functional recovery as well as potential cost mitigation from prehabilitation should be further explored.
Very little description was provided in any review regarding screening to identify indications for conditioning exercise or therapeutic exercise during or after cancer treatment. Screening for deconditioning and early identification of emerging impairments such as cancer-related fatigue, neuropathy, lymphedema, or depression may expedite triage for early therapeutic exercise intervention to mitigate functional decline.71,72 While the body of evidence continues to grow in support of therapeutic exercise and rehabilitative exercise programs for individuals with cancer treatment-related impairments, trials are generally small and poorly controlled making it difficult to provide guidance regarding optimal timing for functional impairment screening and management. The prehabilitation and prospective surveillance models of care should be studied in future research to identify optimal indications and timing for screening as well as triage models that enable application of exercise interventions at the right time and of appropriate intensity.
This review also highlights the importance of timing of exercise and its impact on physiological markers like insulin-like growth factor (IGF) I and II, and other immune protective biomarkers. The current evidence base regarding exercise, in general, supports improvements in critical biomarkers and inflammatory profiles. Reduction of inflammatory markers has significant metabolic and immune protective implications for an individual recovering from cancer treatments. IGF overexpression is linked to breast, prostate, and lung cancers. A recent meta-analysis found that exercise reduced serum levels of IGF I and II in the breast cancer population.73 Further, although exercise has been found to increase local levels of IGF-I after aerobic exercise, which may be helpful to rebuild skeletal muscle, exercise does not impact circulating IGF serum levels nor receptor overexpression.74 This is an important consideration unique to the cancer population which requires further research to investigate optimal timing and dose of exercise to maximize the positive effect on biomarkers of relevance.
Overall the impact of exercise is positive and significantly improves a wide range of functional, psychological, and physiological markers in individuals before, during, and after cancer treatment. The synthesis of these findings enables high level recommendations in the areas of functional and fitness assessment, exercise prescription, and therapeutic exercise throughout the time course of the cancer treatment continuum. Currently no interdisciplinary guidelines exist to provide insight to optimal timing, intensity, duration and frequency of exercise and therapeutic exercise screening and intervention. While the American Cancer Society (ACS) provides general guidelines for physical activity and nutrition for the cancer survivor75 and the American College of Sports Medicine (ACSM) has published general guidelines for exercise in the cancer population1, these efforts fall short of providing specific context for timing of exercise interventions and the necessary screening for side effects, toxicities, and functional impairments that define the specialized needs of the cancer population. Greater attention is needed to promote exercise prescription and future work should focus on developing exercise guidelines that support recommendations at various time points in the cancer care continuum with consideration for the presenting and anticipated treatment side effects, and with regard for the individual’s health status. A suggested framework for exercise prescription is outlined in Table 4 and could serve as the basic construct for future work in guideline development.
Table 4.
A Framework for Cancer Exercise Guidelines
| At Cancer Diagnosis Pre-Treatment/Prehabilitation | During Cancer Treatment | After Cancer Treatment |
|---|---|---|
ALL Patients
|
ALL Patients
|
ALL Patients
|
Prehabilitation Exerciseβ
|
Exercise to Maintain or Improve Enduranceβ
|
Exercise to Maintain or Improve Enduranceβ
|
Therapeutic Exercise
|
Therapeutic Exercise
|
Therapeutic Exercise
|
As recommended by the American College of Sports Medicine’s Exercise Guidelines for Cancer Survivors1all exercise intervention should be preceded by clinical assessment to identify safety concerns, precautions and contraindications.
An interdisciplinary effort to create a set of exercise guidelines would be an important step forward in integrating exercise into the continuum of oncology care and acknowledging the nuances of prescribing exercise for individuals versus sample study populations. Wide stakeholder input would be required for the success of such an effort and should be sought from a variety of disciplines including: oncologic, psychological, psychosocial, exercise, rehabilitation, nutrition, and other supportive services.
Conclusion
The growing population of cancer survivors warrants an urgent need to define clinical interventions that optimize function and survival. Symptom management over the course of cancer treatment and through the lifespan of survivorship is noted to be one of the most significant challenges faced by both patients and health care providers. Based on this review, exercise interventions have a strong evidence base to support their ubiquitous inclusion in every individual’s cancer care plan. The exercise plan of care is ideally designed in the context of known disease treatments and anticipated side effects of treatment and is overseen by a health care provider with specialized knowledge and skills in cancer-specific exercise and cancer rehabilitation. Despite a robust and growing body of evidence to support myriad exercise interventions across various cancer disease states and cancer treatment-related impairments, the supporting infrastructure for exercise planning and implementation for the cancer population is essentially absent. Efforts to strengthen uniformity in clinical trial reporting, develop clinical practice guidelines, and integrate exercise and rehabilitation services into the cancer delivery system are needed.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Leighton Chan MD, Director of the Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, Maryland for his support of this project.
Footnotes
Disclaimer:
The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and science in sports and exercise. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 2.Midtgaard J, Hammer NM, Andersen C, Larsen A, Bruun D-M, Jarden M. Cancer survivors’ experience of exercise-based cancer rehabilitation - a meta-synthesis of qualitative research. Acta Oncologica. 2015;54(5):609–617. doi: 10.3109/0284186X.2014.995777. [DOI] [PubMed] [Google Scholar]
- 3.Silver JK, Gilchrist LS. Cancer rehabilitation with a focus on evidence-based outpatient physical and occupational therapy interventions. American journal of physical medicine & rehabilitation. 2011;90(5 Suppl 1):S5–15. doi: 10.1097/PHM.0b013e31820be4ae. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–177. doi: 10.1016/j.suponc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demark-Wahnefried W, Rogers LQ, Alfano CM, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. Ca-a Cancer Journal for Clinicians. 2015;65(3):167–189. doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
- 6.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and Physical Activity Guidelines for Cancer Survivors. Ca-a Cancer Journal for Clinicians. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 7.Moore SC, Lee IM, Weiderpass E, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016;176(6):816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA: a cancer journal for clinicians. 2013;63(5):295–317. doi: 10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 9.Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer Rehabilitation. Physical Medicine and Rehabilitation Clinics. 2017;28(1):1–17. doi: 10.1016/j.pmr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 10.MacAuley D, Bauman A, Frémont P. Exercise: not a miracle cure, just good medicine. British journal of sports medicine. 2016;50(18):1107–1108. doi: 10.1136/bmj.h1416. [DOI] [PubMed] [Google Scholar]
- 11.Smaradottir A, Smith AL, Borgert AJ, Oettel KR. Are We on the Same Page? Patient and Provider Perceptions About Exercise in Cancer Care: A Focus Group Study. J Natl Compr Canc Netw. 2017;15(5):588–594. doi: 10.6004/jnccn.2017.0061. [DOI] [PubMed] [Google Scholar]
- 12.Stout NL, Silver JK, Raj VS, et al. Toward a National Initiative in Cancer Rehabilitation: Recommendations From a Subject Matter Expert Group. Arch Phys Med Rehabil. 2016;97(11):2006–2015. doi: 10.1016/j.apmr.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Chipperfield K, Brooker J, Fletcher J, Burney S. The impact of physical activity on psychosocial outcomes in men receiving androgen deprivation therapy for prostate cancer: A systematic review. Health Psychology. 2014;33(11):1288–1297. doi: 10.1037/hea0000006. [DOI] [PubMed] [Google Scholar]
- 16.Babatunde OA, Adams SA, Orekoya O, Basen-Engquist K, Steck SE. Effect of Physical Activity on Quality of Life as Perceived by Endometrial Cancer Survivors: A Systematic Review. International Journal of Gynecological Cancer. 2016;26(9):1727–1740. doi: 10.1097/IGC.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 17.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 18.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36(2):185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. Journal of cancer survivorship : research and practice. 2010;4(3):187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 20.Cramer H, Lauche R, Klose P, Dobos G, Langhorst J. A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. European journal of cancer care. 2014;23(1):3–14. doi: 10.1111/ecc.12093. [DOI] [PubMed] [Google Scholar]
- 21.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database of Systematic Reviews. 2012;(11) doi: 10.1002/14651858.CD006145.pub3. N.PAG-N.PAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel JL, Bress K, Popichak L, et al. A Systematic Review of Randomized Controlled Trials Testing the Efficacy of Psychosocial Interventions for Gastrointestinal Cancers. Journal of gastrointestinal cancer. 2014 doi: 10.1007/s12029-014-9605-z. [DOI] [PubMed] [Google Scholar]
- 23.Van Dijck S, Nelissen P, Verbelen H, Tjalma W, Gebruers N. The effects of physical self-management on quality of life in breast cancer patients: A systematic review. Breast (Edinburgh, Scotland) 2016;28:20–28. doi: 10.1016/j.breast.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ (Clinical research ed) 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of Supervised Multimodal Exercise Interventions on Cancer-Related Fatigue: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2015;2015:328636. doi: 10.1155/2015/328636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Backer IC, Schep G, Backx FJ, Vreugdenhil G, Kuipers H. Resistance training in cancer survivors: A systematic review. International journal of sports medicine. 2009;30(10):703–712. doi: 10.1055/s-0029-1225330. [DOI] [PubMed] [Google Scholar]
- 27.Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with Non-small cell lung cancer: A systematic review. Lung cancer (Amsterdam, Netherlands) 2011;72(2):139–153. doi: 10.1016/j.lungcan.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Guinan EM, Connolly EM, Hussey J. Exercise training in breast cancer survivors: a review of trials examining anthropometric and obesity-related biomarkers of breast cancer risk. Physical Therapy Reviews. 2013;18(2):79–89. [Google Scholar]
- 29.Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: a systematic review of the contemporary literature. J Cancer Surviv. 2011;5(4):320–336. doi: 10.1007/s11764-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 30.Keogh JW, Macleod RD. Body Composition, Physical Fitness, Functional Performance, Quality of Life, and Fatigue Benefits of Exercise for Prostate Cancer Patients: A Systematic Review. J Pain Symptom Manage. 2011 doi: 10.1016/j.jpainsymman.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Bourke L, Homer KE, Thaha MA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database of Systematic Reviews. 2013;(9) doi: 10.1002/14651858.CD010192.pub2. N.PAG-N.PAG. [DOI] [PubMed] [Google Scholar]
- 32.Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8:CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheema B, Gaul CA, Lane K, Fiatarone Singh MA. Progressive resistance training in breast cancer: a systematic review of clinical trials. Breast cancer research and treatment. 2008;109(1):9–26. doi: 10.1007/s10549-007-9638-0. [DOI] [PubMed] [Google Scholar]
- 34.Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23(3):486–497. doi: 10.1093/icvts/ivw152. [DOI] [PubMed] [Google Scholar]
- 35.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Capozzi LC, Nishimura KC, McNeely ML, Lau H, Nicole Culos-Reed S. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: A systematic review. British journal of sports medicine. 2016;50(6):325–338. doi: 10.1136/bjsports-2015-094684. [DOI] [PubMed] [Google Scholar]
- 38.D’Souza V, Daudt H, Kazanjian A. Survivorship care plans for people with colorectal cancer: Do they reflect the research evidence? Current Oncology. 2016;23(5):e488–e498. doi: 10.3747/co.23.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mustian KM, Alfano CM, Heckler C, et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergenthal N, Will A, Streckmann F, et al. Aerobic physical exercise for adult patients with haematological malignancies. The Cochrane database of systematic reviews. 2014;(11):Cd009075. doi: 10.1002/14651858.CD009075.pub2. [DOI] [PubMed] [Google Scholar]
- 41.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–111. doi: 10.1016/j.maturitas.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Egan MY, McEwen S, Sikora L, Chasen M, Fitch M, Eldred S. Rehabilitation following cancer treatment. Disabil Rehabil. 2013 doi: 10.3109/09638288.2013.774441. [DOI] [PubMed] [Google Scholar]
- 43.Larkin D, Lopez V, Aromataris E. Managing cancer-related fatigue in men with prostate cancer: a systematic review of non-pharmacological interventions. Int J Nurs Pract. 2014;20(5):549–560. doi: 10.1111/ijn.12211. [DOI] [PubMed] [Google Scholar]
- 44.Hackshaw-McGeagh LE, Perry RE, Leach VA, et al. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control. 2015;26(11):1521–1550. doi: 10.1007/s10552-015-0659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: A systematic review and meta-analysis. BMC cancer. 2012:12. doi: 10.1186/1471-2407-12-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shneerson C, Taskila T, Gale N, Greenfield S, Chen YF. The effect of complementary and alternative medicine on the quality of life of cancer survivors: A systematic review and meta-analyses. Complementary therapies in medicine. 2013;21(4):417–429. doi: 10.1016/j.ctim.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Zimmer P, Baumann FT, Oberste M, et al. Effects of Exercise Interventions and Physical Activity Behavior on Cancer Related Cognitive Impairments: A Systematic Review. Biomed Res Int. 2016;2016:1820954. doi: 10.1155/2016/1820954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan Y, Yang K, Wang Y, Zhang L, Liang H. Could yoga practice improve treatment-related side effects and quality of life for women with breast cancer? A systematic review and meta-analysis. Asia-Pacific journal of clinical oncology. 2015 doi: 10.1111/ajco.12329. [DOI] [PubMed] [Google Scholar]
- 49.Harder H, Parlour L, Jenkins V. Randomised controlled trials of yoga interventions for women with breast cancer: A systematic literature review. Supportive Care in Cancer. 2012;20(12):3055–3064. doi: 10.1007/s00520-012-1611-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhu G, Zhang X, Wang Y, Xiong H, Zhao Y, Sun F. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails. Onco Targets Ther. 2016;9:2153–2168. doi: 10.2147/OTT.S97864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Löf M, Bergström K, Weiderpass E. Physical activity and biomarkers in breast cancer survivors: A systematic review. Maturitas. 2012;73(2):134–142. doi: 10.1016/j.maturitas.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Bradt J, Potvin N, Kesslick A, et al. The impact of music therapy versus music medicine on psychological outcomes and pain in cancer patients: a mixed methods study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(5):1261–1271. doi: 10.1007/s00520-014-2478-7. [DOI] [PubMed] [Google Scholar]
- 53.Carvalho AP, Vital FM, Soares BG. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database of Systematic Reviews. 2012;(4) doi: 10.1002/14651858.CD008693.pub2. N.PAG-N.PAG. [DOI] [PubMed] [Google Scholar]
- 54.Visser WS, Te Riele WW, Boerma D, Van Ramshorst B, Van Westreenen HL. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: A systematic review. Annals of coloproctology. 2014;30(3):109–114. doi: 10.3393/ac.2014.30.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheifetz O, Haley L. Management of secondary lymphedema related to breast cancer. Can Fam Physician. 2010;56(12):1277–1284. [PMC free article] [PubMed] [Google Scholar]
- 56.McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. The Cochrane database of systematic reviews. 2010;(6):Cd005211. doi: 10.1002/14651858.CD005211.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Scott DA, Mills M, Black A, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database of Systematic Reviews. 2013;(3) doi: 10.1002/14651858.CD007730.pub2. N.PAG-N.PAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh F, Newton RU, Galvao DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. Surg Oncol. 2013;22(2):92–104. doi: 10.1016/j.suronc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Annals of Oncology. 2014;25(7):1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 60.Fu MR, Deng J, Armer JM. Putting Evidence Into Practice: Cancer-Related Lymphedema. Clinical journal of oncology nursing. 2014;18:68–79. doi: 10.1188/14.CJON.S3.68-79. [DOI] [PubMed] [Google Scholar]
- 61.Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, et al. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review with Meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(7):1009–1017. doi: 10.1158/1055-9965.EPI-15-1061. [DOI] [PubMed] [Google Scholar]
- 62.Otto SJ, Korfage IJ, Polinder S, et al. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Supportive Care in Cancer. 2015;23(5):1237–1250. doi: 10.1007/s00520-014-2480-0. [DOI] [PubMed] [Google Scholar]
- 63.Fontein DBY, de Glas NA, Duijm M, et al. Age and the effect of physical activity on breast cancer survival: A systematic review. Cancer treatment reviews. 2013;39(8):958–965. doi: 10.1016/j.ctrv.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: A review of the literature. British journal of cancer. 2011;105(SUPPL 1):S52–S73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smits A, Lopes A, Das N, Bekkers R, Massuger L, Galaal K. The effect of lifestyle interventions on the quality of life of gynaecological cancer survivors A systematic review and meta-analysis. Gynecologic oncology. 2015;139(3):546–552. doi: 10.1016/j.ygyno.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database of Systematic Reviews. 2017;2017(1) doi: 10.1002/14651858.CD010802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao Y-FC, Chen S-Y, Lan C, Lai J-S. The cardiorespiratory response and energy expenditure of Tai-Chi-Qui-Gong. The American journal of Chinese medicine. 2002;30(04):451–461. doi: 10.1142/S0192415X02000636. [DOI] [PubMed] [Google Scholar]
- 68.Larson-Meyer DE. A Systematic Review of the Energy Cost and Metabolic Intensity of Yoga. Medicine and science in sports and exercise. 2016;48(8):1558–1569. doi: 10.1249/MSS.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 69.Rhodes RE, Courneya KS, Jones LW. The theory of planned behavior and lower-order personality traits: Interaction effects in the exercise domain. Personality and Individual Differences. 2005;38(2):251–265. [Google Scholar]
- 70.Jones LW, Guill B, Keir ST, et al. Using the theory of planned behavior to understand the determinants of exercise intention in patients diagnosed with primary brain cancer. Psycho-Oncology. 2007;16(3):232–240. doi: 10.1002/pon.1077. [DOI] [PubMed] [Google Scholar]
- 71.Jones LW, Eves ND, Peppercorn J. Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol. 2010;11(10):914–916. doi: 10.1016/S1470-2045(10)70184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stout NL, Binkley JM, Schmitz KH, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl):2191–2200. doi: 10.1002/cncr.27476. [DOI] [PubMed] [Google Scholar]
- 73.Meneses-Echavez JF, Jimenez EG, Rio-Valle JS, Correa-Bautista JE, Izquierdo M, Ramirez-Velez R. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2016;16(1):682. doi: 10.1186/s12885-016-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hambrecht R, Schulze PC, Gielen S, et al. Effects of exercise training on insulin-like growth factor-I expression in the skeletal muscle of non-cachectic patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2005;12(4):401–406. doi: 10.1097/01.hjr.0000173106.68485.b7. [DOI] [PubMed] [Google Scholar]
- 75.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA: a cancer journal for clinicians. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.