Abstract
Alcohol use disorder (AUD) is highly comorbid with chronic pain (CP). Evidence has suggested that neuroadaptive processes characterized by reward deficit and stress surfeit are involved in the development of AUD and pain chronification. Neurological data suggest that shared genetic architecture associated with the reward and stress systems may contribute to the comorbidity of AUD and CP. This monograph first delineates the prevailing theories of the development of AUD and pain chronification focusing on the reward and stress systems. It then provides a brief summary of relevant neurological findings followed by an evaluation of evidence documented by molecular genetic studies. Candidate gene association studies have provided some initial support for the genetic overlap between AUD and CP, however these results must be interpreted with caution until studies with sufficient statistical power are conducted and replications obtained. Genome-wide association studies have suggested a number of genes (e.g., TBX19, HTR7, and ADRA1A) that are either directly or indirectly related to the reward and stress systems in the AUD and CP literature. Evidence reviewed in this monograph suggests that shared genetic liability underlying the comorbidity between AUD and CP, if present, is likely to be complex. As the advancement in molecular genetic methods continues, future studies may show broader central nervous system involvement in AUD-CP comorbidity.
Keywords: alcohol use disorder, chronic pain, comorbidity, candidate association gene studies, genome-wide association studies
1. Introduction
Approximately 15.1 million American adults suffer from alcohol use disorder (AUD) (NIAAA, 2016) and 100 million suffer from chronic pain (CP) (IMNA, 2011). Importantly, the co-occurrence of AUD and CP is common though their relation is complex. For example, one epidemiological study reported that 27% of patients with CP used alcohol as a pain-reliever at least “sometimes” (Riley and King, 2009), whereas a prospective study conducted with NESARC data suggested that drinking cessation significantly predicted reduction in bodily pain (Imtiaz et al., 2017). Given this complexity, increasing attention has been paid to the study of AUD-CP comorbidity in recent years due to their considerable public health significance.
Both AUD and CP are multidimensional and heterogeneous conditions involving multiple biopsychosocial systems. A concatenation of genetic, neurophysiological, and behavioral studies has provided evidence supporting the roles of reward and stress systems in understanding AUD and CP. Unfortunately, research on each condition has proceeded independently with little consideration given to their overlap. In a seminal work, Egli, Koob, and Edwards (2012) attempted to bring the two fields together by delineating the overlapping neural substrates implicated in the reward and stress systems and their relevance to both alcohol dependence and pain processing. Moreover, they highlighted the importance of using molecular genetic approaches to further our understanding of the neurophysiological dysregulation of reward and stress systems common to AUD and CP (Egli et al., 2012).
This monograph summarizes the research on the molecular genetic predispositions associated with the dysregulation of the reward and stress systems associated with both AUD and CP; and how shared features of this dysregulation might contribute to the development of AUD-CP comorbidity. We initially describe the prevailing theories regarding the development of AUD and CP. Next, we discuss the substantial overlap in the neurological underpinnings of AUD and CP from the reward deficit and stress surfeit perspective. Third, we review and aggregate results from genetic association studies that have focused on genes implicated in the neurophysiological mechanisms in the reward and stress systems contributing to both AUD and CP. Lastly, we propose directions for future research to elucidate the genetic influences underlying AUD-CP comorbidity.
2. Theories of Development of Alcohol Use Disorder (AUD)
The cycle of alcohol addiction comprises three stages: repeated intoxication, withdrawal, and craving (Koob and Volkow, 2010), and the cyclic repetition of these stages, escalating over time results in the development of AUD (Koob, 2016). As AUD develops, this cycle becomes characterized by three major pathological characteristics – compulsive alcohol seeking, uncontrollable alcohol consumption, and overwhelming negative affective when alcohol is inaccessible (Koob and Le Moal, 1997). Research suggests that the development of AUD can be explained, in part, by the opponent process theory of motivation, which is characterized by a motivational shift in drinking behaviors from positive to negative reinforcement (Koob, 2003).
According to the opponent process theory of motivation, systems within the central nervous system (CNS) function to maintain equilibrium among affective states. Thus, once a change in affective state is detected, the CNS automatically responds by activating opposing motivational sub-processes to maintain homeostasis (Solomon and Corbit, 1974). Koob and Le Moal (2008) have used this theory to describe the underlying processes involved with the development and perpetuation of addiction. In this model, acute consumption of a drug (e.g., alcohol) by nondependent users induces a rewarding state called the a-process. This rewarding state has a rapid onset and offset, and is thought to positively reinforce early drinking (Koob, 2016). Following the termination of the a-process, an opponent process, the b-process, induces a stressful, anti-rewarding state, thus counteracting the rewarding state induced by the a-process. Compared to the a-process, the b-process, has a slow onset and a sluggish decay. Among nondependent users, the intensities of the reward and stress elicited by the a- and b-processes are consistent with the amount of consumption, and this is sufficient to maintain affective homeostasis.
Repeated exposure to a drug (e.g., alcohol) eventually results in the dysregulation of the reward and stress systems seen among dependent users. Over time, the CNS becomes desensitized to the a-process and the rewarding state it once produced is now attenuated, thus increasing the reward threshold for the a-process and increasing tolerance for the drug (George et al., 2012). Simultaneously, the b-process becomes sensitized, protracted, and intensifies, while the stress threshold decreases, thus resulting in withdrawal (George et al., 2012). The changing of the thresholds, or set points, of the reward and stress systems, and their inability to achieve affective and physiological stability, is referred to as allostasis (Sterling and Eyer, 1988). In this model, the repeated exposure to the drug (e.g., alcohol) over time, forces the b-process to become dominant, and as a result, continued alcohol consumption is negatively reinforced through its temporary alleviation of negative affect (Koob, 2016). This reward and stress dysregulation, often referred to as a reward deficit and stress surfeit disorder (Koob, 2013), results in a state of allostatic load, and manifests as the pathological state of addiction (Koob et al., 2014).
3. Theories of Pain Chronification
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” by the International Association for the Study of Pain (IASP) (Merskey and Boduk, 1994). Aside from the sensory experience, this definition highlights the significance of the cognitive and affective dimensions associated with the subjective experience of pain by including the higher-order brain structures that receive peripheral pain information from the dorsal root ganglia and spinal cord and integrate this information with various pain-related processes (e.g., cognitive, affective, etc.) (Mitsi and Zachariou, 2016).
Chronic pain has been defined as continuous pain, which persists for at least three months (Merskey and Boduk, 1994). Despite their etiologic heterogeneity, CP conditions share some commonalities: (1) pharmacological options for chronic pain conditions and pain management have limited efficacy (e.g., McNicol et al., 2013), (2) the challenges in finding efficacious pharmacological treatments are partly attributable to the high comorbidities between CP conditions and psychopathologies, such as, depression, anxiety, anhedonia, and/or addiction (e.g., Borsook et al., 2016), and (3) clinical and pharmacological evidence collectively suggests the involvement of dysregulation in the reward and stress neural circuitries across many CP conditions (e.g., Apkarian et al., 2013).
To elaborate, the transition from an acute to chronic pain state, via self-sustaining and escalating pain-related processes is called pain chronification, and has been hypothesized to share some key features with the development of AUD. For example, Borsook and colleagues (2016) propose a model of pain chronification that they define as culminating in a state of 'reward deficiency' (RD) and 'anti-reward' (AR). More specifically, they propose that, under healthy conditions, activation of an aversion/stress neural circuit by acute pain is followed by activation of the reward system, particularly in response to the alleviation of pain. Prolonged, recurrent acute pain episodes lead to pain chronification in which pain-related cues increase in salience leading to hypersensitivity of the aversion/stress system and hyposensitivity of the reward system. As a consequence, previous levels of experienced pain are insufficient to activate endogenous pain relief pathways in the reward systems and stronger pharmacological pain relievers are required to activate the reward systems for pain alleviation. This leads to what the authors define as a 'feed-forward loop' that produces an escalating disruption of the homeostatic balance between reward and stress/aversion pathways (Borsook et al., 2013). Thus, while these opponent-process models of AUD and CP propose different patterns of progression towards an allostatic load state, the neural mechanisms underlying both models significantly overlap.
4. Dysregulation of Reward System in Development of AUD and Pain Chronification
Dopamine is released in the mesolimbic pathway – nucleus accumbens (NAc) and ventral tegmental area (VTA). The limbic system and medial prefrontal cortex encode and process the reward value of a stimulus through the dynamic interplay between phasic and tonic dopamine levels (Grace, 2000; Knutson and Cooper, 2005). Tonic dopamine levels are characterized by a slow and low-volume of release that is induced by glutamate and degraded by catechol-O-methyltransferase (COMT) (Mannisto and Kaakkola, 1999). The tonic level of dopamine is a proxy for the amount of reward one generally expects from any given stimuli (Evers et al., 2017). In contrast, phasic dopamine levels are characterized by an instant high-volume release that is quickly attenuated by D2-autoreceptors that feedback to inhibit further dopamine release and activate the dopamine reuptake transporter to remove excess dopamine from the extracellular space (Ford, 2014). Phasic levels of dopamine are determined by the difference between the received and expected value of a stimulus, and is the proxy for the net value of reward for any particular stimulus. This net value is then processed by the limbic system and medial prefrontal cortex (Knutson and Cooper, 2005), through which the incentive salience of the rewarding stimulus is summarized and attributed (Robinson and Berridge, 1993).
Notably, the balance between phasic and tonic dopamine levels has been related to the stimulating value of alcohol over time (Gilpin and Koob, 2008). More specifically, tonic levels of dopamine are influenced by the amount of dopamine accumulated in the extracellular space. Among nondependent users, acute alcohol consumption results in increased phasic dopamine release without impacting tonic levels (Weiss et al., 1993). Chronic alcohol exposure, however, results in decreased phasic levels and increased tonic levels due to constant saturation of D2-autoreceptors and an overloaded reuptake process (Ford, 2014). This process has been hypothesized to underlie tolerance observed among dependent users (Melis et al., 2005; Volkow et al., 2007).
In addition to AUD, the phasic-tonic dopamine model has also been implicated in pain chronification given recent studies demonstrating that dopamine is involved in the evaluative processing of aversive stimuli (Taylor et al., 2016). The recent CP literature suggests that a surfeit of glutamate and/or a deficit of COMT may result in excessive tonic dopamine levels that are sufficient to engage the D2-autoreceptors and reduce phasic release (Wood, 2006). Over time, lowering of the phasic/tonic ratio may lead to a reward deficit state that induces pain chronification and its comorbid symptoms relating to lack of interest in approaching naturally rewarding stimuli (Borsook et al., 2016).
Endorphins bind with mu-opioid receptors in the pituitary gland and hypothalamus and can produce a hedonic state through facilitation of dopamine release in the VTA and NAc during acute intoxication in animal studies (Oswald and Wand, 2004). Over time, they are involved in the down-regulation of the dopamine system and the development of alcohol tolerance. In the pain literature, acute pain onset has been associated with the release of endogenous opioids (Holden et al., 2005). However, in CP, data have suggested that the activity of endogenous analgesic systems involving mu-opioid receptors is attenuated (Miranda et al., 2015).
Glutamatergic (excitatory) and GABAergic (inhibitory) mechanisms within the reward system have also been studied. The immediate consequence of acute alcohol intoxication is a decrease in glutamate and increase in GABA activity resulting in a depression of the CNS (Carboni et al., 1993; Gilpin and Koob, 2008). However, chronic alcohol exposure produces a state of allostasis, and leads to the activation of compensatory processes to achieve homeostasis via an increase in glutamate receptor synthesis to induce hyperexcitability to glutamate (Pulvirenti and Diana, 2001). Together, these compensatory mechanisms lead to: (1) increased tonic presence of dopamine in the extracellular space, which decreases phasic dopamine release, and (2) establishment of context-dependent sensitization that aggravates alcohol cravings and makes sustained sobriety difficult (Tzschentke and Schmidt, 2003). During the transition from non-dependent to dependent alcohol use, compensatory processes also contribute to the desensitization of GABA (esp. GABAA) receptors that leads to alcohol dependence and abuse (Liang and Olsen, 2014). Glutamatergic activity is also increased and sensitized during pain chronification. Additionally, increases in GABA activity inhibit the intercalated cells (ITC) of the amygdala, which block pain signals from reaching the CNS (Neugebauer, 2015). These compensatory mechanisms increase the salience of aversive and noxious stimuli leading to eventual hyperalgesia (Borsook et al., 2016).
5. Dysregulation of Stress System in Development of AUD and Pain Chronification
Having discussed the pathways involved in the reward deficit component, we now discuss how excessive stress contributes to the development of AUD and CP.
Corticotropin-releasing factor receptor 1 (CRF1 receptor) activated in hypothalamus and amygdala has been associated with stress, anxiety, and hyperalgesia during alcohol withdrawal (Egli et al., 2012; Koob, 2013). Consuming additional alcohol negates these effects further encouraging AUD development via negative reinforcement. In pain chronification, CRF1 released in the amygdala has been related to enhanced nociception (Ji and Neugebauer, 2008), which contributes to the establishment and maintenance of hyperalgesia (Tracey and Dunckley, 2004).
Dynorphins that bind to Kappa-opioid receptors are widely represented in the CNS (Watson et al., 1982) and are released during stress (Knoll and Carlezon, 2010). Activation of Kappa-opioid receptors inhibits dopamine release (Margolis et al., 2003), and increases the expression of CRF (Veer et al., 2012), both of which contribute to the experience of dysphoria, anxiety, agitation, and depression (Knoll and Carlezon, 2010) that characterize alcohol withdrawal. In pain chronification, dynorphins also activate the release of bradykinin, which has been linked to inflammation and hyperalgesia, and thus contribute to the development and maintenance of CP (Lai et al., 2006; Wang et al., 2005).
Neuropeptide Y (NPY) is highly expressed in the ventral striatum and amygdala (de Quidt and Emson, 1986) and has been implicated in an anti-stress system that acts in counter to the effects of CRF1 by producing an anxiolytic effect (Heilig and Koob, 2007). Suppression of this anti-stress system, and of NPY specifically, is hypothesized to be involved in both AUD development and pain chronification. With respect to AUD, NPY is actively suppressed during alcohol withdrawal, which enhances the stress that characterizes the withdrawal state (Koob and Le Moal, 2001). In pain chronification, NPY is upregulated in the dorsal horn of the spinal cord following inflammation or injuries and inhibits pain signals (Brumovsky et al, 2004; Wakisaka et al., 1991). In animal models, knocking out NPY induces hyperalgesia (Solway et al., 2011). While we await evidences from human studies, it is reasonable to hypothesize that genetic variation in the NPY gene or any epigenetic changes associated with low NPY expression may play a role in pain chronification.
In sum, the described mechanisms associated with the reward and stress systems indicate that there are overlapping neural mechanisms associated with reward deficit and stress surfeit that contribute to the development and maintenance of AUD and CP. Below, we postulate that shared genetic predispositions may underlie some of this overlap in the neurobiology of AUD and CP.
6. Genetic Studies of AUD and CP
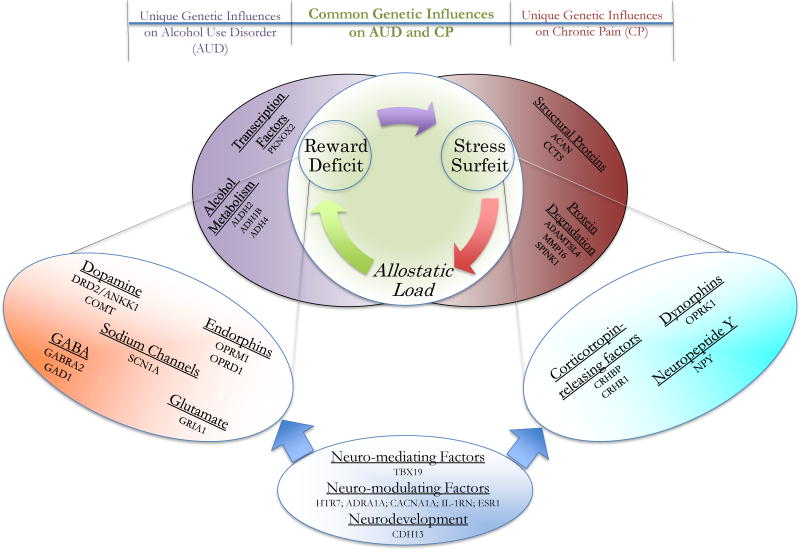
Both AUD and CP have a strong genetic component. Reviews of twin studies have reported that 43% - 53% and 25% - 50% of the variability in AUD and CP, respectively, are attributable to genetic factors (Nielsen et al., 2012; Verhulst et al., 2015). Quantitative genetic studies estimating the genetic correlation between AUD and CP have not yet been conducted. Nonetheless, it is reasonable to theorize that AUD and CP would share some genetic variants that contribute to their risk, based on the substantial heritability estimates reported for each condition and their substantial overlap at the neurobiological level. When discussing genetic contributions to AUD and CPCs, three classes of genetic involvement should be considered: genes that influence (1) both AUD and CPCs (e.g., COMT gene variation associated with the reward pathway, Stallings et al., 2016; Zorina-Lichtenwalter, et al., 2016), (2) AUD independent of CPCs (e.g., ALDH2 gene variation associated with alcohol metabolism, Stallings et al., 2016), and (3) CPCs independent of AUD (e.g., ACAN gene variation associated with damage of structural protein in pain, Zorina-Lichtenwalter et al., 2016). Figure 1 provides a conceptual overview of how genetic variation might influence AUD, CP, and their comorbidity. Though prototypical examples of each class are offered in the figure, oftentimes the boundaries across classes may be fuzzy due to the intricate effects of mediation and modulation among genes on phenotypes.
Figure 1. Unique and common genetic influences to AUD and CP.
Three classes of genetic involvement should be considered: possible genes that influence (1) both AUD and CP (genes in tangerine ellipse are associated with the reward pathways & genes in turquoise ellipse are associated with the stress pathways), (2) AUD independent of CP (in purple ellipse), and (3) CP independent of AUD (in red ellipse). Furthermore, there are genes that mediate or modulate the reward and stress systems, or broadly affect the central nervous system (in blue ellipse). The gene groups and corresponding genes in each group in this figure serve as examples and do not represent an exhaustive list. Among them, some have been supported by existing empirical findings, whilst others await further investigations.
Association designs have been widely employed to study phenotype-genotype relations in molecular genetic studies in recent decades. These studies typically rely on a case-control design to examine whether a specific allele is more likely to be found among “cases” than “controls” at the population level (Gizer et al., 2015). Currently, the two primary types of association studies are the candidate gene and genome-wide association studies. Through such studies, significant insights have been gained in understanding the genetic contributions to AUD and CP individually. Yet, to our knowledge, no studies have been published examining genetic variants that might contribute to their comorbidity. Thus, we review published findings of genetic variants associated with AUD and CP individually to shed light on the possibility of genetic overlap associated with neurotransmitter systems involved in reward and stress dysregulation.
6. A. Candidate gene association studies (CGAS)
Early endeavors in genetic association research were dominated by CGAS due to constraints in genotyping technologies prohibiting the interrogation of large sets of genetic variants (Gizer et al., 2015). CGAS rely on a theory-driven perspective using a priori knowledge regarding the function of a specific gene and its relation to the phenotype of interest to restrict the search to a single or small set of gene(s). A substantial number of CGAS have been conducted to identify genetic variants associated with AUD and CP (e.g., reviews by Buhler et al., 2015; Stallings et al., 2016; Zorina-Lichtenwalter et al., 2016). This monograph does not aim to provide an exhaustive list of findings from both literatures, but rather, to offer the most commonly studied examples highlighting the possible overlap in genetic influences underlying AUD and CP as they relate to the reward and stress systems (Table 1). It should be noted, however, that almost all published CGAS have been statistically underpowered, and there is evidence to suggest that a substantial proportion of findings from these studies represent false positives (Siontis et al., 2010).
Table 1.
Genes Implicated in Alcohol Use Disorder, Chronic Pain, or Both
| Gene | Study Citation(s) for Alcohol Use Disorder | Study Citation(s) for Chronic Pain |
|---|---|---|
| Dysregulation of Brain Reward System | ||
| Dopaminergic Mechanisms | ||
| ANKK1 (ankyrin repeat and kinase domain containing 1) | Gelernter et al., 1993; Goldman, 1993; Le Foll et al., 2009; Munafo et al., 2007; Smith et al., 2008 | Migraine: Del Zompo et al., 1998; Ghosh et al., 2013; Peroutka et al., 1998 |
| COMT (catechol-o-methyltransferase) | Enoch et al., 2006; Sery et al., 2006; Tiihonen et al., 1999; Voisey et al., 2011 | Fibromyalgia (FM): Barbosa et al., 2012; Cohen et al., 2009; Fernandez-de-las Penas et al., 2012; Finan et al., 2011; Martinez-Jauand et al., 2013; Matsuda et al., 2010; Vargas-Alarcon et al., 2007 // Low Back Pain (LBP): Jacobsen et al., 2012; Omair et al., 2012; Omair et al., 2015; Rut et al., 2014 // Migraine: Cargnin et al., 2013 // Post-Operative Pain (POP): Rut et al. (2014) // Temporomandibular Joint Disorders (TMD): Diatchenko et al., 2005; Erdal et al., 2003; Meloto et al., 2015; Michelotti et al., 2014; Smith et al., 2011 |
| DBH (dopamine beta-hydroxylase) | Cubells et al., 2004 | Migraine: Fernandez et al., 2006; Fernandez et al., 2009; Ghosh et al., 2013; Lea et al., 2000 |
| DRD1 (dopamine receptor 1) | Kim et al., 2007 | |
| DRD2 (dopamine beta-receptor 2) | Chen et al., 2011; Goldman et al., 1998; Meyers et al., 2013; Wiesbeck et al., 2006 | Migraine: Del Zompo et al., 1998; Ghosh et al., 2013; Peroutka et al., 1998 |
| DRD4 (dopamine beta-dopamine 4) | George et al., 1993; Le Foll et al., 2009; Paterson et al., 1999 | Migraine: de Sousa et al., 2007; Del Zompo et al., 1998; Mochi et al., 2003 // TMD: Aneiros-Guerrero et al., 2011 |
| MAOA (monoamine oxidase A) | Tikkanen et al., 2010 | FM: Gursoy et al., 2008 // TMD: Mutlu et al., 2005 |
| Opioidergic Mechanisms | ||
| OPRM1 (opioid receptor mu 1) | Arias et al., 2006; Bart et al., 2005; Schwantes-An et al., 2016 | LBP: Hasvik et al., 2014; Omair et al., 2015 // POP: Janicki et al., 2006; Kolesnikov et al., 2013; Olsen et al., 2012 |
| Glutamate Mechanisms | ||
| GAD1 (glutamate decarboxylase 1) | Loh et al., 2006; Tabakoff et al., 2009 | |
| GRIA1 (glutamate receptor ionotropic receptor AMPA type subunit 1) | Migraine: Cargnin et al., 2014; Formicola et al., 2010; Maher et al., 2013 | |
| GRIA3 (glutamate receptor ionotropic receptor AMPA type subunit 3) | Migraine: Formicola et al., 2010; Maher et al., 2013 | |
| GRIN2 (glutamate receptor ionotropic receptor NMDA type subunit 2) | Domart et al., 2012 | |
| LRP1 (low density lipoprotein receptor-related protein 1) | Migraine: Esserlind et al., 2015; Ghosh et al., 2013 | |
| MEF2D (myocyte enhancer factor 2D) | Migraine: Esserlind et al., 2015 | |
| Dysregulation of Brain Reward System | ||
| GABA Mechanisms | ||
| GABRA2 (gama-aminobutyric acid type A receptor subunit alpha-2) | Covault et al., 2004; Drgon et al., 2006; Edenberg et al., 2004; Lappalainen et al., 2005; Matthews et al., 2007; Soyka et al., 2008 | |
| GABRB3 (gama-aminobutyric acid type A receptor subunit beta-3) | FM: Smith et al., 2012 // Migraine: Netzer et al., 2008; Oswell et al., 2008; Russo et al., 2005 | |
| GABRG3 (gama-aminobutyric acid type A receptor subunit gamma-3) | Dick et al., 2004 | |
| SCN1A (sodium voltage-gated channel alpha subunit 1) | Migraine: Persico et al., 2015 | |
| Dysregulation of Brain Stress System | ||
| Corticotropin-Releasing Mechanisms | ||
| CRHBP (corticotropin releasing hormone binding protein) | FM: Holliday et al., 2010 | |
| CRHR1 (corticotropin releasing hormone receptor 1) | Treutlein et al., 2006 | |
| MC2R (melanocortin receptor type 2) | FM: Holliday et al., 2010 | |
| Opioidergic Mechanisms | ||
| OPRK1 (opioid receptor kappa 1) | Gerra et al., 2007; Xuei et al., 2006 | |
| Neuropeptide Y (Anit-Stress / Stress-Buffering) Mechanisms | ||
| NPY (neuropeptide Y) | Kovanen et al., 2010; Lappalainen et al., 2002 | |
Polymorphisms located in genes encoding for proteins involved in dopaminergic mechanisms have shown the strongest overlap with respect to AUD and CP. One of the most widely studied polymorphisms in relation to AUD and CP is the Taq1A polymorphism (rs1800497) located in the Ankyrin repeats and kinase domain containing 1 (ANKK1) gene. Although this polymorphism is a nonsynonymous polymorphism located in exon 8 of ANKK1, it was originally thought to be located in the dopamine D2 receptor gene (DRD2). Functional studies have suggested that this variant results in reduced dopamine synthesis via its impact on ANKK1 function (Mrazek, 2010), and has also been related to altered dopamine D2 receptor density in the striatum (Jonsson et al., 1999). CGAS have related the minor A1 allele (vs. A2 major allele) to risk for both AUD and migraine (e.g., Le Foll et al., 2009 and Ghosh et al., 2013, respectively). While robust support for the association with AUD was demonstrated in a recent meta-analysis (Wang et al., 2013), a similar meta-analysis has yet to be conducted for CP phenotypes. Additionally, the potential influence of the Taq1A polymorphism on the ANKK1 and DRD2 proteins has made it difficult to determine which gene is likely to exhibit a causal relation with each disorder. Nonetheless, a second variant in DRD2, an NcoI polymorphism (rs6275) located in the 7th exon of DRD2 has been associated with a reduction in dopamine-activated upregulation of D2 receptor expression (Duan et al., 2003). The minor T allele (vs. the C major allele) has been associated with increased risk for AUD (Meyers et al., 2013), but reduced risk for migraine (Peroutka et al., 1998). The opposite directions of effect for this variant on risk for AUD and migraine requires further study. It could be a reflection of meaningful differences in how this genetic variant contributes to the etiology of each condition, or it could be that the relation with one or both of these conditions represents a false positive.
The COMT gene, and the functional rs4680 polymorphism in exon 4 of this gene, has also received substantial attention with respect to AUD and CP. The minor A allele (vs. G major allele) of rs4680 causes an amino acid change from valine (Val) to methionine (Met) (Buhler et al., 2015), with the Val allele associated with higher rates of dopamine degradation. CGAS of AUD have yielded conflicting results, however, as some have related the Val allele to increased risk for AUD (e.g., Sery et al., 2006), while other studies have reported that the low degradation activity Met allele was associated with increased risk for AUD (e.g., Tiihonen et al., 1999). Association studies of rs4680 and CP have been somewhat more consistent. The low activity Met allele has been implicated in many CP conditions, such as low back pain (e.g., Jacobsen et al., 2012) and fibromyalgia (e.g., Finan et al., 2011), with recent meta-analyses suggesting robust associations with migraine (Chen et al., 2015) as well as fibromyalgia and chronic widespread pain (Tammimaki and Mannisto, 2012). Nonetheless, the conflicting pattern of results for AUD make it difficult to speculate how this variant and COMT might relate to a shared genetic component underlying AUD and CP.
In addition to dopaminergic genes, genes related to the endogenous opioid system have also been widely studied in the AUD and CP literatures. The most widely studied gene is the opioid receptor mu 1 (OPRM1) gene. The polymorphism, rs1799971 (A major vs. G minor allele), is located within exon 1 of OPRM1, and involves an A to G substitution causing an amino acid change from asparagine (Asn) to aspartic acid (Asp) that enhances beta-endorphin binding (Bond et al., 1998). This polymorphism has been consistently implicated in AUD with a recent meta-analysis that included more than 28,000 participants suggesting that the G (Asp) allele is associated with reduced risk for substance use disorders, including AUD (Schwantes-An et al., 2016). In the CP literature, some studies have also found a link between rs1799971 and pain. For example, Janicki and colleagues (2006) found that the frequency of the G allele was significantly lower in a group of CP patients relative to a comparison group of postoperative patients with acute pain, suggesting that the G allele may be associated with reduced risk for CP. Thus, the G allele of rs1799971 appears to be associated with protection against the development of both AUD and CP.
Thus far, to our knowledge, genetic overlap between AUD and CP has not been found among other reward and stress mechanisms in CGAS using human samples. A few studies using animal models of these conditions have suggested overlapping genetic signals between AUD and CP. For example, in the stress system, variants in OPRK1 have been implicated in AUD in human samples (Gerra et al., 2007; Xuei et al., 2006), while animal studies have suggested variation in this gene is related to pain sensitivity in mice (Fukagawa et al., 2014). Similarly, variation in NPY has been implicated in AUD in human studies (Kovanen et al., 2010), and has been related to pain sensitivity in mice (Solway et al., 2011). These data have led to speculations that genetic overlap in the stress system is plausible, thus meriting a closer examination of OPRK1 and NPY variation in human research.
In sum, CGAS provide some indication that there is overlap in the genes that contribute to the development of AUD and CP, though whether these genes contribute to their comorbidity has yet to be addressed. Additionally, the pitfalls of CGAS, such as poor replication rates and inflated false positive rates suggest researchers need to interpret the reviewed findings with caution and employ other methods, such as meta-analysis, to buttress the findings from CGAS. It is also important to note that while these studies highlight potential areas of genetic overlap, they also serve to highlight the current dearth of knowledge regarding the genetic etiology of these traits. Specifically, genes involved in dopamine and opioid systems represent obvious targets for both, but are likely to represent a very restricted view of their overlap.
6. B. Genome-wide association studies (GWAS)
In contrast to CGAS, GWAS use a data driven approach to individually test for associations with millions of SNPs across the genome. P-values are corrected for multiple testing such that associations below 5e-8 threshold are considered significant. For small-effect, common variants to reach this threshold a minimum of 30,000 participants are required to achieve sufficient statistical power (PGC, 2009). Consequently, it is important to note that many of the current GWAS of AUD and CP are underpowered, leading to the development of large-scale consortia to achieve the required sample sizes. Currently, however, consortia studying these conditions are in their infancy. As a result, we provide a brief review of published findings to date, with a focus on describing how future GWAS efforts might help us better understand those facets of the reward and stress systems that influence AUD, CP and their comorbidity. Studies published between 2000–2016 were identified using search strategies adapted from two recent, comprehensive reviews of molecular genetic studies of AUD (Buhler et al., 2015) and CP (Zorina-Lichtenwalter et al., 2016). The top association signals from these studies as well as lists of suggestive results, as defined by the authors of the individual studies, when available were reviewed. Genes and variants that showed suggestive or significant associations with both AUD and CP are presented in Table 2. Nonetheless, it should be noted that the goal of this monograph is not to provide a comprehensive review of genetic overlap between AUD and CP, but rather to provide examples that afford insights for studying comorbidity of AUD and CP. Thus, only a small number of findings are described in detail.
Table 2.
SNPs near the Overlapping Genes Extracted from Genome-wide Association Data on Alcohol Use Disorder (AUD) and Chronic Pain (CP)
| Overlapping Gene |
SNPs reported in previous AUD literature | Authors (AUD) | SNPs reported in previous CP literature | Authors (CP) |
|---|---|---|---|---|
| ABO | rs657152 | Heath et al., 2011 | rs647800 | Gormley et al., 2016 |
| ADAMTSL1 | rs10756991 | Gelernter et al., 2014 | rs1539000, rs4977338 | Cox et al., 2012 |
| ADRA1A | chr8:26608070, rs10101664, rs10105967, rs17088266, rs61670721 | Kapoor et al., 2013 | rs1048101 | Peters et al., 2013 |
| AJAP1 | rs66989617 | Gelernter et al., 2014 | rs10915528 | Cook-Sather et al., 2014 |
| AKAP6 | rs17412116 | Wang et al., 2012 | rs981524 | Docampo et al., 2014 |
| AP2A1 | rs1001281 | Kapoor et al., 2013 | rs1273644, rs1674139 | Gormley et al., 2016 |
| AUTS2 | rs6943555 | Schumann et al., 2011 | chr7:69991038:I | Gormley et al., 2016 |
| C10orf11 | rs10762705 | Wang et al., 2013 | rs11001775 | Cox et al., 2012 |
| C1D | rs9807970, rs13385536, rs17034884 | Gelernter et al., 2014 | rs1503245 | Cook-Sather et al., 2014 |
| C2 | rs644045, rs685031 | Gelernter et al., 2014 | rs36221133, rs184265581 | Gormley et al., 2016 |
| C7orf10 | rs142530316, rs17620991, rs17688247, rs17688571, rs34994199, rs66651482, rs67648979 | Mbarek et al., 2015 | rs186166891, rs10234636, rs77410344, rs4723954, rs12533531, rs12532479, rs12670267, rs144002785, rs11531504, rs10435164, rs80157425, rs17171694, rs77024938, rs141208879, rs147040642, rs10951637, rs17171696, rs138268186, rs1319467, rs12669577 | Gormley et al., 2016 |
| CACNA1A | rs16050 | Gelernter et al., 2014 | rs10405121, rs11669746, rs12611029, rs2302080, rs4926144, rs77626158, rs8112530 | Gormley et al., 2016 |
| CACNA2D1 | rs3216242 | Gelernter et al., 2014 | rs11971008 | Docampo et al., 2014 |
| CDH13 | rs11640875 | Biernacka et al., 2013; Treutlein et al., 2009 | rs1559437 | Gormley et al., 2016 |
| CNTNAP2 | rs851712, rs115707642, rs181335610, rs4726948, rs76164032, rs76687983, rs7811006 | Gelernter et al., 2014; Lind et al., 2010 | rs10277969 | Cook-Sather et al., 2014 |
| COX7C | rs6888626 | Zuo et al., 2012 | rs2195349 | Cook-Sather et al., 2014 |
| CSMD1 | rs11783062, rs79575989, rs34123713, rs35473038, rs35542721, rs4105690, rs6993088, rs7461469 | Gelernter et al., 2014; Kapoor et al., 2013; Wang et al., 2013 | rs1545821 | Cook-Sather et al., 2014 |
| DOK6 | rs11151517, rs7230078, rs112429852, rs143744549, rs147997033, rs59387400, rs73970243, rs73970248 | Gelernter et al., 2014 | rs8083908 | Cook-Sather et al., 2014 |
| DSCAML1 | rs553637, rs117266385 | Gelernter et al., 2014; Wang et al., 2013 | rs528431 | Cox et al., 2012 |
| ESR1 | rs6902771 | Biernacka et al., 2013; Treutlein et al., 2009 | rs7745737 | Cook-Sather et al., 2014 |
| FAM155A | rs142728746, rs146932814, rs16971278, rs16971285, rs74612847, rs76569829, rs79922539, rs7993103, rs9520352, rs9555412, rs9555413, rs9559170, rs9587393 | Gelernter et al., 2014 | rs9301200, rs9558976 | Cox et al., 2012 |
| FREM1 | rs3747534 | Gelernter et al., 2014 | rs1032474, rs4415414 | Cox et al., 2012 |
| FUT9 | rs4132260 | Wang et al., 2013 | rs7775100, rs9485644, rs2493357, rs7753933, rs716192, rs7746369, rs4304170, rs78878034, rs4563725, rs2387151, rs4002794, rs9390810, rs1570774, rs9322635, rs9404179, rs9498927, rs9498926, rs7740119, rs7745151, rs57583838 | Gormley et al., 2016 |
| GRIA4 | rs2508467 | Karpyak et al., 2012 | rs10502058, rs10895837, rs17104711, rs2510177, rs642544 | Peters et al., 2013 |
| GRK5 | rs12780837 | Gelernter et al., 2014 | rs7071131, rs7076992, rs7077224, rs7090417, rs928670 | Gormley et al., 2016 |
| HLA-DRA | rs9268628, rs9268644, rs2239803, rs9268644 | Gelernter et al., 2014 | rs116618786 | Gormley et al., 2016 |
| HTR7 | rs7916403 | Zlojutro et al., 2011; Zuo et al., 2014 | rs2800143 | Cox et al., 2012 |
| IL-1RN | rs2232354 | Gelernter et al., 2014 | rs315952, rs454078 | Peters et al., 2013 |
| KIAA0040 | rs1057239, rs1057302, rs1894709, rs6425323, rs6701037, rs1057239, rs1057302, rs1894709, rs1057239, rs1894709 | Zuo et al., 2012 | rs10798341, rs10912903, rs12084108, rs12143530, rs2205603, rs2205604, rs2272784, rs3766681, rs3766683, rs3766684, rs3766685, rs3766687, rs3766688, rs3766692, rs3766694, rs6674635, rs6677931, rs6677935, rs760486 | Gormley et al., 2016 |
| KLHL29 | rs150875373 | Gelernter et al., 2014 | rs4665597, rs13011734 | Gormley et al., 2016 |
| MAGI2 | rs990527 | Gelernter et al., 2014 | rs11979133 | Cox et al., 2012 |
| MRPS21 | rs12747669, rs140449835, rs12747669, rs140449835, rs1694374, rs494041, rs573351 | Gelernter et al., 2014 | rs1694374, rs580159, rs497128, rs1260398, rs8006, rs471738, rs11205359, rs1776273, rs543179, rs471657, rs494952, rs573351, rs1776275, rs11811885, rs494041, rs2762860, rs496203, rs3818978, rs500812, rs1694375 | Gormley et al., 2016 |
| MYO3B | rs741283 | Gelernter et al., 2014 | rs6730459, rs76973106 | Cox et al., 2012, Gormley et al., 2016 |
| NCOR2 | rs1244096 | Gelernter et al., 2014 | rs11057627, rs12231617, rs1271309, rs3782266, rs4765566, rs951976 | Gormley et al., 2016 |
| NOS1AP | rs4480334 | Gelernter et al., 2014 | rs16859092 | Cook-Sather et al., 2014 |
| NOTCH4 | rs2071279, rs2071280, rs9267820, rs9267830, rs9267831, rs9267832, rs9267833 | Gelernter et al., 2014 | rs6936346 | Cox et al., 2012 |
| OXR1 | rs6996005, rs7819358 | Kapoor et al., 2013 | rs1453227, rs6469074 | Yamaguchi et al., 2014 |
| PCSK2 | rs112910132 | Gelernter et al., 2014 | rs6514790, rs8120978, rs8121922 | Gormley et al., 2016 |
| PIP5K1B | rs3812537 | Gelernter et al., 2014 | rs11144442 | Gormley et al., 2016 |
| PRDM16 | rs760568 | Gelernter et al., 2014 | rs10218452, rs10797381, rs7518255, rs2075968, rs10909886, rs2376495, rs61759167, rs11587518, rs28594467, rs61759161, rs1393064, rs56304645, rs61759163, rs12745073, rs1393065, rs2493212, rs12136643, rs12038657, rs2651899, rs207195 | Gormley et al., 2016 |
| PRPF3 | rs1260401, rs1776276 | Gelernter et al., 2014 | rs2794680, rs698918, rs1260406, rs1776265, rs2794684, rs2794682, rs1694364, rs4926422, rs1260404, rs3125808, rs1260407, rs1260409, rs698914, rs1097066, rs834225, rs1260385, rs1260403, rs698917, rs1776272, rs1260408 | Gormley et al., 2016 |
| PTPRD | rs10816057, rs10977546 | Gelernter et al., 2014 | rs2039331 | Cox et al., 2012 |
| RBFOX1 | rs1507026, rs1507027, rs1507029, rs28581832, rs4786880, rs7186335, rs7193674, rs7203593, rs76931803, rs8044875, rs9925723 | Gelernter et al., 2014 | rs1955388 | Gormley et al., 2016 |
| RORA | rs79271390 | Gelernter et al., 2014 | rs4238351 | Cook-Sather et al., 2014 |
| RPRD2 | rs1260419, rs1776270 | Gelernter et al., 2014 | rs13374465, rs35784258, rs1617829, rs834241, rs1260392, rs828783, rs4926395, rs1260419, rs11205373, rs832621, rs834234, rs1566225, rs834242, rs11205375, rs12736375, rs7513182, rs11205382, rs1313570, rs10888583, rs834243 | Gormley et al., 2016 |
| SEMA6D | rs11856716 | Gelernter et al., 2014 | rs10519138 | Gormley et al., 2016 |
| SERINC2 | rs1039630, rs10914386, rs2275435, rs4478858, rs4949400, rs4949402, rs4478858, rs2275436, rs4478858, rs2275435, rs2275436, rs4478858, rs4949400, rs4949402 | Biernacka et al., 2013; Zuo et al., 2012; Zuo et al., 2014 | rs140137989 | Gormley et al., 2016 |
| SERPINC1 | rs1799876 | Xu et al., 2015 | rs12137553, rs12746886 | Gormley et al., 2016 |
| SKIV2L | rs419788, rs437179, rs440454 | Gelernter et al., 2014 | rs115867030, rs116336668 | Gormley et al., 2016 |
| SLC9B1 | rs66466366 | Gelernter et al., 2014 | rs10006076 | Gormley et al., 2016 |
| TBX19 | rs1003502 | Yu et al., 2008 | rs2268550 | Cook-Sather et al., 2014 |
| TCERG1L | rs11017811, rs11017815 | Gelernter et al., 2014 | rs1176493 | Cook-Sather et al., 2014 |
| TMEM132D | rs1000118, rs2218917, rs4759995 | Gelernter et al., 2014; Zuo et al., 2012 | rs4759808, rs10847807 | Cox et al., 2012 |
| TNXB | rs2071293, rs3096695, rs3117181, rs3134954, rs7766862 | Heath et al., 2011; Kapoor et al., 2013 | rs150223621, rs182129909 | Gormley et al., 2016 |
| USP24 | rs497159, rs1165238 | Biernacka et al., 2013 | rs12566055 | Cook-Sather et al., 2014 |
Note. SNPs reported here were limited to the top 20 of each gene
As noted, GWAS of AUD and CP conducted to date have been relatively underpowered. Thus, there are few significant results and no overlap among them. Nonetheless, there has been overlap in the suggestive findings reported for each group of disorders. Looking at individual SNPs, thirteen have yielded suggestive associations with both AUD and CP. The SNPs are located in or near the PRPF3 (rs1260401, rs1776276, rs519126), RPRD2 (rs1260419, rs1776270), MRPS21 (rs12747669, rs1694374, rs494041, rs573351), and ONECUT2 (rs4940804, rs4940805, rs4940806) genes and one in an intergenic region (rs2263190 - 6q16.1) (Gelernter et al., 2014; Gormley et al., 2016; Wang et al., 2013). None of these common genes or regions has shown a genome-wide significant relation with AUD or CP and do not fall into the reward or stress systems, suggesting that any substantive interpretation of these results would be premature and require further replication.
In contrast, after annotating each SNP associated with AUD or CP to its nearest gene, there is overlap among the implicated genes, including genes that are hypothesized to play a role in the reward or stress systems (Table 2). These genes can be broken down into three broad categories, namely those hypothesized to be directly involved in either the reward or stress systems, those that may be involved in mechanisms that play a modulatory role or interact with the reward and/or stress systems, and those critical for central nervous system organization and activity in general but whose specific relations to the reward or stress system have yet to be delineated. We provide a brief discussion of genes from each of these categories in the following paragraphs.
Although none of the identified genes in Table 2 were related directly to the reward system, one belonging to the stress system is of particular interest – T-Box 19 (TBX19). A SNP in TBX19 (rs1003502) was identified in an early GWAS of alcohol dependence (Yu et al., 2008), while a different SNP (rs2268550) was identified in a GWAS of postoperative pain (Cook-Sather et al., 2014). Although acute postoperative pain does not belong to the classic CP conditions, it provides insight on pain sensitivity among individuals who may be more susceptible to developing CP in the future (an endophenotype that will be defined below) and has been frequently studied in the CP literature. Of relevance to the present review, TBX19 has been shown to regulate the expression of the adrenocorticotropic hormone (ACTH) precursor, pro-opiomelanocortin (POMC), in the pituitary gland (Liu et al., 2001), and functional mutations in TBX19 result in Isolated Adrenocorticotropin Deficiency (Vallette-Kasic et al., 2005). Thus, it appears to play a critical regulatory role in the HPA system and stress regulation, suggesting a possible shared mechanism through which variation in this gene could contribute to the development of AUD and CP.
Examples of genes showing suggestive associations with AUD and CP and hypothesized to play a modulatory role and interact with the reward and stress systems, as can be seen in Table 2, cover many physiological systems. They included serotonin receptors (e.g. HTR7), adrenergic receptors (e.g. ADRA1A), calcium channel regulators (e.g. CACNA1A), interleukin receptors (e.g. IL-1RN), hormonal receptors (e.g. ESR1), and so on. It is beyond the scope of this monograph to describe them all in detail. Thus, we use two examples, HTR7 and ADRA1A, to illustrate the involvement of these genes in AUD and pain. Two variants, rs7916403 and rs2800143, in the 5-hydroxytryptamine (serotonin) 7 receptor (5-HT7) gene (HTR7) have been respectively associated with susceptibility to alcohol dependence (Zlojutro et al., 2011; Zuo et al., 2014) and increased risk of migraine (Cox et al, 2012). The interaction of serotoninergic and dopaminergic neurons plays a major role in reinforcement learning and memory that subsequently determine incentive salience and motivation (Cools et al., 2011). Moreover, 5-HT7 receptors modulate the stress-related activity of the HPA system (Garcia-Iglesias et al., 2013; Terron, 2014) and are involved in the endogenous pain inhibitory system via mediating the effect of opioid and endocannabinoid-induced analgesia in response to acute stress in animal models (Yesilyurt et al., 2015). SNPs in the ADRA1A gene that interact with the stress system have also been implicated in GWA studies of AUD (Kapoor et al., 2013; chr8: 26608070, rs10101664, rs10105967, rs17088266, and rs61670721) and CP (Peters et al., 2013; rs1048101). These receptors bind with catecholamines mediating the sympathetic nervous system's response to acute stress, including stress that occurs during alcohol withdrawal and pain (Piascik and Perez, 2001). Thus, there is strong evidence supporting the regulatory role of HTR7 and ADRA1A in the reward and stress systems, supporting a certain degree of etiological overlap between AUD and CP.
Finally, there are also overlapping genes described in Table 2 with broad expression across the central nervous system, though their roles in the neural structures critical to the reward and stress systems have yet to be fully described. For example, a SNP in cadherin 13 (CDH13), rs11640875 has been implicated in a GWAS of AUD (Biernacka et al., 2013; Treutlein et al., 2009), and another SNP in the same gene, rs1559437 has been associated with migraine in a GWA study (Gormley et al., 2016). CDH13 is highly expressed in the central nervous system (Takeuchi et al., 2000) where it encodes for a protein, T-cadherin, that is involved in cell adhesion (Cho et al., 2015). Animal studies have suggested that T-cadherin regulates GABAergic inhibitory interneurons in hippocampus (Rivero et al., 2015). Additional work has shown that adiponectin binds with T-cadherin in order to regulate oxidative stress (Kasahara et al., 2013). Alcohol consumption and migraine have been linked to increases in oxidative stress (Albano, 2006; Borkum, 2016), and thus, dysregulation in oxidative stress may further exacerbate the development of AUD and migraine, providing another potential mechanism through which CDH13 might influence AUD and CP. This evidence suggests further genetic influences that may be shared between AUD and CP and that this shared etiology may involve neurological systems beyond the reward and stress pathways, which are the primary focus of the present review.
7. Commentary and Future Directions
This monograph provides an extensive look at the genetic overlap between AUD and CP. CGAS of human samples have documented several potentially important overlapping genetic signals in the dopaminergic and opioidergic neurotransmitter systems, including the ANKK1-DRD2 gene region, COMT, and OPRM1, but given the small sample sizes involved in these studies, these findings require further replication. An additional limitation of these CGAS is that they are restricted to a small set of genes and variants that have been studied across many phenotypes, and thus provide a very limited examination of the potential genetic influences contributing to these conditions. Though still in the early stages, the findings obtained from GWAS of AUD have begun to yield suggestive results identifying novel genes that may be contribute to the etiology of AUD and CP (e.g., AUD: Gelernter et al., 2014, Pain: Anttila et al., 2013). However, GWAS are not without limitations either. Most of the studies conducted for AUD and CP have been underpowered. Thus, while the present review identified some variants and genes with suggestive relations with AUD and CP, it is plausible that many additional variants that exert a significant effect on the disorders and their comorbidity have yet to be identified. As additional studies are conducted and data are contributed to large-scale meta-analytic efforts, statistical power of these studies will increase, allowing for the discovery of additional variants and the genes that they impact. As discussed earlier, evidence from studies using animal models also suggests that genetic overlap exists between AUD and CP outside this small number of genes. Notably, as more robust evidence implicating specific genes in the etiology of AUD and CP accumulates, animal models in which a specific gene or variant can be altered will provide additional insights into how these genes contribute to the development of AUD and CP.
In addition to single variant association analysis, pathway- and network-based approaches have the potential to further our understanding of the genetic factors involved in the etiologies of AUD, CP, and possibly their comorbidity. Pathway analyses focus on the joint analysis of variants within gene sets that are created based on the known function of gene products (e.g., G-protein-coupled receptor activity, cell adhesion) or demonstrated interactions between gene products in the case of protein-protein interaction (PPI) networks (Lin et al., 2016). Additionally, data mining approaches that incorporate data from multiple sources (e.g., association studies, PPI studies, transcriptomics and other omics-level studies) can be used to develop disease/disorder-specific networks, which then can be compared across phenotypes to identify areas of overlap (Kontou et al., 2016). As these types of data accumulate, both approaches will likely expand our understanding of the genetic influences that contribute to the reward and stress systems and how individual differences in these systems contribute to the development of AUD and CP.
Notably, GWAS data also have the potential to inform our understanding of the overlap between these conditions beyond gene discovery. For example, several recently developed approaches allow researchers to aggregate the effects of variants and genes on disorders in ways that allow for the estimation of genetic correlations between traits, and in some cases, test hypotheses regarding the causal pathways underlying them. For example, LD Hub is a centralized database containing an extensive amount of GWAS summary-level data associated with mental and physical disorders (Zheng et al., 2017). It contains a web interface for conducting LD score regression, which allows the estimation of genetic correlations between different complex disorders from GWAS summary-level results (Bulik-Sullivan et al., 2015). Similarly, polygenic risk score approaches have been developed to investigate the relations between complex phenotypes and have been used in some instances to test causal relations among psychiatric phenotypes using Mendelian Randomization designs (e.g., Richmond et al., 2014).
While the described approaches have the potential to significantly expand our understanding of the etiology of AUD and CP, there are some issues that will require special consideration. First, due to the heterogeneity of AUD and CP, endophenotypes or sets of endophenotypes that index one or more transdiagnostic mechanisms that undergird both disorders may be more powerful than using manifest diagnoses as phenotypes for gene identification. Endophenotypes of a disorder are heritable traits associated with the disorder of interest, manifested in preclinical individuals, and co-segregate with the disorder in pedigree analysis (Gottesman and Gould, 2003). Examples of endophenotypes for comorbidity of AUD and CP may include hyperkatifeia or hyperalgesia; however, the selection of such endophenotypes may be limited to our current knowledge regarding the biological underpinnings of each disorder. Thus, the application of phenome-wide association study (PheWAS) methods to genes and variants robustly associated with both AUD and CP may be considered to identify novel phenotypes or endophenotypes that may be used in future studies to identify genetic variants that predispose to the comorbidity of AUD and CP (Polimanti et al, 2016).
Additionally, it is important to mention that genetic influences do not act in isolation. Thus, it is crucial to consider the joint effect of genetic and environmental factors in studying the comorbidity of AUD and CP. As mentioned above, oftentimes, inconsistent findings across studies are attributable to the interaction between genetic and environmental factors. For example, gene-environment interactions (GxE) can be tested at the candidate gene or genome-wide level (GxE GWAS) to investigate whether specific environmental factors, such as social stress (e.g., Singh et al., 2015), moderate the influence of individual variants on AUD and CP. Further, network-based approaches using machine-learning algorithms have been developed to not only identify gene-networks as described above, but also to identify networks that incorporate the moderating effects of environmental variables as well. Notably, one recent example developed such a network to quantify GxE effects on AUD (e.g., Zollanvari et al., 2017), and could be extended to the study of CP and potentially AUD-CP comorbidity. Finally, it will also be important to study the mechanisms through which environmental influences exert their force on genetic influences. For example, through DNA methylation, environmental influences can impact DNA expression by modifying the activity of a DNA sequence without changing the sequence itself. Thus, the transactional nature of gene-environment relations will need to be incorporated into the molecular genetic study of these conditions in order to fully understand their etiology.
8. Conclusion
Neurobiological evidence has suggested that similar dysregulation of reward and stress pathways contribute to AUD and CP, and there is some suggestion that genetic influences on these pathways may contribute to both conditions. CGAS have provided initial evidence to support this premise, but should be evaluated with caution. A number of SNPs suggestively associated with AUD and CP have been identified from GWAS that studied AUD and CP independently, and several of these SNPs have been located in or near a common set of genes. These common genes are either directly or indirectly related to the reward and stress systems, as well as from genes that are more broadly involved with the CNS, whose links to reward and stress systems have yet to be studied. This monograph points to the fact that, if a shared genetic architecture underlies AUD-CP comorbidity, it is likely to implicate systems beyond reward and stress mechanisms and involve instances of mediation and modulation among these systems. Advancement in research designs and methods should help further elucidate the potential genetic overlap between AUD and CP. If shared genetic contributions to AUD and CP can be identified, such knowledge can be used to inform our understanding of the underlying mechanisms that contribute to the etiologies of each disorder and their co-occurrence, and thus has the potential to refine and improve the diagnosis and treatment of AUD and CP.
Acknowledgments
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (T32AA013526) to Kenneth J. Sher and National Institute of Nursing Research (1R01NR015314-01A1) to Jason G. Craggs.
Footnotes
No conflict of interest to declare.
References
- Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- Aneiros-Guerrero A, Lendinez AM, Palomares AR, Perez-Nevot B, Aguado L, Mayor-Olea A, Ruiz-Galdon M, Reyes-Engel A. Genetic polymorphisms in folate pathway enzymes, DRD4 and GSTM1 are related to temporomandibular disorder. BMC Med Genet. 2011;12:75–83. doi: 10.1186/1471-2350-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, Medland SE, Todt U, McArdle WL, Quaye L, Koiranen M, Ikram MA, Lehtimäki T, Stam AH, Ligthart L, Wedenoja J, Dunham I, Neale BM, Palta P, Hamalainen E, Schürks M, Rose LM, Buring JE, Ridker PM, Steinberg S, Stefansson H, Jakobsson F, Lawlor DA, Evans DM, Ring SM, Färkkilä M, Artto V, Kaunisto MA, Freilinger T, Schoenen J, Frants RR, Pelzer N, Weller CM, Zielman R, Heath AC, Madden PAF, Montgomery GW, Martin NG, Borck G, Göbel H, Heinze A, Heinze-Kuhn K, Williams FMK, Hartikainen A-L, Pouta A, van den Ende J, Uitterlinden AG, Hofman A, Amin N, Hottenga J-J, Vink JM, Heikkilä K, Alexander M, Muller-Myhsok B, Schreiber S, Meitinger T, Wichmann HE, Aromaa A, Eriksson JG, Traynor B, Trabzuni D, North American Brain Expression C, Consortium UKBE. Rossin E, Lage K, Jacobs SBR, Gibbs JR, Birney E, Kaprio J, Penninx BW, Boomsma DI, van Duijn C, Raitakari O, Jarvelin M-R, Zwart J-A, Cherkas L, Strachan DP, Kubisch C, Ferrari MD, van den Maagdenberg AMJM, Dichgans M, Wessman M, Smith GD, Stefansson K, Daly MJ, Nyholt DR, Chasman D, Palotie A, for the International Headache Genetics Consortium Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S. Neural mechanisms of pain and alcohol dependence. Pharmacology Biochemistry and Behavior. 2013;112:34–41. doi: 10.1016/j.pbb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the µ-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83:262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Barbosa FR, Matsuda JB, Mazucato M, de Castro França S, Zingaretti SM, Da Silva LM, Martinez-Rossi NM, Júnior MF, Marins M, Fachin AL. Influence of catechol-O-methyltransferase (COMT) gene polymorphisms in pain sensibility of Brazilian fibromialgia patients. Rheumatol Int. 2012;32:427–430. doi: 10.1007/s00296-010-1659-z. [DOI] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased Attributable Risk Related to a Functional [mu]-Opioid Receptor Gene Polymorphism in Association with Alcohol Dependence in Central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- Biernacka JM, Geske JR, Schneekloth TD, Frye MA, Cunningham JM, Choi D-S, Tapp CL, Lewis BR, Drews MS, Pietrzak TL, Colby CL, Hall-Flavin DK, Loukianova LL, Heit JA, Mrazek DA, Karpyak VM. Replication of Genome Wide Association Studies of Alcohol Dependence: Support for Association with Variation in ADH1C. PLoS One. 2013;8:e58798. doi: 10.1371/journal.pone.0058798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: Possible implications for opiate addiction. Proceedings of the National Academy of Sciences. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache: The Journal of Head and Face Pain. 2016;56:12–35. doi: 10.1111/head.12725. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: The value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–297. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Bergman E, Liu H-X, Hökfelt T, Villar MJ. Effect of a graded single constriction of the rat sciatic nerve on pain behavior and expression of immunoreactive NPY and NPY Y1 receptor in DRG neurons and spinal cord. Brain Res. 2004;1006:87–99. doi: 10.1016/j.brainres.2003.09.085. [DOI] [PubMed] [Google Scholar]
- Bühler KM, Giné E, Echeverry-Alzate V, Calleja-Conde J, Fonseca FR, López-Moreno JA. Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict Biol. 2015;20:845–871. doi: 10.1111/adb.12204. [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, ReproGen C, Psychiatric Genomics C, Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control C. Duncan L, Perry JRB, Patterson N, Robinson EB, Daly MJ, Price AL, Neale BM. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni S, Isola R, Gessa GL, Rossetti ZL. Ethanol prevents the glutamate release induced by N-methyl-D-aspartate in the rat striatum. Neurosci Lett. 1993;152:133–136. doi: 10.1016/0304-3940(93)90501-b. [DOI] [PubMed] [Google Scholar]
- Cargnin S, Magnani F, Viana M, Tassorelli C, Mittino D, Cantello R, Sances G, Nappi G, Canonico PL, Genazzani AA, Raffaeli W, Terrazzino S. An opposite-direction modulation of the COMT Val158Met polymorphism on the clinical response to intrathecal morphine and triptans. The Journal of Pain. 2013;14:1097–1106. doi: 10.1016/j.jpain.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Cargnin S, Viana M, Mittino D, Bellomo G, Tassorelli C, Nappi G, Canonico PL, Terrazzino S. Lack of association between GRIA1 polymorphisms and haplotypes with migraine without aura or response to triptans. Neurol Sci. 2014;35:421–427. doi: 10.1007/s10072-013-1535-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Liu F, Shang Q, Song X, Miao X, Wang Z. Association between polymorphisms of DRD2 and DRD4 and opioid dependence: evidence from the current studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156:661–670. doi: 10.1002/ajmg.b.31208. [DOI] [PubMed] [Google Scholar]
- Chen H, Ji C-X, Zhao L-L, Kong X-J, Zeng X-T. Association Between Polymorphisms of DRD2, COMT, DBH, and MAO-A Genes and Migraine Susceptibility: A Meta-Analysis. Medicine. 2015;94:e2012. doi: 10.1097/MD.0000000000002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C-H, Lee H-J, Woo HG, Choi J-H, Greenwood TA, Kelsoe JR. CDH13 and HCRTR2 may be associated with hypersomnia symptom of bipolar depression: a genome-wide functional enrichment pathway analysis. Psychiatry Investig. 2015;12:402–407. doi: 10.4306/pi.2015.12.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Neumann L, Glazer Y, Ebstein RP, Buskila D. The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val158met and fibromyalgia. Clin Exp Rheumatol. 2009;27:S51–S56. [PubMed] [Google Scholar]
- Consortium IHG. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22. 1. Nat Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook-Sather SD, Li J, Goebel TK, Sussman EM, Rehman MA, Hakonarson H. TAOK3, a novel genome-wide association study locus associated with morphine requirement and postoperative pain in a retrospective pediatric day surgery population. Pain. 2014;155:1773–1783. doi: 10.1016/j.pain.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Cox HC, Lea RA, Bellis C, Carless M, Dyer TD, Curran J, Charlesworth J, Macgregor S, Nyholt D, Chasman D, Ridker PM, Schürks M, Blangero J, Griffiths LR. A genome-wide analysis of 'Bounty' descendants implicates several novel variants in migraine susceptibility. Neurogenetics. 2012;13:261–266. doi: 10.1007/s10048-012-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine β-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- De Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system—II. Immunohistochemical analysis. Neuroscience. 1986;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- de Sousa SC, Karwautz A, Wöber C, Wagner G, Breen G, Zesch H-E, Konrad A, Zormann A, Wanner C, Kienbacher C, Collier DA, Wöber-Bingöl Ç. A dopamine D4 receptor exon 3 VNTR allele protecting against migraine without aura. Ann Neurol. 2007;61:574–578. doi: 10.1002/ana.21140. [DOI] [PubMed] [Google Scholar]
- Del Zompo M, Cherchi A, Palmas MA, Ponti M, Bocchetta A, Gessa GL, Piccardi MP. Association between dopamine receptor genes and migraine without aura in a Sardinian sample. Neurology. 1998;51:781–786. doi: 10.1212/wnl.51.3.781. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Dick DM, Edenberg HJ, Xuei X, Goate A, Kuperman S, Schuckit M, Crowe R, Smith TL, Porjesz B, Begleiter H, Foroud T. Association of GABRG3 With Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2004;28:4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- Docampo E, Escaramís G, Gratacòs M, Villatoro S, Puig A, Kogevinas M, Collado A, Carbonell J, Rivera J, Vidal J, Alegre J, Estivill X, Rabionet R. Genome-wide analysis of single nucleotide polymorphisms and copy number variants in fibromyalgia suggest a role for the central nervous system. Pain. 2014;155:1102–1109. doi: 10.1016/j.pain.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Domart M-C, Benyamina A, Lemoine A, Bourgain C, Blecha L, Debuire B, Reynaud M, Saffroy R. Association between a polymorphism in the promoter of a glutamate receptor subunit gene (GRIN2A) and alcoholism. Addict Biol. 2012;17:783–785. doi: 10.1111/j.1369-1600.2011.00321.x. [DOI] [PubMed] [Google Scholar]
- Drgon T, D'Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, Gejman PV. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. The American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. Sex differences in the influence of COMT Val158Met on alcoholism and smoking in plains American Indians. Alcoholism: Clinical and Experimental Research. 2006;30:399–406. doi: 10.1111/j.1530-0277.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Erdal ME, Herken H, Mutlu MN, Bayazit YA. Significance of catechol-O-methyltransferase gene polymorphism in myofacial pain syndrome. The Pain Clinic. 2003;15:309–313. [Google Scholar]
- Esserlind A-L, Christensen AF, Steinberg S, Grarup N, Pedersen O, Hansen T, Werge T, Hansen TF, Husemoen LLN, Linneberg A, Budtz-Jorgensen E, Westergaard ML, Stefansson H, Olesen J. The association between candidate migraine susceptibility loci and severe migraine phenotype in a clinical sample. Cephalalgia. 2015;36:615–623. doi: 10.1177/0333102415570492. [DOI] [PubMed] [Google Scholar]
- Evers EA, Stiers P, Ramaekers JG. High reward expectancy during methylphenidate depresses the dopaminergic response to gain and loss. Soc Cogn Affect Neurosci. 2017;12:311–318. doi: 10.1093/scan/nsw124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Colson N, Quinlan S, MacMillan J, Lea RA, Griffiths LR. Association between migraine and a functional polymorphism at the dopamine β-hydroxylase locus. Neurogenetics. 2009;10:199–208. doi: 10.1007/s10048-009-0176-2. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Lea RA, Colson NJ, Bellis C, Quinlan S, Griffiths LR. Association between a 19 bp deletion polymorphism at the dopamine beta-hydroxylase (DBH) locus and migraine with aura. J Neurol Sci. 2006;251:118–123. doi: 10.1016/j.jns.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Fernández-de-las-Peñas C, Ambite-Quesada S, Gil-Crujera A, Cigarán-Méndez M, Peñacoba-Puente C. Catechol-O-methyltransferase Val158Met polymorphism influences anxiety, depression, and disability, but not pressure pain sensitivity, in women with fibromyalgia syndrome. J Pain. 2012;13:1068–1074. doi: 10.1016/j.jpain.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152:300–307. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formicola D, Aloia A, Sampaolo S, Farina O, Diodato D, Griffiths LR, Gianfrancesco F, Di Iorio G, Esposito T. Common variants in the regulative regions of GRIA1 and GRIA3 receptor genes are associated with migraine susceptibility. BMC Med Genet. 2010;11:103–114. doi: 10.1186/1471-2350-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa H, Koyama T, Fukuda K. κ-Opioid receptor mediates the antinociceptive effect of nitrous oxide in mice. Br J Anaesth. 2014;113:1032–1038. doi: 10.1093/bja/aeu254. [DOI] [PubMed] [Google Scholar]
- García-Iglesias BB, Mendoza-Garrido ME, Gutiérrez-Ospina G, Rangel-Barajas C, Noyola-Díaz M, Terrón JA. Sensitization of restraint-induced corticosterone secretion after chronic restraint in rats: involvement of 5-HT 7 receptors. Neuropharmacology. 2013;71:216–227. doi: 10.1016/j.neuropharm.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Goldman D, Risch N. The A1 allele at the D2 dopamine receptor gene and alcoholism: a reappraisal. JAMA. 1993;269:1673–1677. [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA. Genome-wide association study of alcohol dependence: significant findings in African-and European-Americans including novel risk loci. Mol Psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Cheng R, Nguyen T, Israel Y, O’dowd BF. Polymorphisms of the D4 dopamine receptor alleles in chronic alcoholism. Biochem Biophys Res Commun. 1993;196:107–114. doi: 10.1006/bbrc.1993.2222. [DOI] [PubMed] [Google Scholar]
- Gerra G, Leonardi C, Cortese E, D'Amore A, Lucchini A, Strepparola G, Serio G, Farina G, Magnelli F, Zaimovic A, Mancini A, Turci M, Manfredini M, Donnini C. Human kappa opioid receptor gene (OPRK1) polymorphism is associated with opiate addiction. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144:771–775. doi: 10.1002/ajmg.b.30510. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Pradhan S, Mittal B. Identification of a novel ANKK1 and other dopaminergic (DRD2 and DBH) gene variants in migraine susceptibility. Neuromolecular Med. 2013;15:61–73. doi: 10.1007/s12017-012-8195-9. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Otto JM, Ellingson JM. Molecular genetic approaches to studying the externalizing spectrum. In: Beauchaine TP, Hinshaw SP, editors. The Oxford Handbook of Externalizing Spectrum Disorders. Oxford Library of Psychology; New York: 2015. pp. 125–148. [Google Scholar]
- Goldman D. The DRD2 dopamine receptor and the candidate gene approach in alcoholism. Alcohol and alcoholism (Oxford, Oxfordshire) Supplement. 1993;2:27–29. [PubMed] [Google Scholar]
- Goldman D, Urbanek M, Guenther D, Robin R, Long JC. A functionally deficient DRD2 variant [Ser311Cys] is not linked to alcoholism and substance abuse. Alcohol. 1998;16:47–52. doi: 10.1016/s0741-8329(97)00176-6. [DOI] [PubMed] [Google Scholar]
- Gormley P, Anttila V, Winsvold BS, Palta P, Esko T, Pers TH, Farh K-H, Cuenca-Leon E, Muona M, Furlotte NA, Kurth T, Ingason A, McMahon G, Ligthart L, Terwindt GM, Kallela M, Freilinger TM, Ran C, Gordon SG, Stam AH, Steinberg S, Borck G, Koiranen M, Quaye L, Adams HHH, Lehtimaki T, Sarin A-P, Wedenoja J, Hinds DA, Buring JE, Schurks M, Ridker PM, Hrafnsdottir MG, Stefansson H, Ring SM, Hottenga J-J, Penninx BWJH, Farkkila M, Artto V, Kaunisto M, Vepsalainen S, Malik R, Heath AC, Madden PAF, Martin NG, Montgomery GW, Kurki MI, Kals M, Magi R, Parn K, Hamalainen E, Huang H, Byrnes AE, Franke L, Huang J, Stergiakouli E, Lee PH, Sandor C, Webber C, Cader Z, Muller-Myhsok B, Schreiber S, Meitinger T, Eriksson JG, Salomaa V, Heikkila K, Loehrer E, Uitterlinden AG, Hofman A, van Duijn CM, Cherkas L, Pedersen LM, Stubhaug A, Nielsen CS, Mannikko M, Mihailov E, Milani L, Gobel H, Esserlind A-L, Christensen AF, Hansen TF, Werge T, International Headache Genetics C. Kaprio J, Aromaa AJ, Raitakari O, Ikram MA, Spector T, Jarvelin M-R, Metspalu A, Kubisch C, Strachan DP, Ferrari MD, Belin AC, Dichgans M, Wessman M, van den Maagdenberg AMJM, Zwart J-A, Boomsma DI, Smith GD, Stefansson K, Eriksson N, Daly MJ, Neale BM, Olesen J, Chasman DI, Nyholt DR, Palotie A. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48:856–866. doi: 10.1038/ng.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95:119–128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Gürsoy S, Erdal E, Sezgin M, Barlas İÖ, Aydeniz A, Alaşehirli B, Şahin G. Which genotype of MAO gene that the patients have are likely to be most susceptible to the symptoms of fibromyalgia? Rheumatol Int. 2008;28:307–311. doi: 10.1007/s00296-007-0454-y. [DOI] [PubMed] [Google Scholar]
- Hasvik E, Schistad EI, Grøvle L, Haugen AJ, Røe C, Gjerstad J. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex-OPRM1 A118G polymorphism interaction: a prospective 1-year observational study. BMC Musculoskelet Disord. 2014;15:161–167. doi: 10.1186/1471-2474-15-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou Y-L, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PAF, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JE, Jeong Y, Forrest JM. The endogenous opioid system and clinical pain management. AACN Adv Crit Care. 2005;16:291–301. doi: 10.1097/00044067-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Holliday KL, Nicholl BI, Macfarlane GJ, Thomson W, Davies KA, McBeth J. Genetic variation in the hypothalamic-pituitary-adrenal stress axis influences susceptibility to musculoskeletal pain: results from the EPIFUND study. Ann Rheum Dis. 2010;69:556–560. doi: 10.1136/ard.2009.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz S, Loheswaran G, Le Foll B, Rehm J. Longitudinal alcohol consumption patterns and health-related quality of life: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and alcohol review. 2017 doi: 10.1111/dar.12503. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. [Accessed April 22, 2017];Relieving pain in America. A blueprint for transforming prevention, care, education and research. [The National Academies Press Web site] 2011 Available at: https://www.nap.edu/read/13172/chapter/2 ( https://www.nap.edu/read/13172/chapter/2) [PubMed]
- Jacobsen LM, Schistad EI, Storesund A, Pedersen LM, Rygh LJ, Røe C, Gjerstad J. The COMT rs4680 Met allele contributes to long-lasting low back pain, sciatica and disability after lumbar disc herniation. European Journal of Pain. 2012;16:1064–1069. doi: 10.1002/j.1532-2149.2011.00102.x. [DOI] [PubMed] [Google Scholar]
- Janicki PK, Schuler G, Francis D, Bohr A, Gordin V, Jarzembowski T, Ruiz-Velasco V, Mets B. A genetic association study of the functional A118G polymorphism of the human µ-opioid receptor gene in patients with acute and chronic pain. Anesth Analg. 2006;103:1011–1017. doi: 10.1213/01.ane.0000231634.20341.88. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro-and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol. 2008;99:1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Wang J-C, Wetherill L, Le N, Bertelsen S, Hinrichs AL, Budde J, Agrawal A, Bucholz K, Dick D, Harari O, Hesselbrock V, Kramer J, Nurnberger JI, Rice J, Saccone N, Schuckit M, Tischfield J, Porjesz B, Edenberg HJ, Bierut L, Foroud T, Goate A. A meta-analysis of two genome-wide association studies to identify novel loci for maximum number of alcoholic drinks. Hum Genet. 2013;132:1141–1151. doi: 10.1007/s00439-013-1318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpyak VM, Geske JR, Colby CL, Mrazek DA, Biernacka JM. Genetic variability in the NMDA-dependent AMPA trafficking cascade is associated with alcohol dependence. Addict Biol. 2012;17:798–806. doi: 10.1111/j.1369-1600.2011.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara DI, Williams AS, Benedito LA, Ranscht B, Kobzik L, Hug C, Shore SA. Role of the adiponectin binding protein, T-cadherin (cdh13), in pulmonary responses to subacute ozone. PLoS One. 2013;8:e65829. doi: 10.1371/journal.pone.0065829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-J, Park BL, Yoon S, Lee H-K, Joe K-H, Cheon Y-H, Gwon D-H, Cho S-N, Lee HW, NamGung S, Shin HD. 5′ UTR polymorphism of dopamine receptor D1 (DRD1) associated with severity and temperament of alcoholism. Biochem Biophys Res Commun. 2007;357:1135–1141. doi: 10.1016/j.bbrc.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Gabovits B, Levin A, Veske A, Qin L, Dai F, Belfer I. Chronic pain after lower abdominal surgery: do catechol-O-methyl transferase/opioid receptor µ-1 polymorphisms contribute? Mol Pain. 2013;9:19–26. doi: 10.1186/1744-8069-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontou PI, Pavlopoulou A, Dimou NL, Pavlopoulos GA, Bagos PG. Network analysis of genes and their association with diseases. Gene. 2016;590:68–78. doi: 10.1016/j.gene.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Frontiers in Psychiatry. 2013;4:1–18. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of reward and stress and its relevance for understanding drug-seeking and dependence symptomatology. In: Sher KJ, editor. The Oxford Handbook of Substance Use and Substance Use Disorders. Vol. 1. Oxford Library of Psychology; New York: 2016. pp. 166–191. [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lönnqvist J, Partonen T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol. 2010;45:303–311. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- Lai J, Luo M-C, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9:1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck C, Rosenheck RA, Cramer J, Southwick S, Charney D, Krystal J, Gelernter J. A functional neuropeptide y leu7pro polymorphism associated with alcohol dependence in a large population sample from the united states. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, Somberg LK, Covault J, Kranzler HR, Krystal JH, Gelernter J. Association Between Alcoholism and γ-Amino Butyric Acid α2 Receptor Subtype in a Russian Population. Alcoholism: Clinical and Experimental Research. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Lea RA, Dohy A, Jordan K, Quinlan S, Brimage PJ, Griffiths LR. Evidence for allelic association of the dopamine β-hydroxylase gene (DBH) with susceptibility to typical migraine. Neurogenetics. 2000;3:35–40. doi: 10.1007/pl00022977. [DOI] [PubMed] [Google Scholar]
- Liang J, Olsen RW. Alcohol use disorders and current pharmacological therapies: the role of GABAA receptors. Acta Pharmacol Sin. 2014;35:981–993. doi: 10.1038/aps.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P-L, Yu Y-W, Chung R-H. Pathway Analysis Incorporating Protein-Protein Interaction Networks Identified Candidate Pathways for the Seven Common Diseases. PLoS One. 2016;11:e0162910. doi: 10.1371/journal.pone.0162910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Vink JM, Pergadia ML, Hansell NK, de Moor MHM, Smit AB, Hottenga J-J, Richter MM, Heath AC, Martin NG, Willemsen G, de Geus EJC, Vogelzangs N, Penninx BW, Whitfield JB, Montgomery GW, Boomsma DI, Madden PAF. A genomewide association study of nicotine and alcohol dependence in Australian and Dutch populations. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2010;13:10–29. doi: 10.1375/twin.13.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang H-P, Tsai M-J, Rosenfeld MG. Tbx19, a tissue-selective regulator of POMC gene expression. Proceedings of the National Academy of Sciences. 2001;98:8674–8679. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh EW, Lane HY, Chen CH, Chang PS, Ku LW, Wang KHT, Cheng ATA. Glutamate decarboxylase genes and alcoholism in Han Taiwanese men. Alcoholism: Clinical and Experimental Research. 2006;30:1817–1823. doi: 10.1111/j.1530-0277.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- Maher BH, Lea RA, Follett J, Cox HC, Fernandez F, Esposito T, Gianfrancesco F, Haupt LM, Griffiths LR. Association of a GRIA3 Gene Polymorphism With Migraine in an Australian Case-Control Cohort. Headache: The Journal of Head and Face Pain. 2013;53:1245–1249. doi: 10.1111/head.12151. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. κ-Opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Jauand M, Sitges C, Rodriguez V, Picornell A, Ramon M, Buskila D, Montoya P. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. European Journal of Pain. 2013;17:16–27. doi: 10.1002/j.1532-2149.2012.00153.x. [DOI] [PubMed] [Google Scholar]
- Matsuda JB, Barbosa FR, Morel LJF, França SdC, Zingaretti SM, Silva LMd, Pereira AMS, Marins M, Fachin AL. Serotonin receptor (5-HT 2A) and catechol-O-methyltransferase (COMT) gene polymorphisms: triggers of fibromyalgia? Revista brasileira de reumatologia. 2010;50:141–145. [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: within-family association and linkage analyses. Journal of Studies on Alcohol and Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbarek H, Milaneschi Y, Fedko IO, Hottenga J-J, de Moor MHM, Jansen R, Gelernter J, Sherva R, Willemsen G, Boomsma DI, Penninx BW, Vink JM. The genetics of alcohol dependence: Twin and SNP-based heritability, and genome-wide association study based on AUDIT scores. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015;168:739–748. doi: 10.1002/ajmg.b.32379. [DOI] [PubMed] [Google Scholar]
- McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2013;8:CD006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Meloto CB, Segall SK, Smith S, Parisien M, Shabalina SA, Rizzatti-Barbosa CM, Gauthier J, Tsao D, Convertino M, Piltonen MH, Slade GD, Fillingim RB, Greenspan JD, Ohrbach R, Knott C, Maixner W, Zaykin D, Dokholyan NV, Reenila I, Mannisto PT, Diatchenko L. COMT gene locus: new functional variants. Pain. 2015;156:2072–2083. doi: 10.1097/j.pain.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of chronic pain. 2. IASP Press; Washington: 1994. [Google Scholar]
- Meyers JL, Nyman E, Loukola A, Rose RJ, Kaprio J, Dick DM. The Association between DRD2/ANKK1 and Genetically Informed Measures of Alcohol Use and Problems. Addict Biol. 2013;18:523–536. doi: 10.1111/j.1369-1600.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti A, Liguori R, Toriello M, D’Antò V, Vitale D, Castaldo G, Sacchetti L. Catechol-O-methyltransferase (COMT) gene polymorphisms as risk factor in temporomandibular disorders patients from southern Italy. The Clinical journal of pain. 2014;30:129–133. doi: 10.1097/AJP.0b013e318287a358. [DOI] [PubMed] [Google Scholar]
- Miranda J, Lamana SMS, Dias EV, Athie M, Parada CA, Tambeli CH. Effect of pain chronification and chronic pain on an endogenous pain modulation circuit in rats. Neuroscience. 2015;286:37–44. doi: 10.1016/j.neuroscience.2014.10.049. [DOI] [PubMed] [Google Scholar]
- Mitsi V, Zachariou V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience. 2016;338:81–92. doi: 10.1016/j.neuroscience.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochi M, Cevoli S, Cortelli P, Pierangeli G, Soriani S, Scapoli C, Montagna P. A genetic association study of migraine with dopamine receptor 4, dopamine transporter and dopamine-beta-hydroxylase genes. Neurol Sci. 2003;23:301–305. doi: 10.1007/s100720300005. [DOI] [PubMed] [Google Scholar]
- Mrazek D. Psychiatric Pharmacogenomics. Oxford University Press; New York: 2010. [Google Scholar]
- Munafo MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Mutlu N, Emin Erdal M, Herken H, Ozkaya M, Erdal N, Oz G, Bayazit YA. Monoamine oxidase-A gene promoter polymorphism in temporomandibular joint pain and dysfunction. The Pain Clinic. 2005;17:39–44. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. [Accessed April 22, 2017];Understanding alcohol’s impact on health [NIAAA Web site] 2016 Sep; Available at: https://pubs.niaaa.nih.gov/publications/impactsfactsheet/impactsFactSheet.pdf ( https://pubs.niaaa.nih.gov/publications/impactsfactsheet/impactsFactSheet.pdf)
- Netzer C, Freudenberg J, Toliat MR, Heinze A, Heinze-Kuhn K, Thiele H, Goebel I, Nürnberg P, Ptáček LJ, Göbel H, Todt U, Kubisch C. Genetic association studies of the chromosome 15 GABA-A receptor cluster in migraine with aura. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:37–41. doi: 10.1002/ajmg.b.30560. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Amygdala pain mechanisms. Pain Control. 2015;227:261–284. doi: 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CS, Knudsen GP, Steingrimsdottir OA. Twin studies of pain. Clin Genet. 2012;82:331–340. doi: 10.1111/j.1399-0004.2012.01938.x. [DOI] [PubMed] [Google Scholar]
- Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Røe C, Gjerstad J. Pain intensity the first year after lumbar disc herniation is associated with the A118G polymorphism in the opioid receptor mu 1 gene: evidence of a sex and genotype interaction. J Neurosci. 2012;32:9831–9834. doi: 10.1523/JNEUROSCI.1742-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omair A, Lie BA, Reikeras O, Holden M, Brox JI. Genetic contribution of catechol-O-methyltransferase variants in treatment outcome of low back pain: a prospective genetic association study. BMC Musculoskelet Disord. 2012;13:76–85. doi: 10.1186/1471-2474-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omair A, Mannion AF, Holden M, Fairbank J, Lie BA, Hägg O, Fritzell P, Brox JI. Catechol-O-methyltransferase (COMT) gene polymorphisms are associated with baseline disability but not long-term treatment outcome in patients with chronic low back pain. Eur Spine J. 2015;24:2425–2431. doi: 10.1007/s00586-015-3866-5. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Oswell G, Kaunisto MA, Kallela M, Hämäläinen E, Anttila V, Kaprio J, Färkkilä M, Wessman M, Palotie A. No association of migraine to the GABA-A receptor complex on chromosome 15. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:33–36. doi: 10.1002/ajmg.b.30566. [DOI] [PubMed] [Google Scholar]
- Paterson AD, Sunohara GA, Kennedy JL. Dopamine D4 receptor gene: novelty or nonsense? Neuropsychopharmacology. 1999;21:3–16. doi: 10.1016/S0893-133X(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Price SC, Wilhoit TL, Jones KW. Comorbid migraine with aura, anxiety, and depression is associated with dopamine D2 receptor (DRD2) NcoI alleles. Mol Med. 1998;4:14–21. [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Verdecchia M, Pinzone V, Guidetti V. Migraine genetics: current findings and future lines of research. Neurogenetics. 2015;16:77–95. doi: 10.1007/s10048-014-0433-x. [DOI] [PubMed] [Google Scholar]
- Peters MJ, Broer L, Willemen HLDM, Eiriksdottir G, Hocking LJ, Holliday KL, Horan MA, Meulenbelt I, Neogi T, Popham M, Schmidt CO, Soni A, Valdes AM, Amin N, Dennison EM, Eijkelkamp N, Harris TB, Hart DJ, Hofman A, Huygen FJPM, Jameson KA, Jones GT, Launer LJ, Kerkhof HJM, de Kruijf M, McBeth J, Kloppenburg M, Ollier WE, Oostra B, Payton A, Rivadeneira F, Smith BH, Smith AV, Stolk L, Teumer A, Thomson W, Uitterlinden AG, Wang K, van Wingerden SH, Arden NK, Cooper C, Felson D, Gudnason V, Macfarlane GJ, Pendleton N, Slagboom PE, Spector TD, Völzke H, Kavelaars A, van Duijn CM, Williams FMK, van Meurs JBJ. Genome-wide association study meta-analysis of chronic widespread pain: evidence for involvement of the 5p15.2 region. Ann Rheum Dis. 2013;72:427–436. doi: 10.1136/annrheumdis-2012-201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PGC. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry. 2009;166:540–556. doi: 10.1176/appi.ajp.2008.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piascik MT, Perez DM. α1-Adrenergic receptors: new insights and directions. J Pharmacol Exp Ther. 2001;298:403–410. [PubMed] [Google Scholar]
- Polimanti R, Kranzler HR, Gelernter J. Phenome-wide association study for alcohol and nicotine risk alleles in 26394 women. Neuropsychopharmacology. 2016;41:2688–2696. doi: 10.1038/npp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti L, Diana M. Drug dependence as a disorder of neural plasticity: focus on dopamine and glutamate. Rev Neurosci. 2001;12:141–158. doi: 10.1515/revneuro.2001.12.2.141. [DOI] [PubMed] [Google Scholar]
- Richmond RC, Smith GD, Ness AR, Den Hoed M, McMahon G, Timpson NJ. Assessing causality in the association between child adiposity and physical activity levels: a Mendelian randomization analysis. PLoS Med. 2014;11:e1001618. doi: 10.1371/journal.pmed.1001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain. 2009;10:944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero O, Selten MM, Sich S, Popp S, Bacmeister L, Amendola E, Negwer M, Schubert D, Proft F, Kiser D, Schmitt AG, Gross C, Kolk SM, Strekalova T, van den Hove D, Resink TJ, Nadif Kasri N, Lesch KP. Cadherin-13, a risk gene for ADHD and comorbid disorders, impacts GABAergic function in hippocampus and cognition. Transl Psychiatry. 2015;5:e655. doi: 10.1038/tp.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Russo L, Mariotti P, Sangiorgi E, Giordano T, Ricci I, Lupi F, Chiera R, Guzzetta F, Neri G, Gurrieri F. A New Susceptibility Locus for Migraine with Aura in the 15q11-q13 Genomic Region Containing Three GABA-A Receptor Genes. Am J Hum Genet. 2005;76:327–333. doi: 10.1086/427521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rut M, Machoy-Mokrzyńska A, Ręcławowicz D, Słoniewski P, Kurzawski M, Droździk M, Safranow K, Morawska M, Białecka M. Influence of variation in the catechol-O-methyltransferase gene on the clinical outcome after lumbar spine surgery for one-level symptomatic disc disease: a report on 176 cases. Acta Neurochir (Wien) 2014;156:245–252. doi: 10.1007/s00701-013-1895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, Desrivières S, Aliev FA, Khan AA, Amin N, Aulchenko YS, Bakalkin G, Bakker SJ, Balkau B, Beulens JW, Bilbao A, de Boer RA, Beury D, Bots ML, Breetvelt EJ, Cauchi S, Cavalcanti-Proença C, Chambers JC, Clarke T-K, Dahmen N, de Geus EJ, Dick D, Ducci F, Easton A, Edenberg HJ, Esko T, Fernández-Medarde A, Foroud T, Freimer NB, Girault J-A, Grobbee DE, Guarrera S, Gudbjartsson DF, Hartikainen A-L, Heath AC, Hesselbrock V, Hofman A, Hottenga J-J, Isohanni MK, Kaprio J, Khaw K-T, Kuehnel B, Laitinen J, Lobbens S, Luan Ja, Mangino M, Maroteaux M, Matullo G, McCarthy MI, Mueller C, Navis G, Numans ME, Núñez A, Nyholt DR, Onland-Moret CN, Oostra BA, O'Reilly PF, Palkovits M, Penninx BW, Polidoro S, Pouta A, Prokopenko I, Ricceri F, Santos E, Smit JH, Soranzo N, Song K, Sovio U, Stumvoll M, Surakk I, Thorgeirsson TE, Thorsteinsdottir U, Troakes C, Tyrfingsson T, Tönjes A, Uiterwaal CS, Uitterlinden AG, van der Harst P, van der Schouw YT, Staehlin O, Vogelzangs N, Vollenweider P, Waeber G, Wareham NJ, Waterworth DM, Whitfield JB, Wichmann EH, Willemsen G, Witteman JC, Yuan X, Zhai G, Zhao JH, Zhang W, Martin NG, Metspalu A, Doering A, Scott J, Spector TD, Loos RJ, Boomsma DI, Mooser V, Peltonen L, Stefansson K, van Duijn CM, Vineis P, Sommer WH, Kooner JS, Spanagel R, Heberlein UA, Jarvelin M-R, Elliott P. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proceedings of the National Academy of Sciences. 2011;108:7119–7124. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwantes-An T-H, Zhang J, Chen L-S, Hartz SM, Culverhouse RC, Chen X, Coon H, Frank J, Kamens HM, Konte B, Kovanen L, Latvala A, Legrand LN, Maher BS, Melroy WE, Nelson EC, Reid MW, Robinson JD, Shen P-H, Yang B-Z, Andrews JA, Aveyard P, Beltcheva O, Brown SA, Cannon DS, Cichon S, Corley RP, Dahmen N, Degenhardt L, Foroud T, Gaebel W, Giegling I, Glatt SJ, Grucza RA, Hardin J, Hartmann AM, Heath AC, Herms S, Hodgkinson CA, Hoffmann P, Hops H, Huizinga D, Ising M, Johnson EO, Johnstone E, Kaneva RP, Kendler KS, Kiefer F, Kranzler HR, Krauter KS, Levran O, Lucae S, Lynskey MT, Maier W, Mann K, Martin NG, Mattheisen M, Montgomery GW, Müller-Myhsok B, Murphy MF, Neale MC, Nikolov MA, Nishita D, Nöthen MM, Nurnberger J, Partonen T, Pergadia ML, Reynolds M, Ridinger M, Rose RJ, Rouvinen-Lagerström N, Scherbaum N, Schmäl C, Soyka M, Stallings MC, Steffens M, Treutlein J, Tsuang M, Wall TL, Wodarz N, Yuferov V, Zill P, Bergen AW, Chen J, Cinciripini PM, Edenberg HJ, Ehringer MA, Ferrell RE, Gelernter J, Goldman D, Hewitt JK, Hopfer CJ, Iacono WG, Kaprio J, Kreek MJ, Kremensky IM, Madden PAF, McGue M, Munafò MR, Philibert RA, Rietschel M, Roy A, Rujescu D, Saarikoski ST, Swan GE, Todorov AA, Vanyukov MM, Weiss RB, Bierut LJ, Saccone NL. Association of the OPRM1 Variant rs1799971 (A118G) with Non-Specific Liability to Substance Dependence in a Collaborative de novo Meta-Analysis of European-Ancestry Cohorts. Behav Genet. 2016;46:151–169. doi: 10.1007/s10519-015-9737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šerý O, Didden W, Mikeš V, Pitelová R, Znojil V, Zvolský P. The association between high-activity COMT allele and alcoholism. Neuroendocrinology Letters. 2006;27:231–235. [PubMed] [Google Scholar]
- Singh A, Babyak MA, Nolan DK, Brummett BH, Jiang R, Siegler IC, Kraus WE, Shah SH, Williams RB, Hauser ER. Gene by stress genome-wide interaction analysis and path analysis identify EBF1 as a cardiovascular and metabolic risk gene. Eur J Hum Genet. 2015;23:854–862. doi: 10.1038/ejhg.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siontis KCM, Patsopoulos NA, Ioannidis JPA. Replication of past candidate loci for common diseases and phenotypes in 100 genome-wide association studies. Eur J Hum Genet. 2010;18:832–837. doi: 10.1038/ejhg.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. Am J Epidemiol. 2008;167:125–138. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- Smith SB, Maixner D, Greenspan J, Dubner R, Fillingim R, Ohrbach R, Knott C, Slade G, Bair E, Gibson DG, Zaykin DV, Weir B, Maixner W, Diatchenko L. Potential Genetic Risk Factors for Chronic TMD: Genetic Associations from the OPPERA Case Control Study. The Journal of Pain. 2011;12:T92–101. doi: 10.1016/j.jpain.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Maixner DW, Fillingim RB, Slade G, Gracely RH, Ambrose K, Zaykin DV, Hyde C, John S, Tan K, Maixner W, Diatchenko L. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum. 2012;64:584–593. doi: 10.1002/art.33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proceedings of the National Academy of Sciences. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42:184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Gizer IR, Young-Wolff KC. Genetic epidemiology and molecular genetics. In: Sher KJ, editor. The Oxford Handbook of Substance Use and Substance Use Disorders. Vol. 1. Oxford Library of Psychology; New York: 2016. pp. 192–272. [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress Cognition and Health. John Wiley & Sons; New York: 1988. pp. 629–649. [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, Hübner N, Heinig M, Pravenec M, Mangion J, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders J, Grant B, Hoffman PL, Alcoholism WISoSaTMo Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, Ohtsuki Y. Expression of T-Cadherin (CDH13, H-Cadherin) in Human Brain and Its Characteristics as a Negative Growth Regulator of Epidermal Growth Factor in Neuroblastoma Cells. J Neurochem. 2000;74:1489–1497. doi: 10.1046/j.1471-4159.2000.0741489.x. [DOI] [PubMed] [Google Scholar]
- Tammimäki A, Männistö PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012;22:673–691. doi: 10.1097/FPC.0b013e3283560c46. [DOI] [PubMed] [Google Scholar]
- Taylor AMW, Becker S, Schweinhardt P, Cahill C. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain. 2016;157:1194–1198. doi: 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrón JA. Novel insights into the potential involvement of 5-HT7 receptors in endocrine dysregulation in stress-related disorders. Rev Neurosci. 2014;25:439–449. doi: 10.1515/revneuro-2014-0017. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, Salonen JT, Ryynänen OP, Koulu M, Karvonen MK, Phjalainen T, Syvälahti E, Hietala J. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type. Mol Psychiatry. 1999;4:286–289. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- Tikkanen R, Ducci F, Goldman D, Holi M, Lindberg N, Tiihonen J, Virkkunen M. MAOA Alters the Effects of Heavy Drinking and Childhood Physical Abuse on Risk for Severe Impulsive Acts of Violence Among Alcoholic Violent Offenders. Alcoholism: Clinical and Experimental Research. 2010;34:853–860. doi: 10.1111/j.1530-0277.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Dunckley P. Importance of anti-and pro-nociceptive mechanisms in human disease. Gut. 2004;53:1553–1555. doi: 10.1136/gut.2004.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Maier W, Moessner R, Gaebel W, Dahmen N, Fehr C, Scherbaum N, Steffens M, Ludwig KU, Frank J, Wichmann HE, Schreiber S, Dragano N, Sommer WH, Leonardi-Essmann F, Lourdusamy A, Gebicke-Haerter P, Wienker TF, Sullivan PF, Nöthen MM, Kiefer F, Spanagel R, Mann K, Rietschel M. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Vallette-Kasic S, Brue T, Pulichino A-M, Gueydan M, Barlier A, David M, Nicolino M, Malpuech G, Déchelotte P, Deal C, Van Vliet G, De Vroede M, Riepe FG, Partsch C-J, Sippell WG, Berberoglu M, Atasay Bm, de Zegher F, Beckers D, Kyllo J, Donohoue P, Fassnacht M, Hahner S, Allolio B, Noordam C, Dunkel L, Hero M, Pigeon B, Weill J, Yigit S, Brauner R, Heinrich JJ, Cummings E, Riddell C, Enjalbert A, Drouin J. Congenital Isolated Adrenocorticotropin Deficiency: An Underestimated Cause of Neonatal Death, Explained by TPIT Gene Mutations. The Journal of Clinical Endocrinology & Metabolism. 2005;90:1323–1331. doi: 10.1210/jc.2004-1300. [DOI] [PubMed] [Google Scholar]
- Vargas-Alarcón G, Fragoso J-M, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadóniga J-I, García-Fructuoso F, Ramos-Kuri M, Hernández F, Springall R, Bojalil R, Vallejo M, Martínez-Lavín M. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Res Ther. 2007;9:R110–R116. doi: 10.1186/ar2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer AVT, Yano JM, Carroll FI, Cohen BM, Carlezon WA. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the κ-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45:1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisey J, Swagell CD, Hughes IP, Lawford BR, Young RMD, Morris CP. A novel SNP in COMT is associated with alcohol dependence but not opiate or nicotine dependence: a case control study. Behavioral and Brain Functions. 2011;7:51–56. doi: 10.1186/1744-9081-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennett GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Kohno T, Amaya F, Brenner GJ, Ito N, Allchorne A, Ji R-R, Woolf CJ. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J Neurosci. 2005;25:7986–7992. doi: 10.1523/JNEUROSCI.2393-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Foroud T, Hinrichs AL, Le NXH, Bertelsen S, Budde JP, Harari O, Koller DL, Wetherill L, Agrawal A, Almasy L, Brooks AI, Bucholz K, Dick D, Hesselbrock V, Johnson EO, Kang S, Kapoor M, Kramer J, Kuperman S, Madden PAF, Manz N, Martin NG, McClintick JN, Montgomery GW, Nurnberger JI, Rangaswamy M, Rice J, Schuckit M, Tischfield JA, Whitfield JB, Xuei X, Porjesz B, Heath AC, Edenberg HJ, Bierut LJ, Goate AM. A genome-wide association study of alcohol-dependence symptom counts in extended pedigrees identifies C15orf53. Mol Psychiatry. 2013;18:1218–1224. doi: 10.1038/mp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K-S, Liu X, Zhang Q, Wu L-Y, Zeng M. Genome-wide association study identifies 5q21 and 9p24. 1 (KDM4C) loci associated with alcohol withdrawal symptoms. J Neural Transm. 2012;119:425–433. doi: 10.1007/s00702-011-0729-z. [DOI] [PubMed] [Google Scholar]
- Wang F, Simen A, Arias A, Lu Q-W, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Hum Genet. 2013;132:347–358. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Khachaturian H, Akil H, Coy DH, Goldstein A. Comparison of the distribution of dynorphin systems and enkephalin systems in brain. Science. 1982;218:1134–1136. doi: 10.1126/science.6128790. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wiesbeck GA, Dürsteler-MacFarland KM, Wurst FM, Walter M, Petitjean S, Müller S, Wodarz N, Böning J. No association of dopamine receptor sensitivity in vivo with genetic predisposition for alcoholism and DRD2/DRD3 gene polymorphisms in alcohol dependence. Addict Biol. 2006;11:72–75. doi: 10.1111/j.1369-1600.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Wood PB. Mesolimbic dopaminergic mechanisms and pain control. Pain. 2006;120:230–234. doi: 10.1016/j.pain.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, Zhao H, Farrer LA, Gelernter J. Genomewide association study for maximum number of alcoholic drinks in European Americans and African Americans. Alcoholism: Clinical and Experimental Research. 2015;39:1137–1147. doi: 10.1111/acer.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, Goate A, Bucholz K, Schuckit M, Nurnberger J, Jr, Tischfield J, Kuperman S, Porjesz B, Begleiter H, Foroud T, Edenberg HJ. Association of the [kappa]-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nakaoka H, Yamamoto K, Fujikawa T, Kim YI, Yano K, Haga S, Katayama K, Shibusawa T, Park SB, Maki K, Kimura R, Inoue I. Genome-wide association study of degenerative bony changes of the temporomandibular joint. Oral Dis. 2014;20:409–415. doi: 10.1111/odi.12141. [DOI] [PubMed] [Google Scholar]
- Yesilyurt O, Seyrek M, Tasdemir S, Kahraman S, Deveci MS, Karakus E, Halici Z, Dogrul A. The critical role of spinal 5-HT 7 receptors in opioid and non-opioid type stress-induced analgesia. Eur J Pharmacol. 2015;762:402–410. doi: 10.1016/j.ejphar.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kranzler HR, Panhuysen C, Weiss RD, Poling J, Farrer LA, Gelernter J. Substance dependence low-density whole genome association study in two distinct American populations. Hum Genet. 2008;123:495–506. doi: 10.1007/s00439-008-0501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, Hemani G, Tansey K, Laurin C, Pourcain BS, Warrington NM, Finucane HK, Price AL, Bulik-Sullivan BK, Anttila V, Paternoster L, Gaunt TR, Evans DM, Neale BM. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–279. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Rice JP, Schuckit MA, Foroud T, Edenberg HJ, Porjesz B, Almasy L. Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156:44–58. doi: 10.1002/ajmg.b.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollanvari A, Alterovitz G. SNP by SNP by environment interaction network of alcoholism. BMC Syst Biol. 2017;11:19–29. doi: 10.1186/s12918-017-0403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorina-Lichtenwalter K, Meloto CB, Khoury S, Diatchenko L. Genetic predictors of human chronic pain conditions. Neuroscience. 2016;338:36–62. doi: 10.1016/j.neuroscience.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Zuo L, Gelernter J, Zhang CK, Zhao H, Lu L, Kranzler HR, Malison RT, Li C-SR, Wang F, Zhang X-Y, Deng H-W, Krystal JH, Zhang F, Luo X. Genome-Wide Association Study of Alcohol Dependence Implicates KIAA0040 on Chromosome 1q. Neuropsychopharmacology. 2012;37:557–566. doi: 10.1038/npp.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Lu L, Tan Y, Pan X, Cai Y, Wang X, Hong J, Zhong C, Wang F, Zhang X-Y, Vanderlinden LA, Tabakoff B, Luo X. Genome-wide association discoveries of alcohol dependence. The American Journal on Addictions. 2014;23:526–539. doi: 10.1111/j.1521-0391.2014.12147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]