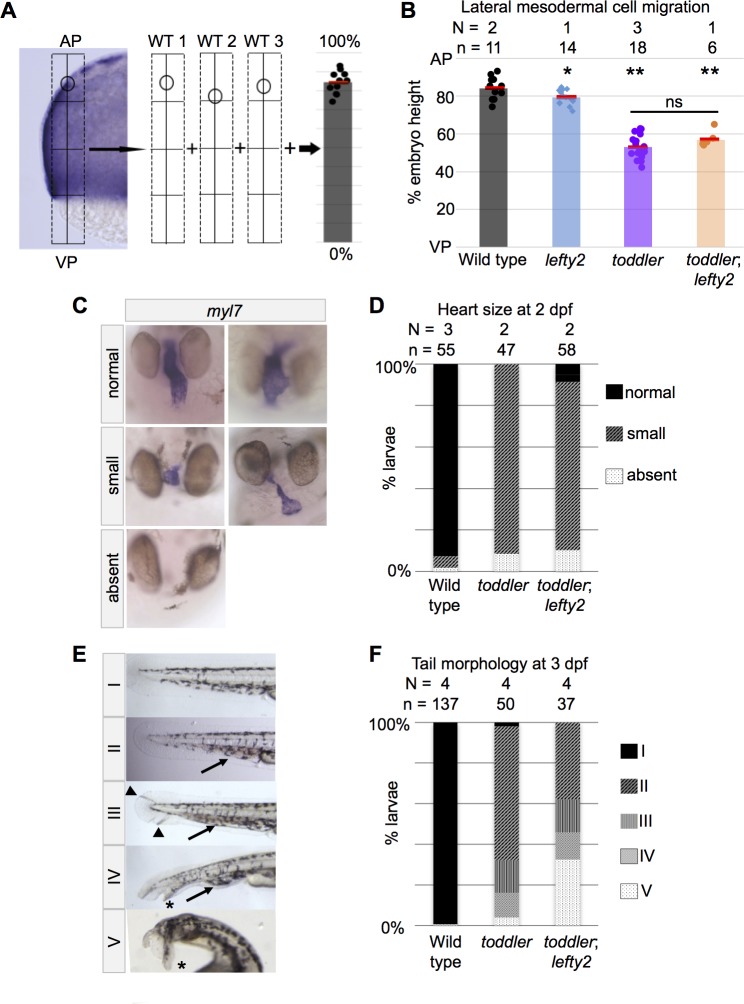
Figure 2. Mesodermal cell migration and larval phenotypes are not rescued by increased numbers of endodermal cells.
(A) Schematic representation of experimental measurements shown in B. Densely packed, fn1a + lateral mesodermal cells are viewed by cross section. Location of the most animally migrated lateral cells of individual embryos are measured relative to AP-VP axis, then consolidated across embryos. AP = Animal pole; VP = vegetal pole. (B) Defects in animal-pole directed migration of mesodermal cells are still present in toddler;lefty2 double mutants. Each point represents a single embryo. Red bars are averages. p-Values for pairwise comparison with wild type unless indicated otherwise. *p=0.04, **p<0.001; ns: p=0.08; unpaired two-tailed t-test. (C–D) toddler;lefty2 double mutants resemble toddler single mutant siblings in respect to heart phenotypes at 2 days post fertilization (dpf). Hearts were classified as small if shortened by more than half of normal length and/or excessively narrow or thin. Hearts that were neither thin nor short but appeared to have looping defects or other patterning defects were classified as normal. Heart phenotypes were scored blind to genotype for toddler and toddler;lefty2 mutants. (C) Representative images of phenotypic classes after in situ hybridization for myl7. (D) Quantification of C. (E–F) toddler;lefty2 double mutants are more poorly patterned than toddler single mutant siblings in respect to tail phenotypes at 3 dpf. (E) Representative images of phenotypic classes. Phenotype classes II-V lack circulation. Arrow: accumulated blood. Arrowhead: defects in mesenchyme. * Duplicated tail tip. (F) Quantification of E. (B,D,F) N = number of independent experiments; n = number of embryos.