Abstract
The basolateral amygdala (BL) is involved in fear and anxiety but it is currently unclear how the same network supports these two states. To address this question, we trained rats on appetitive and aversive conditioning in different contexts. Distinct groups of BL neurons displayed increased activity during appetitive (CS-R) vs. aversive (CS-S) conditioned stimuli (R-cells and S-cells, respectively) and they were typically inhibited by the other CS. When the CS-S was presented in the safe context, rats entered a long-lasting anxiety-like state characterized by increased inter-CS freezing and impaired reward-seeking. During this state, a subset of BL cells (‘state-cells’) showed sustained shifts in baseline activity whose time course matched that of the behavioral changes. Many state-cells with increased firing rates were S-cells whereas R-cells only included state-cells with reduced firing rates. Thus, anxiety involves persistent activity changes that are differentially expressed by subsets of valence-specific BL neurons.
Fear and anxiety are defensive responses with distinct triggers and durations (Davis et al., 2010). Fear arises in situations where organisms are confronted with imminent threats, resulting in brief defensive behaviors like immobility, escape, or attack, depending on threat proximity. In contrast, anxiety is a longer-lasting state of increased vigilance and apprehension that develops in the anticipation of uncertain or unpredictable perils.
While functional imaging studies have implicated the human amygdala in fear and anxiety (Ipser et al., 2013), animal work on the amygdala has focused on fear, particularly classically conditioned fear. These studies, as well as related investigations on appetitive conditioning, have revealed that the acquisition of conditioned emotional responses involves activity-dependent plasticity in the lateral amygdala (LA) (Duvarci and Pare, 2014). As a result, some LA neurons develop potentiated responses to conditioned stimuli (CSs) that predict aversive or rewarding outcomes (Maren and Quirk, 2004; Paton et al., 2006).
In the basolateral nucleus of the amygdala (BL), a recipient of LA inputs, neurons also develop responses to aversive and appetitive CSs (Amano et al., 2011; Herry et al., 2008; Lee et al., 2016; Sangha et al., 2013). In turn, these cells would drive defensive or approach conditioned responses (CRs) via their various targets (Beyeler et al., 2016; Namburi et al., 2015; Stuber et al., 2011). Indeed, inactivation of LA or BL causes deficit in the acquisition and expression of CRs (Amano et al., 2011; Ambroggi et al., 2008; Herry et al., 2008; Stuber et al., 2011). However, the incidence of (and overlap between) neurons with excitatory or inhibitory responses to appetitive and aversive CSs is still unclear, as prior studies yielded inconsistent results.
Furthermore, the amygdala, and especially BL, has been implicated in the genesis of anxiety (Davis and Walker, 2013; Felix-Ortiz et al., 2013; Tye et al., 2011). For example, presentation of multiple unpredictable footshocks causes a persistent enhancement of baseline startle and this anxiety-like state is blocked by inactivating BL (Davis and Walker, 2013). Also, optogenetic excitation of BL projections to the ventral hippocampus induce an anxiety-like state (Felix-Ortiz et al., 2013). However, the pattern of BL activity associated with anxiety remains to be characterized. It is still unclear whether the expression of anxiety involves the sustained activation of cells with excitatory responses to aversive CSs or the recruitment of a different group of dedicated neurons. Also unknown is how anxiety affects the subset of BL neurons that supports appetitive CRs (Paton et al., 2006; Stuber et al., 2011). The present study addressed these questions.
RESULTS
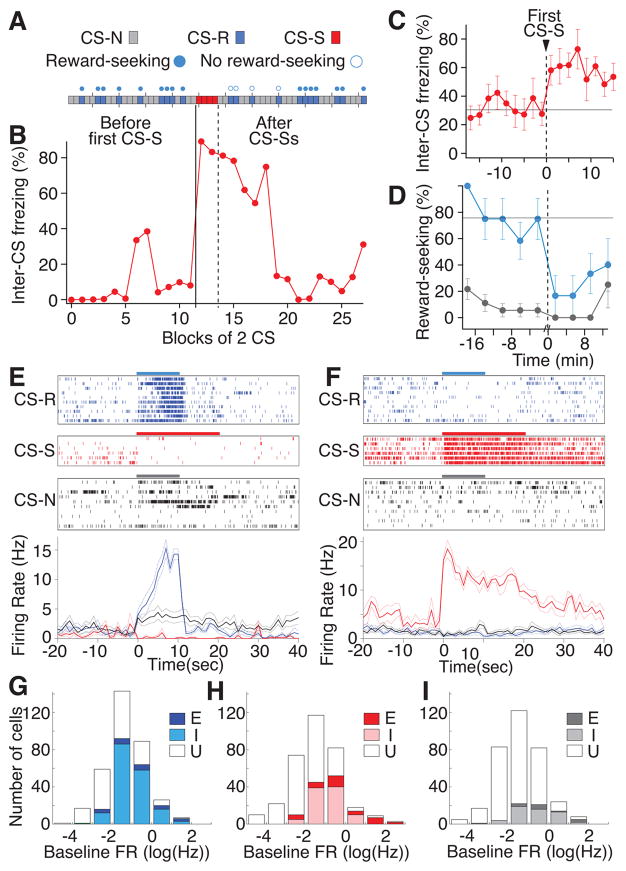
To elicit anxiety-like states, we subjected rats to a mixed appetitive/aversive conditioning paradigm where rats first learned in one environment (A) that distinct auditory stimuli predicted neutral (CS-N) or rewarding (CS-R) outcomes. Then in a second context (B), rats learned that a third cue (CS-S) predicted the delivery of foot-shocks. Next, in context A (the “safe” context), rats were first presented with a mixture of CS-Rs and CS-Ns followed by a series of CS-Ss. Presentations of the threatening CS in the safe context produced an anxiety-like state that continued long after the last CS-S (Fig. 1A–D). This behavioral state was characterized by persistently increased freezing levels in the absence of immediate threat (Fig. 1B,C, inter-CS freezing), and impaired reward-seeking behavior in response to later CS-Rs (Fig. 1A,D). Below, we first characterize the various types of responses elicited in BL neurons by the different CSs and then examine how these transient responses map onto the sustained activity patterns present during anxiety.
Figure 1.
Behavioral manifestations of anxiety and response of PNs to differently valenced CSs. Blue, red and gray are used for the CS-R, CS-S, and CS-N, respectively. (A) Example of reward-seeking behavior before and after presentations of 4 CS-Ss. Each rectangle represents a CS. Filled and empty circles above the rectangles mark trials followed by reward-seeking or not, respectively. (B) Example of persistent increase in freezing during inter-CS periods following CS-Ss (vertical dashed line) in the safe context. Average ± SEM inter-CS freezing (C) and reward-seeking (D) behavior (all six recall sessions), before and after CS-Ss in safe context at time indicated by vertical dashed line. Inter-CS freezing was significantly increased (ranksum test, p<0.001) whereas reward-seeking was significantly decreased (ranksum test, p<0.001) after the CS-Ss. Note that the CS-S presentation period was excluded in D. Activity of representative R-cell (E) and S-cell (F) during the three types of CSs (colored horizontal bars). Top, rasters; bottom, average FR during all available trials (± SEM). PN shown in E had a significantly increased FR during the CS-R and CS-N and a significant activity reduction during the CS-S (rank-sum tests, p’s<0.005). PN shown in F had a significantly increased FR during the CS-S and a significant activity reduction in response to the CS-R. (G–I) Frequency distributions of CS response types as a function of baseline FR (natural log scale). E, excited; I, inhibited; U, unresponsive.
Segregation and overlap between BL neurons responsive to CSs of different valences
Based on spike width and mean firing rate (FR; Supplementary Figs. 1–2), recorded cells (n=371) were classified as putative principal neurons (PNs; n=343; Figs. 1E–I,2; Supplementary Fig. 3) or fast-spiking interneurons (ITNs; n=28; Supplementary Fig. 4). After conditioning, a minority of PNs showed significant increases in FRs during the CS-R (‘R-cells’; 7.0%; Fig. 1E,G) or CS-S (‘S-cells’; 10.5%; Fig. 1F,H; rank-sum tests, p<0.005; Supplementary Fig. 3). In contrast, the incidence of PNs with reduced FRs during these two CSs was much higher (51.0 and 27.4% of cells with the CS-R and CS-S, respectively; Fig. 1G,H). Supplementary Fig. 5 shows where CS-responsive PNs are located.
Figure 2.
Segregation and overlap among PNs responding to appetitive and aversive CSs. (A,B) Ratio (natural log scale) of FRs during CS-S and baseline (y-axis) as a function of that between CS-R and baseline (x-axis) for CS-R responsive (A) or CS-S responsive (B) PNs. Filled circles, PNs responsive to both CS-R and CS-S; blue empty circle, PNs responsive to CS-R; red empty circle, PNs responsive to CS-S. Grey empty circles, unresponsive PNs. (c) Responses of PNs excited (left) or inhibited (right) by one CS to the other CS (of opposite valence). Number of PNs are plotted. Black, excited by other CS; grey, inhibited by other CS; white, unresponsive to other CS.
To examine whether individual PNs respond to CSs of opposite valence in similar or different ways, we plotted their responses to the CS-R against that to the CS-S. This analysis revealed that while there was little overlap between PNs excited by the CS-R or CS-S (4 cells), cells inhibited by both were common (Fig. 2A,B). Furthermore, R- and S-cells often displayed inhibitory responses to the other CS (Fig. 2C), suggesting that there is an antagonistic relationship between them. Among PNs inhibited during the CS-R (n=175) or the CS-S (n=94), respectively 31% and 58% reduced their FR during the other CS (Fig. 2C). Consistent with the neutral outcome of the CS-N, the incidence of PNs with altered FRs during this CS was lower than with the other two CSs (Chi-square=242.7, p<0.0001; increase, 3.8%; decrease, 15.7%; Fig. 1I) and these firing changes had a lower amplitude than with the other CSs (Supplementary Fig. 3). Last, irrespective of CS identity, unresponsive and inhibited cells were more common among PNs with low FRs and conversely for CS-excited cells (Fig. 1G–I). Overall, these results indicate that CS-excited cells are comprised of two largely independent subsets of PNs with opposite response profiles. In contrast, PNs inhibited by the different CSs overlap extensively.
Relative to PNs, a higher proportion of presumed ITNs showed significant increases in FRs (rank-sum tests, p<0.005) during the various CSs (Supplementary Fig. 4): 57% of ITNs were activated during at least one CS, compared to 17.2% of PNs (Chi-square=25.6, p<0.0001). However, the incidence of inhibitory responses did not differ (Chi-square=1.47, p=0.23, respectively 53.6 and 65.0% of ITNs and PNs were inhibited during at least one CS). As a result, a similar proportion of ITNs showed reductions vs. increases in FRs during the various CSs (Supplementary Fig. 4F–H), with 25% exhibiting responses of opposite polarities during the CS-R and CS-S (Supplementary Fig. 4K). Finally, dissimilar to PNs, there was no relationship between baseline FRs and CS response types among ITNs (Supplementary Fig. 4F–H).
Relation between CS responsiveness and anxiety-related activity
Upon presentation of the first CS-S in the safe context (A), a subset of PNs (11.4%) and ITNs (18%), hereafter termed ‘state-cells’, showed persistent increases or decreases in baseline FRs, as assessed in between the CSs (Kruskall-Wallis ANOVA, p<0.05; Fig. 3). These cells were distributed among the various types of CS-responsive neurons described above. Supplementary figure 6 shows their location. Examples of state PNs can be seen in figure 3 (S-cells, Fig. 3A,B; R-cells, Fig. 3C,D, and PNs with reduced activity during the CS-R and CS-S -termed RiSi-, Fig. 3E,F). Additional state-cells are illustrated in Supplementary figure 7. As apparent in these representative examples, the change in baseline activity typically began right after the first CS-S, and persisted long after the last CS-S, paralleling the persistent increase in freezing levels (Fig. 1C) and decrease in reward-seeking (Figs. 1D, 3A–F) associated with anxiety. Of note, none of the PNs excited by both, the CS-R and CS-S, were state-cells.
Figure 3.
Presentation of CS-S in safe context causes long-lasting changes in baseline FRs. Examples of state-cells: S-cells (A,B), R-cells (C,D), and RiSi-cells (E,F). Top, rasters; bottom average FR during all available trials (± SEM). Blue and red horizontal bars at top of rasters indicate when CS-R and CS-S were presented, respectively. Stack of colored rectangles on right of each raster indicates sequence of CS presented (gray, CS-N; blue, CS-R; red, CS-S). Filled and empty circles mark CS-R trials where rat sought the reward or not, respectively. Relative to baseline, all cells shown in A-F had significantly increased (A,B) or decreased (C–F) inter-CS FRs upon introduction of the CS-Ss (Kruskal-Wallis one-way ANOVA; see Methods). The spike waveforms of these cells are shown in Supplementary Figure 1. (G) Proportion of PNs with significant increases (red) or decrease (blue) baseline FR after CS-S presentation among different classes of cells (labels by each bar). The lower-case letters u, i or e, following R or S indicate unresponsive, inhibited or excited by the corresponding CS (R or S), respectively. Dashed lines indicate average proportion of cells with significantly increased (top) or decreased (bottom) baseline FR across entire sample of PNs (n=343). (H) Average (solid line) (±SEM, dashed lines) baseline FRs of all available R-cells (blue, n=20), S-cells (red, n=32), RuSi cells (gray, n=33), and RiSi-cells (black, n=54). Note that PNs excited by both CSs (n=4) are not included here. Time zero corresponds to start of phase 2, when CS-S are presented.
The incidence of anxiety-related neurons varied significantly between the different classes of PNs we delineated based on their activity during valenced CSs (Fig. 3G; Chi-Square=17.45, p=0.004). In particular, state-cells consisted of ~30% of S-cells compared to ~9% of the other cell classes combined. Moreover, the incidence of PNs with increased, decreased or unchanged activity during anxiety differed significantly between S-cells and R-cells (Chi-Square=8.46, p=0.015) and there was a strong correspondence between the polarity of the cells’ responses to the different CSs and the polarity of the changes in baseline FRs they displayed during anxiety. Indeed, S-cells were ten times more likely to exhibit increased than decreased baseline FRs during anxiety (Fig. 3G). In contrast, all state-cells found among R-cells showed a decrease in baseline activity during anxiety (Fig. 3G).
Consistent with these findings, separately averaging the baseline activity of all PNs (not only the state-cells) within the various subclasses delineated in figure 3G revealed that S-cells and R-cells, underwent sustained alterations in activity during anxiety (Fig. 3H). On average S-cells displayed a ~5-fold increase in baseline FR (Fig. 3H, red; signed-rank test, p<0.001). Conversely, R-cells displayed a ~60% reduction in baseline activity (Fig. 3H, blue; signed-rank test, p=0.03). No change in baseline activity was detected in the other classes of PNs. Overall, these results suggest that anxiety recruits two types of state-cells, with decreased or increased baseline FRs, and that they are differentially represented among R- and S-cells.
So far, state-cells were classified using changes in baseline FRs without considering the temporal structure of their activity. Thus, we next tested whether the ensemble activity of state-cells correlated with moment-to-moment variations in freezing behavior, using a decoder analysis on recording sessions where multiple state-cells were simultaneously recorded. Six of fourteen recording sessions from five rats met this criterion (recall sessions, n=2; extinction training sessions, n=4). During these sessions, we simultaneously recorded an average of 7.2 ± 1.7 state-cells (range 2 to 13). Examples are shown in figure 4A,B. In all six tested sessions, there was a significant correlation between predicted and observed values (range of r: 0.35 to 0.85; average 0.56 ± 0.08). Figure 4C–E illustrates three such examples with significant (p<0.05) freezing correlations of 0.85, 0.73, and 0.7, respectively. To examine whether state-cells only encode freezing, we calculated the proportion of FR variance explained by freezing vs. state using an ANOVA. State accounted for a higher proportion of FR variance than freezing (Supplementary Fig. 8A). Comparing the FR of state-cells during periods devoid of freezing within vs. outside the anxiety phase also showed significant FR changes (Supplementary Fig. 8B), again indicating that state-cells encode state even when the contribution of freezing is excluded. In fact, we found that not only state-cells, but also cells that responded to the CS-S with increased or decreased firing rates do not only encode freezing as they showed a variable relation to this index of fear (Supplementary Fig. 9).
Figure 4.
Decoder analysis. (A,B) Two subsets of simultaneously recorded PNs that developed significantly altered baseline FRs as a result of CS-S presentations. During the epochs shown in A and B, a total of 48 and 27 cells were simultaneously recorded; respectively 13 and 12 were state-cells. Top, sequence of CS presentations, as indicated by colored circles (red, CS-S; blue, CS-R; black, CS-N). Filled and empty circles mark CS-R or CS-N trials where rats sought the reward or not, respectively. Middle: histograms indicate % time freezing (y-axis) during successive baseline periods (x-axis). Dashed line indicates average inter-CS freezing during entire depicted period. Bottom: each horizontal line is a different cell. Each bin represents the average FR of a cell during a baseline period (z-scored within each cell to visually harmonize different cells). Cells that developed significantly increased (top) or decreased (bottom) baseline FRs as a result of CS-S presentations are grouped. (C–E) Three examples of observed and predicted inter-CS freezing from cross-validation epochs. Grey bars represent observed inter-CS freezing. Red lines correspond to inter-CS freezing predicted by linear decoder based on FRs of ensemble of simultaneously recorded state-cells. Inter-CS freezing of (C) and (E) correspond to (A) and (B), respectively.
The presence of state-cells with increased or decreased FRs among PNs and ITNs raised the possibility that connectivity between specific subsets of state-cells contributes to shape the activity patterns seen during the anxiety-like state. To test this possibility, we carried out cross-correlation analyses between all pairs of simultaneously recorded PNs and ITNs (Supplementary Tables 1–4). Although these analyses revealed that connections between specific subtypes of PNs and ITNs contribute to shape their CS responsiveness (Supplementary Tables 3–4), we found no evidence that monosynaptic connections between relevant groups of state PNs and ITNs contribute to generate the activity patterns seen in BL during anxiety (Supplementary Tables 1–2).
DISCUSSION
Functional imaging and lesion studies in humans suggest that the basolateral amygdala is involved in the genesis of anxiety. For instance, amygdala activation correlates positively with symptom severity in post-traumatic stress disorder (Rauch et al., 2000). Moreover, the incidence of this disorder is much lower in war veterans who suffered localized damage to the amygdala than to other brain regions (Koenigs et al., 2008). However, the neuronal activity patterns that are expressed during anxiety are unknown. The present study aimed to shed light on this question. We found that anxiety is associated with the differential recruitment of competing valence-related BL neurons. The significance of these findings is considered below.
Reward- and threat-predicting cues recruit different subsets of basolateral amygdala neurons
Previously, it was reported that BL modulates anxiety-related behaviors (Davis and Walker, 2013; Felix-Ortiz et al., 2013; Tye et al., 2011) and is involved in the acquisition and expression of appetitive and aversive associative memories (Amano et al., 2011; Ambroggi et al., 2008; Muller et al., 1997; Sierra-Mercado et al., 2011; Stuber et al., 2011). In mixed appetitive-aversive conditioning paradigms, a variety of CS response types were observed, including cells selectively excited or inhibited by positively or negatively valenced CSs as well as neurons exhibiting responses of the same or opposite polarities to these CSs (Beyeler et al., 2016; Paton et al., 2006; Sangha et al., 2013; Shabel and Janak, 2009). However, the incidence of, and overlap between, these various types of neurons in BL remained unclear. Indeed, prior studies yielded inconsistent results, possibly because they used different species and combined data obtained in different nuclei of the amygdala and cell types. Moreover, it remained unknown whether these various subgroups of valence-related BL cells are differentially recruited during anxiety.
In BL, we found that only ~27% of PNs remained unresponsive to the CS-R and CS-S after conditioning. Nearly 60% of PNs developed inhibitory responses to one or both of these CSs. A minority (~16%) displayed higher FRs during the CS-R or CS-S, with cells excited by both being very rare (~1%). Instead, PNs excited by the CS-R or CS-S exhibited inhibitory responses during the other CS or were unresponsive to the other CS. While a higher proportion of presumed ITNs increased their FRs during at least one valenced CS (54%), they too displayed a significant degree of separation as only 11% of interneurons were excited by both CSs. Together, our results indicate that in BL, largely non-overlapping subsets of PNs and ITNs are excited by positively or negatively valenced CSs.
These results raise the question of what factors determine the identity of the PNs recruited in aversive and appetitive memories? A first possibility is connectivity. BL contains multiple subgroups of PNs that project to diverse sites (Pitkanen A, 2000) and recent studies have shown that specific BL projections are associated with different behaviors (Ambroggi et al., 2008; Felix-Ortiz et al., 2013; Namburi et al., 2015; Stuber et al., 2011). The valence-specific subsets of BL neurons observed here may correspond to the distinct BL output circuits evidenced in these prior studies. However, another possible explanation stems from the observation that recruitment of neurons into memory traces is influenced by dynamic factors, like intrinsic neuronal excitability at the time of training, such that different subsets of neurons are recruited at different times (Han et al., 2007; Rashid et al., 2016; Yiu et al., 2014). Together, these two lines of evidence suggest that BL neurons are flexibly recruited to emotional memories depending on their intrinsic excitability and related molecular expression, but that they are selected among pools of neurons that are pre-wired to generate specific behaviors (Ziv et al., 2013). In this conceptual framework, valence would still be an important factor for the separation of R- and S-cells. Consistent with this possibility, we previously found that the activity of R-cells correlated more strongly with reward-seeking behavior than the CS-R itself (Lee et al., 2016). Since reward-seeking is not an expected conditioned response to a CS-S, this predicts that the majority of R-cells should not overlap with S-cells regardless of time or the specific sensory features of the CSs and USs. Further support for the notion that BL contains distinct valence-specific networks comes from activity-based cell labeling or Ca2+-imaging studies with unconditioned stimuli (Gore et al., 2015; Grewe et al., 2017).
Another important question raised by our findings is the significance of the widespread inhibitory responses to the CSs. Inhibitory responses were reported in several prior unit recording studies, but in varying proportions (Sangha et al., 2013; Beyeler et al., 2016). Similarly, bi-directional changes in CS-induced Ca2+ responses were observed in LA after fear conditioning (Grewe et al., 2017), indicating that learning produces bidirectional changes in neural activity in the amygdala. In the present study, unlike BL cells excited by the CSs, a large proportion of inhibited cells were inhibited by the CS-R and CS-S (RiSi cells). Due to the lack of valence-specificity, these cells are probably not involved in reward-seeking or defensive behaviors, but might drive other BL functions or be associated with valence-independent components. BL appears to contain multiple subsets of PNs contributing differentiated projections to distinct effector networks. Presumably, for a behavioral tendency to be expressed, all other incompatible tendencies must be suppressed, explaining why a high number of cells must be inhibited for a specific CR to be expressed. Additionally, it is possible that valence-specific cells inhibited by only one CS regulate behavior via a disinhibitory mechanism at target sites, as demonstrated in central amygdala (Tye et al., 2011).
Relation between responses to reward- and threat-predicting cues and the sustained activity patterns present during anxiety
Prior animal studies on anxiety have focused on fear generalization, a hallmark of anxiety disorders (Laufer et al., 2016). In the basolateral amygdala, fear conditioning enhances the detection of threatening auditory CSs by increasing the responses of neurons whose preferred frequency is near that of the CS. In parallel, fear conditioning widens the tuning curves of cells whose preferred frequency is distant from the CS (Resnik and Paz, 2015), promoting the generalization of fear responses to safe cues (Ghosh and Chattarji, 2015).
However, anxiety is not always triggered by sensory stimuli, and when it is, it can persist long after the precipitating event has vanished. In our results for instance, freezing and the suppression of reward-seeking continued long after the last CS-S was presented. Paralleling this protracted time course, a subset of BL neurons (state-cells) showed long-lasting increases or decreases in activity, which correlated with temporal variations in anxiety-related behaviors. It seems implausible that such persistent changes could result from the potentiated responses of BL neurons to transient CS-S inputs. More likely possibilities include long-lasting neuromodulatory effects within BL or continuous synaptic inputs from other structures.
Related to this, stress paradigms that promote anxiety-like states leave an enduring trace in the amygdala: they reduce inhibitory transmission while increasing the intrinsic excitability and spine density of principal neurons (reviewed in Chattarji et al., 2015). While these findings indicate that anxiety is associated with widespread changes in the excitability of BL neurons, such shifts do not necessarily influence all neurons in the same way.
In principle, one could test whether state-cells drive anxiety-related behaviors by selectively manipulating their activity. Unfortunately, this could not be done here because selectively targeting inhibitory or excitatory opsins to particular subtypes of state-cells is not possible in the current state of knowledge. However, given that many prior studies found that manipulating BL activity alters manifestations of anxiety (Davis and Walker, 2013; Felix-Ortiz et al., 2013; Tye et al., 2011), it is likely that many state-neurons are “upstream” of the behaviors associated with anxiety. State-cells with increased baseline activity during anxiety were frequently found among S-cells, suggesting that partially overlapping BL circuits support the expression of anxiety and acute fear. In contrast, given that many state-cells with decreased activity during anxiety overlap with RiSi and R-cells, these state-cells are likely related to valence-independent aspects (like attention or arousal) or other functions that must be suppressed during anxiety (like reward-seeking). Together, these findings suggest that anxiety involves distributed but cell-type specific changes in neuronal activity in BL and related structures.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Denis Pare (pare@andromeda.rutgers.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Procedures were approved by the Institutional Animal Care and Use Committee of Rutgers University, in compliance with the Guide for the Care and Use of Laboratory Animals (DHHS). We used naïve male Sprague-Dawley rats (10–11 weeks old and 310–360 g at the beginning of experiments, Charles River Laboratories, New Field, NJ). All subjects (n=10) underwent the same initial behavioral training. Then, a subset of five rats that mastered the task, was implanted with multi-shank silicon probes in BL.
METHOD DETAILS
Overview of the experimental timeline
Rats were maintained on a 12 h light/dark cycle. Experiments were performed during the light phase. After habituation to the animal facility and handling, ten rats were subjected to a food restriction protocol. Then, they received daily training sessions in a reward-seeking task. Rats that mastered the task (n=5) were implanted with multi-shank silicon probes in BL. Following recovery from the surgery, they were retrained on the reward-seeking task. Once they regained their proficiency, rats were fear conditioned in a different context. BL neurons were recorded while rats were presented with appetitive, neutral, and aversive CSs in the same sessions.
Behavioral apparatus
The conditioning chamber for the appetitive task (Context A) was a rectangular box made of black polyvinyl chloride (width, length, and height: 28, 29, and 38 cm, respectively) located inside a sound-attenuating box (Coulbourn Instruments, Holliston, MA). One wall featured a water-port located above the floor. The chamber was dimly illuminated (~2 Lux) by a light located 29 cm above the floor and another one near the water-port. An additional house light was installed over the water-port zone to be used as neutral unconditioned stimulus. Two speakers that delivered auditory CSs were attached to the ceiling. Fear conditioning was performed in a standard rodent conditioning chamber (context B) with a metal grid floor and Plexiglas walls. It was enclosed in a sound-attenuating box. This conditioning chamber was dimly illuminated by a single house light.
Appetitive conditioning
Rats were trained to associate two CSs (CS-R or CS-N) with a rewarding US (20% (w/v) sucrose solution; 30 μl) or neutral US (2 sec continuous dim light), respectively. To ensure proper motivation during conditioning, daily access to food was restricted so that the rats’ bodyweight was maintained at ~85% of their free-feeding weight. In each rat, the CS-R and CS-N were randomly selected among three different pulsing sounds at 75 dB: 4 kHz (0.2 s on, 0.2 s off), 12 kHz (1.4 s on, 0.2 s off) and white noise (1 s on, 0.4 s off). The third sound was used as CS-S for fear conditioning. The CS-R and CS-N lasted 10 s and the CS-S, 20 s.
Termination of the CS-R or CS-N coincided with delivery of the reward or light US, respectively. The sucrose solution was dispensed in the water-port using a solenoid pinch valve. If not ingested by the rat, it was removed from the port by a peristaltic pump, 7 s after delivery. Initially, rats were trained with the CS-R only (60 min daily training sessions with 30 trials each; the inter-CS interval was varied pseudorandomly but averaged 110 s). Rats that reached criterion (consumed ≥80% of rewards) were further trained for discrimination between the CS-R and CS-N until water-port approach ratio in response to the CS-R became ≥4 times higher than that seen in response to the CS-N. Rats that successfully discriminated the CS-R from the CS-N (n=5) next underwent stereotaxic surgery.
Surgery
Rats were anesthetized with isoflurane and administered atropine sulfate (0.05 mg/kg, i.m.) to aid breathing. In aseptic conditions, rats were mounted in a stereotaxic apparatus with non-puncture ear bars. A local anaesthetic (bupivacaine) was injected in the scalp. Fifteen minutes later, the scalp was incised and a craniotomy performed above the amygdala. Then, 64-channel multi-shank silicon probes (Buzsaki64L, Neuronexus, Ann Arbor, MI) were stereotaxically aimed at the BL using the following coordinates for the initial location: anteroposterior (AP) −2.2 to −3.6, mediolateral (ML) 5 to 5.3, and dorsoventral (DV) 8.4 (in mm, relative to bregma). Silicon probes consisted of eight shanks (inter-shank distance of 200 μm), each with eight recording leads (de-insulated area of 160 μm2) separated by ~20 μm dorsoventrally (140 μm between top and bottom lead). They were attached to microdrives, allowing us to modify their position. A craniotomy was also performed above nucleus accumbens (nAc) and the medial prefrontal cortex (mPFC), and pairs of tungsten stimulating electrodes (inter-tip spacing of 1–1.7 mm) were stereotaxically inserted in these two structures (mPFC: AP 2.7–3.7, ML 0.5, DV 3.6–5.2; NAc: AP 1.5, ML 1.35, DV 6.7). Electrical stimuli (≤0.2 ms; 0.1–0.6 mA) delivered through these electrodes helped us localize BL, by monitoring the unit responses they evoked.
Fear conditioning
After recovery from the surgery, rats were re-trained to criterion in the appetitive task over two to three days. Then, on two consecutive days, rats were first habituated to the CS-S (5 unpaired presentations in Context A) and then to Context B (20 min; no CS-S). On the next day, while in Context B, rats received 4 additional unpaired CS-S presentations followed by 5 presentations of CS-S (20 sec, inter-CS interval 100 sec), each immediately followed by a foot shock (0.5 mA, 1 sec). After ~2 min, rats were removed from the chamber.
Recording sessions
BL unit activity was recorded during two kinds of behavioral sessions termed recall and extinction sessions, performed on different days, but both in Context A. Recall sessions happened 1 or 2 days after fear conditioning. They involved multiple presentations of the CS-R and CS-N in random order (Phase 1, n=20–67, 42 ± 4 s inter-trial interval), followed by a block of 4 or 5 CS-Ss (Phase 2, 100 s inter-CS interval), and then additional presentations of the CS-R and CS-N in random order (Phase 3). Except where noted, presentations of the CS-R were always reinforced whereas CS-S presentations were not. CS-Ns were ~1.6 times more frequent than CS-Rs.
Extinction sessions were conducted 1 to 3 days after the recall sessions. This variation was needed because some rats showed decreased reward-seeking after fear conditioning and required additional training on the appetitive portion of task to return their performance to criterion. Extinction sessions unfolded like recall sessions except for a higher number of CS-Ss (20–23) during phase 2. All other aspects of the extinction sessions were identical to the recall sessions, including the inter-trial intervals, ratio of CS-R to CS-N, and CS durations.
To increase sample sizes, additional recording sessions were performed in four rats one week or more later. Prior to these sessions, rats were re-trained on the appetitive portion of the task and they were fear conditioned one or two days later. A recall session was conducted on the next day, and an extinction session, one to three days later. Silicon probes were moved 35–120 μm between the recall and extinction session and ≥140 μm before the second conditioning session, thus avoiding counting some cells twice.
Analysis of freezing and reward-seeking
Two digital cameras (15 Hz frame rate) recorded the rats’ behavior: one located above the water-port and the other above the center of the conditioning chamber. Scoring of behavior was performed blind to the corresponding unit activity. Time spent freezing (immobility with the exception of breathing) was measured automatically, using a previously validated custom MATLAB script (Haufler et al., 2013) that computed movement levels, defined as absolute differences in luminosity values between corresponding pixels in successive video frames. Differences in luminosity were always distributed bimodally and the breakpoint between the two modes was used as cutoff between immobility and movement. When inter-CS freezing levels were plotted as a function of time, we calculated the mean freezing levels of the sampled baseline periods within each time bin. To avoid scoring sleep-related immobility as freezing, we excluded periods of slow-wave sleep, identified as epochs of high power in the 2–20 Hz band of the local field potentials (LFP) recorded in BL (Haufler et al., 2013). This spectral analysis was done using Chronux (http://chronux.org) with a 5-s window size sliding in 2-s increments. LFP power was distributed bimodally and the breakpoint between the two modes was used as cutoff between wakefulness and slow-wave sleep, which was common at the end of the extinction sessions.
Reward-seeking was measured by examining whether rats approached the water port in response to CS-R. Reward approach analyses involved frame-by-frame video inspection of behavior, which was done manually. Reward-seeking behavior was scored as positive when rats positioned their snout over the water-port during a 1-s time window before CS termination or remained there for >2 s during the CS period.
Histology
At the end of the experiments, rats were anesthetized with isoflurane. On each shank, one of the recording sites was marked with a small electrolytic lesion (10 μA between a channel and the animals’ tail for 10 sec). One day later, under deep isoflurane anesthesia, rats were perfused-fixed through the heart, their brains extracted, cut on a vibrating microtome, and the sections counterstained with cresyl violet. We only considered neurons that were histologically-determined to have been recorded in BL.
Data acquisition and analysis
Signals were sampled at 25 kHz and stored on a hard drive. The data was first high-pass filtered using a median filter (window size of 1.1 ms), then thresholded to extract spikes. We next ran PCA on the spikes. Using KlustaKwik (http://klustakwik.sourceforge.net/), spikes were automatically sorted into single units using the first three principal components from each of the eight electrodes of each shank. Spike clusters were then refined manually using Klusters (Hazan et al., 2006). The reliability of cluster separation was verified by inspecting auto- and cross-correlograms. Auto-correlograms had to display a refractory period of at least 2 ms. Cross-correlograms should not show evidence of a refractory period, as this feature betrays overlap between clusters. Units with unstable spike shapes were excluded. In all cases of units kept for the whole recording session, spike shapes remained stable throughout the recording sessions. Supplementary figure 1 shows an example of raw data (eight leads from one shank), clustering of the same data, and evidence of recording stability. Slight spike amplitude changes occurred when tonic firing rate shifted strongly, as described previously (Harris et al., 2000).
BL neurons (110 from recall sessions, and 393 from extinction sessions) from five rats were recorded in the mixed tasks. The proportion of CS-excited or -inhibited cells to CS-R and CS-S are not significantly different between recall sessions and extinction sessions. Thus, we combined the two datasets to examine the proportions of CS-responsive cells. When combining them, we excluded from the extinction session, neurons that had also been recorded during the recall sessions. To identify these neurons, we first noted the position of the recording lead where spikes for a given neuron had the highest amplitude. If the lead position shifted dorsoventrally by the same amount as the silicon probe’s displacement (35–120 μm), the cell was eliminated to avoid double counting, yielding a sample of 375 BL neurons.
BL cells were classified as presumed projection cells or interneurons on the basis of their baseline firing rates and spike duration (though to peak interval). To determine spike duration, we first selected the channel where, for a given cell, action potentials had the largest peak to trough amplitude. We then measured spike duration as the time between spike trough and peak (Barthó et al., 2004). Cells with firing rates <6 Hz and spike peak-to-through times >0.5 ms were classified as PNs. Units with firing rates ≥2.5 Hz and peak-to-through spike times ≤0.5 ms, as ITNs. Four units that only met one of the two criteria were considered unclassified (Supplementary Fig. 2). However, classifying these cells as PNs or ITNs did not alter the study’s conclusions.
QUANTIFICATION AND STATISTICAL ANALYSES
All data are reported as average ± SEM. All statistical tests were two-sided unless stated otherwise. In all cases, all available cells, trials, and subjects were included in the statistical analyses, as appropriate. All of the statistical details of experiments can be found in the figure legends and text of the Results section. The Kolmogorov–Smirnov test was used to assess whether samples were normally distributed. When they were not, we used non-parametric statistical tests, as described below. MATLAB was used for statistical analyses.
To assess whether individual cells exhibited significant changes in FRs during CS presentations, we binned (0.5 s) the baseline period (20 sec before CS onset) and CS period, then calculated average bin values across trials. Then, we compared the averaged bin values of the baseline period and CS period using a rank-sum test with a significance threshold of p < 0.005. For the CS-R and CS-N, all available trials were used. For the CS-S, all available trials were used during recall sessions, but only the first 6 trials in the extinction sessions (to avoid the influence of extinction). It should be noted that the number of R-cells and S-cells did not change appreciably with more liberal significance thresholds. The analysis of responses to the various CSs revealed cells that were unresponsive (u), inhibited (i) or excited (e) by one or more CS, delineating several subtypes of neurons. In the main text, to designate these various classes of neurons, we use the lower case letters u, i, or e following the upper case letter R (for CS-R) or S (for CS-S). For instance, RuSi indicates cells unresponsive to the CS-R and inhibited by the CS-S. Conversely, RiSu indicates cells inhibited by the CS-R and unresponsive to the CS-S, and so on.
To determine whether individual cells exhibited significant changes in baseline FRs upon introduction of the CS-S, we compared their baseline activity (mean FR during 20 s just prior to onset of CSs) in ‘reward-neutral phase’ and ‘fear phase’. For the ‘fear phase’, the time period from the onset of the first to the eleventh CS-S was used in the extinction sessions. In the recall sessions that have a lower number of CS-Ss (4–5), the time period from the onset of the first CS-S to that of the first CS-R after the last CS-S was classified as the ‘fear phase’. And additional 20 s period was sampled during each inter CS-S period to compensate for reduced statistical power due to small number of CS-Ss in the recall sessions.
For ‘reward-neutral phase’, we only used the time period from phase 1 unless an insufficient number of data points were available. In such cases, we also considered phase 3. We added one more consideration for the statistical assessment of the data. We noticed that rats showed more exploration upon entering the test chamber, raising the possibility that differences in locomotor activity and arousal might lead to the spurious detection of significant changes in activity. To avoid this confound, we divided phase 1 in two (early and late parts). The baseline FRs in the early and late parts of phase 1 as well as in ‘fear phase’ were then compared using a Kruskal-Wallis one-way ANOVA followed by post-hoc Tukey’s honest significant difference tests (p < 0.05 with Bonferonni correction). When, for a given cell, the baseline FR of ‘fear phase’ was significantly different from both, the early and late parts of phase 1, and in the same direction, the cell was deemed ‘state-modulated’.
For most recall sessions (4 out of 6), phase 1 featured many CSs (34–65), and was divided in two halves of equal durations for the state analyses. In contrast, the other two recall sessions featured few CSs (19–24) in phase 1, requiring a different approach. For these two sessions, we compared ‘fear phase’ to the entire phase 1 and phase 3 (excluding the first 10 min after the last CS-S). The same approach was used for extinction sessions since they also featured a low number of CSs in phase 1. It should be noted that for the four recall sessions with a high number of CS-Rs and CS-Ns, we obtained nearly identical results when, instead of the above approach, we compared baseline FRs in ‘fear phase’ to that in the entire phase 1 and phase 3. For graphs that plot baseline FRs against time, mean of baseline FRs of sampled baseline periods located in each time bin was calculated and plotted.
To determine whether the incidence of cells with reduced, enhanced, or unchanged baseline FRs varied significantly in each group relative to the rest of our sample, we performed post-hoc Chi-square tests with a significance threshold of 0.05.
To assess whether inter-CS freezing or reward-seeking behavior exhibited significant changes after CS-S presentation, we binned (2 min) the recording session around the onset of the first CS-S, then calculated mean value of available data points (inter-CS freezing or reward approach) in each time bin. For inter-CS freezing, we considered the 20 min period before the first CS-S and the 12 min period after the first CS-S. For reward-seeking behavior, we used the 20 min period before the first CS-S, and 14 min period after the end of the last CS-S. Then, we averaged bin values across trials, and compared the averaged bin values of the ‘before’ and ‘after’ period using a rank-sum test with a significance threshold of p < 0.05.
The proportion of variance in the firing rate of state-cells explained by behavior, CS identity, or state was calculated using the omega squared method: (SSfactor - (df *MSE))/(SST+MSE) where SSFactor stands for sum of squares for factor, MSE for mean square error, SST for sum of squares total and df for degree of freedom of factor. Prior to calculating the ANOVA, we normalized the data by linearly interpolating FRs between spikes, smoothed the data with 4 sec windows, and then sampled the linear interpolation at a rate of 150 Hz to match the video acquisition frame rate. The factors considered in the ANOVA included behavior, CS-identity, and state, as defined below. Behavior was classified as very low freezing (0–20% freezing), low freezing (20–40%), medium freezing (40–60%), high freezing (60–80%) and very high freezing (80–100%). CS identity was classified as no-CS, CS-R, CS-S, and CS-N. State consisted of the anxiety period, as defined above, and outside the anxiety period. The ANOVA was calculated with bins of 1, 2, 5, and 10 s. Periods of slow-wave sleep were excluded from the analyses.
Decoder analysis
For the decoder analysis, we selected recording sessions in which two or more state cells (including both PNs and ITNs) were simultaneously recorded, to test whether freezing is encoded by the ensemble activity of state-cells. A total of six sessions, including two recall sessions and four extinction sessions met this criterion. During each of these sessions, 2 to 13 state-cells were simultaneously recorded (average of 7.2 ± 1.7 for a total of 43). All these state-cells, including PNs and ITNs, were used for the decoder analysis. Because all available state-cells were recorded during these sessions, they were distributed among the various classes of CS-responsive neurons exactly as depicted in figure 3G. To test whether the FRs of BL neurons can predict inter-CS freezing levels, a generalized linear model was constructed using inter-CS freezing and FRs of simultaneously recorded state-cells at each time bin. Because CSs are relatively evenly distributed across the recording session, they approximate the time dimension. Thus, the linear model is formulated as
where CSm is m-th presented CS, zFRn (CSm) is the z-scored mean baseline FR of n-th cell (calculated during the 20 sec period preceding CSm onset), inter-CS freezing(CSm) is the fraction of time that animal spent freezing during the same baseline period, and βn is weight for the n-th cell. For recall sessions, we used the entire recording periods. For extinction sessions, we used a sub-period that included phases 1 and 2. The FR of individual neurons was z-scored using the mean and standard deviation of baseline FRs of a fixed number of inter-CS periods during phase 1 and 3. L is a link function from MATLAB, which assumes a binomial distribution of inter-CS freezing, resulting in predicted inter-freezing values between 0 and 100%. However, binomial and normal distributions yielded qualitatively identical results.
We used a regularized regression of generalized linear model (‘lassoglm’ in MATLAB) with cross-validation. First, the optimal regularization parameter was determined by choosing a value that generated minimal deviance with the given dataset. After optimization, the decoder analysis was performed with a 10-fold cross-validation. To this end, the entire time period (the combination of all the inter-CS periods) was divided randomly into 10 similarly sized groups. For predicting inter-CS freezing from the FRs of time bins (CSs) in each group, the decoder was trained with the remaining groups (90% of the inter-CS freezing and FRs) and tested on the target group (10% of FRs). This process was repeated 100 times to obtain averaged (± SEM) predicted inter-CS freezing. Finally, for each session, we computed a Pearson correlation between averaged predicted freezing and observed freezing (significance threshold of p < 0.05).
Cross-correlation analyses
The spikes of all simultaneously recorded cells were cross-correlated for a total of 7300 cell couples that included 6243 couples of PNs, 1009 couples of PNs and INTs, and 48 couples of ITNs. Since the probability of connections between pairs of PNs was very low (0.67%), we focused on couples that included a PN and an ITN. Each cross-correlogram (CCG) was computed with a bin size of 1 ms in windows of ± 29.5 ms. To determine whether peaks or troughs in the CCGs were significant, we used the procedure described in Fujisawa et al., (2008).
First, for each couple, the spikes of the reference cell were jittered randomly within an interval of ± 5 ms. Then, the jittered spike train from the reference cell and the original spike train from the target cell were cross-correlated. This procedure was repeated 1000 times to compute 1000 surrogate jittered CCGs. The maximum and minimum values of each of the surrogate CCGs were extracted to compute a distribution of 1000 maximum and 1000 minimum values. In order to be considered significant, the peak of the actual CCG had to exceed 99% of the surrogate maximum values. For inhibitory connections, the trough of the actual CCG had to be lower than the bottom 1% of the surrogate minimum values for at least two consecutive bins. In addition, the peak or trough of the actual CCG had to be located within 0.5 to 5.5 ms of the zero time. For couples in which cells were from the same shank, spike count at single bin at 0 ms was assigned as zero because clustering excluded overlapping spikes from the same shank. This was not compensated, and may bias detection threshold toward lower values for those pairs.
In addition to studying the incidence of connections between the various classes of cells described in the results, we also examined whether the efficacy of the putative monosynaptic connections differed significantly during vs. outside the anxiety period. In this case, only for cell couples deemed connected using the above method, we computed two CCGs based either on their activity during anxiety period or outside of it. Then, the values were normalized to the average spike counts before time zero and a signed rank test was used to determine whether a specific class of putative monosynaptic connections showed different efficacies in the two conditions.
DATA AND SOFTWARE AVAILABILITY
The full dataset and custom MATLAB code will be made available upon request.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Sprague Dawley rat | Charles River Laboratories | Sprague Dawley rat |
| Software and Algorithms | ||
| MATLAB | MathWorks | MATLAB R2014b |
| KlustsKwik | http://klustakwik.sourceforge.net/ | Not applicable |
| Klusters | Hazan et al., 2006 | Not applicable |
| Chronux | http://chronux.org | Not applicable |
| Graphic state | Coulbourne Instrument | Not applicable |
| Other | ||
| Silicon probe | NeuoNexus | Buzsaki64L |
| Plexon amplifier | Plexon | Not applicable |
| Behavior control system: Habitest | Coulbourne Instrument | Not applicable |
| Deposited Data | ||
| The full dataset and custom MATLAB code will be made available upon request. | ||
Supplementary Material
Acknowledgments
This material is based upon work supported by NIMH grant R01 MH107239 to Denis Paré.
Footnotes
Author Contributions
SCL and DP designed the experiments. SCL conducted most of the experiments, performed most of the analyses and histological controls. He also contributed to writing the manuscript. AA conducted some of the experiments and performed some of the analyses. DH contributed to the data analyses and statistics. DP contributed to the data analysis, made the figures, and wrote most of the manuscript.
Competing financial interests statement
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral Amygdala Neurons Facilitate Reward-Seeking Behavior by Exciting Nucleus Accumbens Neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Luck R, Wildes CP, Tye KM, Calhoon GG. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Article. Neuron. 2016:1–14. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 2015;18:1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL. Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct Funct. 2013:1–14. doi: 10.1007/s00429-013-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala Microcircuits Controlling Learned Fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genud-Gabai R, Klavir O, Paz R. Safety signals in the primate amygdala. J Neurosci. 2013;33:17986–17994. doi: 10.1523/JNEUROSCI.1539-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci. 2015;18:112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- Gore F, Schwartz EC, Brangers BC, Aladi S, Stujenske JM, Likhtik E, Russo MJ, Gordon JA, Salzman CD, Axel R. Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses. Cell. 2015;162:134–145. doi: 10.1016/j.cell.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, et al. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543:670–675. doi: 10.1038/nature21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal Competition and Selection During Memory Formation. Science (80-) 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan L, Zugaro M, Buzsáki G. Klusters, NeuroScope, NDManager: A free software suite for neurophysiological data processing and visualization. J Neurosci Methods. 2006;155:207–216. doi: 10.1016/j.jneumeth.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci. 2013;67:311–322. doi: 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer O, Israeli D, Paz R. Behavioral and Neural Mechanisms of Overgeneralization in Anxiety. Curr Biol. 2016;26:713–722. doi: 10.1016/j.cub.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Lee SC, Amir A, Headley DB, Haufler D, Pare D. Basolateral amygdala nucleus responses to appetitive conditioned stimuli correlate with variations in conditioned behaviour. Nat Commun. 2016;7:12275. doi: 10.1038/ncomms12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry. 2014;75:909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton John P., editor. The Amygdala: A Functional Analysis. New York: Oxford University Press; 2000. pp. 31–116. [Google Scholar]
- Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, et al. Competition between engrams influences fear memory formation and recall. Science (80-) 2016;353:383–387. doi: 10.1126/science.aaf0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Resnik J, Paz R. Fear generalization in the primate amygdala. Nat Neurosci. 2015;18:188–190. doi: 10.1038/nn.3900. [DOI] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, Janak PH. Safety encoding in the basal amygdala. J Neurosci. 2013;33:3744–3751. doi: 10.1523/JNEUROSCI.3302-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci U S A. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HLL, Pressey J, Mahadevan V, Tran MM, Kushner SA, et al. Neurons Are Recruited to a Memory Trace Based on Relative Neuronal Excitability Immediately before Training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.