Disruptions in breast cancer are common for patients with schizophrenia and are associated with adverse outcomes. The aim of this study was to examine the proportion of patients with schizophrenia who received stage‐appropriate breast cancer care, identify and categorize care processes that may interfere with receiving stage‐appropriate care defined as care disruptions, and determine potentially modifiable predictors of care disruptions.
Keywords: Breast neoplasms, Health care disparities, Mental disorders, Schizophrenia, Time‐to‐treatment
Abstract
Background.
Patients with schizophrenia experience markedly increased breast cancer mortality, yet reasons for this disparity are poorly understood. We sought to characterize disruptions in breast cancer care for patients with schizophrenia and identify modifiable predictors of those disruptions.
Materials and Methods.
We performed a medical record review of 95 patients with schizophrenia and breast cancer treated at an academic cancer center between 1993 and 2015. We defined cancer care disruptions as processes that interfere with guideline‐concordant cancer care, including delays to diagnosis or treatment, deviations from stage‐appropriate treatment, and interruptions in treatment. We hypothesized that lack of psychiatric treatment at cancer diagnosis would be associated with care disruptions.
Results.
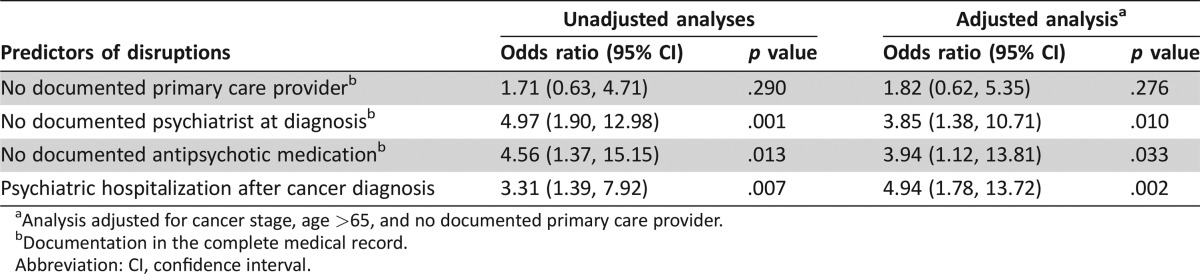
Half of patients with schizophrenia experienced at least one breast cancer care disruption. Deviations in stage‐appropriate treatment were associated with breast cancer recurrence at 5 years (p = .045). Patients without a documented psychiatrist experienced more delays (p = .016), without documented antipsychotic medication experienced more deviations (p = .007), and with psychiatric hospitalizations after cancer diagnosis experienced more interruptions (p < .0001). Independent of stage, age, and documented primary care physician, lack of documented antipsychotic medication (odds ratio [OR] = 4.97, 95% confidence interval [CI] = 1.90, 12.98) and psychiatric care (OR = 4.56, 95% CI = 1.37, 15.15) predicted cancer care disruptions.
Conclusion.
Disruptions in breast cancer care are common for patients with schizophrenia and are associated with adverse outcomes, including cancer recurrence. Access to psychiatric treatment at cancer diagnosis may protect against critical disruptions in cancer care for this underserved population.
Implications for Practice.
Disruptions in breast cancer care are common for patients with schizophrenia, yet access to mental health treatment is rarely integrated into cancer care. When oncologists documented a treating psychiatrist and antipsychotic medication, patients had fewer disruptions in breast cancer care after adjusting for age, cancer stage, and access to primary care. Addressing psychiatric comorbidity at breast cancer diagnosis may increase the likelihood that patients with schizophrenia receive timely, stage‐appropriate cancer treatment. Comanagement of schizophrenia and breast cancer at cancer diagnosis may be one key strategy to decrease inequities in cancer treatment and improve cancer survival in this underserved population.
Introduction
Approximately 2.5 million people are living with schizophrenia in the U.S. [1]. Individuals with schizophrenia die 15–25 years earlier than the general population; 80% of that morality gap is due to medical illness, and cancer is the second leading cause of death [2], [3], [4]. Patients with schizophrenia are more than twice as likely to die from breast, colorectal, lung, and oral cancers, in part due to differences in receipt of stage‐appropriate treatment [5], [6], [7], [8]. Despite recent policy statements from the American Society of Clinical Oncology and Institute of Medicine calling for research examining inequities in cancer outcomes [9], [10], [11], disparities in cancer mortality experienced by individuals with schizophrenia are frequently unrecognized and relatively understudied. Patients with schizophrenia are commonly excluded from cancer clinical trials [12].
Although women with schizophrenia have the same incidence of breast cancer as women without mental illness, they experience two‐ to threefold greater breast cancer mortality [5], [7]. Women with schizophrenia undergo mammography at lower rates, are diagnosed with more advanced stage cancer [13], [14], [15], and are less likely to receive stage‐appropriate treatment [6], [8], [13], [16]. Patient, provider, and systems‐based factors contribute to inequities in treatment and survival. High rates of smoking, medical comorbidity, and poverty impact cancer outcomes but do not account fully for disparities in cancer survival [7], [16], [17]. Severe psychiatric symptoms, including cognitive deficits and disorganization, can make it challenging to understand a cancer diagnosis and urgently start treatment, leading to delays. Given mental health care is segregated from cancer care, oncologists may need to make treatment decisions without a complete medical and psychiatric history.
While the delivery of oncology care for patients with schizophrenia presents distinct challenges, studies have not identified remediable drivers of inequities in their cancer treatment. Prior studies have involved small samples. Researchers from the U.S. Department of Veterans Affairs reported high rates of treatment refusal and patient behaviors that could disrupt breast cancer care in their predominately male population. They recommended psychiatric consultation and advocated for use of mastectomy for early‐stage breast cancer given anticipated nonadherence with radiation [18], [19]. In contrast, a U.K. study conducted at an academic cancer center found no need to alter breast cancer treatment or exclude patients with schizophrenia from clinical trials. Interestingly, all patients in the U.K. study were prescribed antipsychotic medications [20]. Previous research has not elucidated which factors contribute to cancer care disruptions for individuals with schizophrenia.
Using clinical data from a large health system, which included a National Cancer Institute (NCI)‐designated cancer center and comprehensive psychiatric treatment, we aimed to (a) examine the proportion of patients with schizophrenia who received stage‐appropriate breast cancer care, (b) identify and categorize care processes that may interfere with receiving stage‐appropriate care defined as care disruptions, and (c) determine potentially modifiable predictors of care disruptions. We hypothesized that patients with schizophrenia who were engaged in psychiatric treatment at cancer diagnosis would have fewer disruptions in cancer care.
Materials and Methods
Study Design
We conducted a retrospective cohort study of patients with schizophrenia and breast cancer diagnosed between 1993 and 2015 and treated at the Dana‐Farber/Harvard Cancer Center (DF/HCC) and the Partners Healthcare System (PHS), including Massachusetts General Hospital, Dana‐Farber Cancer Institute, Brigham and Women's Hospital, and community affiliates. Patients were identified through the PHS's Research Patient Data Registry (RPDR), a centralized data warehouse that aggregates inpatient and outpatient records. This study was approved by the DF/HCC Institutional Review Board.
Sample
We queried the RPDR for the ICD‐9 diagnoses of schizophrenia spectrum disorder, including schizophrenia, schizoaffective disorder, and schizophreniform disorder (295.0–295.9), and invasive breast cancer (174, 175.0, and 175.9). Inclusion criteria included: schizophrenia spectrum disorder diagnosed at least 1 year prior to breast cancer, first invasive breast cancer diagnosed between 1993 and 2015 confirmed by pathology or oncologist documentation, and at least one oncology note describing cancer care at cancer diagnosis. Since treatment with tamoxifen for 5 years became standard of care in the early 1990s [21], we included patients with breast cancer diagnosed after 1993.
Study Measures
Patient Characteristics.
Demographic and clinical variables were extracted from the electronic health record (EHR) at cancer diagnosis, including age, race, marital status, housing, insurance type, medical comorbidity measured by the Charlson Comorbidity Index (CCI), body mass index, and smoking status. When oncology consultation notes were not available in the EHR, we obtained paper records. Breast cancer characteristics included breast mass size, hormone and HER2/neu receptor status, stage, and grade. We recorded breast cancer treatment recommended to and received by the patient, including details of cancer recurrence.
Mental Health Treatment and Systems Factors.
First, we recorded whether there was any note from a psychiatrist or primary care provider (PCP) in the PHS EHR prior to cancer diagnosis. Next, we assessed documentation of psychiatric treatment in the PHS EHR at cancer diagnosis. This EHR review included documentation of a psychiatrist, antipsychotic medication, and psychiatric hospitalizations from the following sources: notes from primary care, mental health, and/or oncology clinicians in outpatient, inpatient, and emergency settings within the PHS. Finally, we assessed oncologist documentation of mental health factors at cancer diagnosis. To do so, we reviewed initial medical, radiation, and surgical oncology consultation notes for documentation of (a) mental illness, (b) psychiatric care, (c) consideration of mental illness when planning cancer treatment, and (d) consultation of psychiatry. We defined consideration of mental illness in cancer treatment planning as the oncologist's documentation of adapting, adjusting, or maintaining the cancer treatment plan considering the patient's schizophrenia. For example, regarding the decision not to recommend chemotherapy, one medical oncologist wrote, “the benefit to chemotherapy in this situation is measured in single digits and after discussion with her family and caregivers, we felt it would [be] difficult for her to go through, therefore, will probably not recommend it” (oncology consultation note in the EHR).
Breast Cancer Care.
An interdisciplinary team of two psychiatrists who specialize in psycho‐oncology (KEI, WFP) and a medical oncologist and radiation oncologist who specialize in breast cancer (JAS, AGT) established criteria to evaluate stage‐appropriate breast cancer care. These criteria were informed by national guidelines for optimal cancer care including the National Comprehensive Cancer Network, the Quality Oncology Practice Initiative, and the National Quality Forum, and accounted for changes in breast cancer treatment during the study period (supplemental online Appendix) [22], [23], [24]. Given oncologists may weigh the implications of medical and psychiatric comorbidities such as schizophrenia in cancer treatment planning, we used conservative criteria for guideline‐concordant cancer treatment, which may have led to underestimating care disruptions.
To assess screening, we examined whether female patients age ≥42 had a documented screening mammogram within the 2 years prior to diagnosis. To assess timeliness of diagnosis and treatment, we examined the intervals from (a) positive screening mammogram to biopsy, (b) biopsy to initial treatment (surgery or neoadjuvant chemotherapy), (c) time between different treatment modalities received (surgery, chemotherapy, radiation), and (d) diagnosis to endocrine therapy.
Identification and Categorization of Care Disruptions.
To understand the processes that can affect the delivery of breast cancer care for patients with schizophrenia, the interdisciplinary team reviewed a subset of charts (n = 20). First, the team identified the processes by which patients with schizophrenia did not receive timely, stage‐appropriate breast cancer care, which we operationalized as care disruptions. Next, the team categorized the types of care disruptions, including (a) delays (lack of recommended screening, delays to diagnosis or initiation of treatment), (b) deviations (changes from stage‐appropriate cancer treatment recommended by the oncologist or accepted by the patient or caregiver at the time of cancer diagnosis), and (c) interruptions (breaks in planned cancer treatment, including surgery, chemotherapy, radiation, or endocrine therapy). Examples of deviations include changes to surgery (e.g., mastectomy or avoiding a sentinel lymph node biopsy) and not recommending radiation, chemotherapy, and endocrine therapy.
Subsequently, all charts were independently coded by two members of the interdisciplinary team (psychiatrists [KEI, JBT] and trained research assistants [LEF, HPK]). Coders identified all patients with overall care disruptions (yes/no) with high inter‐rater reliability (kappa = 0.86) and recorded the types of disruptions experienced. Patients could experience more than one type of disruption. To strengthen the clinical relevance of identified disruptions, all discrepancies were resolved by consensus review by an interdisciplinary team including a medical oncologist specializing in breast cancer (JAS). Additionally, an investigator (JBT) reviewed 20% of the charts comprehensively to ensure that all care disruptions were captured during the EHR review.
Statistical Analysis
Statistical tests were conducted in the Statistical Package for the Social Sciences (SPSS Version 22.0, IBM, https://www.ibm.com/analytics/us/en/technology/spss). We used measures of central tendency to describe the clinical and demographic characteristics of the sample and rates and types of disruptions in breast cancer care. Rates of oncologist documentation of mental health factors were compared using Fisher's exact tests. Patients were categorized based on whether they experienced disruptions in their cancer care (had disruptions vs. no disruptions). We used the following categories to conceptualize overall care disruptions: (a) delays, (b) deviations, and (c) interruptions. We used chi‐square tests and point biserial correlations to examine univariate associations between patient characteristics and care disruptions.
We then conducted multivariate logistic regressions to examine whether lack of psychiatric treatment (documentation of a psychiatrist and/or antipsychotic medication) at cancer diagnosis was associated with disruptions after adjusting for potential confounding factors at diagnosis, such as age, cancer stage, health insurance type (Medicaid vs. other), and medical comorbidity (CCI dichotomized to 0 vs. ≥1).
Finally, we used Cox proportional hazards models [25] to examine associations between deviations from stage‐appropriate care and disease‐free survival, adjusting for stage of disease (early vs. advanced). This analysis was conducted in a subsample of patients with stage I–III breast cancer who were diagnosed between 1993 and 2011 and therefore had 5‐year follow‐up data. Patients who were deceased or lost to follow‐up were censored using the date of last contact. All statistical tests were two‐sided with a p value of .05 as the threshold for statistical significance.
Results
We retrieved 675 cases for review from the RPDR query for breast cancer and schizophrenia treated between 1993 and 2015. We excluded cases with no documentation of first invasive breast cancer between 1993 and 2015 (n = 291), no oncology notes at diagnosis (n = 124), and no diagnosis of schizophrenia spectrum disorder at least 1 year prior to cancer diagnosis (n = 165), yielding a final sample of 95 eligible cases.
Patient Characteristics
Patient characteristics are described in Table 1. The majority of patients in this sample were white (65.3%) and 17.9% were black, with an average age of 58.6 years of age (SD = 11.5; range = 34–84 years). Over half (54.7%) were unmarried; 18.1% lived in supportive housing (group home, assisted living, or nursing home), 6.3% had a history of homelessness, and 76.8% had Medicaid insurance. Half were obese, and more than a third were current smokers. Although 44.2% of patients were diagnosed with stage I breast cancer, 11.6% were initially diagnosed with metastatic cancer. Ninety percent of patients had hormone receptor‐positive, HER2/neu receptor‐negative disease.
Table 1. Patient and clinical characteristics at cancer diagnosis.
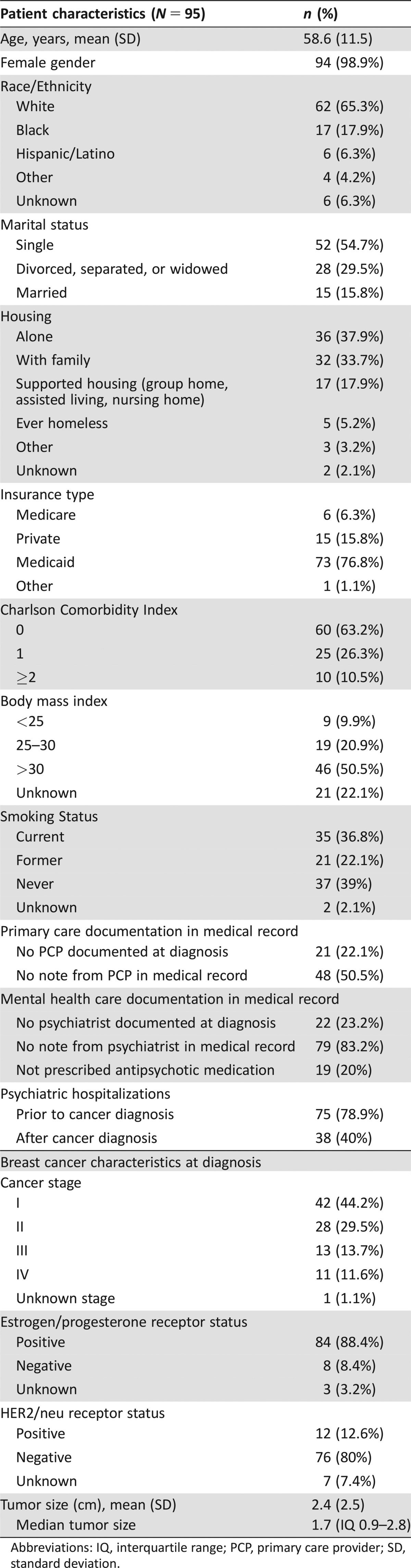
Abbreviations: IQ, interquartile range; PCP, primary care provider; SD, standard deviation.
Mental Health Treatment and Systems Factors
Prior to cancer diagnosis, 50.5% of patients had no primary care note in their EHR, and 83.2% had no note from a psychiatrist. At diagnosis, 23.2% of patients had no documented psychiatrist in their EHR and 20% were not documented to be on antipsychotic medication. Psychiatric hospitalizations after cancer diagnosis were documented in 40% of patients; 19% of those hospitalizations occurred within the first year after cancer diagnosis.
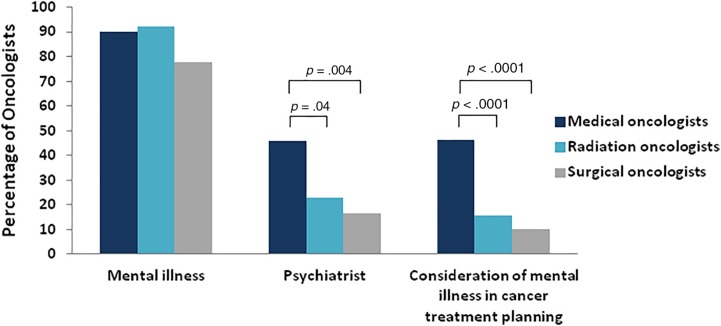
In their initial consultations, oncologists documented a history of mental illness for most patients with schizophrenia (77.9%–92.2%), yet less than one half of medical oncologists and less than one quarter of radiation oncologists documented a treating psychiatrist (Fig. 1). Medical oncologists were more likely than radiation (p = .04) or surgical oncologists (p = .004) to document a psychiatrist and document consideration of schizophrenia in cancer treatment planning (Fig. 1, p < .0001). Across specialties, oncologists rarely documented consulting psychiatry (1%–10%).
Figure 1.
Oncologist documentation of mental health factors in initial consultation note. Forty‐six percent (23/50) of medical oncologists documented a psychiatrist compared with 22.9% (8/35) of radiation oncologists (Fisher's exact test, p = .04) and 16.7% (7/42) surgical oncologists (p = .004). Forty‐six percent (38/82) of medical oncologists documented consideration of schizophrenia in cancer treatment planning compared with 15.6% (10/64) of radiation oncologists and 10.1% (7/69) of surgical oncologists (p < .0001).
Breast Cancer Care
Thirty‐two women with schizophrenia age ≥42 had a screening mammogram documented in the 2 years prior to breast cancer diagnosis (36.8% of overall sample vs. 49.1% of women with PCPs within the PHS). The median time from positive screening mammogram to biopsy was 1.15 months (interquartile range 0.7–1.95); 24.6% had more than 2 months from screening mammogram to biopsy.
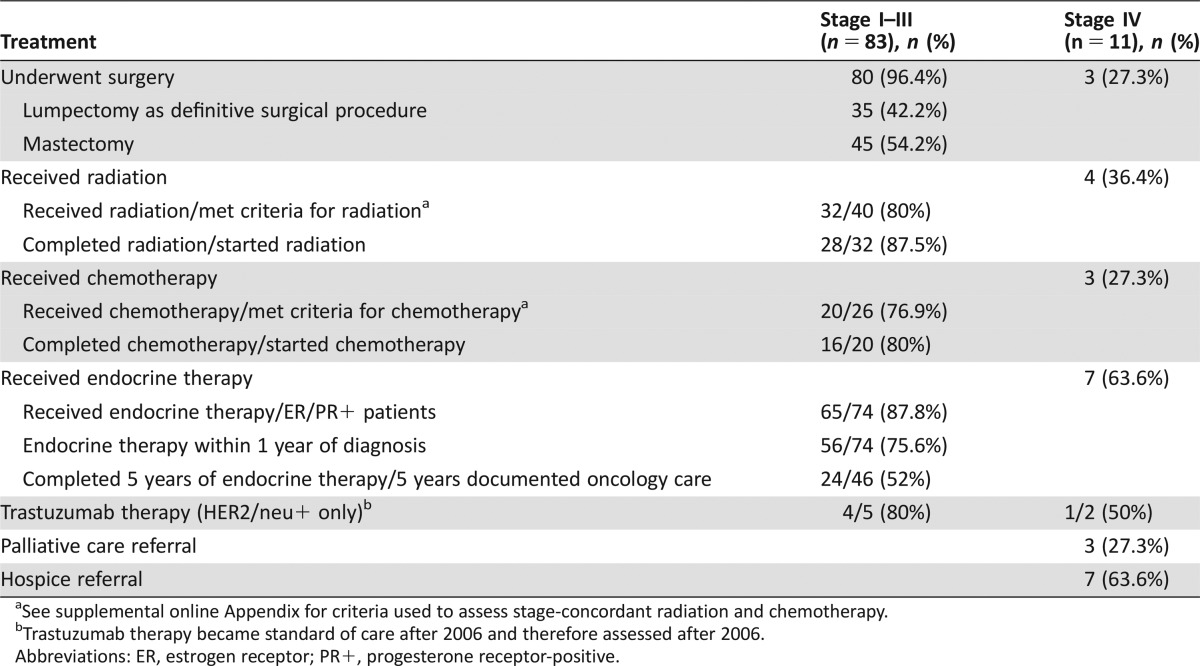
Ninety‐six percent of patients with stage I–III disease underwent surgery, and 54.2% had mastectomies (Table 2). Fourteen percent of patients had surgery or neoadjuvant chemotherapy >90 days after diagnosis (Fig. 2). Nearly one third of patients had radiation >84 days after surgery or chemotherapy. Among the 40 patients who met criteria for radiation following lumpectomy or mastectomy, 32 (80%) started radiation, and 28/32 (87.5%) completed the recommended radiation course. Among the 26 patients who met criteria for chemotherapy, 20 (76.9%) received chemotherapy, and 16/20 (80%) completed treatment. Thus, a significant minority of patients did not complete recommended cancer treatment: 30% did not complete recommended radiation, and 39.5% of patients did not complete recommended chemotherapy. The majority of hormone receptor‐positive patients (77%) were prescribed endocrine therapy <1 year from cancer diagnosis. Among stage I–III patients with 5 years of follow‐up, 24/46 (52%) of patients completed the recommended 5 years of endocrine therapy. Oncologists documented worsening psychiatric symptoms as the most common reason for patients to self‐discontinue endocrine therapy (n = 7). Regarding end‐of‐life care, 34 patients (35% of the overall sample) had died. Thirteen patients (38%) had palliative care consultations (all but one in the inpatient setting), and 15 patients (44%) had documented hospice referrals prior to their death.
Table 2. Cancer treatment received.
See supplemental online Appendix for criteria used to assess stage‐concordant radiation and chemotherapy.
Trastuzumab therapy became standard of care after 2006 and therefore assessed after 2006.
Abbreviations: ER, estrogen receptor; PR+, progesterone receptor‐positive.
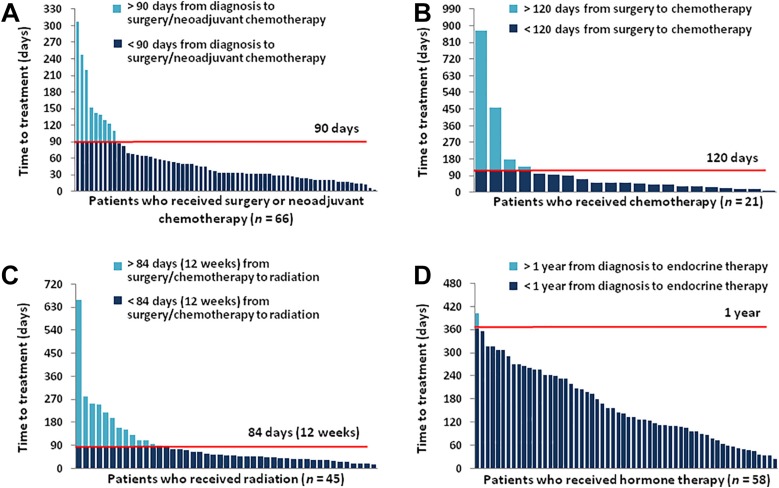
Figure 2.
Proportion of patients meeting national guidelines for time to breast cancer treatment. (A): 13.6% of the patients who underwent surgery or neoadjuvant chemotherapy received treatment >90 days after diagnosis. (B): 19% of the patients who received chemotherapy started treatment >120 days after surgery. (C): 31.1% of the patients who received radiation started treatment >84 days (12 weeks) after surgery. (D): In contrast, 98.3% of patients who received endocrine therapy started treatment within 1 year of diagnosis.
Types and Rates of Disruptions in Cancer Care
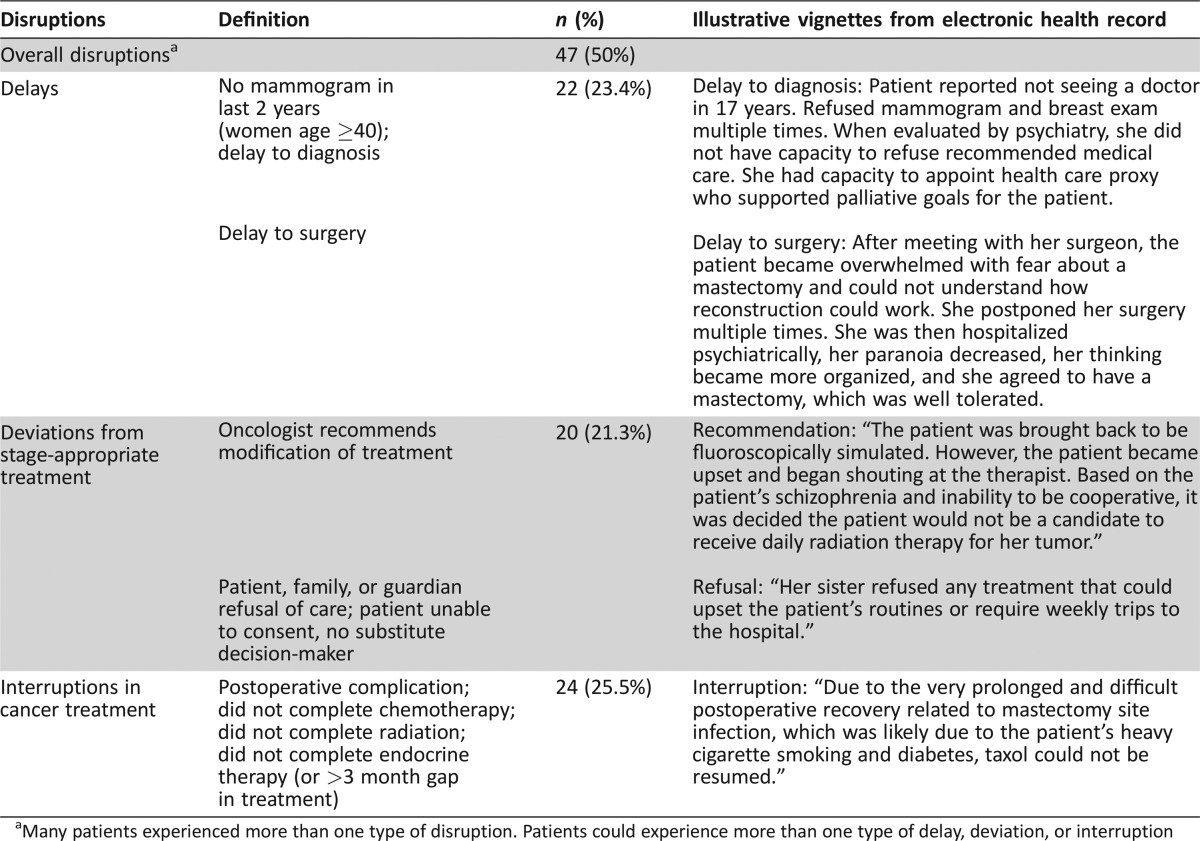
One half of patients with schizophrenia and breast cancer experienced at least one disruption in cancer care. Specifically, patients frequently experienced (a) delays to diagnosis or treatment (n = 22, 23.4%), (b) deviations from stage‐appropriate treatment (n = 20, 21.3%), and (c) interruptions in treatment (n = 24, 25.5%). Sixteen patients (17%) experienced more than one type of care disruption. Examples of delays and changes from stage‐appropriate treatment, (e.g., surgery, radiation, chemotherapy, and endocrine therapy recommended and/or accepted at cancer diagnosis) are provided in Table 3. For example, one surgical oncologist noted in his initial consultation, “The patient was not a good candidate for 6 weeks of radiation, chemotherapy, or even hormonal therapy because of her mental status. It was therefore decided that a simple mastectomy would be advisable.”
Table 3. Disruptions in cancer care (n = 94).
Many patients experienced more than one type of disruption. Patients could experience more than one type of delay, deviation, or interruption
Clinical Relevance of Disruptions in Cancer Care.
Delays were associated with cancer stage at diagnosis; delays occurred in 55% of patients with metastatic disease compared with 18.1% of patients with stage I–III disease (odds ratio [OR] = 5.44, 95% confidence interval [CI] = 1.46, 20.20, p = .006). In patients with stage I–III disease diagnosed between 1993 and 2011 (n = 64), those who experienced deviations from stage‐appropriate care were more likely to recur sooner compared with patients who did not have deviations, over and above the effects of cancer stage (OR = 4.17, 95% CI = 1.03, 16.81, p = .045).
Predictors of Disruptions in Cancer Care
Older patients with schizophrenia (age >65) experienced more disruptions (OR = 3.05, 95% CI = 1.24, 7.51, p = .015). Higher medical comorbidity (CCI) was associated with fewer care disruptions (OR = 0.364, 95% CI = 0.15, 0.88, p = .023). Cancer stage, private versus public insurance, smoking status, obesity, housing status (living alone or with family vs. living in supportive housing) and documentation of a PCP at cancer diagnosis were not associated with disruptions. Conversely, lack of psychiatric treatment (no documentation of psychiatrist and no antipsychotic medication) was associated with disruptions in cancer care (Table 4). Additionally, psychiatric hospitalization after cancer diagnosis was associated with overall cancer care disruptions. These associations remained significant after adjusting for cancer stage, age >65, and documented PCP.
Table 4. Predictors of disruptions in breast cancer care in individuals with schizophrenia.
Analysis adjusted for cancer stage, age >65, and no documented primary care provider.
Documentation in the complete medical record.
Abbreviation: CI, confidence interval.
In exploring the three types of disruptions in cancer care, we found that patients with no documented psychiatrist at diagnosis experienced more delays (OR = 3.97, 95% CI = 1.30, 12.13, p = .016); patients with no antipsychotic medication at cancer diagnosis were more likely to have deviations from stage‐appropriate treatment (OR = 4.33, 95% CI = 1.53, 15.49, p = .007); and patients who were psychiatrically hospitalized after cancer diagnosis had more interruptions in planned cancer treatment (OR = 10.07, 95% CI = 3.09, 33.55, p < .0001). All analyses were adjusted for stage.
Discussion
In this study, we comprehensively describe the trajectory of cancer care for a cohort of patients with schizophrenia and breast cancer. In an NCI‐designated comprehensive cancer center, one half of patients experienced clinically meaningful care disruptions, including delays to diagnosis or treatment, deviations from stage‐appropriate treatment, and interruptions in planned treatment. One in five patients with schizophrenia experienced deviations from guideline‐concordant care due to oncologist recommendation and/or patient or caregiver refusal; these patients had an increased risk of breast cancer recurrence at 5 years independent of cancer stage. The clinical relevancy of these disruptions may partially account for the increased breast cancer mortality experienced by patients with schizophrenia.
Recent analyses using national databases have underscored the importance of avoiding delays to breast cancer treatment [26], [27]. Each additional 60 days to surgery was associated with worse breast cancer‐specific survival (BCSS). Only 1.5% of patients in these national cohorts had >90 days from diagnosis to surgery versus 22.7% of our sample [26]. Furthermore, >90 days from chemotherapy to surgery was associated with worse BCSS; 9.8% had >90 days to chemotherapy compared with one third of our sample [27]. The significant delays experienced by a subset of patients with schizophrenia may increase their risk of dying from breast cancer.
Additionally, patients with schizophrenia and early‐stage breast cancer were nearly twice as likely to have a mastectomy as their initial surgical procedure than patients with early‐stage breast cancer treated at academic cancer centers in the Northeast: 38% of patients in our sample compared with 20% at similar cancer centers [28], [29], [30]. Furthermore, patients in our sample had low rates of receiving adjuvant chemotherapy and radiation; 25% of patients with schizophrenia who met clear criteria for adjuvant radiation failed to receive radiation compared with 5% in national samples [31]. Mastectomy likely makes sense for a subset of patients who are at high risk for not completing radiation [18]. However, many patients with schizophrenia may benefit from less complicated surgical procedures, which are associated with decreased morbidity, particularly given the increased risk of postoperative complications, increased length of stay, and in‐hospital mortality in this population [32]. Specifically, patients with schizophrenia have two to three times greater risk of postoperative infection, pulmonary embolus/deep venous thrombosis, respiratory failure, and delirium compared with patients without mental illness [33], [34]. Contributing factors likely include high rates of smoking, obesity, and medical comorbidity, factors that also predict poor outcomes from breast reconstruction [35]. Furthermore, patients with schizophrenia may have increased risk of emergent surgery and experience challenges communicating their symptoms with clinicians [33].
Compared with patients who have breast‐conserving therapy, patients with early‐stage cancer who have mastectomies have longer delays to adjuvant chemotherapy and radiation and lower rates of BCSS [36], [37]. In contrast, rates of discontinuation of endocrine therapy were similar to national cohorts; 50% of patients nationally, and 48% in our sample, with schizophrenia with estrogen receptor/progesterone receptor‐positive breast cancer had discontinued endocrine therapy at 5 years [38], [39].
Although delays and inequities in cancer care are common in patients with schizophrenia, oncologists have limited information to inform their decisions regarding cancer treatment. Therefore, we identified potentially modifiable risk factors for disruptions in cancer care in this vulnerable population. Rather than cancer stage, public insurance, or lack of primary care physician, lack of mental health treatment predicted disruptions in breast cancer care for patients with schizophrenia. At cancer diagnosis, patients without a documented psychiatrist and antipsychotic medication were four to five times more likely to have disruptions. Patients who were psychiatrically hospitalized after cancer diagnosis also had significantly more disruptions. Treatment of schizophrenia at cancer diagnosis may be critical to facilitate the delivery of cancer care.
Although there is increasing recognition of the need to incorporate psychosocial care into cancer treatment [40], [41], cancer care and mental health care remain fragmented, particularly for patients with severe mental illness. Mental illness may not be recognized at the time of cancer diagnosis and oncologists may not assess whether mental illness is treated. Despite a previously documented diagnosis of schizophrenia, in our sample, oncologists rarely documented if patients had psychiatrists. Surgical and radiation oncologists, who may be the only oncologists involved for patients with early‐stage breast cancer, had particularly low rates of documentation of mental health factors. Collaborative care models that integrate mental health and cancer care are effective for patients with cancer who develop depression [42] and may be adapted to meet the specialty care needs of patients with schizophrenia and cancer [17]. Addressing psychiatric comorbidity at diagnosis may have the potential to prevent disruptions in cancer care by controlling psychiatric symptoms and supporting oncologists in adapting the cancer treatment plan [43]. For example, if psychiatric symptoms are sufficiently treated and psychiatric consultation is available, the oncology team may feel more comfortable recommending breast‐conserving therapy followed by adjuvant chemotherapy or radiation. Decreasing deviations from stage‐appropriate treatment at diagnosis may be one key strategy to improve cancer outcomes in this underserved population.
Although this is the largest medical record review of patients with schizophrenia and breast cancer, the findings are limited given that the population is from a single academic center and the lack of a control group of patients without mental illness. Our primary purpose was to identify potential predictors within the group of patients with schizophrenia and breast cancer to elucidate potential factors that may increase the risk of cancer care disruptions. Future studies will be strengthened by including a control group of patients without mental illness treated at the same cancer center. Although restricting our cohort to a single academic center limits generalizability, focusing on patients who are receiving treatment within an NCI‐designated cancer center likely underestimates the magnitude of the disparity given the resources necessary to receive outpatient oncology care and the relatively greater availability of psychosocial services at academic medical centers compared with community sites. It will be important for future research to include community cancer centers and utilize a prospective study design. Finally, although documentation of mental illness in the medical record is incomplete, this is the extent of the information available to the practicing oncologist and therefore has face validity. Moreover, we focused on the time of cancer diagnosis, when documentation of cancer and comorbid medical conditions should be most comprehensive.
Conclusion
Individuals with schizophrenia have worse cancer outcomes, yet little is known about the factors driving those disparities. In patients with schizophrenia and breast cancer, we identified frequent disruptions that could adversely affect cancer outcomes, including survival. Moreover, we identified potentially modifiable risk factors (e.g., access to psychiatric treatment at cancer diagnosis) that should be evaluated in prospective studies. In particular, optimizing psychiatric treatment when breast cancer is diagnosed may increase the likelihood that patients with schizophrenia receive timely, stage‐appropriate treatment. Furthermore, findings suggest that the receipt of stage‐appropriate treatment protects against risk of recurrence for these patients. Comanagement of schizophrenia and breast cancer starting at cancer diagnosis may be one key strategy that can decrease inequities in treatment and therefore improve cancer survival in this underserved population. Research investigating how to integrate mental health and cancer care is urgently needed to improve cancer outcomes for people with schizophrenia.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Helen P. Knight for data collection and chart review. This research was supported by the Dupont‐Warren Fellowship, Harvard Medical School; the Program in Cancer Outcomes Research Training Fellowship; and the National Cancer Institute, NCI K24 (ERP).
Author Contributions
Conception/Design: Kelly E. Irwin, Elyse R. Park, William F. Pirl
Collection and/or assembly of data: Kelly E. Irwin, Lauren E. Fields, John B. Taylor
Data analysis and interpretation: Kelly E. Irwin, Elyse R. Park, Jennifer A. Shin, Lauren E. Fields, Jamie M. Jacobs, Joseph A. Greer, Alphonse G. Taghian, Oliver Freudenreich, William F. Pirl
Manuscript writing: Kelly E. Irwin, Elyse R. Park, Jennifer A. Shin, Lauren E. Fields, Jamie M. Jacobs, Joseph A. Greer, John B. Taylor, Oliver Freudenreich, William F. Pirl
Final approval of manuscript: Kelly E. Irwin, Elyse R. Park, Jennifer A. Shin, Lauren E. Fields, Jamie M. Jacobs, Joseph A. Greer, John B. Taylor, Alphonse G. Taghian, Oliver Freudenreich, David P. Ryan, William F. Pirl
Disclosures
Alphonse G. Taghian: VisionRT (C/A); David P. Ryan: MPM Capital (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.National Institute of Mental Health. Schizophrenia. Available at http://www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtml. Accessed June 21, 2016.
- 2. Parks J, Svendsen D, Singer P et al, eds. NASMHPD Medical Directors NASMHPD Medical Directors Council Technical Report: Morbidity and mortality in people with serious mental illness. Alexandria, VA, 2006.
- 3. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–1131. [DOI] [PubMed] [Google Scholar]
- 4. Olfson M, Gerhard T, Huang C et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172–1181. [DOI] [PubMed] [Google Scholar]
- 5. Tran E, Rouillon F, Loze JY et al. Cancer mortality in patients with schizophrenia: An 11‐year prospective cohort study . Cancer 2009;115:3555–3562. [DOI] [PubMed] [Google Scholar]
- 6. Chang TS, Hou SJ, Su YC et al. Disparities in oral cancer survival among mentally ill patients. PLoS One 2013;8:e70883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunningham R, Sarfati D, Stanley J et al. Cancer survival in the context of mental illness: A national cohort study. Gen Hosp Psychiatry 2015;37:501–506. [DOI] [PubMed] [Google Scholar]
- 8. Crump C, Winkleby M, Sundquist K et al. Comorbidities and mortality in persons with schizophrenia: A Swedish national cohort study. Am J Psychiatry 2013;170:324–333. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: The National Academies Press, 2001. [PubMed]
- 10. Goss E, Lopez AM, Brown CL et al. American Society of Clinical Oncology policy statement: Disparities in cancer care. J Clin Oncol 2009;27:2881–2885. [DOI] [PubMed] [Google Scholar]
- 11. Moy B, Polite BN, Halpern MT et al. American Society of Clinical Oncology policy statement: Opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol 2011;29:3816–3824. [DOI] [PubMed] [Google Scholar]
- 12.National Library of Medicine. ClinicalTrials.gov. Available at https://clinicaltrials.gov/ct2/home. Accessed July 29, 2016.
- 13. Kisely S, Crowe E, Lawrence D. Cancer‐related mortality in people with mental illness. JAMA Psychiatry 2013;70:209–217. [DOI] [PubMed] [Google Scholar]
- 14. Chochinov HM, Martena PJ, Prior HJ et al. Does a diagnosis of schizophrenia reduce rates of mammography screening? A Manitoba population‐based study. Schizophr Res 2009;113:95–100. [DOI] [PubMed] [Google Scholar]
- 15. Folsom DP, McCahill M, Bartels SJ et al. Medical comorbidity and receipt of medical care by older homeless people with schizophrenia or depression. Psychiatr Serv 2002;53:1456–1460. [DOI] [PubMed] [Google Scholar]
- 16. Bergamo C, Sigel K, Mhango G et al. Inequalities in lung cancer care of elderly patients with schizophrenia: An observational cohort study. Psychosom Med 2014;76:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Irwin KE, Henderson DC, Knight HP et al. Cancer care for individuals with schizophrenia. Cancer 2014;120:323–334. [DOI] [PubMed] [Google Scholar]
- 18. Farasatpour M, Janardhan R, Williams CD et al. Breast cancer in patients with schizophrenia. Am J Surg 2013;206:798–804. [DOI] [PubMed] [Google Scholar]
- 19. Hwang M, Farasatpour M, Williams CD et al. Adjuvant chemotherapy for breast cancer in patients with schizophrenia. Oncol Lett 2012;3:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma A, Ngan S, Nandoskar A et al. Schizophrenia does not adversely affect the treatment of women with breast cancer: A cohort study. Breast 2010;19:410–412. [DOI] [PubMed] [Google Scholar]
- 21.Early Breast Cancer Trialists' Collaborative Group . Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992;339:1–15. [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network. Breast Cancer (Version 2.2016). Available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 20, 2016.
- 23.ASCO Institute for Quality. QOPI Measures Overview. Available at http://www.instituteforquality.org/qopi/measures. Accessed June 20, 2016.
- 24.National Quality Forum. Measures, reports & tools. Available at http://www.qualityforum.org/measures_reports_tools.aspx. Accessed June 20, 2016.
- 25. Cox DR. Regression models and life‐tables. J Roy Stat Soc B Met 1972;34:187–220. [Google Scholar]
- 26. Bleicher RJ, Ruth K, Siqurdson E et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol 2016;2:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chavez‐MacGregor M, Clarke CA, Lichtensztajn DY et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2016;2:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kummerow KL, Du L, Penson DF et al. Nationwide trends in mastectomy for early‐stage breast cancer. JAMA Surg 2015;150:9–16. [DOI] [PubMed] [Google Scholar]
- 29. Vaz‐Luis I, Hughes ME, Cronin A et al. Trends in the use of mastectomy in women with small node‐negative breast cancer treated as US academic centers. Breast Cancer Res Treat 2016;155:569–578. [DOI] [PubMed] [Google Scholar]
- 30. Mahmood U, Hanlon A, Koshy M et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol 2013;20:1436–1443. [DOI] [PubMed] [Google Scholar]
- 31. Jagsi R, Abrahamse P, Morrow M et al. Patterns and correlates of adjuvant radiotherapy receipt after lumpectomy and after mastectomy for breast cancer. J Clin Oncol 2010;28:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daumit G, Pronovost P, Anthony CB et al. Adverse events during medical and surgical hospitalizations for persons with schizophrenia. Arch Gen Psychiatry 2006;63:267–272. [DOI] [PubMed] [Google Scholar]
- 33. Liao CC, Shen WW, Chang CC et al. Surgical adverse outcomes in patients with schizophrenia: A population‐based study. Ann Surg 2013;257:433–438. [DOI] [PubMed] [Google Scholar]
- 34. Copeland LA, Zeber JE, Pugh MJ et al. Postoperative complications in the seriously mentally ill: A systematic review of the literature. Ann Surg 2008;248:31–38. [DOI] [PubMed] [Google Scholar]
- 35. Nelson JA, Fischer JP, Chung C et al. Risk of readmission following immediate breast reconstruction: Results from the 2011 American College of Surgeons National Surgical Quality Improvement Program data sets. Plast Reconstr Surg 2014;134:193e–201e. [DOI] [PubMed] [Google Scholar]
- 36. Agarwal S, Pappas L, Neumayer L et al. Effect of breast conservation therapy vs mastectomy on disease‐specific survival for early‐stage breast cancer. JAMA Surg 2014;149:267–274. [DOI] [PubMed] [Google Scholar]
- 37. Hartmann‐Johnsen OJ, Kåresen R, Schlichting E et al. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: A registry‐based follow‐up study of Norwegian women primary operated between 1998 and 2008. Ann Surg Oncol 2015;22:3836–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greer JA, Amoyal N, Nisotel L et al. A systematic review of adherence to oral antineoplastic therapies. The Oncologist 2016;21:354–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hershman DL, Kushi LH, Shao T et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early‐stage breast cancer patients. J Clin Oncol 2010;28:4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pirl WF, Fann JR, Greer JA et al. Recommendations for the implementation of distress screening programs in cancer centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer 2014;120:2946–2954. [DOI] [PubMed] [Google Scholar]
- 41. Jacobsen PB, Wagner LI. A new quality standard: The integration of psychosocial care into routine cancer care. J Clin Oncol 2012;30:1154–1159. [DOI] [PubMed] [Google Scholar]
- 42. Sharpe M, Walker J, Holm Hansen C et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology‐2): A multicentre randomized controlled effectiveness trial. Lancet 2014;384:1099–1108. [DOI] [PubMed] [Google Scholar]
- 43. Irwin KE, Freudenreich O, Peppercorn J et al. Case records of the Massachusetts General Hospital. Case 30–2016. A 63‐year‐old woman with bipolar disorder, cancer, and worsening depression. N Engl J Med 2016;375:1270–1281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.