The NABRAX study was designed to evaluate the antitumor activity and safety of single‐agent weekly nab‐paclitaxel as neoadjuvant treatment of estrogen receptor‐positive/HER2‐negative breast cancer patients. This article reports on the trial and efforts to define the role of this drug and biomarkers of activity in this particular subtype of breast cancer.
Keywords: Estrogen receptor‐positive breast cancer, Nanoparticle albumin‐bound Paclitaxel, Neoadjuvant treatment, Residual cancer burden, Luminal breast cancer
Abstract
Background.
Nanoparticle albumin‐bound paclitaxel (nab‐Paclitaxel) is an alternative to standard taxanes for breast cancer (BC) treatment. We evaluated nab‐Paclitaxel efficacy as neoadjuvant treatment for early estrogen receptor‐positive (ER+), human epidermal growth factor receptor 2‐negative (HER2‐) disease.
Materials and Methods.
Women with ER+, HER2‐, stage II–III BC were treated preoperatively with four cycles of weekly nab‐Paclitaxel (150 mg/m2), 3 weeks on and 1 week off. We hypothesized that poor pathological response rate (residual cancer burden [RCB] III; Symmans criteria) would be ≤16%.
Results.
Eighty‐one patients with a median age of 47 years were treated; 64.2% were premenopausal, and 69% of tumors were stage II. Residual cancer burden III rate was 28.4% (95% confidence interval [CI]: 18.6%–38.2%), RCB 0+I (good response) rate was 24.7% (95% CI: 15.3%–34.1%) and RCB 0 (complete response) rate was 7.4% (95% CI: 1.7%–13.1%). Objective response rate by magnetic resonance imaging was 76.5% and rate of conversion to breast conserving surgery was 40.0%. The most frequent grade 3 and 4 toxicity was neutropenia (12.3% and 3.7% of patients, respectively), without any febrile neutropenia. Sensory neuropathy grade 2 and 3 were seen in 25.9% and 2.5% of patients, respectively. Tumor secreted protein, acidic, cysteine‐rich (SPARC) overexpression was significantly associated with RCB 0 (odds ratio: 0.079; 95% CI: 0.009–0.689; p = .0216).
Conclusion.
Despite failing to confirm an RCB III rate ≤16% in nab‐Paclitaxel‐treated patients, the RCB 0+I rate indicates a significant drug antitumor activity with low rates of grade 3–4 toxicity. Our exploratory biomarker analysis suggests a potential predictive role of complete response for SPARC. Confirmatory analyses are warranted, adapting dose and schedule to decrease peripheral neurotoxicity. (Trial registration: European Clinical Trials Database study number: 2011‐004476‐10; ClinicalTrials.gov: NCT01565499).
Implications for Practice.
The pathological response rate (residual cancer burden [RCB]; Symmans criteria) of nanoparticle albumin‐bound paclitaxel administered as neoadjuvant treatment for early estrogen receptor‐positive, human epidermal growth factor receptor 2‐negative disease was evaluated. Whereas poor response (RCB III) was 24.7%, similar to that for docetaxel, good response (RCB 0+I) reached 23.0%, far superior to the 13% for docetaxel, while keeping toxicity low. Exploratory biomarker analysis suggests secreted protein, acidic, cysteine‐rich overexpression in tumor cells as a potential predictor of complete response (RCB 0). Findings point to an encouraging single‐agent neoadjuvant treatment with low toxicity, which warrants future research and development.
摘要
背景.纳米微粒白蛋白结合型紫杉醇(nab‐紫杉醇)是乳腺癌(BC)标准紫杉烷类药物治疗的替代疗法。研究评价了nab‐紫杉醇作为新辅助疗法治疗早期雌激素受体‐阳性(ER+)、人表皮生长因子受体2‐阴性(HER2‐)的疗效。
材料与方法.罹患ER+、HER2‐、II–III期BC的女性在术前接受4个周期的nab‐紫杉醇(150 mg/m2, 每周一次)治疗, 每个周期用药3周, 停药1周。假设缓解率≤16%为病理学缓解不佳 [残癌负荷(RCB)III级, Symmans标准]。
结果:81名患者(中位年龄为47岁)接受治疗;64.2%的患者为绝经前女性, 69%为II期乳腺癌患者。RCB III级的比率为28.4%[95%置信区间(CI):18.6%–38.2%], RCB 0+I级(明显缓解)的比率为24.7%(95% CI:15.3%–34.1%), RCB 0级(完全缓解)的比率为7.4%(95% CI:1.7%–13.1%)。经磁共振成像确定的客观缓解率为76.5%, 转为接受乳腺癌保乳手术的比率为40.0%。最常见的3‐4级毒性反应为中性粒细胞减少症(分别为12.3%及3.7%), 未发生中性粒细胞减少性发热。2‐3级感觉神经病变分别见于25.9%及2.5%的患者。肿瘤富含半胱氨酸酸性分泌蛋白(SPARC)过表达与RCB 0级显著相关(比值比:0.079, 95% CI:0.009–0.689; p = 0.0216)。
结论.虽然未能证实nab‐紫杉醇治疗的患者的RCB III级比率≤16%, 但RCB 0+I级比率表明该药物明显具有抗肿瘤活性, 且3‐4级毒性的发生率较低。探索性生物标志物分析表明, SPARC对完全缓解具有潜在预测作用。需要进行验证性分析, 通过调整给药剂量和时间来减轻周围神经毒性。(试验注册:欧洲临床试验数据库研究编号:2011‐004476‐10; Clinical‐Trials.gov:NCT01565499)
Introduction
Neoadjuvant chemotherapy of hormone receptor (HR)‐positive/human epidermal growth factor receptor 2 (HER2)‐negative breast cancer (BC) can produce tumor shrinkage in the majority of patients, allowing for a more conservative surgery (i.e., breast‐preserving surgery) in some of them [1]. In addition, a good pathological response to neoadjuvant therapy is associated with better outcomes [2]. However, the prognostic value of obtaining a pathological complete response (pCR) in this subtype is probably less marked than in others, like triple negative (TN) and HER2‐positive/HR‐negative [3].
In addition, the neoadjuvant setting is an excellent model for testing the activity of new compounds and searching for predictive biomarkers.
Taxanes, paclitaxel and docetaxel, are among the most effective treatments for BC [4] and are considered a significant component of neoadjuvant BC regimens [5]. However, the hydrophobic nature of conventional taxanes mandates the concomitant use of synthetic solvents, which may be responsible for some of their toxicity [6], [7], and may limit their efficacy by reducing the intratumoral concentrations of the active drug [8].
Nanoparticle albumin‐bound paclitaxel (nab‐Paclitaxel) is a formulation of paclitaxel that consists of nanometer‐range particles of paclitaxel, bound to human serum albumin. This union increases drug solubility, improving its delivery to tumor cells and thus avoiding the need for solvents [9].
In the pivotal phase III trial of nab‐Paclitaxel monotherapy versus solvent‐based paclitaxel, the objective response rate (ORR) and time to progression were significantly increased in all patients treated with nab‐Paclitaxel. Overall survival (OS) was also increased for patients treated in second or subsequent lines [10]. Nab‐Paclitaxel has also been evaluated in the neoadjuvant setting of BC patients [11], [12], [13], [14], [15], [16], [17], [18].
All of these neoadjuvant trials administered nab‐Paclitaxel in combination with other drugs and usually in mixed BC subtypes. We designed a trial (the NABRAX study) to evaluate the antitumor activity and safety of single‐agent weekly nab‐Paclitaxel as neoadjuvant treatment of estrogen receptor (ER)‐positive/HER2‐negative BC patients. Our main aims are to further define the role this drug plays in this particular subtype of BC and to search for biomarkers of activity.
Materials and Methods
Study Design
This is a multicenter, single‐arm, phase II trial. All eligible patients were administered intravenous nab‐Paclitaxel weekly (150 mg/m2) on days 1, 8, and 15, every 4 weeks, for four cycles. Upon completion of nab‐Paclitaxel, patients underwent mastectomy or breast‐conserving surgery (BCS), plus axillary lymph node dissection (unless previous sentinel lymph node biopsy had been negative) within a maximum of 6 weeks after the last dose of the study drug. Adjuvant treatment was left at the investigator criteria.
This trial was approved by all the participating institutions’ Ethical Review Boards and the Spanish Health Authorities and registered in European Clinical Trials Database (2011‐004476‐10) and ClinicalTrial.gov (NCT01565499). It was conducted in compliance with Good Clinical Practices and the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients before study entry.
Eligibility Criteria
Patients aged >18 years, with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) <2, were eligible for the trial if they had histologically proven primary unilateral invasive early BC through a core biopsy, and their largest tumor size in the breast was ≥2 cm, or <2 cm if positive for axillary involvement. Tumors had to be ER‐positive (>1%) and HER2‐negative (immunohistochemistry [IHC] 0 or 1+, or negative gene amplification by fluorescence/chromogenic in situ hybridization [ISH]) by local determinations. Patients were required to have adequate bone marrow, renal, and liver functions and be oncology treatment‐naïve. For potentially fertile women, adequate contraception and a negative pregnancy test were required.
Patients were excluded if they had inflammatory BC (T4d), supraclavicular lymph nodes (N3), synchronous contralateral or multicentric BC, evidence of metastatic disease or a pre‐existing neurotoxicity grade ≥2 (based on the National Cancer Institute‐Common Terminology Criteria for Adverse Events [NCI‐CTCAE] version 4.0, http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). Finally, patients were also excluded if they had a previous history of cancer other than adequately treated carcinoma in situ of the cervix, stage I colon cancer, skin carcinoma (non‐melanoma), or other malignant tumors treated more than 5 years before the study entry with no subsequent evidence of recurrence.
Assessments and Endpoints
The primary endpoint of the study was to determine the rate of poor pathological response (residual cancer burden [RCB] III). Secondary endpoints included rate of good pathological response (RCB 0+I), ORR, rate of conversion to BCS, invasive disease‐free survival, and toxicity. Exploratory analyses of tumor and blood samples for potentially predictive biomarkers were centrally performed.
The following baseline procedures were performed before study entry: (a) breast tumor assessment by physical exam; (b) bilateral breast mammogram and magnetic resonance imaging (MRI); (c) lymph node status determination by axillary ultrasound (with pathological confirmation in case of suspicion); (d) ECOG PS evaluation; (e) hematology; (f) serum chemistry; (g) pregnancy test (potentially fertile women only); (h) electrocardiogram; and (i) local determination of ER/progesterone receptor (PgR)/HER2 expression levels. Eastern Cooperative Oncology Group Performance Status evaluation, hematology, and serum chemistry were repeated before each cycle and the breast tumor assessment was repeated before surgery.
Pathological response was centrally assessed at surgery following the Symmans classification criteria [2]. Objective response rate was evaluated according to the Response Evaluation Criteria in Solid Tumors 1.1 criteria [19] before surgery. Toxicities were assessed after each cycle and graded according to the NCI‐CTCAE version 4.0.
Putative Predictive Biomarker Assessment
We analyzed data from patients with available centralized biomarker determination and pathological response information.
Biomarker analysis by IHC and/or ISH in Formalin‐fixed paraffin‐embedded tumor samples was carried out at a central laboratory by a pathologist blinded to clinical parameters (see additional information in supplemental online Appendix 2). The assessment of ER, PgR, HER2, and Ki67 was performed following the American Society of Clinical Oncology and the College of American Pathologists guidelines [20], [21], [22]. The cut‐off considered for Ki67 expression was 20% of positively stained tumor cells [23]. Secreted protein, acidic, cysteine‐rich was evaluated for both tumor and stroma as described previously [24]. Its expression was categorized as negative when the intensity was absent‐to‐weak (+), or moderate (++)‐to‐strong (+++) with a proportion of stained cells <10%. Immunolabeling was positive if the intensity was moderate (++)‐to‐strong (+++) and the extent of staining was ≥10%. Caveolin (Cav)‐1 was evaluated in the stroma and its expression was categorized in low, moderate, or high. The high expression of Cav‐1 was considered as positive. Molecular subtypes were classified according to St. Gallen criteria 2013 [23] and Prat et al. [25] into Luminal A (ER+, PgR >20%, HER2‐, Ki67 <14%), Luminal B1 (ER+, HER2‐, PgR ≤20% and/or Ki67 ≥14%), Luminal B2 (ER+, HER2+, any PgR, any Ki67), TN (ER‐, PgR‐, HER2‐), and HER2‐enriched (ER‐, PgR‐, HER2+) subtypes.
Statistical Considerations
The sample size was calculated based on the null hypothesis of a poor pathological response (RCB III) of 33% (seen in patients with luminal BC when treated with single‐agent docetaxel [26]) and an alternative hypothesis (H1) of RCB III of 16% or less with single‐agent nab‐Paclitaxel. For significance (alpha) of 0.05 and a two‐tailed test, 70 evaluable patients had to be included in the study to yield a statistically significant result with a power of 91.8%. Assuming a dropout rate of 10%, 78 was the total number of patients to be recruited into this study.
The efficacy and safety variables were analyzed in the intent‐to‐treat population (defined as study patients receiving at least one dose of the study drug).
The number and proportion of patients experiencing the different grades of pathological response (III, 0, 0+I), and their corresponding two‐sided 95% CIs, were calculated.
Univariate analyses and multivariate logistic regression analyses were used to study the association of biomarkers with pathological response. The biomarkers were also analyzed by Fisher's exact test.
All analyses were performed using SAS Enterprise Guide 5.1 software (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html).
Results
Patient Characteristics
Between April 2012 and January 2013, 83 patients were registered in 15 Spanish sites. Two of them never received treatment and were excluded from the analysis. The median age was 47 years (range: 28–75 years), 64% of patients were premenopausal, 97.5% had an ECOG PS of 0, and 69.0% and 22.0% were diagnosed with stage II and III, respectively. Most tumors (91.4%) had histology of invasive ductal carcinoma, 48% were histological grade 2, 32% were grade 3, and 21% were PgR negative.
Dose Administration
The median relative dose‐intensity for nab‐Paclitaxel was 98.5%. Of the 81 patients analyzed, six discontinued treatment early; thus, 75 patients completed the treatment as planned. Reasons for discontinuation included adverse events (five patients, three of them due to sensory neuropathy) and consent withdrawal (one patient). Concerning dose modifications, 5.3% of the cycles were omitted, another 5% of the cycles were delayed, and 10.6% of nab‐Paclitaxel doses had to be reduced. Main reasons for dose modification were neutropenia (in 25.9% of patients) and sensory neuropathy (in 18.5% of patients).
Toxicity
Table 1 summarizes all frequent grade 2–4 toxicities per patient and per cycle. The most frequent grade 3–4 toxicities were neutropenia in 13 patients (16%) and sensory neuropathy in two patients (2.5%). Two serious cases of adverse events related with nab‐Paclitaxel were reported; both were grade 3 sensory neuropathy that descended to grade 1 following treatment discontinuation. However, these adverse effects were still present at the two‐year follow‐up.
Table 1. Grade 2–4 related adverse events (toxicity) based on the National Cancer Institute‐Common Terminology Criteria for Adverse Events version 4.0.
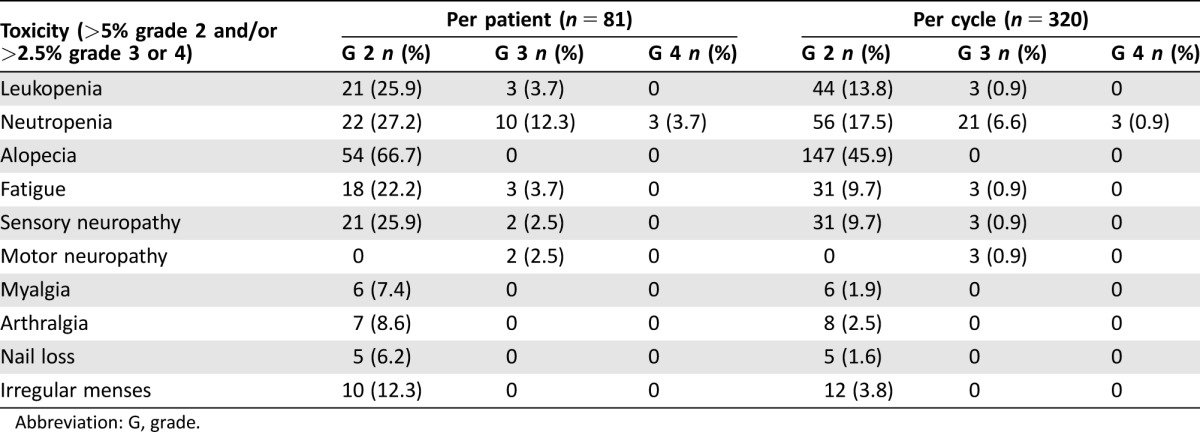
Abbreviation: G, grade.
Efficacy
The RCB evaluation was available for 80 of the 81 treated patients (one patient received three cycles but refused further treatment and withdrew the informed consent). The RCB III rate in the 81 treated patients was 28.4% (95% CI: 18.6%–38.2%); 24.7% (95% CI: 15.3%–34.1%) of patients achieved a good pathological response (RCB 0+I), with six patients (7.4%; 95% CI: 1.7%–13.1%) achieving pathological complete response (RCB 0).
Three patients included in the trial were reclassified as TN by the central laboratory (see Tumor Biomarkers and Clinical‐Pathological Variables Predictive of Efficacy section). Two of them achieved pCR and one achieved an RCB type I response. When excluding these three patients, the rates of RCB III and RCB 0+I were 29.7% and 23.0%, respectively.
The ORR measured by MRI was 76.5% (95% CI: 67.3%–85.7%) and 60.5% (95% CI: 49.9%–71.1%) when measured by mammography.
Breast‐conserving surgery was performed in 49 patients (60.5%). The rate of conversion to BCS in patient candidates for a mastectomy at time of diagnosis was 40%.
Tumor Biomarkers and Clinical‐Pathological Variables Predictive of Efficacy
Of the 81 patients, 77 had available pretreatment tumor sample for biomarker analysis (ER, PgR, HER2, Ki67, Cav‐1 and SPARC) in a central laboratory. The distribution by BC subtypes following St. Gallen 2013 and Prat et al. criteria [23], [25] was as follows: Luminal A (25%), Luminal B1 (71%), and TN (4%). Of these 77 patients, five were excluded from biomarker analysis (three patients [4%] due to central TN phenotype, one due to missing pathological response data, and one due to incomplete central biomarker data).
Residual cancer burden 0, 0+I, and RCB III were correlated with biomarker expression in 72 patients (Tables 2, 3, and 4, respectively). In the univariate analysis, there was no correlation between RCB III and any biomarker or subtype (data not shown). Tumor size <20 mm (p = .0029), absence of regional lymph node metastases (p = .0005), and Cav‐1 overexpression in stroma (p = .0320) seemed to be associated with RCB 0+I (supplemental online Table 1). In the multivariate analysis, only tumor size (odds ratio [OR]: 0.070; 95% CI: 0.008–0.635; p = .0181) and lymph node metastases (OR: 0.070; 95% CI: 0.012–0.394; p = .0026) were significantly associated with RCB (0+I; supplemental online Table 2). Finally, tumor SPARC overexpression was associated with RCB 0 in the univariate analyses (OR: 0.079; 95% CI: 0.009–0.689; p = .0216; supplemental online Table 3).
Table 2. Correlation between individual biomarkers and clinicopathological parameters and pathological complete response (RCB 0).

7th Edition AJCC Cancer Staging Atlas (2010).
Bolded values indicate statistically significant.
Abbreviations: AJCC, American Joint Committee on Cancer; Cav‐1, caveolin‐1; NA, not applicable; PgR, progesterone receptor; pN, pathological lymph nodes; pT, pathological tumor size; RCB, residual cancer burden; SPARC, tumor secreted protein, acidic, cysteine‐rich.
Table 3. Correlation between individual biomarkers and clinico‐pathological parameters and good pathological response (RCB 0+I).
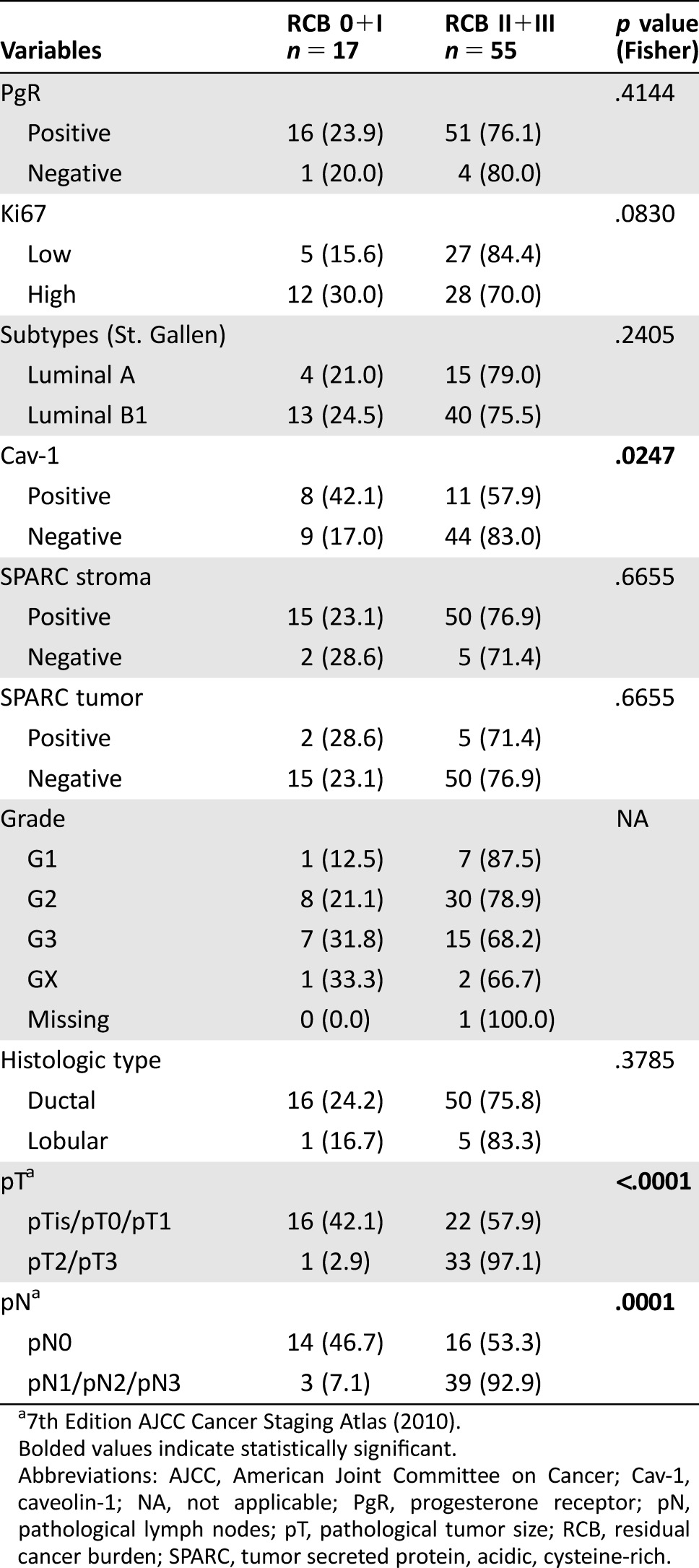
7th Edition AJCC Cancer Staging Atlas (2010).
Bolded values indicate statistically significant.
Abbreviations: AJCC, American Joint Committee on Cancer; Cav‐1, caveolin‐1; NA, not applicable; PgR, progesterone receptor; pN, pathological lymph nodes; pT, pathological tumor size; RCB, residual cancer burden; SPARC, tumor secreted protein, acidic, cysteine‐rich.
Table 4. Correlation between individual biomarkers and clinico‐pathological parameters and poor pathological response (RCB III).
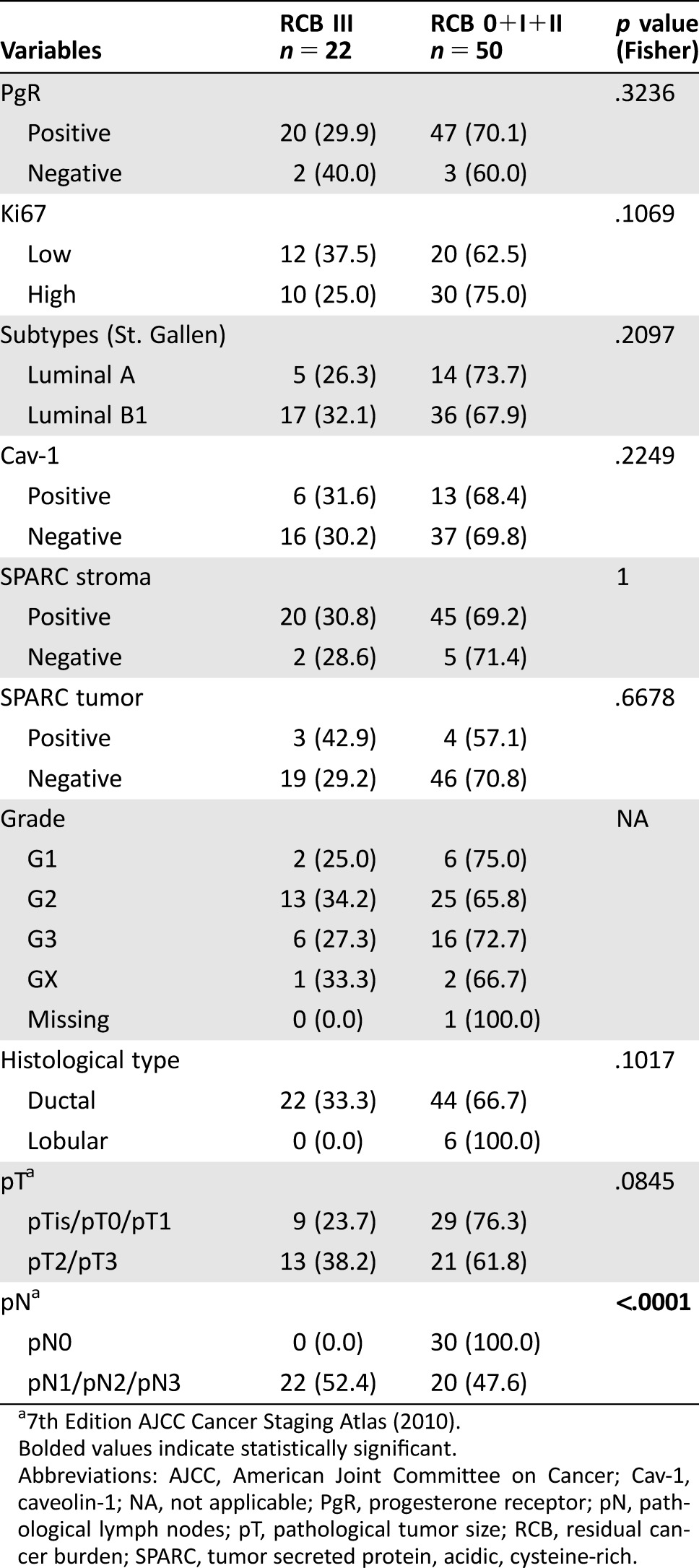
7th Edition AJCC Cancer Staging Atlas (2010).
Bolded values indicate statistically significant.
Abbreviations: AJCC, American Joint Committee on Cancer; Cav‐1, caveolin‐1; NA, not applicable; PgR, progesterone receptor; pN, pathological lymph nodes; pT, pathological tumor size; RCB, residual cancer burden; SPARC, tumor secreted protein, acidic, cysteine‐rich.
Discussion
The NABRAX study failed to confirm the a priori antitumor activity hypothesis (a Symmans RCB III rate of 16% or less in ER‐positive/HER2‐negative BC) because the observed rate was 28.4% (95% CI: 18.6%–38.2%). We have no evidence of any difference in the poor response rate experienced by patients receiving nab‐Paclitaxel and the 33.0% poor response observed in historical controls treated with docetaxel (100 mg/m2) [26].
However, we found a remarkable rate of good pathological response with nab‐Paclitaxel (Symmans RCB 0+I). Twenty of the 81 treated patients, (24.7%, 95% CI: 15.3%–34.1%) achieved RCB 0+I and six patients (7.4%, 95% CI: 1.7%–13.1%) had a pathological complete response (RCB 0). Excluding those three patients reclassified as having TN phenotype BC in the central review, the rate of RCB 0+I was 23.0%. In the historical control series treated with docetaxel, upon which the statistical assumption of the NABRAX study was based [26], the rate of RCB 0+I seen in Luminal A and B patients was 13%, apparently inferior to that observed in the NABRAX trial, although inter‐study comparisons should be interpreted with extreme caution. In support of the good antitumor activity of nab‐Paclitaxel in this population, the ORR by MRI in the NABRAX trial was 76.5% (95% CI: 67.3%–85.7%) and the rate of conversion to BCS in patients who are candidates for mastectomy at diagnosis reached 40%.
Two studies have studied nab‐Paclitaxel in comparison with conventional paclitaxel in the neoadjuvant setting in early‐stage high‐risk BC patients, with inconclusive results. The GEPARSEPTO study found that nab‐Paclitaxel was superior to conventional paclitaxel in achieving pCR (p = .00065) [16]. In that trial, both nab‐Paclitaxel and conventional paclitaxel were administered in 12 weekly doses in consecutive weeks and followed by sequential anthracyclines. This trial started with a dose of 150 mg/m2 of nab‐Paclitaxel but, after a preplanned safety interim analysis, the dose had to be reduced to 125 mg/m2 due to toxicity. The Evaluating Treatment with Neoadjuvant Abraxane trial showed a trend to a superiority of nab‐Paclitaxel but it was not statistically significant (p = .127). In that trial, nab‐Paclitaxel was administered weekly (125 mg/m2; 3 weeks on and 1 week off, for four cycles) and followed by sequential anthracyclines [11]. In the NABRAX study, the schedule administered (150 mg/m2; 3 weeks on and 1 week off, for four cycles) allowed a better tolerance as shown by the median relative dose‐intensity of nab‐Paclitaxel (98.5%) and the low rates of toxicity, apart from grade 2 + 3 peripheral neuropathy that was reported by 28.5% of patients, causing early discontinuation in three patients.
Neoadjuvant hormonotherapy for HR‐positive/HER2‐negative tumors, an alternative approach to neoadjuvant chemotherapy, is gaining ground in some countries and in the research setting [27], [28], [29], [30]. In some studies comparing neoadjuvant hormones versus neoadjuvant chemotherapy in ER‐positive tumors, the clinical response rates were similar with both therapies [31], [32]. However, these results cannot rule out that some of these patients (i.e., those with high Ki67) could benefit from the use of both therapies in sequence. Therefore, neoadjuvant hormonal therapy (which is not usually followed by adjuvant chemotherapy after surgery) should be reserved for patients whose tumors are not candidates for chemotherapy based on proliferation index or genomic profiling.
Previous findings in lung cancer support that higher Cav‐1 levels in tumor‐associated stroma are associated with better ORR and OS [33]. Similarly, our biomarker analysis found that high expression of Cav‐1 was correlated with good pathological response in the univariate analysis but not in the multivariate analysis. Stromal Cav‐1 is a protein that favors biomechanical remodeling of the microenvironment and tumor invasion [34].
The role of SPARC in cancer is controversial. Most studies suggest a protumorigenic role, but others suggest an antitumorigenic activity [35]. In the NABRAX study, SPARC expression in tumor was associated with pathological complete response (RCB 0) in the univariate analyses. Similarly to other proteins involved in extracellular matrix remodeling and invasion in human BC, expression of SPARC in tumor cells has been associated with reduction of characteristic epithelial markers and acquisition of an aggressive and stem cell‐related phenotype in these tumors [36]. Similar findings have been reported in other epithelial tumor types (lung, prostate, ovarian, or endometrial), suggesting the involvement of SPARC present in tumor cells in cancer growth, apoptosis and metastasis, cell migration, and stroma formation [37], [38]. Moreover, SPARC tumor expression and stem cell‐related phenotype have been extensively correlated with response to systemic therapies. In BC, SPARC has been associated with a higher chance of achieving a pathological complete remission after Docetaxel/Doxorubicine/Cyclophosphamide or TAC + Four Cycles of Vinorelbine and Capecitabine (NX) chemotherapy [39]. It has also been hypothesized that binding of SPARC to albumin in the tumor microenvironment may enrich the concentration of nab‐Paclitaxel in head and neck tumors, enhancing its antitumor activity [40]. Furthermore, in a phase I/II trial in pancreas cancer patients treated with gemcitabine and nab‐Paclitaxel, the high expression of SPARC was correlated with improved OS. Patients with high levels of SPARC had a median survival of 17.8 months and patients with low SPARC expression had a median survival of 8.1 months [41]. However, results from the phase III MPACT trial of nab‐Paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic pancreatic cancer showed that there was no association between stromal, tumor epithelial, or plasma SPARC levels and efficacy in terms of OS [24]. This result is in line with GEPARSEPTO trial [16]. The role of SPARC in tumor cells warrants further study. The interest of Cav‐1 and SPARC as predictors of response to nab‐Paclitaxel should be validated in other, larger, cohorts of patients.
Conclusion
Our patient response rate related to nab‐Paclitaxel failed to support its superiority as a neoadjuvant treatment, in terms of reduction of poor pathological response rate, with respect to historical controls treated with docetaxel. However, it showed an encouraging single‐agent activity in patients with ER‐positive/HER2‐negative BC that warrants future development in the neoadjuvant setting, maybe adapting dose and schedule to decrease peripheral neurotoxicity.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We acknowledge the investigators, pathology departments and other site staff of the 15 participant sites, the patients, GEICAM staff, Celgene International Sàrl, Switzerland, and FEDER (European Regional Development Fund).
We thank Hosanna Soler Vila, Ph.D., for her contribution to this manuscript; as medical writer, she contributed by editing this article and ensuring appropriate English redaction. This was funded by GEICAM. This work was supported by an unrestricted grant from Celgene International Sàrl, Switzerland, which also supplied the nab‐Paclitaxel. Dr. Martín, Dr. Barnadas, and Dr. Palacios have research grants CIBERONC‐CB16/12/00471 and RTICC RD12/0036/0076. The study was presented as a poster at the 13th St. Gallen International Breast Cancer Conference in St. Gallen, Switzerland, March 2013; as a poster at the 36th San Antonio Breast Cancer Symposium (SABCS) in San Antonio, Texas, December 2013; and as a poster at the 50th American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, May 2014.
Footnotes
For Further Reading: Priyanka Sharma. Biology and Management of Patients With Triple‐Negative Breast Cancer. The Oncologist 2016;21:1050–1062; first published on July 11, 2016.
Implications for Practice: Triple‐negative breast cancer (TNBC) is an aggressive subtype that is associated with poor outcomes. This article reviews clinical features and discusses the molecular diversity of this unique subtype. Current treatment paradigms, the role of germline testing, and platinum agents in TNBC are reviewed. Results and observations from pertinent clinical trials with potential implications for patient management are summarized. This article also discusses the clinical development and ongoing clinical trials of novel promising therapeutic agents in TNBC.
Author Contributions
Conception/design: Miguel Martín
Provision of study material or patients: Miguel Martín, César Rodríguez‐Martín, Rosalía Caballero, María I. Casas, Federico Rojo, Eva Carrasco
Collection and/or assembly of data: José I. Chacón, Antonio Antón, Arrate Plazaola, Elena García‐Martínez, Miguel A. Seguí, Pedro Sánchez‐Rovira, José Palacios, Lourdes Calvo, Carmen Esteban, Enrique Espinosa, Agusti Barnadas, Norberto Batista, Angel Guerrero, Montserrat Muñoz, Estefania Romio, Silvia Antolín
Data analysis and interpretation: Miguel Martín, César Rodríguez‐Martín, Rosalía Caballero, Federico Rojo, Eva Carrasco
Manuscript writing: Miguel Martín, José I. Chacón, Antonio Antón, Arrate Plazaola, Elena García‐Martínez, Miguel A. Seguí, Pedro Sánchez‐Rovira, José Palacios, Lourdes Calvo, Carmen Esteban, Enrique Espinosa, Agusti Barnadas, Norberto Batista, Angel Guerrero, Montserrat Muñoz, Estefania Romio, César Rodríguez‐Martín, Rosalía Caballero, María I. Casas, Federico Rojo, Eva Carrasco, Silvia Antolín
Final approval of manuscript: Miguel Martín, José I. Chacón, Antonio Antón, Arrate Plazaola, Elena García‐Martínez, Miguel A. Seguí, Pedro Sánchez‐Rovira, José Palacios, Lourdes Calvo, Carmen Esteban, Enrique Espinosa, Agusti Barnadas, Norberto Batista, Angel Guerrero, Montserrat Muñoz, Estefania Romio, César Rodríguez‐Martín, Rosalía Caballero, María I. Casas, Federico Rojo, Eva Carrasco, Silvia Antolín
Disclosures
Montserrat Muñoz: AstraZeneca (SAB), Roche (ET). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2012;30:1747–1749. [DOI] [PubMed] [Google Scholar]
- 2. Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 3. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 4. Aapro MS, Von Minckwitz G. Molecular basis for the development of novel taxanes in the treatment of metastatic breast cancer. EJC Suppl 2008;6:3–11. [Google Scholar]
- 5. Thompson AM, Moulder‐Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol 2012;23(suppl 10):x231–x236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gelderblom H, Verweij J, Nooter K et al. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001;37:1590–1598. [DOI] [PubMed] [Google Scholar]
- 7. Weiss RB, Donehower RC, Wiernik PH et al. Hypersensitivity reactions from taxol. J Clin Oncol 1990;8:1263–1268. [DOI] [PubMed] [Google Scholar]
- 8. ten Tije AJ, Verweij J, Loos WJ et al. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin Pharmacokinet 2003;42:665–685. [DOI] [PubMed] [Google Scholar]
- 9. Desai N, Trieu V, Yao Z et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor‐free, albumin‐bound paclitaxel, ABI‐007, compared with cremophor‐based paclitaxel. Clin Cancer Res 2006;12:1317–1324. [DOI] [PubMed] [Google Scholar]
- 10. Gradishar WJ, Tjulandin S, Davidson N et al. Phase III trial of nanoparticle albumin‐bound paclitaxel compared with polyethylated castor oil‐based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794–7803. [DOI] [PubMed] [Google Scholar]
- 11. Gianni L, Mansutti M, Anton A et al. ETNA (Evaluating Treatment with Neoadjuvant Abraxane) randomized phase III study comparing neoadjuvant nab‐paclitaxel (nab‐P) versus paclitaxel (P) both followed by anthracycline regimens in women with HER2‐negative high‐risk breast cancer: A MICHELANGO study. Paper presented at: ASCO; 2016; Chicago, IL. [Google Scholar]
- 12. Mehta RS. In vivo response‐adapted dose‐dense (dd) doxorubicin and cyclophosphamide (AC) ‐> weekly carboplatin and albumin‐bound paclitaxel (nab‐TC) plus trastuzumab (H) or bevacizumab (B) in patients with large and inflammatory breast cancer (BC): A phase II study. Paper presented at: ASCO; 2008; Chicago, IL.
- 13. Mrozek E, Lustberg MB, Knopp MV et al. Phase II trial of neoadjuvant chemotherapy (NCT) with weekly nanoparticle albumin‐bound paclitaxed (Nab‐P), carboplatin (CBP), and bevacizumab (BEV) in women with clinical stages II‐III breast cancer (BC): Pathologic response prediction by changes in angiogenic volume (AV) by dynamic contrast magnetic resonance imaging (DCE‐MRI). Paper presented at: ASCO; 2010; Chicago, IL. [Google Scholar]
- 14. Paz IB, Lau S, Garberoglio C et al. Nab‐paclitaxel and carboplatin with or without trastuzumab (trast) as part of neoadjuvant chemotherapy (NCT) in patients (pts) with stage II‐III breast cancer (BC). Paper presented at: ASCO; 2008; Chicago, IL. [Google Scholar]
- 15. Robidoux A, Buzdar AU, Quinaux E et al. A phase II neoadjuvant trial of sequential nanoparticle albumin‐bound paclitaxel followed by 5‐fluorouracil/epirubicin/cyclophosphamide in locally advanced breast cancer. Clin Breast Cancer 2010;10:81–86. [DOI] [PubMed] [Google Scholar]
- 16. Untch M, Jackisch C, Schneeweiss A et al. Nab‐paclitaxel versus solvent‐based paclitaxel in neoadjuvant chemotherapy for early breast cancer (geparsepto‐gbg 69): A randomised, phase 3 trial. Lancet Oncol 2016;17:345–356. [DOI] [PubMed] [Google Scholar]
- 17. Veerapaneni A, Boisvert M, Choi A et al. Capecitabine and ABI‐007 chemotherapy as neoadjuvant treatment of locally advanced breast cancer. Paper presented at: ASCO; 2008; Chicago, IL. [Google Scholar]
- 18. Yardley DA, Zubkus J, Daniel B et al. A phase II trial of dose‐dense neoadjuvant gemcitabine, epirubicin, and albumin‐bound paclitaxel with pegfilgrastim in the treatment of patients with locally advanced breast cancer. Clin Breast Cancer 2010;10:367–372. [DOI] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris L, Fritsche H, Mennel R et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287–5312. [DOI] [PubMed] [Google Scholar]
- 22. Wolff AC, Hammond ME, Hicks DG et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 23. Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013;24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hidalgo M, Plaza C, Musteanu M et al. SPARC expression did not predict efficacy of nab‐paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT trial. Clin Cancer Res 2015;21:4811–4818. [DOI] [PubMed] [Google Scholar]
- 25. Prat A, Cheang MC, Martin M et al. Prognostic significance of progesterone receptor‐positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013;31:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin M, Romero A, Cheang MC et al. Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat 2011;128:127–136. [DOI] [PubMed] [Google Scholar]
- 27. Ellis MJ, Tao Y, Luo J et al. Outcome prediction for estrogen receptor‐positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spring LM, Gupta A, Reynolds KL et al. Neoadjuvant endocrine therapy for estrogen receptor‐positive breast cancer: A systematic review and meta‐analysis. JAMA Oncol 2016;2:1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellis MJ, Suman VJ, Hoog J et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor‐rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50‐based intrinsic subtype–ASOSOG Z1031. J Clin Oncol 2011;29:2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eiermann W, Paepke S, Appfelstaedt J et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double‐blind multicenter study. Ann Oncol 2001;12:1527–1532. [DOI] [PubMed] [Google Scholar]
- 31. Alba E, Calvo L, Albanell J et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: Results from the GEICAM/2006‐03, a multicenter, randomized, phase‐II study. Ann Oncol 2012;23:3069–3074. [DOI] [PubMed] [Google Scholar]
- 32. Semiglazov VF, Semiglazov VV, Dashyan GA et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor‐positive breast cancer. Cancer 2007;110:244–254. [DOI] [PubMed] [Google Scholar]
- 33. Bertino EM, Williams TM, Nana‐Sinkam SP et al. Stromal caveolin‐1 is associated with response and survival in a phase II trial of nab‐paclitaxel with carboplatin for advanced NSCLC patients. Clin Lung Cancer 2015;16:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goetz JG, Minguet S, Navarro‐ Lerida I et al. Biomechanical remodeling of the microenvironment by stromal caveolin‐1 favors tumor invasion and metastasis. Cell 2011;146:148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Podhajcer OL, Benedetti LG, Girotti MR et al. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev 2008;27:691–705. [DOI] [PubMed] [Google Scholar]
- 36. Park SY, Lee HE, Li H et al. Heterogeneity for stem cell‐related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res 2010;16:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen J, Wang M, Xi B et al. SPARC is a key regulator of proliferation, apoptosis and invasion in human ovarian cancer. PLoS One 2012;7:e42413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yusuf N, Inagaki T, Kusunoki S et al. SPARC was overexpressed in human endometrial cancer stem‐like cells and promoted migration activity. Gynecol Oncol 2014;134:356–363. [DOI] [PubMed] [Google Scholar]
- 39. Lindner JL, Loibl S, Denkert C et al. Expression of secreted protein acidic and rich in cysteine (SPARC) in breast cancer and response to neoadjuvant chemotherapy. Ann Oncol 2015;26:95–100. [DOI] [PubMed] [Google Scholar]
- 40. Desai N, Trieu V, Damascelli B et al. SPARC expression correlates with tumor response to albumin‐bound paclitaxel in head and neck cancer patients. Transl Oncol 2009;2:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Von Hoff DD, Ramanathan RK, Borad MJ et al. Gemcitabine plus nab‐paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol 2011;29:4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


