Soft tissue sarcomas (STS) are a heterogeneous group of rare mesenchymal tumors, accounting for approximately 1% of all adult malignancies. About 60% of all STS arise in the extremities. This article reports on the clinical behavior of extremity STS and prognostic indicators of survival.
Keywords: Soft tissue sarcoma, Margins, Width, Survival, Recurrence, Metastasis
Abstract
Background.
Soft tissue sarcomas (STS) arising in the extremities pose a therapeutic challenge due to concerns of functional morbidity. Resections with negative margins are the mainstay of therapy, but the prognostic significance of surgical margins remains controversial. The purpose of this study was to determine the prognostic impact of surgical margins and clear margin widths in patients with STS of the extremities.
Materials and Methods.
We assessed the relationship between local recurrence‐free (LRFS), disease‐specific (DSS), and metastasis‐free survival (MFS) and potential prognostic factors retrospectively in a consecutive series of 643 patients treated at our institution between 1996 and 2016. Potential prognostic factors were assessed using univariate and multivariate analyses.
Results.
The median follow‐up time after primary diagnosis was 5.4 years (95% confidence interval [CI]: 4.8–6.0). The five‐year estimates of the DSS, LRFS, and MFS rates in the entire cohort were 85.3% (95% CI: 81.6–88.3), 65.3% (95% CI: 60.8–69.5) and 78.0% (95% CI: 74.1–81.4), respectively. Histological grade and the quality of surgical margins were independent prognostic factors of all three survival endpoints (LRFS, DSS, MFS) in multivariate analyses. Within the R0 subgroup, univariate and multivariate analyses of categorized (≤1 mm vs. 1–5 mm vs. >5 mm) and non‐categorized margin widths revealed that close and wide negative margins led to similar outcomes. Adjuvant radiation improved local control independently, but not DSS and MFS.
Conclusion.
Microscopically negative margins were associated with better LRFS, DSS, and MFS regardless of whether adjuvant radiation was applied. Here, surgical margins can be close as long as the resected tumor has no ink on it.
Implications for Practice.
In the present retrospective analysis of 643 patients with primary soft issue sarcomas of the extremities, surgical margins could be identified as independent predictors of local recurrence‐free, disease‐specific, and metastasis‐free survival. Given the diminished outcome of patients left with positive margins, surgical efforts should aim to achieve microscopically negative margins whenever feasible. It is noteworthy that only the quality of surgical margins, but not the negative margin width attained, had an influence on the prognosis. Our findings suggest that surgical margins can be close as long as the resected tumor has no ink on it.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of rare mesenchymal tumors, accounting for approximately 1% of all adult malignancies [1]. About 60% of all STS arise in the extremities [2], [3].
The standard curative treatment for extremity STS still remains surgical resection with negative margins and radiotherapy for intermediate and high‐grade sarcomas [4], [5], [6]. There have been several analyses of the prognostic factors influencing survival in patients with extremity STS to date. Among these factors, histological grade, tumor size, depth, and histological subtype are considered the most significant. Although negative surgical margins have been determined to be an important factor for improving local recurrence‐free survival (LRFS), their impact on disease‐specific survival (DSS) is still a subject of debate. Large single‐institutional studies investigating the prognostic significance of surgical margins in extremity STS patients have presented inconsistent results [2], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. However, the implementation of radical surgery in the extremities is often difficult. The attainment of negative margins may require extensive surgery and could result in considerable impairment of extremity function, particularly in cases of large tumor size or localization adjacent to critical anatomic structures. It is, therefore, important to determine whether local disease control has a prognostic influence on DSS and to define the optimal margins that are necessary to ensure the best outcome.
The aim of this study was to gain more insight into the clinical behavior of extremity STS and to identify prognostic indicators of survival reviewing our institutional experience. We focused particularly on the effects of surgical margins and clear margin widths on disease outcome.
Patients and Methods
Patients
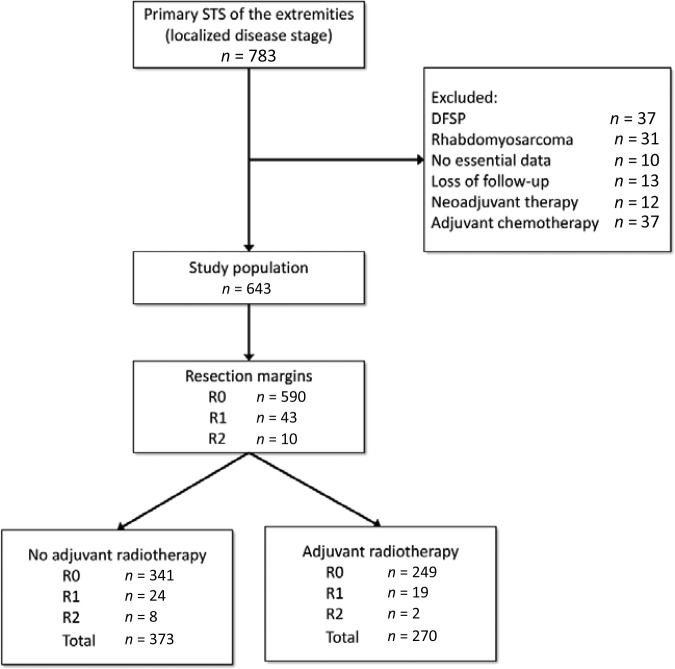
A total of 783 patients with primary STS of the extremities were treated consecutively with curative intent at our institution between 1996 and 2016. Only patients with no simultaneous distant metastases at the time of primary diagnosis were included in the study. We excluded dermatofibrosarcoma protuberans (37), because this STS subtype has a low metastatic potential. Furthermore, we excluded patients treated with adjuvant chemotherapy (37) and preoperative radiotherapy (12), and patients with rhabdomyosarcoma (31), to maintain a homogenous study population. Ten patients were excluded because essential data regarding the initial surgical procedure, such as tumor size or margin status, were not available. Thirteen patients, including those from foreign countries, were lost to follow‐up. Thus, we restricted the analyses to 643 participants with full information available on the outcome, histology, and surgical margins for the initial procedures (Fig. 1). Patient follow‐up results were obtained from our database, medical records, and patient correspondence. The study was approved by the ethics committee of the Medical Faculty of the Ruhr‐University Bochum with the registration number 15–5411.
Figure 1.
Flow chart of patients treated for extremity STS with reasons for exclusion and the study population.
Abbreviations: DFSP, dermatofibrosarcoma protuberans; STS, soft tissue sarcomas.
Treatment
Contrast‐enhanced magnetic resonance imaging (MRI) of the tumor site was routinely performed preoperatively. The goal of surgical treatment for all patients was resection of the primary tumor with negative margins.
In line with the guidelines of the European Society for Medical Oncology for STS treatment, the indication for radiotherapy was given for all G2 and G3 tumors as well as for R1‐resected G1 tumors [6].
After surgical treatment, the follow‐up management for all patients included clinical examinations, chest x‐rays, and contrast‐enhanced MRIs every 3 months in the first 2 years, and then every 6 months for 3 more years. The decision of whether follow‐up MRIs and chest x‐rays would be continued every 6 or 12 months after 5 years was based on previous tumor behavior and the decision of the informed patient.
Histopathological Classification
All STS were diagnosed and classified according to the latest World Health Organization guidelines and the French Federation of Cancer Centers [18], [19]. Surgical margins were assessed after fixation of the pathologic specimen with formalin and dyeing the surface with ink. All pathology slides and the according surgical margin widths were analyzed or reviewed for consensus diagnosis by an experienced soft tissue pathologist from our institution.
Statistical Analysis
All patients were analyzed retrospectively regarding possible prognostic factors influencing survival. Disease‐specific survival was defined as the time period from the date of surgery for primary disease to the date of disease‐specific death or the date of the last follow‐up assessment in living patients. The LRFS and metastasis‐free survival (MFS) were calculated from the date of surgery for the primary disease to the date of first local or distant recurrence or the date of the last follow‐up assessment in recurrence‐free or metastasis‐free patients. Survival rates were estimated according to the Kaplan‐Meier method with respective 95% confidence intervals (CIs) and were compared using the log‐rank test. Multivariate analyses and regression analysis of surgical margin widths were performed using the Cox proportional hazards model and the Wald test. Variables that were associated with p < .05 in the univariate analysis were included in the multivariate regression to assess independent prognostic factors for LRFS, DSS, and MFS. A p value of <.05 was considered statistically significant. Data analyses were performed using the statistical program Stata (Version 11.2, StataCorp, College Station, TX, http://www.stata.com).
Results
Patient and Tumor Characteristics
The median age for the entire cohort at the time of primary diagnosis was 60.7 years (range 14.8–98.4). There were 314 female (48.8%) and 329 male (51.2%) individuals. A total of 192 patients (29.9%) had at least one local recurrence. The median time between primary diagnosis and local recurrence was 1.4 years (95% CI: 1.4–1.9). Over time, 130 patients (20.2%) developed distant metastases. The median time between primary diagnosis and metastasis was 1.3 years (95% CI: 0.8–1.6), while the median survival time after diagnosis of the initial metastasis was 1.5 years (95% CI: 1.1–3.0).
The distribution of the histological grading was as follows: G1 in 147 cases (22.9%), G2 in 247 cases (38.4%), and G3 in 249 cases (38.7%). Primary tumors were located epifascially in 245 patients (38.1%), while 398 patients (61.9%) presented with subfascial tumors. The primary tumors in 420 patients (65.3%) were larger than 5 cm. The most frequent histotypes among the entire cohort were NOS (sarcoma not otherwise specified; 168; 26.1%), liposarcoma (162; 25.2%), leiomyosarcoma (136; 21.2%), myxofibrosarcoma (46; 7.2%), and synovial sarcoma (36; 5.6%).
Treatment Characteristics
Surgical resection of the primary tumor in one or two steps led to microscopically negative margins (R0) in 590 patients (91.8%), whereas 43 patients (6.7%) were left with microscopically positive margins (R1), and 10 patients (1.6%) with macroscopically positive margins (R2).
Amputations were performed in 27 patients (4.2%) presenting with primary tumors. After the resection of the primary tumor, soft tissue defects had to be covered with local flaps in 113 patients (17.6%) and with free flaps in 34 patients (5.3%), while 33 patients (5.1%) underwent transplantation with split‐thickness skin grafts because of mere skin defects.
A total of 270 patients (42.0%) received adjuvant radiotherapy after resection of their primary tumor, with a median overall dose of 60.0 Gray (range: 25.0–79.0). From the 192 (29.9%) patients with local recurrences, 184 underwent surgical resection: negative margins were obtained in 131 (71.2%), 30 (16.3%) were left with R1 margins, and 23 (12.5%) with R2 margins.
Follow‐Up
As of August 2016 (cut‐off date), the median follow‐up was 4.6 years for surviving patients and the reverse Kaplan‐Meier estimate of the median follow‐up after primary diagnosis was 5.4 years (95% CI: 4.8–6.0) [20], [21]. At the cut‐off date, 453 patients (70.5%) had no evidence of disease, whereas 32 patients (5.0%) were alive with residual localized disease and 36 (5.6%) with metastatic disease. During follow‐up, 88 patients (13.7%) died from disease and 34 patients (5.3%) died from other causes.
Univariate Analysis of LRFS
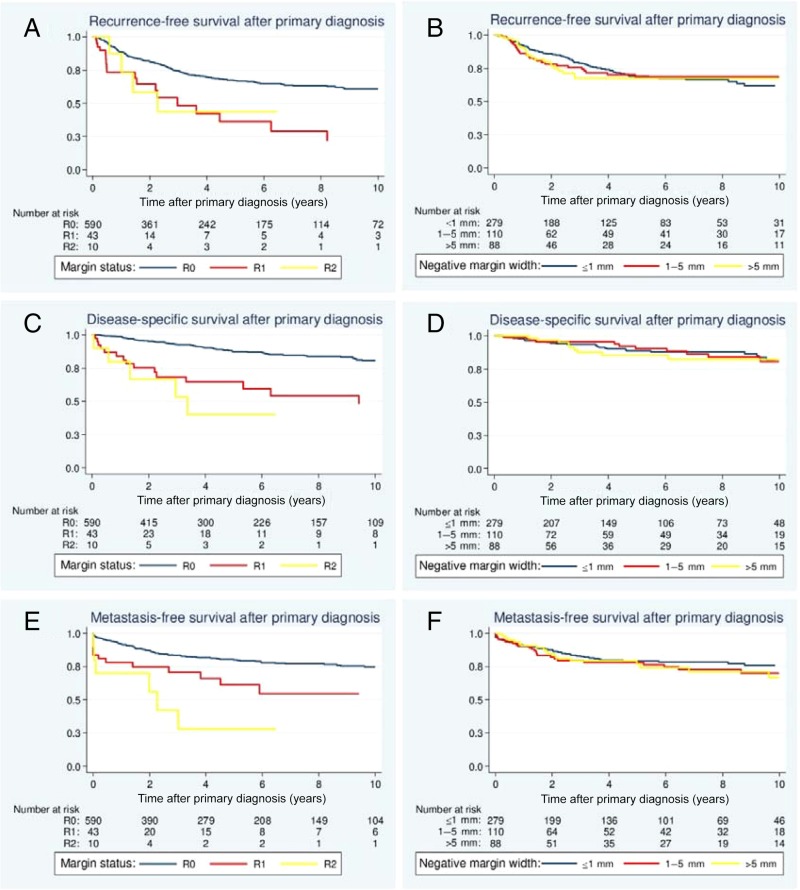
The five‐year rate of LRFS was 65.3% (95% CI: 60.8–69.5) for the entire cohort. Patients >50 years old at the time of primary diagnosis displayed a significantly decreased LRFS compared with younger patients (p = .002; Table 1). Regarding the histological grade, G1 tumors had a more favorable local outcome than G2 and G3 lesions (p < .003). Myxofibrosarcomas (p = .040) were associated with a significantly diminished LRFS (Table 1). By contrast, liposarcomas displayed the lowest rates of local recurrence (p < .001). When analyzing the treatment characteristics, the surgically attained margin status had a significant impact on LRFS (p < .001; Fig. 2A). The impact of negative margin widths was additionally assessed in the subgroup of patients with R0 margins. The closest negative margin width was analyzed histologically at our institution for 477 of the 590 patients (80.8%) with R0‐resected tumors. Univariate analysis of the margin widths categorized revealed that close and wide negative margins led to similar local outcomes (p = .950; Table 1; Fig. 2B). Notably, adjuvant radiation improved LRFS significantly (p = .002). R0 resections were associated with significantly better local outcomes in patients treated with adjuvant radiation as well as patients that did not receive postoperative radiation (Table 1). Here, the negative margin width did not exhibit any prognostic significance in the subset of irradiated patients and patients that did not undergo radiation for their primary tumor.
Table 1. Results of the univariate analyses to determine factors predictive of LRFS.
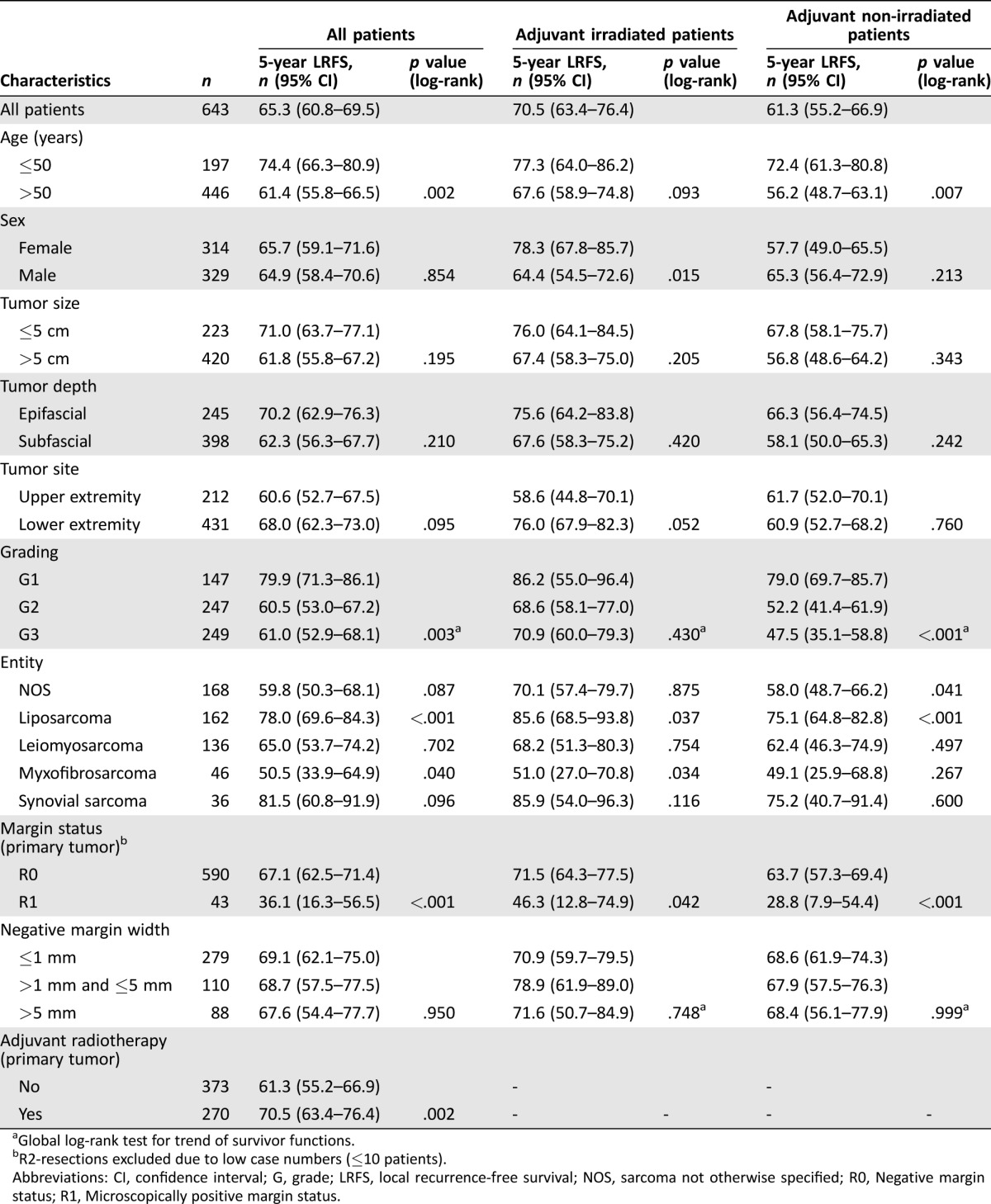
Global log‐rank test for trend of survivor functions.
R2‐resections excluded due to low case numbers (≤10 patients).
Abbreviations: CI, confidence interval; G, grade; LRFS, local recurrence‐free survival; NOS, sarcoma not otherwise specified; R0, Negative margin status; R1, Microscopically positive margin status.
Figure 2.
Effects of surgical margins and clear margin widths on local recurrence‐free survival (A, B), disease‐specific survival (C, D), and metastasis‐free survival (E, F).
Multivariate Analysis of LRFS
Significant and independent prognostic factors for the local outcome in the Cox model were age, histological grade, myxofibrosarcoma subtype, margin status, and adjuvant radiation (Table 4). The hazard ratio (HR) for local recurrence was 2.16 (95% CI: 1.42–3.28; p < .001) for G2 and 1.89 (95% CI: 1.19–3.00; p = .007) for G3 lesions. The myxofibrosarcoma subtype displayed an unfavorable HR of 1.70 (95% CI: 1.01–2.72; p = .048). Regarding the treatment characteristics, positive margins were associated with significantly increased risk of local recurrence (HR: 2.66 [1.68–4.22]; p < .001), while adjuvant radiation improved local control remarkably (HR: 0.45 [0.33–0.62]; p < .001).
Univariate Analysis of DSS
The five‐year estimate of the DSS rate in the entire series was 85.3% (95% CI: 81.6–88.3). Patients >50 years had a significantly diminished DSS compared with younger patients (p = .017; Table 2). Tumor size >5 cm (p = .026) and subfascial localization (p = .008) were also associated with a significantly reduced DSS. Patients with G1 and G2 lesions had more favorable prognoses than patients with G3 lesions (p < .001). Regarding the different histological subsets, patients with liposarcomas had a more favorable DSS (p < .001), while patients with leiomyosarcoma (p = .017) had a diminished survival.
Table 2. Results of multivariate analysis on LRFS, DSS, and MFS according to Cox proportional hazard model.
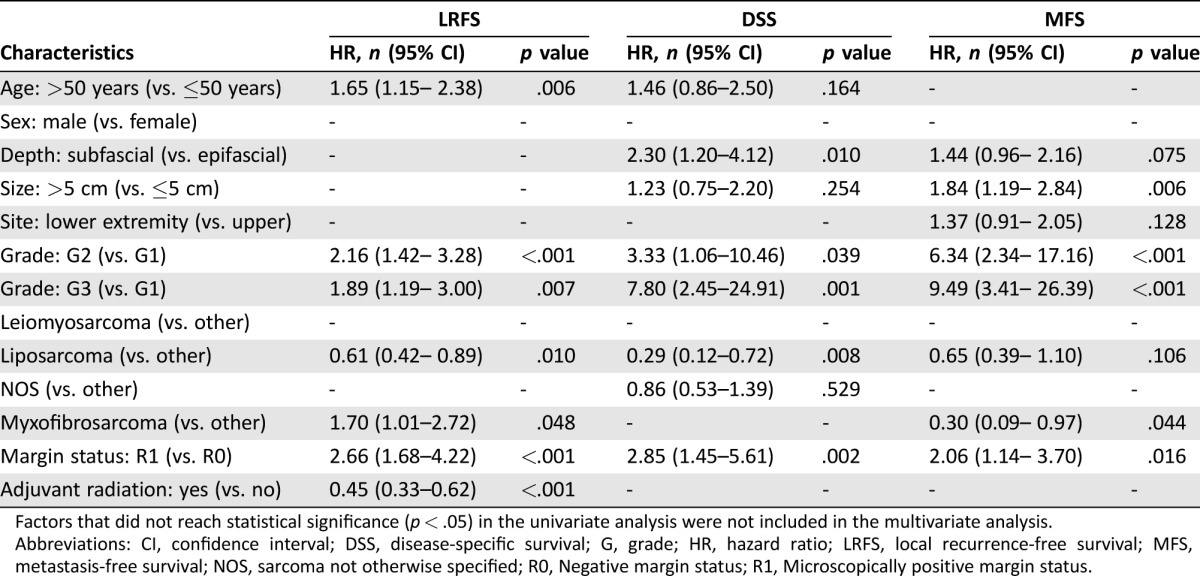
Factors that did not reach statistical significance (p < .05) in the univariate analysis were not included in the multivariate analysis.
Abbreviations: CI, confidence interval; DSS, disease‐specific survival; G, grade; HR, hazard ratio; LRFS, local recurrence‐free survival; MFS, metastasis‐free survival; NOS, sarcoma not otherwise specified; R0, Negative margin status; R1, Microscopically positive margin status.
The surgical margin status following treatment of the primary tumor reached prognostic significance in the univariate analysis. Patients with R0 margins had a significantly better DSS than patients with positive margins (p < .001; Fig. 2C). Similar to findings for LRFS, the margin widths assessed quantitatively had no influence on DSS (p = .570; Fig. 2D). The negative margin width did not have an impact on DSS in the subset of patients that did not receive radiation (p = .676) or in the subset of irradiated patients (p = .990). Regarding the surgical treatment of lesions recurring already, the surgical margin status was a statistically significant indicator of DSS. Complete resection of the last local recurrence with negative margins resulted in a significantly more favorable DSS (five‐year DSS after last resection: R0 71.6% [59.2–80.8] vs. R1/R2 37.2% [17.3–57.2]; p < .001). In contrast to findings for LRFS, adjuvant radiation had no impact on DSS (p = .214).
Multivariate Analysis of DSS
Multivariate analysis revealed tumor depth, histological grade, liposarcoma subtype, and margin status as independent prognostic factors of DSS (Table 4). Regarding the prognostic significance of histological grade, the HR for death was 7.80 (95% CI: 2.45–24.91; p = .001) for G3 tumors when compared with G1 lesions. Liposarcomas presented an HR of 0.29 (95% CI: 0.12–0.72; p = .008). Similar to findings for LRFS, positive margins were also found to be of independent prognostic significance for DSS, with an HR of 2.85 (95% CI: 1.45–5.61; p = .002).
Table 4. Results of the univariate analyses to determine factors predictive of MFS.
Global log‐rank test for trend of survivor functions.
R2‐resections excluded due to low case numbers (≤10 patients).
Abbreviations: CI, confidence interval; G, grade; MFS, metastasis‐free survival; NOS, sarcoma not otherwise specified; R0, Negative margin status; R1, Microscopically positive margin status.
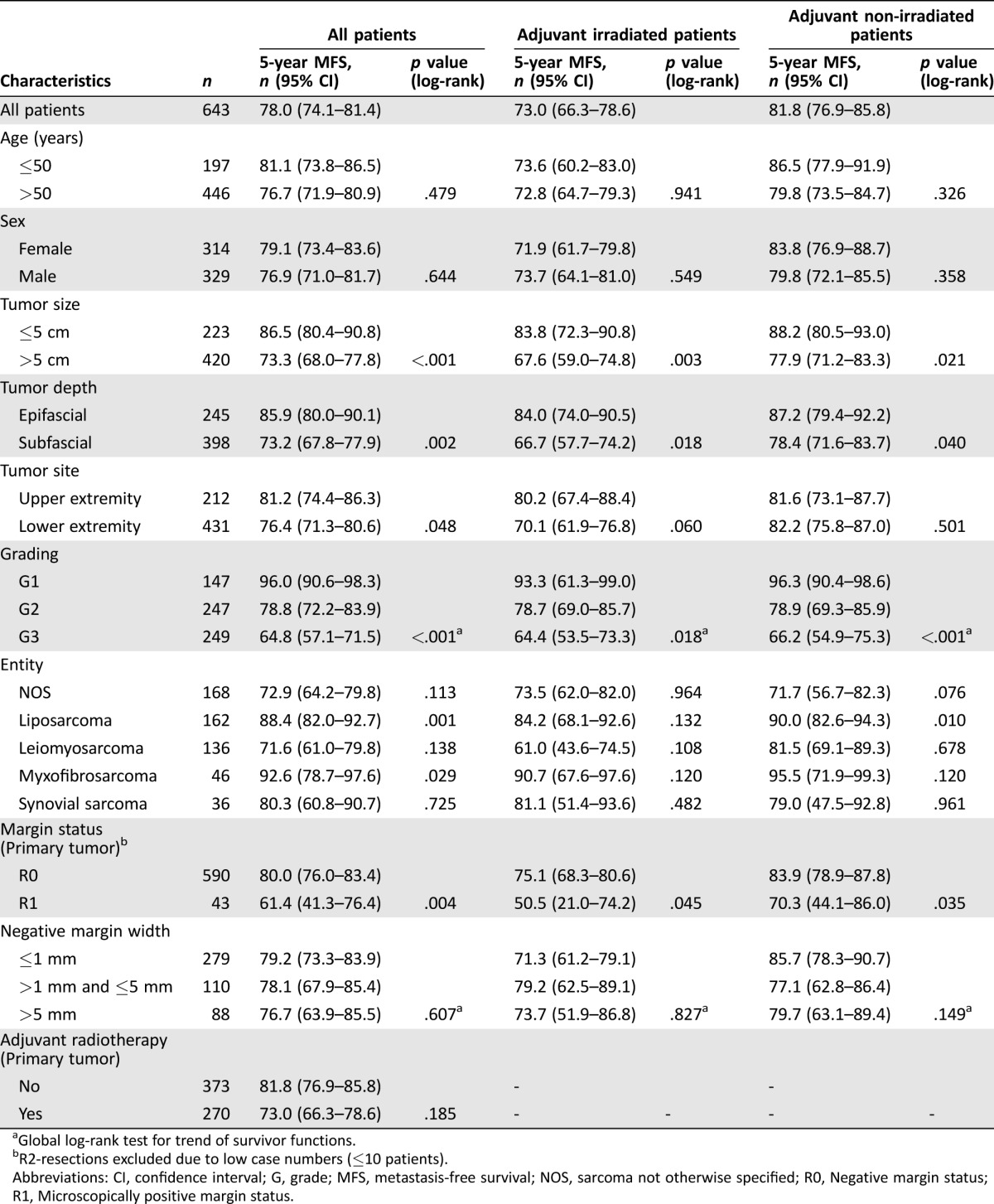
Univariate Analysis of MFS
In accordance with the findings for DSS, tumor size ≤5 cm (p < .001), epifascial localization (p = .002), low‐grade histology (p < .001), liposarcoma subtype (p = .001), and negative margins (p = .004) were associated with a favorable MFS in the univariate analysis (Table 3, Figs. 2E–F). Furthermore, upper extremity lesions (p = .048) and myxofibrosarcomas (p = .029) had a more favorable MFS.
Table 3. Results of the univariate analyses to determine factors predictive of DSS.
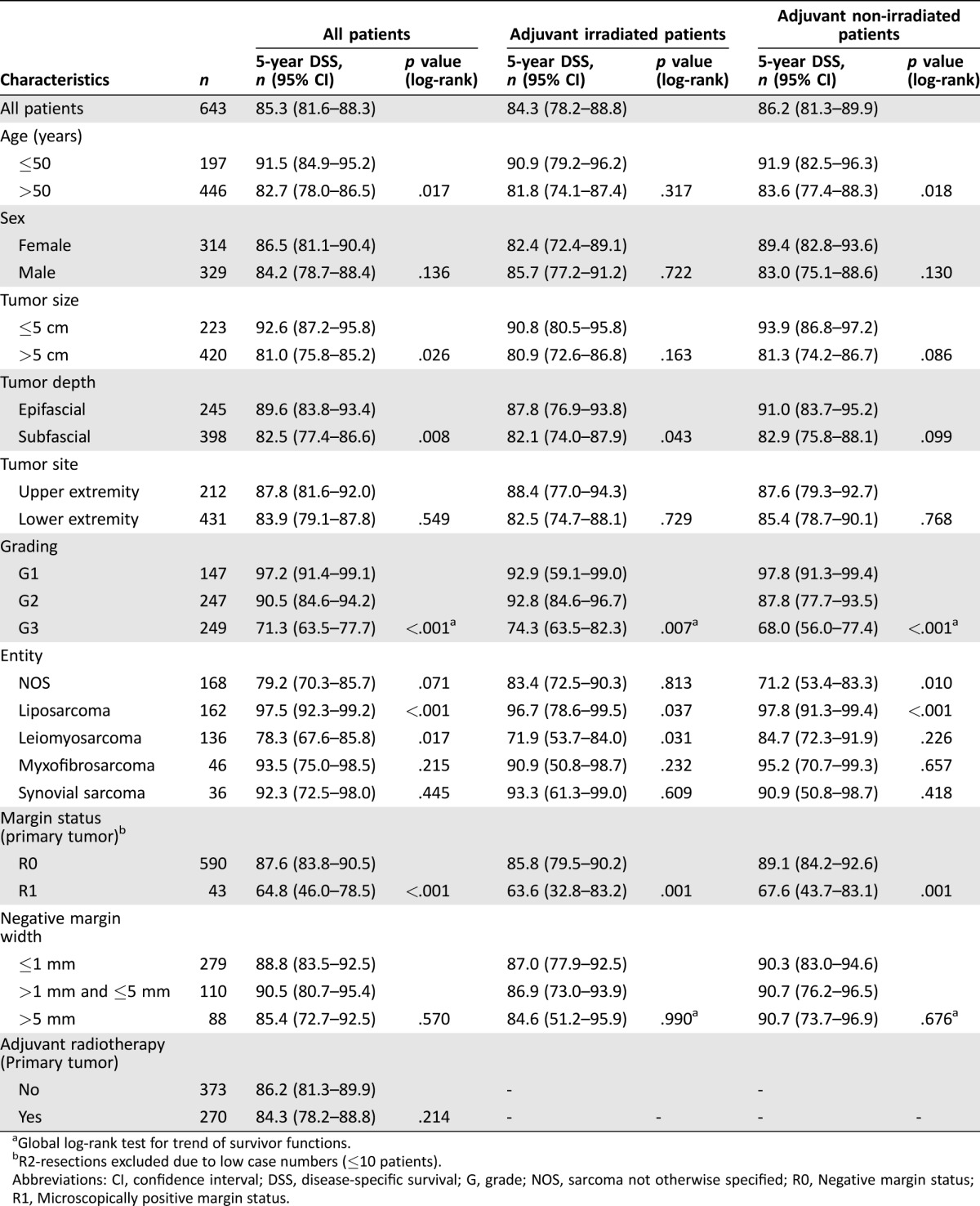
Global log‐rank test for trend of survivor functions.
R2‐resections excluded due to low case numbers (≤10 patients).
Abbreviations: CI, confidence interval; DSS, disease‐specific survival; G, grade; NOS, sarcoma not otherwise specified; R0, Negative margin status; R1, Microscopically positive margin status.
Multivariate Analysis of MFS
Tumor size (p = .006), histological grade, myxofibrosarcoma subtype (p = .044), and surgical margin status (p = .016) emerged as independent prognostic factors in the multivariate analysis of MFS (Table 4).
Regression Analysis of Non‐Categorized Surgical Margin Width
We performed Cox regression analyses to evaluate the prognostic significance of non‐categorized clear margin widths in the R0 subgroup and found that the closest surgical margin width did not influence the outcome. The HR for disease‐related death according to the Wald test was 1.02 (95% CI: 0.67–1.55) for wide margins, which failed to reach statistical significance (p = .943). Both LRFS and MFS were unaffected by the closest surgical margin width. The HR for recurrence and for metastasis were 1.11 (95% CI: 0.80–1.54; p = .523) and 1.10 (95% CI: 0.76–1.57; p = .622), respectively. Thus, close and wide negative margins led to similar DSS, LRFS, and MFS. The regression analyses were also performed separately for the subset of irradiated and non‐irradiated patients. Similar to the univariate findings, the negative margin widths did not have an influence on DSS, LRFS, or MFS in both subsets (data not shown).
Discussion
In the present study, we analyzed the outcome of 643 patients who underwent surgical resection of primary extremity STS with curative intent. The most frequent histological subtypes in our series were NOS (26.1%), liposarcoma (25.2%), and leimyosarcoma (21.2%). The majority of the tumors were high‐grade (G3; 38.7%), large (>5 cm; 65.3%), and subfascially localized (61.9%). Surgical treatment of the primary tumor led to microscopic negative margins in 91.8% of all patients. Despite surgical resection, 20.2% developed distant metastases during the disease course, and the median survival time after the diagnosis of metastasis was 1.5 years. The histological grade and the surgical margin status in the multivariate analyses were the only factors that reached independent prognostic significance for all three survival endpoints assessed.
When reviewing the literature, there were several large studies that analyzed the prognostic significance of surgical margins. Unfortunately, they presented inconsistent findings questioning the prognostic impact of the quality of surgery in extremity STS. Taken together, the results can be subdivided roughly into two possible constellations (Table 5). Several single‐institutional studies demonstrated that surgical margins indeed influenced the local outcome, but did not affect survival [8], [10], [14], [15]. On the other hand, there are several well‐characterized studies that underscored the prognostic significance of surgical margins for both LRFS and DSS [11], [16], [17]. However, none of these studies could establish an association between margins and MFS, which is interesting because one would expect that most of the sarcoma patients die from distant metastases rather than from extensive local recurrences. A multicenter study that was only recently published confirmed that distant metastases are almost the only cause of disease‐related deaths in patients with extremity STS [22]. The surgical margin attained in the present study was not only an independent prognostic factor for LRFS and DSS, but also for the MFS. Hence, the presence of positive margins following curative surgical resection was an indicator for an aggressive tumor that had the potential to recur locally and to metastasize, and, therefore, to impair survival substantially. However, we are not able to assess whether the achievement of negative margins at any cost would have improved the outcome in those patients with positive margins. On the other hand, we cannot estimate the outcome of those patients with negative margins if they had been treated less radically with inadequate margins. Nonetheless, given the diminished outcome of patients left with positive margins, it seems reasonable that surgical efforts should aim at complete resections with negative margins whenever feasible.
Table 5. Overview of retrospective analyses on extremity STS.
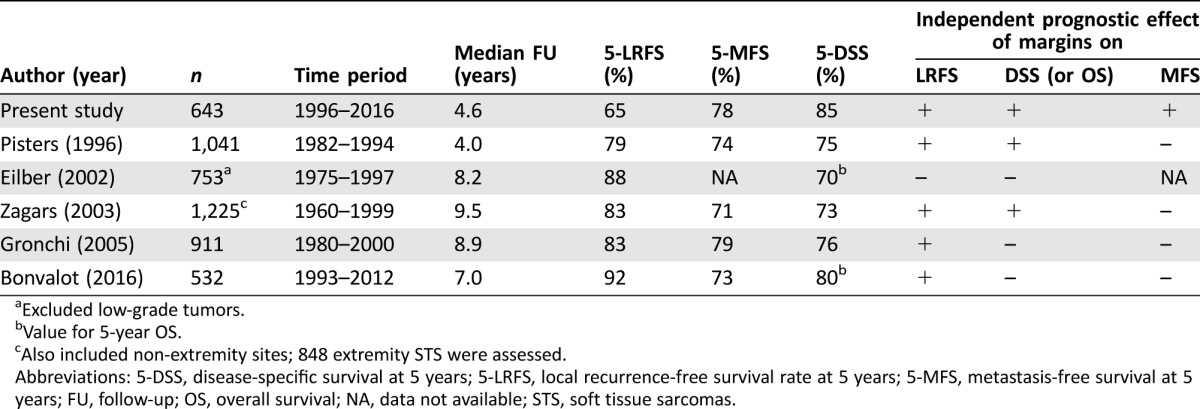
Excluded low‐grade tumors.
Value for 5‐year OS.
Also included non‐extremity sites; 848 extremity STS were assessed.
Abbreviations: 5‐DSS, disease‐specific survival at 5 years; 5‐LRFS, local recurrence‐free survival rate at 5 years; 5‐MFS, metastasis‐free survival at 5 years; FU, follow‐up; OS, overall survival; NA, data not available; STS, soft tissue sarcomas.
As already stated above, the impact of surgical margins assessed qualitatively on disease outcome has been studied extensively, but the amount of normal tissue around the resected tumor that constitutes an optimal negative margin is still not defined. How wide should the optimal clear margin width be? This important question has already been addressed in more frequent malignancies such as breast cancer. Based on a meta‐analysis of more than 20,000 breast cancer patients, the American Society of Clinical Oncology recommends close margins as long as the tumor has no ink on it [23]. Therefore, resections with even very close R0 margins are accepted in the surgical treatment of breast cancer when adjuvant radiation is applied, although subclinical foci of cancer cells are frequently present at large distances (several cm) from the primary tumor site [24]. Due to the rarity of STS, such large analyses cannot be expected and studies on surgical margin widths are sparse [25], [26]. Only recently published and to date the largest study on this issue, Ahmad et al. analyzed the impact of categorized negative margin widths in 235 patients with R0‐resected extremity and truncal STS [25]. They could not find any differences regarding the local control or survival rates based on the quantitative margin widths. In the present study, we analyzed the prognostic influence of categorized and noncategorized surgical margin widths in 477 patients with R0‐resected tumors. We could not detect any beneficial influence of wide margins. Although cross‐analyses revealed that larger tumors were generally resected with smaller margins and vice versa (supplemental online Table 1), close and wide negative margins led to similar LRFS, DSS, and MFS rates. Hence, our findings provide a less radical approach, with the goal of negative margins, even if close, instead of wide safety margins.
Regarding adjuvant treatment modalities, radiation improved local control significantly, while DSS and MFS were not altered in our series. This is in line with the findings of a published randomized, prospective study conducted by the National Cancer Institute in Bethesda in 2014, which included 141 patients with extremity STS [27]. This study revealed that patients who underwent limb‐sparing surgery with adjuvant radiation had a significantly improved LRFS when compared with patients who underwent surgery without radiation. Notably, overall survival was not improved in the radiation treatment group. However, it seems reasonable to include adjuvant radiation, especially in cases of large, high‐grade tumors, to minimize the risk of local recurrence and to ensure the best survival outcomes.
Finally, it has to be stated that the LRFS rates in our study were strikingly low when compared with other large studies on extremity STS. On the other hand, the MFS rates are similar and the DSS rates of our patient population are even a bit higher than in those studies (Table 5). The reason for this discrepancy is unclear. Although most of these studies focused on extremity STS, they are not very comparable. The median age of our patient population was much higher than in the other studies, but the differences in the patient, tumor, and treatment characteristics do not justify such a discrepancy regarding the local control and DSS rates (Table 6). However, a potential reason why LRFS rates were significantly lower in our series might be found in our follow‐up schedule involving frequent contrast‐enhanced MRIs with the intent to detect subclinical recurrences at an early disease stage where curative surgery should be more probable. Unfortunately, the other centers did not delineate their follow‐up strategies. Our assumption that frequent local imaging might lead to an earlier recurrence detection is in contrast to the findings of several retrospective studies that could not reveal a benefit for systematic MRI studies and reported that most of the local recurrences were detected by the patients themselves [28], [29]. Retrospectively, we are not able to assess how many of the local recurrences in our series were definitely diagnosed by clinical examination, MRI imaging, or the patients themselves. Therefore, we have no data to support a comment on the benefit of regular MRIs and clinical examinations.
Table 6. Patient, tumor, and treatment characteristics of retrospective analyses on extremity soft tissue sarcomas.
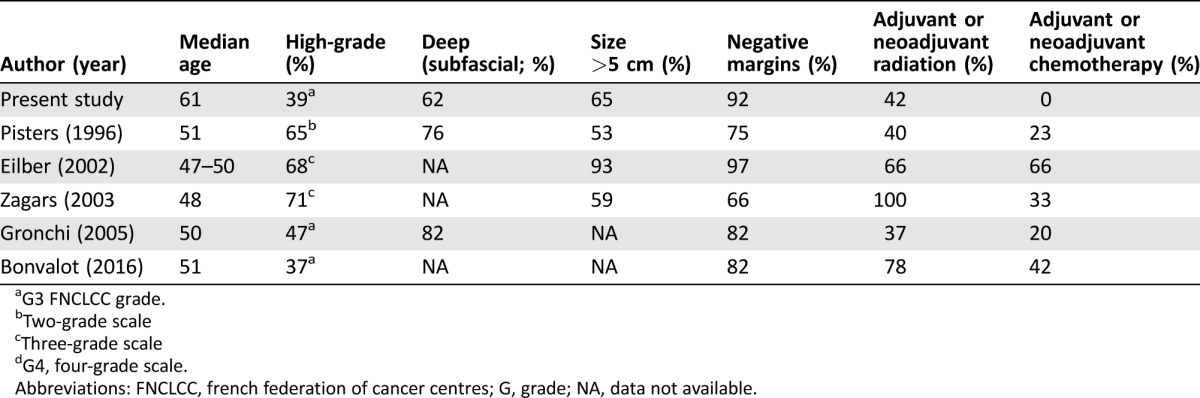
G3 FNCLCC grade.
Two‐grade scale
Three‐grade scale
G4, four‐grade scale.
Abbreviations: FNCLCC, french federation of cancer centres; G, grade; NA, data not available.
Despite the high rates of local recurrence, our patient population actually displayed a quite favorable survival, with a five‐year DSS rate of 84%. This might be due to the fact that most of the patients with local recurrences were suitable for further surgical treatment. Within the subset of patients with local recurrences, 184 of the 192 patients (95.8%) could undergo surgical resection of their locally recurring tumor, and residual disease was found in all pathological specimens. Negative margins could be attained in 71.2% and were associated with a significantly improved DSS, indicating the importance of surgical margins even after recurrence (five‐year DSS after resection of recurrence: 71.6% vs. 37.2%; p < .001). This finding is in line with a previous work by our institution that focused on the long‐term outcome of STS patients after recurrence [30].
Finally, the reservation must be made that our series only included patients with primary STS that were suitable for further surgical treatment with curative intent. Patients presenting with distant metastases or extensive tumors that could not be approached surgically because of rapid disease progression were not assessed in this study. Thus, our findings are only applicable to the selected group of patients for which further surgical treatment was possible and not to all patients with STS of the extremities. This implies a study selection bias that must be acknowledged. Furthermore, our study has only focused on the quantitatively assessed margin widths but not on the quality of the negative margins (fascia, muscle, fat, etc.). However, especially in the subgroup of patients with very close margins, it would be interesting to see whether the quality of margins (fascia, muscle, fat, etc.) is relevant.
Conclusion
This study could underscore the prognostic significance of surgical margins attained at the surgical treatment of extremity STS. As independent prognostic factors, histological grade and surgical margins influenced the local outcome, occurrence of distant metastases, and survival. Given the diminished outcome of patients left with positive margins, surgical efforts should aim to achieve microscopically negative margins whenever feasible. It is noteworthy that only the quality of surgical margins, but not the negative margin width attained, had an influence on the prognosis. A radical surgical approach with the goal of wide clear margins cannot be justified by the data presented. Our findings suggest that surgical margins can be close as long as the resected tumor has no ink on it.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by the FoRUM grant (K090‐15) of the Ruhr‐University Bochum, Germany.
Footnotes
For Further Reading: Rima Ahmad, Alex Jacobson, Francis Hornicek et al. The Width of the Surgical Margin Does Not Influence Outcomes in Extremity and Truncal Soft Tissue Sarcoma TreatedWith Radiotherapy. The Oncologist 2016;21:1269‐1276.
Implications for Practice: In patients undergoing radiation therapy and limb‐sparing surgery for soft tissue sarcoma, the quantitative width of the negative margin does not influence outcome, and so attempts at wide margins of resection appear to be unnecessary, especially when such attempts compromise the functional outcome. Importantly, the conclusions drawn from this study must not be applied to those patients undergoing surgery alone as the local treatment of their soft tissue sarcoma, in which case wider margins of resection may be necessary.
Author Contributions
Conception/design: Kamran Harati, Ole Goertz, Andreas Pieper, Adrien Daigeler, Ingo Stricker, Marcus Lehnhardt
Provision of study material or patients: Andreas Pieper, Hamid Joneidi‐Jafari, Ingo Stricker
Collection and/or assembly of data: Kamran Harati, Andreas Pieper, Hiltrud Niggemann, Ingo Stricker
Data analysis and interpretation: Kamran Harati, Adrien Daigeler, Hiltrud Niggemann, Marcus Lehnhardt
Manuscript writing: Kamran Harati, Marcus Lehnhardt
Final approval of manuscript: Kamran Harati, Adrien Daigeler, Marcus Lehnhardt
Disclosures
The authors indicated no financial relationships.
References
- 1. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 2. Stojadinovic A, Leung DH, Hoos A et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002;235:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernebro J, Bladstrom A, Rydholm A et al. Increased risk of malignancies in a population‐based study of 818 soft‐tissue sarcoma patients. Br J Cancer 2006;95:986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaushal A, Citrin D. The role of radiation therapy in the management of sarcomas. Surg Clin North Am 2008;88:629–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer S, Demetri GD, Baldini EH et al. Management of soft‐tissue sarcomas: An overview and update. Lancet Oncol 2000;1:75–85. [DOI] [PubMed] [Google Scholar]
- 6.ESMO/European Sarcoma Network Working Group . Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii102–112. [DOI] [PubMed] [Google Scholar]
- 7. Novais EN, Demiralp B, Alderete J et al. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res 2010;468:3003–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trovik CS, Bauer HC, Alvegard TA et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically‐treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer 2000;36:710–716. [DOI] [PubMed] [Google Scholar]
- 9. Eilber FC, Rosen G, Nelson SD et al. High‐grade extremity soft tissue sarcomas: Factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg 2003;237:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gronchi A, Casali PG, Mariani L et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol 2005;23:96–104. [DOI] [PubMed] [Google Scholar]
- 11. Pisters PW, Leung DH, Woodruff J et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 12. Lewis JJ, Leung D, Casper ES et al. Multifactorial analysis of long‐term follow‐up (more than 5 years) of primary extremity sarcoma. Arch Surg 1999;134:190–194. [DOI] [PubMed] [Google Scholar]
- 13. Potter BK, Hwang PF, Forsberg JA et al. Impact of margin status and local recurrence on soft‐tissue sarcoma outcomes. J Bone Joint Surg Am 2013;95:e151. [DOI] [PubMed] [Google Scholar]
- 14. Willeumier J, Fiocco M, Nout R et al. High‐grade soft tissue sarcomas of the extremities: Surgical margins influence only local recurrence not overall survival. Int Orthop 2015;39:935–941. [DOI] [PubMed] [Google Scholar]
- 15. Bonvalot S, Levy A, Terrier P et al. Primary extremity soft tissue sarcomas: Does local control impact survival? Ann Surg Oncol 2016;24:194–201. [DOI] [PubMed] [Google Scholar]
- 16. Zagars GK, Ballo MT, Pisters PW et al. Prognostic factors for patients with localized soft‐tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003;97:2530–2543. [DOI] [PubMed] [Google Scholar]
- 17. Gronchi A, Lo Vullo S, Colombo C et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: Local control directly impacts survival. Ann Surg 2010;251:506–511. [DOI] [PubMed] [Google Scholar]
- 18. Fletcher CD. The evolving classification of soft tissue tumours ‐ An update based on the new 2013 WHO classification. Histopathology 2014;64:2–11. [DOI] [PubMed] [Google Scholar]
- 19. Coindre JM. Grading of soft tissue sarcomas: Review and update. Arch Pathol Lab Med 2006;130:1448–1453. [DOI] [PubMed] [Google Scholar]
- 20. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 21. Clark TG, Bradburn MJ, Love SB et al. Survival analysis part I: Basic concepts and first analyses. Br J Cancer 2003;89:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Callegaro D, Miceli R, Bonvalot S et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft‐tissue sarcomas of the extremities: A retrospective analysis. Lancet Oncol 2016;17:671–680. [DOI] [PubMed] [Google Scholar]
- 23. Moran MS, Schnitt SJ, Giuliano AE et al. Society of Surgical Oncology‐American Society for Radiation Oncology consensus guideline on margins for breast‐conserving surgery with whole‐breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507–1515. [DOI] [PubMed] [Google Scholar]
- 24. Holland R, Veling SH, Mravunac M et al. Histologic multifocality of Tis, T1‐2 breast carcinomas. Implications for clinical trials of breast‐conserving surgery. Cancer 1985;56:979–990. [DOI] [PubMed] [Google Scholar]
- 25. Ahmad R, Jacobson A, Hornicek F et al. The width of the surgical margin does not influence outcomes in extremity and truncal soft tissue sarcoma treated with radiotherapy. The Oncologist 2016;21:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. King DM, Hackbarth DA, Kirkpatrick A. Extremity soft tissue sarcoma resections: How wide do you need to be? Clin Orthop Relat Res 2012;470:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beane JD, Yang JC, White D et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20‐year follow‐up of a randomized prospective trial. Ann Surg Oncol 2014;21:2484–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labarre D, Aziza R, Filleron T et al. Detection of local recurrences of limb soft tissue sarcomas: Is magnetic resonance imaging (MRI) relevant? Eur J Radiol 2009;72:50–53. [DOI] [PubMed] [Google Scholar]
- 29. Cheney MD, Giraud C, Goldberg SI et al. MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol 2014;109:593–596. [DOI] [PubMed] [Google Scholar]
- 30. Daigeler A, Zmarsly I, Hirsch T et al. Long‐term outcome after local recurrence of soft tissue sarcoma: A retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Cancer 2014;110:1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.