Tumor infiltrating lymphocytes are associated with good clinical outcome in many types of cancer, including breast cancer. Tertiary lymphoid structures are associated with anti‐tumor immune responses and prolonged patient survival. This study analyzed characteristics of tertiary lymphoid structures and their prognostic value related to different invasive breast cancer molecular subgroups.
Keywords: Breast cancer, Tertiary lymphoid organ, Tumor infiltrating lymphocyte, Human epidermal growth receptor 2 positive cancers
Abstract
Background.
The presence of tumor infiltrating lymphocytes (TIL) is associated with favorable prognosis. Recent evidence suggested that not only their density, but also the spatial organization as tertiary lymphoid structures (TLS), play a key role in determining patient survival.
Materials and Methods.
In a cohort of 248 breast cancers, the clinicopathologic association and prognostic role of TLS was examined.
Results.
Tertiary lymphoid structures were associated with higher tumor grade, apocrine phenotype, necrosis, extensive in situ component, lymphovascular invasion (LVI), and high TIL. For biomarkers, TLS were associated with hormone receptors negativity, HER2 positivity, and c‐kit expression. Tertiary lymphoid structures were significantly related to better disease‐free survival (DFS) in HER2 positive (HER2+) breast cancers (log‐rank = 4.054), which was not dependent on high TIL status. The combined TLS and TIL status was an independent favorable factor associated with DFS in those cases. Interestingly, tumor cell infiltration into the TLS was found in 41.9% of TLS positive cases. It was associated with LVI in HER2 negative (HER2−) TLS positive (particularly estrogen receptor positive [ER+] HER2−) cases. In the ER+ HER2− cases, tumor cell infiltration into TLS was also associated with increased pathologic nodal stage (pN) stage and nodal involvement.
Conclusion.
Tertiary lymphoid structures showed a similar relationship with clinicopathologic features and biomarkers as TIL. The presence of TLS, irrespective of TIL level, could be an important favorable prognostic indicator in HER2+ breast cancer patients. Given the significance of TLS in promoting effective antitumor immunity, further understanding of its organization and induction may provide new opportunities to improve the current immunotherapy strategies.
Implications for Practice.
Despite recent interest on the clinical value of tumor infiltrating lymphocyte (TIL), little was known on the clinical significance on their spatial organization as tertiary lymphoid structures (TLS). Although TLS showed similar relationships with clinicopathologic features and biomarkers as TIL, the prognostic value of TLS, particularly in HER2 positive cancers, was independent of TIL. Moreover, tumor infiltration could be present in TLS which appears to be related to tumor invasion in HER2 negative cancers. Overall, the results demonstrated the additional value for TLS in HER2 cancer subtypes. Further investigations and its standardized evaluation will enhance its use as standard practice.
摘要
背景.肿瘤浸润淋巴细胞(TIL)与良好的预后有关。最新证据表明, TIL的密度和空间组织, 如三级淋巴结构(TLS), 在决定患者生存方面均具有关键作用。
材料与方法.在一个包含248名乳腺癌患者的队列中检查了TLS的临床病理学相关性和预后作用。
结果.三级淋巴结构与较高的肿瘤分级、顶浆分泌表型、坏死、广泛的原位癌成分、淋巴管浸润(LVI)和高TIL相关。对于生物标志物, TLS与激素受体阴性、HER2阳性和c‐kit表达相关。三级淋巴结构与HER2阳性(HER2+)乳腺癌(log‐rank=4.054)患者的无病生存期(DFS)更长显著相关, 而这与高TIL水平无关。在HER2+乳腺癌患者中, TLS和TIL状态是与DFS相关的独立有利因素。有趣的是, 在41.9%的TLS阳性患者中发现了肿瘤细胞浸润到TLS中的情况, 这与HER2阴性(HER2‐)TLS阳性 [特别是雌激素受体阳性(ER+)HER2‐]患者中的LVI相关。在ER+ HER2‐病例中, 肿瘤细胞浸润到TLS也与病理淋巴结分期(pN)升高和淋巴结受累有关。
结论.三级淋巴结构的临床病理特征和生物标志物的关系与TIL相似。无论TIL水平如何, TLS可能是HER2+乳腺癌患者的重要且有利的预后指标。考虑到TLS在促进有效的抗肿瘤免疫方面的重要性, 进一步了解其组织和诱导作用可能为改善目前的免疫疗法策略提供新机会。
Introduction
Invasive breast cancers (IBC) have remarkable heterogeneity, as reflected in the variable tumor biology and outcome. Conventional tumor characteristics such as histologic grade, biomarker expression, and staging may not be adequate to provide precise prognostication. Novel predictive markers are being sought to fine tune the current assessment. The importance of tumor microenvironment in promoting cancer growth has been increasingly recognized [1]. Several lines of studies have reported that the immune system controls oncogenesis and tumor progression under selected circumstances [2]. Tumor infiltrating lymphocytes (TIL) are associated with good clinical outcome in many types of cancer [2]. In triple negative breast cancers (TNBC), a high TIL is associated with favorable prognosis [3], [4] and higher rate of pathologic complete remission in neoadjuvant setting [5]. In addition to the TIL density, recent evidence suggests the spatial organization also plays a key role in determining patient survival [6], [7], [8], [9].
Tertiary lymphoid structures (TLS) are ectopic lymphoid tissues, resembling secondary lymphoid organs (SLO) [9]. They can be found in the tumor microenvironment for various solid cancers, such as melanoma [10], lung [8], colorectal [7], and breast cancers [11], [12], [13], [14], [15]. In TLS, T and B cells are distributed in two discrete but nonrigidly‐defined regions. The B‐cell‐rich area includes the germinal center and a small network of follicular dendritic cells (FDC), while the T‐cell‐rich area is where mature dendritic cells and high endothelial venules (HEV) can be found. Similar to SLO, TLS are believed to be the site of immune response activation against tumor by recruiting and activating TIL, and represent the key to antitumor immune responses [16]. Of note, the presence of TLS was associated with antitumor immune responses and prolonged patient survival [6], [7], [8], [9].
In IBC, the prognostic and predictive values of TIL gained recent attention [17]; nevertheless, there were very few studies on TLS [11], [12], [13], [14], [15]. The clinicopathologic features and prognosis of IBC with TLS have not been extensively analyzed. It is not clear whether analysis of TLS, in addition to TIL, could furnish additional value in the clinical management of IBC. In this study, an IBC cohort was analyzed for the characteristic of TLS and their prognostic value related to different IBC molecular subgroups.
Materials and Methods
Ethics, Consent, and Permissions
The study was performed in accordance with the ethical standards of CUHK‐NETC CREC and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No informed consent has been obtained because only archival materials from pathology tissue bank were retrieved after their use for diagnosis and all cases were non‐identifiable. The research could be permissible without consent. No individual patient data has been reported.
Two hundred forty‐eight patients with histologically confirmed primary IBC from 2003 to 2009 were selected from two of the involved institutions (KWH and TMH). The cases were selected from a consecutive series of IBC. Only those cases with all tumor blocks available for examination were included. All the specimens were formalin fixed, paraffin embedded (FFPE), and routinely processed. The 4 micron slides were stained with hematoxylin and eosin (H&E) and reviewed by two of the authors. The tumors were graded using modified Bloom and Richardson grading [18] and the histologic diagnoses were confirmed (world health organisation (WHO) [19]). Lymphovascular invasion (LVI), apocrine features, extensive in situ components (EIC), and fibrotic focus (FF) were also evaluated as present or absent as previously reported [20]. Patient details and relevant clinical information, including patients’ age, tumor size, pN stage, pT stage, and patient outcome data (overall survival [OS] and disease‐free survival [DFS]), were retrieved from the medical records. Breast cancers were staged according to tumor, node and metastasis (TNM) staging classification. The treatment of the patients conformed to National Comprehensive Cancer Network guidelines. Breast surgery was performed in all cases. For hormone receptor (HR) positive cases, all received adjuvant endocrine therapy with adjuvant chemotherapy. For HR negative cases, adjuvant chemotherapy was given. Patients were treated with Tamoxifen for systemic hormonal therapy. For adjuvant chemotherapy, the patients were mainly treated with either four cycles of AC (doxorubicin and cyclophosphamide), six cycles of CMF (cyclophosphamide, methotrexate and 5‐fluorouracil), or six cycles of FAC (5‐fluorouracil, doxorubicin, and cyclophosphamide). For HER2 positive (HER2+) cases, because the majority of the cases were collected in pre‐trastuzumab era, only four were treated with the targeted therapy and adjuvant chemotherapy. Overall survival was defined as the time interval from the date of initial diagnosis to the date of breast cancer‐related death. Disease‐free survival was defined as the duration between the date of initial diagnosis and the first detection of breast cancer‐specific relapse or death.
TIL and TLS Evaluation
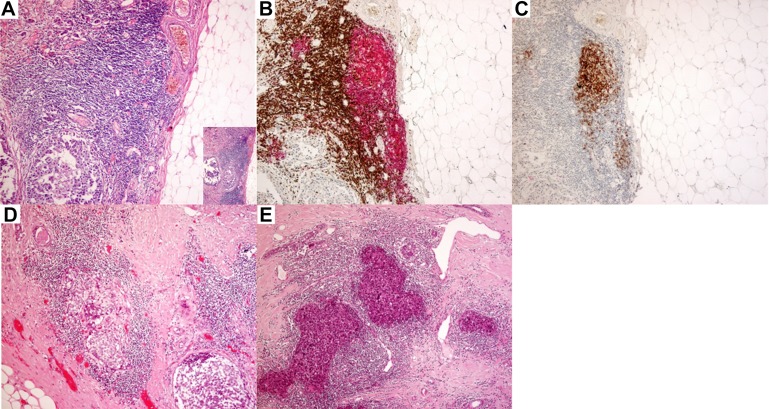
The degree of lymphocytic infiltration was evaluated based on the percentage of tumor stromal area occupied by TIL (International TIL working group) [21]. A cutoff of 20% for TIL, which corresponded to the top 20 percentiles, was applied. Tertiary lymphoid structures within 5 mm from the invasive or in situ tumors were evaluated on whole H&E sections in all available tumor blocks. Tertiary lymphoid structures were identified as highly organized lymphoid aggregates containing vessels that exhibited definite HEV features (plump and cuboidal endothelial cells). In TLS, follicles comprising B lymphocytes and FDC, surrounded by areas of T lymphocytes, dendritic cells, and HEV, could be identified. High endothelial venules possess plump endothelial cells and thin wall (Fig. 1). Tertiary lymphoid structures were confirmed immunohistochemically with cluster of differentiation for cell surface markers (CD) CD3, CD20, and CD23 staining to identify follicles with B lymphocytes (CD20 positive) and FDC (CD23 positive) surrounded by parafollicular zone of T lymphocytes (CD3 positive). In some cases, small tumor cell clusters were observed within the TLS (Fig. 1), and the presence of such tumor clusters was also assessed. The location of these TLS in relation to the IBC was also documented.
Figure 1.
Tertiary lymphoid structures (TLS) shown by hematoxylin and eosin (H&E) staining (insert 40×) (A), with corresponding immunohistochemical staining for CD3 (brown) and CD20 (red) (B), and for CD23 (100×) (C). H&E staining showing tumor cell cluster within TLS (100×) (D, E).
Immunohistochemistry and Scoring
Tumor samples that contained TLS were further assessed by immunohistochemical (IHC) staining using BenchMark XT automated slide‐staining instrument (Ventana Medical Systems, Inc., Tucson, AZ, http://www.ventana.com) with Ultraview Universal DAB Detection Kit. Formalin fixed, paraffin embedded IBC tissue blocks were serially sectioned (4 μm) and stained for CD3 (clone 565), CD20 (clone L26), and CD23 (clone 1B12) after deparaffinization, rehydration, and antigen retrieval. After primary antibody incubation, the sections were incubated with anti‐mouse horseradish peroxidase labeled polymer (Roche, Tucson, AZ, http://www.roche.com) for 30 minutes at room temperature, and then developed with diaminobenzidine. All the slides were counterstained with hematoxylin. The staining for CD3 (T cell), CD20 (B cell), and CD23 (FDC) were all cytoplasmic and membranous. The expression of other biomarkers (estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR), antigen KI‐67 (Ki67), human epidermal growth factor receptor 2 (HER2), Epidermal growth receptor factor (EGFR), cytokeratin 5/6 (CK5/6), cytokeratin 14 (CK14), tumor protein p63 (p63), stem cell growth factor receptor (c‐kit), p‐cadherin, and vimentin) evaluated from tissue microarray (TMA) sections using similar IHC staining protocol were retrieved from our database. Details of the antibodies, antigen retrieval, staining conditions, and scoring are listed in supplemental online Table 1. The tumors were also classified into the five different molecular subtypes using IHC surrogate [22]. The TMA slides were scored for the intensity of staining in the nucleus, cytoplasm, or membrane according to different antibodies by two authors blinded to the clinical information and the staining results of other markers. Any discrepancies were resolved by reviewing at a multi‐head microscope and a consensus was reached.
Statistical Analysis
The findings were analyzed using the statistical software SPSS for Windows, Version 23 (IBM, Armonk, NY, http://www.ibm.com). Chi‐square analysis or Fisher's exact test were used to test for the association of the presence of TLS with tumor grade, FF, LVI, EIC, pN, pT, subtypes, and biomarker expression. Mann‐Whitney U test was used to analyze the differences in patients' age and tumor size with the presence of TLS. Survival data were evaluated with Kaplan‐Meier analysis and Cox regression analysis using the backward Wald method. Statistical significance was established at p < .05.
Results
This cohort included 248 IBC, with 93 (37.5%) having TLS (Fig. 1). The TLS were mainly localized at the peri‐tumoral areas, either at the invasive front or between the in situ and invasive components of the IBC.
Association of TLS with Clinicopathologic Features and Biomarkers
The presence of TLS correlated significantly with higher tumor grade (p < .001), the presence of apocrine feature (p = .001), necrosis (p < .001), LVI (p = .045), EIC (p = .010), and higher TIL (p < .001). There was a trend of association with younger patients’ age (p = .081). There was no correlation with FF, pT stage, pN stage, and tumor size (Table 1). The presence of TLS also varied significantly in different molecular subtypes by IHC surrogates (p < .001). Tertiary lymphoid structures were least common in luminal A cancers (19.7%; 24 out of 113 cases), followed by TNBC (48.9%; 22 out of 45 cases), luminal B cancers (49.1%; 28 out of 57 cases), and were most frequent in HER2‐overexpressing (OE) cancers (56.6%; 17 out of 30 cases; Table 1). For biomarkers, TLS correlated positively with Ki‐67 (p = .021), HER2 (p < .001), c‐kit (p < .001), and p‐cadherin (p < .001) expression, and negatively with ER and PR (p = .001 and p < .001, respectively) expression. A marginal association was found with CK14 (p = .054). There was no correlation with EGFR, AR, CK5/6, p63, and vimentin (Table 2). Multivariate logistic regression analysis showed, among all the correlating factors (with p < .1), high TIL level (OR = 2.650, p < .001), the presence of EIC (OR = 2.712, p = .037), PR status (OR = 0.396, p = .039), and p‐cadherin expression (OR = 2.654, p = .045) were independently associated with TLS (supplemental online Table 2).
Table 1. Correlation of TLS with clinicopathological features.
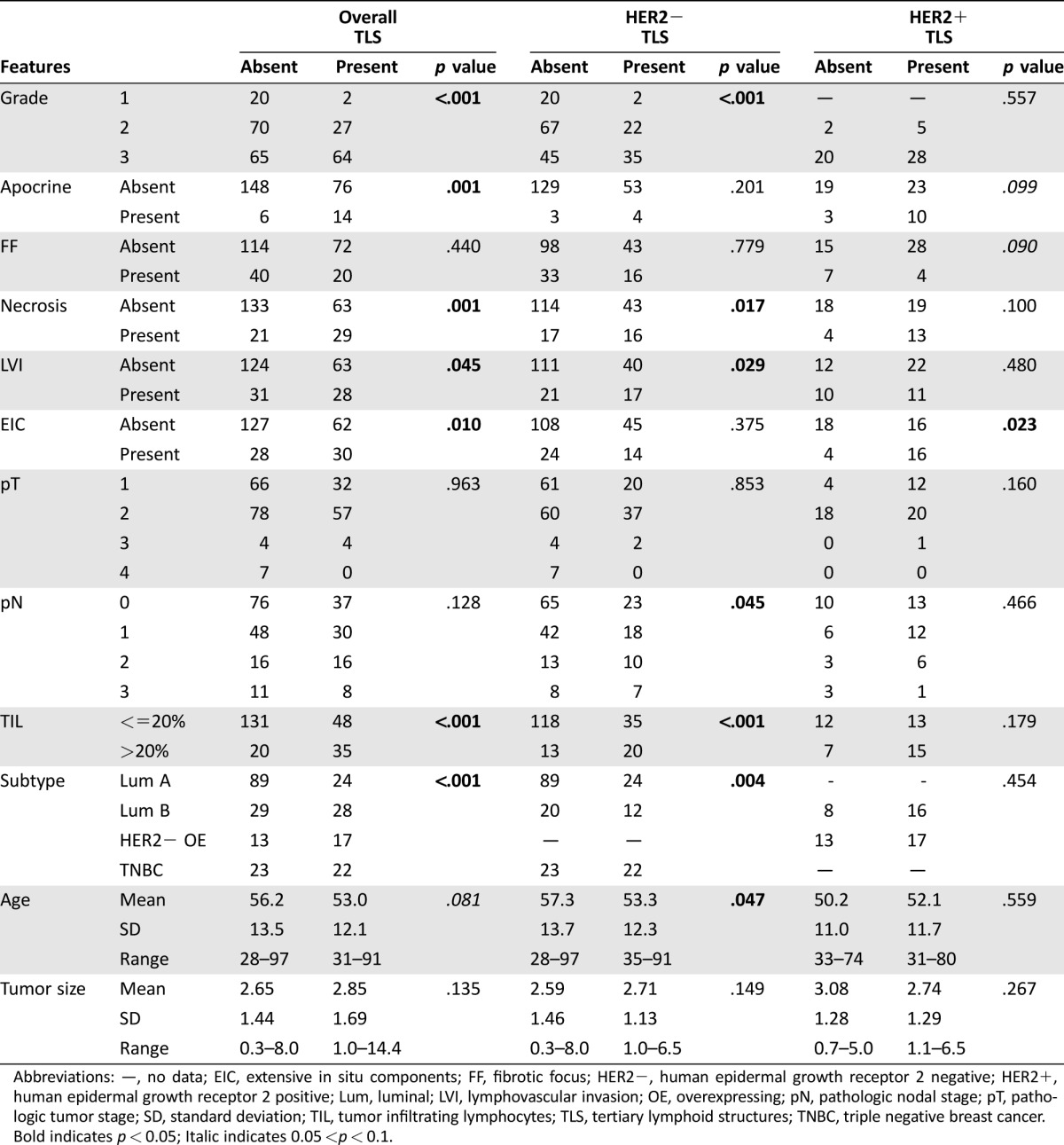
Abbreviations: —, no data; EIC, extensive in situ components; FF, fibrotic focus; HER2−, human epidermal growth receptor 2 negative; HER2+, human epidermal growth receptor 2 positive; Lum, luminal; LVI, lymphovascular invasion; OE, overexpressing; pN, pathologic nodal stage; pT, pathologic tumor stage; SD, standard deviation; TIL, tumor infiltrating lymphocytes; TLS, tertiary lymphoid structures; TNBC, triple negative breast cancer.
Bold indicates p < 0.05; Italic indicates 0.05 <p < 0.1.
Table 2. Correlation of TLS with biomarkers.
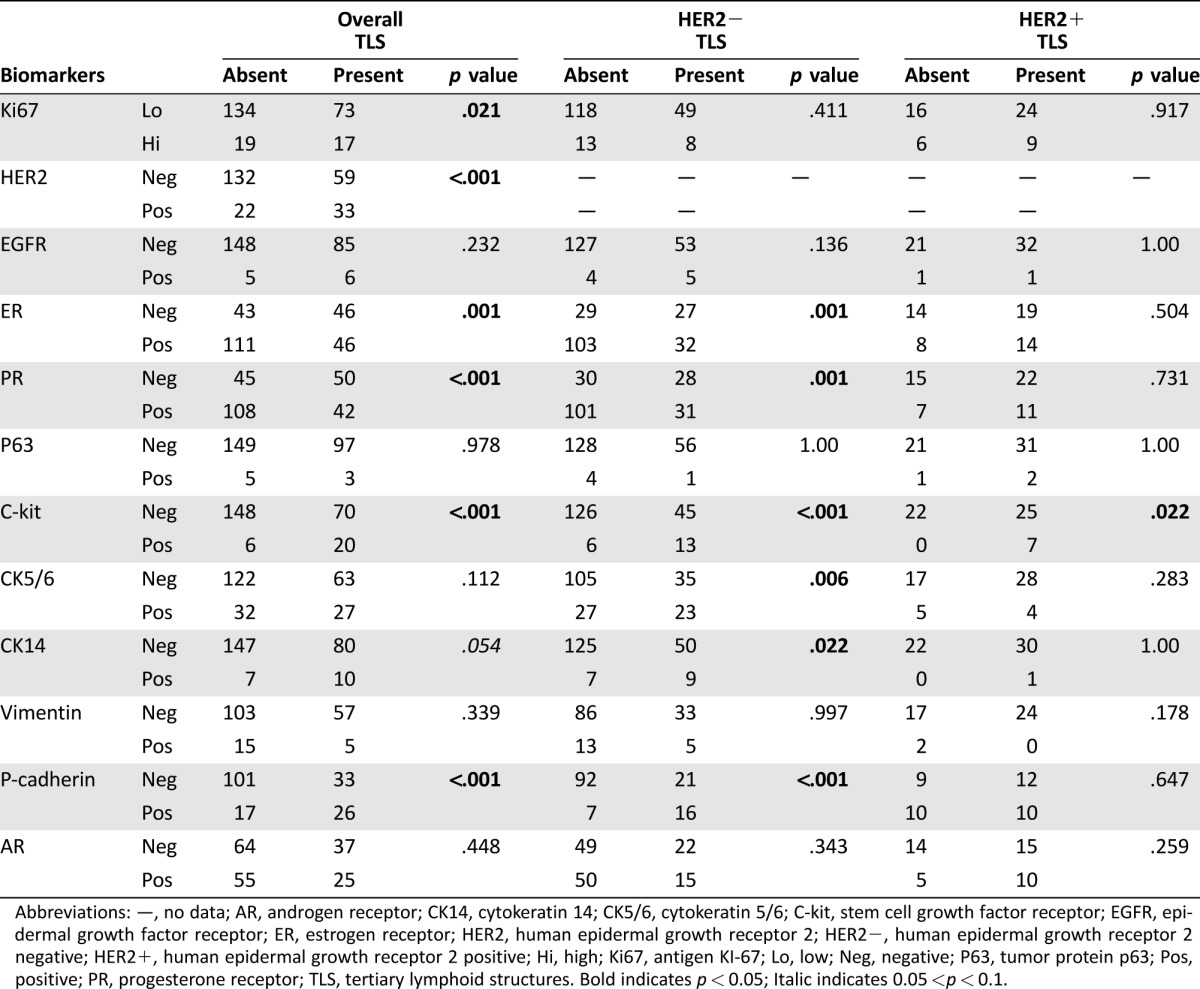
Abbreviations: —, no data; AR, androgen receptor; CK14, cytokeratin 14; CK5/6, cytokeratin 5/6; C‐kit, stem cell growth factor receptor; EGFR, epidermal growth factor receptor; ER, estrogen receptor; HER2, human epidermal growth receptor 2; HER2−, human epidermal growth receptor 2 negative; HER2+, human epidermal growth receptor 2 positive; Hi, high; Ki67, antigen KI‐67; Lo, low; Neg, negative; P63, tumor protein p63; Pos, positive; PR, progesterone receptor; TLS, tertiary lymphoid structures. Bold indicates p < 0.05; Italic indicates 0.05 <p < 0.1.
The association of TLS was further analyzed according to HER2 expression. Tertiary lymphoid structures were found in 59 out of 191 (30.9%) HER2 negative (HER2−) IBC and 33 out of 48 (68.9%) HER2+ IBC. In the HER2− IBC, similar to the entire cohort, TLS were associated with the presence of necrosis (p = .017), higher grade (p < .001), LVI (p = .029), high TIL status (p < .001), and younger age (p = .047; Table 1). Interestingly, there was also significant association with higher pN stage (p = .045; Table 1). For biomarkers, TLS were associated positively with c‐kit (p < .001), CK5/6 (p = .006), CK14 (p = .022), and p‐cadherin (p < .001) expression, and negatively with ER and PR (p = .001 for both) expression (Table 2). In HER2+ IBC, TLS were not associated with any clinicopathologic features and biomarkers, except with EIC (p = .023) and c‐kit (p = .022; Tables 1 and 2).
Association of TLS with Survival
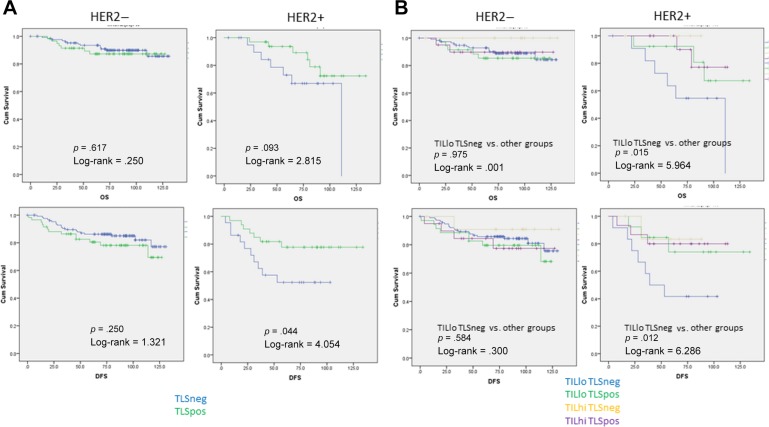
Follow‐up data were available in 245 patients. The mean follow‐up period was 78 months (range, 1–134 months). Fifty cases had breast cancer‐related mortality or relapse. For the entire cohort, neither the presence of TLS nor the level of TIL correlated with patients’ survival (supplemental online Fig. 1). However, in HER2+ IBC, TLS were significantly related to better DFS (log‐rank = 4.054, p = .044), but this was not seen in HER2− IBC. Similar results were observed for OS, with marginal significance (log‐rank = 2.815, p = .093; Fig. 2A). There was a trend of survival advantage for high TIL in HER2+ IBC (data not shown). When the HER2+ IBC were classified by TIL and TLS status (TIL high/low; TLS positive/negative), the worst DFS was observed in the TIL low TLS negative cases (Fig. 2B). In multivariate cox analysis, the combined TLS and TIL status (either with TLS or/and TIL high cases; HR = 0.222, p = .006) together with pN stage (HR = 1.910, p = .027) were independent factors associated with DFS in HER2+ IBC (Table 3).
Figure 2.
Kaplan‐Meier analysis on DFS and OS in HER2+ and HER2− subgroups according to TLS status (A) and combined TIL and TLS status (B).
Abbreviations: DFS, disease‐free survival; HER2−, human epidermal growth receptor 2 negative; HER+, human epidermal growth receptor 2 positive; hi, high; lo, low; neg, negative; OS, overall survival; pos, positive; TIL, tumor infiltrating lymphocytes; TLS, tertiary lymphoid structures.
Table 3. Cox regression in human epidermal growth receptor 2 positive cancers for disease‐free survival (backward Wald method).
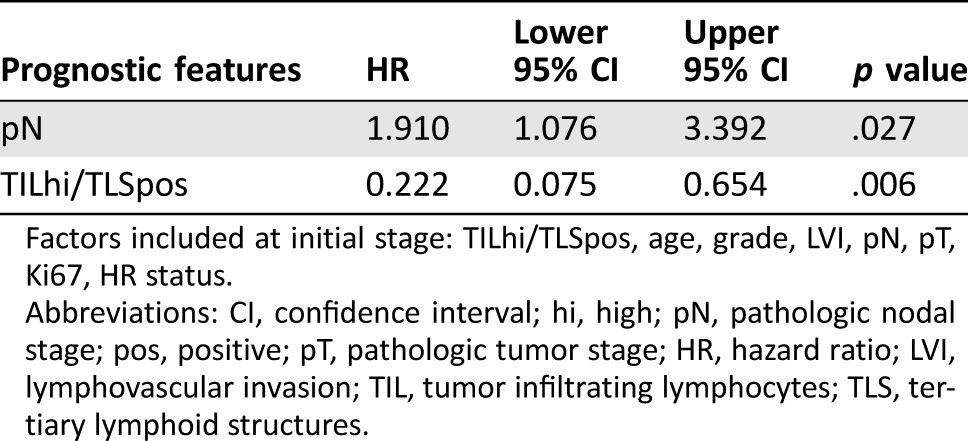
Factors included at initial stage: TILhi/TLSpos, age, grade, LVI, pN, pT, Ki67, HR status.
Abbreviations: CI, confidence interval; hi, high; pN, pathologic nodal stage; pos, positive; pT, pathologic tumor stage; HR, hazard ratio; LVI, lymphovascular invasion; TIL, tumor infiltrating lymphocytes; TLS, tertiary lymphoid structures.
Tumor Cell Infiltration Within TLS
Among 93 TLS positive IBC, tumor cells were found within the TLS in 39 cases (41.9%). In those cases, the presence of tumor cells in TLS was associated with high TIL (p = .045), but not any other clinicopathologic features and biomarkers (supplemental online Table 3). When classified according to HER2 expression, 35.6% (21 out of 59 cases) of HER2− IBC and 47.2% (17 out of 36 cases) of HER2+ IBC showed tumor cell infiltration into the TLS. It is interesting to note that tumor cell infiltration of TLS was associated with the presence of LVI (p = .024) in HER2− IBC, particularly the ER+ HER2− IBC (p = .006). In the ER+ HER2− IBC, tumor cell infiltration also showed an association with increased pN stage (p = .006) and nodal involvement (p = .007; Table 4).
Table 4. Correlation of tumor infiltrated tertiary lymphoid structures according to HER2 subgroups.
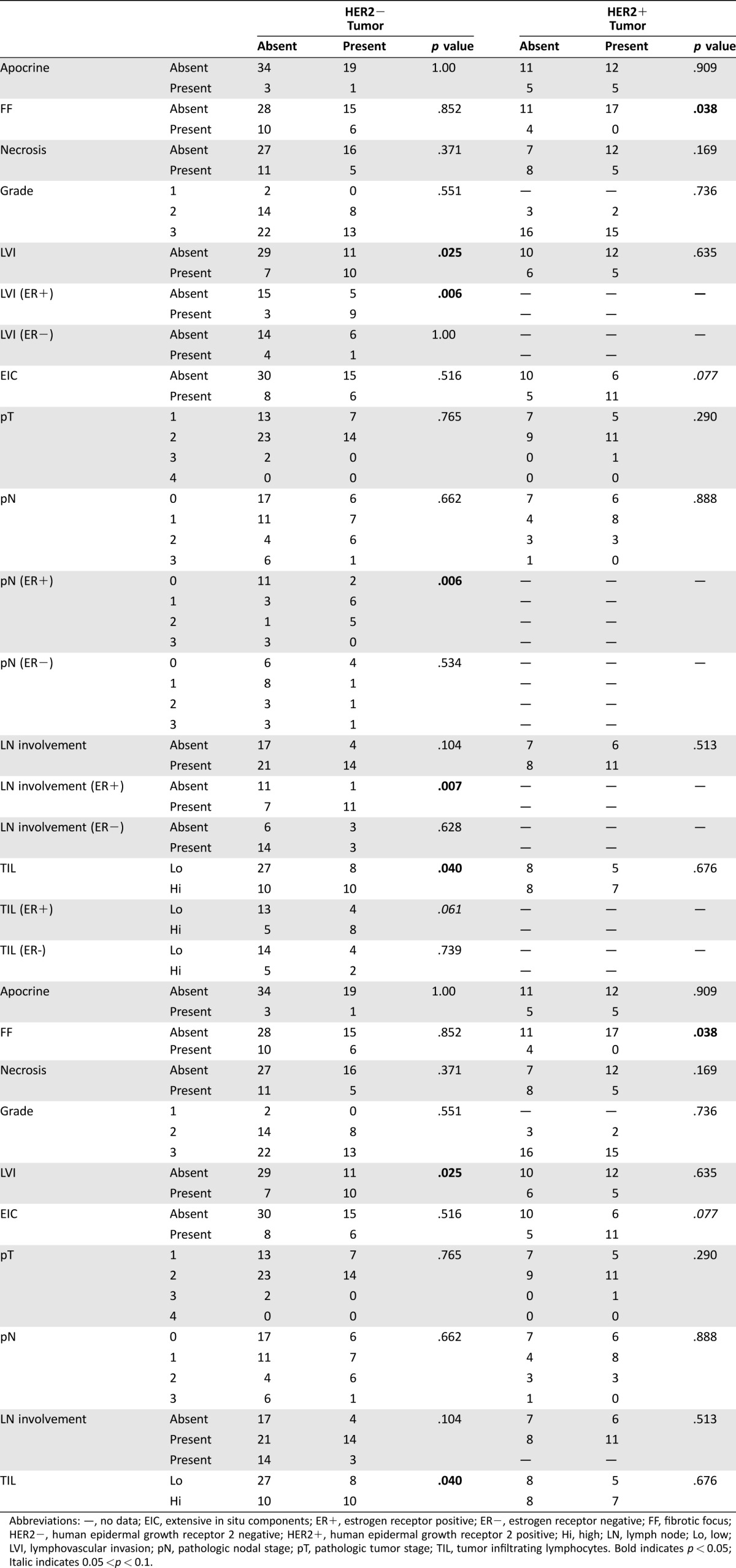
Abbreviations: —, no data; EIC, extensive in situ components; ER+, estrogen receptor positive; ER−, estrogen receptor negative; FF, fibrotic focus; HER2−, human epidermal growth receptor 2 negative; HER2+, human epidermal growth receptor 2 positive; Hi, high; LN, lymph node; Lo, low; LVI, lymphovascular invasion; pN, pathologic nodal stage; pT, pathologic tumor stage; TIL, tumor infiltrating lymphocytes. Bold indicates p < 0.05; Italic indicates 0.05 <p < 0.1.
Discussion
In the current study, the relationship between TLS and various clinicopathologic features in IBC was analyzed. The association of TLS was similar to our previous findings with TIL [23] and from others [15], including an association with higher tumor grade, the presence of necrosis, ER negativity, HER2 positivity, and, not surprisingly, with TIL. Nonetheless, there were additional hitherto unreported TLS‐associated features, namely the presence of EIC. This may be reflected histologically because TLS and components in TLS were more commonly found near the in situ component [12], [14], [24]. There was a reduction of TLS from in situ to invasive components [24]. It is possible that TLS may help to guard against the transition from in situ carcinoma to IBC. The other novel observation is the association of TLS with higher pN and LVI, particularly in HER2− IBC. Interestingly, among HER2− ER+ IBCs, compared with those without infiltration, cases with tumor cell infiltration into TLS were associated with LVI and nodal metastasis. The small invasive tumor cell clusters present inside TLS were similar to those observed in lymph node metastases. Despite the relatively small number of cases involved in the subset, it is tempting to speculate that these tumor cells that penetrated into TLS may metastasize more readily to regional nodes. In fact, the favorable prognostic impact of TLS has not been shown in all studies [12], [25]. Similarly, we have not observed favorable prognosis of TLS in HER2− cases. The relationship of TLS in tumor invasion might account for these findings.
While the prognostic role of TIL has been extensively studied in many (including breast) cancers [2], relatively little has been reported for TLS [12], [13], [14], [15], and these mainly included TLS prognostic features, such as dendriric cell lysosomal associated membrane protein (DC− LAMP)+ DC density [24] and follicular helper T [11]. Some studies evaluated the prognostic value of TLS. However, they were limited to subsets of breast cancers [12], [14]. More comprehensive evaluations of TLS are lacking. In the current study, TLS were associated with significantly better DFS in HER2+ IBC, but not in HER2− IBC. More intriguingly, in the HER2+ IBC, TLS and/or TIL high status was an independent favorable prognostic factor. Our data may indicate the presence of TLS, in addition to TIL, is an important indicator for an active antitumor immune response. Tumoral TLS are surrounded by specific vasculature comprising peripheral lymph node addressin positive blood vessels [26], which may enable direct migration of peripheral blood lymphocytes into TLS. Indeed, naïve T and B cells could be found within TLS in tumors [26]. These cells can escape from the local immunosuppressive influence of tumor milieu, thus promoting more effective antitumor immunity [27]. An active immune response is important for treatment of HER2+ IBC because many of these patients are treated with chemotherapy and/or HER2 targeted therapy; some of the favorable effects were attributed to an active antitumor immunity [5], [12]. In this regard, the presence of TLS could well be an indicator for treatment responses for HER2+ IBC.
The histologic assessment of TLS lacks standardization. The currently recommended TIL assessment for prognostication did not assess areas outside of the tumor borders and around DCIS and normal lobules where TLS were commonly found. In addition, no consensus has been reached for identification and scoring of TLS. Some reports, as in the present study, have detected TLS using a combination of immunohistochemical and histologic methods according to whether TLS has germinal centers and HEV [28]. Others used only surrogate markers [11], [24] or simple morphological criteria [12]. However, their specificity in TLS identification has yet to be examined. Moreover, the areas for evaluated TLS varied. In most studies, the areas for its evaluation and whether the assessment included all tumor slides were not specified. In the current study, we included only cases with all tumor slides available for TLS assessment to minimize the misidentification of TLS positive case as negative. However, there could be some bias in our case selection. Validation of our results with a large cohort is necessary. As assessment of TLS may gain importance, future studies focusing on its standardized evaluation will be needed.
To date, the mechanism governing the induction and maintenance of TLS in tumor microenvironment remains unclear. Our findings indicated that the presence of TLS correlated significantly with higher TIL. Indeed, other studies also reported TIL was the strongest independent factor predicting TLS [12], [14], [15], suggesting high TIL were required for shaping organized immune response. Despite this association, not all high TIL cases showed TLS formation; thus, additional factors could be involved to maintain the high level of organization in TLS. Several reports showed high density of mature FDC associated with TLS structures. Mature FDC may participate in the induction and/or maintenance of HEV in TLS [24]. Furthermore, the expression of lymphoid chemokines (C‐C motif chemokine ligand ((CCL)19, CCL21, and C‐X‐C motif chemokine ligand (CXCL)13) may be essential for TLS formation [11], [29]. The absence of one of these chemokines was sufficient to abrogate the development of TLS in the lungs of mice infected by the influenza virus [30]. These chemokines have also been recognized as both prognostic factors and immunomodulatory therapeutics in the context of cancer. The overexpression of these chemokines has been reported in tumoral TLS [26], [29]. Furthermore, when produced in the tumor microenvironment, these chemokines promoted the recruitment of specialized immune cell subsets in association with TLS formation. However, the precise stimulus initiating the development of TLS in cancers has not been identified. The identification of these factors would be crucial in formulating (and manipulating) antitumor immunity.
Conclusion
Altogether, our results indicated that TLS are present in IBC, and show a relationship with clinicopathologic features and biomarkers similar to TIL. Moreover, the presence of TLS may indicate an active antitumor immune response and favorable patient outcome, particularly for HER2+ IBC patients. Given the significance of TLS in promoting effective antitumor immunity, further understanding in their organization and induction may provide new opportunities to improve the current immunotherapy strategies.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Author Contributions
Conception/design: Gary M. Tse
Provision of study material or patients: Siu Ki Chan, Sai Yin Cheung, Gary M. Tse
Collection and/or assembly of data: Xia Liu, Thazin Hlaing, Yun‐Bi Ni, Siu Ki Chan, Sai Yin Cheung
Data analysis and interpretation: Xia Liu, Julia Y.S. Tsang, Thazin Hlaing, Jintao Hu
Manuscript writing: Xia Liu, Julia Y.S. Tsang, Gary M. Tse
Final approval of manuscript: Xia Liu, Julia Y.S. Tsang, Thazin Hlaing, Jintao Hu, Yun‐Bi Ni, Siu Ki Chan, Sai Yin Cheung, Gary M. Tse
Disclosures
The authors indicated no financial relationships.
References
- 1. Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–322. [DOI] [PubMed] [Google Scholar]
- 2. Giraldo NA, Becht E, Vano Y et al. The immune response in cancer: From immunology to pathology to immunotherapy. Virchows Arch 2015;467:127–135. [DOI] [PubMed] [Google Scholar]
- 3. Adams S, Gray RJ, Demaria S et al. Prognostic value of tumor‐infiltrating lymphocytes in triple‐negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loi S, Sirtaine N, Piette F et al. Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02‐98. J Clin Oncol 2013;31:860–867. [DOI] [PubMed] [Google Scholar]
- 5. Denkert C, von Minckwitz G, Brase JC et al. Tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2‐positive and triple‐negative primary breast cancers. J Clin Oncol 2015;33:983–991. [DOI] [PubMed] [Google Scholar]
- 6. Goc J, Germain C, Vo‐Bourgais TK et al. Dendritic cells in tumor‐associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705–715. [DOI] [PubMed] [Google Scholar]
- 7. Di Caro G, Bergomas F, Grizzi F et al. Occurrence of tertiary lymphoid tissue is associated with T‐cell infiltration and predicts better prognosis in early‐stage colorectal cancers. Clin Cancer Res 2014;20:2147–2158. [DOI] [PubMed] [Google Scholar]
- 8. Dieu‐Nosjean MC, Antoine M, Danel C et al. Long‐term survival for patients with non‐small‐cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008;26:4410–4417. [DOI] [PubMed] [Google Scholar]
- 9. Hiraoka N, Ino Y, Yamazaki‐Itoh R. Tertiary lymphoid organs in cancer tissues. Front Immunol 2016;7:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messina JL, Fenstermacher DA, Eschrich S et al. 12‐Chemokine gene signature identifies lymph node‐like structures in melanoma: Potential for patient selection for immunotherapy? Sci Rep 2012;2:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu‐Trantien C, Loi S, Garaud S et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013;123:2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee HJ, Kim JY, Park IA et al. Prognostic significance of tumor‐infiltrating lymphocytes and the tertiary lymphoid structures in HER2‐positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol 2015;144:278–288. [DOI] [PubMed] [Google Scholar]
- 13. Song IH, Heo SH, Bang WS et al. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple‐negative breast cancer. Cancer Res Treat 2016;49:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee HJ, Park IA, Song IH et al. Tertiary lymphoid structures: Prognostic significance and relationship with tumour‐infiltrating lymphocytes in triple‐negative breast cancer. J Clin Pathol 2016;69:422–430. [DOI] [PubMed] [Google Scholar]
- 15. Figenschau SL, Fismen S, Fenton KA et al. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 2015;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fridman WH, Pages F, Sautes‐Fridman C et al. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- 17. Savas P, Salgado R, Denkert C et al. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol 2016;13:228–241. [DOI] [PubMed] [Google Scholar]
- 18. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long‐term follow‐up. Histopathology 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 19. Lakhani SR, Ellis IO, Schnitee SJ et al. World Health Organisation classification of tumors of the Breast, Fourth Edition. Lyon, France: IARC Press, 2012. [Google Scholar]
- 20. Tsang JY, Ni YB, Chan SK et al. CX3CL1 expression is associated with poor outcome in breast cancer patients. Breast Cancer Res Treat 2013;140:495–504. [DOI] [PubMed] [Google Scholar]
- 21. Salgado R, Denkert C, Demaria S et al. The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsang JY, Hui SW, Ni YB et al. Lymphocytic infiltrate is associated with favorable biomarkers profile in HER2‐overexpressing breast cancers and adverse biomarker profile in ER‐positive breast cancers. Breast Cancer Res Treat 2014;143:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Martinet L, Filleron T, Le Guellec S et al. High endothelial venule blood vessels for tumor‐infiltrating lymphocytes are associated with lymphotoxin beta‐producing dendritic cells in human breast cancer. J Immunol 2013;191:2001–2008. [DOI] [PubMed] [Google Scholar]
- 25. Giraldo NA, Becht E, Pages F et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res 2015;21:3031–3040. [DOI] [PubMed] [Google Scholar]
- 26. de Chaisemartin L, Goc J, Damotte D et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011;71:6391–6399. [DOI] [PubMed] [Google Scholar]
- 27. Dieu‐Nosjean MC, Goc J, Giraldo NA et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014;35:571–580. [DOI] [PubMed] [Google Scholar]
- 28. Hiraoka N, Ino Y, Yamazaki‐Itoh R et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer 2015;112:1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neyt K, Perros F, GeurtsvanKessel CH et al. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 2012;33:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rangel‐Moreno J, Moyron‐Quiroz JE, Hartson L et al. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A 2007;104:10577–10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.